Abstract
Objective
To describe the similarities and differences in the evaluation and treatment of multisystem inflammatory syndrome in children (MIS-C) at hospitals in the US.
Study design
We conducted a cross-sectional survey from June 16 to July 16, 2020, of US children's hospitals regarding protocols for management of patients with MIS-C. Elements included characteristics of the hospital, clinical definition of MIS-C, evaluation, treatment, and follow-up. We summarized key findings and compared results from centers in which >5 patients had been treated vs those in which ≤5 patients had been treated.
Results
In all, 40 centers of varying size and experience with MIS-C participated in this protocol survey. Overall, 21 of 40 centers required only 1 day of fever for MIS-C to be considered. In the evaluation of patients, there was often a tiered approach. Intravenous immunoglobulin was the most widely recommended medication to treat MIS-C (98% of centers). Corticosteroids were listed in 93% of protocols primarily for moderate or severe cases. Aspirin was commonly recommended for mild cases, whereas heparin or low molecular weight heparin were to be used primarily in severe cases. In severe cases, anakinra and vasopressors frequently were recommended; 39 of 40 centers recommended follow-up with cardiology. There were similar findings between centers in which >5 patients vs ≤5 patients had been managed. Supplemental materials containing hospital protocols are provided.
Conclusions
There are many similarities yet key differences between hospital protocols for MIS-C. These findings can help healthcare providers learn from others regarding options for managing MIS-C.
Abbreviations: COVID-19, Coronavirus disease 2019; IVIG, Intravenous immunoglobulin; MIS-C, Multisystem inflammatory syndrome in children; MRI, Magnetic resonance imaging; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
In April 2020, physicians in the United Kingdom and France identified an outbreak of children admitted to the pediatric intensive care unit with a hyperinflammatory condition characterized by fever, cardiovascular shock, and suspected severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: pediatric multisystem inflammatory syndrome – temporally associated with SARS-CoV-2.1, 2, 3 The US Centers for Disease Control and Prevention subsequently released a health advisory in May 2020 for multisystem inflammatory syndrome in children (MIS-C), defining the syndrome as children with fever, laboratory evidence of inflammation, multisystem organ involvement, severe illness, and SARS-CoV-2 infection or exposure.4 Other clinical characteristics include acute myocardial dysfunction, respiratory failure, features of Kawasaki disease, and features of toxic shock syndrome.5 , 6 This rare but life-threatening condition has been reported with increasing frequency in the US, and growing evidence establishes MIS-C as an immune-mediated condition following SARS-CoV-2 infection.7, 8, 9, 10
Given the novelty of this new syndrome, evidence-based guidelines for management of children with MIS-C are lacking. Early reports of MIS-C highlight the variability in the evaluation and management of these patients.2 , 5 , 7 , 8 , 10, 11, 12 The American College of Rheumatology and the American Academy of Pediatrics have released guidance based primarily on expert opinion.13 , 14 In the absence of evidence-based therapies for MIS-C, many centers have created protocols to guide hospital evaluation and management. The purpose of this study is to describe the similarities and differences in the evaluation and treatment of MIS-C at hospitals in the US.
Methods
We conducted a cross-sectional survey of US children's hospitals regarding their protocols for patients with MIS-C. Participants were recruited via e-mails to individuals on pediatric cardiology and infectious diseases list serves and via direct contact to physicians known to be coordinating the MIS-C response at their hospital. The survey was administered from June 16 to July 16, 2020, through the electronic database Research Electronic Data Capture (REDCap) at Children's Healthcare of Atlanta.15 , 16 REDCap is a secure, Web-based software platform designed to support data capture for research studies, providing an intuitive interface for validated data capture; audit trails for tracking data manipulation and export procedures; automated export procedures for seamless data downloads to common statistical packages; and procedures for data integration and interoperability with external sources. No patient data were collected as part of this inquiry, and this study was considered non-human subjects research by the Children's Healthcare of Atlanta institutional review board.
We developed an online questionnaire to learn about the protocol at each center (Appendix 1; available at www.jpeds.com). Elements of the questionnaire included characteristics of the hospital (location, number of pediatric beds, number of patients with MIS-C treated), clinical definition of MIS-C (duration of fever, organ system involvement, evidence of SARS-CoV-2 infection), evaluation (laboratory studies, imaging), treatment (medications and dosages), and follow-up. Participants were invited to share their protocol for inclusion in this publication; Appendix 2 (available at www.jpeds.com) contains the protocols from those who approved publication of the protocols. Participants at centers without a protocol were able to complete the survey but their responses were excluded from the analyses.
We performed descriptive statistics to summarize quantitative elements via SAS 9.4 (SAS Institute, Cary, North Carolina) and Microsoft Excel (Microsoft Corporation, Redmond, Washington). We reviewed the qualitative elements for key themes and summarized the responses as appropriate. We excluded survey responses without sufficient data for analysis. We then performed a subanalysis to compare the quantitative elements comparing the responses of centers in which >5 and ≤5 patients had been treated. In the subanalysis, we conducted χ2 analyses or Fisher exact test where appropriate. For the subanalysis, we performed a sensitivity analysis comparing results for centers in which >10 and ≤10 patients with MIS-C had been treated.
Results
There were 48 surveys completed from participants at 48 unique centers across the US. One record was excluded due to insufficient data submitted, 6 records were excluded because the center did not have a protocol, and 1 record was removed after submission at the request of the contributing center. Thus, survey responses from 40 centers were available for analysis (Figure 1 ). Protocols from 32 centers were submitted with the survey and approved for publication in Appendix 2.
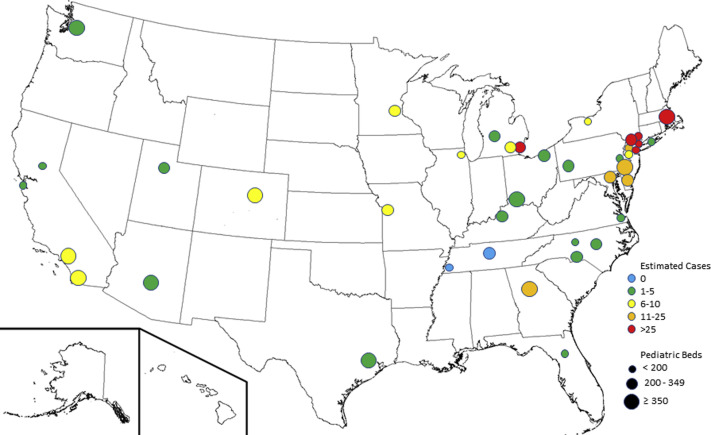
Figure 1.
Individuals from 40 children's hospitals in the US with protocols for the evaluation and management of MIS-C responded to the survey. The hospitals varied in both size (by number of pediatric hospital beds) and experience in treating patients with MIS-C.
Hospital Characteristics
Participating centers varied in size: 15 small pediatric centers (<200 pediatric beds), 15 medium centers (200-<350 pediatric beds), and 10 large centers (≥350 pediatric beds). Experience with treating MIS-C differed between centers: 2 centers had treated 0 patients with MIS-C; 18 centers, 1-5 patients; 9 centers, 6-10 patients; 5 centers, 11-25 patients; and 6 centers, >25 patients. Of the 40 protocols, 21 had been revised since inception.
Definition of MIS-C
All respondents indicated that fever is required as part of the definition of MIS-C; however, the required duration and degree of fever varied. Overall, 21 of the 40 centers required only 1 day of fever, 2 centers required at least 2 days, 15 centers required at least 3 days, and 2 centers required at least 4 days of fever. Of the 22 centers that specified a minimum temperature for fever, 20 set 38.0°C as the minimum. Almost all (38/40) centers specified the presence of certain organ system involvement; of these, 3 required only 1 organ system, 31 required at least 2 organ systems, and 4 required at least 3 organ systems involved. In 36 of the 40 protocols, abnormal laboratory markers of inflammation were required to meet MIS-C inclusion criteria; 31 of 40 centers did not require laboratory evidence of current or previous SARS-CoV-2 infection. Instead, previous exposure to someone with coronavirus disease 2019 (COVID-19) in the 4 weeks preceding the onset of symptoms sufficed to meet inclusion criteria. Three centers commented that, given the high prevalence of COVID-19 in their community, the requirement of a known exposure was waived as all children are assumed to have had previous exposure to someone with COVID-19 in the preceding 4 weeks. One center commented that the working definition for MIS-C was too broad, often resulting in unnecessary testing and, in at least one case, delayed diagnosis of perforated appendicitis.
Evaluation of MIS-C
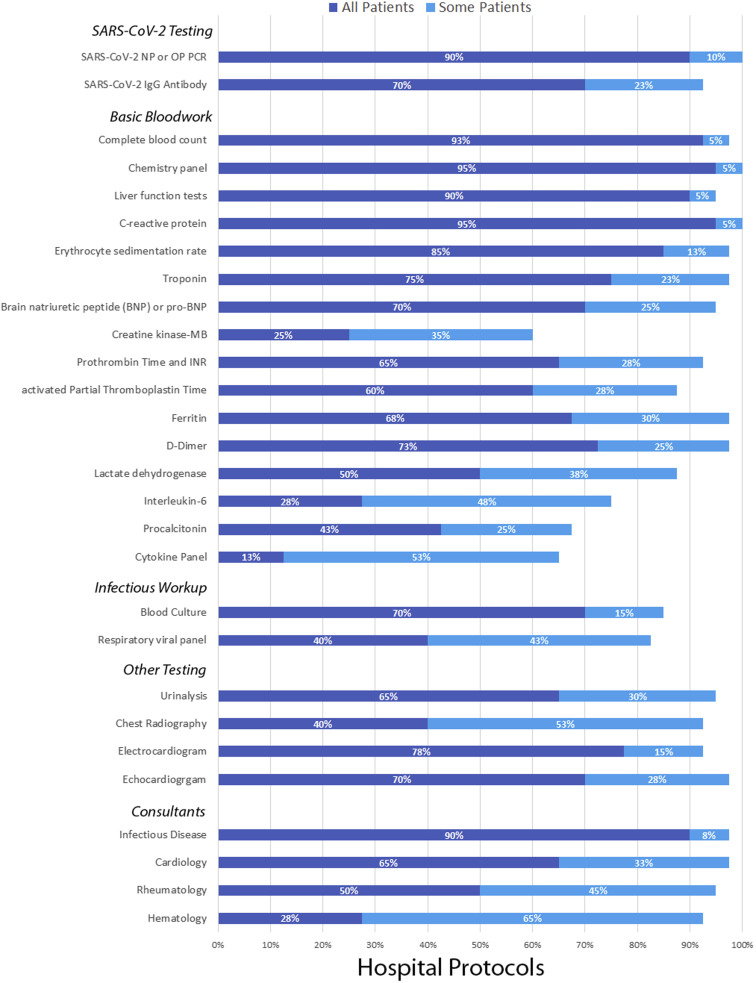
In the evaluation of patients with possible MIS-C, there often was a tiered approach, with some centers recommending performing initial laboratory tests on all patients and then further tests only on patients with high suspicion of MIS-C or with relevant symptoms (Figure 2 ). For the identification of SARS-CoV-2, all centers performed polymerase chain reaction testing from a nasopharyngeal or oropharyngeal sample. Most centers also tested for SARS-CoV-2 antibody in all possible patients with MIS-C. Routine bloodwork included complete blood count, basic metabolic panel, hepatic panel, C-reactive protein, and erythrocyte sedimentation rate. Further bloodwork including investigation for inflammation, cardiac involvement, and abnormal anticoagulation were often recommended. Further recommended testing was common and included electrocardiogram, echocardiogram, urinalysis, and chest radiograph. Pursuit of evidence of potential alternative causes or co-infection was routinely recommended, eg, by blood culture or respiratory viral panel testing. For admitted patients, it was recommended almost always that infectious diseases be consulted, followed by cardiology, rheumatology, and hematology.
Figure 2.
The protocols for evaluation of MIS-C varied between centers for SARS-CoV-2 testing, basic bloodwork, infectious diseases evaluations, ancillary testing, and consultant services. Some protocols included certain aspects for all patients with potential MIS-C, whereas others performed portions for only some patients.
Treatment of MIS-C
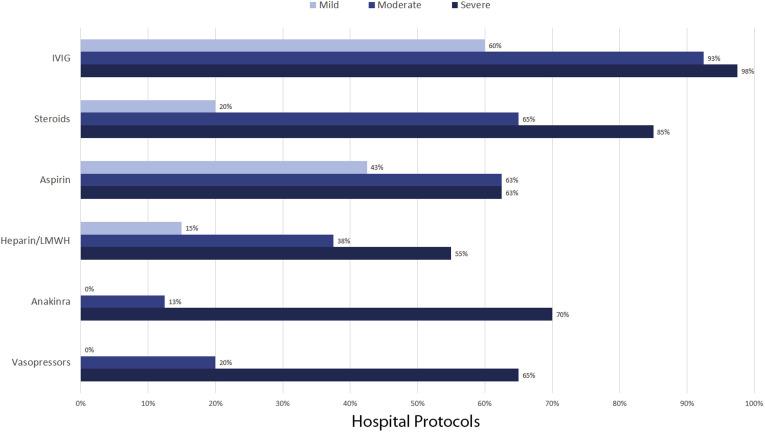
Some centers had a similar treatment approach for all patients, whereas others varied the approach by severity of illness (Figure 3 ). Severity of illness was defined specifically at each center, with no uniform definition. Submitted criteria for severity of illness included vasoactive-inotropic score, location in the hospital (intensive care unit vs general medical unit), degree of hyperinflammation, and presence of shock or cardiac involvement.
Figure 3.
Medical management of MIS-C often varied by severity, with severity being defined differently by each center. For centers that recommended IVIG, 54% recommended a second dose for patients who were refractory to the first dose. Medications used by ≤20 of the 40 centers are not shown. LMWH, low molecular weight heparin.
Intravenous immunoglobulin (IVIG) was the most widely used medication to treat MIS-C, with 98% of centers including IVIG in their recommendations and 60% recommending the use of IVIG regardless of severity. Of the 39 protocols that mentioned any use of IVIG, 21 recommended a second dose of IVIG for cases that were refractory to the first dose. Corticosteroids were listed in 93% of protocols, although corticosteroid therapy tended to be reserved primarily for moderate or severe cases. Aspirin was commonly included in protocols, even for mild cases, whereas heparin or low molecular weight heparin were used primarily in severe cases. In severe cases, anakinra and vasopressors were recommended frequently. Other medications that were recommended in fewer than 25 of the 40 protocols included clopidogrel (3 centers), warfarin (3 centers), remdesivir (10 centers), and tocilizumab or infliximab (13 centers); these medications were reserved primarily for severe or refractory cases. Hydroxychloroquine was not recommended in any of the protocols included in the study.
Follow-up of Patients with MIS-C
Although there was no standardized follow-up plan for patients with MIS-C, 26 participants responded that their protocol recommends follow-up similar to that of the American Heart Associations for Kawasaki disease. Nearly all centers (39/40) recommended follow-up with cardiology, but they differed as to the timing and performance of echocardiogram. Seven centers arranged follow-up in 1 week, 22 centers planned for 2 weeks, and 9 centers for 1 month (with 1 participant not providing a time of follow-up). Almost all centers (36/40) included aspirin as a discharge medication, with 26 centers including aspirin regardless of degree or presence of coronary involvement. Use of cardiac magnetic resonance imaging (MRI) was mentioned in protocols of 22 centers, primarily for evaluation and follow-up of cardiac dysfunction. Protocols at 2 centers included MRI during the initial inpatient hospitalization, 9 centers recommended MRI during outpatient follow-up (6 at 1-3 months, 3 at 3-6 months), and 10 centers deferred to cardiologist's opinion regarding when to obtain MRI. Other common specialty follow-up visits included rheumatology by protocols at 24 centers, infectious diseases at 20, and hematology at 8 centers.
Subanalysis
In the subanalysis, there were similar findings among almost all components of the protocols for evaluation and management of MIS-C for centers in which >5 patients compared with ≤5 patients had been treated (Table ). The only significant difference was that centers in which >5 patients had been treated were more likely to arrange follow-up in infectious diseases clinic (70% vs 30%). In the sensitivity analysis comparing findings for those centers in which >10 patients with MIS-C vs ≤10 patients had been treated, there were likewise similar results (data not shown); the only significant difference was that for centers in which >10 patients had been treated anakinra was less likely to be included in their protocols (36% vs 83%, P = .008).
Table.
Comparison of protocol recommendations for the evaluation and management of patients with MIS-C based on center experience
| Protocol components | Centers in which ≤5 patients with MIS-C treated (n = 20) | Centers in which >5 patients with MIS-C treated (n = 20) | P value |
|---|---|---|---|
| Definition of MIS-C | |||
| Only 1 d of fever | 10 (50%) | 11 (55%) | 1.00 |
| At least 2 organ systems involved | 17 (85%) | 14 (70%) | .45 |
| Require laboratory markers of inflammation | 18 (90%) | 18 (90%) | 1.00 |
| Evaluation of patients with MIS-C (for either some or all patients) | |||
| SARS-CoV-2 testing | |||
| SARS-CoV-2 nasopharyngeal or oropharyngeal PCR | 20 (100%) | 20 (100%) | 1.00 |
| SARS-CoV-2 IgG antibody | 19 (95%) | 18 (90%) | 1.00 |
| Basic bloodwork | |||
| Complete blood count | 19 (95%) | 20 (100%) | 1.00 |
| Chemistry panel | 20 (100%) | 20 (100%) | 1.00 |
| Hepatic screening | 19 (95%) | 19 (95%) | 1.00 |
| C-reactive protein level | 20 (100%) | 20 (100%) | 1.00 |
| Erythrocyte sedimentation rate | 20 (100%) | 19 (95%) | 1.00 |
| Troponin level | 19 (95%) | 20 (100%) | 1.00 |
| BNP or pro-BNP level | 18 (90%) | 20 (100%) | .49 |
| Creatine kinase-MB level | 11 (55%) | 13 (65%) | .52 |
| Prothrombin time and INR | 18 (90%) | 19 (95%) | 1.00 |
| Activated partial thromboplastin time | 17 (85%) | 18 (90%) | 1.00 |
| Ferritin level | 19 (95%) | 20 (100%) | 1.00 |
| D-dimer level | 19 (95%) | 20 (100%) | 1.00 |
| Lactate dehydrogenase level | 17 (85%) | 18 (90%) | 1.00 |
| Interleukin-6 level | 14 (70%) | 16 (80%) | .47 |
| Procalcitonin level | 15 (75%) | 12 (60%) | .31 |
| Cytokine panel | 14 (70%) | 12 (60%) | .51 |
| Infectious diseases workup | |||
| Blood culture | 16 (80%) | 18 (90%) | .66 |
| Respiratory virus panel PCR | 15 (75%) | 18 (90%) | .41 |
| Other testing | |||
| Urinalysis | 18 (90%) | 20 (100%) | .49 |
| Chest radiography | 19 (95%) | 18 (90%) | 1.00 |
| Electrocardiogram | 19 (95%) | 18 (90%) | 1.00 |
| Echocardiogram | 20 (100%) | 19 (95%) | 1.00 |
| Consultants | |||
| Infectious diseases | 19 (95%) | 20 (100%) | 1.00 |
| Cardiology | 19 (95%) | 20 (100%) | 1.00 |
| Rheumatology | 18 (90%) | 20 (100%) | .49 |
| Hematology | 19 (95%) | 18 (90%) | 1.00 |
| Medical management of patients with MIS-C (for at least 1 type of severity) | |||
| IVIG | 20 (100%) | 19 (95%) | 1.00 |
| Corticosteroids | 19 (95%) | 18 (90%) | 1.00 |
| Aspirin | 14 (70%) | 14 (70%) | 1.00 |
| Heparin/LMWH | 12 (60%) | 13 (65%) | .74 |
| Anakinra | 16 (80%) | 12 (60%) | .17 |
| Vasopressor agents | 13 (65%) | 13 (65%) | 1.00 |
| Follow-up of patients with MIS-C | |||
| In accordance with American Heart Association Kawasaki guidelines | 13 (65%) | 13 (65%) | 1.00 |
| Aspirin | 16 (80%) | 20 (100%) | .11 |
| Subspecialty follow-up | |||
| Cardiology | 19 (95%) | 20 (100%) | 1.00 |
| Infectious disease | 6 (30%) | 14 (70%) | .01 |
| Rheumatology | 13 (65%) | 11 (55%) | .52 |
| Hematology | 5 (25%) | 3 (15%) | .69 |
BNP, Brain natriuretic peptide; INR, international normalized ratio; LMWH, low molecular weight heparin; PCR, polymerase chain reaction.
Bold indicates P < .05.
Discussion
This survey of the protocols for the evaluation and treatment of MIS-C in US children's hospitals highlights major similarities and differences among centers. These findings can inform centers considering creation or modification of MIS-C protocols. Protocols of most centers adhered to the MIS-C definition put forth by Centers for Disease Control and Prevention in May 2020. However, some protocols require 3 days of fever instead of 1, and protocols of centers in areas with high prevalence of COVID-19 do not require positive SARS-CoV-2 test results or a known exposure to someone with the disease. In the evaluation of patients for MIS-C, most protocols begin with a tiered approach that is standard for the workup of a febrile illness, with further testing often dictated by symptoms or initial laboratory test results. The findings from this survey underscore the collaborative effort to optimally manage MIS-C, as most protocols recommend consultation of multiple subspecialists. IVIG is a mainstay of treatment at most centers, with corticosteroids, aspirin, and heparin often used as well. Anakinra and vasopressor agents are used frequently in children with severe illness. Almost all children are recommended at discharge to receive low-dose aspirin and follow-up with cardiology.
Many of the elements of the protocols for MIS-C are similar to those for Kawasaki disease.2 , 5 , 7 , 8 As cases of MIS-C were emerging, the patients were noted to have some clinical signs and symptoms overlapping with Kawasaki disease, left ventricular systolic dysfunction as seen in Kawasaki disease shock syndrome, and occasional coronary dilation. The current American Heart Association Kawasaki disease guidelines recommend 2 g/kg IVIG after diagnosis and consideration of a 2- to 3-week course of tapering corticosteroids for patients at high risk for coronary artery aneurysms. For Kawasaki disease, administration of a second dose of IVIG, high-dose intravenous methylprednisolone, and other immunomodulatory agents are considered if the patient continues to be febrile 36 hours after completion of the initial dose of IVIG. Low-dose aspirin is recommended until 4-6 weeks after onset of illness and normal follow-up echocardiogram, and systemic anticoagulation with low molecular weight heparin or warfarin is recommended for rapidly progressing coronary aneurysms or those with z score >10.17 Our survey revealed that treatment for MIS-C among US children's hospitals roughly correlated with these Kawasaki disease recommendations. A large diversion from the Kawasaki disease guidelines was the inclusion of systemic anticoagulation in some MIS-C protocols. This choice was potentially made due to elevated d-dimers, frequent deep venous thromboses and pulmonary emboli seen in acutely ill adults with COVID-19, and a small number of reported MIS-C cases with thrombosis.7 , 18 The current choice of therapeutic agents appear reasonable, as many patients have recovery of left ventricular systolic function at the time of discharge.3 , 5 Until long-term data are obtained, it is likely reasonable to continue low-dose aspirin in the acute 4- to 6-week period as in Kawasaki disease. However, this approach is not without risk given the concern for Reye syndrome, and the benefit in MIS-C may be less than that in Kawasaki disease as patients with MIS-C are less likely to have elevated platelet counts or coronary artery involvement.7 , 8 , 11 , 19
Optimal evaluation and management of MIS-C clearly is evolving, as evidenced by more than one-half of the centers modifying their protocols rapidly following inception. This iterative process has similarly been seen in the management of adults with COVID-19. For instance, recent data from the RECOVERY (Randomised Evaluation of COVID-19 Therapy) trial indicate that dexamethasone may improve mortality in hospitalized adults with severe COVID-19 illness.20 It remains to be seen whether such treatment would be useful in children. We anticipate frequent revisions to hospital protocols as evidence regarding SARS-CoV-2 and MIS-C management emerges.
These findings have important implications during the current pandemic. In the US, cases of COVID-19 continue to rise, especially among the younger age group.21 As a result, we anticipate that cases of MIS-C will increase as well. The findings of this survey can help hospitals with little or no experience to date with these patients to better prepare for their evaluation and management. Around the world, the COVID-19 pandemic continues in many countries. In some developing nations, certain treatment options such as IVIG are not readily available. These survey results can help identify other potential options in resource-limited settings.
Our study has several limitations. First, we did not provide a definition for severity of illness, and determination of severity may differ between institutions. Therefore, what may be considered a moderate case at one center may be severe at another; such differences should be considered when interpreting the recommended treatment options. Second, there was a wide variation in experience in managing MIS-C; there were 6 centers with experience treating >25 patients, and 2 centers with no experience treating patients with MIS-C. Thus, some protocols may be based on experience whereas others may be based on personal opinion. We attempted to overcome this limitation by comparing the protocols at centers with more and less experience. Finally, it is important to recognize that this study captures what centers have recommended for the evaluation and management of MIS-C at their institution, not what has actually been done for those patients. Furthermore, consensus on use or non-use of evaluation or management tools is not equivalent to optimal practice. Indeed, protocols may serve as a framework for management of patients with MIS-C, but care should be individualized as dictated by patient signs, symptoms, response to treatment, and evolving medical evidence.
Acknowledgments
We thank the additional individuals who contributed data to this survey, including Eva Cheung, MD (NewYork-Presbyterian Morgan Stanley Children's Hospital of Columbia Irving Medical Center); Lauren Henderson, MD, MMSc (Boston Children's Hospital); Whitnee J. Hogan, MD (UCSF Benioff Children's Hospital); Sean Lang, MD (Cincinnati Children's Hospital Medical Center); Jennifer Schuster, MD, MSCI (Children's Mercy Kansas City); Renata Shih, MD (Congenital Heart Center, University of Florida); Dongngan T. Truong, MD, MS (University of Utah/Primary Children's Hospital); Rajiv Verma, MD (Children's Hospital of New Jersey); and Justin P. Zachariah, MD, MPH (Texas Children's Hospital, Baylor College of Medicine). We also thank the multidisciplinary teams that helped to create MIS-C protocols at the children's hospitals in this study, without whose collaborative efforts this study would not have been possible.
Footnotes
The authors declare no conflicts of interest.
Portions of this study were presented during the Centers for Disease Control Communication Outreach and Communication Activity (CDC COCA), July 16, 2020, virtual meeting (based out of Atlanta, Georgia).
Appendix
Survey for the treatment of multisystem inflammatory syndrome in children
Submitted hospital-based protocols for the treatment of multisystem inflammatory syndrome in children
References
- 1.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimaud M., Starck J., Levy M., Marais C., Chareyre J., Khraiche D. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020;10:69. doi: 10.1186/s13613-020-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19): US Centers for Disease Control and Prevention; 2020. https://emergency.cdc.gov/han/2020/han00432.asp Accessed August 31, 2020.
- 5.Belhadjer Z., Meot M., Bajolle F., Khraiche D., Legendre A., Abakka S. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 6.Godfred-Cato S., Bryant B., Leung J., Oster M.E., Conklin L., Abrams J. COVID-19-associated multisystem inflammatory syndrome in children—United States, March-July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dufort E.M., Koumans E.H., Chow E.J., Rosenthal E.M., Muse A., Rowlands J. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung E.W., Zachariah P., Gorelik M., Boneparth A., Kernie S.G., Orange J.S. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020;324:294–296. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belot A., Antona D., Renolleau S., Javouhey E., Hentgen V., Angoulvant F. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.22.2001010. 2001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toubiana J., Poirault C., Corsia A., Bajolle F., Fourgeaud J., Angoulvant F. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pediatrics AAo Multisystem Inflammatory Syndrome in Children (MIS-C) Interim Guidance. https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/multisystem-inflammatory-syndrome-in-children-mis-c-interim-guidance/ Accessed August 31, 2020.
- 14.Henderson L.A., Canna S.W., Friedman K.G., Gorelik M., Lapidus S.K., Bassiri H. American College of Rheumatology Clinical Guidance for Pediatric Patients with Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with SARS-CoV-2 and Hyperinflammation in COVID-19. Version 1. Arthritis Rheumatol. 2020 doi: 10.1002/art.41454. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O'Neal L. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCrindle B.W., Rowley A.H., Newburger J.W., Burns J.C., Bolger A.F., Gewitz M. Diagnosis, treatment, and long-term management of Kawasaki Disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 18.Nahum J., Morichau-Beauchant T., Daviaud F., Echegut P., Fichet J., Maillet J.M. Venous thrombosis among critically ill patients with coronavirus disease 2019 (COVID-19) JAMA Netw Open. 2020;3:e2010478. doi: 10.1001/jamanetworkopen.2020.10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi M., Mason W. Kawasaki syndrome, Reye syndrome, and aspirin. Pediatrics. 1986;77:616–617. [PubMed] [Google Scholar]
- 20.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.COVIDView: A Weekly Surveillance Summary of US COVID-19 Activity: US Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html Accessed August 31, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survey for the treatment of multisystem inflammatory syndrome in children
Submitted hospital-based protocols for the treatment of multisystem inflammatory syndrome in children