Abstract
Because of the Coronavirus Disease 2019 (COVID-19) pandemic, we were forced to cancel scheduled visits for nearly 150 patients followed in our heart failure (HF) outpatient clinic. Therefore, we structured a telephone follow-up, developing a standardized 23-item questionnaire from which we obtained the Covid-19-HF score. The questionnaire was built to reproduce our usual clinical evaluation investigating a patient's social and functional condition, mood, adherence to pharmacological and nonpharmacological recommendations, clinical and hemodynamic status, pharmacological treatment, and need to contact emergency services. The score was used as a clinical tool to define patients' clinical stability and timing of the following telephone contact on the basis of the assignment to progressively increasing risk score groups: green (0–3), yellow (4–8), and red (≥9).
Here we present our experience applying the score in the first 30 patients who completed the 4-week follow-up, describing baseline clinical characteristics and events that occurred in the period of observation.
Keywords: Chronic heart failure, outpatient management, Covid-19, telehealth, phone support, score
By the end of 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) produced a rapidly expanding epidemic in Wuhan, China.1 The World Health Organization termed this illness Coronavirus Disease 2019 (COVID-19). Because of the numerous cases that occurred in Italy, on March 9, 2020, the Italian government imposed a total lockdown and social isolation. After this decision, physicians were forced to review their outpatient activity, limiting ambulatory visits to exceptional cases, trying to reduce the risk derived from interpersonal contact, especially in older and frail patients. Telehealth has therefore gained greater prominence in the management of chronic conditions, with the purpose to delivery care remotely. Randomized clinical trials on telehealth interventions for chronic heart failure (HF) suggested that either tele-monitoring,2, 3, 4 or telephone support by structured questionnaires5, 6, 7, 8, 9 did not reduce hospital readmissions or mortality. More recently, however, a pilot study addressing the potential of audio/video interaction (known as Virtual Visits, VVs) compared with in-person visits in post-discharge care of HF showed promising results, and other studies reported improved symptom control and quality of life with tele-monitoring of patients with HF.10, 11, 12 Due to overall weak evidence, the European Society of Cardiology guidelines do not recommend any specific telehealth instrument in the management of HF.13 Nevertheless, as the exceptional COVID-19 pandemic briskly led to reduced clinical control, we developed a standardized questionnaire suitable for telephone administration to older patients with HF and/or caregivers.
Transition From a HF Outpatient Clinic to a Structured Telephone Follow-up
Since 2017, we have structured a multidisciplinary HF clinic as a hospital-based outpatient unit in a tertiary-level academic hospital, managed by a team of cardiologists, geriatricians, and HF-trained nurses. The main mission of this unit is to assist patients within 2 weeks of hospital discharge after acute HF, through follow-up programs tailored individually to severity of disease and clinical stability.
Due to the emergent COVID-19 pandemic, several “nonurgent” outpatient services like ours were suddenly closed. We were taking care of almost 150 patients with follow-up visits already scheduled over the following weeks.
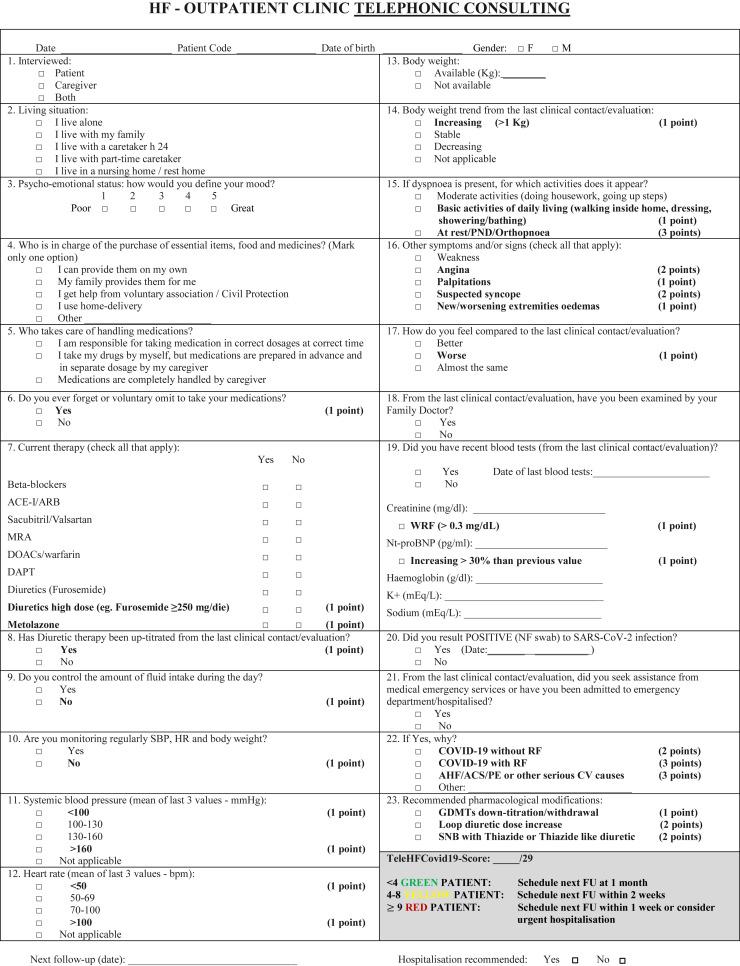
Here we present the development and implementation of a standardized questionnaire, currently administered to our HF patients and/or caregivers during scheduled telephone contacts to evaluate clinical stability and to remotely assist patients. An English version of the questionnaire is shown in Figure 1 . In accordance with current Italian privacy laws, the questionnaire was anonymized, and patients were identified by a numeric code, date of birth, and gender. The 23-item questionnaire was designed for rapid administration during telephone interview, with a median call duration of 6 minutes. It was designed to investigate 7 domains: (1) social and functional condition; (2) mood; (3) adherence to pharmacological and nonpharmacological recommendations (blood pressure, heart rate, weight monitoring and fluid intake control); (4) clinical and hemodynamic status; (5) recording of laboratory tests; (6) current pharmacological treatment; (7) recent evaluation by family physician or need to contact emergency services followed or not by hospitalization, and reasons for these medical contacts. General and pharmacological recommendations as well as the following telephone contact were finally recorded.
Fig. 1.
COVID-19-HF outpatient follow-up questionnaire. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; GDMTs, guidelines directed medical treatments; MRA, mineralocorticoid receptor antagonist; RI respiratory insufficiency; SBP, systolic blood pressure; SNB, sequential nephron blockade; WRF, worsening renal function. A downloadable PDF of this form is available at www.sciencedirect.com.
We decided to evaluate mood using a numerical scale. This approach was chosen instead of applying more complex and validated questionnaires14 , 15 to spare overall time of interview. Similarly, we limited functional status assessment to 2 questions regarding the need of assistance to purchase food and medicines and needed help with therapy, which are fundamental aspects of basic care. Adherence to pharmacotherapy was evaluated by using 1 single question instead of more complex and time-consuming validated tools.16 Current pharmacotherapy (guideline-directed medical therapies [GDMTs] and the need of high-dose loop diuretics, such as furosemide ≥250 mg daily, alone or in combination with metolazone), as well as the need of incremental dose since previous contact were also recorded. Then, we investigated the adherence to prior patient education of self-monitored parameters and nonpharmacological recommendations on which all of our patients have been trained. We further investigated the clinical status by evaluating the presence/severity of dyspnea and association with other signs/symptoms (asthenia, angina, palpitations, unexplained fall/syncope, new/worsening extremity edemas), together with patient's perception of his or her clinical status compared with the previous week. Finally, patients or caregivers were encouraged to e-mail us the results of blood tests commonly used in the follow-up of HF outpatients and significant variations in N-terminal pro B-type natriuretic peptide and creatinine were recorded. Finally, recommendations to increase diuretic therapy or need for withdraw or down-titrate GDMTs were also documented.
To determine the timing of the next telephonic evaluation, we decided to weight questions regarding clinical and hemodynamic status, adherence to pharmacological and nonpharmacological recommendations, therapeutic changes, and need for hospitalization by scoring the answers (from 1 to 3) to build a score. This was done on clinical judgment and review of current HF literature, after collegial discussion and final agreement of all authors. The sum of individual scores represented the novel TeleHFCovid19-score, ranging from 0 to 29. Based on such score, 3 groups of patients were identified by arbitrary cutoff levels (Figure 1): the green (score <4), the yellow (score 4–8), and the red (score ≥9) group, for which next telephonic evaluation was planned respectively after 4, 2, and 1 week, respectively. Alternatively, the red group could receive recommendation for urgent hospital evaluation.
Implementation Evaluation
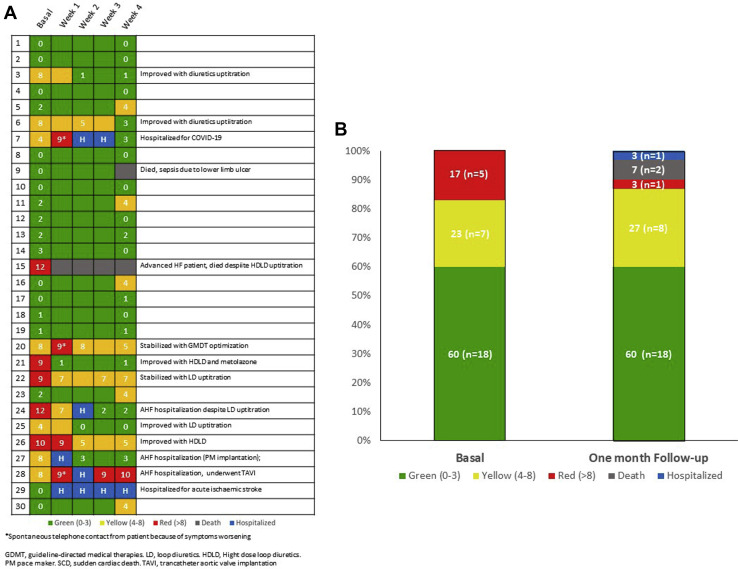
Here we report our experience with the first 30 patients who completed the 4 weeks of follow-up. Baseline epidemiological and clinical characteristics of these patients divided according to the score assigned during the first call in the 3 color groups are shown in Table 1 . Sixty percent of patients were assigned to the hypothetically low-risk green group, 17% to the high-risk red group, and the remaining to the intermediate-risk yellow group. Mean age of our study population was 84.4 years and slightly higher, although nonsignificantly, in the red group. Nearly half of this sample of patients presented HF with reduced ejection fraction and just more than half had an ischemic etiology. Patients in the yellow and red groups were more frequently treated with high-dose loop diuretics. Figure 2 A shows score variation and change of group as well as events occurring during the first month for each patient. We observed 2 deaths (6.7%), 1 in the red group and 1 in the green group, and 5 hospital admissions (16.7%): 3 in the yellow group and 1 each in the green and red group, respectively. Causes of hospitalization are reported in Table 1. Figure 2B shows color group variation at the end of the observation period: at 4 weeks, 3 patients had died, 1 patient was still hospitalized, the percentage of “green patients” remained stable (60%), whereas “red patients” decreased from 17% to 3%, and the percentage of “yellow patients” increased from 23% to 27%.
Table 1.
Baseline Clinical Characteristics and Events During the First 4-Week Observation Period
| Total Population, n = 30 | Green, n = 18 | Yellow, n = 7 | Red, n = 5 | P value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, y, mean ± SD | 84.4 ± 7.7 | 84.2 ± 8.0 | 82.3 ± 9.0 | 88.2 ± 3.7 | .43 |
| Females, n (%) | 12 (40.0) | 7 (38.9) | 2 (28.6) | 3 (60.0) | .54 |
| Lives alone, n (%) | 4 (13.3) | 2 (11.1) | 2 (28.6) | 0 (0.0) | .32 |
| Functional characteristics | |||||
| Independent in IADL, n (%) | 5 (16.7) | 4 (22.2) | 1 (14.3) | 0 (0.0) | .49 |
| Independent in medical treatment management, n (%) | 17 (56.7) | 12 (66.7) | 4 (57.1) | 1 (20.0) | .18 |
| Medical history | |||||
| Diabetes mellitus, n (%) | 8 (26.7) | 5 (27.8) | 2 (28.6) | 1 (20.0) | .93 |
| Hypertension, n (%) | 20 (66.7) | 12 (66.7) | 4 (57.1) | 4 (80.0) | .71 |
| Paroxysmal/permanent AF, n (%) | 23 (76.7) | 12 (66.7) | 7 (100.0) | 4 (80.0) | .23 |
| CKD (eGFR <60 ml/min/1.73 m2), n (%) | 25 (83.3) | 15 (83.3) | 5 (71.4) | 5 (100.0) | .42 |
| HF ischemic etiology, n (%) | 17 (56.7) | 8 (44.4) | 6 (85.7) | 3 (60.0) | .17 |
| HFrEF, n (%) | 14 (46.7) | 9 (50.0) | 3 (42.9) | 2 (40.0) | .90 |
| Pharmacological treatments | |||||
| ACEI/ARBs, n (%) | 11 (36.7) | 5 (27.8) | 4 (57.1) | 2 (40.0) | .39 |
| Sa/Va, n (%) | 8 (26.7) | 6 (33.3) | 1 (14.3) | 1 (20.0) | .59 |
| BBs, n (%) | 25 (83.3) | 15 (83.3) | 5 (71.4) | 5 (100.0) | .42 |
| MRAs, n (%) | 17 (56.7) | 11 (61.1) | 5 (71.4) | 1 (20.0) | .17 |
| Furosemide, n (%) | 28 (93.3) | 17 (94.4) | 6 (85.7) | 5 (100.0) | .59 |
| Furosemide high dose, n (%) | 5 (16.7) | 0 (0.0) | 3 (42.9) | 2 (40.0) | .011 |
| Events at 4 weeks follow-up | |||||
| Deaths, n (%) | 2 (6.7) | 1 (5.6) | 0 (0.0) | 1 (20.0) | .38 |
| HF-related deaths, n (%) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | .16 |
| Total hospitalization, n (%) | 5 (16.7) | 1 (5.6) | 3 (42.9)∗ | 1 (20.0) | .08 |
| HF hospitalization, n (%) | 3 (60.0) | 0 (0.0) | 2 (66.7) | 1 (100.0) | .33 |
NOTE. bold values show significant differences among the three groups.
ACEI, angiotensin-converting enzyme inhibitors; AF, atrial fibrillation; ARBs, angiotensin receptor blockers; BBs, beta-blockers; CKD, chronic kidney disease; COVID-19, Coronavirus Disease 19; eGFR, estimated glomerular filtration rate; HF, heart failure; HFrEF, HF with reduced ejection fraction; IADL, instrumental activities of daily leaving; MRAs, mineral receptor antagonists; SA/Va, sacubitril/valsartan; SD, standard deviation.
Only 1 hospitalization for COVID-19.
Fig. 2.
(A) Single patient variation of COVID-19-HF score during the observation period and change of groups. GDMT, guideline-directed medical therapies; HDLD, Hight dose loop diuretics; LD, loop diuretics; PM, pacemaker; SCD, sudden cardiac death; TAVI, transcatheter aortic valve implantation. (B) Color groups variation at the end of the observation period (percentage of patients).
Discussion
Because of the rapid lockdown of many outpatient services and of the simultaneous need of re-allocating many health care professionals for the management of severe COVID-19 cases requiring hospitalization, a rapid reorganization of our outpatient clinical activity was necessary, to continue providing specialized medical assistance to older, comorbid patients with HF. John F. Kennedy said once that “When written in Chinese, the word ‘crisis’ is composed of two characters—one represents danger, the other represents opportunity.”17 The impossibility to perform a physical and instrumental examination in a complex clinical syndrome such as HF represents a major challenge. In this regard, the Heart Failure Society of America recently suggested to cope with this emergency by using visual visits, implementing audio contact with a physical video-evaluation.10 Nevertheless, considering the advanced average age of our patients, we argued that not all users could carry out a video-call or feel comfortable with this technology. For these reasons, we opted for a telephone-visit, being aware of its limits.
From this preliminary experience, we may report several positive points. Patients have favorably accepted this telephone follow-up in a difficult moment in which they felt isolated and particularly vulnerable. Furthermore, by optimization of GDMTs and especially by adjusting diuretic dose, we managed to improve symptoms in several patients obtaining a score reduction (and change of color group allocation). Moreover, we were able to identify patients poorly adherent to nonpharmacological recommendations and reinforce the importance of following them. We also managed to correctly select the timing of hospital admission for elective/urgent procedures (eg, pacemaker implantation and percutaneous aortic valve implantation) in progressively worsening patients. Finally, in this difficult and peculiar situation in which we were taking care of complex patients without the possibility to perform a clinical and instrumental examination, it has been helpful to be guided by a comprehensive and standardized questionnaire, and to objectively observe and react to even small score variations, focusing interventions were needed. Nevertheless, some limitations should also be reported. Even if the questionnaire's social and functional characteristics were investigated, the Covid-19-HF score is focused on HF management and does not take in account frailty-related risks and the burden of noncardiovascular comorbidities. Consequently, in this first sample of patients, the score has not been able to identify and prevent non–HF-related complications (eg, cerebrovascular events, sepsis, Covid-19 pneumonia). Second, by using only a telephone interview, we might overestimate or in other cases underestimate the patient's real clinical condition and in selected patients the use of tele-visits could be useful. Furthermore, in some cases, evaluations might have been nonobjective, but the result of caregiver's perception and interpretation.
Comment
The COVID-19-HF score should not be considered a prognostic score at present, because its prognostic power needs to be validated in a longitudinal follow-up, currently ongoing. Until then, it should be considered a potentially useful clinical tool to be used in this emergency situation, which, through a standardized approach, could help physicians maintaining the follow-up of their patients and appropriately scheduling reevaluation though next telephonic contact and identifying patients at greatest risk of imminent instability, who may need urgent hospital evaluation. Furthermore, at the end of the pandemic, this instrument also could represent a useful resource in the management of low-risk HF patients, allowing clinicians to appropriately schedule clinical reevaluation in a moment when, with the reopening of outpatient services, the demand will be very high. Moreover, this type of approach also could be transferred to other chronic conditions whose clinical course is characterized by exacerbations, such as chronic obstructive pulmonary disease in which an early recognition of signs of alert/change of condition may allow the prompt initiation of treatment and preventive strategies.
“Every crucial experience can be regarded either as a setback, or the start of a wonderful new adventure, it depends on your perspective!”
Mary Roberts Rinehart18
The pragmatic innovation described in this article may need to be modified for use by others; in addition, strong evidence does not yet exist regarding efficacy or effectiveness. Therefore, successful implementation and outcomes cannot be assured. When necessary, administrative and legal review conducted with due diligence may be appropriate before implementing a pragmatic innovation.
Acknowledgments
We acknowledge Mrs Marzia Conforti, Mrs Katia Zini, Mrs Adriana Bambi, Mrs Maddalena Ciompi, Mrs Silvia Burchi, Mrs Francesca Valeri, Mrs Francesca Nesti, Mrs Rita Peruzzi, and Mr Damasco Donati for their precious and irreplaceable clinical work in our HF outpatient clinic and for the support in managing the telephone follow-up.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith A.C., Thomas E., Snoswell C.L. Telehealth for global emergencies: Implications for coronavirus disease 2019 (COVID-19) J Telemed Telecare. 2020;26:309–313. doi: 10.1177/1357633X20916567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaudhry S.I., Mattera J.A., Curtis J.P. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363:2301–2309. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koehler F., Winkler S., Schieber M. Telemedical Interventional Monitoring in Heart Failure Investigators. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: The telemedical interventional monitoring in heart failure study. Circulation. 2011;123:1873–1880. doi: 10.1161/CIRCULATIONAHA.111.018473. [DOI] [PubMed] [Google Scholar]
- 5.Van Spall H.G.C., Rahman T., Mytton O. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: A systematic review and network meta-analysis. Eur J Heart Fail. 2017;19:1427–1443. doi: 10.1002/ejhf.765. [DOI] [PubMed] [Google Scholar]
- 6.Jerant A.F., Azari R., Martinez C., Nesbitt T.S. A randomized trial of telenursing to reduce hospitalization for heart failure: Patient-centered outcomes and nursing indicators. Home Health Care Serv Q. 2003;22:1–20. doi: 10.1300/J027v22n01_01. [DOI] [PubMed] [Google Scholar]
- 7.Wakefield B.J., Ward M.M., Holman J.E. Evaluation of home telehealth following hospitalization for heart failure: A randomized trial. Telemed J E Health. 2008;14:753–761. doi: 10.1089/tmj.2007.0131. [DOI] [PubMed] [Google Scholar]
- 8.Cleland J.G., Louis A.A., Rigby A.S. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: The Trans-European Network-Home-Care Management System (TEN-HMS) study. J Am Coll Cardiol. 2005;45:1654–1664. doi: 10.1016/j.jacc.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 9.Dar O., Riley J., Chapman C. A randomized trial of home telemonitoring in a typical elderly heart failure population in North West London: Results of the Home-HF study. Eur J Heart Fail. 2009;11:319–325. doi: 10.1093/eurjhf/hfn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorodeski E.Z., Goyal P., Cox Z.L. Virtual visits for care of patients with heart failure in the era of COVID-19: A statement from the Heart Failure Society of America. J Card Fail. 2020;26:448–456. doi: 10.1016/j.cardfail.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dracup K., Walden J.A., Stevenson L.W., Brecht M.L. Quality of life in patients with advanced heart failure. J Heart Lung Transplant. 1992;11(2 Pt 1):273–279. [PubMed] [Google Scholar]
- 12.Jayaram N.M., Khariton Y., Krumholz H.M. Impact of telemonitoring on health status. Circ Cardiovasc Qual Outcomes. 2017;10:e004148. doi: 10.1161/CIRCOUTCOMES.117.004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 14.Brink T.L., Yesavage J.A., Lum O. Screening tests for geriatric depression. Clin Gerontol. 1982;1:37–43. [Google Scholar]
- 15.Beck A.T., Guth D., Steer R.A. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behav Res Ther. 1997;35:785–791. doi: 10.1016/s0005-7967(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 16.Morisky D.E., Ang A., Krousel-Wood M. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008;10:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Senator John F. Kennedy's speech at the 1959 Convocation of United Negro College Fund in Indianapolis, Indiana. April 12, 1959. Available at: https://www.jfklibrary.org/asset-viewer/archives/JFKSEN/0902/JFKSEN-0902-023. Accessed November 13, 2020.
- 18.Rinehart M.R. Dover Publications; Mineola, NY: 2014. The Circular Staircase. [Google Scholar]