OBJECTIVES:
Endobiliary radiofrequency ablation (RFA) for malignant biliary obstruction is a promising option for improving biliary stent patency, but its efficacy and safety with endoscopic ultrasound (EUS)-guided biliary drainage are uncertain. We examined the feasibility of EUS-guided hepaticoenterostomy with antegrade stenting (EUS-HEAS) and RFA in patients with unresectable malignant biliary obstruction.
METHODS:
This was a prospective, single-center, single-arm, preliminary study. Twenty patients who met the eligibility criteria for inclusion between August 2018 and January 2020 were enrolled. We evaluated the technical success, functional success, recurrent biliary obstruction (RBO), and adverse events other than RBO associated with EUS-HEAS with RFA.
RESULTS:
The technical and functional success rates were both 80% (16/20). The procedure was unsuccessful in a total of 4 patients due to failure to insert the RFA catheter through the fistula (2 patients) or failure to pass the RFA catheter through the stricture (2 patients). Early and late adverse events other than RBO occurred in 10% (2/20) and 13% (2/16) of subjects, respectively. The RBO rate was 25% (4/16), and the median time to RBO was 276 days. The success rate of endoscopic reintervention using hepaticoenterostomy was 100% (4/4).
DISCUSSION:
This preliminary study showed that EUS-HEAS with RFA achieves good results but RFA catheter insertion can be difficult. Further evaluation and device improvements are warranted.
INTRODUCTION
Endoscopic ultrasound (EUS)-guided biliary drainage, including EUS-guided hepaticoenterostomy (EUS-HES), EUS-guided choledocoduodenostomy, and EUS-guided antegrade stenting (EUS-AS), has been developed as an alternative method to manage malignant biliary obstruction (MBO) when the transpapillary approach is difficult (1–3). More recently, a combined technique, which comprises of both EUS-HES and EUS-AS (EUS-HEAS), has been reported to maintain stent patency for a longer duration and exhibit a lower rate of adverse events when compared with EUS-HES or EUS-AS alone (4–8). However, the continued developments in chemotherapy demand more robust stent patency.
Endobiliary radiofrequency ablation (RFA) has been reported to control local tumor growth and improve stent patency under endoscopic retrograde cholangiopancreatography (ERCP) guidance (9,10). Although RFA can also be useful for EUS-guided biliary drainage (11), no study about the effects of RFA under EUS guidance has been conducted. This study aims to examine the feasibility of EUS-HEAS with RFA in patients with unresectable MBO.
METHODS
Study design and patients
This was a prospective, single-center, single-arm preliminary study. The following patients were included in the study: patients who were aged 20 years or older, had obstructive jaundice and/or cholangitis due to unresectable MBO, and had experienced failure or difficulty with the transpapillary approach. Patients exhibiting any of the following criteria were excluded: (i) difficulty applying the EUS-guided approach including massive ascites and tumor extension into the puncture site, (ii) contraindication(s) to RFA, (iii) hilar stricture, (iv) compression stricture by lymph node metastasis, (v) deemed ineligible by the investigator, and/or (vi) unable to provide informed consent.
Aichi Medical University Hospital's institutional review board approved this study in accordance with the principles of the Declaration of Helsinki (Approval number: 2018-H189). The protocol was registered in the University Hospital Medical Information Network Clinical Trial Registry database (identifier: UMIN000033855).
Technique
A GF-UCT260 linear array echoendoscope (Olympus Medical Systems, Tokyo, Japan) was used with an EU-ME2 ultrasound processor (Olympus Medical Systems). The left intrahepatic bile duct was punctured using a 19-gauge aspiration needle (EZ shot 3 Plus; Olympus Medical Systems) from the stomach or intestine. After confirming that the bile duct was correctly punctured on cholangiography, a 0.025-inch guidewire (VisiGlide 2; Olympus Medical Systems) was inserted into the bile duct through the needle. Then, the needle was extracted from the scope, and a tapered catheter (StarTipV; Olympus Medical Systems) was inserted over the guidewire. After the guidewire was advanced through the stricture and the duodenal papilla, tract dilation was performed using a 4-mm balloon catheter (REN Biliary Balloon Dilation Catheter; Kaneka Medix, Osaka, Japan). Then, a Habib EndoHPB catheter (Boston Scientific, Marlborough, MA) was inserted over the guidewire, and ablation of the stricture was performed for 90 seconds at a power of 7 W. After the ablation, an uncovered metal stent with an ultrathin delivery system (Zeo Stent V; Zeon Medical, Tokyo, Japan, Bilerush Selective; Piolax Medical Devices, Kanagawa, Japan, or Niti-S Large Cell SR Slim Delivery; Taewoong Medical, Seoul, Korea) was placed antegradely across the stricture. Finally, a dedicated single-pigtail plastic stent (PS) (Type IT; Gadelius Medical, Tokyo, Japan), originally designed for EUS-HES (12), was placed across the hepaticoenterostomy fistula (Figures 1–2 and see Video 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A408). All procedures were performed by a single endoscopist (T.I.) who was experienced in performing therapeutic ERCP and EUS-guided procedures.
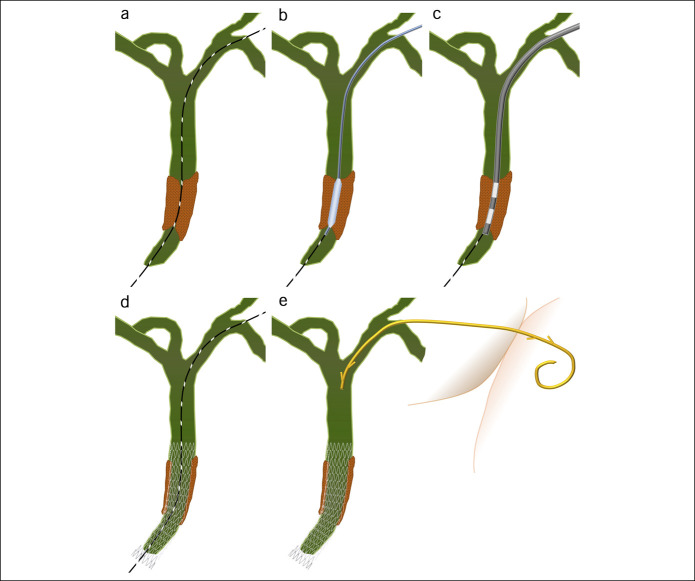
Figure 1.
The bile duct was punctured, and the guidewire was advanced through the stricture (a). Balloon dilation of the stricture and fistula (b) and ablation of the stricture (c) were subsequently performed. A metal stent was placed antegradely across the stricture (d), and a dedicated single-pigtail plastic stent was placed across the hepaticoenterostomy fistula (e).
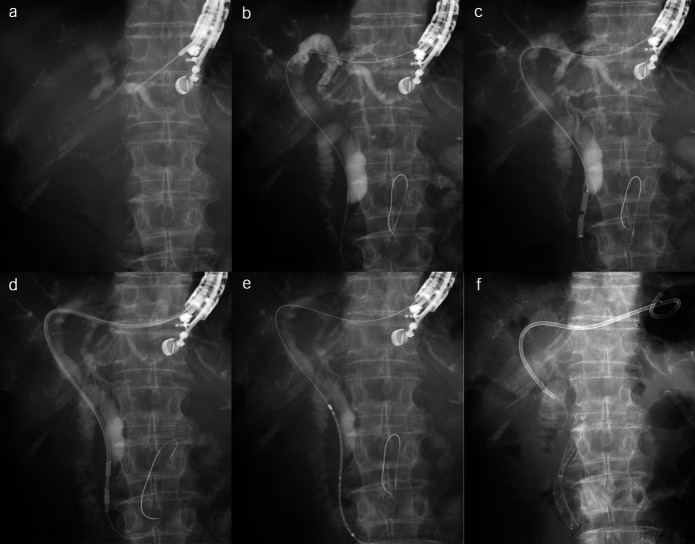
Figure 2.
After the puncture of the left intrahepatic bile duct (a), a guidewire was advanced through the lower bile duct stricture and the duodenal papilla (b). The dilation of the stricture and the fistula was performed using a 4-mm balloon catheter (c). Radiofrequency ablation to the stricture was performed for 90 seconds at a power of 7 W (d). An uncovered metal stent was placed antegradely across the stricture (e). Finally, a dedicated single-pigtail plastic stent was placed from the hepatic duct to the stomach (f).
Reintervention
After insertion of a TJF260V duodenoscope (Olympus Medical Systems), a 0.025-inch guidewire was inserted into the bile duct alongside the previously placed hepaticoenterostomy stent. The stent was subsequently removed through the scope using forceps. Then, an additional metal stent was placed across the stricture (in a stent-in-stent fashion), and the dedicated PS across the hepaticoenterostomy was replaced (see Video 2, Supplementary Digital Content 2, http://links.lww.com/CTG/A409).
Outcomes
The outcomes evaluated included technical and functional success, recurrent biliary obstruction (RBO), adverse events other than RBO, and reintervention associated with EUS-HEAS with RFA. Functional success was defined as a decrease in bilirubin and liver enzyme levels to normal or to <50% of levels within 14 days, and RBO was defined by the recurrence of jaundice and/or cholangitis along with biliary dilation (13). The time to RBO was defined as the period from stent placement to RBO development. Patient follow-up involved examinations and laboratory tests at least once every 4 weeks, and computed tomography was performed when adverse events or RBOs were suspected.
Statistical analyses
Because this study was a pilot study, the sample size was determined by the number of patients we were able to enroll within the study period. Categorical variables are expressed as numbers and percentages, and continuous variables are reported as medians and ranges. The time to RBO was determined using the Kaplan-Meier method. If RBO had not occurred by the time the patient died or by the end of the study period, the data were considered to be censored. All statistical analyses were performed using R version 3.4.1 (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient characteristics
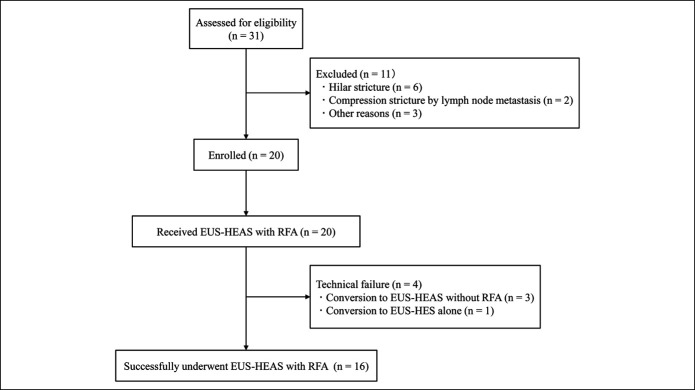
Between August 2018 and January 2020, 20 patients who met the eligibility criteria for study inclusion were enrolled (Figure 3). Table 1 presents the patients' demographics, including sex, age, etiology, surgically altered anatomy, indwelling duodenal stent, location and length of the stricture, bilirubin, and alkaline phosphatase levels, cholangitis, and chemotherapy status. The median follow-up period was 183 days.
Figure 3.
Flowchart of patient enrollment in the study. EUS-HES, endoscopic ultrasound-guided hepaticoenterostomy; EUS-HEAS, endoscopic ultrasound-guided hepaticoenterostomy with antegrade stenting; RFA, radiofrequency ablation.
Table 1.
Baseline characteristics of the patients
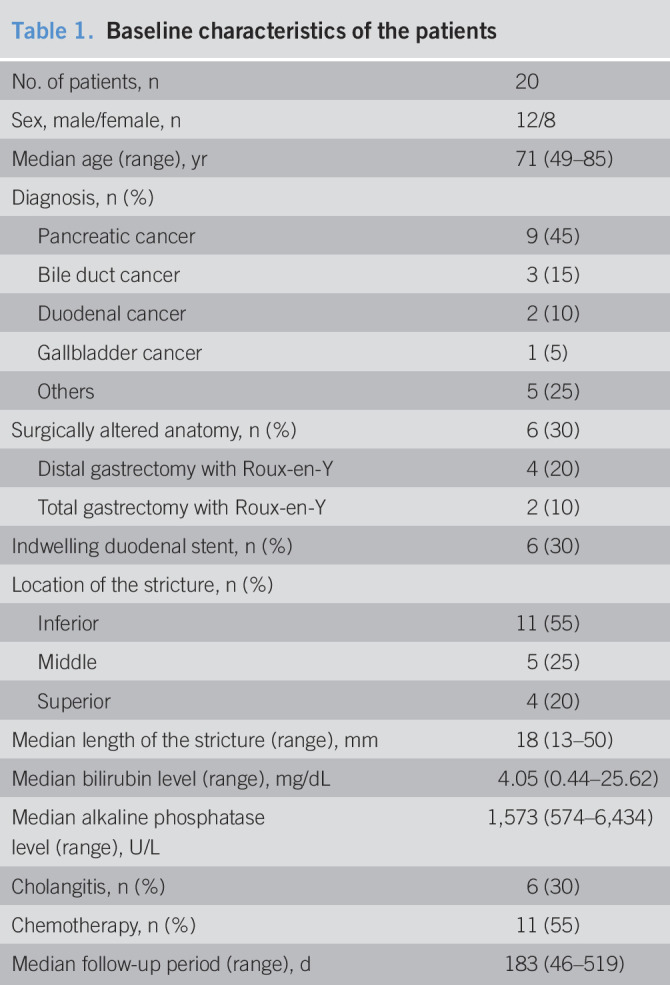
| No. of patients, n | 20 |
| Sex, male/female, n | 12/8 |
| Median age (range), yr | 71 (49–85) |
| Diagnosis, n (%) | |
| Pancreatic cancer | 9 (45) |
| Bile duct cancer | 3 (15) |
| Duodenal cancer | 2 (10) |
| Gallbladder cancer | 1 (5) |
| Others | 5 (25) |
| Surgically altered anatomy, n (%) | 6 (30) |
| Distal gastrectomy with Roux-en-Y | 4 (20) |
| Total gastrectomy with Roux-en-Y | 2 (10) |
| Indwelling duodenal stent, n (%) | 6 (30) |
| Location of the stricture, n (%) | |
| Inferior | 11 (55) |
| Middle | 5 (25) |
| Superior | 4 (20) |
| Median length of the stricture (range), mm | 18 (13–50) |
| Median bilirubin level (range), mg/dL | 4.05 (0.44–25.62) |
| Median alkaline phosphatase level (range), U/L | 1,573 (574–6,434) |
| Cholangitis, n (%) | 6 (30) |
| Chemotherapy, n (%) | 11 (55) |
| Median follow-up period (range), d | 183 (46–519) |
Technical and functional success
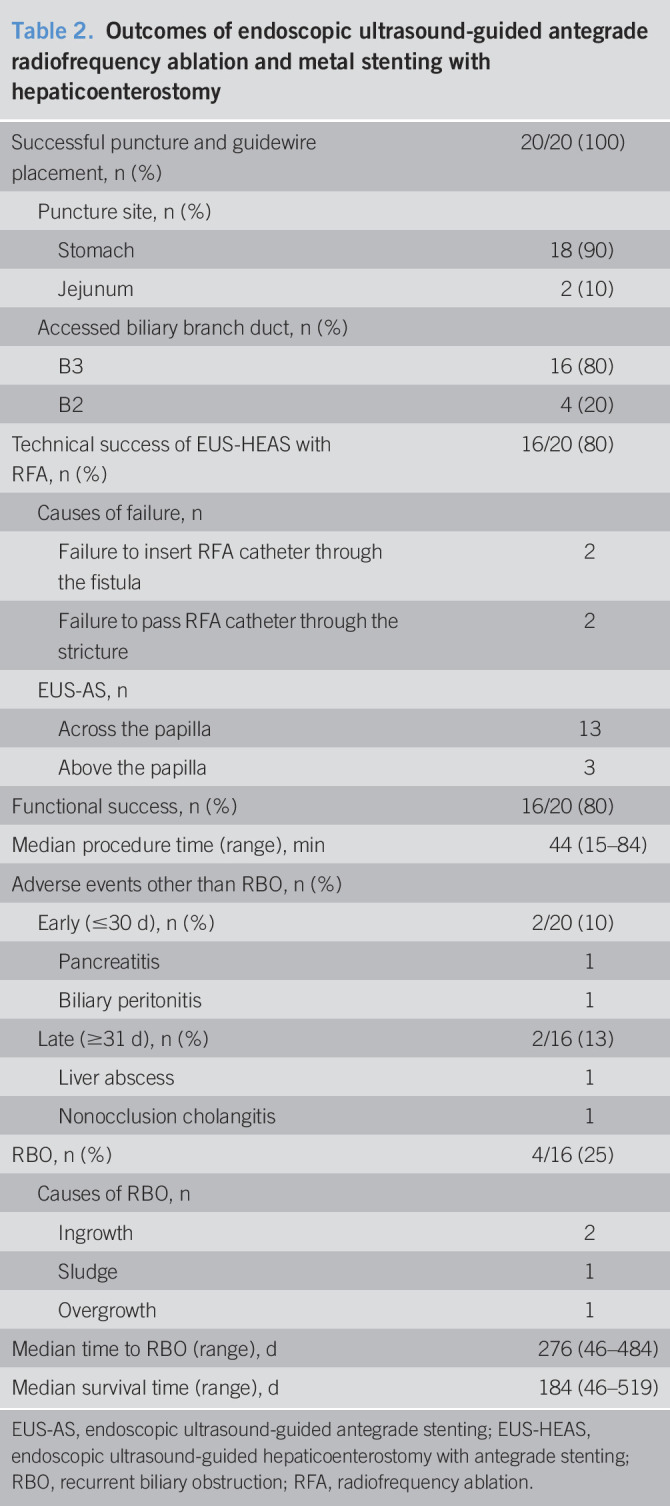
Table 2 shows the outcomes of EUS-HEAS with RFA. The technical success rate was 80% (16/20). The procedure was unsuccessful in a total of 4 patients due to failure to insert the RFA catheter into the fistula (2 patients) or failure to pass the RFA catheter through the stricture (2 patients). Functional success was achieved in all patients who underwent technically successful procedures.
Table 2.
Outcomes of endoscopic ultrasound-guided antegrade radiofrequency ablation and metal stenting with hepaticoenterostomy
| Successful puncture and guidewire placement, n (%) | 20/20 (100) |
| Puncture site, n (%) | |
| Stomach | 18 (90) |
| Jejunum | 2 (10) |
| Accessed biliary branch duct, n (%) | |
| B3 | 16 (80) |
| B2 | 4 (20) |
| Technical success of EUS-HEAS with RFA, n (%) | 16/20 (80) |
| Causes of failure, n | |
| Failure to insert RFA catheter through the fistula | 2 |
| Failure to pass RFA catheter through the stricture | 2 |
| EUS-AS, n | |
| Across the papilla | 13 |
| Above the papilla | 3 |
| Functional success, n (%) | 16/20 (80) |
| Median procedure time (range), min | 44 (15–84) |
| Adverse events other than RBO, n (%) | |
| Early (≤30 d), n (%) | 2/20 (10) |
| Pancreatitis | 1 |
| Biliary peritonitis | 1 |
| Late (≥31 d), n (%) | 2/16 (13) |
| Liver abscess | 1 |
| Nonocclusion cholangitis | 1 |
| RBO, n (%) | 4/16 (25) |
| Causes of RBO, n | |
| Ingrowth | 2 |
| Sludge | 1 |
| Overgrowth | 1 |
| Median time to RBO (range), d | 276 (46–484) |
| Median survival time (range), d | 184 (46–519) |
EUS-AS, endoscopic ultrasound-guided antegrade stenting; EUS-HEAS, endoscopic ultrasound-guided hepaticoenterostomy with antegrade stenting; RBO, recurrent biliary obstruction; RFA, radiofrequency ablation.
Adverse events
The rate of early adverse events was 10% (2/20); pancreatitis and biliary peritonitis, which occurred in 1 patient each, were improved with conservative management. The rate of late adverse events was 13% (2/16), and liver abscess and nonocclusion cholangitis occurred in 1 patient each. The liver abscess was treated by percutaneous drainage, and the nonocclusion cholangitis was treated by conservative management.
RBO and survival
The rate of RBO was 25% (4/16), and it was caused by ingrowth in 2 patients, by sludge in 1, and by overgrowth in 1. The median time to RBO was 276 days (Figure 4), and the median survival time was 184 days (Figure 5).
Figure 4.
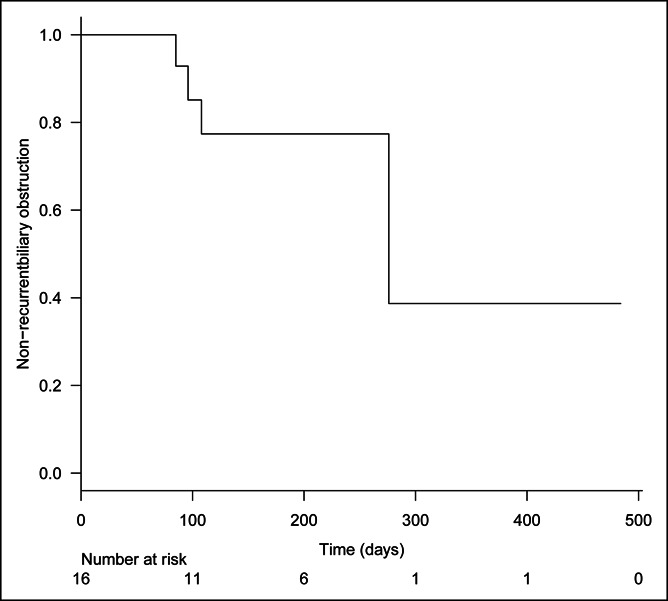
Kaplan-Meier analysis of time to recurrent biliary obstruction. The median time to recurrent biliary obstruction was 276 days.
Figure 5.
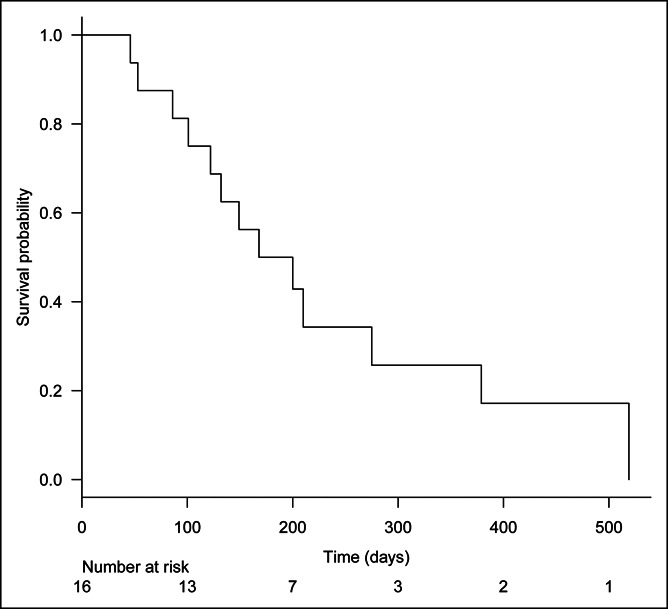
Kaplan-Meier analysis of patient survival. The median survival time was 184 days.
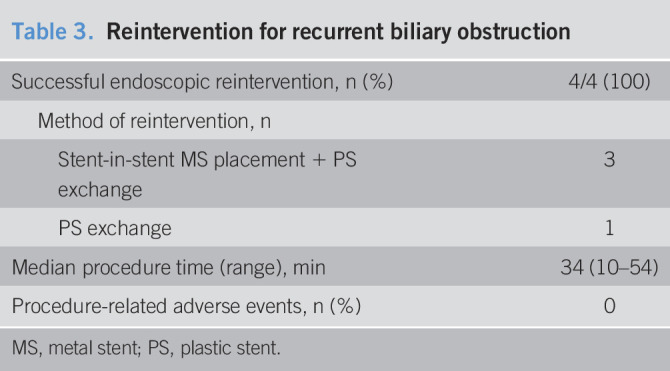
The success rate of endoscopic reintervention using the hepaticoenterostomy was 100% (4/4) (Table 3). Antegrade metal stenting by the stent-in-stent method with an exchange of PS across the hepaticoenterostomy was performed in 3 patients, and only the replacement of PS after cleaning was performed in 1 patient. No reintervention-related adverse event occurred.
Table 3.
Reintervention for recurrent biliary obstruction
| Successful endoscopic reintervention, n (%) | 4/4 (100) |
| Method of reintervention, n | |
| Stent-in-stent MS placement + PS exchange | 3 |
| PS exchange | 1 |
| Median procedure time (range), min | 34 (10–54) |
| Procedure-related adverse events, n (%) | 0 |
MS, metal stent; PS, plastic stent.
DISCUSSION
This study showed that the use of EUS-HEAS with RFA for MBO in which the transpapillary approach was difficult was technically feasible with low adverse event rates and good long-term outcomes. EUS-HEAS is a promising option in patients experiencing difficulty with ERCP because it can achieve longer stent patency and can be associated with fewer procedure-related adverse events compared with EUS-HES or EUS-AS alone. However, even when EUS-HEAS is performed, stent occlusion often occurs (5–8), and reintervention is often more complicated compared with ERCP guidance. Thus, further improvements to stent patency with simple reintervention approaches are required.
For EUS-AS, many institutions opt for an uncovered metal stent (5–7,14) because it has a thin and flexible delivery system that enables advance through the fistula, the bent hilar portion, and MBO. Furthermore, it is likely to prevent stent dislocation or misplacement and pancreatic duct orifice obstruction. However, tumor ingrowth is a significant issue for uncovered stents. The use of RFA has putative tumor-suppressive effects, which allows superior stent patency by preventing tumor ingrowth (10,15). Our experience with EUS-HEAS with RFA reported in this study showed better results regarding time to RBO (276 days) than previous studies without RFA (63–150 days) (5–8). RFA can also improve the stent patency of EUS-AS.
For EUS-HES, the dedicated single-pigtail PS (12) was used in this study. We considered that the long-term effects of drainage could be left to EUS-AS with RFA, and the hepaticoenterostomy stent would function to reduce bile leakage and maintain the fistula for reintervention. Therefore, the dedicated PS seems to be suitable for our aims. Although using a metal stent for EUS-HES achieves superior stent patency compared with PS, it might result in overdilation, occlusion of the intrahepatic bile duct, and tissue hyperplasia caused by stent ends (16), which might complicate reintervention. In this study, reintervention using hepaticoenterostomy with stent removal and replacement was successful in all patients.
However, advancement of RFA catheter was often difficult, and 4 cases (20%) exhibited technical failure in this study. We believe that this was because the current RFA catheter is 8-Fr with an inflexible body and is not tapered at the tip, and thus, device improvement is needed. A novel balloon-catheter RFA (17) might be better for antegrade RFA, although this technology is still being evaluated in animal trials. Because a long procedure time leads to peritonitis due to bile leakage, it should be changed to EUS-HEAS without RFA or EUS-HES alone without challenging too much.
The results of this study should be considered in the context of its limitations, which include its single-center setting, its small sample size, the involvement of a single operator, and its nonrandomized design. Furthermore, the potential for selection bias could not be avoided in this pilot study setting, and the follow-up period was relatively short.
Despite these limitations, this is the first study to investigate and report favorable results regarding the use of EUS-HEAS with RFA. These results should drive further multicenter trials and the development of new RFA catheters.
CONFLICTS OF INTEREST
Guarantor of the article: Tadahisa Inoue, MD, PhD.
Specific author contributions: T.I.: conception and design, data acquisition, analysis and interpretation, and drafting and revising of the manuscript. M.I., R.K., Y.K., T.O., Y.S., Y.N., K.I., and M.Y.: data interpretation and revising of the manuscript.
Financial support: None to report.
Potential competing interests: T. I. received honoraria from Boston Scientific Japan and Japan Lifeline, outside the submitted work. The other authors disclose no financial relationships relevant to this publication.
Clinical trial registry: UMIN000033855.
Statement of ethics: Aichi Medical University Hospital's institutional review board approved this study in accordance with the principles of the Declaration of Helsinki (Approval number: 2018-H189).
Study Highlights.
WHAT IS KNOWN
✓ Endobiliary RFA for MBO is a promising option for improving biliary stent patency, but its efficacy and safety with EUS-guided biliary drainage are uncertain.
WHAT IS NEW HERE
✓ EUS-HEAS with RFA for MBO was technically feasible with low adverse event rates and good long-term outcomes. However, advancement of RFA catheter was often difficult.
TRANSLATIONAL IMPACT
✓ The results should drive further multicenter trials and the development of new RFA catheters.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A408; http://links.lww.com/CTG/A409
REFERENCES
- 1.Giovannini M, Moutardier V, Pesenti C, et al. Endoscopic ultrasound-guided bilio duodenal anastomosis: A new technique for biliary drainage. Endoscopy 2001;33:898–900. [DOI] [PubMed] [Google Scholar]
- 2.Isayama H, Nakai Y, Itoi T, et al. Clinical practice guidelines for safe performance of endoscopic ultrasound/ultrasonography- guided biliary drainage: 2018. J Hepatobiliary Pancreat Sci 2019;26:249–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teoh AYB, Dhir V, Kida M, et al. Consensus guidelines on the optimal management in interventional EUS procedures: Results from the Asian EUS group RAND/UCLA expert panel. Gut 2018;67:1209–28. [DOI] [PubMed] [Google Scholar]
- 4.Mukai S, Itoi T. EUS-guided antegrade procedures. Endosc Ultrasound 2019;8:S7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imai H, Takenaka M, Omoto S, et al. Utility of endoscopic ultrasound-guided hepaticogastrostomy with antegrade stenting for malignant biliary obstruction after failed endoscopic retrograde cholangiopancreatography. Oncology 2017;93(Suppl 1):69–75. [DOI] [PubMed] [Google Scholar]
- 6.Ogura T, Kitano M, Takenaka M, et al. Multicenter prospective evaluation study of endoscopic ultrasound-guided hepaticogastrostomy combined with antegrade stenting (with video). Dig Endosc 2018;30:252–9. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto K, Itoi T, Tsuchiya T, et al. EUS-guided antegrade metal stenting with hepaticoenterostomy using a dedicated plastic stent with a review of the literature (with video). Endosc Ultrasound 2018;7:404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada M, Ogura T, Kamiyama R, et al. EUS-guided antegrade biliary stenting using a novel fully covered metal stent (with video). J Gastrointest Surg 2019;23:192–8. [DOI] [PubMed] [Google Scholar]
- 9.Larghi A, Rimbaş M, Tringali A, et al. Endoscopic radiofrequency biliary ablation treatment: A comprehensive review. Dig Endosc 2019;31:245–55. [DOI] [PubMed] [Google Scholar]
- 10.Sofi AA, Khan MA, Das A, et al. Radiofrequency ablation combined with biliary stent placement versus stent placement alone for malignant biliary strictures: A systematic review and meta-analysis. Gastrointest Endosc 2018;87:944–51. [DOI] [PubMed] [Google Scholar]
- 11.Inoue T, Ito K, Yoneda M. Antegrade readiofrequency ablation and stenting for biliary stricture through endoscopic ultrasound-guided hepaticogastrostomy. Dig Endosc 2018;30:793–4. [DOI] [PubMed] [Google Scholar]
- 12.Umeda J, Itoi T, Tsuchiya T, et al. A newly designed plastic stent for EUS-guided hepaticogastrostomy: A prospective preliminary feasibility study (with videos). Gastrointest Endosc 2015;82:390–6.e2. [DOI] [PubMed] [Google Scholar]
- 13.Isayama H, Hamada T, Yasuda I, et al. TOKYO criteria 2014 for transpapillary biliary stenting. Dig Endosc 2015;27:259–64. [DOI] [PubMed] [Google Scholar]
- 14.Iwashita T, Yasuda I, Mukai T, et al. Endoscopic ultrasound-guided antegrade biliary stenting for unresectable malignant biliary obstruction in patients with surgically altered anatomy: Single-center prospective pilot study. Dig Endosc 2017;29:362–8. [DOI] [PubMed] [Google Scholar]
- 15.Inoue T, Ibusuki M, Kitano R, et al. Endobiliary radiofrequency ablation combined with bilateral metal stent placement for malignant hilar biliary obstruction. Endoscopy 2020;52:595–9. [DOI] [PubMed] [Google Scholar]
- 16.Hara K, Yamao K, Mizuno N, et al. Endoscopic ultrasonography-guided biliary drainage: Who, when, which, and how? World J Gastroenterol 2016;22:1297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue T, Ito K, Yoneda M. Novel balloon catheter-based endobiliary radiofrequency ablation system: An ex-vivo experimental study. Dig Endosc 2020;32:974–8. [DOI] [PubMed] [Google Scholar]