Abstract
Objective:
Patients with Xq26.3 microduplication present early-childhood gigantism due to marked growth hormone (GH) hypersecretion from mixed GH-PRL adenomas and hyperplasia. The microduplication includes GPR101 which is upregulated in patients’ tumor tissue. The GPR101 gene codes for an orphan G protein coupled receptor that is normally highly expressed in the hypothalamus. Our aim was to determine whether GPR101 loss of function mutations or deletions could be involved in patients with congenital isolated GH deficiency (GHD).
Design and methods:
Taking advantage of the cohort of patients from the GENHYPOPIT network, we studied 41 patients with unexplained isolated GHD. All patients had Sanger sequencing of the GPR101 gene and array comparative genome hybridization (aCGH) to look for deletions. Functional studies (cell culture with GH secretion measurements, cAMP response) were performed.
Results:
One novel GPR101 variant, c.589 G>T (p.V197L), was seen in the heterozygous state in a patient with isolated GHD. In silico analysis suggested that this variant could be deleterious. Functional studies did not show any significant difference in comparison with wild type for GH secretion and cAMP response. No truncating, frameshift, or small insertion-deletion (indel) GPR101 mutations were seen in the 41 patients. No deletion or other copy number variation at chromosome Xq26.3 was found on aCGH.
Conclusion:
We found a novel GPR101 variant of unknown significance, in a patient with isolated GH deficiency. Our study did not identify GPR101 abnormalities as a frequent cause of GH deficiency.
Keywords: hypopituitarism, gigantism, X-LAG syndrome, growth hormone deficiency, congenital hypopituitarism
Introduction
Isolated GH deficiency (IGHD) is the most common pituitary hormone deficiency. The incidence of congenital IGHD is estimated to be 1/4000–10000, and as up to 30% are familial, this suggests that genetic causes are likely to be frequent[1, 2]. Genetic forms of IGHD are classified into 4 different types, based on the mode of inheritance (autosomal dominant or recessive) and the severity of the phenotypes [2, 3]. Mutations of GH1, or the genes encoding the GHRH receptor or ghrelin are the most frequent etiologies of genetic forms of IGHD[1]. Of note, GHD can also be the initial clinical manifestation of a wider phenotype of combined pituitary hormone deficiencies; in those cases, mutations of several genes encoding transcription factors (POU1F1, PROP1, LHX3, LHX4) have been described [4, 5]. Altogether, however, the genetic etiologies of <20% of GHD cases are known currently and the cause of the majority of supposedly genetic GHD therefore remains undetermined[1]. Recently, we reported a microduplication on chromosome Xq26.3 in patients with early childhood-onset gigantism [6]. Patients with this condition -termed X-linked acrogigantism (X-LAG) syndrome- present with mixed GH-PRL adenomas with or without hyperplasia that leads to marked GH and IGF-1 hypersecretion [7]. This is usually accompanied by hyperprolactinemia of variable severity. The clinical profile is one of generally normal-sized infants born following unremarkable pregnancies in which dramatically accelerated height and weight gain occurs at a median age of 12 months[6, 7]. The usual presentation is sporadic, but Xq26.3 microduplications also have been found in two familial isolated pituitary adenoma (FIPA) kindreds [6]. The microduplicated region contains four protein-coding genes, among which only GPR101 is upregulated in tumor tissue from X-LAG patients. The GPR101 gene codes for an orphan G protein coupled receptor (GPCR) that is expressed in the hypothalamus [6]. Overexpression studies of a putative activating GPR101 variant in GH3 cells led to increased GH secretion; this GPR101 sequence variant also occurs in a subgroup of patients with acromegaly, usually in tumor tissue [6, 8].
Given the phenotypes involving GPR101 in X-LAG syndrome and acromegaly, we wanted to study whether deletions or loss-of-function mutations of GPR101 could explain cases of congenital isolated GHD of unknown etiology. Taking advantage of the international GENHYPOPIT network[9], aimed at identifying new aetiologies of congenital hypopituitarism, we looked for mutations of GPR101 and deletions on chromosome Xq26.3 in a group of patients with congenital isolated GHD.
Methods
Subjects
The GENHYPOPIT network was launched as a multicenter study involving both French and international pediatric and adult endocrinology centers. The precise characteristics of this network have been previously reported[9]. After written informed consent was given, blood samples were collected from patients and, whenever possible, first-degree relatives. Informed written consent was obtained from the parents, caretakers or guardians on behalf of the minor/children enrolled in the study. The study was approved by the Ethics committees of the University of Aix-Marseille II (France) and the Centre Hospitalier Universitaire de Liege (Belgium).
Endocrine and imaging studies
Hormonal studies and intracranial imaging were performed in all patients at each referring medical center. Structural malformations on magnetic resonance imaging (MRI) were systematically sought and recorded. Patients with a known postnatal cause of acquired hypopituitarism were excluded. Complete GH deficiency was defined by subnormal response (<10 mUI/L) to at least 1 provocative test: insulin test (0.05 U/kg), GHRH infusion test or arginine-insulin test. Partial GH deficiency was defined by a response between 10 and 20 mUI/l to the same provocative tests. In adults, gonadotrope deficiency was defined by low plasma testosterone with non-elevated gonadotropin levels in men, amenorrhea with low plasma estradiol and low or normal gonadotropins in non-menopausal women, and a lack of increased gonadotropins in postmenopausal women. The diagnosis of thyrotrope deficiency was based on low free T4 level with normal or diminished TSH. Corticotrope deficiency was defined by subnormal response of cortisol to an insulin tolerance test (peak < 550 nmol/ liter), associated with basal ACTH levels less than 5 ng/ml at 08:00 h.
GPR101 sequencing
All patients underwent Sanger sequencing of the GPR101 gene using peripheral blood leukocyte DNA. The sequences of the primers for GPR101 are given as supplemental data. Samples underwent PCR (conditions available on request) and were sequenced on an ABI 3130XL (Applied Biosystems). Genetic sequences and variants were called against the human GPR101 reference sequence (NM_054021.1, hg19 NCBI build 37). GH1 sequencing was performed in all patients, and no mutation was identified. LHX4, LHX3 and HESX1 sequencing was performed in patients wit extra-pituitary anomaly, and no mutation was identified.
Array comparative genome hybridization (aCGH)
Patients’ peripheral blood leukocytes DNA underwent aCGH as previously described [6]. Briefly, aCGH analysis used an 8×60K (G4827A-031746; Agilent Technologies, Santa Clara, CA, USA). The arrays were scanned with a G2565CA microarray scanner (Agilent Technologies, Santa Clara, CA, USA) and the images were extracted and analyzed with CytoGenomics software v2.0 (Agilent Technologies, Santa Clara, CA, USA). An ADM-2 algorithm (cut-off 6.0), followed by a filter to select regions with three or more adjacent probes and a minimum average log2 ratio ±0.25, was used to detect copy number changes. The quality of each experiment (log ratio spread) was assessed with CytoGenomics software v2.0. Genomic positions were based on the UCSC February 2009 human reference sequence (hg19 NCBI build 37 reference sequence assembly). Filtering of copy number changes was carried out using the BENCHlab CNV software (Cartagenia, Leuven, Belgium).
In silico analysis
Previously reported GPR101 variants were identified via Exome Aggregation Consortium (ExAC), Cambridge, MA (URL: http://exac.broadinstitute.org) [date accessed (September, 2015)]. Predict SNP (http://loschmidt.chemi.muni.cz/predictsnp) was used to appraise the potential pathogenicity of novel allelic variants. Alignment of amino acid sequences for homo sapiens, mus musculus, rattus norvegicus, bos taurus, chimpanzee, equus caballus and canis familiaris was performed with the Clustal omega program accessible via UniProKB (http://www.uniprot.org).
In vitro characterization studies
The effect of a novel p.V197L GPR101 variant was studied in vitro in the rat somatomammotroph GH3 cell line [6]. For all studies, effects were calculated as the average of two experiments performed in triplicate. Statistical comparisons were performed using a one-way ANOVA as compared to mock/vehicle. Briefly, GH3 cells were grown in Dulbecco’s modified Eagle’s medium (Life Technologies) supplemented with 10% fetal bovine serum (Gemini Bio-Products), and 1% antibiotic-antimycotic (Life Technologies) in a humidified atmosphere at 37°C with 5% CO2. Cells were seeded in 12-well plates at a density of 2 × 105 cells/well. After 24 h, cells were starved with DMEM without serum for 16 h and then transfected with Lipofectamine 2000 (Life Technologies) according to the manufacturer’s protocol, using Opti-MEM I Reduced Serum Medium (Life Technologies) and 1 μg of each vector, alone or in combination. The human wild-type GPR101 (NM_054021.1) coding sequence cloned into the pCMV-XL5 vector was purchased from Origene (SC120214). The p.V197L variant was introduced into the human GPR101wt template using the QuikChange Lightning site-directed mutagenesis kit (Agilent Technologies). The empty pCMV-XL5 vector was used as a negative control. 24 h after transfection supernatants were collected and GH secretion was measured using the Rat/Mouse Growth Hormone ELISA kit (EZRMGH-45K, EMD Millipore). GH secretion values (expressed in ng/ml) were normalized on protein content. For cAMP pathway activation studies, GH3 cells were seeded in 12-well plates at a density of 2 × 105 cells/well. After 24 h, cells were starved with DMEM without serum for 16 h and then transfected with Lipofectamine 2000 (Life Technologies), using Opti-MEM I Reduced Serum Medium (31985–070, Life Technologies), 1 μg of each GPR101 vector (human wild-type GPR101 and p.V197L GPR101), 800 ng of pGL4.29[luc2P/CRE/Hygro] vector containing a cAMP response element (CRE) that drives the transcription of the luciferase reporter gene (Promega), and 40 ng of the Renilla vector (pRL-SV40, Promega). The empty pCMV-XL5 vector was used as negative control. 24h after transfection, a subset of cells was treated with 10 μM forskolin (Sigma-Aldrich) for 1 h and then lysed. Firefly and Renilla luciferase activities were measured consecutively in the same sample using the Dual-Luciferase Reporter Assay System (Promega) following the manufacturer’s protocol. Ratios of Firefly vs. Renilla luminescence signals, serving as a measure for reporter activity normalized for transfection efficiency, were measured using a FLUOstar Omega microplate reader (BMG LABTECH).
Results
Cohort characteristics
Forty-one patients, 27 males and 14 females, with a median age of 12.9 years (min, 2.4 ; max, 44) at last follow-up, were included in this study. Thirty-seven patients (90.2%) presented with complete GH deficiency, whereas the remaining 4 had partial GH deficiency. The median age at diagnosis of GH deficiency was 5 years (min, neonatal ; max, 44). Only one patient was diagnosed as an adult.. Fourteen patients (34.1%) had at least one other family member with GH deficiency.
On pituitary MRI, 17 patients (41.4%) had pituitary hypoplasia whereas one (2.4%) had enlargement suggestive of pituitary hyperplasia. Fourteen patients (34.1%) had stalk interruption syndrome, with an ectopic posterior lobe and a thin or non-visualized pituitary stalk. Five patients also had other disorders such as eye anomalies such as strabismus (n=1) or cataract (n=1), diabetes insipidus (n=1), Chiari syndrome (n=1) or cleft palate (n=1).
Genetic analyses
One previously undescribed variant c.589 G>T (p.V197L) was seen in the heterozygous state in one patient and was further studied in vitro (see below). This caucasian female patient was aged 5 when complete GH deficiency was diagnosed (GH <0.3 ng/ml after one provocative stimulation test; IGF1 57 ng/ml (Normal value, 99–254)). She had no other pituitary hormone deficiency. Pituitary MRI was normal. Sequencing of GH1 did not reveal any anomaly. No clinical or genomic data was available for her parents.
No deletions or other copy number variants were found at chromosome Xq26.3 on aCGH. On sequencing, no truncating, frameshift, or small insertion-deletion (indel) GPR101 mutations were seen in the 43 analyzed patients. A number of previously described common missense variants in GPR101 were seen: c.370G>T (p.V124L), c.712G>A (p.V238I), c.878C>T (p.T293I), and c.1127T>C (p.L376P). A c.166T>C synonymous variant was observed in homozygosis.
In silico analysis
The p.V197L variant was not identified as a known polymorphism in ExAC. The Predict SNP platform identified p.V197L as a deleterious variant with PolyPhen2, MAPP and SNAP algorithms. Alignment of sequences identified the valine in position 197 as a highly conserved amino-acid in all species studied (Figure 1).
Figure 1.
Sequence alignment of GPR101 in several species (extracted from UniProtKB). RAT, rattus norvegicus; MOUSE, mus musculus; BOVIN, bos taurus; PANTR, chimpanzee; CANFA, canis familiaris; HORSE, equus caballus. Black box, Valine in position 197.
In vitro characterization
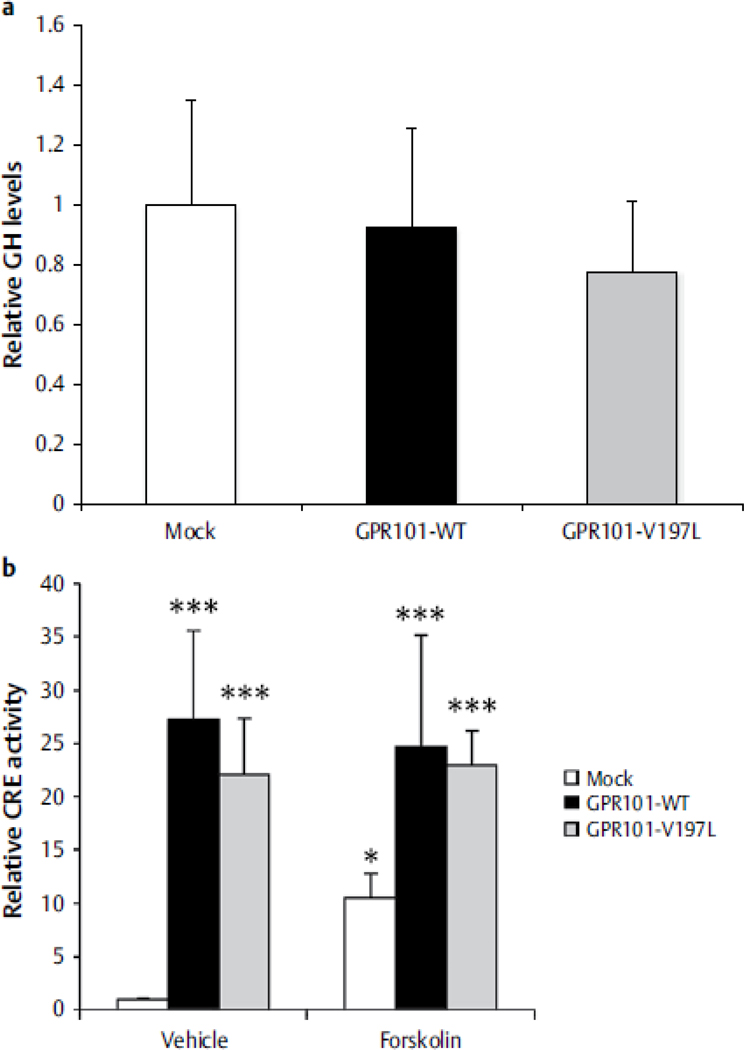
The effect of the c.589 G>T (p.V197L) change was studied further in GH3 cells. As shown in Figure 1, this variant was associated with a not statistically significant decrease in GH secretion as compared with wild-type GPR101. As compared with mock transfection, both wild-type GPR101 and the p.V197L change led to a statistically significant increase in CRE activation; while activation was less with p.V197L than wild-type GPR101, the difference was not statistically significant (both with and without forskolin; Figure 2).
Figure 2.
Panel A. A numerical decrease in GH secretion was seen in GH3 cells expressing the GPR101 p.V197L variant as compared with wild-type GPR101. The difference was not statistically significant. Panel B. There was a significant increase in CRE activity with the p.V197L GPR101 variant relative to mock transfection, however this difference was not statistically significant as compared with wild type GPR101. Results are expressed as mean ± SD of 2 experiments done in triplicate, one-way ANOVA compared to mock/vehicle. *
Discussion and conclusions
Microduplications on chromosome Xq26.3 including GPR101 lead to X-LAG syndrome, a dramatic pediatric-onset gigantism disorder associated with mixed GH-PRL secreting pituitary macroadenomas/hyperplasia that usually occur in the first year of life [6]. X-LAG syndrome represents about 10% of pituitary gigantism cases and differs significantly from other genetic (e.g AIP-related) and unexplained forms of pituitary gigantism[10, 11]. We also reported a GPR101 change (p.E308D) at an apparently increased frequency in patients with acromegaly [6, 8]. In a relevant in vitro model, the rat somatomammotrope GH3 cell, p.E308D led to increases in GH secretion as compared with wild-type GPR101. These data provide evidence for the involvement of GPR101 in the somatotrope axis. However, the precise roles of GPR101 in the pituitary and the brain remain to be studied, even if studies in endometrial models suggest that GnRH(1–5) may act via GPR101 to modulate epidermal growth factor receptor activity[12, 13]. Given the phenotype of marked GH hypersecretion associated with duplications involving GPR101, we were interested in studying the opposite hormonal condition, namely patients with GHD. Also as we previously reported, analysis of public databases have noted cases with short stature that had genomic deletions including chromosome Xq26.3; however most of those cases had large deletions spanning many more genes beyond this specific region6. In fact we show in the current study that no deletions of GPR101 and the surrounding genomic region were seen on aCGH. GPR101 sequencing showed that truncating or other clearly deleterious mutations were not found in our cohort. Taken together, these results suggest that inactivation or deletion of GPR101 is not a frequent cause of unexplained congenital GHD.
In the current study we noted a previously undescribed missense variant in GPR101, p.V197L, present in heterozygosis in one patient. When expressed in GH3 cells, this variant did not lead to a statistically significant change in GH secretion or cAMP activity in comparison with wild-type GPR101. There are nevertheless several strands of indirect evidence for a deleterious effect of this variant: three prediction algorithms suggested that this variant was pathogenic and the amino acid at position 197 is highly conserved across species. It is the first amino acid of the fifth transmembrane helix of GPR101 suggesting that changing this amino-acid could lead to conformational changes of the protein and altered function, as previously shown for mutations of transcription factors involved in corticotroph deficiency, for instance[14]. Finally, this variant has never been described in online databases such as ExAC. These prediction models, however are difficult to interpret if family segregation data are missing ; moreover, some studies indicated that 30% of disease-causing genetic variations cited in the literature based on perdiction models were actually polymorphisms or misinterpreted variants [15]. Family segregation would have given major information on the pathogenicity of this variant ; these data were however not accessible. It is thus highly uncertain whether this GPR101 variant could be responsible for the phenotype of our patients. While highly expressed in the hypothalamus and striatum, the physiological role of GPR101 remains largely obscure and the manner by which it may influence the somatotrope axis is unknown [16–18]. Moreover, it is well recognized that functional studies performed in heterologous cells, an artificial in vitro system, at pharmacological doses that do not necessarily reflect biological processes, may not provide a complete view of the activity of genetic variants (e.g. in functional studies performed with variants of LIM transcription factors [19, 20]). On balance, GPR101 variants in patients with growth disorders should be re-analyzed as more evidence of the function of GPR101 becomes apparent.
The rarity of identified GPR101 anomalies in our cohort may be due to a number of reasons. We may not have selected an ideal group to study, as we chose a group that was generally representative of the GHD population, including one third of familial cases. Moreover, our cohort was mainly including males, whereas X-LAG was mainly observed in females. It may be that by focusing on more severe GHD a greater number of pathological variants could be identified. Concentrating the screening activities on groups with pituitary hypoplasia and other brain malformations could provide useful information on a potential role for this receptor in GHD. The fact that the patient with the p.V197L variant had pure GH deficiency is evidence for considering that further studies should examine a larger number of patients with a pure isolated GHD phenotype. Finally, some of our patients were presenting with extra-pituitary anomaly and isolated GH deficiency: even if the phenotype of patients with GPR101 mutations in X-LAG syndrome was pure pituitary hypersecretion, we decided to screen them as the precise roles of GPR101 during fetal development remains unknown, and the phenotype of GPR101 deficiency might have been more complex than anticipated.
To conclude, this study is the first to analyze the potential involvement of GPR101 deletions and single nucleotide variants in congenital GHD. We did not find an unequivocal link between GPR101 loss of function and GHD. Of note, a rare novel p.V197L GPR101 variant was found in a patient with isolated GHD: its clinical significance remains unknown. This suggests that with growing understanding of the role of GPR101 in somatotrope axis function, the contribution of functional GPR101 variants to GHD and pituitary development should be further explored in a larger number of patients.
References
- 1.Alatzoglou KS, Webb EA, Le Tissier P, Dattani MT. Isolated growth hormone deficiency (GHD) in childhood and adolescence: recent advances. Endocrine reviews 2014;35:376–432. [DOI] [PubMed] [Google Scholar]
- 2.Mullis PE. Genetics of isolated growth hormone deficiency. Journal of clinical research in pediatric endocrinology 2010;2:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mullis PE. Genetic control of growth. European journal of endocrinology / European Federation of Endocrine Societies 2005;152:11–31. [DOI] [PubMed] [Google Scholar]
- 4.Kelberman D, Rizzoti K, Lovell-Badge R, Robinson IC, Dattani MT. Genetic regulation of pituitary gland development in human and mouse. Endocrine reviews 2009;30:790–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castinetti F, Reynaud R, Quentien MH, Jullien N, Marquant E, Rochette C, Herman JP, Saveanu A, Barlier A, Enjalbert A, Brue T. Combined pituitary hormone deficiency: current and future status. Journal of endocrinological investigation 2015;38:1–12. [DOI] [PubMed] [Google Scholar]
- 6.Trivellin G, Daly AF, Faucz FR, Yuan B, Rostomyan L, Larco DO, Schernthaner-Reiter MH, Szarek E, Leal LF, Caberg JH, Castermans E, Villa C, Dimopoulos A, Chittiboina P, Xekouki P, Shah N, Metzger D, Lysy PA, Ferrante E, Strebkova N, Mazerkina N, Zatelli MC, Lodish M, Horvath A, de Alexandre RB, Manning AD, Levy I, Keil MF, Sierra Mde L, Palmeira L, Coppieters W, Georges M, Naves LA, Jamar M, Bours V, Wu TJ, Choong CS, Bertherat J, Chanson P, Kamenicky P, Farrell WE, Barlier A, Quezado M, Bjelobaba I, Stojilkovic SS, Wess J, Costanzi S, Liu P, Lupski JR, Beckers A, Stratakis CA. Gigantism and acromegaly due to Xq26 microduplications and GPR101 mutation. The New England journal of medicine 2014;371:2363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckers A, Lodish MB, Trivellin G, Rostomyan L, Lee M, Faucz FR, Yuan B, Choong CS, Caberg JH, Verrua E, Naves LA, Cheetham TD, Young J, Lysy PA, Petrossians P, Cotterill A, Shah NS, Metzger D, Castermans E, Ambrosio MR, Villa C, Strebkova N, Mazerkina N, Gaillard S, Barra GB, Casulari LA, Neggers SJ, Salvatori R, Jaffrain-Rea ML, Zacharin M, Santamaria BL, Zacharieva S, Lim EM, Mantovani G, Zatelli MC, Collins MT, Bonneville JF, Quezado M, Chittiboina P, Oldfield EH, Bours V, Liu P, WdH W, Pellegata N, Lupski JR, Daly AF, Stratakis CA. X-linked acrogigantism syndrome: clinical profile and therapeutic responses. Endocrine-related cancer 2015;22:353–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daly AF, Trivellin G, Stratakis CA. Gigantism, acromegaly, and GPR101 mutations. The New England journal of medicine 2015;372:1265. [DOI] [PubMed] [Google Scholar]
- 9.Reynaud R, Gueydan M, Saveanu A, Vallette-Kasic S, Enjalbert A, Brue T, Barlier A. Genetic screening of combined pituitary hormone deficiency: experience in 195 patients. The Journal of clinical endocrinology and metabolism 2006;91:3329–36. [DOI] [PubMed] [Google Scholar]
- 10.Beckers A, Aaltonen LA, Daly AF, Karhu A. Familial isolated pituitary adenomas (FIPA) and the pituitary adenoma predisposition due to mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene. Endocrine reviews 2013;34:239–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rostomyan L, Daly AF, Petrossians P, Nachev E, Lila AR, Lecoq AL, Lecumberri B, Trivellin G, Salvatori R, Moraitis AG, Holdaway I, Kranenburg-van Klaveren DJ, Chiara Zatelli M, Palacios N, Nozieres C, Zacharin M, Ebeling T, Ojaniemi M, Rozhinskaya L, Verrua E, Jaffrain-Rea ML, Filipponi S, Gusakova D, Pronin V, Bertherat J, Belaya Z, Ilovayskaya I, Sahnoun-Fathallah M, Sievers C, Stalla GK, Castermans E, Caberg JH, Sorkina E, Auriemma RS, Mittal S, Kareva M, Lysy PA, Emy P, De Menis E, Choong CS, Mantovani G, Bours V, De Herder W, Brue T, Barlier A, Neggers SJ, Zacharieva S, Chanson P, Shah NS, Stratakis CA, Naves LA, Beckers A. Clinical and genetic characterization of pituitary gigantism: an international collaborative study in 208 patients. Endocrine-related cancer 2015;22:745–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho-Clark M, Larco DO, Zahn BR, Mani SK, Wu TJ. GnRH-(1–5) activates matrix metallopeptidase-9 to release epidermal growth factor and promote cellular invasion. Molecular and cellular endocrinology 2015. [DOI] [PubMed] [Google Scholar]
- 13.Cho-Clark M, Larco DO, Semsarzadeh NN, Vasta F, Mani SK, Wu TJ. GnRH-(1–5) transactivates EGFR in Ishikawa human endometrial cells via an orphan G protein-coupled receptor. Mol Endocrinol 2014;28:80–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vallette-Kasic S, Couture C, Balsalobre A, Gauthier Y, Metherell L, Dattani M, Drouin J. The TPIT gene mutation M86R associated with isolated adrenocorticotropin deficiency interferes with protein: protein interactions. The Journal of clinical endocrinology and metabolism 2007;92:3991–9. [DOI] [PubMed] [Google Scholar]
- 15.Boycott KM, Vanstone MR, Bulman DE, MacKenzie AE. Rare-disease genetics in the era of next-generation sequencing: discovery to translation. Nature reviews Genetics 2013;14:681–91. [DOI] [PubMed] [Google Scholar]
- 16.Bates B, Zhang L, Nawoschik S, Kodangattil S, Tseng E, Kopsco D, Kramer A, Shan Q, Taylor N, Johnson J, Sun Y, Chen HM, Blatcher M, Paulsen JE, Pausch MH. Characterization of Gpr101 expression and G-protein coupling selectivity. Brain research 2006;1087:1–14. [DOI] [PubMed] [Google Scholar]
- 17.Lee DK, Nguyen T, Lynch KR, Cheng R, Vanti WB, Arkhitko O, Lewis T, Evans JF, George SR, O’Dowd BF. Discovery and mapping of ten novel G protein-coupled receptor genes. Gene 2001;275:83–91. [DOI] [PubMed] [Google Scholar]
- 18.Reinius B, Blunder M, Brett FM, Eriksson A, Patra K, Jonsson J, Jazin E, Kullander K. Conditional targeting of medium spiny neurons in the striatal matrix. Frontiers in behavioral neuroscience 2015;9:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rochette C, Jullien N, Saveanu A, Caldagues E, Bergada I, Braslavsky D, Pfeifer M, Reynaud R, Herman JP, Barlier A, Brue T, Enjalbert A, Castinetti F. Identifying the Deleterious Effect of Rare LHX4 Allelic Variants, a Challenging Issue. PloS one 2015;10:e0126648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregory LC, Humayun KN, Turton JP, McCabe MJ, Rhodes SJ, Dattani MT. Novel Lethal Form of Congenital Hypopituitarism Associated With the First Recessive LHX4 Mutation. The Journal of clinical endocrinology and metabolism 2015;100:2158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]