Supplemental Digital Content is available in the text.
Keywords: bundle of care, cardiac arrest, cardiopulmonary resuscitation, emergency medical services, resuscitation centers, sudden cardiac death survival
Abstract
Objectives:
To construct a highly detailed yet practical, attainable roadmap for enhancing the likelihood of neurologically intact survival following sudden cardiac arrest.
Design, Setting, and Patients:
Population-based outcomes following out-of-hospital cardiac arrest were collated for 10 U.S. counties in Alaska, California, Florida, Ohio, Minnesota, Utah, and Washington. The 10 identified emergency medical services systems were those that had recently reported significant improvements in neurologically intact survival after introducing a more comprehensive approach involving citizens, hospitals, and evolving strategies for incorporating technology-based, highly choreographed care and training. Detailed inventories of in-common elements were collated from the ten 9-1-1 agencies and assimilated. For reference, combined averaged outcomes for out-of-hospital cardiac arrest occurring January 1, 2017, to February 28, 2018, were compared with concurrent U.S. outcomes reported by the well-established Cardiac Arrest Registry to Enhance Survival.
Interventions:
Most commonly, interventions and components from the ten 9-1-1 systems consistently included extensive public cardiopulmonary resuscitation training, 9-1-1 system-connected smart phone applications, expedited dispatcher procedures, cardiopulmonary resuscitation quality monitoring, mechanical cardiopulmonary resuscitation, devices for enhancing negative intrathoracic pressure regulation, extracorporeal membrane oxygenation protocols, body temperature management procedures, rapid cardiac angiography, and intensive involvement of medical directors, operational and quality assurance officers, and training staff.
Measurements and Main Results:
Compared with Cardiac Arrest Registry to Enhance Survival (n = 78,704), the cohorts from the 10 emergency medical services agencies examined (n = 2,911) demonstrated significantly increased likelihoods of return of spontaneous circulation (mean 37.4% vs 31.5%; p < 0.001) and neurologically favorable hospital discharge, particularly after witnessed collapses involving bystander cardiopulmonary resuscitation and shockable cardiac rhythms (mean 10.7% vs 8.4%; p < 0.001; and 41.6% vs 29.2%; p < 0.001, respectively).
Conclusions:
The likelihood of neurologically favorable survival following out-of-hospital cardiac arrest can improve substantially in communities that conscientiously and meticulously introduce a well-sequenced, highly choreographed, system-wide portfolio of both traditional and nonconventional approaches to training, technologies, and physiologic management. The commonalities found in the analyzed systems create a compelling case that other communities can also improve out-of-hospital cardiac arrest outcomes significantly by conscientiously exploring and adopting similar bundles of system organization and care.
Sudden cardiac arrest (SCA), particularly out-of-hospital cardiac arrest (OHCA), remains a leading cause of premature death worldwide. Despite widespread community-based bystander cardiopulmonary resuscitation (CPR) training and proliferation of advanced care emergency medical services (EMS) systems, the reported likelihood of survival with favorable neurologic function remains less than 10% in most communities (1). Outcomes are probably even worse among EMS systems not reporting their outcomes, likely reflecting a lack of resources including quality assurance programs that gauge performance or simply not choosing to report dismal outcomes. When prudent data collection programs such as the Cardiac Arrest Registry to Enhance Survival (CARES) registry are introduced, SCA outcomes improve (2, 3).
For decades, consensus-forming groups such as the American Heart Association (AHA) or the International Liaison Committee on Resuscitation (ILCOR) have developed well-promulgated guidelines for implementing both basic and advanced interventions for SCA, emphasizing a judicious “evidence-based” process using published peer-reviewed literature (4, 5). These processes have clear value, but they also have their limitations as well. Evaluated publications can convey conflicting information, involve mixed populations without stratification, routinely possess statistical limitations, or can be confounded by a lack of adherence to intended protocols, all leading to inconclusive findings (6).
Furthermore, in cardiac arrest research, the traditional controlled trials that test the effect of a singular intervention may be one of the main reasons for those misleading or conflicting results and subsequent inconclusive consensus (7). Examining simple binary outcomes though interventional trials (effective or not) has been compromised by the profoundly time-dynamic and multivariable nature of SCA cases. A single intervention (e.g., drug, automated defibrillator) that is highly effective when provided within minutes of a SCA may not be helpful when too many minutes have elapsed. Cardiovascular, pulmonary, and resulting hemodynamic abnormalities can rapidly evolve after SCA onset and those temporal considerations need to be addressed in a multidimensional manner.
In addition, basic CPR itself requires conscientious attention to coordinating multiple interdependent and interactive components (e.g. chest compression rate, depth, recoil, number of interruptions) under different circumstances (8). Likewise, assisted breathing components not only require attention to the frequency, force, and speed of delivery of each breath but also the actual volume and the rapid release of each positive pressure breath once delivered. These ventilatory variables significantly impact circulation and thus the effectiveness of other interventions, including medications, and CPR itself (5, 9, 10). In addition, all of these factors need to be well-controlled and adjusted under certain conditions, particularly when flow-enhancing devices are used or when spontaneous circulation or respirations resume (9).
Accordingly, any proscribed ideal target for each of these circulatory and ventilatory components may need to be adjusted and adapted, not only according to the multidimensional and interactive effects of these variables but also according to a myriad of other factors such as time-dependent changes in physiologic conditions and hemodynamics. Such symphonic-like components and movements also require expert conductors who can meticulously anticipate, manage, revise, and adapt a multifaceted, tightly choreographed process instead of employing the same rote fixed protocols and procedures start to finish (11–13).
These complex dynamics over the course of resuscitation efforts may have confounded many of our current “evidence-based” publications, even gold standard clinical trials (10, 14). Specifically, if an otherwise well-designed clinical trial shows a single intervention to be ineffective, that conclusion is likely accurate, but only within the context of that study (10). Experience has now shown investigators that the same intervention might have been demonstrated to be quite effective if it had been introduced using an approach that successfully harmonizes time-appropriate quality CPR performance and/or physiologically sound ventilatory practices (10, 14).
For OHCA, the setting itself may also affect the outcomes. Important variables include the populations served, community residential infrastructure, traffic, geography, climate, dispatch functions, and the frequency and quality of early bystander CPR (bCPR) (15). EMS design alone (system response configuration and resources) can significantly impact the skills of EMS personnel and therefore outcomes (14–17). Receiving facility resources such as extracorporeal membrane oxygenation (ECMO) and percutaneous coronary intervention (PCI) can also influence ultimate outcomes (18). Postresuscitation decision-making (positioning, method/degree of therapeutic cooling, premature withdrawal of support) can all become pivotal considerations as well (19–21).
In essence, many interdependent components forming a longitudinal bundle of management for SCA must be simultaneously optimized, choreographed, and properly implemented with time-appropriate and physiologically driven approaches. Attention to detail must extend from the dispatch center through eventual discharge from the hospital as well as medical follow-up services such as physical and neuropsychiatric rehabilitation (22, 23).
This concept of an interdependent “bundle of care” is not new. Other complex disease conditions also require well-choreographed, multifaceted, interdependent bundles of treatment, not just a single intervention at a time (23). Even the universally recognized metaphor of the “chain of survival” for cardiac arrest (die rettungskette originally described by Fritz Ahnefeld in 1967 [24]) is an early example. Each link in this symbolic chain needs to be present and optimized to enhance survival chances. The common idiom, “only as strong as the weakest link”, applies here even more so given the hundreds of links involved.
Recognizing all of these concerns, several dozen resuscitation practitioners from a multitude of nations, specialties, and agencies recently gathered to form an International Resuscitation Collaborative (IRC) to inventory both current and proposed future “best practices” in OHCA management. The group first queried a number of U.S. EMS agencies that had recently reported significant improvements in SCA outcomes and then analyzed each of the them in detail for common elements that could help to summarize and assimilate a potential “work-in-progress” roadmap that might help to create a more optimal bundled approach for OHCA.
The integral intent was to create a user-friendly, reproducible, and attainable practical roadmap built upon state-of-the art evidence and the collective experience of the veteran participants, particularly those well-versed in the budgetary and logistical challenges/pitfalls of implementing new procedures within EMS systems. The process focused on identifying the common synergistic strategies, technologies, and procedures that could be incorporated and well-integrated across the entire spectrum of management, from enhanced dispatch procedures and community-wide initiatives for public involvement to on-scene patient care management and receiving facility interventions.
A basic tenet of validating classic scientific laboratory research is the ability to accurately communicate and reproduce methods from one investigator’s laboratory to another’s, and, in turn, explicitly reproduce those results. Considering decades of challenges and disappointments posed by traditional clinical trial methodologies, the ultimate purpose here was to construct an alternative approach that could be used to consistently reproduce the research and guide others to enhance neurologically-intact survival following sudden cardiac arrest.
METHODS
Participants
A multidisciplinary collaboration was established by a well-recognized group of cardiologists, emergency and internal medicine physicians, paramedics, and fire rescue leaders in the United States, Canada, and Europe, largely comprised of those who had published or reported new approaches to CPR with associated high rates of resuscitation and neurologically intact survival for both OHCA and in-hospital cardiac arrest. Adopting the moniker, IRC, the majority of the collaborative members have also participated in the processes for developing the related evidence-based management guidelines for the AHA, the European Resuscitation Council, or ILCOR.
Setting
The IRC participants assembled voluntarily, without financial support and sponsorship, in Oakland, CA, in late September 2018 (n = 32) and subsequently in Paris, France, in October 2019 (n = 28). The meeting and invitations to participants were organized and facilitated through the nonprofit organizations, Take Heart America, the French Resuscitation Council, and the Metropolitan EMS Medical Directors Alliance (23).
Prior to their first convocation, a prospective plan was placed into motion in terms of how the evaluation would be conducted and how the IRC participants would report the results. As a first step, the participants prospectively chose to review, analyze, and collate inventories from 10 EMS systems (EMS-10). The systems chosen were not only those that had reported exceptional improvements in outcomes after introduction of novel or expedited approaches to OHCA care but also those that were able to provide complete process-reporting datasets equivalent to those within the CARES registry (25). The EMS-10 was also derived from 10 geographically diverse U.S. counties including agencies in Alaska, California, Florida, Ohio, Minnesota, Utah, and Washington. Representatives from selected agencies were invited to attend and present their information under the direct scrutiny, interrogatories, and commentaries of the IRC participants.
At both conferences, the prospective plan for the sessions was to first have introductory reports from each system. The introductory comments from participants were delivered in traditional abstract presentation formats later followed by several sessions of modified Delphi roundtables. After detailed inventories of the strategies, interventions, protocols, and procedural approaches, the findings were tabulated to identify commonalities, differences, and complementary elements. Findings were then presented, reviewed, and discussed by IRC members in-person to better delineate and understand the various components identified and, in turn, to create a recommended bundle of management distilled from those discussions.
In contrast to the approach often used by other organizations and the concerns expressed in the Introduction, this deliberative process did not focus on a grading system for any individual intervention. Instead, it focused on distilling the comprehensive inventories derived and tabulated from these high-performance EMS-10 systems. Step 1 was to identify the readily acceptable common elements found in each of these high-functioning systems to create the basic foundation of the proposed bundle of care. Step 2 was to sequentially identify other best practices and innovative procedures from individual systems that were mutually considered to be suitable and complementary additions to the proposed bundle of care roadmap after thoughtfully considering the applicable literature, feasibility, safety, and outcome data. After formulating a comfort level and agreement on those additional components, Step 3 was an additional exercise to identify potential avenues of improvement that could be explored in the future such as prehospital implementation of ECMO, an intervention that has already been introduced into some other agencies, but not among the EMS-10. Of note, training tools, procedural efficiencies, and the impact of resuscitation efforts on personnel were reviewed in great detail. Team-building implementation solutions were also part of the dialogue. Despite numerous academic debates within the deliberations, the interactions were exceptionally congenial with little remaining controversy by the end of the process.
Outcomes Measurements
Although the primary goal of the analysis was to identify the common elements and best practices associated with these high-performance systems, it was recognized that the significant improvements in outcomes originally reported by the EMS-10 were based on historical controls within each of the individual communities. Therefore, for reference purposes, the IRC also obtained and combined the OHCA outcomes during the prior 12-month calendar year (2017, or a more proximate 12-mo period, March 2017 to February 2018). These aggregate 12-month data were then compared with the concurrent (2017) calendar year outcome data reported by the respected CARES project. CARES, a voluntary registry launched as a collaborative effort between the U.S. Centers for Disease Control and Prevention and Emory University, has become a reliable marker of outcomes among many progressive U.S. EMS systems conscientiously monitoring their OHCA outcomes (2).
Data collected and reported through CARES include process data such as the time elapsed from the first call to 9-1-1 (or equivalent) until initiation of basic CPR by first responders or time of return of spontaneous circulation (ROSC) as applicable as well as documentation of whether the OHCA was witnessed and if CPR was performed by bystanders. It also includes what the presenting cardiac rhythm was and, when applicable, the eventual neurologic status using the Cerebral Performance Category (CPC) score at the time of hospital discharge (HD).
The primary analysis reference point for the current evaluation was the mean rate of HD with a favorable neurologic outcome defined as a CPC score of 1 or 2 (HD CPC 1–2) and, secondarily, the frequency of ROSC, regardless of the first recorded cardiac rhythm (2, 23, 26).
Interventions Evaluated
The interventions evaluated are best defined by the in-common elements and components identified among the EMS-10 and reported in the inventories obtained. The in-common practices among those systems were supported by the best available preclinical and clinically relevant evidence. The inventories considered detailed best practices and tools for training and implementing both conventional and novel interventions. However, among participants, the interventions originally assessed within individual systems were considered the most important components when finally examining and tabulating the respective bundles of care that resulted in outcome improvements.
Statistical Analyses
Statistical comparisons between the concurrent CARES and EMS-10 outcomes included two-tailed Fisher exact test and calculations of the odds ratio (OR) with 95% CIs (95% CI). Data are expressed as a mean ± sd with p value of less than 0.05 defining statistical significance.
RESULTS
The location of the analyzed EMS-10 systems and the interventions used in each are shown in Table 1 and Figure 1. Collectively, these agencies provide 9-1-1 responses for residential populations of approximately 4 million persons. A total of 2,911 OHCA patients were treated by the EMS-10 during the respective 12-month analysis periods. Figure 1 includes those interventions deployed by each or all (bolded) of the EMS-10 agencies and their receiving hospitals. Community response components included techniques to increase the frequency of bCPR such as lay rescuer training in schools, just-in-time dispatch-assisted CPR including “no-no-go” dispatch protocols, and smart phone applications (27–30). The mean frequency of bCPR across the 10 systems was 43.7%.
TABLE 1.
Outcomes From 10 High-Functioning Emergency Medical Services Systems, Both Individually and Combined, As Compared With Cardiac Arrest Registry to Enhance Survival Outcomes
| 9-1-1 System Jurisdictions | Total Cases, n | Bystander Cardiopulmonary Resuscitation, % | Return of Spontaneous Circulation (%) | HD, % (n) | HD With CPC 1, 2 (n) | HD With CPC 1, 2 (%) | Utstein 2 Casesa, % (n) |
|---|---|---|---|---|---|---|---|
| Alameda County, CA | 1043 | 38 | 33.20 | 11.3 (118) | 85 | 8.10 | 48 (20/42) |
| Anchorage, AK | 249 | 73 | 37.30 | 16.1 (40) | 39 | 15.70 | 32.3 (10/31) |
| Anoka County, MN | 211 | 31.30 | 33.60 | 16.1 (34) | 32 | 15.20 | 37.5 (7/18) |
| St. John’s County, FL | 155 | 52 | 46.50 | 12.9 (20) | 17 | 11 | 26 (7/27) |
| Lucas County, OH | 510 | 43.30 | 31.80 | 11.6 (59) | 43 | 8.40 | 37 (15/41) |
| Minneapolis-St. Paul, MN | 387 | 36.80 | 47 | 14.5 (56) | 39 | 10.10 | 44 (15/34) |
| Palm Beach County, FL | 41 | 48.80 | 58.50 | 12.2 (5) | 5 | 12 | 25 (1/4) |
| Rialto, CA | 90 | 41 | 46 | 18 (16) | 12 | 12.80 | 40 (4/10) |
| Salt Lake City, UT | 131 | 64 | 44 | 21 (28) | 26 | 19.80 | 58 (15/26) |
| Whatcom County, WA | 94 | 45.70 | 41.50 | 19.1 (18) | 14 | 14.90 | 75 (6/8) |
| Emergency Medical Services-10 | 2,911 | 43.70 | 37.4 | 13.5 (394) | 312 | 10.70 | 41.6 (100/241) |
| Cardiac Arrest Registry to Enhance Survival b | 78,704 | 44.9 | 31.5 | 10.4 (8,223) | 6,622 | 8.4 | 29.2 (2,540/8,689) |
| p | Nonsignificant | < 0.0001 | < 0.0001 | 0.0002 | 0.0002 | 0.0001 |
CPC = Cerebral Performance Category, HD = hospital discharge.
aRate of HD with favorable neurologic function among patients with the greatest likelihood of survival, namely those with a witnessed arrest, a first recorded rhythm of either ventricular tachycardia or ventricular fibrillation and those who received bystander cardiopulmonary resuscitation (CPR).
bCardiac Arrest Registry to Enhance Survival rates of survival to hospital discharge with good neurologic function rates remained constant in 2017 and 2018.
Patients with a CPC score of 1 or 2 were considered to have a favorable neurologic outcome. Secondary clinical outcomes included rates of return of spontaneous circulation, overall frequency of hospital discharge, and Utstein 2 Cases. Bystander CPR rates are from 2017. Collective outcome data for the 10 Emergency Medical Services systems are in bold.
Figure 1.
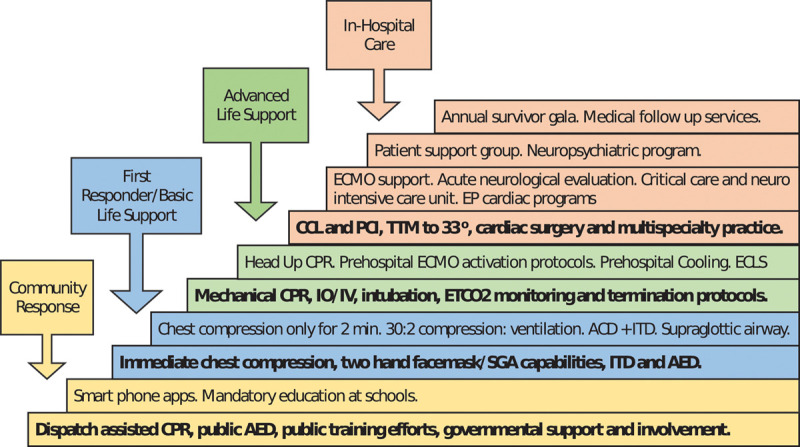
The bundle of care. No single intervention is effective in the treatment of cardiac arrest. Key interventions that are similar across all 10 emergency medical services systems are in bold. ACD = active compression decompression, AED = automated external defibrillator, CCL = cardiac catheterization laboratory, CPR = cardiopulmonary resuscitation, ECLS = extracorporeal life support program, ECMO = extracorporeal membrane oxygenation, EP = electrophysiologic programs, ETCo2 = end-tidal Co2, IO = intraosseous infusion, ITD = impedance threshold device, PCI = percutaneous coronary intervention, SGA = supraglottic airway, TTM = therapeutic temperature management.
In addition to phone applications, other evolving technologies were common as shown in Figure 1 and Supplemental Digital Content 1 (http://links.lww.com/CCX/A314). Supplementary Table 1, a–d (Supplemental Digital Content 1, http://links.lww.com/CCX/A314) displays the key first responder, basic life support, and advanced life support interventions provided on-scene, including the commonality of feedback tools for quality CPR performance. There was in-common practice of applying an impedance threshold device (ITD) whether using a facemask, supraglottic airway, or endotracheal intubation for ventilation (14, 22, 26). As shown in Supplementary Table 1, a–d (Supplemental Digital Content 1, http://links.lww.com/CCX/A314) and Supplemental Digital Content 2 (http://links.lww.com/CCX/A315), the ITD was combined with automated mechanical CPR devices employing an active compression-decompression (ACD) component in eight of the 10 systems (22, 26). Automated CPR was not only used for transport safety but also used during on-scene resuscitation to standardize chest compression rate, depth, and recoil and to better enhance intrathoracic pressure regulation (26, 31, 32). With its ability to generate even more negative intrathoracic pressure during the decompression phase, a manual ACD CPR pump was used prior, or as alternative, to using automated CPR in six of those eight systems (26, 31, 32).
All EMS-10 systems had designated resuscitation centers providing rapid access to PCI and targeted (core) temperature management (TTM) with most centers aiming for 33°C for 24 hours minimally (33). As indicated in the tables, ECMO protocols were commonly used or soon to be implemented in those centers. Three systems had implemented technology to gradually raise the head and thorax to a specified degree during CPR which had consistently augmented local resuscitation rates incrementally (22, 31, 34). Another consistent attribute overall was intensive involvement of medical directors, quality assurance officers, operations chiefs, and training staff.
Stratified by pediatric and adult OHCA, a more comprehensive IRC description of the underlying rationale for any specific individual intervention was detailed within the Supplemental Digital Content 1 (http://links.lww.com/CCX/A314) and Supplemental Digital Content 2 (http://links.lww.com/CCX/A315).
Based upon these inventory analyses and discussions, the IRC assimilated “seven pillars of care” (Fig. 2) to be considered when implementing a more comprehensive resuscitation bundle. Interactions between these seven domains had emerged not only as being quite codependent but also synergistic in terms of improved outcomes (22). Beyond specifying that all core elements be included in the roadmap, the IRC stipulated emphatically that the many fine details outlined for training and execution were pivotal to success as was close attention to the less tangible aspects of monitoring and team building (Figs. 3 and 4).
Figure 2.
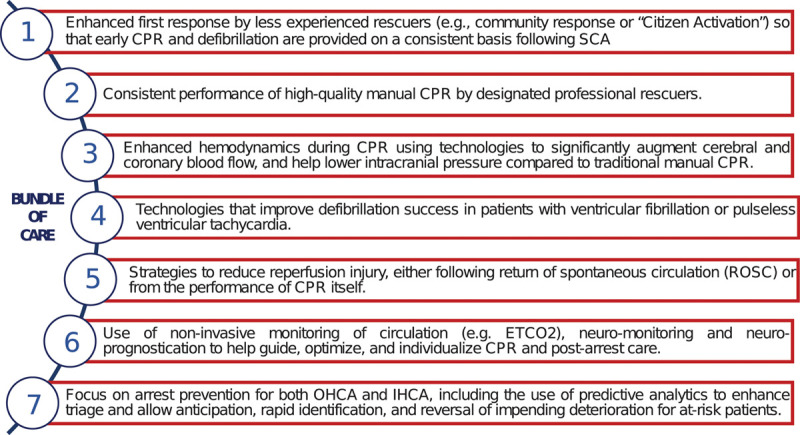
Seven pillars of care. All 10 emergency medical services systems first focused on the rapid delivery of chest compressions and performance of high-quality manual cardiopulmonary resuscitation (CPR) by first responders. Several included the use of one or more CPR feedback tools or guides. All of these systems implemented one or more of the recent technology and procedural advances such as expedited dispatch-assisted CPR instructions or smart phone apps, mechanical CPR, active compression decompression CPR, impedance threshold device airway attachments, elevation of the head and thorax during CPR, targeted temperature management strategies, extracorporeal membrane oxygenation CPR, and rapid percutaneous coronary intervention as part of the bundle. ETCo2 = end-tidal Co2, IHCA = in-hospital cardiac arrest, OHCA = out-of-hospital cardiac arrest, ROSC = return of spontaneous circulation, SCA = sudden cardiac arrest.
Figure 3.
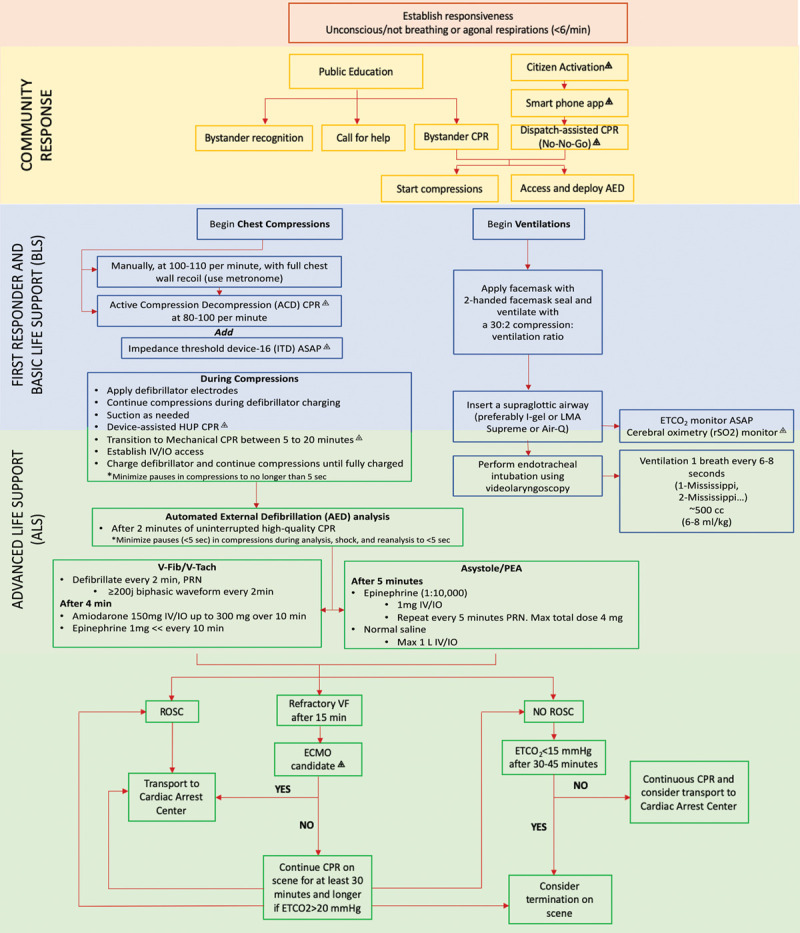
Optimal bundle of care. ⨹Not currently part of the standard American Heart Association or the International Liaison Committee on Resuscitation. Each intervention requires adequate personnel and, in some cases, special devices and technology. Interventions that may be performed by either basic life support or advanced life support (ALS) personnel are found in bullet points that span both groups of rescuers. ACD = active compression decompression, AED = automated external defibrillator, ASAP = as soon as possible, CPR = cardiopulmonary resuscitation, ECMO = extracorporeal membrane oxygenation, ETCo2 = end-tidal carbon dioxide, HUP = head up CPR, IO = intraosseous infusion, IV = IV access, ITD = impedance threshold device, LMA = laryngeal mask airway, Max = maximum, PEA = pulseless electrical activity, PRN = pro re nata, ROSC = return of spontaneous circulation, rSO2 = regional oxygen saturation from cerebral oximetry, VF, V-Fib = ventricular fibrillation, V-Tach = ventricular tachycardia.
Figure 4.
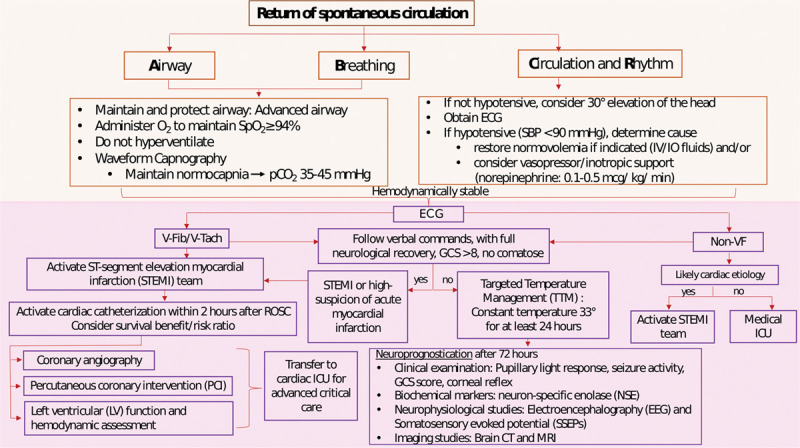
In-hospital cardiac arrest care. ECG = electrocardiogram, EEG = electroencephalography, GCS = Glasgow Coma Scale, IO = intraosseous infusion, IV = IV access, LV = left ventricular, NSE = neuron specific enolase, PCI = percutaneous coronary intervention, ROSC = return of spontaneous circulation, SBP = systolic blood pressure, SpO2 = oxygen saturation from pulse oximetry, SSEPs = somatosensory evoked potentials, STEMI = ST-segment elevation myocardial infarction, TTM = therapeutic temperature management, VF, V-Fib = ventricular fibrillation, V-Tach = ventricular tachycardia.
In terms of comparative outcomes, the mean ROSC rates for all cases, regardless of presenting cardiac rhythm, ranged from 35% to 40% (Table 1). The overall frequency of HD CPC 1–2 (favorable neurologic outcome), even including the large percentage of patients presenting with asystole, was 10.7% (range 8.1–19.8%), and there were no significant differences identified across the various systems. Stratifying for the bystander-witnessed cases presenting with ventricular fibrillation/ventricular tachycardia (VF/VT) for which a bystander performed basic CPR and/or used an automated external defibrillator, the mean HD CPC 1–2 was 41.6% (range 25–75%).
For comparative reference, mean ROSC and HD CPC 1–2 rates were significantly higher than CARES counterparts (Table 1). Overall, combined mean HD CPC 1–2 frequency was nearly 30% higher in the EMS-10 group (10.7%) versus 8.4% for CARES (p < 0.002; OR = 1.30, 95% CI = 1.159–1.473). For the subgroup of VF/VT bystander-witnessed arrests with bystander intervention, HD CPC 1–2 was 41.6% versus 29.2% in CARES (p < 0.001). The frequencies of bCPR were similar (EMS-10: 43.7% vs CARES: 44.9%) as were all other comparison data reviewed (e.g., age, sex, EMS response intervals).
As a supplemental evaluation, investigators analyzed the annualized cost of adding all of the proposed equipment. The evaluation included a 3-year amortization analysis of the costs for a standard 12-lead monitor-defibrillator with pulse oximetry, ETCO2 detectors, and quality CPR monitors, as well as the less conventional adjunct technologies such as automated CPR devices, ACD pumps, the ITD, intraosseous infusion supplies, equipment for device-assisted controlled sequential elevation (DACSE) of the head and thorax during CPR, and the cost of other consumables (e.g., medications, airways, personal protective equipment, electrocardiographic pads, IV fluids). The final amortization, including the cost of the standard monitor-defibrillator, was roughly 13,000 U.S. dollars a year for each response vehicle (ambulance). That figure represents an approximate 1–2% increment in the annual cost of operating an ambulance when one considers the major overriding costs of personnel salary and benefits, the amortized cost of the vehicle itself, and all of the other emergency equipment, supplies, and consumables, including fuel, oxygen, and many levels of disposables.
DISCUSSION
For decades, traditional gold-standard trials testing singular interventions for OHCA have yielded disappointing results. Most of those trials were typically perplexed by the pivotal interactive nature of various interdependent variables. In addition, underrecognized confounding factors such as suboptimal quality of CPR, overzealous positive pressure ventilation, time delays to interventions, and many other systematic factors played large roles (9, 10, 14, 17, 19, 21, 23, 31, 35–38). Low survival rates create further statistical quandaries, especially in essential subgroup analyses. Overall, those trials provided useful observational data collection, but the inherent realities and limitations of OHCA studies have stymied progress in validating life-saving interventions.
In this current analysis, survival rate improvements were independently reported by 10 independent EMS systems concomitantly introducing an evolving and very similar “bundled” management approach to OHCA. The analysis purposefully involved this selection bias using those systems that had adopted novel approaches and subsequently reported significant and fairly rapid improvements in outcomes. Although the agencies each possessed diverse attributes in terms of agency size, population components, variable response configurations, and geographically diverse jurisdictions, the analyses revealed that, despite these differences, each had adopted similar, comprehensive strategies as reported here and each had similar results. Portfolio inventories consistently featured highly integrated procedural and technological interventions that were physiologically driven and intensely choreographed across the seven pillars of care (12, 19, 20, 23, 26, 28, 31, 39, 40).
The identified elements were largely based on quality-improvement initiatives independently conducted in each jurisdiction over a short period of time during which the more traditional system factors (%VF/VT, response intervals, early defibrillation) remained largely constant. Although acknowledging the typical limitations of historically controlled observations, the IRC did emphasize that many of the outcome improvements were not only clear and sustained incremental improvements that appeared soon after introducing the new elements, but that they were also considered relatively reproducible findings across these independent and diverse agencies working in parallel of each other.
Beyond attention to the interdependent bundled approach, most improvements had their roots in well-designed preclinical trials and several compelling clinical feasibility/safety studies while also paying close attention to physiologically driven CPR technologies and procedures (22). With the many overlapping common elements, the participating IRC members readily constructed what they believed to be a feasible, relatively inexpensive, and largely reproducible roadmap for improving OHCA outcomes (Figs. 2 and 3).
Most commonly, the majority of these EMS-10 systems had 1) included the use of new technological tools for bCPR and streamlined dispatch procedures (41, 42); 2) introduced the “combination” of an ITD with highly choreographed and closely monitored quality CPR, usually expedited by mechanical CPR devices (10, 14, 26, 39); and 3) they also used designated receiving facilities that provided TTM and rapid access to PCI (18–20, 33). Several systems had yet to implement technologies such as ECMO (in-hospital or prehospital) or DACSE of the head and thorax during CPR (22, 31, 43–47). However, all of the participants, including non-U.S. attendees, clearly indicated intentions to eventually incorporate both of these as well and monitor the outcomes (18, 22).
Many of the proposed technologies and interventions carry fiscal impact in terms of the devices, software, training, and monitoring needs (48). However, it was agreed that the consistent improvements indicated cost-worthiness and the reported subanalysis indicated only a very small incremental increase in the annualized cost of operations.
IRC participants did emphasize that for the proposed bundle of care to be effective (23), it required meticulous attention to detail and conscientious training incorporating procedural efficiencies for rapid and highly choreographed, physiologic sound interventions (14, 23, 31, 38, 49, 50). They also expressed concern for harm if interventions are not implemented correctly. Although positive pressure ventilation can be used to achieve adequate lung inflation (to enhance oxygenation and transpulmonary blood flow during CPR), it can also detrimentally obstruct circulation if provided too frequently (9). Likewise, the ITD can significantly improve survival chances, but only with optimal combinations of minimally interrupted chest compressions (rate, depth, and full chest recoil) properly coordinated with ventilation (14, 17, 31, 36, 38, 39). Conversely, they stressed that using novel procedures with simpler “system-1 thinking” strategies, such as age-based pediatric dosing, will not only expedite delivery of care but will also diminish errors (12). Finally, singular interventions instituted for the purposes of making improvements will not alter outcomes without coordinating and including all of the other interdependent components.
CONCLUSIONS
Several EMS agencies independently demonstrated significantly improved OHCA outcomes within their respective jurisdictions after each had introduced a very similar portfolio of interdependent clinical, procedural, and technological interventions. A proposed “bundle” of conventional and innovative care techniques for OHCA assimilated from these agencies can be used to reproduce the likelihood of favorable neurologic function for other communities (23, 40). As EMS-10 survival frequencies were, on average, nearly 30% higher than the national average of prudent EMS systems measuring and reporting outcomes, these findings further create a compelling argument that methodical, conscientious quality-improvement approaches to SCA management, such as the assimilated bundled care strategies compiled here, are reproducible and that they can further improve cardiac arrest outcomes, even within other high-performing EMS systems.
ACKNOWLEDGMENTS
The primary authors acknowledge the many direct and indirect contributions to these efforts from numerous others including the quality improvement officers, medical directors, training and operational chiefs, dispatchers, and the multitude of component personnel within each of the 10 evaluated emergency medical services systems. Through their team efforts, they have advanced the life-saving capabilities for their respective communities. Also, those among them who carefully tracked and documented those outcomes using Cardiac Arrest Registry to Enhance Survival or other equivalent systems need to be recognized for facilitating this important communication for other communities.
International Resuscitation Collaborative Members were Paul E. Pepe, MD, MPH, MCCM (Dallas County Fire Rescue, Dallas, TX; Palm Beach County Fire Rescue, West Palm Beach, FL; Department of Fire Rescue, Broward Sheriff’s Office, Fort Lauderdale, FL; Department of Management, Policy and Community Health, School of Public Health, University of Texas Health Sciences Center, Houston, TX; Metropolitan EMS Medical Directors Global Alliance, Dallas, TX); Tom P. Aufderheide, MD, MS (Department of Emergency Medicine, Medical College of Wisconsin, Milwaukee, WI); Lionel Lamhaut, MD, PhD (Department of Cardiology, Université Paris Descartes-Sorbonne Paris Cité, Paris, France; Intensive Care Unit – SAMU 75, Necker-Enfants-Malades Hospital, APHP, Paris, France); Daniel P. Davis, MD (Riverside County Fire Department, Riverside, CA); Charles J. Lick, MD (Allina Transportation, St. Paul, MN); Kees H. Polderman, MD (Department of Intensive Care, The Essex Cardiothoracic Centre, Basildon University Hospital, Basildon, Essex, England, United Kingdom; Department of Critical Care Medicine, United General Hospital, Houston, TX); Kenneth A. Scheppke, MD (Palm Beach County Fire Rescue, West Palm Beach, FL; Department of Fire Rescue, Broward Sheriff ’s Office, Fort Lauderdale, FL); Charles D. Deakin, MD (National Institute for Health Research [NIHR] Southampton Respiratory Biomedical Research Unit, Southampton, United Kingdom); Brian J. O’Neil, MD (Department of Emergency Medicine, Wayne State University, Detroit, MI); Hans van Schuppen, MD (Department of Anesthesiology, Amsterdam University Medical Center and the University of Amsterdam, Amsterdam, The Netherlands); Michael K. Levy, MD (Anchorage Fire Department, Anchorage, AK); Marvin A. Wayne, MD (Department of Emergency Medicine, PeaceHealth St. Joseph Medical Center and Whatcom County Emergency Medical Services, Bellingham, WA); Scott T. Youngquist, MD, MS (Salt Lake City Fire Department, Salt Lake City, UT; Division of Emergency Medicine, Department of Surgery, University of Utah, University of Utah School of Medicine, Salt Lake City, UT); Johanna C. Moore, MD, MS (Department of Emergency Medicine, Hennepin Healthcare Research Institute, Minneapolis, MN; Department of Emergency Medicine, University of Minnesota, Minneapolis, MN); Keith G. Lurie, MD (Department of Emergency Medicine, Hennepin Healthcare Research Institute, Minneapolis, MN; Department of Emergency Medicine, University of Minnesota, Minneapolis, MN; Department of Medicine, Cardiovascular Division, University of Minnesota, MN); Jason A. Bartos, MD, PhD (Department of Medicine, Cardiovascular Division, University of Minnesota, MN); Kerry M. Bachista, MD, EMT-P (Department of Emergency Medicine, University of Florida College of Medicine, Jacksonville, FL); Michael J. Jacobs, EMT-P (Alameda County EMS, San Leandro, CA); Carolina Rojas-Salvador, MD (Department of Emergency Medicine, Hennepin Healthcare Research Institute, Minneapolis, MN; Department of Emergency Medicine, University of Minnesota, Minneapolis, MN); Sean T. Grayson, MS, EMT-P (City of Rialto Fire Department, Rialto, CA); James E. Manning, MD (Department of Emergency Medicine, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC); Michael C. Kurz, MD (Department of Emergency Medicine, University of Alabama School of Medicine, Birmingham, AL); Guillaume Debaty, MD, PhD (Department of Emergency Medicine, Grenoble Alpes University Hospital, Grenoble, France); Nicolas Segal, MD, PhD (Department of Emergency Medicine, University of New Mexico Hospital, Albuquerque, NM); Peter M. Antevy, MD (Palm Beach County Fire Rescue, West Palm Beach, FL); David A. Miramontes, MD (Department of Surgery, The University of Texas Health Science Center, San Antonio, TX; The Southwest Texas Regional Advisory Council, San Antonio, TX); Sheldon Cheskes, MD (Department of Family and Community Medicine, Division of Emergency Medicine, University of Toronto, ONT, Canada); Joseph E. Holley, MD (Department of Emergency Medicine, University of Tennessee, Memphis, TN; Memphis Fire Department, Memphis TN; Division of Emergency Medical Services, State of Tennessee Department of Health, Nashville, TN); Ralph J. Frascone, MD (Department of Emergency Medicine, Regions Hospital, St. Paul, MN); Brent Parquette, NREMT-P (Lucas County Emergency Medical Services, Toledo, OH); Raymond L. Fowler, MD (University of Texas Southwestern Medical Center, Dallas, TX); Demetris Yannopoulos, MD (University of Texas Southwestern Medical Center, Dallas, TX); Brent A. Parquette, NREMT-P, Lucas County Emergency Medical Services, Toledo, OH; Ganesh Raveendran, MD, MPH (Department of Medicine Cardiovascular Division & Lillehei Heart Institute, University of Minnesota, Minneapolis, MN); Alice Hutin, MD, PhD (Service d’Aide Médicale Urgente [SAMU] in Paris, France); Renaud Tissier, DVM, PhD (Mondor Institute for Biomedical Research and National Veterinary School of Alfort, Paris, France); Robert Niskanen, MSEE (Take Heart America and Resurgent Biomedical Consulting, Shoreline, WA); James H. Logan, EMT-P (Shelby County Department of Health, Memphis, TN); Debbie Gillquist, EMT-P (Take Heart America, Minneapolis, MN).
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Dr. Lurie who is a coinventor of multiple CPR devices and founder of Advanced CPR Solutions LLC, that develops novel resuscitation technologies. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Contributor Information
Collaborators: Paul E. Pepe, Tom P. Aufderheide, Lionel Lamhaut, Daniel P. Davis, Charles J. Lick, Kees H. Polderman, Kenneth A. Scheppke, Charles D. Deakin, Brian J. O’Neil, Hans van Schuppen, Michael K. Levy, Marvin A. Wayne, Scott T. Youngquist, Johanna C. Moore, Keith G. Lurie, Jason A. Bartos, Kerry M. Bachista, Michael J. Jacobs, Carolina Rojas-Salvador, Sean T. Grayson, James E. Manning, Michael C. Kurz, Guillaume Debaty, Nicolas Segal, Peter M. Antevy, David A. Miramontes, Sheldon Cheskes, Joseph E. Holley, Ralph J. Frascone, Brent Parquette, Raymond L. Fowler, Demetris Yannopoulos, Brent A. Parquette, Ganesh Raveendran, Alice Hutin, Renaud Tissier, Robert Niskanen, James H. Logan, and Debbie Gillquist
REFERENCES
- 1.Benjamin EJ, Muntner P, Alonso A, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2019 update: A report from the American Heart Association. Circulation. 2019; 139:e56–e528 [DOI] [PubMed] [Google Scholar]
- 2.Abrams HC, McNally B, Ong M, et al. A composite model of survival from out-of-hospital cardiac arrest using the cardiac arrest registry to enhance survival (CARES). Resuscitation. 2013; 84:1093–1098 [DOI] [PubMed] [Google Scholar]
- 3.Chan PS, McNally B, Tang F, et al. ; CARES Surveillance Group. Recent trends in survival from out-of-hospital cardiac arrest in the United States. Circulation. 2014; 130:1876–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perkins GD, Neumar R, Monsieurs KG, et al. ; International Liaison Committee on Resuscitation. The international liaison committee on resuscitation-review of the last 25 years and vision for the future. Resuscitation. 2017; 121:104–116 [DOI] [PubMed] [Google Scholar]
- 5.Soar J, Donnino MW, Maconochie I, et al. ; ILCOR Collaborators. 2018 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations summary. Circulation. 2018; 138:e714–e730 [DOI] [PubMed] [Google Scholar]
- 6.Nas J, Te Grotenhuis R, Bonnes JL, et al. Meta-analysis comparing cardiac arrest outcomes before and after resuscitation guideline updates. Am J Cardiol. 2020; 125:618–629 [DOI] [PubMed] [Google Scholar]
- 7.Sinha SS, Sukul D, Lazarus JJ, et al. Identifying important gaps in randomized controlled trials of adult cardiac arrest treatments: A systematic review of the published literature. Circ Cardiovasc Qual Outcomes. 2016; 9:749–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Travers AH, Perkins GD, Berg RA, et al. Part 3: Adult basic life support and automated external defibrillation: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2015; 13216 Suppl 1S51–S83 [DOI] [PubMed] [Google Scholar]
- 9.Aufderheide TP, Lurie KG. Death by hyperventilation: A common and life-threatening problem during cardiopulmonary resuscitation. Crit Care Med. 2004; 32:S345–S351 [DOI] [PubMed] [Google Scholar]
- 10.Pepe PE, Aufderheide TP. EBM vs. EBM: Combining evidence-based and experienced-based medicine in resuscitation research. Curr Opin Crit Care. 2017; 23:199–203 [DOI] [PubMed] [Google Scholar]
- 11.Caffrey SL, Willoughby PJ, Pepe PE, et al. Public use of automated external defibrillators. N Engl J Med. 2002; 347:1242–1247 [DOI] [PubMed] [Google Scholar]
- 12.Banerjee PR, Ganti L, Pepe PE, et al. Early on-scene management of pediatric out-of-hospital cardiac arrest can result in improved likelihood for neurologically-intact survival. Resuscitation. 2019; 135:162–167 [DOI] [PubMed] [Google Scholar]
- 13.Kudenchuk PJ, Brown SP, Daya M, et al. ; Resuscitation Outcomes Consortium Investigators. Resuscitation outcomes consortium-amiodarone, lidocaine or placebo study (ROC-ALPS): Rationale and methodology behind an out-of-hospital cardiac arrest antiarrhythmic drug trial. Am Heart J. 2014; 167:653–9.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duval S, Pepe PE, Aufderheide TP, et al. Optimal combination of compression rate and depth during cardiopulmonary resuscitation for functionally favorable survival. JAMA Cardiol. 2019; 4:900–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stout J, Pepe PE, Mosesso VN, Jr. All-advanced life support vs tiered-response ambulance systems. Prehosp Emerg Care. 2000; 4:1–6 [DOI] [PubMed] [Google Scholar]
- 16.Curka PA, Pepe PE, Ginger VF, et al. Emergency medical services priority dispatch. Ann Emerg Med. 1993; 22:1688–1695 [DOI] [PubMed] [Google Scholar]
- 17.Pepe PE, Roppolo LP, Fowler RL. Prehospital endotracheal intubation: Elemental or detrimental? Crit Care. 2015; 19:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yannopoulos D, Bartos JA, Raveendran G, et al. Coronary artery disease in patients with out-of-hospital refractory ventricular fibrillation cardiac arrest. J Am Coll Cardiol. 2017; 70:1109–1117 [DOI] [PubMed] [Google Scholar]
- 19.Yannopoulos D, Bartos JA, Aufderheide TP, et al. ; American Heart Association Emergency Cardiovascular Care Committee. The evolving role of the cardiac catheterization laboratory in the management of patients with out-of-hospital cardiac arrest: A scientific statement from the American Heart Association. Circulation. 2019; 139:e530–e552 [DOI] [PubMed] [Google Scholar]
- 20.Anderson KB, Poloyac SM, Kochanek PM, et al. Effect of hypothermia and targeted temperature management on drug disposition and response following cardiac arrest: A comprehensive review of preclinical and clinical investigations. Ther Hypothermia Temp Manag. 2016; 6:169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gold B, Puertas L, Davis SP, et al. Awakening after cardiac arrest and post resuscitation hypothermia: Are we pulling the plug too early? Resuscitation. 2014; 85:211–214 [DOI] [PubMed] [Google Scholar]
- 22.Pepe PE, Scheppke KA, Antevy PM, et al. Confirming the clinical safety and feasibility of a bundled methodology to improve cardiopulmonary resuscitation involving a head-up/torso-up chest compression technique. Crit Care Med. 2019; 47:449–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lick CJ, Aufderheide TP, Niskanen RA, et al. Take Heart America: A comprehensive, community-wide, systems-based approach to the treatment of cardiac arrest. Crit Care Med. 2011; 39:26–33 [DOI] [PubMed] [Google Scholar]
- 24.Ahnefeld FW, Frey R, Fritsche P, et al. Die glieder der rettungskette. Munch Med Wochenschr. 1967; 109:2157–2161 [PubMed] [Google Scholar]
- 25.McNally B, Vellano K, Sinkfield T, et al. Cardiac arrest registry to enhance survival. Annual report. CARES Survival Report. 2017, Atlanta, GA: Emory University. pp 5–38 [Google Scholar]
- 26.Aufderheide TP, Frascone RJ, Wayne MA, et al. Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: A randomised trial. Lancet. 2011; 377:301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasson C, Meischke H, Abella BS, et al. ; American Heart Association Council on Quality of Care and Outcomes Research, Emergency Cardiovascular Care Committee, Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, Council on Clinical Cardiology, and Council on Cardiovascular Surgery and Anesthesia. Increasing cardiopulmonary resuscitation provision in communities with low bystander cardiopulmonary resuscitation rates: A science advisory from the American Heart Association for healthcare providers, policymakers, public health departments, and community leaders. Circulation. 2013; 127:1342–1350 [DOI] [PubMed] [Google Scholar]
- 28.Lewis M, Stubbs BA, Eisenberg MS. Dispatcher-assisted cardiopulmonary resuscitation: Time to identify cardiac arrest and deliver chest compression instructions. Circulation. 2013; 128:1522–1530 [DOI] [PubMed] [Google Scholar]
- 29.Ringh M, Rosenqvist M, Hollenberg J, et al. Mobile-phone dispatch of laypersons for CPR in out-of-hospital cardiac arrest. N Engl J Med. 2015; 372:2316–2325 [DOI] [PubMed] [Google Scholar]
- 30.Kirkbright S, Finn J, Tohira H, et al. Audiovisual feedback device use by health care professionals during CPR: A systematic review and meta-analysis of randomised and non-randomised trials. Resuscitation. 2014; 85:460–471 [DOI] [PubMed] [Google Scholar]
- 31.Lurie KG, Nemergut EC, Yannopoulos D, et al. The physiology of cardiopulmonary resuscitation. Anesth Analg. 2016; 122:767–783 [DOI] [PubMed] [Google Scholar]
- 32.Wang HE, Schmicker RH, Daya MR, et al. Effect of a strategy of initial laryngeal tube insertion vs endotracheal intubation on 72-hour survival in adults with out-of-hospital cardiac arrest: A randomized clinical trial. JAMA. 2018; 320:769–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirkegaard H, Søreide E, de Haas I, et al. Targeted temperature management for 48 vs 24 hours and neurologic outcome after out-of-hospital cardiac arrest: A randomized clinical trial. JAMA. 2017; 318:341–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rojas-Salvador C, Moore JC, Salverda B, et al. Effect of controlled sequential elevation timing of the head and thorax during cardiopulmonary resuscitation on cerebral perfusion pressures in a porcine model of cardiac arrest. Resuscitation. 2020; 149:162–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeung J, Matsuyama T, Bray J, et al. Does care at a cardiac arrest centre improve outcome after out-of-hospital cardiac arrest? - a systematic review. Resuscitation. 2019; 137:102–115 [DOI] [PubMed] [Google Scholar]
- 36.Aufderheide TP, Kudenchuk PJ, Hedges JR, et al. ; ROC Investigators. Resuscitation outcomes consortium (ROC) PRIMED cardiac arrest trial methods part 1: Rationale and methodology for the impedance threshold device (ITD) protocol. Resuscitation. 2008; 78:179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stiell IG, Callaway C, Davis D, et al. ; ROC Investigators. Resuscitation outcomes consortium (ROC) PRIMED cardiac arrest trial methods part 2: Rationale and methodology for “Analyze Later vs. Analyze Early” protocol. Resuscitation. 2008; 78:186–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yannopoulos D, Aufderheide TP, Abella BS, et al. Quality of CPR: An important effect modifier in cardiac arrest clinical outcomes and intervention effectiveness trials. Resuscitation. 2015; 94:106–113 [DOI] [PubMed] [Google Scholar]
- 39.Sugiyama A, Duval S, Nakamura Y, et al. Impedance threshold device combined with high-quality cardiopulmonary resuscitation improves survival with favorable neurological function after witnessed out-of-hospital cardiac arrest. Circ J. 2016; 80:2124–2132 [DOI] [PubMed] [Google Scholar]
- 40.Hinchey PR, Myers JB, Lewis R, et al. ; Capital County Research Consortium. Improved out-of-hospital cardiac arrest survival after the sequential implementation of 2005 AHA guidelines for compressions, ventilations, and induced hypothermia: The Wake County experience. Ann Emerg Med. 2010; 56:348–357 [DOI] [PubMed] [Google Scholar]
- 41.Van Schuppen H. Community response to cardiac arrest in the Netherlands. Journal of Emergency Medical Services. 2019; 44:19–22 [Google Scholar]
- 42.Roppolo LP, Westfall A, Pepe PE, et al. Dispatcher assessments for agonal breathing improve detection of cardiac arrest. Resuscitation. 2009; 80:769–772 [DOI] [PubMed] [Google Scholar]
- 43.Bartos JA, Nutting L, Carlson C, et al. Early neuroprognostication after refractory VF/VT cardiac arrest requiring ECPR. Circulation. 2019; 140:A10 [Google Scholar]
- 44.Moore JC, Segal N, Lick MC, et al. Head and thorax elevation during active compression decompression cardiopulmonary resuscitation with an impedance threshold device improves cerebral perfusion in a swine model of prolonged cardiac arrest. Resuscitation. 2017; 121:195–200 [DOI] [PubMed] [Google Scholar]
- 45.Moore JC, Holley J, Segal N, et al. Consistent head up cardiopulmonary resuscitation haemodynamics are observed across porcine and human cadaver translational models. Resuscitation. 2018; 132:133–139 [DOI] [PubMed] [Google Scholar]
- 46.Yannopoulos D, Bartos JA, Martin C, et al. Minnesota Resuscitation Consortium’s advanced perfusion and reperfusion cardiac life support strategy for out-of-hospital refractory ventricular fibrillation. J Am Heart Assoc. 2016; 5:32–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hutin A, Abu-Habsa M, Burns B, et al. Early ECPR for out-of-hospital cardiac arrest: Best practice in 2018. Resuscitation. 2018; 130:44–48 [DOI] [PubMed] [Google Scholar]
- 48.Lurie K, Levy M, Swor R, et al. The cost of prehospital cardiac arrest. Journal of Emergency Medical Services. 2017; 12:1–5 [Google Scholar]
- 49.Kloeck W, Cummins R, Chamberlain D, et al. Early defibrillation. An advisory statement from the advance life support working group of the international liaison committee on resuscitation. Circulation. 1997; 95:2183–2184 [DOI] [PubMed] [Google Scholar]
- 50.Van Diepen S, Girotra S, Abella BS, et al. Multistate 5-year initiative to improve care for out-of-hospital cardiac arrest: Primary results from the HeartRescue project. J Am Heart Assoc. 2017; 6:e005716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


