Abstract
Increased heme levels, anemia, and desaturation occur during infection. We aimed to compare the levels of heme, heme oxygenase-1 (HO-1), ferritin, and bilirubin in coronavirus disease 2019 (COVID-19) patients at different saturation levels. Heme and HO-1 enzyme levels significantly increased in the low SpO2 group, but further studies are required.
Keywords: COVID-19, Heme, Heme oxygenase-1, SARS-CoV-2
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS CoV-2) can infect human target sites through the cellular angiotensin-converting enzyme II (ACE2) receptor in the respiratory tract.1 Furthermore, acute respiratory disease, pneumonia, and desaturation develops to threaten human life.2 , 3 Increase in heme levels, breakdown of red blood cells (RBCs), and anemia, which are frequently detected during infection process, deteriorates the oxygen saturation levels. It has been observed that desaturation results in poor outcomes in COVID-19 patients.4 This study aimed to compare the levels of heme, heme oxygenase-1 (HO-1), ferritin, and bilirubin in COVID-19 patients with different oxygen saturation levels, and find the potential prognostic factor in clinical SARS CoV-2 infection.
Patients and methods
This clinical observational study was approved by the Institutional Review Board of the Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (approval number 09-X-041). The study enrolled patients with COVID-19 who were admitted to the Taipei Tzu Chi Hospital between February 1, 2020 and May 15, 2020. For this retrospective observational study, informed consent was signed by the legal agent or the patient after being released from COVID-19 quarantine. The patient privacy rights, including those of individual person's data, in any form (including individual details, images, or videos), were observed. COVID-19 patients were enrolled, each having at least two different dates of laboratory data collection, including for the initial hospital admission and re-examination, four days later. The remaining specimens were stored at −20 °C temporarily. Heme and HO-1 were analyzed after receiving the informed consent.
The inclusion criteria: The clinical diagnosis of COVID-19 was based on a positive SARS-CoV-2 reverse transcription-polymerase chain reaction (RT-PCR) test, detected from the nasopharynx, oropharynx, or sputum samples. The exclusion criteria: patients with any concurrent infection such as bloodstream infection within 7 days of initial hospital admission were excluded.
For the purpose of oxygenation studies, the patients were divided into two groups based on whether or not their lowest oxygen saturation levels were measured by pulse oximetry (SpO2) at the time of hospital admission. Severe type COVID-19 had an oxygen saturation level ≤95% when breathing the ambient air.5 Eight patients had the lowest SpO2 ≤ 95% (severe type) while the other 8 had the lowest SpO2 > 95% (normal group).
Details of the onset date of clinical symptoms, throat swab conversion, course of disease, and laboratory results were collected from the medical records retrospectively, except for heme and HO-1. Influenza rapid test was negative in the study population. The disease course included the duration from symptom initiation to throat swab conversion. Throat swab conversion was the duration from a positive to negative test for SARS CoV-2 in the throat swab.
The quarantine policy, clinical diagnosis, and treatment adhered to the guidelines of the WHO,5 American Thoracic Society,6 and Surviving Sepsis Campaign.7 Each patient was quarantined in a single isolation room until they tested negative for SARS-CoV-2 in the RT-PCR test, three times consecutively.
The collected whole-blood samples were processed under the conditions recommended in the Ficoll–Paque plus (GE, USA). Heme was detected using the Heme assay kit (Abnova, Taiwan). HO-1 was detected using the HO-1 enzyme-linked immunosorbent assay (ELISA) kit (Abcam, USA).
Continuous data were expressed as mean ± standard error. Categorical data were expressed as frequencies and percentages. The clinical characteristics were compared using the Student's t-test for low and normal SpO2 groups. The Fisher's exact test was used for comparing 2 × 2 categorical variables. P < 0.05 was considered to indicate statistical significance.
Results
The SpO2 ≤95% group had no significant differences with the normal group with regard to sex, age, fever episodes, days of fever, and pneumonia (Supplemental Table 1). The low SpO2 group had a significantly higher initial body temperature than the normal SpO2 group (37.08 °C vs. 36.48 °C, p = 0.033). Total bilirubin was significantly higher in the low SpO2 group than in the normal group (0.95 vs. 0.5, p = 0.04) (Supplemental Table 1). Furthermore, the low SpO2 group had higher, but not significant, initial heme levels than the normal SpO2 group, (49.78 vs. 33.79, p = 0.446). HO-1 and ferritin levels were higher in the low SpO2 group than in the normal group. (3175.75 vs. 2255.60 for HO-1; 286.50 vs. 180.38 for ferritin).
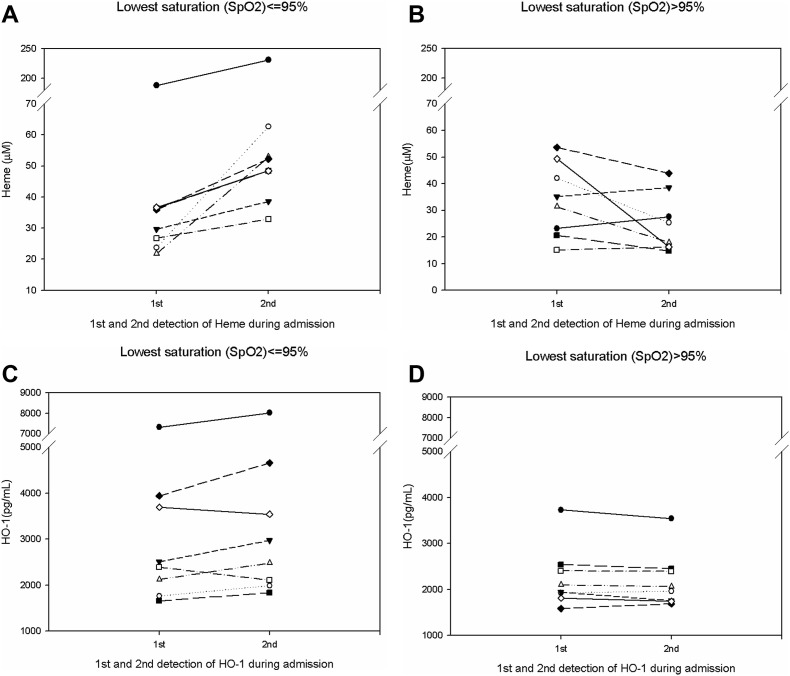
We further studied changes in heme-related parameters, initially and during admission (Table 1 and Fig. 1 ). There were no changes in RBC count, hemoglobin, hematocrit, and serum iron levels in both the groups. However, it was interesting to note that heme levels showed a significant increase in the low SpO2 group than in the normal SpO2 group (21.06 vs. −8.73, p = 0.001), Fig. 1A and B. HO-1 levels also showed a significant increase (274.24 vs. −55.66, p = 0.038), Fig. 1C and D.
Table 1.
Changes in parameters during admission in the two saturation groups.
| Lowest saturation (SpO2) | ≤95% N = 8 |
>95% N = 8 |
p value |
|---|---|---|---|
| Red blood cell (106/μL) | −0.01 ± 0.17 | −0.06 ± 0.13 | 0.833 |
| Hemoglobin (g/dL) | −0.17 ± 0.53 | −0.18 ± 0.38 | 0.985 |
| Hematocrit % | −0.25 ± 1.56 | −0.86 ± 1.22 | 0.763 |
| Serum Iron (μg/dL) | 14.50 ± 7.28 | 8.37 ± 8.71 | 0.598 |
| Heme (μM) | 21.06 ± 5.12 | −8.73 ± 4.43 | 0.001b |
| Heme oxygenase-1 (pg/mL) | 274.24 ± 128.29 | −55.66 ± 35.59 | 0.038a |
| Ferritin (ng/mL) | 79.93 ± 94.23 | −9.55 ± 6.62 | 0.360 |
| Total bilirubin (mg/dL) | 2.17 ± 2.07 | 0.12 ± 0.06 | 0.339 |
p < 0.05.
p < 0.01.
Fig. 1.
(A): Image showing increased heme levels during the admission period in the lowest SpO2 ≤ 95% group. (B): Image showing no change or decrease in the heme levels during the admission period in the lowest SpO2 > 95% group. (C): Image showing increased heme oxygenase-1 (HO-1) levels during the admission period in the lowest SpO2 ≤ 95% group. (D): Image showing no change or decrease in the heme oxygenase-1 (HO-1) levels during the admission period in the lowest SpO2 > 95% group.
Discussion
In our study, the low SpO2 group had a significant increase in heme and HO-1 levels. Oxygen saturation is a prognostic factor for COVID-19, which implied virus attacks at the upper airway, seeding to the lower respiratory tract where it damages the alveoli of the lungs, and finally induces acute respiratory distress syndrome and hypoxemia.2 In traditional infection and sepsis process, heme and free hemoglobin production were found. Later, the HO-1 enzyme breaks down heme to ferritin and biliverdin.8 The biliverdin will further produce bilirubin. This study has some limitations in that the study population is small; hence, the baseline clinical characteristics could not be adjusted for confounding factors. Since HO-1 is an inducible cytoprotective enzyme that copes with oxidative stress, the cytoprotective and anti-inflammatory properties of HO-1 may limit the damage caused by SARS-CoV-2.9 Interestingly, in a prior study in Taiwan, a higher HO-1 expression in the HO-1 (−497A/∗) genotype was associated with the protection from SARS infection.10 The raised HO-1 in the low oxygenation group may reduce inflammation and provide survival benefit in this particular group of patients. We analyzed the correlation between HO-1 and disease outcomes. Due to the small sample sizes in our study, no significant correlation was found and disease severity may influence the disease outcome. We have tried to suggest the possibility that SARS CoV-2 induced inflammation mechanisms and that the mechanisms occur via HO-1 enzyme at the initial diagnosis of COVID-19. Further large study is needed, adjusted for or stratified by disease severity to clarify the correlation between HO-1 and disease outcomes.
Due to the possibility of erythrocyte infection by SARS CoV-2 and the release of more heme into the blood system in COVID-19, there may be more heme and HO-1 than in classic bacterial sepsis for the same disease severity condition. Cell studies will be needed to understand the effect of ACE II receptor on the erythrocytes and possible infection process of the erythrocytes by SARS-CoV-2. Furthermore, clinical comparison study between COVID-19 and classic bacterial sepsis for the same disease severity, will help to establish the different levels of heme and HO-1.
Conclusion
Heme and HO-1 increased in the blood system of severe type COVID-19 patients; meaning that desaturation may relate to heme and HO-1. Further studies are needed to resolve the possibility of HO-1 mechanism in anti-inflammation process during SARS-CoV-2 infection.
Funding
The study was supported by a grant from the Buddhist Tzu Chi Medical Foundation [TCMF-A 109-05 (109)] and the Taipei Tzu Chi Hospital [TCRD-TPE-109-53]. The funding source had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of competing interest
The authors declare that they have no conflicting interests.
Acknowledgments
The authors would like to acknowledge Chih-Yu Chan, MSc, for conducting the analysis of heme and HO-1. He works at the Department of Pathology and Laboratory Medicine and Medical Research center, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei, Taiwan.
We would like to thank Editage (www.editage.com) for English language editing and Publication Support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmii.2020.10.001.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai C.C., Liu Y.H., Wang C.Y., Wang Y.H., Hsueh S.C., Yen M.Y. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect. 2020;53:404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei X.S., Wang X.R., Zhang J.C., Yang W.B., Ma W.L., Yang B.H. A cluster of health care workers with COVID-19 pneumonia caused by SARS-CoV-2. J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li T. Diagnosis and clinical management of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection: an operational recommendation of Peking Union Medical College Hospital (V2.0) Emerg Microb Infect. 2020;9:582–585. doi: 10.1080/22221751.2020.1735265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamil S., Mark N., Carlos G., Cruz C.S.D., Gross J.E., Pasnick S. Diagnosis and management of COVID-19 disease. Am J Respir Crit Care Med. 2020;201:19–20. doi: 10.1164/rccm.2020C1. [DOI] [PubMed] [Google Scholar]
- 7.Alhazzani W., Moller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E. Surviving epsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Crit Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araujo J.A., Zhang M., Yin F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front Pharmacol. 2012;3:119. doi: 10.3389/fphar.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hooper P.L. COVID-19 and heme oxygenase: novel insight into the disease and potential therapies. Cell Stress Chaperones. 2020;25:707–710. doi: 10.1007/s12192-020-01126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh Y.H., Chen C.W., Schmitz S.F., King C.C., Chen W.J., Wu Y.C. Candidate genes associated with susceptibility for SARS-coronavirus. Bull Math Biol. 2010;72:122–132. doi: 10.1007/s11538-009-9440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.