Abstract
Objective
Osteoarthritis causes a significant healthcare burden and the number of total knee arthroplasty (TKA) procedures is predicted to increase significantly in the coming years. We conducted a systematic review to assess the scope and quality of all current TKA cost-effectiveness analysis (CEA) studies, identify trends, and identify areas for improvement.
Methods
An electronic database search of MEDLINE, Embase, the CEA registry and Scopus was used to identify all CEA studies where TKA was used with a comparator. Studies were included from January 1, 1997 to February 2, 2020. The Quality of Health Economic Analysis Studies (QHES) instrument was used to assess their quality. Thirty-three studies were included that offered both a QALY and cost calculation. The main findings, incremental-cost effectiveness ratios and other important study characteristics were then ascertained, and trends identified.
Results
Certain surgical interventions were suggested to be more cost-effective than TKA. This included unicompartmental knee arthroplasty for unicompartmental osteoarthritis, computer-assisted TKA compared to conventional TKA, and resurfacing the patella compared to no resurfacing. TKA was more cost-effective compared to non-operative management regardless of specific patient variables.
Conclusions
The analyses of the CEAs included in the study have to be interpreted with caution. Overall, certain surgical methods within TKA and alternative methods to TKA appear to be favoured for treating particular knee osteoarthritic conditions due to their suggested greater cost-effectiveness but this should be interpreted within local contexts. Our results should help guide future policy-making as healthcare associated costs continue to rise.
Keywords: Total knee arthroplasty, QALY, ICER, Cost-effectiveness analysis
1. Introduction
Osteoarthritis is a common cause of morbidity globally and causes a significant financial burden to healthcare systems.1 Symptomatic knee osteoarthritis occurs in around 10% of men and 13% of women aged 60 or older,1 and this percentage is likely to increase with an ageing population and increasing obesity rates. The age standardised prevalence of knee osteoarthritis for adults aged 45 and older was 19.2% in 1990, and increased to 27.8% in 2008.2 The incidence rate of total knee arthroplasties (TKA) in the USA is the highest globally and even with conservative projection approaches, this is projected to increase up to six-fold by 2030.3,4 There are various published systematic reviews focusing on areas such as sports,5 trauma,6 upper limb7 and foot and ankle surgery8 but despite the high economic impact of arthroplasty surgery, there has been no recent systematic review of the cost-effectiveness of TKA.
This systematic review will assess the scope and quality of all current TKA CEA studies. This review will also identify trends, areas where further CEAs must be performed and where improvement is needed for greater quality. We used the Quality of Health Economic Studies (QHES) instrument9 to measure the quality of the studies and the significance of the findings for clinical practice decision-making. No funding was received for this systematic review.
2. Methods/literature search
2.1. Search strategy
A systematic search was performed to identify all CEA studies that offered an economic evaluation of TKA procedures in MEDLINE, EMBASE, the Cost-Effectiveness Analysis (CEA) Registry (by the Centre for the Evaluation of Value and Risk in Health at Tufts University)10 and Scopus. The First Panel on Cost-Effectiveness in Health and Medicine published their first-consensus-based guidelines for the conduct of CEAs on October 16, 1996 which formed the basis of the rationale for the time limit in our search.11 Search results were therefore restricted from January 1997 (to allow for sufficient time for studies to reflect these guidelines) to February 2020 (date of the search). This systematic review adheres to the checklist reported by PRISMA.12 A detailed description of the search terms used for the electronic databases is included in the supplementary file.
2.2. Study inclusion
All titles and abstracts obtained from the initial search were screened for duplicate articles prior to checking for relevance in terms of economic analysis in TKA. This was carried out by 2 independent reviewers in order to ensure consistency. Full-text articles were retrieved and reviewed for further selection by applying the following inclusion/exclusion criteria.
2.2.1. Inclusion criteria
-
1.
Total knee arthroplasty/replacement as a comparator
-
2.
Other comparator is a non-operative management option, or a different surgical procedure performed on the knee.
-
3.
CEAs of different knee implant/prostheses for TKA
-
4.
Clinical studies only
-
5.
Studies adhering to methodology consistent with CEAs
2.2.2. Exclusion criteria
-
1.
Editorials reviews and conference abstracts
-
2.
Not in English language
-
3.
Studies without a comparator
-
4.
Studies that do not offer both a primary quality metric (e.g. quality-adjusted life-years, QALY) and cost calculation
-
5.
Studies comparing non-surgical factors, management options, or venous thromboprophylaxis
-
6.
Studies published before January 1, 1997
As the included studies list quality-adjusted life-years (QALYs) which require subjective valuations of health states by the patients, this review therefore focuses on cost-utility analyses.5,13 These studies use an incremental cost-effectiveness ratio (ICER), which is calculated by dividing the differences in costs by the differences in QALYs between two management options.11 ICERs are then evaluated against a willingness-to-pay (WTP) threshold, which indicates the maximum a healthcare consumer is willing to pay to obtain an additional QALY.14 Hence, if a management option's ICER falls below the WTP threshold relative to its comparator, it can be considered a cost-effective alternative.
2.3. PRISMA flowchart
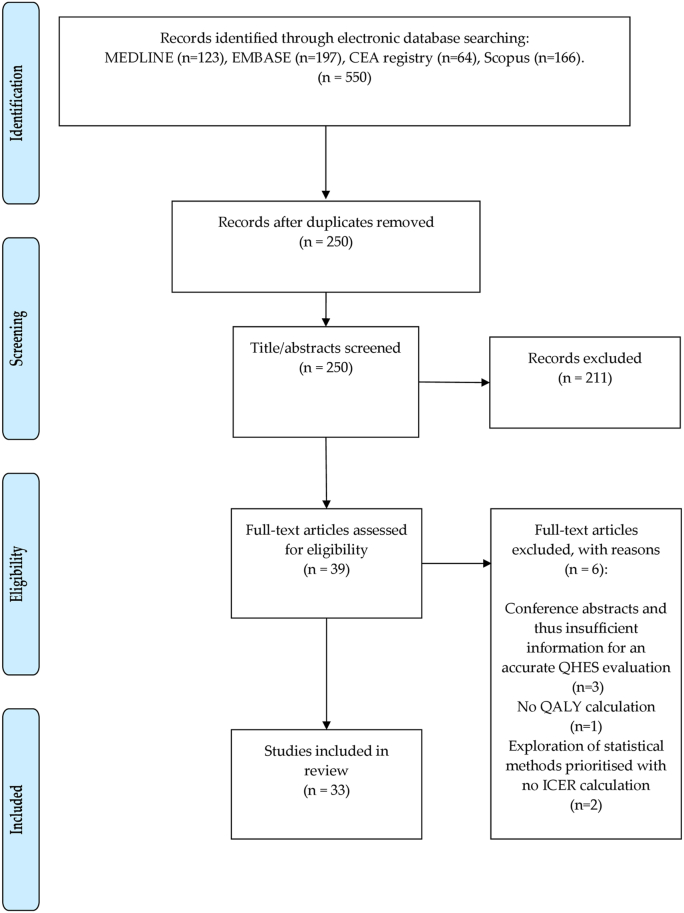
Fig. 1 shows a PRISMA flowchart demonstrating the search process used to identify the 33 studies from the electronic databases.
Fig. 1.
Shows a PRISMA flowchart demonstrating the search process involved in identifying the 33 studies from the electronic databases.
2.4. Quality assessment
Quality assessment of each study was performed using the QHES instrument.9 This score ranges from 0 to 100. Any score from 75 to 90 reflects a study of being good quality and above 90 as excellent quality. The QHES is designed to evaluate the use of suitable methods, valid, transparent results and their comprehensive reporting in each CEA.9 Each of the 33 studies were reviewed by the secondary author (KT) and the primary author (AK) for a 50% random sample using a random number generator to ensure at least a 90% inter-assessor agreement.
3. Results
3.1. Overall summary
An overview of the 33 CEAs is presented in Table 1. The ICER values have been adjusted to the 2020 US Dollar using the Consumer Price Index (CPI),15 2020 United Kingdom Pound using the Office for National Statistics CPI,16 and 2020 Euro from the European Commission and the European Central Bank's Harmonised Index of Consumer prices (HICP).17
Table 1.
Summary of the ICERs and key findings of all 33 CEA studies included in this review, stratified by comparator type.
| Author (year, country) | Study Design | ICERa | WTPa | Key findings |
|---|---|---|---|---|
| UKA vs TKA | ||||
| Soohoo et al. (2006, USA)33 | Decision Tree | Implant survival: | $50,000 | UKA is a cost-effective alternative for unicompartmental OA when the durability and function of the UKA implant is similar to the TKA one and with appropriate patient selection. However, when adjusted for 2020, UKA is no longer cost-effective to TKA for the 17 y vs 20 y category. |
| 12 y vs 15 y: $440, | ||||
| 11 y vs 15 y: TKA dominant, 17 y vs 20 y: $59,556^ | ||||
| Slover et al. (2006, USA)51 | Markov | Base case: UKA dominant | $50,000 | Although UKA was dominant, the average differences in costs and QALYs with TKA were $200 and 0.05 respectively. Thus, both have similar cost-effectiveness in the elderly low-demand population. |
| Peersman et al. (2014, Belgium)52 | Markov | UKA dominant | €10,000, €25,000, €50,000 | UKA offers a clear increase in health outcomes for a smaller cost than TKA for unicompartmental OA. |
| Ghomrawi et al. (2015, USA)34 | Markov | Age at surgery (TKA vs. UKA): | $100,000 | UKA is cost effective compared to TKA in patients over 65 years of age for end-stage unicompartmental OA. However, modest improvements in implant survival could make it cost-effective in younger patients. |
| 45 y: $34,011 | ||||
| 55 y: $71,425 | ||||
| 65 y,75 y,85 y: UKA Dominant | ||||
| Chawla et al. (2017, USA)35 | Markov | PFA vs TKA: | $50,000 | Improvements in implant survival have resulted in PFA being a more cost-effective joint preserving procedure in younger patients. |
| 50 y - $3445 | ||||
| 60 y - $ 3405 | ||||
| Burn et al. (2018, UK)53 | Markov | UKA Dominant | £20,000 | UKA is expected to provide better health outcomes and lower lifetime costs than TKR. However, surgeon usage of UKA has a significant impact on cost-effectiveness. |
| Xie et al. (2010, Canada)54 | Prospective cohort | Perspective (TKA vs. UKA): | $10,000 and $50,000 | There is a 0.4 probability of TKA being cost-effective from the societal or patient's perspective at a $50,000 WTP and 0.7 from the government's at a WTP of $10,000 for unicompartmental OA (without inflation adjustment). Longer study needed. |
| Societal - $79,430 | ||||
| Patient - $73,510 | ||||
| Government - $5917 | ||||
| Beard et al. (2019, UK)55 | RCT | PKR dominant | n/a | During the 5-year study period, PKR offers slightly better outcomes, lower surgical costs and lower follow-up health-care costs compared to TKR for treatment of late-stage isolated medial compartment OA. Hence, it should be first choice for it. |
| Resurfacing vs No Resurfacing in TKA | ||||
| Weeks et al. (2018, Canada)36 | Decision Tree | Resurfacing Dominant | n/a | Resurfacing the patella is cost-effective due to higher revision rates for non-resurfaced TKA. |
| Zmistowski et al. (2019, USA)37 | Decision Tree | Overall: $3,032b, | n/a | It is not cost effective to routinely resurface nonarthritic patella during primary TKA. Selective resurfacing for arthritic patients is vital for cost-effectiveness. |
| Nonarthritic patellae only: $183,584b | ||||
| Computer-Assisted TKA vs Non-Assisted TKA | ||||
| Novak et al. (2007, USA)31 | Decision Tree | $59,033^ | $50,000/ | Computer-assisted implant alignment systems increase the precision of component alignment enough to reduce failure rates and revisions to justify the extra cost vs. mechanical alignment systems during TKA (not true following 2020 ICER adjustment). |
| $100,000 | ||||
| Dong et al. (2006, UK)30 | Markov | CAS dominant vs. Conventional TKA | £30,000 | Computer-assisted TKA is cost effective in the long-term through reducing revision rates and complications via more precise component alignment. |
| Gothesen et al. (2013, Norway)29 | Markov | Conventional TKA dominant vs CAS | 500,000 kr (NOK) | At high operation volume hospitals, CAS needs to improve implant survival in 60 and 75-year-old cohorts just marginally for cost-effectiveness. A more significant increase is needed for low volume hospitals. |
| TKA vs Non-Operative Management | ||||
| Losina et al. (2009, USA)39 | Markov | Overall: $21,123 | $50,000/$100,000 | TKA is cost-effective across all risk groups for perioperative complications. |
| High-risk patients only: $36.415 | ||||
| Ponnusamy et al. (2018, Canada)40 | Markov | Non-obese: $3510, Overweight: $3,002, Obese: $3,118, Severely obese: $3,742, Morbidly obese: $5,853, Super obese: $12,569 | $50,000 | Not opting for TKA care based on BMI is not justified, even in the super obese cohort. |
| Elmallah et al. (2017, USA)56 | Prospective | $47,357 | $50,000 | Following an OA diagnosis, TKA is cost-effective. |
| Dakin et al. (2012, UK)38 | RCT | Baseline patient OKS: | £20,000 -£30,000 | The UK primary trust criteria (2012) restricting TKA to patients with pre-operative OKS <27 is denying a cost-effective treatment to patients above this OKS. |
| <9: £5,768, | ||||
| 9-11: £5,577, | ||||
| 12-13: £5,032, | ||||
| 14-15: £5,152, | ||||
| 16-17: £6,407, | ||||
| 18-19: £8,068, | ||||
| 20-21: £14,735, | ||||
| 22-24: £11,270, | ||||
| 25-27: £13,655, | ||||
| >27: £14,366, | ||||
| Skou et al. (2020, Denmark)57 | RCT | TKR plus non-surgical treatment vs. non-surgical treatment only: | €22,655 | From a 24-month perspective, in patients with moderate to-severe knee OA in secondary care in Denmark, TKR plus non-surgical (exercise, education, diet, insoles and pain medication). |
| Unadjusted base-case: €19,579, Adjusted base-case: €34,519, Adjusted base-case including deaths: €48,984 to 67,964 | treatment is not cost-effective compared with non-surgical treatment with the potential for later TKR if needed when adjusted for the covariates: age, sex and baseline values. | |||
| TKA Implant Type | ||||
| Fennema et al. (2014, Germany)46 | Markov | TKA with advanced low-wear bearings vs standard polyethylene bearing: overall: €18,198, patients <55 y: €722 and 75: €91,687b | €0, €10,000, €25,000, €50,000 | Low-wear articulations may be considered cost-effective overall but it is age-dependent, with the ICER being significantly lower for younger people than for older people, where it no longer becomes cost-effective. |
| Pennington et al. (2016, UK)48 | Markov | AGC Biomet dominated Genesis 2 and Triathlon. PFC Sigma dominated by Nexgen. | £20,000 - £30,000 | AGC Biomet prostheses are the least costly cemented unconstrained fixed brand for TKR but Nexgen prostheses lead to improved patient outcomes, at low additional cost and so should be first choice as they are the most cost-effective. |
| For 70 y men and women, Nexgen vs. AGC Biomet, £2715 and £2667 respectively | ||||
| Suter et al. (2013,USA)47 | Markov | Innovative vs standard TKA implants: ≥ 50% decrease in long-term TKA failure at ≤ 50% increased cost: < $100,000. | $150,000 | Innovative implants must decrease TKA failure by 50–55% or more compared to standard implants to be broadly cost-effective. |
| A 20% decrease in long-term failure at 50% increased cost: < $150,000 (only in healthy 50,59 y) | ||||
| Hamilton et al. (2013, UK)58 | RCT | Over a lifetime and at 1 year, Triathlon TKA dominated Kinemax TKA | £20,000 | The values for money saved per QALY were statistically insignificant and so both implants were of similar value using the SF-6D and QALY methodology. |
| Multiple Surgical Comparator studies | ||||
| Konopka et al. (2015, USA)59 | Markov | 50-60 y: | $50,000/$100,000/$150,000 | In a 50-60 y with unicompartmental medial knee OA, HTO is the most cost-effective management option. TKA is also more cost effective than UKA in the same cohort. |
| TKA vs HTO - $262,908, | ||||
| TKA vs UKA - $14,058c | ||||
| Kazarian et al. (2018, USA)60 | Markov | TKA dominates NST from 40 to 69 years and just over $16,494 at 80 y, UKA dominates TKA for all ages at time of treatment | $50,000 | In unicompartmental OA, using surgical treatments is cost-effective in all age groups. UKA should be prioritised over TKA. |
| Stan et al. (2015, Romania)45 | Prospective | TKA to unoperated knee dominates both TKA after HTO and CM | n/a | Careful patient selection could help optimise the cost-effectiveness of TKA as unoperated knee TKA is dominant to operated knee TKA. |
| Murray et al. (2014, UK)61 | RCT | Patellar resurfacing vs. no resurfacing: >95% probability of being cost-effective at WTP, | £20,000 | Patellar resurfacing is cost-effective, mobile bearings highly cost-effective (however there was considerable uncertainty), all-polyethylene components are poor value for money and should not be used in place of metal-backed components. |
| Mobile bearing vs fixed bearing: £2044 | ||||
| Metal-backed tibial components vs all-polyethylene ones: £43 | ||||
| Hak et al. (2013, UK)44 | n/a | Lifetime + 10-year durability: | £20,000–£30,000 | In the NHS, the KineSpring System is the most cost-effective strategy to treat knee OA. |
| UKA, HTO and KineSpring system dominated TKA, TKA dominated CM. | ||||
| TKA vs no treatment: | ||||
| £1,303b (lifetime) | ||||
| €4,153b (10 year) | ||||
| Marcacci et al. (2013, Italy)41 | n/a | Lifetime + 10 year durability: | €25,000–€30,000 | In the Italian Healthcare system, the KineSpring system offers the lowest cost/QALY and so is the most cost effective option. |
| UKA, HTO and KineSpring system dominated TKA, TKA dominated CM, TKA vs no treatment: | ||||
| €2348b (lifetime) | ||||
| €4,884b (10 year) | ||||
| Li et al. (2013, Germany)42 | n/a | KineSpring System dominated surgical treatments (TKA, UKA and HTO) and conservative management. | € 39,742 | The KineSpring System is the most cost-effective option for knee OA patients in Germany. |
| Surgical treatments vs. no treatment: €10,722 | ||||
| Strain et al. (2015, Spain)43 | n/a | Lifetime + 10-year durability: | €20,000 to €30,000 | Same results achieved in the Spanish Healthcare system as Italian.41 The KineSpring System is the most cost-effective treatment for knee OA. |
| UKA, HTO and KineSpring system dominated TKA, TKA dominated CM. TKA vs no treatment: | ||||
| €2530 (lifetime) | ||||
| €5264 (10 year) | ||||
| Other: | ||||
| Odum et al. (2013, USA)20 | Markov | Simultaneous bilateral TKA dominated staged bilateral TKA | $328,874 | Simultaneous bilateral total knee arthroplasty is more cost-effective with lower costs and greater health outcomes for the average patient. |
| Van der Woude et al. (2016, Netherlands)62 | Markov | KJD dominant vs. TKA | € 20,000 | Treating knee OA with KJD over TKA has a high potential to be cost-effective, which is most likely in the younger population. |
| Clement et al. (2019, UK)63 | Markov | Robot-assisted UKA vs TKA by annual patient case volume (patients/year): | £20,000 | Robot-assisted UKA is cost-effective compared with manual TKA for patients with isolated medial compartment knee OA. Increasing the annual patient case volume and reducing the length of hospital stay decreased the ICER of using rUKA over manual TKA. |
| 10: £7170b, | ||||
| 100: £1395b, | ||||
| 200 (with a 2 day stay): £648b, | ||||
| 200 (with a 1 day stay): £364b | ||||
BMI – Body Mass Index, CAS – Computer assisted surgery, CM – Conservative management, HTO – High Tibial Osteotomy, KJD - Knee Joint distraction, NST – Nonsurgical treatment, OA – Osteoarthritis, OKS – Oxford Knee Score, PFA – Patellofemoral arthroplasty, PKR – Partial Knee replacement, QALY – Quality-adjusted life year, RCT – Randomised controlled trial, SF-6D – Short-Form 6-Dimension health index, UKA - Unicompartmental knee arthroplasty, WTP – Willingness to pay threshold, y = Years of age.
^ Inflation adjustment resulted in ICER increasing above WTP threshold and so the management option no longer being cost effective. This is reflected in the main findings being altered.
All values are given per QALY.
Inflation year not given in study, so 2020 adjustment not performed.
ICER value has been calculated using provided information in study.
3.2. Main study characteristics
The main study characteristics are summarised in Table 2 and include (i) comparison of unicompartmental knee arthroplasty with total knee arthroplasty; (ii) analysis of patella resurfacing; (iii) analysis of computer-assisted arthroplasty; (iv) comparison of arthroplasty with non-operative management and other surgical alternatives; and (v) analysis of various knee arthroplasty implants.
Table 2.
Main study characteristics of cost-effectiveness studies of total knee arthroplasty (n = 33).
| Study characteristic | Number of studies | Percentage contribution to total (% to 1 decimal point) |
|---|---|---|
| Country of study | ||
| United States | 12 | 36.4 |
| United Kingdom | 9 | 27.3 |
| Canada | 3 | 9.1 |
| Other (Europe) | 9 | 27.3 |
| Study design | ||
| Randomised controlled trial | 5 | 15.2 |
| Prospective cohort | 3 | 9.1 |
| Markov | 17 | 51.5 |
| Decision Tree | 4 | 12.1 |
| Not stated | 4 | 12.1 |
| Data mining (health outcomes and costs) explained | ||
| Yes | 33 | 100 |
| No | 0 | 0 |
| Level of evidencea | ||
| I | 12 | 36.4 |
| II | 9 | 27.3 |
| III | 5 | 15.2 |
| IV | 7 | 21.2 |
| Perspective | ||
| Healthcare payer | 18 | 54.5 |
| Societal | 7 | 21.2 |
| Both | 4 | 12.1 |
| Not stated | 4 | 12.1 |
| Time Horizon | ||
| ≤10 years | 9 | 27.3 |
| >10 years | 7 | 21.2 |
| Lifetime | 17 | 51.5 |
| Not stated | 1 | 3.0 |
| Sufficient Time Horizon and Discounting (both costs and health outcomes) present | ||
| Yes | 26 | 78.8 |
| No | 7 | 21.2 |
| Sensitivity Analysis | ||
| Deterministic only | 5 | 15.2 |
| Probabilistic only | 4 | 12.1 |
| Both | 15 | 45.5 |
| Not stated | 8 | 24.2 |
| Unspecified | 1 | 3.0 |
| Statement of funding | ||
| Yes | 19 | 57.6 |
| No | 14 | 42.4 |
| Discussion of potential bias present | ||
| Yes | 8 | 24.2 |
| No | 25 | 75.8 |
“Other” countries include: Belgium, Denmark, Germany, Italy, Netherlands, Norway, Romania, and Spain.
Based on recommendations by Wright et al. (2003) for judging the level of evidence for a primary research question [64].
The study design of choice is the Markov model which was used in 17 of the 33 studies. All studies that originated from the USA were model-based, with nine out of 12 studies using the Markov model. However, those from the UK, Canada and other European countries (denoted as “other” in Table 2) used either model-based or real-life clinical studies indiscriminately. Three of the seven studies from the UK were RCTs.
Interestingly, the most common level of evidence in the studies was Level-I (n = 36.4%). The percentage of studies above Level-II reporting was 63.6% (n = 21) indicating that CEAs tend towards higher levels of evidence. This is important as higher levels of evidence are more convincing to surgeons when tackling clinical dilemmas18 such as the ones in question here. However, it is important to acknowledge that since RCTs are not always possible,19 Level-I evidence may not be available for all clinical scenarios and Level-III or IV evidence may still be of great value to orthopaedic surgeons.
Another important finding is that 54.5% (n = 18) CEAs used exclusively a healthcare perspective in order to derive the costs. Seven studies used societal, four used both and four did not mention such information. Five of the 12 studies (38.5%) from the USA used exclusively a healthcare and another five (38.5%) the societal perspective. This is in contrast to the UK, where studies displayed a slight preference towards the healthcare payer perspective (n = 4, 57.1%). These costs and the associated health outcomes following TKA were calculated across a range of time horizons. There is a trend towards the USA preferring longer time horizons, with 83.3% (n = 10) of their studies declaring either a lifetime or duration greater than 10 years. In contrast, the UK has shown a preference towards time horizons of 10 years and below with 66.7% (n = 6) of their CEAs fitting into this category. No significant trends were observed for the studies from Canada or the European countries.
When the studies were stratified by their country of origin, the USA had the largest range of WTP thresholds between $50,000 per QALY up to $150,000. Furthermore, the study by Odum et al.20 suggested that the WTP for the USA should be no higher than $328,874 per QALY.21 This range was to be expected based on previous evidence. Studies performed in the UK had an expected WTP range of between £20,000 to £30,000 per QALY, which is decided by the National Institute for Health and Care Excellence (NICE).22
There were a few ill-defined trends when stratifying the type of sensitivity analysis used by country of origin. Eight out of the 12 (66.67%) CEAs from the USA incorporated both a deterministic and probabilistic sensitivity analysis. This indicates a slight preference to using both. Canada, the UK and the other European countries showed no reasonable preference with their choice of sensitivity analysis type.
Only 57.6% (n = 19) of the studies disclosed their source of funding. Of these 19 studies, 47.4% (n = 9) received funding from exclusively public sources, 10.5% (n = 2) from exclusively private sources, 26.3% (n = 3) from both and 15.8% (n = 3) reported having received no funding. The remaining 14 CEAs failed to disclose any funding sources in their reports.
3.3. CEA study quality
The mean summed QHES score for all 33 CEAs studies present was 83.7. According to the thresholds set by Tran et al.,23 this would mean the studies were on average of good quality. This is perhaps expected considering that the first CEA studies using TKA as a comparator were first published in 2006, ten years after the First Panel on Cost-Effectiveness in Health and Medicine published their first guidelines for the conduct of CEA studies in 1996.11 The majority of CEAs that failed to achieve higher scores fell short in similar QHES categories including failing to state or justify the perspective used, not discussing the extent of potential biases and not including a statement of funding.
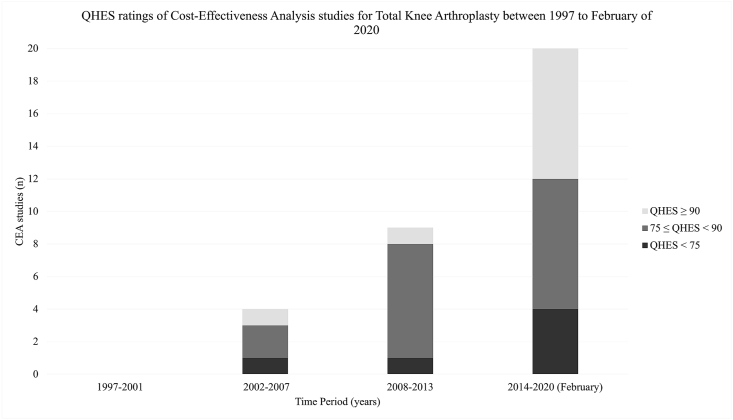
Since 1997, there has been a significant increase in the number of CEAs using TKA as a comparator. Over time, there has been a reduction in the proportion of these CEAs that have scored over 75 in the QHES after an initial increase between 2002-2007 and 2008–2013 (Fig. 2). From 2002 to 2007, 75% (n = 3) of the studies scored greater than 75. This was higher at 88.9% (n = 8) between 2008 and 2013, and 80.0% (n = 16) between 2014–February 2020. However, this result must be acknowledged in the context that there were four, nine and 20 studies in these year groups respectively. This data is illustrated in Fig. 2.
Fig. 2.
Bar graph demonstrating the 33 cost-effectiveness analyses (CEAs) studies identified published over time stratified by their QHES scores. Scores of 90 and above are considered excellent quality, between 75 and 90 of high-quality and below 75 of poor quality. 23
The average QHES scores following stratification of the CEAs via their comparators used are displayed in Table 3.
Table 3.
Mean QHES scores of CEA studies in this review stratified by comparator type (n = 30).
| Comparator Type | Mean QHES (n) |
|---|---|
| UKA vs TKA | 84.8 (8) |
| Resurfacing vs no resurfacing in TKA | 90 (2) |
| Other | 87.3 (3) |
| Computer-assisted TKA vs non-assisted TKA | 86.7 (3) |
| TKA vs non-operative management | 84.8 (5) |
| Multiple surgical comparator studies | 79.6 (8) |
| TKA implant type | 80 (4) |
4. Discussion
Overall, the mean quality of studies as measured by the QHES was high at 83.7. This is comparable to the mean QHES scores observed in the subspecialties of upper-limb orthopaedic surgery,7 trauma6 and sports medicine5 which were 82, 79.25 and 81.8 respectively. This suggests that the findings drawn from such studies are of significant value and of greater applicability to clinical decision-making.
However, when stratified into the comparator type categories mentioned earlier, there is a considerable range in both the quantity of studies published and their respective qualities. The mean values for the CEAs following stratification were all still above 75. Between 2014 and 2020 (February), there was a drop in the proportion of high-quality (QHES>75) studies compared to the time period 2008–2013 from 88.9% to 80%. This trend was also observed for CEA studies in upper-limb orthopaedic surgery,7 where there was a greater proportion of studies demonstrating lower quality (QHES<75) in recent years. This is concerning as it reflects a loss of CEA standards performed across orthopaedics. The Second Panel on Cost-Effectiveness in Health and Medicine has published a new set of guidelines in 201624, which should help to re-establish high-quality methodological CEA study practices to maintain standards.
Despite a majority reporting of the perspective used in the CEAs, it is important for indirect societal and direct healthcare costs to be calculated as they are both important. Patient recovery times for the ability to resume normal daily activities following TKA may extend up to 12 weeks or more. This highlights the importance of any unpaid patient costs of lost productivity and unpaid caregiving. This is emphasised by the Second Panel's recommendation to consider both the healthcare payer and societal perspectives when CEAs are performed.24 This needs to be emphasised so that future CEAs may incorporate both measures and provide a more realistic cost-effective analysis for clinical decision-making.
It is surprising that only five of the 18 studies from the UK and other European countries used RCTs to conduct their CEA. Health-technology assessment (HTA) bodies in Europe emphasise funding for RCT-based research as clinical evidence for CEA studies.25,26 No RCT CEA studies were identified from the USA. This was also observed by Rajan et al.7 who looked at CEAs in upper-limb surgery. There has been significant political resistance to the use of CEA studies in Federal coverage decisions for insurance policies, including Medicare.27 72.2% (n = 13) CEAs from the UK, Canada and other European countries included at least the healthcare perspective to account for the costs in their analyses. This is in agreement with the formal integration by the HTA bodies of these nations to use CEAs to guide policies of whether to fund a particular management option or not. Hence, third-party payer perspectives are prioritised.24,28 In contrast, the USA does not show a preference for their payer perspective despite the United States-based First Panel's recommendation to use societal perspectives.24,28
A limitation of using model-based papers in a review such as this is made apparent with the recommendation for CAS. Based on the papers selected, TKA with CAS was suggested to be more cost-effective as CAS increases the precision of component alignment during TKA, therefore reducing long-term failure rates and complications sufficiently to justify its extra costs.29, 30, 31 However, in the study by Novak et al.,31 under a WTP of the $50,000, the ICER for CAS following adjustment to the 2020 inflation value becomes $59,033. This now suggests it to be no longer cost-effective against conventional mechanical alignment systems at the given WTP. In addition, it was shown that hospital operation volume may influence the cost-effectiveness of CAS with low volume hospitals needing a much greater increase in implant survival for it to be deemed cost-effective compared to high operation volume hospitals.29 However, it must be noted that only three studies looked at TKA with CAS and all of these were model-based.29, 30, 31 CAS may offer theoretical benefit and decrease outliers in some instances, but this conclusion has not been supported in either registry data or smaller prospective studies. The authors of the three papers quoted also recognise this as a shortcoming in their conclusion.
There has been considerable CEA research into the use of UKA over TKA for unicompartmental osteoarthritis (n = 8) and using multiple comparators to TKA such as HTO and UKA (n = 8). However, little research has been undertaken in areas such as the use of patella resurfacing in TKA (n = 2) and computer assisted TKA (n = 3). Modern surgery is more commonly incorporating computer-guidance systems in surgeries such as TKA32 so there is now an expectation that the number of CEAs exploring this field will increase in the future.
If we consider UKA over TKA for unicompartmental osteoarthritis, UKA was suggested to be the more cost-effective option overall according to the reviewed studies due to the associated improved health outcomes, and lower surgical and follow-up costs. However, the studies included suffer from similar modelling data input issues as previously discussed in the CAS subsection and conclusions need to be interpreted with caution. Following inflation adjustment to 2020 values, UKA appeared to no longer be cost-effective in the 17y vs 20y category.33 UKA was suggested to not be cost-effective in younger patients below 65y. The studies suggest that the cost-effectiveness of UKA may improve through increasing the surgeon's usage of UKA and the implant's durability and function.33, 34, 35
Resurfacing the patella was suggested to be cost-effective compared to not resurfacing it during TKA largely due to the associated reduced revision rates.36,37 However, Zmistowski et al. showed that this was perhaps only true when resurfacing was performed on arthritic patella as routinely resurfacing non-arthritic patella was not shown to be cost-effective.37 This finding is also supported by national joint registry data.
When comparing TKA to non-operative management options, TKA was proposed to be more cost-effective regardless of patient factors that could potentially influence decision-making policies such as their OKS,38 risk for perioperative complications39 and BMI.40 These findings are important in ensuring a potentially more cost-effective treatment option is not denied to patients based on these aforementioned metrics.
Regarding the eight CEAs that used multiple comparators in their cost-effective analysis, various findings may be drawn. The KineSpring system dominated other surgical treatment methods in Italy,41 Germany,42 Spain43 and the UK.44 However, the mean QHES score for these studies was the lowest at 79.6 and they failed to disclose their funding source. In addition, careful patient selection can help optimize the cost-effectiveness of TKA; TKA on an unoperated knee is dominant to that after HTO and CM.45
Lastly, regarding the TKA implant type, the key factors that influenced whether certain implant types were deemed cost-effective were suggested to be the age of the patient at surgery46 and the degree to which the given implant reduces the failure rate of TKA. This results in a reduced need for revision surgeries.47 For example, low-wear bearings were shown to be cost-effective compared to polyethylene bearings, but this was age-dependent, with the ICER being significantly higher in the older population where it was no longer cost-effective.46 Furthermore, the Nexgen implant was shown to be dominant over the Triathlon, Genesis 2 and PFC Sigma ones.48 In addition, Nexgen was also indicated as more cost-effective than AGC Biomet by leading to improved patient outcomes at low additional cost.48 However, it is important to acknowledge that these findings are based on large national joint registries and are therefore vulnerable to all their associated shortcomings. They do not control for all the relevant variables involved, which include: the surgeon's skill, technique, and experience, implant design changes over time, bone cement type and implant alignment.
Any systematic review is limited by the quality of the included studies, and studies were not excluded following the results of quality checks such as the QHES. A potential limitation could be that the CEAs included in this review were conducted across 11 countries in total. This may result in problems associated with the generalisability and so transferability of the CEA study findings to other healthcare settings.49,50 Using the QHES to measure study quality is also prone to bias due to the subjective nature of reporting by the assessor. However, we ensured at least a 90% inter-assessor agreement between both reviewers. Lastly, the QHES values study design reporting, and gives equal weight to the different methods without consideration of their respective limitations resulting in analytical bias.
5. Conclusions
Overall, CEA studies in TKA have been increasing in number over the past couple of decades. This reflects the increasing interest and importance of this field within resource-limited healthcare environments and with ageing populations. It is reassuring to see a high mean quality of reporting in CEAs whose findings can therefore certainly be considered in clinical decision making. However, there is a clear need for further studies and a greater quality of reporting in the cost-effectiveness literature for total knee arthroplasty in orthopaedic surgery to ensure that high standards of reporting are not lost over time. Results of CEAs should also be interpreted in local healthcare contexts. Therefore, it will be vital to monitor the ongoing quality of these studies in accordance with Second Panel Recommendations.24
CRediT authorship contribution statement
Achi Kamaraj: Formal analysis, Writing - original draft, contributed to the design and implementation of the research, to the analysis of the results. Kendrick To: Formal analysis, Writing - original draft, contributed to the design and implementation of the research. KT Matthew Seah: Formal analysis, Writing - original draft, contributed to the design and implementation of the research. Wasim S. Khan: Formal analysis, Writing - original draft, contributed to the design and implementation of the research.
Declaration of competing interest
All authors have no conflicts of interest to declare.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jor.2020.10.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhang Y., Jordan J.M. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence R.C., Felson D.T., Helmick C.G. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inacio M.C.S., Paxton E.W., Graves S.E., Namba R.S., Nemes S. Projected increase in total knee arthroplasty in the United States - an alternative projection model. Osteoarthritis Cartilage. 2017;25:1797–1803. doi: 10.1016/j.joca.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Aujla R.S., Esler C.N. Total knee arthroplasty for osteoarthritis in patients less than fifty-five years of age: a systematic review. J Arthroplasty. 2017;32:2598–2603. doi: 10.1016/j.arth.2017.02.069. e2591. [DOI] [PubMed] [Google Scholar]
- 5.Nwachukwu B.U., Schairer W.W., Bernstein J.L., Dodwell E.R., Marx R.G., Allen A.A. Cost-effectiveness analyses in orthopaedic sports medicine: a systematic review. Am J Sports Med. 2014;43:1530–1537. doi: 10.1177/0363546514544684. [DOI] [PubMed] [Google Scholar]
- 6.Nwachukwu B., Schairer W., O'Dea E., McCormick F., Lane M. The Quality of Cost-Utility Analyses in Orthopedic Trauma. J. Orthopedics. 2015;38:e673–e680. doi: 10.3928/01477447-20150804-53. [DOI] [PubMed] [Google Scholar]
- 7.Rajan P.V., Qudsi R.A., Dyer G.S.M., Losina E. Cost-utility studies in upper limb orthopaedic surgery. J. Bone Joint. 2018;100-B:1416–1423. doi: 10.1302/0301-620X.100B11.BJJ-2018-0246.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karhade A.V., Kwon J.Y. Cost-utility analyses in US orthopaedic foot and ankle surgery: a systematic review. Foot Ankle Spec. 2018;11:548–552. doi: 10.1177/1938640018782588. [DOI] [PubMed] [Google Scholar]
- 9.Ofman J.J., Sullivan S.D., Neumann P.J. Examining the value and quality of health economic analyses: implications of utilizing the QHES. J Manag Care Pharm. 2003;9:53–61. doi: 10.18553/jmcp.2003.9.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cost-effectiveness analysis registry. http://healtheconomics.tuftsmedicalcenter.org/cear4/Home.aspx
- 11.Weinstein M.C., Siegel J.E., Gold M.R., Kamlet M.S., Russell L.B. Recommendations of the Panel on cost-effectiveness in health and medicine. J Am Med Assoc. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G. Group, the P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement The PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 13.Brauer C.A., Neumann P.J., Rosen A.B. Trends in cost effectiveness analyses in orthopaedic surgery. Clin Orthop Relat Res. 2007;457:42–48. doi: 10.1097/BLO.0b013e31803372c9. [DOI] [PubMed] [Google Scholar]
- 14.Bertram M.Y., Lauer J.A., De Joncheere K. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ. 2016;94:925–930. doi: 10.2471/BLT.15.164418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Consumer price index inflation calculator. http://www.bls.gov/data/inflation_calculator.htm/ US Department of Labor. Bureau of Labor Statistics.
- 16.UK inflation calculator.” U.S. Official inflation data, alioth finance. 2 Feb 2020. https://www.officialdata.org/uk/inflation/2011?amount=35
- 17.Euro Inflation Calculator.” U.S. Official Inflation Data, Alioth Finance. 2 Feb 2020. https://www.officialdata.org/eu/inflation/2012?amount=2348 [Google Scholar]
- 18.Hurwitz S.R., Slawson D., Shaughnessy A. Orthopaedic information mastery: applying evidence-based information tools to improve patient outcomes while saving orthopaedists' time. J. Bone Jt. Surg. Am. Vol. 2000;82:888–894. doi: 10.2106/00004623-200006000-00020. [DOI] [PubMed] [Google Scholar]
- 19.McLeod R.S., Wright J.G., Solomon M.J., Hu X., Walters B.C., Lossing Al. Randomized controlled trials in surgery: issues and problems. Surgery. 1996;119:483–486. doi: 10.1016/s0039-6060(96)80254-6. [DOI] [PubMed] [Google Scholar]
- 20.Odum S.M., Troyer J.L., Kelly M.P., Dedini R.D., Bozic K.J. A cost-utility analysis comparing the cost-effectiveness of simultaneous and staged bilateral total knee arthroplasty. J Bone Jt Surg Am Vol. 2013;95:1441–1449. doi: 10.2106/JBJS.L.00373. [DOI] [PubMed] [Google Scholar]
- 21.Braithwaite R.S., Meltzer D.O., King J.T., Jr., Leslie D., Roberts M.S. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46 doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 22.Shiroiwa T., Sung Y.-K., Fukuda T., Lang H.-C., Bae S.-C., Tsutani K. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ. 2010;19:422–437. doi: 10.1002/hec.1481. [DOI] [PubMed] [Google Scholar]
- 23.Tran B.X., Nong V.M., Maher R.M., Nguyen P.K., Luu H.N. A systematic review of scope and quality of health economic evaluation studies in Vietnam. PLoS One. 2014;9 doi: 10.1371/journal.pone.0103825. e103825-e103825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanders G.D., Neumann P.J., Basu A. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second Panel on cost-effectiveness in health and MedicineRecommendations from the Second Panel on cost-effectiveness in health and MedicineRecommendations. J Am Med Assoc. 2016;316:1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 25.Health Technology Assessment (HTA) Programme National Institute for health research. National health service (NHS) http://www.nets.nihr.ac.uk/programmes/hta
- 26.Velasco-Garrido M.B.R. European Observatory on Health Systems and Policies. World Health Organization (WHO); 2005. Health technology assessment: an introduction to objectives, role of evidence, and structure in Europe.http://www.euro.who.int/__data/assets/pdf_file/0018/90432/E [Google Scholar]
- 27.Garber A.M., Phelps C.E. Future costs and the future of cost-effectiveness analysis. J Health Econ. 2008;27:819–821. doi: 10.1016/j.jhealeco.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neumann Pj SGDRLBSJEGTG . second ed. Oxford University Press; New York: 2017. Cost Effectiveness in Health and Medicine. [Google Scholar]
- 29.Gothesen O., Slover J., Havelin L. An economic model to evaluate cost-effectiveness of computer assisted knee replacement surgery in Norway. BMC Muscoskel Disord. 2013;14:202. doi: 10.1186/1471-2474-14-202. 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong H., Buxton M. Early assessment of the likely cost-effectiveness of a new technology: a Markov model with probabilistic sensitivity analysis of computer-assisted total knee replacement. Int J Technol Assess Health Care. 2006;22:191–202. doi: 10.1017/S0266462306051014. [DOI] [PubMed] [Google Scholar]
- 31.Novak E.J., Silverstein M.D., Bozic K.J. The cost-effectiveness of computer-assisted navigation in total knee arthroplasty. J Bone Jt Surg Am Vol. 2007;89:2389–2397. doi: 10.2106/JBJS.F.01109. [DOI] [PubMed] [Google Scholar]
- 32.Siddiqi A., Hardaker W., Eachempati K., Sheth N. Advances in computer-aided total knee arthroplasty technology. Orthopedics. 2017;40 doi: 10.3928/01477447-20170831-02. [DOI] [PubMed] [Google Scholar]
- 33.Soohoo N.F., Sharifi H., Kominski G., Lieberman J.R. Cost-effectiveness analysis of unicompartmental knee arthroplasty as an alternative to total knee arthroplasty for unicompartmental osteoarthritis. J Bone Jt Surg Am Vol. 2006;88:1975–1982. doi: 10.2106/JBJS.E.00597. [DOI] [PubMed] [Google Scholar]
- 34.Ghomrawi H.M.H.M., Eggman A.A., Pearle A.D. Effect of age on cost-effectiveness of unicompartmental knee arthroplasty compared with total knee arthroplasty in the U.S. J Bone Jt Surg Am Vol. 2015;97:396–402. doi: 10.2106/JBJS.N.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chawla H., Nwachukwu B.U., van der List J.P. Cost effectiveness of patellofemoral versus total knee arthroplasty in younger patients. J. Bone Joint. 2017;99-B:1028–1036. doi: 10.1302/0301-620X.99B8.BJJ-2016-1032.R1. [DOI] [PubMed] [Google Scholar]
- 36.Weeks C.A., Marsh J.D., MacDonald S.J., Graves S., Vasarhelyi E.M. Patellar resurfacing in total knee arthroplasty: a cost-effectiveness analysis. J Arthroplasty. 2018;33:3412–3415. doi: 10.1016/j.arth.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Zmistowski B.M., Fillingham Y.A., Salmons H.I. Routine patellar resurfacing during total knee arthroplasty is not cost-effective in patients without patellar arthritis. J Arthroplasty. 2019 doi: 10.1016/j.arth.2019.04.040. [DOI] [PubMed] [Google Scholar]
- 38.Dakin H., Gray A., Fitzpatrick R., MacLennan G., Murray D. Rationing of total knee replacement: a cost-effectiveness analysis on a large trial data set. BMJ Open. 2012 doi: 10.1136/bmjopen-2011-000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Losina E., Walensky R.P., Kessler C.L. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169:1113–1121. doi: 10.1001/archinternmed.2009.136. discussion 1121-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ponnusamy K.E., Vasarhelyi E.M., Somerville L. Cost-effectiveness of total knee arthroplasty vs nonoperative management in normal, overweight, obese, severely obese, morbidly obese, and super-obese patients: a Markov model. J Arthroplasty. 2018;33:S32–S38. doi: 10.1016/j.arth.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 41.Marcacci M., Zaffagnini S., Li C.S., Bhandari M. Cost-effectiveness and economic impact of the KineSpring® knee implant system in the treatment of knee osteoarthritis in Italy. J Long Term Eff Med Implants. 2013;23:211–222. doi: 10.1615/jlongtermeffmedimplants.v23.i2-03.90. [DOI] [PubMed] [Google Scholar]
- 42.Li C.S., Seeger T., Auhuber T.C., Bhandari M. Cost-effectiveness and economic impact of the KineSpring® Knee Implant System in the treatment for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2013;21:2629–2637. doi: 10.1007/s00167-013-2427-x. [DOI] [PubMed] [Google Scholar]
- 43.Strain D., Li C.S., Phillips M. Cost effectiveness and economic impact of the KineSpring knee implant system in the treatment of knee osteoarthritis in Spain. J Long Term Eff Med Implants. 2015;25:225–235. doi: 10.1615/jlongtermeffmedimplants.2015012728. [DOI] [PubMed] [Google Scholar]
- 44.Hak A., Li C.S., Bhandari M. Cost-effectiveness and economic impact of the KineSpring Knee Implant System in the treatment of knee osteoarthritis in the United Kingdom. J Long Term Eff Med Implants. 2013;23:199–210. doi: 10.1615/jlongtermeffmedimplants.2013010141. [DOI] [PubMed] [Google Scholar]
- 45.Stan G., Orban H., Orban C. vol. 110. 2015. pp. 368–374. (Cost Effectiveness Analysis of Knee Osteoarthritis Treatment. Chirurgia (Bucharest, Romania : 1990)). [PubMed] [Google Scholar]
- 46.Fennema P., Heyse T.J., Uyl-de Groot C.A. Cost-effectiveness and clinical implications of advanced bearings in total knee arthroplasty: a long-term modeling analysis. Int J Technol Assess Health Care. 2014;30:218–225. doi: 10.1017/S0266462314000129. [DOI] [PubMed] [Google Scholar]
- 47.Suter L.G., Paltiel A.D., Rome B.N. Placing a price on medical device innovation: the example of total knee arthroplasty. PloS One. 2013;8 doi: 10.1371/journal.pone.0062709. e62709-e62709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pennington M., Grieve R., Black N., van der Meulen J.H. Cost-effectiveness of five commonly used prosthesis brands for total knee replacement in the UK: a study using the NJR dataset. PloS One. 2016;11 doi: 10.1371/journal.pone.0150074. e0150074-e0150074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hughes D., Charles J., Dawoud D. Conducting economic evaluations alongside randomised trials: current methodological issues and novel approaches. Pharmacoeconomics. 2016;34:447–461. doi: 10.1007/s40273-015-0371-y. [DOI] [PubMed] [Google Scholar]
- 50.Koopmanschap M.A., Touw K.C., Rutten F.F. Analysis of costs and cost-effectiveness in multinational trials. Health Pol. 2001;58:175–186. doi: 10.1016/s0168-8510(01)00142-7. [DOI] [PubMed] [Google Scholar]
- 51.Slover J., Espehaug B., Havelin LI. Cost-effectiveness of unicompartmental and total knee arthroplasty in elderly low-demand patients: A Markov decision analysis. J Bone Jt Surg - Ser A. 2006;88(11):2348–2355. doi: 10.2106/JBJS.E.01033. [DOI] [PubMed] [Google Scholar]
- 52.Peersman G., Jak W., Vandenlangenbergh T. Cost-effectiveness of unicondylar versus total knee arthroplasty: A Markov model analysis. Knee. 2014;21(S1):S37–S42. doi: 10.1016/S0968-0160%2814%2950008-7. [DOI] [PubMed] [Google Scholar]
- 53.Burn E., Liddle A., Hamilton T., Pandit H., Murray D., Judge A. Cost-utility of unicompartmental compared to total knee replacements: An analysis based on matched routinely-collected data from the United Kingdom. Value Heal. 2016;19(7):A539. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed17&NEWS=N&AN=613235830 [Google Scholar]
- 54.Xie F., Lo N.N., Tarride J.E., O’Reilly D., Goeree R. Total or partial knee replacement? Cost-utility analysis in patients with knee osteoarthritis based on a 2-year observational study. Eur J Heal Econ. 2010;11(1):27–34. doi: 10.1007/s10198-009-0154-5. [DOI] [PubMed] [Google Scholar]
- 55.Beard DJ, Davies LJ, Cook JA. The clinical and cost-effectiveness of total versus partial knee replacement in patients with medial compartment osteoarthritis (TOPKAT): 5-year outcomes of a randomised controlled trial. Greshon A, Holl MG, Lancet (London, editors. Fusco. 2019;394(10200):746–756. doi: 10.1016/S0140-6736(19)31281-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elmallah R.K., Chughtai M., Khlopas A. Determining cost-effectiveness of total hip and knee arthroplasty using the short form-6D utility measure. J Arthropl. 2017;32(2):351–354. doi: 10.1016/j.arth.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 57.Skou S.T., Roos E., Laursen M. Cost-effectiveness of total knee replacement in addition to non-surgical treatment: A 2-year outcome from a randomised trial in secondary care in Denmark. BMJ Open. 2020;10(1) doi: 10.1136/bmjopen-2019-033495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamilton D.F., Clement N.D., Burnett R. Do modern total knee replacements offer better value for money? A health economic analysis. Int Orthop. 2013;37(11):2147–2152. doi: 10.1007/s00264-013-1992-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Konopka J.F., Gomoll A.H., Thornhill T.S. The cost-effectiveness of surgical treatment of medial unicompartmental knee osteoarthritis in younger patients a computer model-based evaluation. J Bone Jt Surg Am. 2015;97(10):807–817. doi: 10.2106/JBJS.N.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kazarian G.S., Lonner J.H., Maltenfort M.G. Cost-effectiveness of surgical and nonsurgical treatments for unicompartmental knee arthritis: Markov model. J Bone Jt Surg Am. 2018;100(19):1653–1660. doi: 10.2106/JBJS.17.00837. [DOI] [PubMed] [Google Scholar]
- 61.Murray D.W., MacLennan G.S., Breeman S., Dakin H.A., Fiddian N., Fitzpatrick R., Grant A.M., Gray A.M., Johnston L., MacLennan G.S., Morris R.W., Murray D.W., Rowley D., Breeman S., Campbell M.K., Campbell S., Ellington J., Grant A.M., Kelaher M., Langston A., MacLennan G.S., McCormack K., Ramsay C., Ross S., Vale C.M.K. A randomised controlled trial of the clinical effectiveness and cost-effectiveness of different knee prostheses: The Knee Arthroplasty Trial (KAT) Health Technol Assess (Rockv) 2014;18(19):1–235. doi: 10.3310/hta18190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Woude J.A.D., Nair S.C., Custers R.J.H. Knee joint distraction compared to total knee arthroplasty for treatment of end stage osteoarthritis: Simulating long-term outcomes and cost-effectiveness. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0155524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clement N.D., Deehan D.J., Patton J.T. Robot-assisted unicompartmental knee arthroplasty for patients with isolated medial compartment osteoarthritis is cost-effective: a markov decision analysis. Bone Joint J. 2019;101-B(9):1063–1070. doi: 10.1302/0301-620X.101B9.BJJ-2018-1658.R1. [DOI] [PubMed] [Google Scholar]
- 64.Wright J.G., Swiontkowski M.F., Heckman J.D. Introducing levels of evidence to the journal. J Bone Joint Surg Am. 2003;85(1):1–3. https://pubmed.ncbi.nlm.nih.gov/12533564 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.