Abstract
Background
New York City (NYC) has endured the greatest burden of COVID-19 infections in the US. Health inequities in South Bronx predisposed this community to a large number of infectious cases, hospitalizations, and mortality. Health care workers (HCWs) are at a high risk of exposure to the infection. This study aims to assess seroprevalence and the associated characteristics of consenting HCWs from an NYC public hospital.
Methods
This cross-sectional study includes serum samples for qualitative SARS-CoV-2 antibody testing with nasopharyngeal swabs for SARS-CoV-2; PCR and completion of an online survey capturing demographics, COVID-19 symptoms during the preceding months on duty, details of healthcare and community exposure, and travel history were collected from consenting participants in May 2020. Participants' risk of exposure to COVID-19 infection in the hospital and in the community was defined based on CDC guidelines. Travel history to high-risk areas was also considered an additional risk. The Odds Ratio with bivariable and multivariable logistic regression was used to assess characteristics associated with seroprevalence.
Results
A total of 500 HCW were tested, 137 (27%) tested positive for the SARS-CoV-2 antibody. Symptomatic participants had a 75% rate of seroconversion compared to those without symptoms. Subjects with anosmia and ageusia had increased odds of seroconversion in comparison to those without these symptoms. Community exposure was 34% among those who had positive antibodies.
Conclusion
Seroprevalence among HCWs was high compared to the community at the epicenter of the pandemic. Further studies to evaluate sustained adaptive immunity in this high-risk group will guide our response to a future surge.
Keywords: Seroprevalence, Health care workers, Antibody, Exposure, SARS-CoV-2 PCR, COVID-19
Introduction
The United States currently has the highest number of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections globally, with the Bronx having the highest proportion of positive cases with an incidence of 850.2 hospitalizations per 100,000 persons in New York City (New York State Department of Health, 2020a). Black and Hispanic residents in the city had higher hospitalization rates and death due to COVID-19 (New York State Department of Health, 2020b). Older age and a higher number of comorbidities like chronic kidney disease, cancer, COPD, immune-compromised state, obesity, congestive heart failure, diabetes, and others increase the risk for adverse outcomes (Center for Disease Control and Prevention, 2020a). While most patients with SARS-CoV-2 infection have clinical presentations ranging from mild to severe respiratory illness, there is compelling evidence of asymptomatic and presymptomatic transmission of this infection, creating a breakdown of public health strategies to control the infection (Savvides and Siegel, 2020).
Antibodies to the spike (S) protein are considered to be the primary target of neutralizing activity following SARS-CoV-2 infection, conferring protective immunity compared to the membrane (M), envelope (E), and nucleocapsid proteins (Buchholz et al., 2004). While there is a better understanding of the immunological response to SARS-CoV-2 infection, there is a lack of serological assays to specifically detect SARS-CoV-2 antibodies. There is data to suggest that in a high prevalence setting, the commonly available commercial assays can miss SARS-CoV-2 antibodies, and the sensitivity of these assays is insufficient to detect the neutralizing capacity of seropositive individuals (Mueller, 2020).
Following the first case of COVID-19 on March 1, 2020, in a matter of weeks, NYC hospitals experienced a surge in infections, straining resources and supplies, especially personal protective equipment (PPE), resulting in sub-optimal patient care situations for Health care workers (HCWs). Data about COVID-19 infections in US HCWs is limited. The CDC reported that about 55% of SARS-CoV-2 PCR positive HCWs reported exposure at work, with most of them being minimally symptomatic or asymptomatic (Center for Disease Control and Prevention, 2020b). Convenience sampling of 3000 people in NY State showed that 13.9–14.9% of the population have COVID-19 antibodies (New York State Department of Health and The Official website of New York State, 2020). A large cohort study of HCW in the greater NYC area showed a seroprevalence of SARS-CoV-2 antibodies at 13.7% (Moscola et al., 2020). A seroprevalence study of a representative sample of HCW from Spain during the pandemic peak showed 9.3% had antibodies to SARS-CoV-2, while a similar hospital-wide screening study from Belgium confirmed only 6.4% of the hospital staff had antibodies (Stadlbauer, 2020, Steensels et al., 2020). We present the results of a cross-sectional study to assess the seroprevalence of the SARS-CoV-2 IgG antibody among HCWs at a heavily impacted community hospital during the COVID-19 pandemic in NYC.
Methods
Study setting and population
The cohort included HCWs across all hospital services who worked at the level one trauma center in the South Bronx during the period from March 1 to May 1, 2020. The study received institutional review board approval (IRB # 20-009).
After informed consent was obtained, participants underwent qualitative serology testing (Abbott Architect SARS-CoV-2 IgG Assay, Abbott Park, IL 60064 USA) (Food and Drug Administration, 2020), a nasopharyngeal swab for SARS-CoV-2 (Bio-Reference Laboratories, Inc., Elmwood Park, NJ, USA) and completed an online survey. The Abbott Architect assay uses a qualitative Chemiluminescent microparticle immunoassay technology targeting the nucleocapsid antigen of the virus with a sensitivity of 100% (CI 95.8–100%) and specificity of 99.6 (CI 99–99.9%) (Food and Drug Administration, 2020). The online survey was accessed by a Unique Identification Number assigned to each participant, blinded to the research team to ensure confidentiality. The survey requested information on age, race/ethnicity, comorbidities, residential zip code, and healthcare and community exposure details. History, timing, and duration of symptoms of COVID-19 infection such as fever, cough, shortness of breath, anosmia, ageusia, myalgia, nausea, and/or diarrhea in the preceding 8–10 weeks were also requested.
The risk of exposures in the healthcare setting and community exposure was determined based on CDC guidelines (Centers for Disease Control and Prevention, 2020). Close contact for healthcare exposures is defined as being within approximately six feet of a person with COVID-19 for a prolonged period (such as caring for the patient or sitting within six feet of the patient in a waiting area or room) or having unprotected direct contact with infectious secretions or excretions of the patient (e.g., being coughed on, touching used tissues with a bare hand). High-risk exposure is defined as HCW who had prolonged close contact with COVID-19 patients who were not wearing a facemask while the HCW's nose and mouth were exposed to material potentially infected with the virus causing COVID-19 or being present in the room for procedures that generate aerosols or during which respiratory secretions are likely to be poorly controlled (e.g., cardiopulmonary resuscitation, intubation, extubation, bronchoscopy, nebulizer therapy, sputum induction) on patients with COVID-19 when the HCP's eyes, nose or mouth were not protected. Medium-risk exposure is defined as HCW who had prolonged close contact with patients with COVID-19 who were not wearing a facemask while the HCW nose and mouth were exposed to material potentially infectious with the virus causing COVID-19. Low-risk exposure includes brief interactions with patients with COVID-19 or prolonged close contact with patients who were wearing a facemask for source control while the HCP was wearing a facemask or a respirator with eye protection, where the addition of a facemask or respirator would further lower the risk of exposure. Individuals outside of healthcare settings with close contact (<6 feet) for ≥15 min to a person with COVID-19 who had symptoms (in the period from two days before symptom onset of a clinically compatible illness) or a person who has tested positive for COVID-19 (laboratory-confirmed), but were asymptomatic in the two days before the date of specimen collection until they met criteria for discontinuing home isolation, has community exposure. High-risk domestic travel areas were identified as California and Washington State, while the international high-risk areas were China, South Korea, Iran, Italy, and Spain.
Statistical analysis
Descriptive statistics were used to summarize the baseline characteristics of the cohort and key study outcome variables. Categorical variables were compared by the Chi–squared test and Student t-test for continuous variables. Missing data were imputed with the mean for continuous variables. Binomial logistic regression was used to calculate odds ratios and 95% CI for evaluating the association with seroprevalence of socio-demographic variables. Significant symptoms and exposure-related variables observed to be associated with seropositivity in a bivariate analysis (cut-off of p < 0.15) were independently assessed by multivariable logistic regression for its association with seropositivity, adjusting for all significant variables as covariates. A p-value of <0.05 was considered significant. All statistical tests were performed using SPSS version 17 (IBM, USA).
Results
Among the 659 participants screened for the study, 500 consented and underwent PCR and antibody testing, and 478 participants completed the survey. The participants who completed the survey were included in the evaluation and analysis of characteristics predisposing to seroprevalence. Overall study participants were female (69%), under the age of 40 (48%), Hispanic (28%), Asian and Caucasian (24%) racial/ethnic distribution, with 33% being physicians followed by nurses (30%). Hypertension, asthma/COPD, and diabetes were common comorbidities. Childhood BCG vaccination was reported by 43% of participants. Most participants lived in NYC boroughs and used either public transport or walked to work.
In our study group, 53% were asymptomatic. Symptomatic participants reported sore throat/sinusitis (67%), myalgia (57%), and fever (36%), with 39% having symptoms for up to two weeks. Most HCWs (85%) had a prior test for SARS-CoV-2 PCR, with 19% of those being positive. Among the HCWs involved in direct patient care, 14% had high/moderate risk exposure. Community exposure due to household contact with COVID-19 was present in 18% of the participants.
Risk factors and seropositivity
In the overall cohort, the prevalence of SARS-CoV-2 IgG antibodies was 27% (137/500). Of the participants that completed the survey, 130 were positive for IgG antibodies. Ninety-eight (75%) of the 130 participants with positive antibodies had COVID-19-like symptoms in the preceding ten weeks. Among the symptomatic participants, 44% (98/225) were seropositive compared to 13% (32/253) without symptoms.
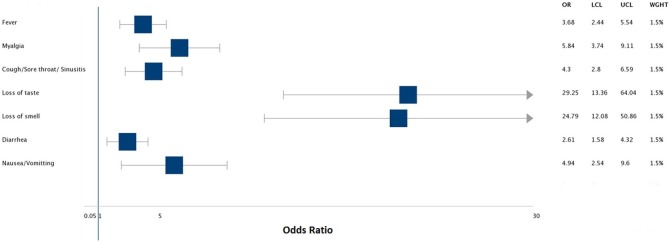
Table 1 shows the distribution of covariates with respect to SARS-CoV-2 IgG positivity. Race was significantly associated with seropositivity with an unadjusted odds ratio of 2.42 (95% CI 1.3−4.5; p = 0.005) for Hispanics and 2.55 (95% CI 1.3−5.02; p = 0.006) for Blacks when compared to Caucasians. The unadjusted odds ratio for seropositivity was significantly higher for anosmia (29.25; CI 13.36−64.04; p < 0.001) and ageusia (24.79; CI 12.08−50.86; p < 0.001) (Figure 1 ). On multivariate analysis (Table 2 ), HCWs who reported symptoms of fever, ageusia, and anosmia were 7.4, 3.3, and 5.5 times, respectively, likely to be seropositive compared to those without the aforesaid symptoms. Participants with symptoms above 14 days were 4.3 times more likely to be seropositive than those with symptoms less than five days. High and moderate risk of exposure was twice as likely to be associated with seropositivity than low risk.
Table 1.
Association of variables with seropositivity.
| Characteristics | Total (N = 478) | IgG+ (N = 130) | IgG- (N = 348) | p | OR (95% CI) |
|---|---|---|---|---|---|
| Age | |||||
| From 20 to 39 | 230 (48%) | 58 (25%) | 172 (75%) | – | Ref |
| From 40 to 59 | 196 (41%) | 60 (30%) | 136 (70%) | 0.210 | 0.76 (0.5−1.16) |
| >60 | 52 (11%) | 12 (23%) | 40 (77%) | 0.740 | 1.12 (0.55−2.28) |
| Gender | |||||
| Female | 329 (69%) | 87 (26%) | 242 (73%) | 0.600 | 0.9 (0.67,1.24) |
| Male | 149 (31%) | 43 (29%) | 106 (31%) | ||
| Ethnicity | |||||
| Hispanic | 132 (28%) | 41 (31%) | 91 (69%) | 0.005 | 2.42 (1.3−4.5) |
| Black | 87 (18%) | 28 (32%) | 59 (68%) | 0.006 | 2.55 (1.3−5.02) |
| Asian | 114 (24%) | 30 (26%) | 84 (74%) | 0.050 | 1.9 (1.00−3.69) |
| Other race | 30 (6%) | 13 (43%) | 17 (57%) | 0.002 | 4.1 (1.7−9.9) |
| Caucasian | 115 (24%) | 18 (15%) | 97 (85%) | – | Ref |
| Living conditions | |||||
| Alone | 116 (24%) | 30 (25%) | 86 (75%) | 0.800 | 0.93 (0.66−1.32) |
| With others | 362 (76%) | 100 (28%) | 262 (72%) | ||
| Housing | |||||
| Apartment/Condo | 269 (56%) | 83 (31%) | 186 (69%) | 0.040 | 1.37 (1.008,1.86) |
| Single family home | 209 (44%) | 47 (23%) | 162 (78%) | ||
| Means of travel to work | |||||
| Private transport | 252 (53%) | 60 (24%) | 192 (76%) | – | Ref |
| Public transportb | 204 (43%) | 59 (29%) | 145 (71%) | 0.200 | 1.3 (0.85−1.98) |
| Walk to work | 22 (4%) | 11 (50%) | 11 (50%) | 0.010 | 3.2 (1.3−7.75) |
| Comorbidities | |||||
| Hypertension | 81 (17%) | 25 (31) | 56 (69%) | 0.400 | 1.06 (0.9,1.2) |
| Diabetes | 31 (7%) | 10 (32%) | 21 (68%) | 0.300 | 1.08 (0.8−1.3) |
| Heart failure | 2 (0.5%) | 1 (50%) | 1 (50%) | 0.400 | 1.4 (0.36−5.83) |
| Copd/Asthma | 46 (10%) | 18 (39%) | 28 (61%) | 0.080 | 1.21 (0.95−1.54) |
| Chronic kidney disease | 3 (0.6%) | 1 (33%) | 2 (67%) | 0.600 | 1.09 (0.49−2.43) |
| Cancer | 3 (0.6%) | 1 (33%) | 2 (67%) | 0.600 | 1.09 (0.49−2.43) |
| Rheumatological diseases | 10 (2%) | 4 (40%) | 6 (60%) | 0.270 | 1.21 (0.73−2.02) |
| History of BCG vaccinec | 204 (43%) | 63 (31%) | 141 (69%) | 0.120 | 1.09 (0.97−1.22) |
| History of symptoms of COVID | |||||
| Fever | 80 (17%) | 62 (78%) | 18 (23%) | <0.001 | 3.68 (2.44−5.54) |
| Myalgias | 128 (27%) | 70 (55%) | 58 (45%) | <0.001 | 5.84 (3.74,9.11) |
| Cough/Sorethroat/Sinusitis | 150 (31%) | 72 (48%) | 78 (52%) | <0.001 | 4.3 (2.8,6.59) |
| Ageusia | 61 (13%) | 53 (87%) | 8 (13%) | <0.001 | 29.25 (13.36−64.04) |
| Anosmia | 65 (14%) | 55 (85%) | 10 (15%) | <0.001 | 24.79 (12.08−50.86) |
| Diarrhea | 78 (16%) | 35 (45%) | 43 (55%) | <0.001 | 2.61 (1.58−4.32) |
| Nausea/Vomiting | 41 (9%) | 25 (61%) | 16 (39%) | <0.001 | 4.94 (2.54,9.6) |
| Asymptomatic | 253 (53%) | 32 (13%) | 221 (87%) | <0.001 | 0.19 (0.12−0.3) |
| Approximate duration of symptoms in days (IQR)a | 7 (3−14) | 14 (5−21) | 5 (3−7) | <0.001 | |
| <5 days | 94 (20%) | 26 (28%) | 68 (72%) | – | Ref |
| 6−14 days | 77 (16%) | 37 (48%) | 40 (52%) | 0.006 | 2.4 (1.2−4.5) |
| >14 days | 47 (10%) | 37 (79%) | 10 (21%) | <0.001 | 9.6 (4.2−22.2) |
| Type of PPE usedd | |||||
| N95 only | 76 (16%) | 19 (25%) | 57 (75%) | 0.300 | 0.87 (0.5,1.54) |
| Surgical mask only | 109 (23%) | 39 (36%) | 70 (64%) | 0.020 | 1.7 (1.08,2.69) |
| N95 and surgical mask | 361 (75%) | 90 (25%) | 271 (75%) | 0.050 | 0.64 (0.41,1) |
| Face shield and goggles | 329 (69%) | 77 (23%) | 252 (77%) | 0.004 | 0.55 (0.36,0.84) |
| None | 2 (0.4%) | 1 (50%) | 1 (50%) | 0.470 | 2.69 (0.17,43.2) |
| Nature of work | |||||
| Physician | 157 (33%) | 39 (25%) | 118 (75%) | – | Ref |
| Nurse | 142 (30%) | 40 (28%) | 102 (72%) | 0.510 | 1.18 (0.7−1.9) |
| Ancillary service | 72 (15%) | 20 (28%) | 52 (72%) | 0.600 | 1.1 (0.62−2.1) |
| Others | 107 (22%) | 31 (29%) | 76 (71%) | 0.450 | 1.2 (0.71−2.1) |
| Health care worker exposuree | |||||
| High and moderate | 65 (14%) | 25 (38%) | 40 (62%) | 0.710 | 1.84 (1.06,3.18) |
| Low | 413 (86%) | 105 (25 %) | 309 (75%) | ||
| Community exposuref | 119 (25%) | 44 (37%) | 75 (63%) | 0.005 | 1.86 (1.19−2.9) |
| Location of residence | |||||
| Manhattan | 79 (17%) | 22 (28%) | 57 (72%) | – | Ref |
| Bronx | 154 (32%) | 41 (27%) | 113 (73%) | 0.840 | 0.94 (0.51−1.7) |
| Brooklyn | 20 (4%) | 7 (35%) | 13 (65%) | 0.530 | 1.39 (0.49−3.95) |
| Queens | 39 (8%) | 12 (31%) | 27 (69%) | 0.740 | 1.15 (0.49−2.66) |
| Long island | 19 (4%) | 6 (32%) | 13 (68%) | 0.740 | 1.19 (0.40−3.5) |
| Upstate NY | 11 (2%) | 2 (18%) | 9 (82%) | 0.500 | 0.57 (0.11−2.87) |
| New Jersey | 47 (10%) | 9 (19%) | 38 (81%) | 0.270 | 0.61 (0.25−1.47) |
| Westchester county NY | 24 (5%) | 7 (29%) | 17 (71%) | 0.900 | 1.06 (0.38−2.92) |
| Rest of the country | 85 (18%) | 24 (28%) | 61 (72%) | 0.900 | 1.01 (0.51−2.01) |
| Travel historyg | |||||
| Domestic (High risk) | 26 (5%) | 3 (12%) | 23 (88%) | 0.040 | 0.18 (0.03−0.96) |
| International (High risk) | 19 (4%) | 5 (26%) | 14 (74%) | 0.340 | 1.9 (0.38−9.44) |
IQR-Interquartile Range.
Public Transport includes: Subway, Taxi or Bus.
BCG Vaccine received in childhood.
PPE: Personal Protective Equipment.
High-Risk HCW defined as: HCP who had prolonged close contact with patients with COVID-19 not wearing a facemask while the HCP nose and mouth were exposed to material potentially infectious with the virus causing COVID-19 OR Being present in the room for procedures that generate aerosols or during which respiratory secretions are poorly controlled when the HCP’s eyes, nose, or mouth were not protected. Moderate Risk HCW was defined as: HCP who had prolonged close contact with patients with COVID-19 who were not wearing a facemask while the HCP nose and mouth were exposed to material potentially infectious with the virus causing COVID-19. Low-Risk HCW was defined as: Brief interactions with patients with COVID-19 or prolonged close contact with patients who were wearing a facemask for source control while the HCP was wearing a facemask or respirator.
Community Exposure defined as: Individual had close contact (<6 feet) for ≥15 min to a person with COVID-19 who had symptoms or a Person who has tested positive for COVID-19 but were asymptomatic.
High-Risk Domestic locations include: California and Washington state. High-Risk International locations include: China, South Korea, Iran, Italy, and Spain.
Figure 1.
Association between symptomatology and seroconversion.
Table 2.
Adjusted Associations of seropositivity with participant characteristics using Multivariate Linear Regression.
| Characteristics | Prevalence (%) | IgG seropositivity | p-value |
|---|---|---|---|
| OR (95% CI) | |||
| Ethnicity | |||
| Hispanic | 31% | 1.32 (0.6−2.89) | 0.480 |
| Black | 32% | 1.5 (0.62−3.58) | 0.360 |
| Asian | 26% | 0.90 (0.39−2.07) | 0.820 |
| Other races | 43% | 2.59 (0.86−7.73) | 0.080 |
| Caucasian | 15% | Ref. | |
| Means of travel | |||
| Public | 29% | 0.84 (0.47−1.52) | 0.580 |
| Walk to work | 50% | 2.29 (0.69−7.54) | 0.170 |
| Private | 24% | Ref. | |
| Healthcare exposure high and moderate risk | 38% | 2.0 (0.99−4.25) | 0.050 |
| Community exposure | 37% | 1.27 (0.68−2.36) | 0.440 |
| Symptoms | |||
| Fever | 78% | 7.43 (3.33−16.57) | <0.001 |
| Cough/Sore throat/Sinusitis | 48% | 1.04 (0.5−2.18) | 0.900 |
| Myalgia | 55% | 1.40 (0.66−2.9) | 0.370 |
| Ageusia | 87% | 3.35 (1.03−10.8) | 0.040 |
| Anosmia | 85% | 5.57 (1.88−16.5) | 0.002 |
| Nausea | 61% | 1.64 (0.57−4.6) | 0.350 |
| Diarrhea | 45% | 0.50 (0.20−1.21) | 0.120 |
| Duration of symptoms | |||
| >14 days | 79% | 4.3 (1.4−12.90 | 0.008 |
| 6 to 14 days | 48% | 1.19 (0.49−2.91) | 0.690 |
| <5 days | 28% | Ref. | |
IgG negative was considered as the reference category for seroconversion.
Correlation of point seroprevalence with SARS-CoV-2 PCR results and prior PCR status
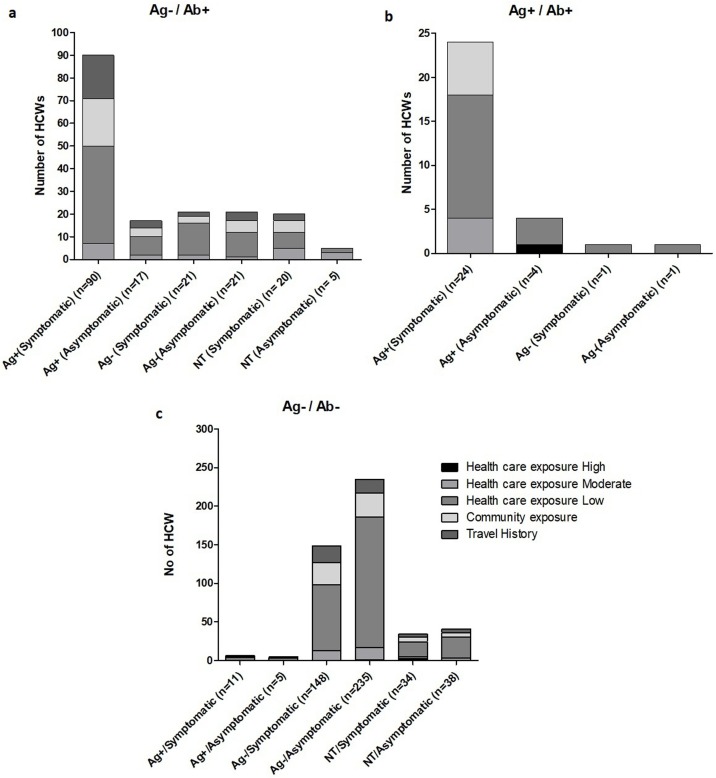
Based on the viral PCR and antibody testing results, three subgroups of the cohort were identified (Figure 2 ). Of the 130 participants who were antibody positive, 105 had a negative SARS-CoV-2 PCR during the study. In this subgroup, 60 subjects had prior PCR positivity, with 50 being symptomatic. Twenty-two of the 25 subjects who were both PCR and antibody-positive at the time of the study had prior PCR positivity, with 18 reporting symptoms of COVID like illness. Among 348 participants who tested negative for viral PCR and anti-SARS-CoV-2 antibody at the time of testing, 285 (82%) did not report any detectable viral RNA anytime in the past three months (study period) with predominantly low-risk healthcare exposure (255/347−73%). This sub-cohort was younger participants less than 40 years of age, without any significant comorbidities, and of these, 98 (35%) reported sore throat/ sinusitis (62/98) and myalgia (46/98). Seven participants with prior positive PCR, including four who were symptomatic, were seronegative at the time of this study. Both groups had low-risk healthcare exposure.
Figure 2.
Antigen (PCR) and antibody (IgG) results compared to the history of prior PCR results and symptoms in HCWs. (a) Ag−/Ab+ groups, N = 144; (b) Ag+/Ab+ groups, N = 30; and (c) Ag−/Ab− groups, N = 426. HWC, healthcare workers.
Discussion
We report the seroprevalence of SARS-CoV-2 in a cross-sectional sample of HCWs who worked on the frontlines in one of NYC’s public hospitals. The 27% seroprevalence in our high-risk cohort is comparable to 24.4% observed in a cross-sectional study of 554 HCWs from a hospital in the UK (Shields, 2020). Recent data from a university hospital in the US support the considerably higher prevalence of COVID-19 infection among HCWs in comparison with non-HCWs (7.3% vs. 0.4%) (Barrett, 2020). In a similar vein, a previous study done during the influenza A (H1N1) pandemic that occurred in 2009, wherein a multicenter study to evaluate seroprevalence before and after vaccination, showed that first-responders that interacted with five or more infected patients mounted a significantly higher seropositivity (Aguilar-Madrid et al., 2015). The evaluation of SARS-CoV2 antibody levels among 40,329 HCW in an extensive hospital system in the greater NYC area showed a 13.7% seroprevalence.
In contrast, another large retrospective cross-sectional analysis of SARS-CoV-2 seroprevalence in a comparative group including patients with COVID-19 infections and a representative sample from the general population confirmed the presence of seropositive samples in late February, suggesting that SARS-CoV-2 infection was present in the NYC metropolitan area before March 1 (Moscola et al., 2020, Stadlbauer, 2020). The representative sample's seroprevalence was 19.3% in late April, suggesting low community seroprevalence (Stadlbauer, 2020). We believe the higher seroprevalence seen in our cohort could be a combination of healthcare and community exposure as our hospital was the “epicenter of the epicenter” during the pandemic, and most of the HCWs lived in and around NYC.
In our analysis, after adjustment for baseline characteristics, on multivariate regression analysis, we did not find any racial/ethnic predisposition to seropositivity among HCW. On the contrary, there has been published data favoring an association of SARS-CoV-2 seropositivity in non-Hispanic blacks and Hispanics when compared to whites in the general population (Biggs, 2020; Feehan, 2020; Killerby, 2020; Menachemi, 2020). The reasons for this disparity could be factors related to social determinants of health, increased comorbidities, and a higher likelihood of people from these communities to be essential workers, increasing their risk of exposure to the infection (Gould and Wilson, 2020).
We observed a difference in seropositivity among prior symptomatic and asymptomatic HCWs (44% vs. 13%), similar to a cross-sectional study of HCWs from the UK (35.8% vs. 17.1%) (Shields, 2020). The persistence of PCR positivity in 22 of the 25 seropositive participants seemed less likely to be infectious, though we do not have viral load estimation to corroborate. As has been shown in previous studies, ageusia and anosmia were positively associated (OR > 24) with seropositivity (Garcia-Bastiero et al., 2020). The lower seropositivity rates among asymptomatic participants suggest that developing the disease following the infection was an important factor influencing the presence of antibodies. Among the 43% (204/478) of participants who had received BCG vaccination in childhood, 63 (31%) developed antibodies to SARS-CoV-2 compared to 27% seropositivity in the entire cohort; the difference was, however, not significant.
Multiple studies on prevalence in the community and among HCW have shown a wide-range of seropositivity for SARS-CoV-2 antibodies in the NYC area ranging from 6.9% to 27%, which is well below the estimated 67% needed to achieve herd immunity to SARS-CoV-2 (Fontanet and Cauchemez, 2020). Hence implementation of protocols and workflows to reduce exposure and transmission among HCWs must be implemented to protect the frontline HCWs during a second surge. Screening and triage of everyone entering the healthcare facility for signs and symptoms of COVID-19, revaluation of admitted patients for signs and symptoms of COVID-19, implementation of universal source control measures including the use of facemasks among all patients/visitors to the hospital, use of facemasks by all HCWs at all times, continued physical distancing and implementation of universal use of personal protective equipment are some of the strategies recommended by CDC. Appropriate use of telemedicine for consultations, primary care visits, and communications with family members of hospitalized patients is a valuable resource during the pandemic (Cianetti et al., 2020).
The Abbott SARS-CoV-2 antibody assay detected the presence of IgG against SARS-CoV-2 nucleocapsid protein. A recent study comparing performance characteristics of various commercially available antibody tests in a high prevalence area has shown that currently available test systems missed a large proportion of antibodies as the sensitivities were insufficient for detecting everyone with neutralizing antibodies (Mueller, 2020).
Our study has several limitations. First, it is a single-center design with voluntary participation and subject characteristics collected via a self-reported online survey. Second, data about symptoms within the previous 8–10 weeks were collected, resulting in the possibility of self-report bias. Third, we were unable to perform a quantitative analysis of SARS-CoV-2 IgG and neutralizing antibodies to complete the evaluation of seroprevalence at this time. Fourth, there remains a possibility that we could have missed seroconversion in a group of subjects based on the timing of the study since IgM tests were not performed, which could reflect more recent infections as compared to IgG. Fifth, we were able to receive only 478 surveys among our tested 500 participants, which could also contribute to bias. Finally, we did not determine dynamic antibody responses at this time, though we plan it for the future.
In conclusion, HCWs tested after the initial surge following the COVID-19 pandemic, have demonstrated a higher seroprevalence compared to the community in NYC. Symptomatic participants had a higher rate of seropositivity compared to those without symptoms. A combination of healthcare and community exposure likely contributed to the seroprevalence. While the present assessment might not be an accurate indicator of immunity to the SARS-CoV-2 virus due to barriers in the testing method, the timing of the testing, etc., further studies are warranted to better understand our adaptive immune response.
Conflict of interest
None declared.
Author contribution
UV, VD and VM conceptualized and visualized the study, in addition to working with the team on data curation, formal analysis, designing the methodology, writing the original draft and review & editing. NJ, SR, MJ, MA, and SM worked on data curation, validation, project administration, formal analysis, and writing - original draft. MAS and AMB were involved in executing the methodology of the study with project administration, use of resources, supervision and validation of data curation, formal analysis, and writing the original draft, with review & editing the manuscript. AP, NS and MK were involved in supervision, validation, methodology, project administration, and writing - review & editing the manuscript. All authors approved the final version of the submission with review and editing.
UV, VD and VM conceptualized and visualized the study, in addition to working with the team on data curation, formal analysis, designing the methodology, writing the original draft and review & editing. NJ, SR, MJ, MA, and SM worked on data curation, validation, project administration, formal analysis, and writing - original draft. MAS and AMB were in-volved in executing the methodology of the study with project administration, use of resources, supervision and validation of data curation, formal analysis, and writing the original draft, with review & editing the manuscript. AP, NS and MK were involved in supervision, validation, methodology, project administration, and writing - review & editing the manuscript. All au-thors approved the final version of the submission with review and editing.
Ethical approval
The study protocol was approved by the Institutional Review Board Committee (IRB# 20-009) on 5/1/2020. All patients provided written, Informed Consent to participate in the study.
Funding source
None declared.
Acknowledgements
We are grateful to all the HCWs for their heroic efforts during the pandemic and to our study participants. We appreciate the continued support of the Department of Internal Medicine, other clinical and non-clinical staff at NYC HHC Lincoln. A special thanks to Suja Abraham for laboratory support. Additionally, we would like to recognize Agnes Ampem-Darko, Nursing Supervisor in Ambulatory Care, and Hector Tavarez, Administrator for Outpatient Clinic, for streamlining the workflow for specimen collection. We thank Tamara Dudley NP, Dorinda Ezell NP, Conekia Kinsey NP, and Alesia McFarlin NP for laboratory coordination, sample collection, and survey follow-up. We thank Dr. Veena Menon, PhD, for her expert advice and Ms. Fabia Edathadathil for support in analyzing the study. To all the First Responders and Healthcare Volunteers that came from across the country to help our patients during the COVID-19 pandemic into the center of the hotspot, thank you.
References
- Aguilar-Madrid G. Seroprevalence of pandemic A(H1N1) pmd09 virus antibodies in Mexican Health Care Workers before and after vaccination. Arch Med Res. 2015;46(2):154–163. doi: 10.1016/j.arcmed.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Barrett E. Prevalence of SARS-CoV-2 infection in previously undiagnosed health care workers at the onset of the US COVID-19 epidemic. medRxiv. 2020;04.20:20072470. doi: 10.1101/2020.04.20.20072470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs H.M. Estimated community seroprevalence of SARS-CoV-2 antibodies–two Georgia counties, April 28–May 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(29):965–970. doi: 10.15585/mmwr.mm6929e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz U.J. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc Natl Acad Sci U S A. 2004;101(26):9804–9809. doi: 10.1073/pnas.0403492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention . 2020. People with Certain Medical Conditions.https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html [Google Scholar]
- Center for Disease Control and Prevention Characteristics Of health care personnel with COVID-19–United States, February 12–April, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:477–481. doi: 10.15585/mmwr.mm6915e6. https://www.cdc.gov/mmwr/volumes/69/wr/mm6915e6.htm 2020. [Accessed 1 June 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2020. Public Health Guidance for Community-Related Exposure.https://www.cdc.gov/coronavirus/2019-ncov/php/public-health-recommendations.html [Google Scholar]
- Cianetti S. Model for taking care of patients with early childhood caries during the SARS-Cov-2 pandemic. Int J Environ Res Public Health. 2020;17(11):3751. doi: 10.3390/ijerph17113751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feehan A.K. Racial and workplace disparities in seroprevalence of SARS-CoV-2 in Baton Rouge, Louisiana, July 15–31, 2020. medRxiv. 2020;08.26:20180968. doi: 10.1101/2020.08.26.20180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanet A., Cauchemez S. COVID-19 herd immunity: where are we? Nat Rev Immunol. 2020;20:583–584. doi: 10.1038/s41577-020-00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration . 2020. SARS-C0V-2 IgG Architect. Abbot Instruction Manual.https://www.fda.gov/media/137383/download [Google Scholar]
- Garcia-Bastiero A. seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun. 2020;11(1):3500. doi: 10.1038/s41467-020-17318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E., Wilson V. Economic Policy Institute; Washington, DC: 2020. Black Workers Face Two of the Most Lethal Preexisting Conditions for Coronavirus—Racism and Economic Inequality.https://www.epi.org/publication/black-workers-covid June 1. [Google Scholar]
- Killerby M.E. Characteristics associated with hospitalization among patients with COVID-19—Metropolitan Atlanta, Georgia, March–April 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):790–794. doi: 10.1101/2020.08.26.20180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menachemi N. Population point prevalence of SARS-CoV-2 infection based on a statewide random sample—Indiana, April 25–29, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(29):960–964. doi: 10.15585/mmwr.mm6929e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscola J. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City Area. JAMA. 2020;324(9):893–895. doi: 10.1001/jama.2020.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller L. Sensitivity of commercial Anti-SARS-CoV-2 serological assays in a high-prevalence setting. medRxiv. 2020;06.11:20128686. doi: 10.1101/2020.06.11.20128686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New York State Department of Health . 2020. New York Department of Health COVID-19 Tracker.https://covid19tracker.health.ny.gov/ [Google Scholar]
- New York State Department of Health . 2020. Addressing Health Inequities in Care During COVID-19.https://www1.nyc.gov/assets/doh/downloads/pdf/covid/providers/covid-19-health-inequities.pdf [Google Scholar]
- New York State Department of Health, The Official website of New York State . 2020. Amid Ongoing COVID-19 Pandemic, Governor Cuomo Announces Phase II Results of Antibody Testing Study Show 14.9% of the Population has COVID-19 Antibodies.https://www.governor.ny.gov/news/amid-ongoing-covid-19-pandemic-governor-cuomo-announces-phase-ii-results-antibody-testing-study [Google Scholar]
- Savvides C., Siegel R. Asymptomatic and presymptomatic transmission of SARS-CoV-2: a systematic review. medRxiv. 2020;06.11:20129072. doi: 10.1101/2020.06.11.20129072. [DOI] [Google Scholar]
- Shields A. SARS-CoV-2 seroconversion in health care workers. medRxiv. 2020;05.18:20105197. doi: 10.1101/2020.05.18.20105197. [DOI] [Google Scholar]
- Stadlbauer D. Seroconversion of a city: longitudinal monitoring of SARS-CoV-2 seroprevalence in New York City. medRxiv. 2020;06.28:20142190. doi: 10.1101/2020.06.28.20142190. [DOI] [Google Scholar]
- Steensels D. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324(2):195–197. doi: 10.1001/jama.2020.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]