Abstract
The bone marrow microenvironment/niche plays a key role in regulating hematopoietic stem and progenitor cell (HSPC) activities, however mechanisms regulating niche cell function are not well understood. In this study, we show that niche intrinsic expression of the CXCR4 chemokine receptor critically regulates HSPC maintenance during study-state, and promotes early hematopoietic regeneration after myeloablative irradiation. At steady-state, chimeric mice with wild-type (WT) HSPC and marrow stroma that lack CXCR4 show decreased HSPC quiescence, and their repopulation capacity was markedly reduced. Mesenchymal stromal cells (MSC) were significantly reduced in the bone marrow (BM) of CXCR4 deficient mice, which was accompanied by decreased levels of the HSPC supporting factors stromal cell-derived factor-1 (SDF-1) and stem cell factor (SCF). CXCR4 also plays a crucial role in survival and restoration of BM stromal cells after myeloablative irradiation, where loss of BM stromal cells was more severe in CXCR4 deficient mice compared to WT mice. In addition, transplantation of WT donor HSPC into CXCR4 deficient recipient mice demonstrated reduced HSPC homing and early hematopoietic reconstitution. We found that CXCR4 signaling attenuates irradiation-induced BM stromal cell loss by up-regulating the expression of the anti-apoptotic protein Survivin via the PI3K pathway. Our study suggests that SDF-1-CXCR4 signaling in the stromal microenvironment cells plays a crucial role in maintenance of HSPCs during homeostasis, and promotes niche regeneration and early hematopoietic reconstitution after transplantation. Modulation of CXCR4 signaling in the HSPC microenvironment could be a means to enhance hematopoietic recovery after clinical hematopoietic cell transplantation.
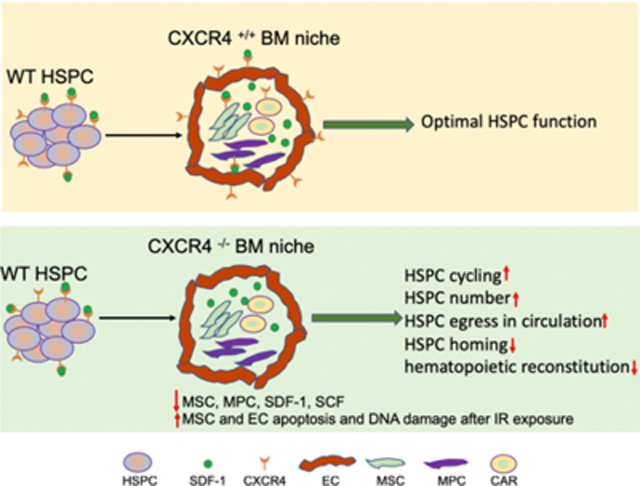
Graphical Abstract
Bone marrow (BM) niche expressed CXCR4 critically regulates the hematopoietic stem and progenitor cell (HSPC) function. CXCR4 gene deletion exclusively in BM niche impairs wild-type HSPC quiescence, retention in BM, and their repopulation ability. These defects in HSPC function are associated with a reduction in HSPC supporting factors in BM due to loss of mesenchymal stem and progenitor cells.
INTRODUCTION
The proliferation and differentiation of hematopoietic stem and progenitor cells (HSPC) provides life-long production of blood and immune cells in adults. HSPC reside in a complex bone marrow (BM) microenvironment/niche formed by blood vessels, perivascular mesenchymal stromal cells (MSC), macrophages and sympathetic nerve fibers 1–5. Niche MSC and endothelial cells (EC) provide pivotal factors required for maintenance of HSPC under steady state, and hematopoietic regeneration after stress 3, 4. Conditional deletion of the cytokines stromal cell-derived factor-1 (SDF-1) or stem cell factor (SCF) in EC and MSC results in a marked reduction in HSPC number and an increase in their trafficking to extramedullary organs 6, 7. Blocking EC angiogenic activity by neutralizing vascular endothelial-cadherin (VE-cadherin) or vascular endothelial growth factor receptor-2 (VEGFR2) impairs hematopoietic stem cell (HSC) self-renewal and long-term repopulation ability 1.
SDF-1 is an essential factor supporting HSC quiescence and their retention in the BM 8–10, and is primarily produced by perivascular MSC that co-localize with HSC 4. The biological activities of SDF-1 are mediated through binding to its cognate G-protein coupled receptor CXCR4 11–14. While it has been shown that SDF-1 signaling through CXCR4 expressed on HSC can regulate their quiescence, maintenance and retention; the role of the SDF-1-CXCR4 signaling pathway in the marrow stromal cells that extrinsically support HSC function remains unclear. In this study we show that BM stromal cells express CXCR4 and that SDF-1-CXCR4 signaling in stromal cells extrinsically regulates HSC maintenance, self-renewal, and hematopoietic reconstitution after BM myeloablation. This identifies a critical unrecognized new level of extrinsic regulation of HSC maintenance and response to stress.
MATERIALS AND METHODS
Mice
C57BL/6 and B6.Cg-Tg(CAG-cre/Esr1)5Amc (Tamoxifen –inducible Cre) mice were purchased from Jackson Laboratories (Bar Harbor, ME). B6.SJL-Ptprca/Pepcb (BoyJ) mice were bred and maintained in the IUSM LARC facility. CXCR4 floxed mice (CXCR4fl/fl) were originally obtained from Dr. Yong-Rui Zou (Feinstein Institute for Medical Research, Manhasset, NY) and maintained in our animal facility. CXCR4 condition KO mice were generated by crossing CXCR4fl/fl to tamoxifen-inducible Cre transgenic mice. B6.Cg-Tg(CAG-cre/Esr1)5Amc and CXCR4fl/fl mice were on the C57BL/6 background. CXCR4 gene deletion in adult mice (9–12 week old) was induced by tamoxifen injections (2 mg/mouse, i.p.) for five consecutive days. Hereafter, we refer to CXCR4fl/fl mice as WT, and after tamoxifen treatment as CXCR4 −/− or CXCR4 KO mice. All animal experiments were approved by the IUSM IACUC.
Reagents
Antibodies against c-kit (clone: 2B8), Sca-1 (clone:D7), lineage (clone:17A2/RB6–8C5/RA3–6B2/Ter-119/M1/70), CD48 (clone: HM48–1), CD45 (clone: 30-F11), anti-Ter119 (clone: ter-119) PDGFR (clone: APB5), CD51 (clone: RMV-7) and CD31 (clone: MEC13.3) were from Biolegend (San Diego, CA). Antibodies against CD150 (clone: mShad150), and VE-cadherin (clone: eBioBV13) were from BD Biosciences (San Diego, CA). Anti-Nestin (clone: 307501) antibody was from R&D Systems (Minneapolis, MN) and anti-Survivin (clone: 71G4B7E) and Phospho-Akt (Ser473) antibodies were from Cell Signaling Technology (Danvers, MA). Antibody against Kap-1 (phospho S824)) was purchased from Abcam (Cambridge, MA). YM155 was purchased from Cayman Chemical (Ann Arbor, Michigan) and LY294002 was from Millipore Sigma (St. Louis, MO).
Flow-cytometry analysis
Whole BM and spleen cells were treated with FcR blocker (BD Biosciences), and then stained with anti-lineage, anti-Sca-1, anti-c-kit, anti-CD150, and anti-CD48 antibodies to measure the phenotypically defined HSPC populations. After staining, cells were fixed with 1% paraformaldehyde and analyzed by flow cytometry. For stromal cell analysis, BM cells were first stained with anti-CD45, anti-ter119, anti-CD51, anti-PDGFRa, anti-CD31 and anti-VE-cadherin antibodies followed by fixation and permeabilization, and then stained with anti-Nestin and anti-phospho Kap-1 antibodies. Active caspase 3 and 7 were detected using CellEvent™ Caspase-3/7 (Invitrogen), and dead cells were excluded using LIVE/DEAD™ Fixable Stain (Invitrogen). Total cell count in the BM, spleen and blood was quantitated by Heska Element HT5 analyzer.
Quantitative RT-PCR
RNA from stromal cells (CD45-Ter119-CD31-) were isolated using QIAGEN RNeasy micro or mini kit. cDNAs were reverse-transcribed from total RNA using SuperScript VILO (Life technologies, Invitrogen), according to the manufacturers’ protocols and subjected to real-time PCR using SYBR Green Supermix (Life technologies) using QuantStudio 6 Real time PCR System (ABI, Foster City, CA). All of the samples were run in triplicate. Amplification of GAPDH was used for sample normalization. qRT–PCR primers were as follows:
Survivin F: ATCGCCACCTTCAAGAACTG; Survivin R: AATCAGGCTCGTTCTCGGTA
CXCR4 F: GGCTGTAGAGCGAGTGTTGC; CXCR4 R AGATGGTGGGCAGGAAGATCC
Chimeric mouse generation
Chimeric mice were generated by transplanting 1×106 whole BM cells from WT mice into lethally irradiated (1150 cGy split dose) congenic WT or Tamoxifen –inducible Cre+ CXCR4fl/fl recipients. Two-month post-transplant, chimeric mice were treated with tamoxifen for 5 consecutive days and HSPC and total hematopoietic content determined by FACS immunophenotype and CFC assay. To evaluate the HSC self-renewal capacity, BM cells (200,000) from chimeric mice (CD45.1+) plus 200,000 competitive whole BM cells from untreated CD45.2+ mice were transplanted into lethally irradiated CD45.2+ recipients. Peripheral blood chimerism and multi-lineage reconstitution were assessed monthly for 20 weeks post-transplant.
HSPC homing and hematopoietic stem cell engraftment
Whole BM cells (2×107) from WT mice (CD45.1+) mice were labeled with 5-(and −6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes, Thermo Fisher), washed, and transplanted into lethally irradiated WT and CXCR4 KO mice (CD45.2+). At 24-hour post transplantation, BM cells were harvested, lineage positive cells depleted using MACS microbeads, and lineage cells were stained for LSK and the total number of CD45.1+CFSE+ LSK cells were determined by flow cytometry. To measure the role of stromal CXCR4 in hematopoietic regeneration, we transplanted 1×106 WT donor cells into lethally irradiated WT or CXCR4−/− mice, and total CD45.1+ cells, LSK and SLAM LSK cells were measured at 15 days and 6 months post transplantation.
Ex vivo culture and treatment
Freshly harvested mouse BM cells were enriched for the CD45neg fraction using anti-CD45 magnetic beads (Miltenyi Biotech), and 1×106 CD45-negative cells were cultured in 6-well adherent tissue culture plates using MesenCult media (Stem Cell Technologies, Vancouver, BC) with 10% FBS. One half of the media was replaced after 7 days and on day 14 cells were stained with Giemsa staining solution (EMD Chemicals, Billerica, MA) and colonies enumerated. For in vitro BM stromal cell culture, CD45-negative BM cells were irradiated at 550 cGy, cultured in mouse MesenCult medium with or without SDF-1 (10 ng/ml) for 3 days, and treated with YM155 (10 ng/ml) or LY294002 (10 nM). To measure the phospho AKT, CD45-negative BM cells were cultured overnight in MesenCult medium, followed by 30 min treatment with SDF-1, and intracellular phospho AKT was measured by flow cytometry. For in vivo experiments, mice were irradiated at 650 cGy, and treated with SDF-1 (100 ug/kg/day) alone or in combination with YM155 (10 mg/kg/day) for 3 days.
ELISA
BM extracellular fluid (BMEF) was obtained by flushing one femur with 1 mL ice-cold PBS followed by centrifugation at 400 x g for 3 minutes. Cell free supernatants were used to measure SDF-1 and SCF by ELISA (R&D Systems). All samples were run in duplicate.
Migration assay
Bone marrow CD45neg cells were placed in upper chambers of transwell inserts (5 um) (Corning, Lowell, MA) and their migration to rm/rhSDF-1 (100 ng/ml; R&D system) was quantitated by flow cytometry after 4 hours. Percentage migration was calculated by dividing total cells migrated to the lower well by the cell input multiplied by 100. MSC cell migration was determined by comparison of the proportion of MSCs in input and migrated populations.
Statistical Analysis:
All WT and KO mice were age and sex matched, and cages were randomly assigned to treatment groups. The number of animals used in the experiments was estimated to give sufficient power (>90%) based on the effect sizes observed in our preliminary data. Grubbs method was used to identify the outliers and no animals were excluded from the analysis. In vivo and ex vitro processing steps were not blinded. All the statistical analyses were performed using Excel (Microsoft Corp, Redmond, WA) or Prism-GraphPad software (GraphPad, San Diego, CA). Statistical significance for binary comparisons was assessed by 2-tailed Student’s t-test; data were normally distributed with sufficiently equivalent variances. For comparison of more than two groups, ANOVA with post-hoc test was used. All data are reported as Mean ± SEM.
RESULTS
Loss of CXCR4 in marrow stromal cells impairs HSPC quiescence and maintenance
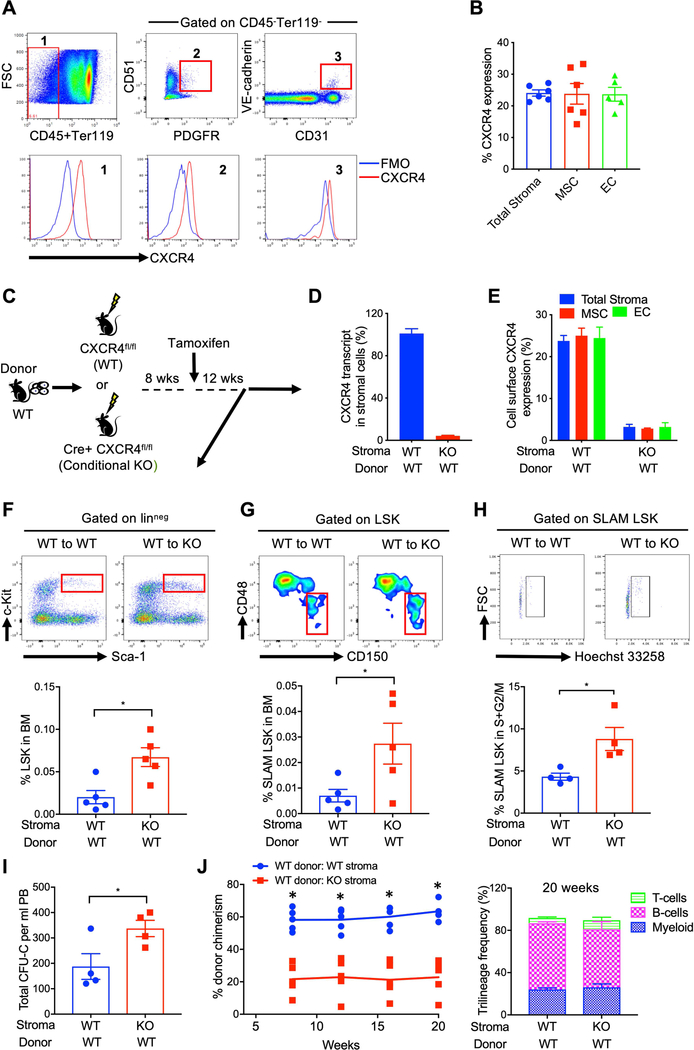
HSPC quiescence and marrow retention has been linked to the interaction between SDF-1 produced extrinsically by perivascular stromal cells and CXCR4 expressed on HSC 4, 8, 9, 15. However, the role of intrinsic SDF-1-CXCR4 signaling in niche stromal cells and its subsequent effects on supporting HSC function is not clear. To evaluate if a niche cell SDF-1-CXCR4 axis contributes to HSPC regulation we first measured CXCR4 expression on different subpopulations of marrow stromal cells. Flow-cytometry analysis showed that approximately 25% of total stromal cells (CD45-Ter119-), 23% of MSCs (CD45-Ter119-CD31- PDGFR+CD51+) and 21% of ECs (CD45-Ter119-CD31+VE-cadherin+) express CXCR4 (Figure 1A & 1B). To evaluate the physiological implication of intrinsic stromal cell CXCR4 signaling in regulation of HSPC function, we created chimeric mice by transplanting WT donor cells (Boy J; CD45.1) into WT (CXCR4fl/fl) or tamoxifen-inducible CXCR4 conditional knockout (Cre-ER-CXCR4fl/fl) recipient mice (C57BL6; CD45.2) (Figure 1C). At 8-weeks post-transplantation, all recipient mice were ≥95% CD45.1+, confirming full donor chimerism. At this time point both sets of chimeric mice received tamoxifen administration and 12 weeks later marrow and peripheral blood (PB) HSPC were quantitated. CXCR4 transcript expression was essentially eliminated in stromal cells of chimeric mice with WT HSPC and CXCR4 knockout (KO) stroma (WT: CXCR4−/− mice) (Figure 1D). Consistent with absence of CXCR4 transcript, expression of cell surface CXCR4 was reduced on total stromal cells (>87%), MSCs (>90%) and ECs (>92%) after tamoxifen treatment (Figure 1E). Interestingly, WT: CXCR4−/− chimeric mice revealed significantly increased total BM LSK and SLAM LSK cells (Figure 1F & 1G) and significantly higher number of SLAM-LSK cells in active cell cycle (S+G2/M) (Figure 1H) compared with chimeric mice with WT HSPC and WT stroma (WT: CXCR4+/+ mice). In addition, peripheral blood colony-forming cells (CFU-C) were increased in WT: CXCR4−/− mice (Figure I). Total leukocytes in BM and PB were similar in both groups (Supplementary Figure 1A &1B). In addition, BM histology in chimeric mice with wild-type or CXCR4 KO stroma was similar to wild-type mice (Supplementary Figure 1C). To measure the long-term repopulating potential of HSC in these chimeric mice, competitive transplantation was performed using BM cells from either WT: CXCR4+/+ or WT: CXCR4−/− chimeric mice as donors and lethally irradiated WT congenic mice as recipients. Bone marrow cells from chimeric mice with CXCR4 deficient stromal cells (WT: CXCR4−/−) displayed markedly reduced hematopoietic repopulating capacity compared to BM cells from chimeric mice with wild-type stroma (WT: CXCR4+/+) (Figure 1J left) measured by blood chimerism. Trilineage distribution (myeloid, B-cells and T-cells) was similar in both types of chimeric mice (Figure 1J right). WT: CXCR4−/− chimeric mice donor cells also showed reduced hematopoietic engraftment in the BM of recipient mice at 20 wks post transplantation (Supplementary Figure 1D). These results strongly suggest that BM microenvironment CXCR4 signaling plays a crucial role in HSPC quiescence, maintenance and function during steady-state.
Figure 1: Loss of CXCR4 in BM stromal cells reduces HSPC retention and function.
(A) Top; Representative flow cytometry plots of freshly isolated mouse BM stromal cells (CD45-Ter119-), MSCs (CD45-Ter119-CD31-CD51+PDGFRα+) and ECs (CD45-Ter119-CD31+VE-cadherin+). Bottom; Representative flow plots showing CXCR4 expression on total stromal cells, MSC and EC. (B) Cell surface CXCR4 expression on mouse BM stromal cells, MSC, and EC (X±SEM; N= 5 mice, each assayed individually). (C) Schematic of chimeric mice generation, CXCR4 deletion and functional analysis. (D-E) CXCR4 transcript expression in CD45-Ter119-stromal cells of chimeric mice with WT stroma or CXCR4 KO stroma (X±SEM; N= 3 mice, each assayed individually) and cell surface CXCR4 expression on BM total stromal cells, MSC and EC of chimeric mice with WT stroma or CXCR4 KO stroma (X±SEM; N= 5 mice, each assayed individually). (F-G) LSK and SLAM-LSK frequency in the BM of chimeric mice with WT donor:CXCR4 KO stroma or WT donor:WT stroma at 12-week post tamoxifen treatment (X±SEM; N= 5 mice per group, assayed individually; *=P<0.05). (H) Cell-cycle status of BM SLAM-LSK from chimeric mice with WT or KO stroma (X±SEM; N= 4 mice per group, assayed individually; *=P<0.05). (I) Peripheral blood total CFU-C (CFU-GM, BFU-E, CFU-GEMM) in both group of mice at 12-week tamoxifen treatment (X±SEM; N= 4 mice per group assayed individually; *=P<0.05). (J) Long-term repopulating ability of HSPC from chimeric mice with WT donor:CXCR4 KO stroma or WT donor:WT stroma. BM cells from chimeric mice (CD45.1+) plus 200,000 competitive whole BM cells from untreated CD45.2+ mice were transplanted into lethally irradiated CD45.2+ recipients. Peripheral blood chimerism and multi-lineage reconstitution was assessed 20 weeks’ post-transplant (X±SEM; N= 4 mice per group, each assayed individually; *=P<0.05).
Enhanced MSC egress and reduced levels of marrow stem cell supporting factors in the absence of CXCR4
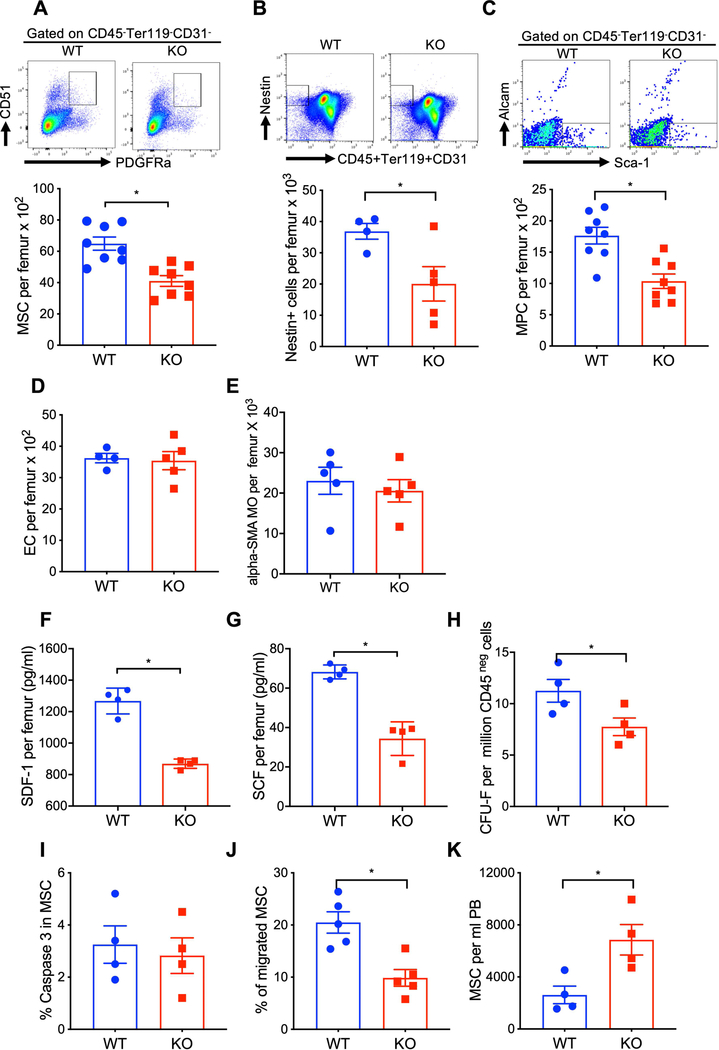
Because CXCR4 deficiency in the BM microenvironment impairs HSPC function, we tested whether CXCR4 signals directly regulate the activity of BM niche cells. At twelve-week post CXCR4 gene deletion, a substantial decrease in MSC (CD45-Ter119-CD31-PDGFR+CD51+), nestin+CD45-Ter119-CD31- cells and mesenchymal progenitor cells (MPC) (CD45-Ter119-CD31-Sca-1+Alcam-) was observed in the BM of CXCR4 KO mice as compared to WT mice (Figure 2A–2C). Of note, BM MSC counts were equivalent in Cre-CXCR4 fl/fl and Cre-CXCR4wt/fl control groups (Supplementary Figure 2A). Total marrow EC and α-SMA+ macrophage numbers were similar in WT and CXCR4 KO mice (Figure 2D & 2E). The HSPC supporting factors SDF-1 and SCF were significantly reduced in the BM of CXCR4 KO mice (Figure 2F & 2G). To further define the effect of CXCR4 signaling on MSC function, we measured MSC proliferation, survival and migration in WT and CXCR4 KO mice. CXCR4 deficient BM stromal cells generated fewer CFU-F colonies compared to WT stromal cells (Figure 2H). However, MSC survival, determined by caspase 3 expression, was similar in both group of mice (Figure 2I). MSC from CXCR4 KO mice demonstrated reduce migration to SDF-1 compared to WT mice MSC (Figure 2J). Moreover, CXCR4 KO mice consistently demonstrated an increase in trafficking of MSC to the peripheral circulation compared to WT mice (Figure 2K). Total BM cellularity was similar in WT and CXCR4 KO mice (Supplementary Figure 2B). These data suggest that CXCR4 signaling regulates MSC retention in the BM, which provides optimal levels of hematopoietic factors for HSC maintenance.
Figure 2: CXCR4 deficiency enhances MSC egress and reduces stem cell supporting factors in the BM.
(A-E) Total BM MSC, Nestin+ cells, MPC, EC and αSMA+ MO in WT and CXCR4 conditional KO mice (X±SEM; N= 4–8 mice per group assayed individually; *=P<0.05). (F-G) SDF-1 and SCF in the BMEF of WT and CXCR4 conditional KO mice at 12-week post tamoxifen treatment(X±SEM; N= 4 mice per group assayed individually in duplicate; *=P<0.05). (H) CFU-F generation from WT and CXCR4 conditional KO mice derived MSC. CD45-negative BM cells from WT and CXCR4 KO mice were cultured for 15 days, and CFU-F were quantitated after Giemsa staining (X±SEM; N= 4 mice per group assayed individually; *=P<0.05). (I) Caspase-3 expression in BM MSC of WT and CXCR4 conditional KO mice at 12-week post tamoxifen treatment. Caspase-3 expression in MSC gated population was determined by flow-cytometry (X±SEM; N= 4 mice per group assayed individually; *=P<0.05). (J) Migration of MSC from WT and CXCR4 conditional KO mice to SDF-1 (100 ng/ml) in vitro in transwell cultures (X±SEM; N= 4 mice per group assayed individually; *=P<0.05). (K) MSC in PB of WT and CXCR4 conditional KO mice (X±SEM; N= 4 mice per group assayed individually; *=P<0.05).
CXCR4 is required for protection of stromal niche cells and hematopoietic regeneration after myeloablative irradiation
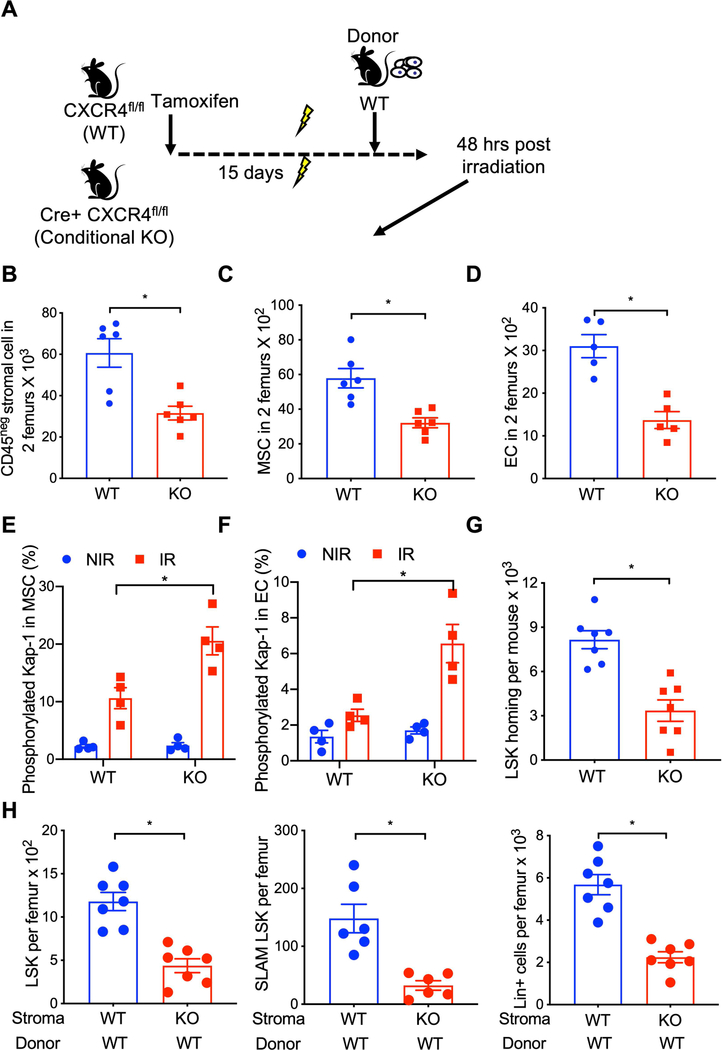
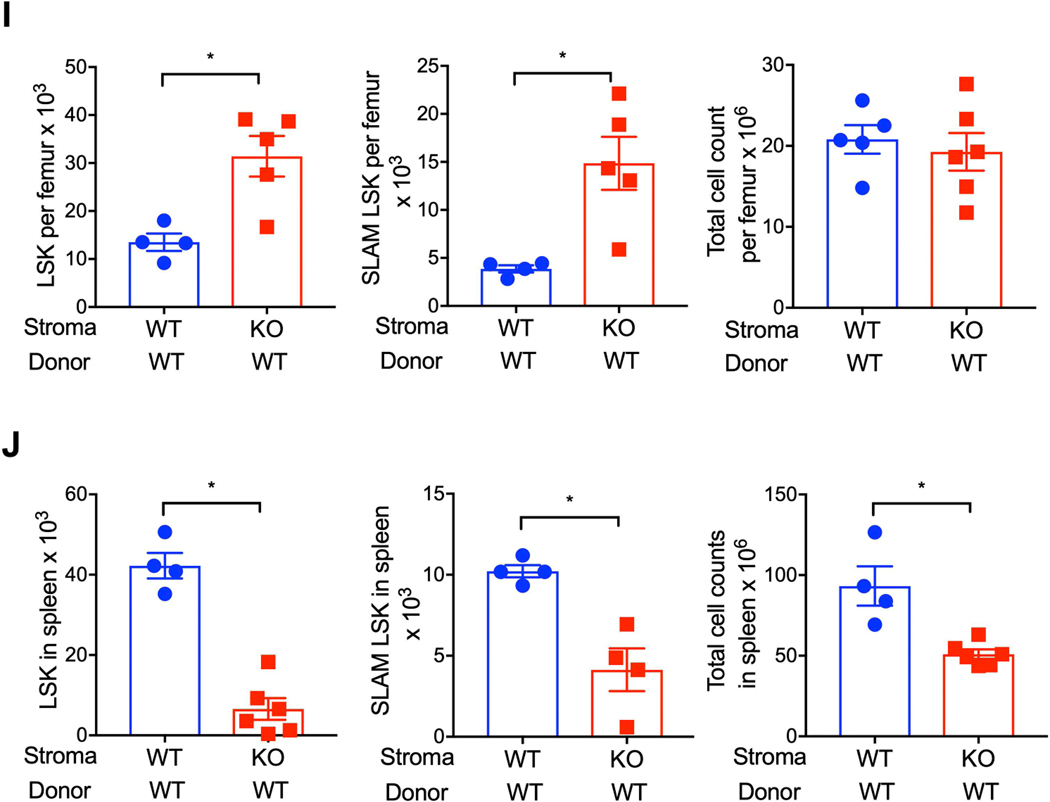
Because myeloablative irradiation severely damages the BM microenvironment16, 17 and hematopoietic regeneration is critically regulated by microenvironment/niche cells, we evaluated whether stromal cell CXCR4 expression contributes to niche protection/restoration and hematopoietic regeneration after lethal irradiation using a transplantation model. Wild-type and CXCR4 conditional KO mice (treated 15 days earlier with tamoxifen) were lethally irradiated, and at 24 hours post-irradiation transplanted with WT whole BM cells (Figure 3A). At 48 hours post-irradiation, BM niche stromal cell populations were evaluated by flow cytometry. Total BM stromal cell, MSC, and EC count substantially lower in irradiated CXCR4 KO mice compared to WT mice (Figures 3B, 3C, 3D & Supplementary Figure 2C). Expression of the DNA damage marker phosphorylated-Kap-1 was significantly higher in MSC and EC of CXCR4 KO mice compared to WT irradiated mice (Figure 3E & 3F). To further determine whether ablation of CXCR4 signaling in the BM niche cells influences their ability to support hematopoietic reconstitution after irradiation, we measured donor HSPC homing and engraftment in lethally irradiated WT or CXCR4 KO recipient mice. Homing of donor LSK cells was substantially lower in CXCR4 KO recipient mice compared to WT recipient mice at 24-hour post-transplantation (Figure 3G). At 15-days post-transplantation, donor derived LSK, SLAM LSK and lineage-positive cells (Figure 3H) were significantly lower in the BM of CXCR4 KO recipient mice compared to WT recipients. While donor LSK and SLAM LSK number were significantly higher in the BM of CXCR4 KO recipient mice at six-month post transplantation compared to WT recipient mice (Figure 3I), overall BM cellularity was similar. Furthermore, spleen LSK, SLAM LSK and total nucleated cells were significantly lower in CXCR4 KO recipient mice compared to WT recipient mice (Figure 3J). In contrast, spleen LSK cell count was higher in CXCR4 KO mice compared to WT mice under normal physiological condition (Supplementary Figure 3A). These findings demonstrate that BM niche cell CXCR4 is essential for stem cell niche protection, early hematopoietic regeneration, and HSPC maintenance after radiation-induced myelosuppression.
Figure 3: CXCR4 is required for the niche protection and hematopoietic regeneration after myeloablative irradiation.
(A) Schematic of WT HSPC transplantation into WT and CXCR4 KO recipient mice. CXCR4 gene deletion was induced by tamoxifen administration. At 15-day post tamoxifen treatment, WT and CXCR4 KO mice were lethally irradiated and transplanted with WT donor cells 24 hours later. Bone marrow niche composition and donor HSPC homing was measured 24 hours post transplantation. (B-D) CD45neg total stromal cell, MSC and EC in the BM (2 femurs) of WT and CXCR4 KO recipient mice transplanted with WT donor cells (X±SEM; N= 5–6 mice per group assayed individually; *=P<0.05). Expression of phosphorylated Kap-1 in gated BM MSC (E) and EC (F) of lethally irradiated WT and CXCR4 KO mice. (X±SEM; N= 4 mice per group assayed individually; *=P<0.05). (G) Homing Wild-type donor HSPC either in WT or CXCR4 KO recipient mice (X±SEM; N= 4 mice per group assayed individually; *=P<0.05). (H) WT donor LSK (left), SLAM-LSK (center) and total nucleated cells (right) in the BM of lethally irradiated WT or CXCR4 KO mice recipient mice 15-days post-transplant (X±SEM; N= 6–7 mice per group assayed individually; *=P<0.05). (I) WT donor LSK (left), SLAM-LSK (center) and total nucleated cells (right) in the BM of lethally irradiated WT or CXCR4 KO mice recipient at 6 month post-transplant (X±SEM; N= 4–6 mice per group assayed individually; *=P<0.05).
(J) Spleen LSK (left), SLAM LSK (center) and total nucleated cellularity (right) at 6 month post-transplant (X±SEM; N= 4–6 mice per group assayed individually; *=P<0.05).
CXCR4 attenuates irradiation induced BM stromal cells loss by regulating Survivin expression
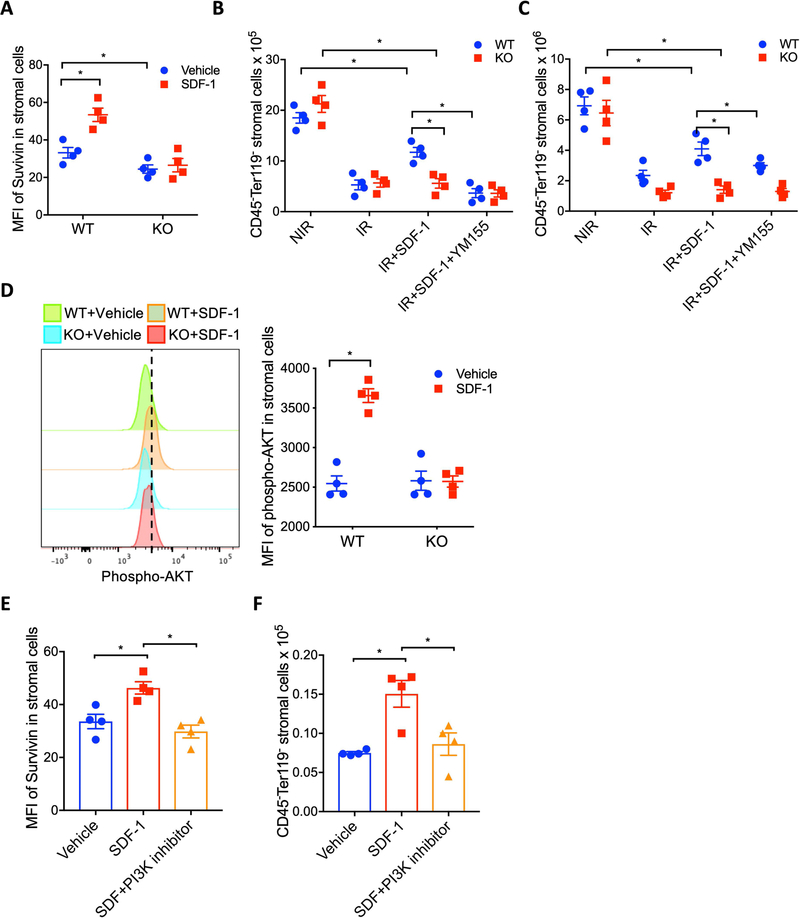
We have previously reported that the anti-apoptotic protein Survivin plays a crucial role in niche MSC survival after irradiation exposure 18. To investigate whether CXCR4 signaling supports HSC niche cell survival after irradiation exposure by regulating Survivin expression, we first measured whether the SDF-1-CXCR4 axis regulates Survivin expression in BM niche stromal cells. Flow cytometry data revealed that ex vivo SDF-1 treatment substantially increased intracellular Survivin protein expression in CD45-Ter119- marrow stromal cells of WT mice, but failed to increase Survivin in CXCR4 KO stromal cells (Figure 4A). Interestingly, the basal stromal cell Survivin level was significantly higher in WT mice compared to CXCR4 KO mice, further linking CXCR4 signaling in stromal cells to Survivin expression. In addition, ex vivo treatment of BM stromal cells with SDF-1 increased Survivin transcript expression (Supplementary Figure 3B).
Figure 4: CXCR4 attenuates irradiation-induced BM stromal cell loss by up-regulating intracellular Survivin.
(A) Survivin expression in BM stromal cells (CD45-Ter119-) cells from WT and CXCR4 KO mice after treatment in vitro with SDF-1 for 24 hours. Data are X±SEM; N= 4 mice per group, assayed individually; *P<0.05). (B-C) Effects of in vitro and in vivo treatment with SDF-1 and Survivin inhibitor (YM155) on WT and CXCR4 KO BM stromal cell recovery after irradiation exposure. Data are X±SEM; N= 4 mice per group, assayed individually; *P<0.05). (D) Phospho-AKT in BM stromal cells of WT and CXCR4 KO mice after in vitro treatment with or without SDF-1. Data are X±SEM; N= 4 experiments; *P<0.05). (E) Intracellular Survivin expression in irradiated BM stromal cells treated with SDF-1 or SDF-1plus LY294002; Data are X±SEM; N= 4 experiments; *P<0.05). (F) Effect of in vitro SDF-1 or SDF-1plus LY294002 treatment on irradiated BM stromal cell recovery; Data are X±SEM; N= 4 experiments; *P<0.05).
To determine the significance of Survivin in CXCR4 signaling mediated niche protection after irradiation, WT and CXCR4 KO mouse BM stromal cells were cultured ex vivo with SDF-1, with or without YM155, a selective Survivin synthesis inhibitor 18, followed by irradiation at 500 cGy. At 48 hours post-irradiation, survival of WT and CXCR4−/− BM stromal cells was significantly reduced compared to non-irradiated controls (Figure 4B). Irradiation-mediated loss of WT BM stromal cells was significantly attenuated in the presence of SDF-1, however SDF-1 was unable to rescue CXCR4 KO BM stromal cells (Figure 4B). The radio protective effect of SDF-1 on WT stromal cell number was abrogated by YM155 treatment. In vivo, irradiation-induced stromal cell loss was more severe in CXCR4 KO mice compared to wild-type mice, and SDF-1 treatment substantially prevented stromal cell loss in WT mice but failed to rescue stromal cell loss in CXCR4 KO mice (Figure 4C). Attenuation of stromal cell loss observed in SDF-1 supplemented WT mice was abrogated by YM155 treatment (Figure 4C). The SDF-1-CXCR4 axis has been implicated in the activation of the P13K/AKT pathway in many neoplastic hematopoietic and solid cancer cells. To determine whether CXCR4 signaling enhances Survivin expression in stromal cells by regulating PI3K/AKT activation, we first measured AKT phosphorylation in SDF-1 treated BM stromal cells from WT and CXCR4 KO mice. SDF-1 treatment substantially increased AKT phosphorylation in WT stromal cells, but not in CXCR4 KO mice (Figure 4D). Interestingly, in vitro treatment of WT BM stromal cells with SDF-1 enhanced Survivin expression, whereas blockade of PI3K activation with LY294002 suppressed the SDF-1-induced increase in Survivin expression (Figure 4E). In addition, SDF-1 prevented irradiation-induced BM stromal loss, however the protective effect of SDF-1 was abrogated by LY294002 (Figure 4F). These data suggest that SDF-1-CXCR4 axis-mediated Survivin expression via PI3K/AKT in BM niche stromal cells is important for their survival after irradiation exposure.
DISCUSSION
Although, prior studies have described roles for intrinsic CXCR4 signaling in the regulation of HSPC quiescence and maintenance 8–10, survival/apoptosis 19, 20 and BM retention 21, whether and how CXCR4 signaling within the stromal cell populations that extrinsically influence HSPC function is not known. In this study we demonstrate for the first time that an SDF-1-CXCR4 axis in the stromal cell populations that comprise the HSPC niche directly regulates niche cell homeostasis and indirectly regulates/influences HSPC activity. The SDF-1 receptor CXCR4 is expressed on BM stroma cells including MSC and EC. Chimeric mice with CXCR4 deficient stroma and WT hematopoietic cells demonstrated defective HSPC quiescence, BM retention and long-term repopulating ability. In addition, HSPC homing and early hematopoietic regeneration is markedly reduced when WT donor cells are transplanted into mice in which CXCR4 was deleted in the marrow stromal cells. Hematopoietic stem cell functional activities has been linked to intrinsic CXCR4 signaling 8. Our findings challenge that HSPC intrinsic signaling alone maintains their quiescence, marrow retention and self-renewal, as selective ablation of CXCR4 in the BM stem cell microenvironment results in HSPC hyper-proliferation, lower self-renewal and reduced BM retention. Mesenchymal stromal cells are required for HSPC maintenance and depletion of these cells enhances HSPC migration to extra extramedullary sites 4. Under steady-state, we now find that CXCR4 signaling in stromal cells serves to regulate MSC retention in the BM, as ablation of CXCR4 on MSC enhanced their egress to the peripheral circulation and reduced their migration to SDF-1 in vitro. Thus, niche CXCR4 signaling likely influences HSPC maintenance by regulating MSC retention in the BM. It is well known that BM microenvironment cells support hematopoiesis through the production of factors that regulate HSPC function. Previous studies have shown that within the BM microenvironment, SDF-1 and SCF are primarily produced by MSC and EC 3, 4. Reduced levels of HSPC supportive factors SDF-1 and SCF in the BM of CXCR4 KO mice strongly suggests that niche intrinsic CXCR4 signaling regulates HSPC function by influencing the production of HSPC supportive factors.
High-dose irradiation commonly used as a preparative regimen for stem cell transplantation severely damages the BM niche and impairs hematopoietic reconstitution 1, 16. Our study show that the niche intrinsic CXCR4-SDF-1 axis protects them from irradiation induced damage and promotes hematopoietic recovery; suggests a new approach to alleviate the potentially life-threatening complications of radiotherapy and myeloablative transplantation. CXCR4 signaling promotes BM stromal cell survival by upregulating the expression of Survivin, an anti-apoptotic protein. In this context using pharmacologic and genetic manipulation, we recently showed that Survivin critically promotes BM MSC survival after irradiation exposure 18. CXCR4 signaling mediated upregulation of Survivin has been shown to mitigate radiotherapy induced cancer cell apoptosis 22. PI3K/AKT and ERK are critical downstream pathways of SDF-1-CXCR4 axis 23 and a number of studies have shown that Survivin expression is upregulated by the PI3K/AKT/p70S6k1 pathway in many neoplastic hematopoietic and solid cancer cells24–27 and notably in normal EC as well 28. Since irradiation causes DNA damage 29 and Survivin is shown to enhance DNA break repair 30, it is possible that CXCR4 signaling promotes BM stromal cell survival through Survivin-mediated enhancement of DNA damage repair. In this context, we found increased expression of phosphorylated kap-1 and reduced levels of Survivin in CXCR4 KO stromal cells compared to WT stromal cells. In hematopoietic progenitor cells, we have previously reported that the anti-apoptotic effects of Survivin require the cyclin dependent kinase inhibitor p21WAF1/Cip1 31. Moreover we have shown that Survivin reduces p53 protein and enhances p53 degradation post-transcriptionally through blocking caspase-mediated Mdm2 cleavage32. In normal HSC 33 and in leukemic stem cells 34, differential mRNA microarray analysis indicates that Survivin impacts DNA-dependent transcription and affects multiple signaling pathways, notably, Src, PI3K, MAPK and EGRF affecting transcription of genes involved in protein localization and translation, cytokine production and DNA damage repair. Thus, there are many potential pathways whereby CXCR4-mediated up regulation of Survivin expression can affect stromal cell survival and proliferation and further study is needed to explore the mechanistic links.
CONCLUSION
Our study suggests that SDF-1-CXCR4 signaling in hematopoietic niche cells plays a crucial role in maintenance of HSPC function during homeostasis, and promotes niche regeneration and recovery of hematopoiesis after transplantation.
Supplementary Material
Figure 1: (A-B) Total leukocytes count in the BM and blood of chimeric mice with WT donor:CXCR4 KO stroma or WT donor:WT stroma at 12-week post tamoxifen treatment (X±SEM; N= 4 mice per group, assayed individually; *=P<0.05), (C) Representative bone marrow histology of chimeric mice with WT donor:CXCR4 KO stroma or WT donor:WT stroma at 12-week post tamoxifen treatment (scale bars: 100 microns; magnification: 200X), (D) BM cells from chimeric mice (CD45.1+) plus 200,000 competitive whole BM cells from untreated CD45.2+ mice were transplanted into lethally irradiated CD45.2+ recipients. BM chimerism was assessed 20 weeks’ post-transplant (X±SEM; N= 4 mice per group, each assayed individually; *=P<0.05).
Figure 2: (A) Total BM MSC in Cre-CXCR4fl/fl, Cre-CXCR4wt/fl and Cre+CXCR4fl/fl mice at 12 weeks after tamoxifen treatment (X±SEM; N= 5 mice per group assayed individually; *=P<0.05), (B) Total cell counts in the BM of WT and CXCR4 conditional KO mice (X±SEM; N= 6-7 mice per group assayed individually; *=P<0.05), (C) WT and CXCR4 KO mice BM cell viability and total cell count/femur at 48 hours post irradiation (X±SEM; N= 4 mice per group assayed individually; *=P<0.05).
Figure 3: (A) LSK cell count in spleen of WT and CXCR4 KO mice under normal physiological condition (X±SEM; N= 4 mice per group assayed individually; *=P<0.05). (B) Survivin transcript expression in BM stromal cells after in vitro treatment with SDF-1 (100 ng/ml) for 24 hours (X±SEM; N= 4 experiments *=P<0.05).
Significance.
Our study identify how the bone marrow niche intrinsic CXCR4-SDF-1 axis supports HSPC maintenance and retention under steady-state, and promotes early hematopoietic recovery after myeloablation. Our findings suggest that targeted modulation of CXCR4 signaling in bone marrow niche may provide a novel approach to alleviate the irradiation/chemotherapy-induced bone marrow stroma damage, to enhance blood cell production in patients undergoing stem cell transplantation for several hematological complications including leukemia, lymphoma and aplastic anemia, as well as metabolic disorders such as diabetes.
ACKNOWLEDGEMENTS
This work was supported by US Public Health Service grants HL096305, CA182947, AG046246 and AI128894 (to LMP) from the National Institutes of Health. Flow cytometry was performed in the Flow Cytometry Resource Facility of the IU Simon Cancer Center (NCI P30 CA082709).
Supported by US Public Health Service grants HL096305, CA182947, AG046246 and AI128894 (to LMP) from the National Institutes of Health.
Footnotes
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
CONFLICT OF INTEREST
The authors have declared that no conflict of interest exists.
REFERENCES
- 1.Butler JM, Nolan DJ, Vertes EL, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow A, Lucas D, Hidalgo A, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208:261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding L, Saunders TL, Enikolopov G, et al. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao M, Tao F, Venkatraman A, et al. N-Cadherin-Expressing Bone and Marrow Stromal Progenitor Cells Maintain Reserve Hematopoietic Stem Cells. Cell Rep. 2019;26:652–669 e656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asada N, Kunisaki Y, Pierce H, et al. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol. 2017;19:214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nie Y, Han YC, Zou YR. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med. 2008;205:777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugiyama T, Kohara H, Noda M, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Depond M, He L, et al. CXCR4/CXCL12 axis counteracts hematopoietic stem cell exhaustion through selective protection against oxidative stress. Sci Rep. 2016;6:37827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foudi A, Jarrier P, Zhang Y, et al. Reduced retention of radioprotective hematopoietic cells within the bone marrow microenvironment in CXCR4−/− chimeric mice. Blood. 2006;107:2243–2251. [DOI] [PubMed] [Google Scholar]
- 12.Ma Q, Jones D, Borghesani PR, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagasawa T. CXC chemokine ligand 12 (CXCL12) and its receptor CXCR4. J Mol Med (Berl). 2014;92:433–439. [DOI] [PubMed] [Google Scholar]
- 14.Peled A, Grabovsky V, Habler L, et al. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J Clin Invest. 1999;104:1199–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbuehl JP, Tatarova Z, Held W, et al. Long-Term Engraftment of Primary Bone Marrow Stromal Cells Repairs Niche Damage and Improves Hematopoietic Stem Cell Transplantation. Cell Stem Cell. 2017;21:241–255 e246. [DOI] [PubMed] [Google Scholar]
- 17.Hooper AT, Butler JM, Nolan DJ, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh P, Fukuda S, Liu L, et al. Survivin Is Required for Mouse and Human Bone Marrow Mesenchymal Stromal Cell Function. Stem Cells. 2018;36:123–129. [DOI] [PubMed] [Google Scholar]
- 19.Broxmeyer HE, Cooper S, Kohli L, et al. Transgenic expression of stromal cell-derived factor-1/CXC chemokine ligand 12 enhances myeloid progenitor cell survival/antiapoptosis in vitro in response to growth factor withdrawal and enhances myelopoiesis in vivo. J Immunol. 2003;170:421–429. [DOI] [PubMed] [Google Scholar]
- 20.Broxmeyer HE, Kohli L, Kim CH, et al. Stromal cell-derived factor-1/CXCL12 directly enhances survival/antiapoptosis of myeloid progenitor cells through CXCR4 and G(alpha)i proteins and enhances engraftment of competitive, repopulating stem cells. J Leukoc Biol. 2003;73:630–638. [DOI] [PubMed] [Google Scholar]
- 21.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D, Jiao C, Zhu Y, et al. Activation of CXCL12/CXCR4 renders colorectal cancer cells less sensitive to radiotherapy via up-regulating the expression of survivin. Exp Biol Med (Maywood). 2017;242:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang P, Wang G, Huo H, et al. SDF-1/CXCR4 signaling up-regulates survivin to regulate human sacral chondrosarcoma cell cycle and epithelial-mesenchymal transition via ERK and PI3K/AKT pathway. Med Oncol. 2015;32:377. [DOI] [PubMed] [Google Scholar]
- 24.Asanuma H, Torigoe T, Kamiguchi K, et al. Survivin expression is regulated by coexpression of human epidermal growth factor receptor 2 and epidermal growth factor receptor via phosphatidylinositol 3-kinase/AKT signaling pathway in breast cancer cells. Cancer Res. 2005;65:11018–11025. [DOI] [PubMed] [Google Scholar]
- 25.Carter BZ, Milella M, Altieri DC, et al. Cytokine-regulated expression of survivin in myeloid leukemia. Blood. 2001;97:2784–2790. [DOI] [PubMed] [Google Scholar]
- 26.Dasgupta P, Kinkade R, Joshi B, et al. Nicotine inhibits apoptosis induced by chemotherapeutic drugs by up-regulating XIAP and survivin. Proc Natl Acad Sci U S A. 2006;103:6332–6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao P, Meng Q, Liu LZ, et al. Regulation of survivin by PI3K/Akt/p70S6K1 pathway. Biochem Biophys Res Commun. 2010;395:219–224. [DOI] [PubMed] [Google Scholar]
- 28.Papapetropoulos A, Fulton D, Mahboubi K, et al. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275:9102–9105. [DOI] [PubMed] [Google Scholar]
- 29.Lomax ME, Folkes LK, O’Neill P. Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin Oncol (R Coll Radiol). 2013;25:578–585. [DOI] [PubMed] [Google Scholar]
- 30.Jiang G, Ren B, Xu L, et al. Survivin may enhance DNA double-strand break repair capability by up-regulating Ku70 in human KB cells. Anticancer Res. 2009;29:223–228. [PubMed] [Google Scholar]
- 31.Fukuda S, Mantel CR, Pelus LM. Survivin regulates hematopoietic progenitor cell proliferation through p21WAF1/Cip1-dependent and -independent pathways. Blood. 2004;103:120–127. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Fukuda S, Pelus LM. Survivin regulates the p53 tumor suppressor gene family. Oncogene. 2004;23:8146–8153. [DOI] [PubMed] [Google Scholar]
- 33.Fukuda S, Hoggatt J, Singh P, et al. Survivin modulates genes with divergent molecular functions and regulates proliferation of hematopoietic stem cells through Evi-1. Leukemia. 2015;29:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukuda S, Abe M, Onishi C, et al. Survivin selectively modulates genes deregulated in human leukemia stem cells. J Oncol. 2011;2011:946936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1: (A-B) Total leukocytes count in the BM and blood of chimeric mice with WT donor:CXCR4 KO stroma or WT donor:WT stroma at 12-week post tamoxifen treatment (X±SEM; N= 4 mice per group, assayed individually; *=P<0.05), (C) Representative bone marrow histology of chimeric mice with WT donor:CXCR4 KO stroma or WT donor:WT stroma at 12-week post tamoxifen treatment (scale bars: 100 microns; magnification: 200X), (D) BM cells from chimeric mice (CD45.1+) plus 200,000 competitive whole BM cells from untreated CD45.2+ mice were transplanted into lethally irradiated CD45.2+ recipients. BM chimerism was assessed 20 weeks’ post-transplant (X±SEM; N= 4 mice per group, each assayed individually; *=P<0.05).
Figure 2: (A) Total BM MSC in Cre-CXCR4fl/fl, Cre-CXCR4wt/fl and Cre+CXCR4fl/fl mice at 12 weeks after tamoxifen treatment (X±SEM; N= 5 mice per group assayed individually; *=P<0.05), (B) Total cell counts in the BM of WT and CXCR4 conditional KO mice (X±SEM; N= 6-7 mice per group assayed individually; *=P<0.05), (C) WT and CXCR4 KO mice BM cell viability and total cell count/femur at 48 hours post irradiation (X±SEM; N= 4 mice per group assayed individually; *=P<0.05).
Figure 3: (A) LSK cell count in spleen of WT and CXCR4 KO mice under normal physiological condition (X±SEM; N= 4 mice per group assayed individually; *=P<0.05). (B) Survivin transcript expression in BM stromal cells after in vitro treatment with SDF-1 (100 ng/ml) for 24 hours (X±SEM; N= 4 experiments *=P<0.05).