Successful treatment of tuberculosis (TB) can be hampered by Mycobacterium tuberculosis populations that are temporarily able to survive antibiotic pressure in the absence of drug resistance-conferring mutations, a phenomenon termed drug tolerance. We summarize findings on M. tuberculosis tolerance published in the past 20 years. Key M. tuberculosis responses to drug pressure are reduced growth rates, metabolic shifting, and the promotion of efflux pump activity.
KEYWORDS: drug tolerance, efflux pumps, lipid metabolism, metabolic shifting, metabolic slowdown, mycobacterial cell wall, Mycobacterium tuberculosis, redox homeostasis, sigma factors, WhiB regulon
SUMMARY
Successful treatment of tuberculosis (TB) can be hampered by Mycobacterium tuberculosis populations that are temporarily able to survive antibiotic pressure in the absence of drug resistance-conferring mutations, a phenomenon termed drug tolerance. We summarize findings on M. tuberculosis tolerance published in the past 20 years. Key M. tuberculosis responses to drug pressure are reduced growth rates, metabolic shifting, and the promotion of efflux pump activity. Metabolic shifts upon drug pressure mainly occur in M. tuberculosis’s lipid metabolism and redox homeostasis, with reduced tricarboxylic acid cycle activity in favor of lipid anabolism. Increased lipid anabolism plays a role in cell wall thickening, which reduces sensitivity to most TB drugs. In addition to these general mechanisms, drug-specific mechanisms have been described. Upon isoniazid exposure, M. tuberculosis reprograms several pathways associated with mycolic acid biosynthesis. Upon rifampicin exposure, M. tuberculosis upregulates the expression of its drug target rpoB. Upon bedaquiline exposure, ATP synthesis is stimulated, and the transcription factors Rv0324 and Rv0880 are activated. A better understanding of M. tuberculosis’s responses to drug pressure will be important for the development of novel agents that prevent the development of drug tolerance following treatment initiation. Such agents could then contribute to novel TB treatment-shortening strategies.
INTRODUCTION
Tuberculosis (TB), an infectious disease caused by Mycobacterium tuberculosis, remains an important global public health problem, with 10 million new cases and 1.5 million TB deaths in 2018 (1). Even though TB is curable, the treatment success rates in 2016 were only 82% for drug-susceptible TB and 55% for multidrug-resistant (MDR) TB (2).
The standard treatment for drug-susceptible TB consists of four drugs (isoniazid [INH], rifampicin [RIF], ethambutol [EMB], and pyrazinamide [PZA]) for 2 months followed by INH and RIF for 4 months (3). The response to this standardized treatment regimen shows interpatient variability, even among patients with confirmed drug-susceptible TB. An important measure of the response to treatment is the time to culture conversion (i.e., the time from treatment initiation to the first of two consecutive negative cultures), which varies between patients, with 95% confidence intervals ranging from 41 to 83 days (4–6). Furthermore, while most patients are cured and remain free of TB upon the completion of a 6-month treatment regimen, some patients relapse even if they were fully adherent to treatment (7). To date, this interpatient variability in treatment responses has primarily been attributed to differences in host factors such as baseline mycobacterial loads, the presence of cavitary lesions, HIV infection, and smoking (8–13).
In addition to these host factors, the heterogeneity of mycobacterial populations may also contribute to the observed interpatient variability in treatment responses. Several studies demonstrated that kill curves of M. tuberculosis under drug pressure are biphasic and possibly even multiphasic (14, 15). Similarly, a recent modeling study of the time to culture positivity (i.e., the time between inoculation of medium and detection of growth) in serial isolates showed that multiple bacterial populations with different growth dynamics and distinctive kill rates are present in a patient’s sputum specimen (16). Taken together, these data suggest that mycobacterial populations are heterogeneous, with more than one bacterial phenotype being present in a single patient (14, 15, 17–20).
Drug tolerance, the phenomenon of M. tuberculosis surviving antibiotic treatment for a prolonged period in the absence of resistance mechanisms (21), is one manifestation of this heterogeneity in mycobacterial populations. While drug tolerance has been extensively studied in other bacterial species such as Staphylococcus aureus, Salmonella spp., and Escherichia coli, it has received less attention in mycobacteria (22). For M. tuberculosis, studies have mainly focused on mycobacterial persisters, a subset of drug-tolerant mycobacteria that arise within inherently heterogeneous populations. Through growth arrest, these persisters survive environmental stress conditions, including stress induced by concentrations of bactericidal drugs that are otherwise lethal (21, 23). While antibiotic persistence and drug tolerance share several characteristics, both differ from drug resistance. Drug resistance, or the ability to replicate in the presence of a drug, is most often specific to a single drug or drug class, is inheritable, and increases the MIC, the lowest concentration of a specific antibiotic needed to prevent growth (21, 24). In contrast, drug tolerance often acts across drug classes, is rarely genetically encoded, and does not affect the MIC but changes the MDK99, the minimum duration of treatment that kills 99% of the mycobacterial population (7, 21, 24). Upon exposure to bactericidal drugs, tolerant mycobacteria are thus killed at a lower rate than the fully susceptible population from which they arose (21). In addition to persistence, tolerance, and drug resistance, M. tuberculosis can also adapt to antibiotic stress by employing “intrinsic resistance” strategies, which include transient mechanisms such as thickening of the mycobacterial cell wall, activation of efflux pumps, and altered expression of transcriptional regulators.
In this review, we summarize and critically appraise the published data on the mechanisms that mycobacteria employ to transiently alter drug sensitivity upon drug pressure. While M. tuberculosis adapts to many stress conditions, including nutrient deficiency, acid stress, and exposure to immune cells, we review here what is known about mechanisms that are activated upon antibiotic drug pressure. Furthermore, while intrinsic resistance mechanisms sensu stricto can be classified as drug resistance, we include these phenomena in our review under the umbrella of the drug-tolerant M. tuberculosis state, as intrinsic resistance is clearly distinct from drug resistance caused by genomic variants in resistance-conferring genes. A better understanding of drug tolerance in M. tuberculosis is important as it may allow the development of novel treatment-shortening strategies aimed at targeting those M. tuberculosis populations that have become tolerant and would otherwise temporarily survive the presence of drugs.
TOLERANCE MECHANISMS
The survival of mycobacteria strongly depends on their ability to quickly respond to stresses. The published literature reveals that several interconnected biological pathways are involved in the emergence and establishment of a drug-tolerant state in response to the stress imposed by an effective drug (25–27). While some of these mechanisms are general, as they are nonspecific and occur upon exposure across drug classes (general tolerance), others are drug-specific tolerance mechanisms and occur only following exposure to one specific drug or drug class (drug-specific tolerance).
Under drug pressure, the expression of hundreds of M. tuberculosis genes changes rapidly. The resulting transcriptional and posttranscriptional gene regulation results in a tolerant phenotype of M. tuberculosis. As shown in Fig. 1, the main mechanisms conferring a drug-tolerant phenotype in M. tuberculosis include (i) metabolic slowdown by reducing the metabolism and growth rate, which is a successful strategy as antibiotics generally target active cellular processes; (ii) metabolic shifting, which consists of rerouting metabolite fluxes as an approach to retain homeostasis when specific pathways are targeted by antibiotic drugs; (iii) cell wall thickening, which reduces the drug concentration in mycobacterial cells; and (iv) the upregulation of efflux pumps, which increases the clearance of antibiotics out of the mycobacterial cell. Whether the observed changes in gene regulation reflect the development of a tolerant M. tuberculosis state in response to antibiotic stress, the selection of a preexisting drug-tolerant mycobacterial subpopulation upon exposure to antibiotics, or a combination of both is not known and difficult to disentangle using currently available techniques.
FIG 1.
Mechanisms for cross-tolerance in Mycobacterium tuberculosis. (A) Under nonstress conditions, cellular processes driving core metabolic pathways are unaffected. (B) Under drug pressure, homeostasis is affected by targeting core metabolic processes and toxic compounds, resulting in cellular damage and eventually cell death. (C) M. tuberculosis (Mtb) adapts to drug pressure through transcriptional and posttranscriptional regulatory mechanisms to develop a drug-tolerant state: metabolic slowdown reduces drug-induced cellular damage (1); metabolic processes are rerouted, and homeostasis is retained through the up- and downregulation of interweaved pathways (2); cell wall thickening limits drug entry into the cell and lowers drug activity (3); and the upregulation of efflux pumps lowers intracellular drug concentrations (4).
General Tolerance Mechanisms
Transcriptional and posttranscriptional gene regulation.
The processes that orchestrate the cellular developments leading to drug tolerance are complex and regulated by highly interconnected metabolic pathways. In this section, we focus on the most frequently reported regulatory mechanisms and describe important transcriptional and posttranscriptional regulatory mechanisms in M. tuberculosis. At the transcriptional level, selected WhiB transcription factors and sigma factors function as stress regulators. Posttranscriptionally, toxin-antitoxin (TA) systems and small regulatory RNAs (srRNAs) coordinate drug tolerance.
whiB genes encode transcriptional regulators involved in multiple cellular processes, including cell division, pathogenesis, and the response to diverse stresses, including exposure to antibiotics (28). In M. tuberculosis, whiB3 and whiB7, two of the seven identified whiB-like genes, have been associated with intrinsic drug resistance (29). For whiB7, studies have shown that its expression is induced upon antibiotic pressure and that whiB7-null mutants are hypersusceptible to antibiotics in vitro (30). In Mycobacterium smegmatis, pretreatment with whiB7 activators reduced drug susceptibility (31). The whiB7 transcriptional regulator may result in a drug-tolerant state by upregulation of drug efflux pumps (tap or Rv1258c) and by readjustment of cellular processes that compensate for the metabolic shifting induced under drug pressure (30). whiB3 expression has also been associated with reduced drug sensitivity, as mice infected with M. tuberculosis whiB3 mutant strains had longer survival times under drug pressure than mice infected with wild-type whiB3 M. tuberculosis strains (32). Because whiB3 encodes a 4Fe-4S redox sensor protein, WhiB3 likely plays a role in modulating drug sensitivity through regulation of the balance between redox and bioenergetic homeostasis, both processes known to affect the response to TB drugs (33–35). Other core metabolic pathways where WhiB3 plays a regulatory role are glycolysis, the pentose phosphate pathway, the tricarboxylic acid (TCA) cycle, and amino acid biosynthesis (35).
Sigma factors also play a crucial role in prokaryote transcription by binding RNA polymerase. The regulatory network of sigma factors is complex and involves multiple posttranslational modifications such as phosphorylation and acetylation via protein kinases and anti-sigma factors. In M. tuberculosis, 13 sigma factors (SigA to SigM) have been identified (36). Upon drug pressure, the expression of SigB, -E, -F, -G, -H, -I, and -J is increased, while the expression of SigA was found to be reduced (27, 37). Studies have shown that SigA, SigB, SigE, and SigF may be involved in M. tuberculosis’s adaptation to antibiotic stress (38–40).
TA systems are two-component systems that permit rapid adaptation of gene expression in bacteria following stress exposure (41). Toxin-antitoxin systems comprise a toxin that can induce growth arrest (or cell death) and an antitoxin that neutralizes the toxin. Under conditions where growth is favored, both the toxin and antitoxin are ubiquitously expressed so that the action of the toxin is neutralized by the antitoxin and normal growth occurs. Under antibiotic stress, growth inhibition can be obtained through the induction of specific proteases that degrade the antitoxin so that the action of the toxin is no longer restricted (42, 43). In M. tuberculosis, 79 TA loci have been identified; both the expression of various TA systems as well as the expression of proteases are altered upon antibiotic pressure and increased upon prolonged exposure to drugs (42–44). While some toxins (e.g., the MazF, Rv1577c, Rv2651c, and Rv0366c family) confer drug tolerance across drug classes, other toxins (e.g., RelE) generate drug-specific antibiotic tolerance, as discussed in the relevant sections below (45–47).
Small RNAs (sRNAs) regulate bacterial gene expression by binding mRNA, thereby repressing mRNA translation and increasing mRNA degradation (48). sRNAs were implicated in the regulation of antibiotic responses by readjusting the expression of genes associated with efflux pumps, metabolic enzymes, transport proteins, cell wall synthesis, and membrane proteins (49–51). Whereas modified in vitro expression of sRNA has been shown to alter antibiotic susceptibility in nonmycobacterial species (52, 53), sRNA regulation in M. tuberculosis is poorly understood, with validation of sRNA-target interactions being limited to a single study (54).
Metabolic slowdown.
Because most antibiotics target metabolically active M. tuberculosis, lowered metabolic activity under drug pressure can successfully generate a drug-tolerant state (Fig. 1C, panel 1) (55–57). For example, the observation that RIF kills significantly more log-phase bacilli than stationary-phase bacteria illustrates that metabolic slowdown via reducing replication is a successful strategy to produce RIF tolerance (58). Furthermore, the accumulation of mutations related to a decreased metabolic state has been associated with long-term treatment courses, further supporting metabolic slowdown as an efficient drug tolerance strategy (59).
In M. tuberculosis, metabolic slowdown occurs through the regulation of multiple interrelated pathways that control energy, carbon, and lipid metabolism. Upon treatment initiation, genes involved in the TCA cycle, aerobic respiration, and ATP synthesis, the main pathways involved in energy metabolism, are downregulated under antibiotic stress (27). Also, mRNA synthesis and protein synthesis, proxies for metabolic activity, are reduced, as evidenced by the lowered expression of genes involved in transcription (tuf, gyrA, and gyrB) and essential translation components (including 16S RNA and genes encoding ribosomal proteins) (27).
Metabolic shifting.
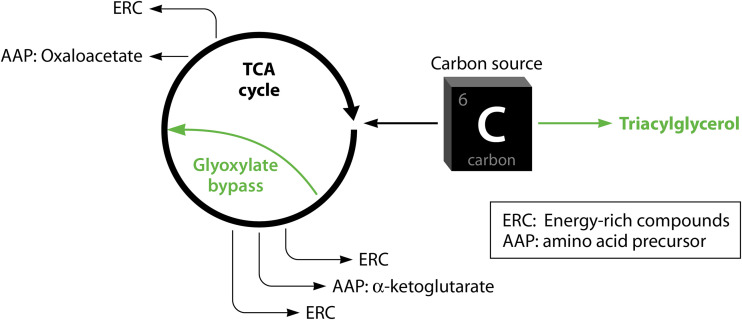
In addition to lowered metabolic activity, M. tuberculosis can also generate drug tolerance by shifting between pathways (Fig. 1C, panel 2). Carbon flux rerouting from energy-generating pathways to energy storage pathways has been associated with growth arrest and reduced drug susceptibility in M. tuberculosis (33, 60, 61). While most of the findings on metabolic shifting are based on gene expression studies, a few differential protein expression studies also found changes consistent with metabolic shifting of lipid metabolism and redox homeostasis in response to drug exposure (62–64). Under stress conditions (including drug pressure), M. tuberculosis can shift from carbon metabolism via the growth-promoting TCA cycle to carbon storage in fatty acids (FAs) via triacylglycerol (TAG) synthesis via the upregulation of tgs1 (gene encoding triacylglycerol synthase) (33, 65). When the TCA cycle is slowed, the turnover of alpha-ketoglutarate, oxaloacetate, and energy-rich reducing agents such as NADH is reduced, which results in reduced amino acid synthesis, protein translation, and metabolism in general. In addition, genes encoding enzymes that catalyze the glyoxylate bypass, such as isocitrate lyases, are found to be upregulated under RIF pressure, which is shown to result in cross-tolerance to INH, RIF, and streptomycin (34). The use of the glyoxylate bypass, an alternative route in the TCA cycle, shortcuts the production of alpha-ketoglutarate and a significant part of the production of energy-rich compounds in the TCA cycle. Alpha-ketoglutarate-derived amino acids can thus form a bottleneck for normal growth and metabolism, while the decreased production of highly energetic reducing agents could limit the production of radical oxygen species (ROS) induced by bactericidal drugs (Fig. 2). Interestingly, the metabolic shifts associated with drug tolerance overlap metabolic shifts observed in dormancy, where M. tuberculosis accumulates TAG lipid bodies that are hydrolyzed during periods of nutrient starvation (66, 67). It is unclear whether the metabolic shifts resulting in the accumulation of energy storage compounds should be regarded as being the result of reduced growth or a more universal and fundamental process that induces both dormancy and drug tolerance.
FIG 2.
Metabolic shifting of carbon fluxes away from the TCA cycle. The promotion of FA synthesis through the upregulation of tgs1 is one strategy adopted by M. tuberculosis to limit carbon uptake in the TCA cycle by competing for mutual carbon sources. Reduced TCA cycle activity results in lower rates of turnover of alpha-ketoglutarate and oxaloacetate, both of which are metabolites required for amino acid synthesis. The upregulation of the glyoxylate bypass further limits the turnover of alpha-ketoglutarate.
Mycobacterial cell wall thickening.
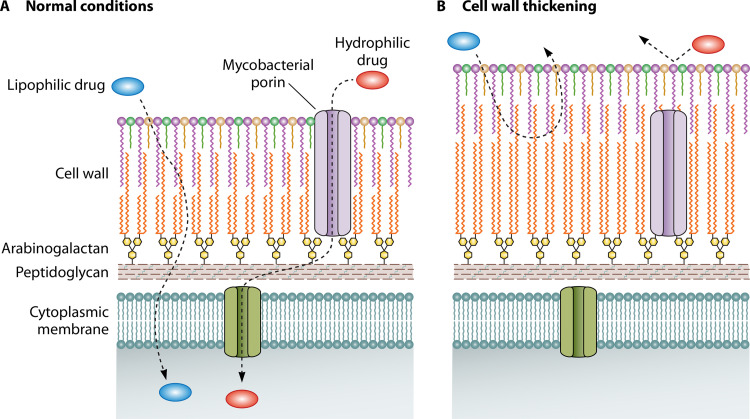
In mycobacteria, the cell wall acts as a natural barrier, and thickening of the cell wall structure in response to antibiotic stress could result in a drug-tolerant state (Fig. 1C, panel 3) (68–71). The thick and viscous bilipid layer of the cell wall slows the uptake of lipophilic drug agents and reduces the entry of hydrophilic drug agents through mycobacterial porins (Fig. 3). The reduced uptake of lipophilic drugs following cell wall thickening is supported by the observation of increased uptake and influx of lipophilic drugs (RIF) and, consequently, enhanced drug susceptibility in M. smegmatis mycolate-deficient mutants that were defective in crucial cell wall synthesis genes (72–77).
FIG 3.
Cell wall thickening. Cell wall thickening is a tolerance mechanism that results in the less efficient transport of lipophilic drugs across the mycobacterial cell wall. The transport of hydrophilic drugs is reduced as fewer porins now extend across the entire thickness of the cell wall.
Mycobacterial cell wall thickening also restricts the entry of hydrophilic drugs, as porins may no longer be able to bridge the increased cell wall width (Fig. 3) (68, 78, 79). Mycobacterial porins may indeed play an important role in regulating drug susceptibility in mycobacteria. For example, M. smegmatis mspA mutants that result in a nonfunctional MspA porin are less sensitive to hydrophilic drugs (ampicillin) and hydrophobic drugs (RIF). Conversely, the overexpression of the MspA porin in M. tuberculosis increases susceptibility to INH, EMB, streptomycin, and β-lactam antibiotics (80, 81), and the expression of Rv1698 and OmpA porins restores drug susceptibility in mspA mutants (81, 82).
As cell wall thickening occurs only under anaerobic or microaerobic stress conditions, cell wall thickening of M. tuberculosis most likely acts as a strategy complementary to the metabolic shift from aerobic energy-related pathways (such as aerobic respiration and ATP synthesis) to anaerobic respiration (27, 83, 84). Similarly, the various adaptations in M. tuberculosis’s lipid metabolism and the altered redox homeostasis upon drug pressure could influence cell wall permeability and contribute to a drug-tolerant state (65).
Upregulation of efflux pumps.
Efflux pumps are proteinaceous transporter channels that pump various compounds, including antibiotics, out of the cell. While specific mutations in efflux-associated genes have been associated with fixed resistance to several TB drugs, the upregulation of efflux pump expression also functions as an adaptive mechanism that can be activated upon drug pressure in genetically susceptible M. tuberculosis strains. Efflux pump expression is condition dependent, reversible, and transient (Fig. 1C, panel 4) (4, 85). Several efflux pumps have been found to be upregulated under antibiotic stress, either a specific drug or multiple structurally unrelated drugs. Similar to the phenomenon where a specific mutation for one drug can cause “cross-resistance” to another drug, the broad target range of efflux pumps can result in reduced sensitivity of M. tuberculosis to multiple TB drugs (86) (Table 1). For example, the upregulation of efflux pump activity and metabolic shifting induced by exposure to RIF may result in cross-tolerance to ofloxacin (87).
TABLE 1.
Efflux pumps upregulated under antibiotic stress
| Efflux pump(s)/gene | Induced upon exposure to: | Reduces susceptibility to: | Reference(s) |
|---|---|---|---|
| Rv1258c (Tap) | RIF, capreomycin | RIF | 27, 30, 31, 154, 136 |
| iniBAC operon | INH, EMB | Multiple drugs | 26, 65, 155, 156 |
| Rv3065 (mmr) | INH, levofloxacin | Conflicting information | 27, 157, 158 |
| efpA | INH, ETH | INH (hypothesized) | 26, 27, 65, 94, 155, 158 |
| Rv0849 | INH or RIF | RIF, amikacin | 157, 158 |
| Rv1218c | No literature found | Multiple drugs | 157 |
| Multiple efflux and transporter genes | RIF | RIF, ofloxacin | 87 |
Genetic adaptations conferring tolerance.
Even though tolerance is a reversible phenotypic state of M. tuberculosis, recent evidence suggests that the presence of specific genetic variants can increase the propensity of M. tuberculosis to enter a drug-tolerant state under antibiotic stress. For example, the presence of mutations in the prpR gene that regulates propionate catabolism conferred drug tolerance when M. tuberculosis was exposed to RIF, INH, and ofloxacin (88). Drug tolerance occurred through altering propionyl-CoA metabolism when bacteria were grown in vitamin B12-free propionate-supplemented medium. Tolerance was reversed upon the addition of vitamin B12, as propionate was then metabolized in the vitamin B12-dependent methylmalonyl-CoA pathway (88). Another example is “high-persisting” (hip) mutants of genes linked to lipid biosynthesis and carbon metabolism pathways that have been isolated under RIF and streptomycin pressure (89). In hip mutants, reduced FadE30 activity is likely to manifest as reduced lipid catabolism, consistent with metabolic shifting of carbon away from the TCA cycle in favor of lipid synthesis. The upregulation of icl and tgs1 in hip mutants is also of interest (89), as this promotes the glyoxylate bypass and the redirection of carbon sources into lipid synthesis.
While genetic changes are usually permanent, transient frameshift mutations in the glpK gene, which encodes a glycerol-3-kinase, are associated with tolerance to INH, RIF, PZA, and moxifloxacin, both in vitro and in clinical isolates (90, 91). It has been hypothesized that glpK frameshift mutations result in drug tolerance through the metabolic changes that result from reduced glycerol metabolism in glpK mutants, which could affect drug effectiveness by reducing growth, impacting the cellular structure, promoting the expression of stress response regulators (such as DosR and SigH), or inducing genes related to TAG synthesis (90, 91).
Drug-Specific Tolerance Mechanisms
Isoniazid.
Isoniazid (INH) is a prodrug that is converted intracellularly by the catalase-peroxidase KatG into isonicotinoyl, which binds NAD+ to form isonicotinoyl-NAD. Isonicotinoyl-NAD negatively affects cell wall integrity by inhibiting InhA, a key enzyme in the synthesis of the bacterial cell wall lipid mycolic acid (Fig. 4). KatG-mediated conversion of INH generates high levels of mutagenic ROS, which contributes to the high bactericidal activity of INH. During the first 2 days of treatment, INH kills about 90% of M. tuberculosis bacteria (65). Thereafter, the bactericidal activity decreases, a phenomenon attributed to INH tolerance (27, 65). The transcriptional response to INH stress occurs rapidly following INH exposure (25, 65, 92–94), with over 100 genes upregulated in the bacilli that remain alive after the initial killing by INH (55, 57, 95, 96). The majority of the INH-regulated genes cluster in metabolic pathways involved in lipid metabolism and cell wall synthesis, reflecting the two main INH tolerance mechanisms: a reduced need for lipid synthesis due to reduced growth and metabolic shifting of lipid metabolism and redox homeostasis pathways to circumvent INH-induced disruption of cell wall integrity (Fig. 4). The involvement of different pathways suggests that M. tuberculosis exploits a wide arsenal of tolerance mechanisms and is consistent with the discovery of cross-talking mechanisms within mycobacterial metabolism.
FIG 4.
Isoniazid (INH)-specific tolerance in Mycobacterium tuberculosis. (A) In the absence of tolerance, INH inhibits mycolic acid synthesis, resulting in insufficient mycolic acid production for growth. (B) Upon INH exposure, M. tuberculosis employs several tolerance mechanisms to overcome the INH-induced inhibition of mycolic acid synthesis: de novo-synthesized fatty acids and degraded high-molecular-weight TAGs fuel mycolic acid synthesis by acting as a source of mycolic acid precursors that enter mycolic acid synthesis (1), genes involved in mycolic acid synthesis (cmaA2, cmrA, mmaA2, mmaA3, mmaA4, umaA1, and fbpC) are upregulated (2), reduced carbon flow toward the TCA cycle leads to metabolic slowdown due to lowered ATP and protein synthesis (3), the promotion of mycolic acid synthesis and the reduced need for mycolic acids in light of reduced growth ensure sufficient mycolic acid production (4), and M. tuberculosis decreases the expression of NADH dehydrogenase to avoid the further accumulation of NAD+ when NAD+ is accumulated upon increased production of fatty acids and mycolic acids and reduced TCA cycle activity (5).
The first important INH tolerance mechanism is the reduced need for lipid synthesis due to reduced growth and metabolic shifting of lipid metabolism, as INH tolerance has been observed in slow-growing and nonreplicating M. tuberculosis (55, 57, 96, 97). Under INH stress, M. tuberculosis likely reduces its lipid dependency by reducing metabolic activity (65). The production of cell wall lipids then becomes less critical as fewer metabolites are required for growth. In vitro experiments further suggest that under INH stress, M. tuberculosis may sense the low concentrations of mature mycolic acids and increased levels of mycolic acid biosynthesis intermediates, resulting in M. tuberculosis adapting its lipid metabolism. For example, M. tuberculosis upregulates multiple genes involved in mycolic acid synthesis (fas, the kas operon, cmaA2, cmrA, mmaA2, mmaA3, mmaA4, umaA1, and fbpC), which could promote mycolic acid synthesis and circumvent the cell wall damage induced by INH (62, 65, 92–94). In addition, genes involved in lipid metabolism not directly related to mycolic acid synthesis are implicated in the INH stress response. For example, tgs1, which metabolically shifts carbon sources from the TCA cycle into TAG lipid synthesis, and genes involved in lipid degradation, such as fabG4, fadE5, fadA, fadB, prpC, fadE24, and fadE23, are upregulated upon treatment with INH (65, 94). The storage lipids produced by the TAG synthesis pathway can be used by M. tuberculosis as a reservoir for fatty acids required for mycolic acid biosynthesis (98). Exposure to INH also increases the TCA cycle’s glyoxylate bypass through upregulating isocitrate lyase, generating lower rates of amino acid precursor turnover and fewer high-energy reducing agents such as NADH, which in turn likely results in decreased metabolic activity and decreased ROS production (34).
Another important INH tolerance mechanism is related to redox homeostasis. Redox homeostasis is affected under INH stress due to the altered lipid metabolism and reduced production of NADH in the TCA cycle, which results in the accumulation of NAD+. Under INH pressure, M. tuberculosis downregulates the expression of NADH dehydrogenase-encoding genes to limit the production of NAD+ (95). This shifts the chemical equilibrium toward reduced NAD+ to isonicotinoyl binding, which reduces InhA inhibition. This reversible adaptation is mechanistically similar to one of the resistance mechanisms that M. tuberculosis employs, where a fixed mutation in the NADH dehydrogenase-encoding ndh gene confers irreversible resistance to INH (99). In addition to the downregulation of NADH catabolism, INH stress is likely to modify M. tuberculosis’s redox homeostasis by inducing the expression of oxidoreductases (fprB, Rv0085, and Rv1885c); Rv3049c, which is presumed to function as a monooxygenase that interacts with NADP; and the oxidative stress response gene ahpC that encodes an antioxidant enzyme that protects mycobacteria against radical oxygen species (27, 63, 65, 94). The role of redox homeostasis in INH tolerance is further supported by the observation that upon host infection, when M. tuberculosis is taken up by macrophages, M. tuberculosis senses the redox changes that result from phagosomal acidification and uses them as a trigger to promote drug tolerance (100). Interestingly, in vivo mouse and guinea pig models suggest that chloroquine, an antimalarial drug that inhibits phagosomal acidification, may result in increased susceptibility of M. tuberculosis to INH, as the coadministration of chloroquine with INH increased the killing of drug-tolerant bacteria, reduced lung damage, and decreased relapse rates (100).
A temporary upregulation of inhA or downregulation of katG may also result in INH tolerance, but studies report conflicting results. One study reported that the histone-like MDP1 DNA binding protein that inhibits katG expression results in phenotypic INH tolerance in M. smegmatis (101). Another study observed that subpopulations with a reduced frequency of katG pulsing are more likely to be INH tolerant (102). However, a third study failed to show any differential inhA or katG expression upon challenge with INH (27).
Finally, the sigma factors SigB and SigE were found to play a role in M. tuberculosis tolerance induced upon exposure to INH, with INH being more active in SigB and SigE loss-of-function M. tuberculosis mutants (38). The role of SigB in INH tolerance has been studied in M. smegmatis, where SigB interacts with RNA polymerase binding protein A (RbpA) to promote the transcription of polyphosphate kinase 1 (ppk1) (103). Increased Ppk1 activity results in the accumulation of polyphosphates, a process that has been associated with tolerance in response to heavy metal or osmotic stress in E. coli, Lactobacillus, and other bacteria (103–105). Furthermore, SigB and SigE may play a role in M. tuberculosis tolerance through the transcriptional repressor MprA, which is bound to conserved motifs in the upstream regions of both SigB and SigE.
Rifampicin.
Rifampicin (RIF) inhibits the transcription of RNA by binding the RNA polymerase B subunit. Mutations in the RNA polymerase B-encoding rpoB gene that result in a reduced binding affinity of RIF have been extensively studied with regard to the acquisition of RIF resistance (106). Transient phenotypic adaptations of M. tuberculosis in response to exposure to RIF have been reported, but the literature is scarce. Studies demonstrated that M. tuberculosis can induce tolerance to RIF exposure through mistranslation of rpoB, upregulation of rpoB, increased efflux pump activity, and metabolic shifting (27, 34, 87, 107, 108).
Mistranslation is a stress-sensitive phenomenon where the translation of mRNA results in a mutated or truncated protein product. In vitro assays have shown that mistranslation of rpoB is a non-genetically-encoded phenotypic phenomenon that reduces the binding affinity between the drug and its target (108). Whether RIF pressure itself promotes the mistranslation of rpoB and paradoxically results in RIF tolerance remains unknown.
In response to low RIF concentrations, the upregulation of rpoB through the transcriptional regulation of M. tuberculosis rpoB promoters I and II occurs exclusively in rpoB wild-type M. tuberculosis populations and decreases the pathogen’s sensitivity to RIF (107). Under normal conditions, rpoB expression from promoter I inhibits rpoB expression from promoter II. Upon exposure to RIF, inhibition of RNA polymerase reduces transcription from promoter I, resulting in reduced inhibition of promoter II and a net increase of rpoB transcription. Interestingly, this mechanism is observed only when M. tuberculosis is exposed to low concentrations of RIF, as exposure to high RIF concentrations leads to the simultaneous inhibition of RNA polymerase activity at both promoters I and II (107).
In addition to the overexpression of the drug target, metabolic slowdown via reducing replication can result in RIF tolerance, as RIF kills significantly more M. tuberculosis bacilli in log phase than M. tuberculosis bacilli in stationary phase (58).
Sigma factors may also play a role in modulating RIF susceptibility, as mutants lacking sigF showed increased RIF susceptibility (40). However, this finding was not replicated in a later study (109).
Ethambutol.
Ethambutol (EMB) exerts its bacteriostatic activity by inhibiting the production of arabinogalactan, a mycobacterial cell wall constituent (110, 111). EMB may further act synergistically with INH by targeting the mycolic acid biosynthesis pathway through inhA repression, suggesting that the M. tuberculosis tolerance mechanisms employed for INH may generate cross-tolerance to EMB (112). While there is limited research on tolerance to EMB, one study found that EMB is highly bactericidal when M. tuberculosis lacks the SigB or SigE sigma factor, suggesting that the sigma factors SigB and SigE could induce tolerance upon EMB exposure (38).
Pyrazinamide.
Pyrazinamide (PZA) is a prodrug that is converted by the amidase encoded by the pncA (Rv2043c) gene into the active compound pyrazinoic acid (POA), which affects the activity of critical cellular enzymes and energy production through acidification of the cytoplasm and destabilization of the proton motive force (113). To date, no studies have investigated PZA-induced tolerance. What has been shown is that while PZA has limited bactericidal activity against actively growing bacteria, PZA is effective against static and metabolically slowed M. tuberculosis bacteria that remain under INH and RIF pressure (113, 114). An explanation for this observation could lie in the fact that POA is pumped out of M. tuberculosis by an efflux pump, a process that is energy dependent. Indeed, conditions such as hypoxic stress that reduce energy production or cotreatment with energy inhibitors increase PZA activity (113). The improved bactericidal activity of PZA under conditions of reduced energy could also explain the strong synergism between PZA and bedaquiline (BDQ), an important TB drug inhibiting ATP synthesis, in mouse models (115) and clinical trials (116). Downregulation of the drug target pncA probably does not contribute to PZA-induced M. tuberculosis tolerance, as pncA downregulation is not observed under PZA exposure (27).
Ethionamide.
Under ethionamide (ETH) stress, M. tuberculosis induces a transcriptional response similar to the one observed under INH stress, which is not surprising as both drugs target mycolic acid synthesis. The TA responses following exposure to INH and ETH are clustered and different from the TA responses induced by RIF or streptomycin (44). Of the 70 genes differentially expressed upon ETH-induced stress, 39 genes behaved similarly under INH stress (92). Nevertheless, M. tuberculosis strains that cannot convert the INH prodrug and thus do not develop a stress response under INH exposure still develop a stress response under ETH exposure (94). The stress response observed under ETH exposure is thus, at least in part, unique to exposure to ETH. Of the genes induced by ETH, 15 were associated with lipid metabolism, and 18 were associated with cell wall synthesis and cell processes (92). Downregulation of ethA, the gene that encodes the ETH drug-activating enzyme, does not seem to play a role in ETH tolerance, as such downregulation is not observed in the first 14 days of ETH exposure (27).
Aminoglycosides.
Aminoglycosides (AGs) inhibit protein synthesis by binding rRNA. Upon AG pressure, whiB7 overexpression affects genes involved in drug efflux (tap) and ribosomal protection (including eis) to reduce sensitivity to all AGs used for the treatment of TB (amikacin, kanamycin, capreomycin, and streptomycin) (30, 39). In contrast to the irreversible mutations in the eis promoter regions that confer resistance to capreomycin, kanamycin, and amikacin (117–120), WhiB7 overexpression and eis upregulation can generate a temporary state of reduced sensitivity to AG (117–120). SigA may play a role in this process as it binds to WhiB7 to form a WhiB7-SigA promoter complex. SigA also interacts with the DevR/DosR dormancy regulon, resulting in decreased metabolic rates, growth arrest, and shifts in carbon, energy, and lipid metabolism (121). While these changes affect nonresponsiveness to drugs, they have to date been reported only under hypoxic and chemical stresses (21).
Another pathway for AG tolerance may be through the sigma factors SigE and SigB, as it has been shown that SigE and SigB loss-of-function mutants showed reduced survival in vitro upon exposure to streptomycin (38).
TA systems are reported to participate in streptomycin tolerance, with the TA response to streptomycin being similar to the TA response following starvation, indicating that M. tuberculosis could make use of several fundamental stress pathways to cope with a wide variety of stresses (44).
Bedaquiline.
Upon exposure to BDQ, which inhibits ATP synthase, M. tuberculosis rapidly adapts its metabolism. The low bactericidal activity of BDQ during the first days following BDQ exposure likely reflects the slow killing that is associated with ATP inhibition, further compromised by a BDQ-induced stress response that triggers metabolic shifting and metabolic slowdown (Fig. 5) (37, 122, 123). Of the 10 known universal stress proteins in M. tuberculosis, 7 are upregulated upon BDQ exposure. In addition, the overexpression of several transcription factors (including SigG, Rv0324, and Rv0880) generates BDQ tolerance by inducing a BDQ stress response (37, 123). Of these, Rv0324 is also overexpressed upon exposure to capreomycin and moxifloxacin, suggesting potential antagonism. Interestingly, Rv0880 expression is reduced upon exposure to pretomanid (PTM), suggesting synergism between pretomanid and BDQ (123).
FIG 5.
Bedaquiline (BDQ)-specific tolerance in Mycobacterium tuberculosis. The effects of BDQ exposure in the absence of tolerance (red) and the presence of tolerance (green) consist of multiple adaptations: efflux pump activity that occurs even in the nontolerant state and may be increased in the tolerant state (A), metabolic slowdown to reduce ATP dependency (B), and promoting ATP synthesis to overcome the ATP shortage induced by BDQ, either directly through the overexpression of genes encoding subunits of the ATP synthase machinery or indirectly by the stimulation of oxidative phosphorylation (C). The latter can be performed by increasing the synthesis of components used during oxidative phosphorylation (e.g., cytochromes) and/or by promoting trehalose metabolism and TCA cycle activity to increase the production of the energy-rich metabolites that power oxidative phosphorylation and generate the proton motive force that is required for ATP synthesis.
The metabolic shifts that drive BDQ tolerance are likely to involve multiple pathways. Reduced metabolic activity through increased expression of the dormancy regulon could contribute to BDQ tolerance, as it reduces the need for ATP synthesis. Increased ATP synthesis through the overexpression of genes encoding ATP synthase subunits, genes involved in oxidative phosphorylation (such as cytochrome-associated genes), and genes associated with the TCA cycle (which generates the reducing agents that enter oxidative phosphorylation) all illustrate how M. tuberculosis employs metabolic shifting to overcome the BDQ-driven pressure on ATP synthesis (37). In addition, M. tuberculosis shifts central carbon metabolism in response to BDQ by upregulating treS, which introduces a shift toward trehalose-fueled metabolism, thereby maintaining ATP and NADPH levels and further contributing to BDQ tolerance (124). The role of trehalose as an internal carbon source in BDQ tolerance is supported by the findings that treS deletion mutants incapable of generating a trehalose-fueled central carbon metabolism showed rapid depletion of ATP and were 144-fold more susceptible to BDQ (124).
Finally, efflux pump expression upon BDQ exposure may contribute to the development of a transient state of BDQ tolerance. This is not surprising given that mutations in Rv0678, a transcriptional repressor of the MmpS5-MmpL5 efflux pump-encoding genes, confer resistance to BDQ and cross-resistance to clofazimine (125). The observation that the efflux pump inhibitors verapamil and reserpine increase BDQ activity in wild-type H37Rv M. tuberculosis suggests that efflux pump activity introduces a basal level of intrinsic BDQ resistance in M. tuberculosis (125, 126). While BDQ-induced overexpression of efflux pump activity could result in BDQ tolerance, this has, to our knowledge, not yet been reported.
DISCUSSION
Two decades ago, Wallis et al. suggested that drug tolerance (defined as delayed in vitro killing of M. tuberculosis) may be of clinical importance, as the presence of drug-tolerant populations in pretreatment isolates was associated with relapse or treatment failure. They also stated that drug therapy can trigger drug tolerance in vivo, as clinical isolates showed increased drug tolerance upon prolonged drug exposure (7). A better understanding of the mechanism that M. tuberculosis uses to generate a state of drug tolerance to either multiple drugs or a specific drug or drug class could result in the identification of novel agents aimed at preventing the development of a drug-tolerant phenotypic state in M. tuberculosis, thus creating a new approach to treatment shortening for TB. Moreover, understanding drug-specific tolerance mechanisms could contribute to improved drug regimen designs where antagonistic drug combinations are avoided.
Since this seminal paper, several experimental studies have provided evidence for the existence of a drug-tolerant phenotype of M. tuberculosis and have explored the mechanisms of general tolerance to multiple drug classes and tolerance to specific drugs. Tolerance, a temporal and reversible phenotypic state of M. tuberculosis that develops in response to stress (including drug pressure), results from adaptive mechanisms that allow M. tuberculosis to survive for a longer period when exposed to stress. The main general mechanisms that mycobacteria use to acquire a temporary drug-tolerant state are slowdown of its metabolism, rearrangement of core metabolic pathways to circumvent the effects of the drugs, upregulation of efflux pumps, and cell wall restructuring. These mechanisms are intertwined through shifting of central carbon pathways, lipid metabolism, and redox homeostasis via transcriptional and posttranscriptional regulators such as sigma factors, WhiB regulons, and toxin-antitoxin systems. With regard to drug-specific tolerance responses, most studies have focused on INH and BDQ, while less attention has been paid to the other drugs used for the treatment of TB.
Potential Clinical Utility and Topics for Further Study
Several studies have made important observations that highlight the clinical relevance and the potential application of an improved understanding of tolerance. First, the presence of tolerance can increase the risk of development of resistance, as has been demonstrated in (in vitro and in vivo) experimental evolutionary studies in E. coli and Staphylococcus aureus (127, 128). In M. tuberculosis, the observation that cell wall thickness increases from pansusceptible to multidrug-resistant and extensively drug-resistant (XDR) M. tuberculosis strains suggests that transient drug tolerance can precede fixed drug resistance. Cell wall thickening results in phenotypically tolerant mycobacteria that are able to survive longer in the presence of antituberculosis drugs. Under these conditions, M. tuberculosis is exposed for prolonged periods to an environment that is mutagenic, thus promoting the acquisition of drug resistance (129). The hypothesis of tolerance preceding drug resistance is further supported by the observation of a trehalose shift in clinical XDR isolates compared to susceptible isolates and the finding that prpR mutations are enriched in resistant strains compared to susceptible strains (88, 124). Second, the discovery that supplementation of INH-containing regimens with drugs that target redox metabolism (such as the malarial drug chloroquine) increases the killing of INH-tolerant bacilli and reduces relapse rates highlights the opportunity to explore the repurposing of existing drugs to optimize the treatment of TB by reducing the development of tolerance (100). Third, the finding that the proportion of RIF-induced tolerant bacteria is inversely related to the dose of RIF used supports the use of RIF high-dose treatment regimens, as this may reduce the development of RIF tolerance (107). Fourth, PZA is highlighted as a complementary and synergistic companion drug in various treatment regimens (113, 115, 116, 130). An understanding of how PZA targets tolerant bacterial populations would be of great value for the development of drugs that target tolerant M. tuberculosis (131). Finally, the observation that the development of tolerance to BDQ may be inhibited by the administration of BDQ in combination with PTM (123) is of interest, especially given the recently approved Nix-TB regimen (PTM plus BDQ and linezolid) for the treatment of difficult-to-treat MDR and XDR strains (132, 133).
A better understanding of the tolerance mechanisms used by M. tuberculosis under drug pressure could thus result in the identification of novel or repurposed drugs that enable intelligent regimen design to avoid tolerance to core drugs used in the treatment of TB. The prevention of the development of drug tolerance could complement current approaches to treatment shortening, as the prevention of the acquisition of tolerance could reduce the duration of infectiousness, decrease relapse rates, and reduce the risk of the development of drug resistance. A number of interesting genes and hypothetical proteins have already been identified (65, 92–94, 134), and future studies could result in the identification of additional novel agents. For example, one could investigate the use of WhiB3 and WhiB7 inhibitors to increase the efficacy of drugs that are currently prone to the effects of WhiB3 and WhiB7, or one could employ antibiotics that cannot be detected by these WhiB regulators (30, 31). Cotreatment with mycobacterial efflux pump inhibitors (such as verapamil) could limit intrinsic resistance induced by efflux pumps (87, 126, 135–138), and drugs that target sigma factors may also be of interest for the optimization of TB treatment (139). Finally, the use of repurposed drugs that target slow-growing and dormant bacteria, such as nonsteroidal anti-inflammatory drugs and nitroimidazopyrans, should be investigated (140, 141).
Limitations of Current Studies
The experimental studies that we review share several limitations. First, while the hypothesis of the existence of a tolerant phenotypic state in M. tuberculosis is driven by the clinical observation of heterogeneous treatment response dynamics between patients, most studies have been performed on laboratory M. tuberculosis strains. The few studies that used clinical samples to study the adaptation of M. tuberculosis to antibiotic stress included a small number of patients (n < 12) (59, 142–145), limiting the ability to examine the diverse responses that M. tuberculosis exhibits in response to drug pressure and restricting assessments of the relative importance of pathogen and host factors such as HIV status, smoking status, and the presence of lung cavities in the response to treatment. Second, most studies performed transcriptomics experiments. Studying a transient phenomenon such as tolerance using transcriptomics is difficult, as the results of RNA expression profiling analyses likely depend on environmental and growth conditions, and transcriptional profiles observed under culture conditions might not be representative of what is truly happening in the host. Conflicting data between transcriptomic studies may thus be due to the conditions under which the experiments were performed. For example, the conflicting data on the differential expression of katG upon INH exposure could be due to the study of M. tuberculosis in the stationary phase in one study compared to the study of extracted RNA from sputum samples conserved at −80°C within 2 h after collection in another study (27, 101). Third, disentangling whether the observed mycobacterial transcriptional responses upon drug pressure are generated by drug pressure, immune pressure, or the selection of preexisting bacterial subpopulations is challenging. Furthermore, other environmental triggers such as biofilm formation, hypoxic stress, and nutrient starvation have been associated with drug tolerance and might share underlying mechanisms provoked by drug exposure (146, 147). Further experimental work investigating the role of different microenvironments in the selection versus the creation of slow-growing subpopulations upon drug pressure is thus of interest. Fourth, most studies applied a single omics approach, mainly transcriptomics. This limits our understanding of the relative contributions of genetics, epigenetics, and proteomics to tolerance. Methylation-mediated pathway regulation has been shown to play a role in antibiotic stress survival in E. coli (148). The contribution of epigenetic mechanisms to the acquisition of tolerance in M. tuberculosis has not yet been investigated, as most epigenetic research in the field of tuberculosis has focused on host epigenetics and has linked host epigenetics to M. tuberculosis survival in the host and drug resistance (149–151). Few studies have assessed the complement, i.e., how the M. tuberculosis epigenome is used as a mechanism to evade bacterial killing (149–151). In addition to epigenetics, proteomics could be important for the study of tolerance, as the functional changes in expressed proteins ultimately result in the phenotypically tolerant state (152). Nevertheless, only a few investigators have applied a proteomics approach to the study of drug tolerance in M. tuberculosis (62–64, 153). Finally, while mechanisms such as thickening of the cell wall and increased efflux pump activity are sensu stricto mechanisms of resistance as they increase the MIC, we include these types of innate or intrinsic resistance under mechanisms that generate a drug-tolerant phenotype as they are very different from the classical mechanisms of drug resistance conferred by genomic variants in resistance-conferring genes such as rpoB, katG, gyrA, and others.
CONCLUSION
When the bacillus is under drug pressure, a myriad of mechanisms is used by M. tuberculosis to adapt to the stress of exposure to drugs and acquire a drug-tolerant M. tuberculosis state across drug classes or specific to one drug. The main mechanisms are metabolic slowdown, metabolic shifting, cell wall thickening, and upregulation of efflux pumps, whereas drug-specific tolerance mechanisms aim to overcome or counteract the action of the drug. Future research, including multi-omics approaches and clinical translational studies of the fundamental processes of M. tuberculosis tolerance, is needed. The knowledge generated could then result in the identification of novel strategies that target or prevent the acquisition of drug-tolerant M. tuberculosis populations. This could accelerate bacillary clearance, which in turn would lead to a shorter duration of infectiousness, a lower risk of the acquisition of drug resistance, and reduced inflammation and lung tissue damage. Such novel tolerance-targeting approaches could complement the treatment-shortening strategies that are already being investigated.
ACKNOWLEDGMENTS
This work was supported by the Research Foundation Flanders (FWO) under grant no. G0F8316N (FWO Odysseus). S.L.S. is funded by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation (NRF) of South Africa under award number UID 86539. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NRF.
We thank members of the international TORCH consortium for their valuable comments. In particular, we thank the reviewers for their feedback and acknowledge the help of our art enhancer Patrick Lane, who contributed significantly to the quality of our work.
Biographies

Sander N. Goossens is a Ph.D. student at the University of Antwerp under the supervision of his promotor Prof. Annelies Van Rie. He obtained his master’s in cell, genetic, and molecular biology at Vrije Universiteit Brussel (VUB), Belgium, with greatest distinction in 2014. His thesis was on the application of the then highly novel CRISPR/Cas technology for the generation of knockout mouse embryonic stem cell lines that could be used for studying early embryonal development. Before starting his Ph.D., he was a biology and physics teacher at the International Montessori School in Brussels. Since his childhood, he has been passionate about the natural processes that govern our world and is particularly interested in how they translate clinically.

Samantha L. Sampson studied Medical Biochemistry and obtained her Ph.D., titled “Mycobacterium tuberculosis: Genetic and Phenotypic Comparison,” at Stellenbosch University (South Africa). After being a postdoc at the Harvard School of Public Health, she became a research associate at the Centre for Molecular Microbiology and Infection, Imperial College London, United Kingdom, followed by a position as an associate professor and eventually a professor at the Faculty of Medicine and Health Sciences at the University of Stellenbosch. Prof. Sampson has been involved in tuberculosis research for over 20 years. Her interest in tuberculosis, an infectious disease that not only presents intriguing biological questions but also has major public health and socioeconomic impacts in resource-poor settings (including South Africa), is focused on advancing our understanding of the host-pathogen interface in tuberculosis disease, with a particular interest in drug tolerance and persistence.

Annelies Van Rie studied pediatrics at the University of Leuven, Belgium, and obtained her Ph.D. on the “Molecular Epidemiology of Drug-Resistant Tuberculosis” at the University of Stellenbosch, South Africa. She started as a Medical Epidemiologist at Global Medical Affairs, Aventis Pasteur, became Assistant Professor of Epidemiology (and eventually full professor) at the School of Public Health, University of North Carolina at Chapel Hill, and is now Professor of Epidemiology at the University of Antwerp, Belgium. For over 20 years, her work has focused on clinical, epidemiological, and translational tuberculosis research, with a special emphasis on the molecular epidemiology of drug-resistant TB, evaluation of new diagnostics, optimized use of existing and new anti-TB drugs, and implementation of research to measure the impact of public health interventions. The goal of her research activities is to improve TB management and control in resource-poor high-burden as well as low-TB-burden settings.
REFERENCES
- 1.WHO. 2019. Global tuberculosis report 2019. WHO, Geneva, Switzerland: https://www.who.int/tb/publications/global_report/en/. [Google Scholar]
- 2.WHO. 2018. Global tuberculosis report 2018. WHO, Geneva, Switzerland: https://www.who.int/tb/publications/global_report/en/. [Google Scholar]
- 3.Grace AG, Mittal A, Jain S, Tripathy JP, Satyanarayana S, Tharyan P, Kirubakaran R. 2018. Shortened treatment regimens versus the standard regimen for drug-sensitive pulmonary tuberculosis. Cochrane Database Syst Rev 12:CD012918. doi: 10.1002/14651858.CD012918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yihunie Akalu T, Muchie KF, Alemu Gelaye K. 2018. Time to sputum culture conversion and its determinants among multi-drug resistant tuberculosis patients at public hospitals of the Amhara Regional State: a multicenter retrospective follow up study. PLoS One 13:e0199320. doi: 10.1371/journal.pone.0199320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillespie SH, Crook AM, McHugh TD, Mendel CM, Meredith SK, Murray SR, Pappas F, Phillips PP, Nunn AJ, REMoxTB Consortium. 2014. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 371:1577–1587. doi: 10.1056/NEJMoa1407426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boeree MJ, Heinrich N, Aarnoutse R, Diacon AH, Dawson R, Rehal S, Kibiki GS, Churchyard G, Sanne I, Ntinginya NE, Minja LT, Hunt RD, Charalambous S, Hanekom M, Semvua HH, Mpagama SG, Manyama C, Mtafya B, Reither K, Wallis RS, Venter A, Narunsky K, Mekota A, Henne S, Colbers A, van Balen GP, Gillespie SH, Phillips PPJ, Hoelscher M, PanACEA Consortium. 2017. High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis 17:39–49. doi: 10.1016/S1473-3099(16)30274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallis RS, Patil S, Cheon SH, Edmonds K, Phillips M, Perkins MD, Joloba M, Namale A, Johnson JL, Teixeira L, Dietze R, Siddiqi S, Mugerwa RD, Eisenach K, Ellner JJ. 1999. Drug tolerance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 43:2600–2606. doi: 10.1128/AAC.43.11.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Afzal A, Rathore R, Butt NF, Randhawa FA. 2018. Efficacy of vitamin D supplementation in achieving an early sputum conversion in smear positive pulmonary tuberculosis. Pak J Med Sci 34:849–854. doi: 10.12669/pjms.344.14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Visser ME, Stead MC, Walzl G, Warren R, Schomaker M, Grewal HM, Swart EC, Maartens G. 2012. Baseline predictors of sputum culture conversion in pulmonary tuberculosis: importance of cavities, smoking, time to detection and W-Beijing genotype. PLoS One 7:e29588. doi: 10.1371/journal.pone.0029588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musteikienė G, Miliauskas S, Zaveckienė J, Žemaitis M, Vitkauskienė A. 2017. Factors associated with sputum culture conversion in patients with pulmonary tuberculosis. Medicina (Kaunas) 53:386–393. doi: 10.1016/j.medici.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Unsematham S, Kateruttanakul P. 2013. Factors predicting sputum smear conversion and treatment outcomes in new smear-positive pulmonary tuberculosis. J Med Assoc Thai 96:644–649. [PubMed] [Google Scholar]
- 12.Aliyu MH, Salihu HM, Ratard R. 2003. HIV infection and sputum-culture conversion in patients diagnosed with Mycobacterium tuberculosis: a population-based study. Wien Klin Wochenschr 115:340–346. doi: 10.1007/BF03041486. [DOI] [PubMed] [Google Scholar]
- 13.Olaru ID, Heyckendorf J, Grossmann S, Lange C. 2014. Time to culture positivity and sputum smear microscopy during tuberculosis therapy. PLoS One 9:e106075. doi: 10.1371/journal.pone.0106075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad Z, Klinkenberg LG, Pinn ML, Fraig MM, Peloquin CA, Bishai WR, Nuermberger EL, Grosset JH, Karakousis PC. 2009. Biphasic kill curve of isoniazid reveals the presence of drug-tolerant, not drug-resistant, Mycobacterium tuberculosis in the guinea pig. J Infect Dis 200:1136–1143. doi: 10.1086/605605. [DOI] [PubMed] [Google Scholar]
- 15.Burger DA, Schall R. 2015. A Bayesian nonlinear mixed-effects regression model for the characterization of early bactericidal activity of tuberculosis drugs. J Biopharm Stat 25:1247–1271. doi: 10.1080/10543406.2014.971170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chigutsa E, Patel K, Denti P, Visser M, Maartens G, Kirkpatrick CM, McIlleron H, Karlsson MO. 2013. A time-to-event pharmacodynamic model describing treatment response in patients with pulmonary tuberculosis using days to positivity in automated liquid mycobacterial culture. Antimicrob Agents Chemother 57:789–795. doi: 10.1128/AAC.01876-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barr DA, Kamdolozi M, Nishihara Y, Ndhlovu V, Khonga M, Davies GR, Sloan DJ. 2016. Serial image analysis of Mycobacterium tuberculosis colony growth reveals a persistent subpopulation in sputum during treatment of pulmonary TB. Tuberculosis (Edinb) 98:110–115. doi: 10.1016/j.tube.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keren I, Minami S, Rubin E, Lewis K. 2011. Characterization and transcriptome analysis of Mycobacterium tuberculosis persisters. mBio 2:e00100-11. doi: 10.1128/mBio.00100-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant SS, Kaufmann BB, Chand NS, Haseley N, Hung DT. 2012. Eradication of bacterial persisters with antibiotic-generated hydroxyl radicals. Proc Natl Acad Sci U S A 109:12147–12152. doi: 10.1073/pnas.1203735109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burger DA, Schall R. 2018. Robust fit of Bayesian mixed effects regression models with application to colony forming unit count in tuberculosis research. Stat Med 37:544–556. doi: 10.1002/sim.7529. [DOI] [PubMed] [Google Scholar]
- 21.Balaban NQ, Helaine S, Lewis K, Ackermann M, Aldridge B, Andersson DI, Brynildsen MP, Bumann D, Camilli A, Collins JJ, Dehio C, Fortune S, Ghigo JM, Hardt WD, Harms A, Heinemann M, Hung DT, Jenal U, Levin BR, Michiels J, Storz G, Tan MW, Tenson T, Van Melderen L, Zinkernagel A. 2019. Definitions and guidelines for research on antibiotic persistence. Nat Rev Microbiol 17:441–448. doi: 10.1038/s41579-019-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trastoy R, Manso T, Fernandez-Garcia L, Blasco L, Ambroa A, Perez Del Molino ML, Bou G, Garcia-Contreras R, Wood TK, Tomas M. 2018. Mechanisms of bacterial tolerance and persistence in the gastrointestinal and respiratory environments. Clin Microbiol Rev 31:e00023-18. doi: 10.1128/CMR.00023-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gollan B, Grabe G, Michaux C, Helaine S. 2019. Bacterial persisters and infection: past, present, and progressing. Annu Rev Microbiol 73:359–385. doi: 10.1146/annurev-micro-020518-115650. [DOI] [PubMed] [Google Scholar]
- 24.Brauner A, Fridman O, Gefen O, Balaban NQ. 2016. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol 14:320–330. doi: 10.1038/nrmicro.2016.34. [DOI] [PubMed] [Google Scholar]
- 25.Boshoff HI, Myers TG, Copp BR, McNeil MR, Wilson MA, Barry CE III. 2004. The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism: novel insights into drug mechanisms of action. J Biol Chem 279:40174–40184. doi: 10.1074/jbc.M406796200. [DOI] [PubMed] [Google Scholar]
- 26.Briffotaux J, Liu S, Gicquel B. 2019. Genome-wide transcriptional responses of Mycobacterium to antibiotics. Front Microbiol 10:249. doi: 10.3389/fmicb.2019.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walter ND, Dolganov GM, Garcia BJ, Worodria W, Andama A, Musisi E, Ayakaka I, Van TT, Voskuil MI, de Jong BC, Davidson RM, Fingerlin TE, Kechris K, Palmer C, Nahid P, Daley CL, Geraci M, Huang L, Cattamanchi A, Strong M, Schoolnik GK, Davis JL. 2015. Transcriptional adaptation of drug-tolerant Mycobacterium tuberculosis during treatment of human tuberculosis. J Infect Dis 212:990–998. doi: 10.1093/infdis/jiv149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng F, Long Q, Xie J. 2012. The function and regulatory network of WhiB and WhiB-like protein from comparative genomics and systems biology perspectives. Cell Biochem Biophys 63:103–108. doi: 10.1007/s12013-012-9348-z. [DOI] [PubMed] [Google Scholar]
- 29.Geiman DE, Raghunand TR, Agarwal N, Bishai WR. 2006. Differential gene expression in response to exposure to antimycobacterial agents and other stress conditions among seven Mycobacterium tuberculosis whiB-like genes. Antimicrob Agents Chemother 50:2836–2841. doi: 10.1128/AAC.00295-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris RP, Nguyen L, Gatfield J, Visconti K, Nguyen K, Schnappinger D, Ehrt S, Liu Y, Heifets L, Pieters J, Schoolnik G, Thompson CJ. 2005. Ancestral antibiotic resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 102:12200–12205. doi: 10.1073/pnas.0505446102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burian J, Ramon-Garcia S, Sweet G, Gomez-Velasco A, Av-Gay Y, Thompson CJ. 2012. The mycobacterial transcriptional regulator whiB7 gene links redox homeostasis and intrinsic antibiotic resistance. J Biol Chem 287:299–310. doi: 10.1074/jbc.M111.302588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steyn AJ, Collins DM, Hondalus MK, Jacobs WR Jr, Kawakami RP, Bloom BR. 2002. Mycobacterium tuberculosis WhiB3 interacts with RpoV to affect host survival but is dispensable for in vivo growth. Proc Natl Acad Sci U S A 99:3147–3152. doi: 10.1073/pnas.052705399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baek SH, Li AH, Sassetti CM. 2011. Metabolic regulation of mycobacterial growth and antibiotic sensitivity. PLoS Biol 9:e1001065. doi: 10.1371/journal.pbio.1001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nandakumar M, Nathan C, Rhee KY. 2014. Isocitrate lyase mediates broad antibiotic tolerance in Mycobacterium tuberculosis. Nat Commun 5:4306. doi: 10.1038/ncomms5306. [DOI] [PubMed] [Google Scholar]
- 35.Saini V, Cumming BM, Guidry L, Lamprecht DA, Adamson JH, Reddy VP, Chinta KC, Mazorodze JH, Glasgow JN, Richard-Greenblatt M, Gomez-Velasco A, Bach H, Av-Gay Y, Eoh H, Rhee K, Steyn AJC. 2016. Ergothioneine maintains redox and bioenergetic homeostasis essential for drug susceptibility and virulence of Mycobacterium tuberculosis. Cell Rep 14:572–585. doi: 10.1016/j.celrep.2015.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou P, Wang X, Zhao Y, Yuan W, Xie J. 2018. Sigma factors mediated signaling in Mycobacterium tuberculosis. Future Microbiol 13:231–240. doi: 10.2217/fmb-2017-0127. [DOI] [PubMed] [Google Scholar]
- 37.Miryala SK, Anbarasu A, Ramaiah S. 2019. Impact of bedaquiline and capreomycin on the gene expression patterns of multidrug-resistant Mycobacterium tuberculosis H37Rv strain and understanding the molecular mechanism of antibiotic resistance. J Cell Biochem 120:14499–14509. doi: 10.1002/jcb.28711. [DOI] [PubMed] [Google Scholar]
- 38.Pisu D, Provvedi R, Espinosa DM, Payan JB, Boldrin F, Palu G, Hernandez-Pando R, Manganelli R. 2017. The alternative sigma factors SigE and SigB are involved in tolerance and persistence to antitubercular drugs. Antimicrob Agents Chemother 61:e01596-17. doi: 10.1128/AAC.01596-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burian J, Yim G, Hsing M, Axerio-Cilies P, Cherkasov A, Spiegelman GB, Thompson CJ. 2013. The mycobacterial antibiotic resistance determinant WhiB7 acts as a transcriptional activator by binding the primary sigma factor SigA (RpoV). Nucleic Acids Res 41:10062–10076. doi: 10.1093/nar/gkt751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen P, Ruiz RE, Li Q, Silver RF, Bishai WR. 2000. Construction and characterization of a Mycobacterium tuberculosis mutant lacking the alternate sigma factor gene, sigF. Infect Immun 68:5575–5580. doi: 10.1128/IAI.68.10.5575-5580.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar A, Alam A, Bharadwaj P, Tapadar S, Rani M, Hasnain SE. 2019. Toxin-antitoxin (TA) systems in stress survival and pathogenesis, p 257–274. In Hasnain S, Ehtesham N, Grover S (ed), Mycobacterium tuberculosis: molecular infection biology, pathogenesis, diagnostics and new interventions. Springer, New Delhi, India: 10.1007/978-981-32-9413-4_15. [DOI] [Google Scholar]
- 42.Slayden RA, Dawson CC, Cummings JE. 2018. Toxin-antitoxin systems and regulatory mechanisms in Mycobacterium tuberculosis. Pathog Dis 76:fty039. doi: 10.1093/femspd/fty039. [DOI] [PubMed] [Google Scholar]
- 43.Kim Y, Choi E, Hwang J. 2016. Functional studies of five toxin-antitoxin modules in Mycobacterium tuberculosis H37Rv. Front Microbiol 7:2071. doi: 10.3389/fmicb.2016.02071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta A, Venkataraman B, Vasudevan M, Gopinath Bankar K. 2017. Co-expression network analysis of toxin-antitoxin loci in Mycobacterium tuberculosis reveals key modulators of cellular stress. Sci Rep 7:5868. doi: 10.1038/s41598-017-06003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh R, Barry CE III, Boshoff HI. 2010. The three RelE homologs of Mycobacterium tuberculosis have individual, drug-specific effects on bacterial antibiotic tolerance. J Bacteriol 192:1279–1291. doi: 10.1128/JB.01285-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tandon H, Sharma A, Sandhya S, Srinivasan N, Singh R. 2019. Mycobacterium tuberculosis Rv0366c-Rv0367c encodes a non-canonical PezAT-like toxin-antitoxin pair. Sci Rep 9:1163. doi: 10.1038/s41598-018-37473-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tiwari P, Arora G, Singh M, Kidwai S, Narayan OP, Singh R. 2015. Corrigendum: MazF ribonucleases promote Mycobacterium tuberculosis drug tolerance and virulence in guinea pigs. Nat Commun 6:7273. doi: 10.1038/ncomms8273. [DOI] [PubMed] [Google Scholar]
- 48.Storz G, Vogel J, Wassarman KM. 2011. Regulation by small RNAs in bacteria: expanding frontiers. Mol Cell 43:880–891. doi: 10.1016/j.molcel.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Felden B, Cattoir V. 2018. Bacterial adaptation to antibiotics through regulatory RNAs. Antimicrob Agents Chemother 62:e02503-17. doi: 10.1128/AAC.02503-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dersch P, Khan MA, Muhlen S, Gorke B. 2017. Roles of regulatory RNAs for antibiotic resistance in bacteria and their potential value as novel drug targets. Front Microbiol 8:803. doi: 10.3389/fmicb.2017.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan H, Ho J, Liu X, Zhang L, Wong SH, Chan MT, Wu WK. 2017. Potential and use of bacterial small RNAs to combat drug resistance: a systematic review. Infect Drug Resist 10:521–532. doi: 10.2147/IDR.S148444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramos CG, Grilo AM, Sousa SA, Feliciano JR, da Costa PJ, Leitao JH. 2014. Regulation of Hfq mRNA and protein levels in Escherichia coli and Pseudomonas aeruginosa by the Burkholderia cenocepacia MtvR sRNA. PLoS One 9:e98813. doi: 10.1371/journal.pone.0098813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim T, Bak G, Lee J, Kim KS. 2015. Systematic analysis of the role of bacterial Hfq-interacting sRNAs in the response to antibiotics. J Antimicrob Chemother 70:1659–1668. doi: 10.1093/jac/dkv042. [DOI] [PubMed] [Google Scholar]
- 54.Gerrick ER, Barbier T, Chase MR, Xu R, Francois J, Lin VH, Szucs MJ, Rock JM, Ahmad R, Tjaden B, Livny J, Fortune SM. 2018. Small RNA profiling in Mycobacterium tuberculosis identifies MrsI as necessary for an anticipatory iron sparing response. Proc Natl Acad Sci U S A 115:6464–6469. doi: 10.1073/pnas.1718003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karakousis PC, Williams EP, Bishai WR. 2008. Altered expression of isoniazid-regulated genes in drug-treated dormant Mycobacterium tuberculosis. J Antimicrob Chemother 61:323–331. doi: 10.1093/jac/dkm485. [DOI] [PubMed] [Google Scholar]
- 56.Deb C, Lee CM, Dubey VS, Daniel J, Abomoelak B, Sirakova TD, Pawar S, Rogers L, Kolattukudy PE. 2009. A novel in vitro multiple-stress dormancy model for Mycobacterium tuberculosis generates a lipid-loaded, drug-tolerant, dormant pathogen. PLoS One 4:e6077. doi: 10.1371/journal.pone.0006077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vilcheze C, Hartman T, Weinrick B, Jain P, Weisbrod TR, Leung LW, Freundlich JS, Jacobs WR Jr. 2017. Enhanced respiration prevents drug tolerance and drug resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 114:4495–4500. doi: 10.1073/pnas.1704376114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu Y, Mangan JA, Dhillon J, Sole KM, Mitchison DA, Butcher PD, Coates AR. 2000. Detection of mRNA transcripts and active transcription in persistent Mycobacterium tuberculosis induced by exposure to rifampin or pyrazinamide. J Bacteriol 182:6358–6365. doi: 10.1128/jb.182.22.6358-6365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meumann EM, Globan M, Fyfe JAM, Leslie D, Porter JL, Seemann T, Denholm J, Stinear TP. 2015. Genome sequence comparisons of serial multi-drug-resistant Mycobacterium tuberculosis isolates over 21 years of infection in a single patient. Microb Genom 1:e000037. doi: 10.1099/mgen.0.000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daniel J, Deb C, Dubey VS, Sirakova TD, Abomoelak B, Morbidoni HR, Kolattukudy PE. 2004. Induction of a novel class of diacylglycerol acyltransferases and triacylglycerol accumulation in Mycobacterium tuberculosis as it goes into a dormancy-like state in culture. J Bacteriol 186:5017–5030. doi: 10.1128/JB.186.15.5017-5030.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi L, Sohaskey CD, Pfeiffer C, Datta P, Parks M, McFadden J, North RJ, Gennaro ML. 2010. Carbon flux rerouting during Mycobacterium tuberculosis growth arrest. Mol Microbiol 78:1199–1215. doi: 10.1111/j.1365-2958.2010.07399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hughes MA, Silva JC, Geromanos SJ, Townsend CA. 2006. Quantitative proteomic analysis of drug-induced changes in mycobacteria. J Proteome Res 5:54–63. doi: 10.1021/pr050248t. [DOI] [PubMed] [Google Scholar]
- 63.Jia L, Coward L, Gorman GS, Noker PE, Tomaszewski JE. 2005. Pharmacoproteomic effects of isoniazid, ethambutol, and N-geranyl-N′-(2-adamantyl)ethane-1,2-diamine (SQ109) on Mycobacterium tuberculosis H37Rv. J Pharmacol Exp Ther 315:905–911. doi: 10.1124/jpet.105.087817. [DOI] [PubMed] [Google Scholar]
- 64.Sharma P, Kumar B, Singhal N, Katoch VM, Venkatesan K, Chauhan DS, Bisht D. 2010. Streptomycin induced protein expression analysis in Mycobacterium tuberculosis by two-dimensional gel electrophoresis & mass spectrometry. Indian J Med Res 132:400–408. [PubMed] [Google Scholar]
- 65.Tudo G, Laing K, Mitchison DA, Butcher PD, Waddell SJ. 2010. Examining the basis of isoniazid tolerance in nonreplicating Mycobacterium tuberculosis using transcriptional profiling. Future Med Chem 2:1371–1383. doi: 10.4155/fmc.10.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garton NJ, Waddell SJ, Sherratt AL, Lee SM, Smith RJ, Senner C, Hinds J, Rajakumar K, Adegbola RA, Besra GS, Butcher PD, Barer MR. 2008. Cytological and transcript analyses reveal fat and lazy persister-like bacilli in tuberculous sputum. PLoS Med 5:e75. doi: 10.1371/journal.pmed.0050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deb C, Daniel J, Sirakova TD, Abomoelak B, Dubey VS, Kolattukudy PE. 2006. A novel lipase belonging to the hormone-sensitive lipase family induced under starvation to utilize stored triacylglycerol in Mycobacterium tuberculosis. J Biol Chem 281:3866–3875. doi: 10.1074/jbc.M505556200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jarlier V, Nikaido H. 1994. Mycobacterial cell wall: structure and role in natural resistance to antibiotics. FEMS Microbiol Lett 123:11–18. doi: 10.1111/j.1574-6968.1994.tb07194.x. [DOI] [PubMed] [Google Scholar]
- 69.Rastogi N, Frehel C, Ryter A, Ohayon H, Lesourd M, David HL. 1981. Multiple drug resistance in Mycobacterium avium: is the wall architecture responsible for exclusion of antimicrobial agents? Antimicrob Agents Chemother 20:666–677. doi: 10.1128/aac.20.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith T, Wolff KA, Nguyen L. 2013. Molecular biology of drug resistance in Mycobacterium tuberculosis. Curr Top Microbiol Immunol 374:53–80. doi: 10.1007/82_2012_279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nguyen L. 2016. Antibiotic resistance mechanisms in M. tuberculosis: an update. Arch Toxicol 90:1585–1604. doi: 10.1007/s00204-016-1727-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao LY, Laval F, Lawson EH, Groger RK, Woodruff A, Morisaki JH, Cox JS, Daffe M, Brown EJ. 2003. Requirement for kasB in Mycobacterium mycolic acid biosynthesis, cell wall impermeability and intracellular survival: implications for therapy. Mol Microbiol 49:1547–1563. doi: 10.1046/j.1365-2958.2003.03667.x. [DOI] [PubMed] [Google Scholar]
- 73.Philalay JS, Palermo CO, Hauge KA, Rustad TR, Cangelosi GA. 2004. Genes required for intrinsic multidrug resistance in Mycobacterium avium. Antimicrob Agents Chemother 48:3412–3418. doi: 10.1128/AAC.48.9.3412-3418.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh A, Gupta R, Vishwakarma RA, Narayanan PR, Paramasivan CN, Ramanathan VD, Tyagi AK. 2005. Requirement of the mymA operon for appropriate cell wall ultrastructure and persistence of Mycobacterium tuberculosis in the spleens of guinea pigs. J Bacteriol 187:4173–4186. doi: 10.1128/JB.187.12.4173-4186.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh A, Jain S, Gupta S, Das T, Tyagi AK. 2003. mymA operon of Mycobacterium tuberculosis: its regulation and importance in the cell envelope. FEMS Microbiol Lett 227:53–63. doi: 10.1016/S0378-1097(03)00648-7. [DOI] [PubMed] [Google Scholar]
- 76.Nguyen L, Chinnapapagari S, Thompson CJ. 2005. FbpA-dependent biosynthesis of trehalose dimycolate is required for the intrinsic multidrug resistance, cell wall structure, and colonial morphology of Mycobacterium smegmatis. J Bacteriol 187:6603–6611. doi: 10.1128/JB.187.19.6603-6611.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu J, Nikaido H. 1999. A mutant of Mycobacterium smegmatis defective in the biosynthesis of mycolic acids accumulates meromycolates. Proc Natl Acad Sci U S A 96:4011–4016. doi: 10.1073/pnas.96.7.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Danilchanka O, Pavlenok M, Niederweis M. 2008. Role of porins for uptake of antibiotics by Mycobacterium smegmatis. Antimicrob Agents Chemother 52:3127–3134. doi: 10.1128/AAC.00239-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sarathy JP, Dartois V, Lee EJ. 2012. The role of transport mechanisms in Mycobacterium tuberculosis drug resistance and tolerance. Pharmaceuticals (Basel) 5:1210–1235. doi: 10.3390/ph5111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mailaender C, Reiling N, Engelhardt H, Bossmann S, Ehlers S, Niederweis M. 2004. The MspA porin promotes growth and increases antibiotic susceptibility of both Mycobacterium bovis BCG and Mycobacterium tuberculosis. Microbiology 150:853–864. doi: 10.1099/mic.0.26902-0. [DOI] [PubMed] [Google Scholar]
- 81.Stephan J, Mailaender C, Etienne G, Daffe M, Niederweis M. 2004. Multidrug resistance of a porin deletion mutant of Mycobacterium smegmatis. Antimicrob Agents Chemother 48:4163–4170. doi: 10.1128/AAC.48.11.4163-4170.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Siroy A, Mailaender C, Harder D, Koerber S, Wolschendorf F, Danilchanka O, Wang Y, Heinz C, Niederweis M. 2008. Rv1698 of Mycobacterium tuberculosis represents a new class of channel-forming outer membrane proteins. J Biol Chem 283:17827–17837. doi: 10.1074/jbc.M800866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cunningham AF, Spreadbury CL. 1998. Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and localization of the 16-kilodalton alpha-crystallin homolog. J Bacteriol 180:801–808. doi: 10.1128/JB.180.4.801-808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Velayati AA, Farnia P, Masjedi MR, Zhavnerko GK, Merza MA, Ghanavi J, Tabarsi P, Farnia P, Poleschuyk NN, Ignatyev G. 2011. Sequential adaptation in latent tuberculosis bacilli: observation by atomic force microscopy (AFM). Int J Clin Exp Med 4:193–199. [PMC free article] [PubMed] [Google Scholar]
- 85.Machado D, Coelho TS, Perdigao J, Pereira C, Couto I, Portugal I, Maschmann RA, Ramos DF, von Groll A, Rossetti MLR, Silva PA, Viveiros M. 2017. Interplay between mutations and efflux in drug resistant clinical isolates of Mycobacterium tuberculosis. Front Microbiol 8:711. doi: 10.3389/fmicb.2017.00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wiuff C, Zappala RM, Regoes RR, Garner KN, Baquero F, Levin BR. 2005. Phenotypic tolerance: antibiotic enrichment of noninherited resistance in bacterial populations. Antimicrob Agents Chemother 49:1483–1494. doi: 10.1128/AAC.49.4.1483-1494.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Louw GE, Warren RM, Gey van Pittius NC, Leon R, Jimenez A, Hernandez-Pando R, McEvoy CR, Grobbelaar M, Murray M, van Helden PD, Victor TC. 2011. Rifampicin reduces susceptibility to ofloxacin in rifampicin-resistant Mycobacterium tuberculosis through efflux. Am J Respir Crit Care Med 184:269–276. doi: 10.1164/rccm.201011-1924OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hicks ND, Yang J, Zhang X, Zhao B, Grad YH, Liu L, Ou X, Chang Z, Xia H, Zhou Y, Wang S, Dong J, Sun L, Zhu Y, Zhao Y, Jin Q, Fortune SM. 2018. Clinically prevalent mutations in Mycobacterium tuberculosis alter propionate metabolism and mediate multidrug tolerance. Nat Microbiol 3:1032–1042. doi: 10.1038/s41564-018-0218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Torrey HL, Keren I, Via LE, Lee JS, Lewis K. 2016. High persister mutants in Mycobacterium tuberculosis. PLoS One 11:e0155127. doi: 10.1371/journal.pone.0155127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Safi H, Gopal P, Lingaraju S, Ma S, Levine C, Dartois V, Yee M, Li L, Blanc L, Ho Liang H-P, Husain S, Hoque M, Soteropoulos P, Rustad T, Sherman DR, Dick T, Alland D. 2019. Phase variation in Mycobacterium tuberculosis glpK produces transiently heritable drug tolerance. Proc Natl Acad Sci U S A 116:19665–19674. doi: 10.1073/pnas.1907631116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bellerose MM, Baek SH, Huang CC, Moss CE, Koh EI, Proulx MK, Smith CM, Baker RE, Lee JS, Eum S, Shin SJ, Cho SN, Murray M, Sassetti CM. 2019. Common variants in the glycerol kinase gene reduce tuberculosis drug efficacy. mBio 10:e00663-19. doi: 10.1128/mBio.00663-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fu LM. 2006. Exploring drug action on Mycobacterium tuberculosis using Affymetrix oligonucleotide genechips. Tuberculosis (Edinb) 86:134–143. doi: 10.1016/j.tube.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Betts JC, McLaren A, Lennon MG, Kelly FM, Lukey PT, Blakemore SJ, Duncan K. 2003. Signature gene expression profiles discriminate between isoniazid-, thiolactomycin-, and triclosan-treated Mycobacterium tuberculosis. Antimicrob Agents Chemother 47:2903–2913. doi: 10.1128/aac.47.9.2903-2913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wilson M, DeRisi J, Kristensen HH, Imboden P, Rane S, Brown PO, Schoolnik GK. 1999. Exploring drug-induced alterations in gene expression in Mycobacterium tuberculosis by microarray hybridization. Proc Natl Acad Sci U S A 96:12833–12838. doi: 10.1073/pnas.96.22.12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jeeves RE, Marriott AA, Pullan ST, Hatch KA, Allnutt JC, Freire-Martin I, Hendon-Dunn CL, Watson R, Witney AA, Tyler RH, Arnold C, Marsh PD, McHugh TD, Bacon J. 2015. Mycobacterium tuberculosis is resistant to isoniazid at a slow growth rate by single nucleotide polymorphisms in katG codon Ser315. PLoS One 10:e0138253. doi: 10.1371/journal.pone.0138253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Herbert D, Paramasivan CN, Venkatesan P, Kubendiran G, Prabhakar R, Mitchison DA. 1996. Bactericidal action of ofloxacin, sulbactam-ampicillin, rifampin, and isoniazid on logarithmic- and stationary-phase cultures of Mycobacterium tuberculosis. Antimicrob Agents Chemother 40:2296–2299. doi: 10.1128/AAC.40.10.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saito K, Warrier T, Somersan-Karakaya S, Kaminski L, Mi J, Jiang X, Park S, Shigyo K, Gold B, Roberts J, Weber E, Jacobs WR Jr, Nathan CF. 2017. Rifamycin action on RNA polymerase in antibiotic-tolerant Mycobacterium tuberculosis results in differentially detectable populations. Proc Natl Acad Sci U S A 114:E4832–E4840. doi: 10.1073/pnas.1705385114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cabruja M, Mondino S, Tsai YT, Lara J, Gramajo H, Gago G. 2017. A conditional mutant of the fatty acid synthase unveils unexpected cross talks in mycobacterial lipid metabolism. Open Biol 7:160277. doi: 10.1098/rsob.160277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vilcheze C, Weisbrod TR, Chen B, Kremer L, Hazbon MH, Wang F, Alland D, Sacchettini JC, Jacobs WR Jr. 2005. Altered NADH/NAD+ ratio mediates coresistance to isoniazid and ethionamide in mycobacteria. Antimicrob Agents Chemother 49:708–720. doi: 10.1128/AAC.49.2.708-720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mishra R, Kohli S, Malhotra N, Bandyopadhyay P, Mehta M, Munshi M, Adiga V, Ahuja VK, Shandil RK, Rajmani RS, Seshasayee ASN, Singh A. 2019. Targeting redox heterogeneity to counteract drug tolerance in replicating Mycobacterium tuberculosis. Sci Transl Med 11:eaaw6635. doi: 10.1126/scitranslmed.aaw6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Niki M, Niki M, Tateishi Y, Ozeki Y, Kirikae T, Lewin A, Inoue Y, Matsumoto M, Dahl JL, Ogura H, Kobayashi K, Matsumoto S. 2012. A novel mechanism of growth phase-dependent tolerance to isoniazid in mycobacteria. J Biol Chem 287:27743–27752. doi: 10.1074/jbc.M111.333385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wakamoto Y, Dhar N, Chait R, Schneider K, Signorino-Gelo F, Leibler S, McKinney JD. 2013. Dynamic persistence of antibiotic-stressed mycobacteria. Science 339:91–95. doi: 10.1126/science.1229858. [DOI] [PubMed] [Google Scholar]
- 103.Wang Z, Cumming BM, Mao C, Zhu Y, Lu P, Steyn AJC, Chen S, Hu Y. 2018. RbpA and sigma(B) association regulates polyphosphate levels to modulate mycobacterial isoniazid-tolerance. Mol Microbiol 108:627–640. doi: 10.1111/mmi.13952. [DOI] [PubMed] [Google Scholar]
- 104.Chen J, Su L, Wang X, Zhang T, Liu F, Chen H, Tan C. 2016. Polyphosphate kinase mediates antibiotic tolerance in extraintestinal pathogenic Escherichia coli PCN033. Front Microbiol 7:724. doi: 10.3389/fmicb.2016.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alcantara C, Coll-Marques JM, Jadan-Piedra C, Velez D, Devesa V, Zuniga M, Monedero V. 2018. Polyphosphate in Lactobacillus and its link to stress tolerance and probiotic properties. Front Microbiol 9:1944. doi: 10.3389/fmicb.2018.01944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zaw MT, Emran NA, Lin Z. 2018. Mutations inside rifampicin-resistance determining region of rpoB gene associated with rifampicin-resistance in Mycobacterium tuberculosis. J Infect Public Health 11:605–610. doi: 10.1016/j.jiph.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 107.Zhu JH, Wang BW, Pan M, Zeng YN, Rego H, Javid B. 2018. Rifampicin can induce antibiotic tolerance in mycobacteria via paradoxical changes in rpoB transcription. Nat Commun 9:4218. doi: 10.1038/s41467-018-06667-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Javid B, Sorrentino F, Toosky M, Zheng W, Pinkham JT, Jain N, Pan M, Deighan P, Rubin EJ. 2014. Mycobacterial mistranslation is necessary and sufficient for rifampicin phenotypic resistance. Proc Natl Acad Sci U S A 111:1132–1137. doi: 10.1073/pnas.1317580111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hartkoorn RC, Sala C, Magnet SJ, Chen JM, Pojer F, Cole ST. 2010. Sigma factor F does not prevent rifampin inhibition of RNA polymerase or cause rifampin tolerance in Mycobacterium tuberculosis. J Bacteriol 192:5472–5479. doi: 10.1128/JB.00687-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Takayama K, Kilburn JO. 1989. Inhibition of synthesis of arabinogalactan by ethambutol in Mycobacterium smegmatis. Antimicrob Agents Chemother 33:1493–1499. doi: 10.1128/aac.33.9.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang L, Zhao Y, Gao Y, Wu L, Gao R, Zhang Q, Wang Y, Wu C, Wu F, Gurcha SS, Veerapen N, Batt SM, Zhao W, Qin L, Yang X, Wang M, Zhu Y, Zhang B, Bi L, Zhang X, Yang H, Guddat LW, Xu W, Wang Q, Li J, Besra GS, Rao Z. 2020. Structures of cell wall arabinosyltransferases with the anti-tuberculosis drug ethambutol. Science 368:1211–1219. doi: 10.1126/science.aba9102. [DOI] [PubMed] [Google Scholar]
- 112.Zhu C, Liu Y, Hu L, Yang M, He ZG. 2018. Molecular mechanism of the synergistic activity of ethambutol and isoniazid against Mycobacterium tuberculosis. J Biol Chem 293:16741–16750. doi: 10.1074/jbc.RA118.002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang Y, Shi W, Zhang W, Mitchison D. 2013. Mechanisms of pyrazinamide action and resistance. Microbiol Spectr 2:MGM2-0023-2013. doi: 10.1128/microbiolspec.MGM2-0023-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mitchison DA. 2004. The search for new sterilizing anti-tuberculosis drugs. Front Biosci 9:1059–1072. doi: 10.2741/1293. [DOI] [PubMed] [Google Scholar]
- 115.Ibrahim M, Andries K, Lounis N, Chauffour A, Truffot-Pernot C, Jarlier V, Veziris N. 2007. Synergistic activity of R207910 combined with pyrazinamide against murine tuberculosis. Antimicrob Agents Chemother 51:1011–1015. doi: 10.1128/AAC.00898-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Diacon AH, Dawson R, von Groote-Bidlingmaier F, Symons G, Venter A, Donald PR, van Niekerk C, Everitt D, Winter H, Becker P, Mendel CM, Spigelman MK. 2012. 14-day bactericidal activity of PA-824, bedaquiline, pyrazinamide, and moxifloxacin combinations: a randomised trial. Lancet 380:986–993. doi: 10.1016/S0140-6736(12)61080-0. [DOI] [PubMed] [Google Scholar]
- 117.Zaunbrecher MA, Sikes RD Jr, Metchock B, Shinnick TM, Posey JE. 2009. Overexpression of the chromosomally encoded aminoglycoside acetyltransferase eis confers kanamycin resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 106:20004–20009. doi: 10.1073/pnas.0907925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Houghton JL, Green KD, Pricer RE, Mayhoub AS, Garneau-Tsodikova S. 2013. Unexpected N-acetylation of capreomycin by mycobacterial Eis enzymes. J Antimicrob Chemother 68:800–805. doi: 10.1093/jac/dks497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen W, Biswas T, Porter VR, Tsodikov OV, Garneau-Tsodikova S. 2011. Unusual regioversatility of acetyltransferase Eis, a cause of drug resistance in XDR-TB. Proc Natl Acad Sci U S A 108:9804–9808. doi: 10.1073/pnas.1105379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Houghton JL, Biswas T, Chen W, Tsodikov OV, Garneau-Tsodikova S. 2013. Chemical and structural insights into the regioversatility of the aminoglycoside acetyltransferase Eis. Chembiochem 14:2127–2135. doi: 10.1002/cbic.201300359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gautam US, Sikri K, Vashist A, Singh V, Tyagi JS. 2014. Essentiality of DevR/DosR interaction with SigA for the dormancy survival program in Mycobacterium tuberculosis. J Bacteriol 196:790–799. doi: 10.1128/JB.01270-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Koul A, Vranckx L, Dhar N, Gohlmann HW, Ozdemir E, Neefs JM, Schulz M, Lu P, Mortz E, McKinney JD, Andries K, Bald D. 2014. Delayed bactericidal response of Mycobacterium tuberculosis to bedaquiline involves remodelling of bacterial metabolism. Nat Commun 5:3369. doi: 10.1038/ncomms4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Peterson EJR, Ma S, Sherman DR, Baliga NS. 2016. Network analysis identifies Rv0324 and Rv0880 as regulators of bedaquiline tolerance in Mycobacterium tuberculosis. Nat Microbiol 1:16078. doi: 10.1038/nmicrobiol.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee JJ, Lee SK, Song N, Nathan TO, Swarts BM, Eum SY, Ehrt S, Cho SN, Eoh H. 2019. Transient drug-tolerance and permanent drug-resistance rely on the trehalose-catalytic shift in Mycobacterium tuberculosis. Nat Commun 10:2928. doi: 10.1038/s41467-019-10975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Andries K, Villellas C, Coeck N, Thys K, Gevers T, Vranckx L, Lounis N, de Jong BC, Koul A. 2014. Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS One 9:e102135. doi: 10.1371/journal.pone.0102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gupta S, Cohen KA, Winglee K, Maiga M, Diarra B, Bishai WR. 2014. Efflux inhibition with verapamil potentiates bedaquiline in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58:574–576. doi: 10.1128/AAC.01462-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu J, Gefen O, Ronin I, Bar-Meir M, Balaban NQ. 2020. Effect of tolerance on the evolution of antibiotic resistance under drug combinations. Science 367:200–204. doi: 10.1126/science.aay3041. [DOI] [PubMed] [Google Scholar]
- 128.Levin-Reisman I, Ronin I, Gefen O, Braniss I, Shoresh N, Balaban NQ. 2017. Antibiotic tolerance facilitates the evolution of resistance. Science 355:826–830. doi: 10.1126/science.aaj2191. [DOI] [PubMed] [Google Scholar]
- 129.Velayati AA, Farnia P, Ibrahim TA, Haroun RZ, Kuan HO, Ghanavi J, Farnia P, Kabarei AN, Tabarsi P, Omar AR, Varahram M, Masjedi MR. 2009. Differences in cell wall thickness between resistant and nonresistant strains of Mycobacterium tuberculosis: using transmission electron microscopy. Chemotherapy 55:303–307. doi: 10.1159/000226425. [DOI] [PubMed] [Google Scholar]
- 130.McCune RM Jr, Tompsett R. 1956. Fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. I. The persistence of drug-susceptible tubercle bacilli in the tissues despite prolonged antimicrobial therapy. J Exp Med 104:737–762. doi: 10.1084/jem.104.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gopal P, Gruber G, Dartois V, Dick T. 2019. Pharmacological and molecular mechanisms behind the sterilizing activity of pyrazinamide. Trends Pharmacol Sci 40:930–940. doi: 10.1016/j.tips.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.US Food and Drug Administration. 2019. FDA approves new drug for treatment-resistant forms of tuberculosis that affects the lungs. US Food and Drug Administration, Silver Spring, MD. [Google Scholar]
- 133.Conradie F, Diacon AH, Ngubane N, Howell P, Everitt D, Crook AM, Mendel CM, Egizi E, Moreira J, Timm J, McHugh TD, Wills GH, Bateson A, Hunt R, Van Niekerk C, Li M, Olugbosi M, Spigelman M, Nix-TB Trial Team. 2020. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med 382:893–902. doi: 10.1056/NEJMoa1901814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vashisht R, Bhat AG, Kushwaha S, Bhardwaj A, OSDD Consortium, Brahmachari SK. 2014. Systems level mapping of metabolic complexity in Mycobacterium tuberculosis to identify high-value drug targets. J Transl Med 12:263. doi: 10.1186/s12967-014-0263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Louw GE, Warren RM, Gey van Pittius NC, McEvoy CR, Van Helden PD, Victor TC. 2009. A balancing act: efflux/influx in mycobacterial drug resistance. Antimicrob Agents Chemother 53:3181–3189. doi: 10.1128/AAC.01577-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Szumowski JD, Adams KN, Edelstein PH, Ramakrishnan L. 2013. Antimicrobial efflux pumps and Mycobacterium tuberculosis drug tolerance: evolutionary considerations. Curr Top Microbiol Immunol 374:81–108. doi: 10.1007/82_2012_300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pule CM, Sampson SL, Warren RM, Black PA, van Helden PD, Victor TC, Louw GE. 2016. Efflux pump inhibitors: targeting mycobacterial efflux systems to enhance TB therapy. J Antimicrob Chemother 71:17–26. doi: 10.1093/jac/dkv316. [DOI] [PubMed] [Google Scholar]
- 138.Chen C, Gardete S, Jansen RS, Shetty A, Dick T, Rhee KY, Dartois V. 2018. Verapamil targets membrane energetics in Mycobacterium tuberculosis. Antimicrob Agents Chemother 62:e02107-17. doi: 10.1128/AAC.02107-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.El-Mowafi SA, Sineva E, Alumasa JN, Nicoloff H, Tomsho JW, Ades SE, Keiler KC. 2015. Identification of inhibitors of a bacterial sigma factor using a new high-throughput screening assay. Antimicrob Agents Chemother 59:193–205. doi: 10.1128/AAC.03979-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Maitra A, Bates S, Kolvekar T, Devarajan PV, Guzman JD, Bhakta S. 2015. Repurposing—a ray of hope in tackling extensively drug resistance in tuberculosis. Int J Infect Dis 32:50–55. doi: 10.1016/j.ijid.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 141.Maitra A, Bates S, Shaik M, Evangelopoulos D, Abubakar I, McHugh TD, Lipman M, Bhakta S. 2016. Repurposing drugs for treatment of tuberculosis: a role for non-steroidal anti-inflammatory drugs. Br Med Bull 118:138–148. doi: 10.1093/bmb/ldw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Eldholm V, Norheim G, von der Lippe B, Kinander W, Dahle UR, Caugant DA, Mannsaker T, Mengshoel AT, Dyrhol-Riise AM, Balloux F. 2014. Evolution of extensively drug-resistant Mycobacterium tuberculosis from a susceptible ancestor in a single patient. Genome Biol 15:490. doi: 10.1186/s13059-014-0490-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Trauner A, Liu Q, Via LE, Liu X, Ruan X, Liang L, Shi H, Chen Y, Wang Z, Liang R, Zhang W, Wei W, Gao J, Sun G, Brites D, England K, Zhang G, Gagneux S, Barry CE III, Gao Q. 2017. The within-host population dynamics of Mycobacterium tuberculosis vary with treatment efficacy. Genome Biol 18:71. doi: 10.1186/s13059-017-1196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu Q, Via LE, Luo T, Liang L, Liu X, Wu S, Shen Q, Wei W, Ruan X, Yuan X, Zhang G, Barry CE III, Gao Q. 2015. Within patient microevolution of Mycobacterium tuberculosis correlates with heterogeneous responses to treatment. Sci Rep 5:17507. doi: 10.1038/srep17507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Seraphin MN, Norman A, Rasmussen EM, Gerace AM, Chiribau CB, Rowlinson MC, Lillebaek T, Lauzardo M. 2019. Direct transmission of within-host Mycobacterium tuberculosis diversity to secondary cases can lead to variable between-host heterogeneity without de novo mutation: a genomic investigation. EBioMedicine 47:293–300. doi: 10.1016/j.ebiom.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sarathy JP, Via LE, Weiner D, Blanc L, Boshoff H, Eugenin EA, Barry CE III, Dartois VA. 2018. Extreme drug tolerance of Mycobacterium tuberculosis in caseum. Antimicrob Agents Chemother 62:e02266-17. doi: 10.1128/AAC.02266-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Richards JP, Cai W, Zill NA, Zhang W, Ojha AK. 2019. Adaptation of Mycobacterium tuberculosis to biofilm growth is genetically linked to drug tolerance. Antimicrob Agents Chemother 63:e01213-19. doi: 10.1128/AAC.01213-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cohen NR, Ross CA, Jain S, Shapiro RS, Gutierrez A, Belenky P, Li H, Collins JJ. 2016. A role for the bacterial GATC methylome in antibiotic stress survival. Nat Genet 48:581–586. doi: 10.1038/ng.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Marimani M, Ahmad A, Duse A. 2018. The role of epigenetics, bacterial and host factors in progression of Mycobacterium tuberculosis infection. Tuberculosis (Edinb) 113:200–214. doi: 10.1016/j.tube.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 150.Shell SS, Prestwich EG, Baek SH, Shah RR, Sassetti CM, Dedon PC, Fortune SM. 2013. DNA methylation impacts gene expression and ensures hypoxic survival of Mycobacterium tuberculosis. PLoS Pathog 9:e1003419. doi: 10.1371/journal.ppat.1003419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen L, Li HC, Chen T, Yu L, Guo HX, Chen YH, Chen M, Li ZY, Wu ZH, Wang XZ, Zhao J, Yan HM, Wang XC, Zhou L, Zhou J. 2018. Genome-wide DNA methylation and transcriptome changes in Mycobacterium tuberculosis with rifampicin and isoniazid resistance. Int J Clin Exp Pathol 11:3036–3045. [PMC free article] [PubMed] [Google Scholar]
- 152.Banaei-Esfahani A, Nicod C, Aebersold R, Collins BC. 2017. Systems proteomics approaches to study bacterial pathogens: application to Mycobacterium tuberculosis. Curr Opin Microbiol 39:64–72. doi: 10.1016/j.mib.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lata M, Sharma D, Kumar B, Deo N, Tiwari PK, Bisht D, Venkatesan K. 2015. Proteome analysis of ofloxacin and moxifloxacin induced Mycobacterium tuberculosis isolates by proteomic approach. Protein Pept Lett 22:362–371. doi: 10.2174/0929866522666150209113708. [DOI] [PubMed] [Google Scholar]
- 154.Adams KN, Takaki K, Connolly LE, Wiedenhoft H, Winglee K, Humbert O, Edelstein PH, Cosma CL, Ramakrishnan L. 2011. Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell 145:39–53. doi: 10.1016/j.cell.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Colangeli R, Helb D, Vilcheze C, Hazbon MH, Lee CG, Safi H, Sayers B, Sardone I, Jones MB, Fleischmann RD, Peterson SN, Jacobs WR Jr, Alland D. 2007. Transcriptional regulation of multi-drug tolerance and antibiotic-induced responses by the histone-like protein Lsr2 in M. tuberculosis. PLoS Pathog 3:e87. doi: 10.1371/journal.ppat.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Alland D, Kramnik I, Weisbrod TR, Otsubo L, Cerny R, Miller LP, Jacobs WR Jr, Bloom BR. 1998. Identification of differentially expressed mRNA in prokaryotic organisms by customized amplification libraries (DECAL): the effect of isoniazid on gene expression in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 95:13227–13232. doi: 10.1073/pnas.95.22.13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Balganesh M, Dinesh N, Sharma S, Kuruppath S, Nair AV, Sharma U. 2012. Efflux pumps of Mycobacterium tuberculosis play a significant role in antituberculosis activity of potential drug candidates. Antimicrob Agents Chemother 56:2643–2651. doi: 10.1128/AAC.06003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li G, Zhang J, Guo Q, Jiang Y, Wei J, Zhao LL, Zhao X, Lu J, Wan K. 2015. Efflux pump gene expression in multidrug-resistant Mycobacterium tuberculosis clinical isolates. PLoS One 10:e0119013. doi: 10.1371/journal.pone.0119013. [DOI] [PMC free article] [PubMed] [Google Scholar]