Abstract
Cushing syndrome (CS) describes the signs and symptoms caused by exogenous or endogenous hypercortisolemia. Endogenous CS is caused by either ACTH-dependent sources (pituitary or ectopic) or ACTH-independent (adrenal) hypercortisolemia. Several genes are currently known to contribute to the pathogenesis of CS. Germline gene defects, such as MEN1, AIP, PRKAR1A and others, often present in patients with pituitary or adrenal involvement as part of a genetic syndrome. Somatic gene defects, such as USP8, TP53, and others, also underlie a large percentage of patients with CS, and give insight in pathways involved in pituitary or adrenal tumorigenesis.
Keywords: Gene, pituitary, adrenal, Cushing, tumor
Introduction
Cushing syndrome (CS) refers to the constellation of signs and symptoms resulting from excessive levels of cortisol.(1) Although exogenous CS is a relatively common diagnosis, endogenous CS accounts for only 2.3–3.2 new cases per million people per year; 10% of those present in children.(2–4) The etiology of endogenous CS involves either an ACTH-dependent source, most commonly pituitary adenomas (PAs), described as Cushing disease (CD), or less often ectopic CRH and/or ACTH secretion, or ACTH-independent (adrenal-related) hypercortisolemia (Table 1).(1) Several subclassifications of the various types of CS exist based on their clinical, histological and macroscopic presentation (Table 1) and may give clues to their etiology.
Table 1.
Classification of types of Cushing syndrome.
| Etiology | Mechanism | |
|---|---|---|
| Exogenous | Iatrogenic | Exogenous administration of supraphysiologic doses of glucocorticoids as part of therapeutic scheme for a medical condition (autoimmune, rheumatologic, malignant etc) |
| Endogenous | ACTH-dependent | Pituitary ACTH secreting tumors (ACTH-secreting pituitary adenomas or carcinomas, pituitary blastomas) |
| Ectopic ACTH and/or CRH secretion (bronchial, thymic, or pancreatic neuroendocrine tumors, and others) | ||
| ACTH-independent | Adrenocortical carcinomas | |
| Cortisol-Producing Adrenocortical adenomas (CPAs) | ||
| Bilateral adrenocortical hyperplasia (BAH) - Micronodular (most nodules < 1cm) • Pigmented • Primary Pigmented Nodular Adrenocortical Disease (PPNAD) in the context of Carney complex • Isolated Primary Pigmented Nodular Adrenocortical Disease (PPNAD) • Isolated Micronodular Adrenocortical Disease (iMAD) - Macronodular (most nodules > 1cm) • Bilateral Macroadenomatous hyperplasia (BMAH): adenomas with internodular atrophy • Massive Macronodular Adrenocortical Disease (MMAD): adenomas with internodular hyperplasia | ||
This review focuses on old and new germline or somatic gene defects of CS and provides a description of accompanying features in these patients (Table 2). These can be used for guidance in selecting appropriate genetic testing. Germline genetic defects in patients with CD are uncommon and explain less than 5% of all cases currently.(5) On the other hand, germline gene defects in patients with ACTH-independent CS may explain more than half of cases depending on the subtype of adrenal pathology identified. In either case, several lessons can be taught by the gene defects found about the possible pathways that may be affected and lead to hypercortisolemia. Malignant tumors, and specifically adrenocortical carcinomas, are not extensively reviewed. Other genomic mechanisms potentially involved in pathogenesis of CS, such as variable expression of components of certain pathways, methylation changes or miRNA changes, also contribute to the genetic background of CS.
Table 2.
Characteristics and presentation of patients with genetic syndromes associated with Cushing syndrome.
| Genetic syndrome | Type of CS | Gene(s) | Gene locus | Approximate frequency of CS in patients with the genetic syndrome | Clinical presentation |
|---|---|---|---|---|---|
| Multiple Endocrine Neoplasia type 1 (MEN1) | Cushing disease, ACTH-independent CS or ectopic CS | MEN1 | 11q13.1 | Cushing disease: 1% ACTH-independent CS: 2.5–5.5% | Tumors of anterior pituitary gland, parathyroid glands, pancreatic islet cells and others |
| Multiple Endocrine Neoplasia type 2A/2B (MEN2A/2B) | Cushing disease or ectopic CS | RET | 10q11.21 | Rare case reports | Medullary thyroid carcinoma with pheochromocytoma and hyperparathyroidism (MEN2A) or mucosal neuromas and intestinal ganglioneuromas (MEN2B) |
| Multiple Endocrine Neoplasia type 4 (MEN4) | Cushing disease | CDKN1B | 12p13.1 | 1 case reported | MEN1-like syndrome |
| Familial Isolated Pituitary Adenomas (FIPA) | Cushing disease | AIP (15–30% of cases) | 11q13.2 | 5% of FIPA patients | Presence of at least two family members with PAs, with or without other abnormalities |
| Carney complex (CNC) | ACTH-independent CS, rare cases of Cushing disease | PRKAR1A | 17q24.2 | ACTH-independent CS: 60% CD: Rare case reports | Myxomas, spotty skin pigmentation, endocrine overactivity and other tumors |
| McCune-Albright (MAS) | ACTH-independent CS, rare cases of Cushing disease | GNAS | 20q13.32 | Up to 7% | Polyostotic fibrous dysplasia, café-au-lait macules, and precocious puberty, and other endocrine disorders |
| Beckwith-Wiedemann syndrome (BWS) | ACTH-independent CS, rare cases of Cushing disease | IGF2, H9 and CDKI | 11p15 | Rare case reports | Hemihypertrophy, macrosomia, macroglossia, predisposition to embryonic tumors and others |
| Li-Fraumeni syndrome (LFS) | ACTH-independent CS | TP53 | 17p13.1 | 6–13% of patients (frequency referring to adrenocortical carcinomas) | Predisposition to various tumors at early age, including soft tissue sarcoma, osteosarcoma, brain tumors, breast cancer, melanoma and others |
| DICER1 | Cushing disease | DICER1 | 14q32.13 | Rare | Pleuropulmonary blastomas, cystic nephromas, Sertoli-Leydig cell tumors, multinodular goiter, and other tumors |
| 3 P association (3PA) | Cushing disease | SDHx, VHL, MEN1, RET and MAX | Various | 1 case reported | 3Ps: Pituitary adenomas, Pheochromocytomas and/or Paragangliomas |
| Tuberous sclerosis (TS) | Cushing disease | TSC1, TSC2 | 9q34.13 (TSC1) 16p13.3 (TSC2) | Rare case report | Hamartomas, epilepsy and mental retardation |
| USP8 germline syndrome | Cushing disease | USP8 | 15q21.2 | 1 patient reported (100% penetrance hypothesized) | Cushing disease, developmental delay, dysmorphic features, skin defects, chronic lung disease, chronic kidney disease, dilated cardiomyopathy with congestive heart failure (CHF) and others |
Well established genetic causes of CS
Multiple Endocrine Neoplasia (MEN)
Multiple endocrine neoplasia (MEN) syndromes are commonly associated with CS.(6) MEN type 1 (MEN1) (OMIM#131100) is caused by genetic defects in MEN1 which functions as a tumor suppressor gene at the endocrine tissues.(7) PAs present in 40% of patients with MEN1. However, ACTH-secreting pituitary tumors account for only 3–4% of them.(8, 9) They present at the third to fourth decade of life, although patients as young as 9 years of age have been reported.(8–10) Multiple synchronous or metachronous PAs of similar or different functional status may present in patients with MEN1, and clinicians should review carefully imaging and biochemical studies to avoid unnecessary interventions.(11)
Only few case reports of ACTH-secreting PAs have been reporting in the context of the other MEN syndromes, types 2 and 4 (MEN2 and MEN4, respectively): RET [MEN2A (OMIM#171400) and MEN2B (OMIM#162300)] and CDKN1B gene mutations [MEN4 (OMIM#610755)] suggesting that these syndromes play a less important role in pituitary tumorigenesis. (12–14)
Of note, patients with MEN syndromes may present with adrenal or ectopic CS.(15) In ACTH-independent CS, MEN1 has been involved in the development of unilateral or bilateral adrenal disorders, including isolated nodules and carcinomas, or bilateral adrenocortical hyperplasia.(16) In studies where the adrenal presentation and function of patients with MEN1 was investigated, 20–50% of them were found to have abnormal radiographic presentation of adrenals, but only 2.5–5.5% patients presented with CS.(17–19) Ectopic CS in the context of MEN1 is not infrequent and most common sources are neuroendocrine tumors, thymic and others.(20, 21) In MEN2 and MEN4, ectopic CS may be associated with ectopic ACTH secretion from medullary thyroid carcinomas or pheochromocytomas, and rarely from other tumors.(22, 23)
Clinicians should follow carefully the diagnostic evaluation for the diagnosis and identification of the source of hypercortisolemia, without assuming a pituitary source in the case of elevated ACTH.(24, 25) Since PAs are infrequently associated with ACTH secretion, while non-functioning PAs are common, the presence of ACTH-dependent CS with the imaging identification of an adenoma, should be followed by workup for confirmation of pituitary versus ectopic source, such as CRH stimulation test, high-dose dexamethasone suppression test or Bilateral Inferior Petrosal Sinus Sampling (IPSS) as appropriate.(25)
Familial Isolated Pituitary Adenomas (FIPA)/AIP
The term Familial isolated pituitary adenomas (FIPA) describes the presence of at least two family members with PAs, with or without other abnormalities.(26) Although the presence of family history and the inheritance pattern implies a genetic etiology for this syndrome, specific gene defects have been identified in half of the cases.(26) Fifteen percent of patients carry an aryl hydrocarbon receptor-interacting protein (AIP) gene mutation.(26–28) The AIP protein is probably involved in the synthesis of cAMP. Thus, AIP gene defects cause increased cAMP production leading to aberrant cell proliferation (29).
Approximately 5% of patient with FIPA have ACTH-producing adenomas, but most of them do not have an identified genetic background.(26, 30) Only few patients with CD and AIP mutations have been reported in either familial or sporadic cases, with the youngest patient presenting at 6 years of age. (31, 32) Other genetic causes of FIPA, such as GPR101 (causing X-Linked Acrogigantism or X-LAG) are not found in patients with CD.(33, 34)
Carney complex and PKA in the pathogenesis of CS
Carney complex (CNC) presents with the constellation of myxomas, spotty skin pigmentation, and endocrine overactivity.(35) CNC is caused by mutations of the PRKAR1A gene in 70% of cases, whereas a second locus on chromosome 2p16 has been identified for some of the remaining cases.(36, 37) PRKAR1A encodes the type 1 alpha regulatory subunit of the protein kinase A (PKA), and gene defects lead to increased free catalytic subunits which lead to increased downstream activity of PKA. (35)
CNC presents with primary pigmented nodular adrenocortical disease (PPNAD) in approximately 60% of patients.(38) Patients may have radiographically normal-looking adrenals or adrenals with multiple nodules (often <1 cm in largest diameter).(39) CS in the context of PPNAD may be atypical or cyclical, but most patients have a pathognomonic paradoxical response to dexamethasone during Liddle’s test when performed during the active state.(40)
More recently, two cases of corticotropinomas in patients with pathogenic variants in PRKAR1A have been reported, expanding the spectrum of the known pituitary involvement in CNC, previously limited to growth hormone and/or prolactin secreting PAs and somato(mamo)troph hyperplasia.(41, 42)
TSC and the mTOR//PI3K/Akt pathway
Tuberous sclerosis (TS) is an autosomal dominant syndrome presenting with hamartomas, mental retardation and epilepsy. Neuroendocrine tumors may be a rare presentation of TS and few cases of ACTH-secreting PAs have been reported in the literature. (43–45) Two genes are involved in the pathogenesis of the syndrome: TSC1 (OMIM#191100) and TSC2 (OMIM#613254). The genes are part of the mTOR/PI3K/Akt pathway that regulates cell growth and proliferation.(46) Of note, few cases of corticotropinomas with somatic mutations or amplifications of PIK3CA, another component of the mTOR/PI3K/Akt pathway, have been reported especially in more aggressive tumors.(47, 48)
Adrenocortical carcinomas
Although this review is mainly focused on non-malignant causes of CS, since cancerous causes often have multiple genetic gene defects as well as chromosomal rearrangements that render them aggressive and malignant, it is important to mention the main genetic regulators of adrenocortical carcinomas. One of the most important genes involved in adrenocortical tumorigenesis is TP53 which is identified in up to 70% of cases, especially in children.(49, 50) TP53 is a tumor suppressor gene coding for p53 protein which regulates cell cycle. Li Fraumeni syndrome (LFS) is an autosomal dominant condition caused by germline defect of TP53 gene represents one is the extreme of the spectrum of the p53 defects. Patients with LFS have predisposition for several tumors, including soft tissue sarcomas, osteosarcomas and adrenocortical carcinomas.(51)
Wnt signaling pathway/b-catenin
Beta-catenin is part of the Wnt signaling pathway, involved in cell differentiation and several cancers. Wnt signaling is activated during development and later downregulated. Activation of the canonical Wnt pathway through binding to Frizzled receptor leads to decreased degradation of intracellular b-catenin which after accumulation in the cytoplasm translocates to the nucleus to activate TCF/LEF-type transcription factors of several genes involved in cell cycle regulation (such as cyclins and c-MYC).(52)
Activation of the Wnt signaling pathway has been reported as a frequent event in adrenocortical carcinomas and adenomas. Somatic mutations of b-catenin gene (CTNNB1) explain up to half of the these cases where Wnt signaling is found increased.(53) APC gene, responsible for familial adrenomatous polyposis (FAP) when mutations are present in the germline, is another member of the Wnt pathway and regulates the ubiquitination of b-catenin acting as a suppressor of its effects.(54) Inactivation of APC has been reported in cases of adrenocortical adenomas and hyperplasia.(55)
Neonatal CS in the context of genetic syndromes
CS in the neonatal period is extremely rare.(56) When present, its association with underlying genetic syndromes is common. Of the identified genetic causes of neonatal CS, McCune-Albright syndrome (MAS) and Beckwith-Wiedemann syndrome (BWS) may be associated with neonatal ACTH-independent CS.(57, 58) MAS is caused by postzygotic somatic mutations of the GNAS gene, which codes for the Gsa subunit of G-protein coupled receptor, leading to constitutive activation and increased intracellular cAMP.(59) Most cases of neonatal CS and MAS are caused by unilateral or bilateral adrenocortical hyperplasia. BWS is caused by defects of the 11p15 locus involving IGF2, H9 and CDKI genes.(60) Patients with BWS present with adrenocortical tumors or bilateral adrenocortical hyperplasia. Of note, GNAS gene defects and BWS have been recently reported in association with CD, expanding our understanding of the effects of these genes.(61–65)
In 2014, de Kock et al published an important paper reinvestigating the cause of previously reported cases of neonatal CD. The authors reported that the reported tumors actually fit the histologic diagnosis of pituitary blastomas.(66) They further identified DICER1 variants in the available samples, with or without loss of heterozygosity at the tumor level.(66) DICER1 codes for a small RNA processing endoribonuclease that is involved in siRNA and miRNA production.(67) DICER1 syndrome involves among other presentations pleuropulmonary blastomas, cystic nephromas, Sertoli-Leydig cell tumors, multinodular goiter and other tumors.(68)
Ectopic Cushing syndrome
Ectopic CS may present in the context of various cancers including breast, colon, pancreas and others, however the genetic cause of aberrant POMC expression and ACTH secretion has not been extensively studied.(69) Most of the cases of ectopic CS with an identified genetic cause correspond to MEN1 or RET gene mutations. Some additional causes of ectopic CS that have been reported in the literature include BRAF and TP53 mutations, in neuroendocrine tumors of the colon, and a case report of ectopic CS in the context of a pancreatic neuroendocrine tumor in a patient with VHL mutation. (70, 71)
Newly identified genes in the germline or somatic state
USP8
The USP8 gene codes for a deubiquitinase protein involved in the recycling of epidermal growth factor receptor (EGFR). In 2015, two independent groups reported somatic variants in the USP8 gene in corticotropinomas.(72, 73) All variants were located in a hot spot, the 14–3-3 binding motif (between amino acids 713 and 720), and led to gain of function activation of the gene. Increased deubiquitinase activity led to increased EGFR levels and consequently elevated proopiomelanocortin (POMC) gene expression. Since then additional groups have reported a frequency of 20–60% of USP8 mutations in CD, and have studied potential implication in patient prognosis, with some reporting a more aggressive behavior of these tumors.(74–77) Recently, Cohen et al reported the first patient with a germline USP8 gene defect. The patient presented with recurrent severe CD, and multiple other medical problems that highlight the involvement of USP8 in several tissues. (78)
USP8-negative corticotropinomas have been recently linked to somatic variants in other genes of the MAPK pathway (USP48 and BRAF), which may explain up to 20% of all cases. (79, 80)
ARMC5
In 2013, Assie et al reported the association of ARMC5 with the pathogenesis of massive macronodular adrenocortical disease (MMAD). ARMC5 gene defects were identified in 55% of all cases.(81) Biallelic inactivation was present in all cases, with patients carrying a germline mutation and a second “hit” (another inactivating variant or deletion at the other allele) occurring at the tumor level.(81) ACRM5 protein is involved in dedifferentiation and apoptosis signaling of adrenocortical cells, resulting in reduced steroidogenesis and mass formation.(81, 82) The end result of this defect is cell overgrowth and mass production, leading to excessive hormone secretion and CS.(82)
PKA subunits and Phosphodiesterase (PDE)
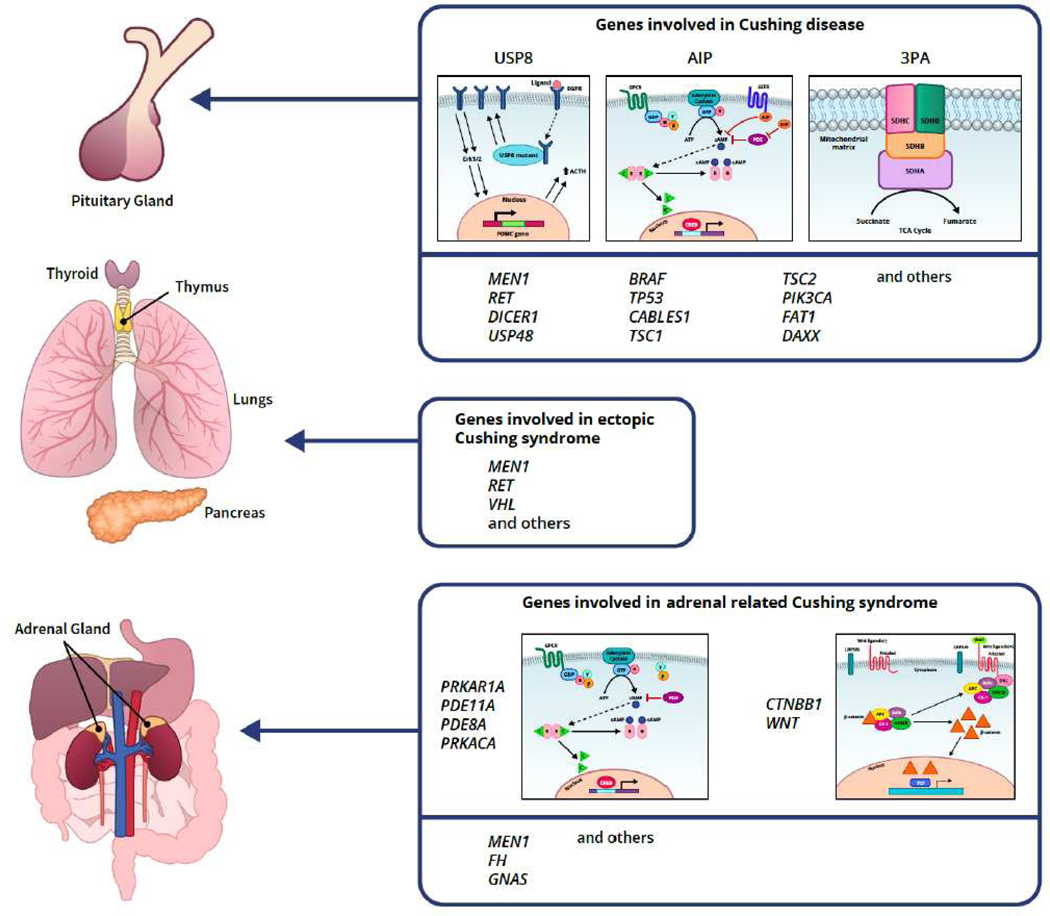
The description of PRKAR1A gene defects in adrenal CS and the involvement of abnormal cAMP-PKA activity in several adrenal disorders leading to excess cortisol production, led to identification of additional genes of the cAMP-PKA pathway which are causative or contributory to adrenal-related hypercortisolemia.(83) PDE11A (and possibly PDE8B) mutations contribute to a variety of pathologic adrenal lesions.(84, 85) Phosphodiesterases (PDEs) are enzymes involved in the hydrolysis of cAMP and defects in these genes lead to increased levels of cAMP and aberrant PKA signaling (Figure 1). Amplifications and activating mutations of the PRKACA gene, coding for the C alpha catalytic subunit of PKA, have also been involved in the pathogenesis of adrenal CS, caused by either isolated cortisol producing adenomas (CPAs) or adrenocortical hyperplasia.(86, 87)
Figure 1.
Genes involved in various subtypes of CS. (Graph credit to Nichole Jonas)
TP53 in CD
After initial reports of rare TP53 mutations in PAs and their association mainly with aggressive tumors, a recent study and metanalysis of corticotropinomas changed this assumption and reported that up to 12% of tumors may have a somatic TP53 mutation.(88–90) Authors reported TP53 mutations in 33% of USP8-negative tumors and hypothesized that p53 protein is important for regulation of apoptosis (one of the main pathways affected in corticotropinomas) or the BRCA1 mediated DNA-repair in corticotroph cells.(80, 91) Further studies are needed to 245 confirm this finding.
SDH
The “three P association” or 3PAs describes the combination of pituitary adenomas, pheochromocytomas (PHEO) and/or paragangliomas (PGL) at members of the same family.(92) Most cases are caused by SDH-related genes (SDHA, SDHAF2, SDHB, SDHC, SDHD), while additional genes have been more recently reported (VHL, MEN1, RET and MAX). Succinate dehydrogenase (SDH) is part of the complex II of the mitochondria involved in energy production and the respiratory chain. (93) Although PAs is a mandatory component of the syndrome only one case of a patient with ACTH-secreting adenoma has been reported in the literature.(94) Of note, SDHx genes may be associated with adrenocortical carcinomas which commonly present with ACTH-independent Cushing syndrome. (95)
Food-dependent CS
Food-dependent CS is a rare variant of ACTH-independent CS, where cortisol secretion is stimulated by the post-prandial state, most probably by the effect of glucose-dependent insulinotropic polypeptide (GIP).(96, 97) Although ectopic GIP receptors (GIPRs) in adrenal tumor cells were described many years ago, it was in 2017 when researchers identified the genetic basis of this rare variant of adrenal CS, and reported somatic duplications in chromosome 19q13.3 containing GIPR gene in 3 patients with food-dependent CS.(96, 98) In 2/3 cases there was chromosomal rearrangement that moved the GIPR gene under the effect of glucocorticoid-responsive elements, leading to increased expression of GIPR in tumor cells which then react with increased hormone production under the effect of meals.(98)
Other genes
CABLES1 was recently identified as a rare cause of corticotropinomas.(99) The gene codes for a protein that interacts with cyclin-dependent kinase, and inactivating mutations of the gene result in aberrant cell proliferation.(99)
Recommendations for genetic testing of patients with CS
When considering offering genetic testing to patients outside the research setting, practitioners should understand the diagnostic yield of the test and the effect on treatment options, if any. We always review carefully every patient’s personal and family history for evidence of diagnoses that could provide clues to a specific genetic syndrome. Specifically, personal history of other medical diagnoses (such as cancer, especially in young age), physical characteristics (such as freckling or other birthmarks) or pertinent family history (members of the family with pituitary or adrenal disorders, history of nephrolithiasis or calcium problems, type and age at diagnosis of cancers in the family, early onset obesity or diabetes and others) form our pretest probability for an underlying genetic defect. If we suspect a well characterized genetic syndrome, we discuss with patient and/or parents (if children) the value of the test. For certain conditions (like MEN), there are already established guidelines on diagnosis and genetic screening.(100) For newer conditions, advise on genetic screening should be provided by physicians familiar with the conditions and able to provide appropriate counseling.
Practice points
The etiology of endogenous CS involves either an ACTH-dependent source, most commonly pituitary adenomas (PAs), described as Cushing disease (CD), or less often ectopic CRH and/or ACTH secretion, or ACTH-independent (adrenal-related) hypercortisolemia
Germline genetic defects in patients with CD are uncommon and explain less than 5% of all cases. On the other hand, germline gene defects in patients with ACTH-independent CS may explain high percentage of cases depending on the subtype of adrenal pathology identified.
Patient’s phenotyping, family history and histologic evaluation of the source of hypercortisolemia provide necessary clues to direct targeted genetic testing whenever possible.
Research agenda
Currently available extensive genetic testing may provide more data on the underlying genetic causes of CS, but challenges present with the volume of data produced from these techniques and the capacity of bioinformatic analysis.
Additional genomic mechanisms, such as methylation and miRNA changes, potentially contribute to the pathogenesis or the variable phenotype of patients with CS.
Acknowledgments
Funding: The work was supported by the Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), National Institutes of Health.
Footnotes
Disclosure Statement: Dr. Stratakis holds patents on technologies involving PRKAR1A, PDE11A, GPR101 genes and/or their function; his laboratory has received research funding support by Pfizer Inc. for work unrelated to this project.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing’s syndrome. Lancet 2015, 386(9996):913–927. [DOI] [PubMed] [Google Scholar]
- 2.Wengander S, Trimpou P, Papakokkinou E, Ragnarsson O. The incidence of endogenous Cushing’s syndrome in the modern era. Clin Endocrinol (Oxf) 2019, 91(2):263–270. [DOI] [PubMed] [Google Scholar]
- 3.Lindholm J, Juul S, Jorgensen JO, Astrup J, Bjerre P, Feldt-Rasmussen U, Hagen C, Jorgensen J, Kosteljanetz M, Kristensen L, Laurberg P, Schmidt K, Weeke J. Incidence and late prognosis of cushing’s syndrome: a population-based study. J Clin Endocrinol Metab 2001, 86(1):117–123. [DOI] [PubMed] [Google Scholar]
- 4.Stratakis CA. An update on Cushing syndrome in pediatrics. Ann Endocrinol (Paris) 2018, 79(3):125–131. [DOI] [PubMed] [Google Scholar]
- 5.Marques P, Korbonits M. Genetic Aspects of Pituitary Adenomas. Endocrinol Metab Clin North Am 2017, 46(2):335–374. [DOI] [PubMed] [Google Scholar]
- 6.Falchetti A, Marini F, Luzi E, Tonelli F, Brandi ML. Multiple endocrine neoplasms. Best Pract Res Clin Rheumatol 2008, 22(1):149–163. [DOI] [PubMed] [Google Scholar]
- 7.Matkar S, Thiel A, Hua X. Menin: a scaffold protein that controls gene expression and cell signaling. Trends Biochem Sci 2013, 38(8):394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *8.de Laat JM, Dekkers OM, Pieterman CR, Kluijfhout WP, Hermus AR, Pereira AM, van der Horst-Schrivers AN, Drent ML, Bisschop PH, Havekes B, de Herder WW, Valk GD. Long-Term Natural Course of Pituitary Tumors in Patients With MEN1: Results From the DutchMEN1 Study Group (DMSG). J Clin Endocrinol Metab 2015, 100(9):3288–3296. [DOI] [PubMed] [Google Scholar]
- 9.Verges B, Boureille F, Goudet P, Murat A, Beckers A, Sassolas G, Cougard P, Chambe B, Montvernay C, Calender A. Pituitary disease in MEN type 1 (MEN1): data from the France-Belgium MEN1 multicenter study. J Clin Endocrinol Metab 2002, 87(2):457–465. [DOI] [PubMed] [Google Scholar]
- 10.Makri A, Bonella MB, Keil MF, Hernandez-Ramirez L, Paluch G, Tirosh A, Saldarriaga C, Chittiboina P, Marx SJ, Stratakis CA, Lodish M. Children with MEN1 gene mutations may present first (and at a young age) with Cushing disease. Clin Endocrinol (Oxf) 2018, 89(4):437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schorr M, Zhang X, Zhao W, Abedi P, Lines KE, Hedley-Whyte ET, Swearingen B, Klibanski A, Miller KK, Thakker RV, Nachtigall LB. Two Synchronous Pituitary Adenomas Causing Cushing Disease and Acromegaly. AACE Clin Case Rep 2019, 5(5):e276–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito T, Miura D, Taguchi M, Takeshita A, Miyakawa M, Takeuchi Y. Coincidence of multiple endocrine neoplasia type 2A with acromegaly. Am J Med Sci 2010, 340(4):329–331. [DOI] [PubMed] [Google Scholar]
- 13.Heinlen JE, Buethe DD, Culkin DJ, Slobodov G. Multiple endocrine neoplasia 2a presenting with pheochromocytoma and pituitary macroadenoma. ISRN Oncol 2011, 2011:732452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ezzat T, Paramesawaran R, Phillips B, Sadler G. MEN 2 syndrome masquerading as MEN 1. Ann R Coll Surg Engl 2012, 94(6):e206–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simonds WF, Varghese S, Marx SJ, Nieman LK. Cushing’s syndrome in multiple endocrine neoplasia type 1. Clin Endocrinol (Oxf) 2012, 76(3):379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drougat L, Espiard S, Bertherat J. Genetics of primary bilateral macronodular adrenal hyperplasia: a model for early diagnosis of Cushing’s syndrome? Eur J Endocrinol 2015, 173(4):M121–131. [DOI] [PubMed] [Google Scholar]
- 17.Waldmann J, Bartsch DK, Kann PH, Fendrich V, Rothmund M, Langer P. Adrenal involvement in multiple endocrine neoplasia type 1: results of 7 years prospective screening. Langenbecks Arch Surg 2007, 392(4):437–443. [DOI] [PubMed] [Google Scholar]
- 18.Langer P, Cupisti K, Bartsch DK, Nies C, Goretzki PE, Rothmund M, Roher HD. Adrenal involvement in multiple endocrine neoplasia type 1. World J Surg 2002, 26(8):891–896. [DOI] [PubMed] [Google Scholar]
- 19.Gatta-Cherifi B, Chabre O, Murat A, Niccoli P, Cardot-Bauters C, Rohmer V, Young J, Delemer B, Du Boullay H, Verger MF, Kuhn JM, Sadoul JL, Ruszniewski P, Beckers A, Monsaingeon M, Baudin E, Goudet P, Tabarin A. Adrenal involvement in MEN1. Analysis of 715 cases from the Groupe d’etude des Tumeurs Endocrines database. Eur J Endocrinol 2012, 166(2):269–279. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Su J, Zhao L, Wu J, Ding X, Fang F, Wu Y, Sun H, Peng Y. Familial Cushing syndrome due to thymic carcinoids in a multiple endocrine neoplasia type 1 kindred. Endocrine 2014, 47(1):183–190. [DOI] [PubMed] [Google Scholar]
- 21.Karageorgiadis AS, Papadakis GZ, Biro J, Keil MF, Lyssikatos C, Quezado MM, Merino M, Schrump DS, Kebebew E, Patronas NJ, Hunter MK, Alwazeer MR, Karaviti LP, Balazs AE, Lodish MB, Stratakis CA. Ectopic adrenocorticotropic hormone and corticotropin-releasing hormone co-secreting tumors in children and adolescents causing cushing syndrome: a diagnostic dilemma and how to solve it. J Clin Endocrinol Metab 2015, 100(1):141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendonca BB, Arnhold IJ, Nicolau W, Avancini VA, Boise W. Cushing’s syndrome due to ectopic ACTH secretion by bilateral pheochromocytomas in multiple endocrine neoplasia type 2A. N Engl J Med 1988, 319(24):1610–1611. [DOI] [PubMed] [Google Scholar]
- 23.Zaydfudim V, Stover DG, Caro SW, Phay JE. Presentation of a medullary endocrine neoplasia 2A kindred with Cushing’s syndrome. Am Surg 2008, 74(7):659–661. [PubMed] [Google Scholar]
- 24.Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2008, 93(5):1526–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tatsi C, Stratakis C: Cushing Disease: Diagnosis and Treatment In: Pituitary Disorders of Childhood Diagnosis and Clinical Management. edn. Edited by Kohn B: Springer Nature Switzerland AG; 2019: 89114. [Google Scholar]
- 26.Stiles CE, Korbonits M: Familial Isolated Pituitary Adenoma In: Endotext. edn. Edited by Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P et al. South Dartmouth (MA); 2000. [Google Scholar]
- *27.Vierimaa O, Georgitsi M, Lehtonen R, Vahteristo P, Kokko A, Raitila A, Tuppurainen K, Ebeling TM, Salmela PI, Paschke R, Gundogdu S, De Menis E, Makinen MJ, Launonen V, Karhu A, Aaltonen LA. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science 2006, 312(5777):1228–1230. [DOI] [PubMed] [Google Scholar]
- 28.Daly AF, Vanbellinghen JF, Khoo SK, Jaffrain-Rea ML, Naves LA, Guitelman MA, Murat A, Emy P, Gimenez-Roqueplo AP, Tamburrano G, Raverot G, Barlier A, De Herder W, Penfornis A, Ciccarelli E, Estour B, Lecomte P, Gatta B, Chabre O, Sabate MI, Bertagna X, Garcia Basavilbaso N, Stalldecker G, Colao A, Ferolla P, Wemeau JL, Caron P, Sadoul JL, Oneto A, Archambeaud F, Calender A, Sinilnikova O, Montanana CF, Cavagnini F, Hana V, Solano A, Delettieres D, Luccio-Camelo DC, Basso A, Rohmer V, Brue T, Bours V, Teh BT, Beckers A. Aryl hydrocarbon receptor-interacting protein gene mutations in familial isolated pituitary adenomas: analysis in 73 families. J Clin Endocrinol Metab 2007, 92(5):1891–1896. [DOI] [PubMed] [Google Scholar]
- 29.Tuominen I, Heliovaara E, Raitila A, Rautiainen MR, Mehine M, Katainen R, Donner I, Aittomaki V, Lehtonen HJ, Ahlsten M, Kivipelto L, Schalin-Jantti C, Arola J, Hautaniemi S, Karhu A. AIP inactivation leads to pituitary tumorigenesis through defective Galphai-cAMP signaling. Oncogene 2015, 34(9):1174–1184. [DOI] [PubMed] [Google Scholar]
- 30.Daly AF, Jaffrain-Rea ML, Ciccarelli A, Valdes-Socin H, Rohmer V, Tamburrano G, Borson-Chazot C, Estour B, Ciccarelli E, Brue T, Ferolla P, Emy P, Colao A, De Menis E, Lecomte P, Penfornis F, Delemer B, Bertherat J, Wemeau JL, De Herder W, Archambeaud F, Stevenaert A, Calender A, Murat A, Cavagnini F, Beckers A. Clinical characterization of familial isolated pituitary adenomas. J Clin Endocrinol Metab 2006, 91(9):3316–3323. [DOI] [PubMed] [Google Scholar]
- 31.Stratakis CA, Tichomirowa MA, Boikos S, Azevedo MF, Lodish M, Martari M, Verma S, Daly AF, Raygada M, Keil MF, Papademetriou J, Drori-Herishanu L, Horvath A, Tsang KM, Nesterova M, Franklin S, Vanbellinghen JF, Bours V, Salvatori R, Beckers A. The role of germline AIP, MEN1, PRKAR1A, CDKN1B and CDKN2C mutations in causing pituitary adenomas in a large cohort of children, adolescents, and patients with genetic syndromes. Clin Genet 2010, 78(5):457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cazabat L, Bouligand J, Salenave S, Bernier M, Gaillard S, Parker F, Young J, Guiochon-Mantel A, Chanson P. Germline AIP mutations in apparently sporadic pituitary adenomas: prevalence in a prospective singlecenter cohort of 443 patients. J Clin Endocrinol Metab 2012, 97(4):E663–670. [DOI] [PubMed] [Google Scholar]
- 33.Trivellin G, Correa RR, Batsis M, Faucz FR, Chittiboina P, Bjelobaba I, Larco DO, Quezado M, Daly AF, Stojilkovic SS, Wu TJ, Beckers A, Lodish M, Stratakis CA. Screening for GPR101 defects in pediatric pituitary corticotropinomas. Endocr Relat Cancer 2016, 23(5):357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trivellin G, Daly AF, Faucz FR, Yuan B, Rostomyan L, Larco DO, Schernthaner-Reiter MH, Szarek E, Leal LF, Caberg JH, Castermans E, Villa C, Dimopoulos A, Chittiboina P, Xekouki P, Shah N, Metzger D, Lysy PA, Ferrante E, Strebkova N, Mazerkina N, Zatelli MC, Lodish M, Horvath A, de Alexandre RB, Manning AD, Levy I, Keil MF, Sierra Mde L, Palmeira L, Coppieters W, Georges M, Naves LA, Jamar M, Bours V, Wu TJ, Choong CS, Bertherat J, Chanson P, Kamenicky P, Farrell WE, Barlier A, Quezado M, Bjelobaba I, Stojilkovic SS, Wess J, Costanzi S, Liu P, Lupski JR, Beckers A, Stratakis CA. Gigantism and acromegaly due to Xq26 microduplications and GPR101 mutation. N Engl J Med 2014, 371(25):2363–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stratakis CA. Carney complex: A familial lentiginosis predisposing to a variety of tumors. Rev Endocr Metab Disord 2016, 17(3):367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *36.Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet 2000, 26(1):89–92. [DOI] [PubMed] [Google Scholar]
- 37.Kamilaris CDC, Faucz FR, Voutetakis A, Stratakis CA. Carney Complex. Exp Clin Endocrinol Diabetes 2019, 127(2–03):156–164. [DOI] [PubMed] [Google Scholar]
- 38.Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R, Rene-Corail F, Stergiopoulos S, Bourdeau I, Bei T, Clauser E, Calender A, Kirschner LS, Bertagna X, Carney JA, Stratakis CA. Mutations in regulatory subunit type 1A of cyclic adenosine 5’-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab 2009, 94(6):2085–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groussin L, Cazabat L, Rene-Corail F, Jullian E, Bertherat J. Adrenal pathophysiology: lessons from the Carney complex. Horm Res 2005, 64(3):132–139. [DOI] [PubMed] [Google Scholar]
- 40.Stratakis CA, Sarlis N, Kirschner LS, Carney JA, Doppman JL, Nieman LK, Chrousos GP, Papanicolaou DA. Paradoxical response to dexamethasone in the diagnosis of primary pigmented nodular adrenocortical disease. Ann Intern Med 1999, 131(8):585–591. [DOI] [PubMed] [Google Scholar]
- 41.Hernandez-Ramirez LC, Tatsi C, Lodish MB, Faucz FR, Pankratz N, Chittiboina P, Lane J, Kay DM, Valdes N, Dimopoulos A, Mills JL, Stratakis CA. Corticotropinoma as a Component of Carney Complex. J Endocr Soc 2017, 1(7):918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiefer FW, Winhofer Y, Iacovazzo D, Korbonits M, Wolfsberger S, Knosp E, Trautinger F, Hoftberger R, Krebs M, Luger A, Gessl A. PRKAR1A mutation causing pituitary-dependent Cushing disease in a patient with Carney complex. Eur J Endocrinol 2017, 177(2):K7–K12. [DOI] [PubMed] [Google Scholar]
- 43.Dworakowska D, Grossman AB. Are neuroendocrine tumours a feature of tuberous sclerosis? A systematic review. Endocr Relat Cancer 2009, 16(1):45–58. [DOI] [PubMed] [Google Scholar]
- 44.Nandagopal R, Vortmeyer A, Oldfield EH, Keil MF, Stratakis CA. Cushing’s syndrome due to a pituitary corticotropinoma in a child with tuberous sclerosis: an association or a coincidence? Clin Endocrinol (Oxf) 2007, 67(4):639–641. [DOI] [PubMed] [Google Scholar]
- 45.Tigas S, Carroll PV, Jones R, Bingham E, Russell-Jones D, Powell M, Scobie IN. Simultaneous Cushing’s disease and tuberous sclerosis; a potential role for TSC in pituitary ontogeny. Clin Endocrinol (Oxf) 2005, 63(6):694–695. [DOI] [PubMed] [Google Scholar]
- 46.Rosner M, Hanneder M, Siegel N, Valli A, Fuchs C, Hengstschlager M. The mTOR pathway and its role in human genetic diseases. Mutat Res 2008, 659(3):284–292. [DOI] [PubMed] [Google Scholar]
- 47.Murat CB, Braga PB, Fortes MA, Bronstein MD, Correa-Giannella ML, Giorgi RR. Mutation and genomic amplification of the PIK3CA proto-oncogene in pituitary adenomas. Braz J Med Biol Res 2012, 45(9):851–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin Y, Jiang X, Shen Y, Li M, Ma H, Xing M, Lu Y. Frequent mutations and amplifications of the PIK3CA gene in pituitary tumors. Endocr Relat Cancer 2009, 16(1):301–310. [DOI] [PubMed] [Google Scholar]
- *49.Zheng S, Cherniack AD, Dewal N, Moffitt RA, Danilova L, Murray BA, Lerario AM, Else T, Knijnenburg TA, Ciriello G, Kim S, Assie G, Morozova O, Akbani R, Shih J, Hoadley KA, Choueiri TK, Waldmann J, Mete O, Robertson AG, Wu HT, Raphael BJ, Shao L, Meyerson M, Demeure MJ, Beuschlein F, Gill AJ, Sidhu SB, Almeida MQ, Fragoso M, Cope LM, Kebebew E, Habra MA, Whitsett TG, Bussey KJ, Rainey WE, Asa SL, Bertherat J, Fassnacht M, Wheeler DA, Cancer Genome Atlas Research N, Hammer GD, Giordano TJ, Verhaak RGW. Comprehensive Pan-Genomic Characterization of Adrenocortical Carcinoma. Cancer Cell 2016, 29(5):723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lodish M. Cushing’s syndrome in childhood: update on genetics, treatment, and outcomes. Curr Opin Endocrinol Diabetes Obes 2015, 22(1):48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Latronico AC, Pinto EM, Domenice S, Fragoso MC, Martin RM, Zerbini MC, Lucon AM, Mendonca BB. An inherited mutation outside the highly conserved DNA-binding domain of the p53 tumor suppressor protein in children and adults with sporadic adrenocortical tumors. J Clin Endocrinol Metab 2001, 86(10):4970–4973. [DOI] [PubMed] [Google Scholar]
- 52.Taciak B, Pruszynska I, Kiraga L, Bialasek M, Krol M. Wnt signaling pathway in development and cancer. J Physiol Pharmacol 2018, 69(2). [DOI] [PubMed] [Google Scholar]
- 53.Tissier F, Cavard C, Groussin L, Perlemoine K, Fumey G, Hagnere AM, Rene-Corail F, Jullian E, Gicquel C, Bertagna X, Vacher-Lavenu MC, Perret C, Bertherat J. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res 2005, 65(17):7622–7627. [DOI] [PubMed] [Google Scholar]
- 54.Hankey W, Frankel WL, Groden J. Functions of the APC tumor suppressor protein dependent and independent of canonical WNT signaling: implications for therapeutic targeting. Cancer Metastasis Rev 2018, 37(1):159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaujoux S, Pinson S, Gimenez-Roqueplo AP, Amar L, Ragazzon B, Launay P, Meatchi T, Libe R, Bertagna X, Audebourg A, Zucman-Rossi J, Tissier F, Bertherat J. Inactivation of the APC gene is constant in adrenocortical tumors from patients with familial adenomatous polyposis but not frequent in sporadic adrenocortical cancers. Clin Cancer Res 2010, 16(21):5133–5141. [DOI] [PubMed] [Google Scholar]
- 56.Tatsi C, Stratakis CA. Neonatal Cushing Syndrome: A Rare but Potentially Devastating Disease. Clin Perinatol 2018, 45(1):103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carney JA, Young WF, Stratakis CA. Primary bimorphic adrenocortical disease: cause of hypercortisolism in McCune-Albright syndrome. Am J Surg Pathol 2011, 35(9):1311–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown RJ, Kelly MH, Collins MT. Cushing syndrome in the McCune-Albright syndrome. J Clin Endocrinol Metab 2010, 95(4):1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med 1991, 325(24):1688–1695. [DOI] [PubMed] [Google Scholar]
- 60.Gicquel C, Bertagna X, Schneid H, Francillard-Leblond M, Luton JP, Girard F, Le Bouc Y. Rearrangements at the 11p15 locus and overexpression of insulin-like growth factor-II gene in sporadic adrenocortical tumors. J Clin Endocrinol Metab 1994, 78(6):1444–1453. [DOI] [PubMed] [Google Scholar]
- 61.Brioude F, Nicolas C, Marey I, Gaillard S, Bernier M, Das Neves C, Le Bouc Y, Touraine P, Netchine I. Hypercortisolism due to a Pituitary Adenoma Associated with Beckwith-Wiedemann Syndrome. Horm Res Paediatr 2016, 86(3):206–211. [DOI] [PubMed] [Google Scholar]
- 62.Carney JA, Ho J, Kitsuda K, Young WF Jr., Stratakis CA Massive neonatal adrenal enlargement due to cytomegaly, persistence of the transient cortex, and hyperplasia of the permanent cortex: findings in Cushing syndrome associated with hemihypertrophy. Am J Surg Pathol 2012, 36(10):1452–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.MacFarland SP, Mostoufi-Moab S, Zelley K, Mattei PA, States LJ, Bhatti TR, Duffy KA, Brodeur GM, Kalish JM. Management of adrenal masses in patients with Beckwith-Wiedemann syndrome. Pediatr Blood Cancer 2017, 64(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williamson EA, Ince PG, Harrison D, Kendall-Taylor P, Harris PE. G-protein mutations in human pituitary adrenocorticotrophic hormone-secreting adenomas. Eur J Clin Invest 1995, 25(2):128–131. [DOI] [PubMed] [Google Scholar]
- 65.Riminucci M, Collins MT, Lala R, Corsi A, Matarazzo P, Gehron Robey P, Bianco P. An R201H activating mutation of the GNAS1 (Gsalpha) gene in a corticotroph pituitary adenoma. Mol Pathol 2002, 55(1):58–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Kock L, Sabbaghian N, Plourde F, Srivastava A, Weber E, Bouron-Dal Soglio D, Hamel N, Choi JH, Park SH, Deal CL, Kelsey MM, Dishop MK, Esbenshade A, Kuttesch JF, Jacques TS, Perry A, Leichter H, Maeder P, Brundler MA, Warner J, Neal J, Zacharin M, Korbonits M, Cole T, Traunecker H, McLean TW, Rotondo F, Lepage P, Albrecht S, Horvath E, Kovacs K, Priest JR, Foulkes WD. Pituitary blastoma: a pathognomonic feature of germ-line DICER1 mutations. Acta Neuropathol 2014, 128(1):111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Solarski M, Rotondo F, Foulkes WD, Priest JR, Syro LV, Butz H, Cusimano MD, Kovacs K. DICER1 gene mutations in endocrine tumors. Endocr Relat Cancer 2018, 25(3):R197–R208. [DOI] [PubMed] [Google Scholar]
- 68.Foulkes WD, Priest JR, Duchaine TF. DICER1: mutations, microRNAs and mechanisms. Nat Rev Cancer 2014, 14(10):662–672. [DOI] [PubMed] [Google Scholar]
- 69.Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing’s syndrome due to ectopic corticotropin secretion: twenty years’ experience at the National Institutes of Health. J Clin Endocrinol Metab 2005, 90(8):4955–4962. [DOI] [PubMed] [Google Scholar]
- 70.Mokhtar A, Arnason T, Gaston D, Huang WY, MacKenzie H, Al-Hazmi R, Vaninetti N, Tugwell B, Rayson D. ACTH-Secreting Neuroendocrine Carcinoma of the Cecum: Case Report and Review of the Literature. Clin Colorectal Cancer 2019, 18(1):e163–e170. [DOI] [PubMed] [Google Scholar]
- 71.Benitez Velazco A, Pacheco Capote C, Latre Romero JM. [Ectopic Cushing’s syndrome caused by a functioning pancreatic neuroendocrine tumour in a patient with von Hippel-Lindau disease]. Rev Esp Med Nucl 2008, 27(1):29–33. [DOI] [PubMed] [Google Scholar]
- *72.Reincke M, Sbiera S, Hayakawa A, Theodoropoulou M, Osswald A, Beuschlein F, Meitinger T, Mizuno-Yamasaki E, Kawaguchi K, Saeki Y, Tanaka K, Wieland T, Graf E, Saeger W, Ronchi CL, Allolio B, Buchfelder M, Strom TM, Fassnacht M, Komada M. Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat Genet 2015, 47(1):31–38. [DOI] [PubMed] [Google Scholar]
- *73.Ma ZY, Song ZJ, Chen JH, Wang YF, Li SQ, Zhou LF, Mao Y, Li YM, Hu RG, Zhang ZY, Ye HY, Shen M, Shou XF, Li ZQ, Peng H, Wang QZ, Zhou DZ, Qin XL, Ji J, Zheng J, Chen H, Wang Y, Geng DY, Tang WJ, Fu CW, Shi ZF, Zhang YC, Ye Z, He WQ, Zhang QL, Tang QS, Xie R, Shen JW, Wen ZJ, Zhou J, Wang T, Huang S, Qiu HJ, Qiao ND, Zhang Y, Pan L, Bao WM, Liu YC, Huang CX, Shi YY, Zhao Y. Recurrent gain-of-function USP8 mutations in Cushing’s disease. Cell Res 2015, 25(3):306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Faucz FR, Tirosh A, Tatsi C, Berthon A, Hernandez-Ramirez LC, Settas N, Angelousi A, Correa R, Papadakis GZ, Chittiboina P, Quezado M, Pankratz N, Lane, Dimopoulos A, Mills JL, Lodish M, Strataki CA. Somatic USP8 Gene Mutations Are a Common Cause of Pediatric Cushing Disease. J Clin Endocrinol Metab 2017, 102(8):2836–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perez-Rivas LG, Theodoropoulou M, Ferrau F, Nusser C, Kawaguchi K, Stratakis CA, Faucz FR, Wildemberg LE, Assie G, Beschorner R, Dimopoulou C, Buchfelder M, Popovic V, Berr CM, Toth M, Ardisasmita AI, Honegger J, Bertherat J, Gadelha MR, Beuschlein F, Stalla G, Komada M, Korbonits M, Reincke M. The Gene of the Ubiquitin-Specific Protease 8 Is Frequently Mutated in Adenomas Causing Cushing’s Disease. J Clin Endocrinol Metab 2015, 100(7):E997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ballmann C, Thiel A, Korah HE, Reis AC, Saeger W, Stepanow S, Kohrer K, Reifenberger G, KnobbeThomsen CB, Knappe UJ, Scholl UI. USP8 Mutations in Pituitary Cushing Adenomas-Targeted Analysis by Next-Generation Sequencing. J Endocr Soc 2018, 2(3):266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Losa M, Mortini P, Pagnano A, Detomas M, Cassarino MF, Pecori Giraldi F. Clinical characteristics and surgical outcome in USP8-mutated human adrenocorticotropic hormone-secreting pituitary adenomas. Endocrine 2019, 63(2):240–246. [DOI] [PubMed] [Google Scholar]
- *78.Cohen M, Persky R, Stegemann R, Hernandez-Ramirez LC, Zeltser D, Lodish MB, Chen A, Keil MF, Tatsi C, Faucz F, Buchner D, Stratakis CA, Tiosano D. Germline USP8 mutation associated with pediatric Cushing disease and other clinical features: a new syndrome. J Clin Endocrinol Metab 2019, 104(10):4676–4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *79.Chen J, Jian X, Deng S, Ma Z, Shou X, Shen Y, Zhang Q, Song Z, Li Z, Peng H, Peng C, Chen M, Luo C, Zhao D, Ye Z, Shen M, Zhang Y, Zhou J, Fahira A, Wang Y, Li S, Zhang Z, Ye H, Li Y, Shen J, Chen H, Tang F, Yao Z, Shi Z, Chen C, Xie L, Wang Y, Fu C, Mao Y, Zhou L, Gao D, Yan H, Zhao Y, Huang C, Shi Y. Identification of recurrent USP48 and BRAF mutations in Cushing’s disease. Nat Commun 2018, 9(1):3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sbiera S, Perez-Rivas LG, Taranets L, Weigand I, Flitsch J, Graf E, Monoranu CM, Saeger W, Hagel C, Honegger J, Assie G, Hermus AR, Stalla GK, Herterich S, Ronchi CL, Deutschbein T, Reincke M, Strom TM, Popov N, Theodoropoulou M, Fassnacht M. Driver mutations in USP8 wild type Cushing’s disease. Neuro Oncol 2019, 21(10):1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *81.Assie G, Libe R, Espiard S, Rizk-Rabin M, Guimier A, Luscap W, Barreau O, Lefevre L, Sibony M, Guignat L, Rodriguez S, Perlemoine K, Rene-Corail F, Letourneur F, Trabulsi B, Poussier A, Chabbert-Buffet N, Borson-Chazot F, Groussin L, Bertagna X, Stratakis CA, Ragazzon B, Bertherat J. ARMC5 mutations in macronodular adrenal hyperplasia with Cushing’s syndrome. N Engl J Med 2013, 369(22):2105–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Espiard S, Drougat L, Libe R, Assie G, Perlemoine K, Guignat L, Barrande G, Brucker-Davis F, Doullay F, Lopez S, Sonnet E, Torremocha F, Pinsard D, Chabbert-Buffet N, Raffin-Sanson ML, Groussin L, Borson-Chazot F, Coste J, Bertagna X, Stratakis CA, Beuschlein F, Ragazzon B, Bertherat J. ARMC5 Mutations in a Large Cohort of Primary Macronodular Adrenal Hyperplasia: Clinical and Functional Consequences. J Clin Endocrinol Metab 2015, 100(6):E926–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bimpaki EI, Nesterova M, Stratakis CA. Abnormalities of cAMP signaling are present in adrenocortical lesions associated with ACTH-independent Cushing syndrome despite the absence of mutations in known genes. Eur J Endocrinol 2009, 161(1):153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *84.Horvath A, Boikos S, Giatzakis C, Robinson-White A, Groussin L, Griffin KJ, Stein E, Levine E, Delimpasi G, Hsiao HP, Keil M, Heyerdahl S, Matyakhina L, Libe R, Fratticci A, Kirschner LS, Cramer K, Gaillard RC, Bertagna X, Carney JA, Bertherat J, Bossis I, Stratakis CA. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet 2006, 38(7):794–800. [DOI] [PubMed] [Google Scholar]
- 85.Horvath A, Giatzakis C, Tsang K, Greene E, Osorio P, Boikos S, Libe R, Patronas Y, Robinson-White A, Remmers E, Bertherat J, Nesterova M, Stratakis CA. A cAMP-specific phosphodiesterase (PDE8B) that is mutated in adrenal hyperplasia is expressed widely in human and mouse tissues: a novel PDE8B isoform in human adrenal cortex. Eur J Hum Genet 2008, 16(10):1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *86.Cao Y, He M, Gao Z, Peng Y, Li Y, Li L, Zhou W, Li X, Zhong X, Lei Y, Su T, Wang H, Jiang Y, Yang L, Wei W, Yang X, Jiang X, Liu L, He J, Ye J, Wei Q, Li Y, Wang W, Wang J, Ning G. Activating hotspot L205R mutation in PRKACA and adrenal Cushing’s syndrome. Science 2014, 344(6186):913–917. [DOI] [PubMed] [Google Scholar]
- 87.Lodish MB, Yuan B, Levy I, Braunstein GD, Lyssikatos C, Salpea P, Szarek E, Karageorgiadis AS, Belyavskaya E, Raygada M, Faucz FR, Izzat L, Brain C, Gardner J, Quezado M, Carney JA, Lupski JR, Stratakis CA. Germline PRKACA amplification causes variable phenotypes that may depend on the extent of the genomic defect: molecular mechanisms and clinical presentations. Eur J Endocrinol 2015, 172(6):803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Levy A, Hall L, Yeudall WA, Lightman SL. p53 gene mutations in pituitary adenomas: rare events. Clin Endocrinol (Oxf) 1994, 41(6):809–814. [DOI] [PubMed] [Google Scholar]
- 89.Herman V, Drazin NZ, Gonsky R, Melmed S. Molecular screening of pituitary adenomas for gene mutations and rearrangements. J Clin Endocrinol Metab 1993, 77(1):50–55. [DOI] [PubMed] [Google Scholar]
- 90.Tanizaki Y, Jin L, Scheithauer BW, Kovacs K, Roncaroli F, Lloyd RV. P53 gene mutations in pituitary carcinomas. Endocr Pathol 2007, 18(4):217–222. [DOI] [PubMed] [Google Scholar]
- 91.Sbiera S, Kunz M, Weigand I, Deutschbein T, Dandekar T, Fassnacht M. The New Genetic Landscape of Cushing’s Disease: Deubiquitinases in the Spotlight. Cancers (Basel) 2019, 11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *92.Xekouki P, Pacak K, Almeida M, Wassif CA, Rustin P, Nesterova M, de la Luz Sierra M, Matro J, Ball E, Azevedo M, Horvath A, Lyssikatos C, Quezado M, Patronas N, Ferrando B, Pasini B, Lytras A, Tolis G, Stratakis CA. Succinate dehydrogenase (SDH) D subunit (SDHD) inactivation in a growth-hormoneproducing pituitary tumor: a new association for SDH? J Clin Endocrinol Metab 2012, 97(3):E357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moosavi B, Berry EA, Zhu XL, Yang WC, Yang GF. The assembly of succinate dehydrogenase: a key enzyme in bioenergetics. Cell Mol Life Sci 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xekouki P, Szarek E, Bullova P, Giubellino A, Quezado M, Mastroyannis SA, Mastorakos P, Wassif CA, Raygada M, Rentia N, Dye L, Cougnoux A, Koziol D, Sierra Mde L, Lyssikatos C, Belyavskaya E, Malchoff C, Moline J, Eng C, Maher LJ 3rd, Pacak K, Lodish M, Stratakis CA. Pituitary adenoma with paraganglioma/pheochromocytoma (3PAs) and succinate dehydrogenase defects in humans and mice. J Clin Endocrinol Metab 2015, 100(5):E710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Else T, Lerario AM, Everet J, Haymon L, Wham D, Mullane M, Wilson TL, Rainville I, Rana H, Worth AJ, Snyder NW, Blair IA, McKay R, Kilbridge K, Hammer G, Barletta J, Vaidya A. Adrenocortical carcinoma and succinate dehydrogenase gene mutations: an observational case series. Eur J Endocrinol 2017, 177(5):439–444. [DOI] [PubMed] [Google Scholar]
- 96.Lacroix A, Ndiaye N, Tremblay J, Hamet P. Ectopic and abnormal hormone receptors in adrenal Cushing’s syndrome. Endocr Rev 2001, 22(1):75–110. [DOI] [PubMed] [Google Scholar]
- 97.Lacroix A, Bolte E, Tremblay J, Dupre J, Poitras P, Fournier H, Garon J, Garrel D, Bayard F, Taillefer R, et al. Gastric inhibitory polypeptide-dependent cortisol hypersecretion--a new cause of Cushing’s syndrome. N Engl J Med 1992, 327(14):974–980. [DOI] [PubMed] [Google Scholar]
- 98.Lecoq AL, Stratakis CA, Viengchareun S, Chaligne R, Tosca L, Demeocq V, Hage M, Berthon A, Faucz FR, Hanna P, Boyer HG, Servant N, Salenave S, Tachdjian G, Adam C, Benhamo V, Clauser E, Guiochon-Mantel A, Young J, Lombes M, Bourdeau I, Maiter D, Tabarin A, Bertherat J, Lefebvre H, de Herder W, Louiset E, Lacroix A, Chanson P, Bouligand J, Kamenicky P. Adrenal GIPR expression and chromosome 19q13 microduplications in GIP-dependent Cushing’s syndrome. JCI Insight 2017, 2(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hernandez-Ramirez LC, Gam R, Valdes N, Lodish MB, Pankratz N, Balsalobre A, Gauthier Y, Faucz FR, Trivellin G, Chittiboina P, Lane J, Kay DM, Dimopoulos A, Gaillard S, Neou M, Bertherat J, Assie G, Villa C, Mills JL, Drouin J, Stratakis CA. Loss-of-function mutations in the CABLES1 gene are a novel cause of Cushing’s disease. Endocr Relat Cancer 2017, 24(8):379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A, Lips CJ, Lombardi G, Mannelli M, Pacini F, Ponder BA, Raue F, Skogseid B, Tamburrano G, Thakker RV, Thompson NW, Tomassetti P, Tonelli F, Wells SA Jr., Marx SJ Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 2001, 86(12):5658–5671. [DOI] [PubMed] [Google Scholar]