Patients and physicians worldwide are facing tremendous health care hazards that are caused by the ongoing severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) pandemic. Remdesivir (GS-5734) is the first approved treatment for severe coronavirus disease 2019 (COVID-19). It is a novel nucleoside analog with a broad antiviral activity spectrum among RNA viruses, including ebolavirus (EBOV) and the respiratory pathogens Middle East respiratory syndrome coronavirus (MERS-CoV), SARS-CoV, and SARS-CoV-2.
KEYWORDS: COVID-19, MERS-CoV, SARS-CoV, SARS-CoV-2, antiviral, coronavirus, ebolavirus, remdesivir
SUMMARY
Patients and physicians worldwide are facing tremendous health care hazards that are caused by the ongoing severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) pandemic. Remdesivir (GS-5734) is the first approved treatment for severe coronavirus disease 2019 (COVID-19). It is a novel nucleoside analog with a broad antiviral activity spectrum among RNA viruses, including ebolavirus (EBOV) and the respiratory pathogens Middle East respiratory syndrome coronavirus (MERS-CoV), SARS-CoV, and SARS-CoV-2. First described in 2016, the drug was derived from an antiviral library of small molecules intended to target emerging pathogenic RNA viruses. In vivo, remdesivir showed therapeutic and prophylactic effects in animal models of EBOV, MERS-CoV, SARS-CoV, and SARS-CoV-2 infection. However, the substance failed in a clinical trial on ebolavirus disease (EVD), where it was inferior to investigational monoclonal antibodies in an interim analysis. As there was no placebo control in this study, no conclusions on its efficacy in EVD can be made. In contrast, data from a placebo-controlled trial show beneficial effects for patients with COVID-19. Remdesivir reduces the time to recovery of hospitalized patients who require supplemental oxygen and may have a positive impact on mortality outcomes while having a favorable safety profile. Although this is an important milestone in the fight against COVID-19, approval of this drug will not be sufficient to solve the public health issues caused by the ongoing pandemic. Further scientific efforts are needed to evaluate the full potential of nucleoside analogs as treatment or prophylaxis of viral respiratory infections and to develop effective antivirals that are orally bioavailable.
INTRODUCTION
In December 2019, a novel coronavirus (nCoV), severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2), emerged in Wuhan, central China. Like in SARS-CoV, infections with the closely related SARS-CoV-2 cause a respiratory disease that can progress to viral pneumonia and acute respiratory distress syndrome (ARDS) (1). Because of its onset in December 2019, the associated disease was called coronavirus disease 2019 (COVID-19). Through 26 June 2020, the ongoing pandemic caused more than 9 million confirmed COVID-19 cases and nearly 500,000 deaths globally (2). In the light of an uncontrolled expansion and the steadily increasing COVID-19 fatalities in January and February 2020, huge efforts were put into the identification of effective antiviral agents against COVID-19. Nucleoside/nucleotide analogs are one of the most promising antiviral drug classes in general, and significant drug discoveries emerged from this class that today form the basis for treatments against infections with several herpesviruses, human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV). Remdesivir or GS-5734 is a prodrug of a nucleoside analog with direct antiviral activity against several single-stranded RNA viruses, including SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV). The first cell-based studies of remdesivir also showed antiviral activity against the novel SARS-CoV-2 (3, 4). In the absence of any effective treatment options against COVID-19, remdesivir has been applied under compassionate use. Recently, preliminary data from a randomized placebo-controlled clinical trial showed that remdesivir reduces the time to recovery in patients with COVID-19 (5), leading to an emergency-use authorization (EUA) by the U.S. Food and Drug Administration (FDA) only 2 days after the first press release from the National Institute of Allergy and Infectious Diseases (NIAID) (6). On 3 July, the European Medicines Agency (EMA) granted a conditional marketing authorization for remdesivir, now being the first approved antiviral treatment against COVID-19 (7) (Fig. 1). Here, we provide a comprehensive review of results from preclinical and clinical studies on this important novel antiviral drug to understand its clinical significance. In addition, we briefly describe the discovery and molecular mechanism of viral inhibition.
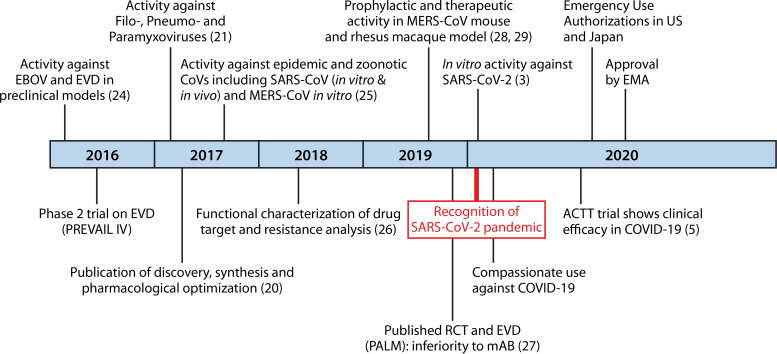
FIG 1.
Milestones in the discovery of remdesivir as an anti-COVID-19 treatment. Shown is a chronological summary of important achievements in the discovery and preclinical and clinical evaluations of remdesivir (GS-5734). Achievements appear according to the year of manuscript publication or reception at a peer-reviewed journal. COVID-19, coronavirus disease 2019; EBOV, ebolavirus; EMA, European Medicines Agency; EVD, ebolavirus disease; MERS-CoV, Middle East respiratory syndrome coronavirus; SARS-CoV(-2), severe acute respiratory distress syndrome coronavirus (2); RCT, randomized controlled clinical trial; mAB, monoclonal antibody. (See references 3, 5, 20, 21, and 24–29.)
DISCOVERY OF REMDESIVIR (GS-5734)
Nucleoside Analogs as Antiviral Agents
Nucleoside and nucleotide analogs as small-molecule-based antivirals have been explored for many years and form the backbone of treatment against viral infections, including HIV, hepatitis B virus, and herpesvirus infections (8–10). In 2013, the nucleotide analog sofosbuvir was approved by the FDA for the treatment of chronic hepatitis C virus infections. The novel compound that targets the RNA-dependent viral polymerase (NS5B) revolutionized HCV treatment, as it is able to cure the formerly lifelong chronic progressive disease when combined with other antivirals (11). In the past years, nucleoside/nucleotide analogs were increasingly recognized as potential antivirals targeting other positive-stranded RNA viruses such as members of the Flaviviridae, Picornaviridae, Caliciviridae, and Coronaviridae families, as they share relevant amino acid sequences with HCV (12), and the RNA-dependent polymerases are closely related phylogenetically (13, 65). This supported the assembly of antiviral compound libraries that could be screened against emerging RNA viruses. In the past years, several pharmacological advances in the development of nucleoside analogs were made based on structure-to-activity relationship (SAR) studies that improved pharmacokinetics, antiviral activity, and selectivity (14–16). A comprehensive overview of the medicinal chemistry and pharmacological evolution of antiviral nucleoside analogs can be found elsewhere (17, 18). Nucleoside analogs require intracellular activation by phosphorylation in order to become their active metabolites. One of the most important milestones was the addition of a monophosphate prodrug to the nucleoside, which significantly improved intracellular delivery and activation (19–21). This so-called ProTide approach, developed by McGuigan et al. (22, 23), was also used to optimize the precursor of remdesivir named GS-441524.
A Broad-Spectrum Antiviral Inhibits Ebolavirus
The parent molecule of remdesivir, GS-441524, was derived from a small-molecule library of around 1,000 diverse nucleoside and nucleoside phosphonate analogs that were assembled over many years of antiviral research based on their potential ability to target emerging RNA viruses such as SARS-CoV and MERS-CoV of the Coronaviridae or Zika and dengue viruses of the Flaviviridae family (20). Following the ebolavirus (EBOV) epidemic in West Africa from 2013 to 2016, a selection of promising leads from this library underwent intensive testing against different types of EBOV in collaboration with the Centers for Disease Control and Prevention (CDC) and the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID), which included studies in nonhuman primates (NHPs) (24). These efforts finally led to the identification of GS-5734, a monophosphate prodrug version of GS-441524, as the most promising lead against EBOV. GS-5734, later renamed remdesivir, had a broad antiviral spectrum, including EBOV, Marburg virus, respiratory syncytial virus (RSV), HCV, and several paramyxoviruses (20, 21, 24), in vitro. In addition, it demonstrated activity against MERS-CoV (24–26) and SARS-CoV (25, 26). Favorable in vitro results stimulated further evaluation in EBOV-infected macaques, where remdesivir suppressed viral replication and improved survival, clinical signs of the disease, and pathophysiological blood markers (24). After its discovery, remdesivir was administered under compassionate use to patients with ebolavirus disease (EVD) but stopped after an interim analysis of the first randomized controlled clinical trial (RCT) showed an inferiority of remdesivir to treatments with monoclonal antibodies (MAb114 and REGN-EB3). The trial evaluated the efficacies of different investigational therapeutics against EVD. Following the interim analysis, the remdesivir arm was halted for the remainder of the trial (27).
Lead Candidate against COVID-19
In December 2019, a novel coronavirus, SARS-CoV-2, emerged and caused a pandemic that is still ongoing. There were strong arguments for the antiviral effect of remdesivir against coronaviruses emerging from multiple cell-based in vitro models, including primary human airway epithelial (HAE) cell cultures (25), and, for MERS-CoV, from a mouse model of pulmonary infection (28). In addition, in a rhesus macaque model of MERS-CoV infection, remdesivir demonstrated strong prophylactic properties, and administration was associated with clinical benefits for treated subjects (29). The global hazards caused by the pandemic with the novel SARS-CoV-2 prompted the identification of potential treatment options. Given the solid preclinical data, remdesivir was considered one of the most promising candidates that went into clinical testing against COVID-19.
MECHANISM OF ACTION
Remdesivir is a monophosphoramidate nucleoside prodrug that undergoes intracellular metabolic conversion to its active metabolite nucleoside triphosphate (NTP). As described for several other direct-acting antivirals, the active metabolite of remdesivir (remdesivir triphosphate [remdesivir-TP] or GS-443902) subsequently targets the machinery responsible for the replication of the viral RNA genome, a highly conserved element of the viral life cycle. Nucleoside analogs are synthetic compounds that work by competition with endogenous natural nucleoside pools for incorporation into replicating viral RNA. While these compounds mimic their physiological counterparts, the incorporation of the analog molecule disrupts subsequent molecular processes. The drug target and the exact processes that lead to the inhibition of viral replication have been studied extensively in ebolavirus (24, 30). The suggested drug target, the EBOV RNA-dependent RNA polymerase (RdRp) complex, was only recently biochemically purified, which allowed for in-depth molecular analyses. Viral RdRp is the target protein for the active metabolite remdesivir-TP. Remdesivir-TP acts as the substrate for RdRp where it competes with ATP for incorporation into new strands. Inhibition of EBOV RdRp most probably results from delayed chain termination, a mechanism that is known from approved antivirals against human immunodeficiency virus type 1 (HIV-1) and HBV (31–34). In the case of EBOV, the incorporation of remdesivir-TP into replicating RNA was observed to cause chain termination predominantly at five positions downstream (i + 5) (30). Importantly, the activity of human RNA polymerase is not inhibited in the presence of remdesivir-TP (24).
In SARS-CoV and MERS-CoV, remdesivir-TP interferes with the nsp12 polymerase, which is a multisubunit RNA synthesis complex of viral nonstructural proteins (nsp’s) produced as cleavage products of viral polyproteins. As nsp12 is highly conserved across the coronavirus family, it is most likely that the mechanism of action (MOA) of remdesivir does not differ significantly among CoVs (35, 36). Like in EBOV, remdesivir-TP efficiently inhibits the replication of SARS-CoV and MERS-CoV by causing delayed chain termination when being incorporated into the replicating RNA (26). A recent biochemical analysis revealed that in SARS-CoV-2, remdesivir-TP causes the termination of RNA synthesis at three positions after the position where it is incorporated (i + 3). This mechanism was nearly identical in RdRps of SARS-CoV and MERS-CoV (37). The premature termination of RNA synthesis ultimately abrogates further transcriptional and translational processes needed for the generation of new virions (Fig. 2). The resulting antiviral effects of remdesivir have been studied in different cell-based models.
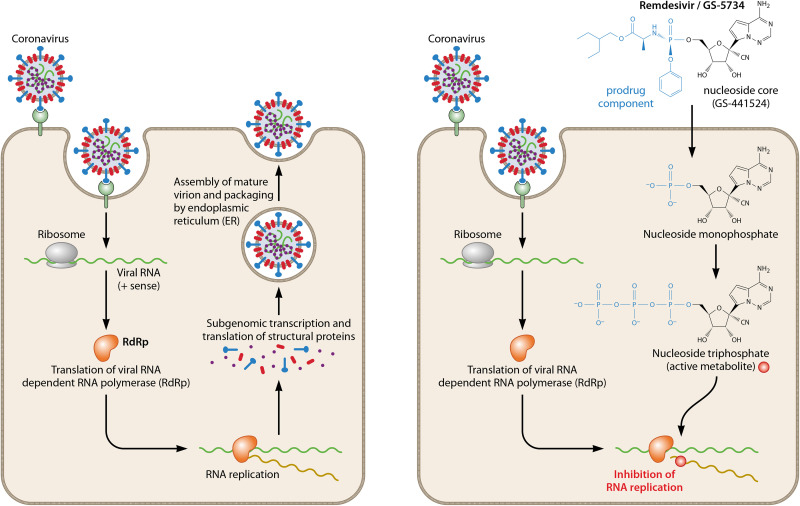
FIG 2.
Intracellular activation of remdesivir (GS-5734) and inhibition of coronavirus replication. Passage through the cell membrane by remdesivir is facilitated by the prodrug component attached to the nucleoside core. Upon entering the target cell, the pronucleotide undergoes further phosphorylation steps to become the active triphosphate metabolite that effectively inhibits viral RNA replication. Delayed chain termination is caused by the following processes: (i) misintegration of nucleoside triphosphate (NTP) into replicating RNA by RdRp, (ii) prevention of further chain elongation after NTP plus 3 additional nucleosides, and (iii) premature termination of RNA synthesis.
PRECLINICAL STUDIES
General Considerations
Remdesivir has been tested against various viruses in different cell-based systems and target cells (Table 1). When comparing the results of antiviral assays, one has to take into account that different assay methodologies and parameters such as the target cell type and virus input used may have significant impacts on the efficacy outcome (38). A variety of methods are available to assess the antiviral activity of candidate compounds in cell-based models. Simplified, susceptible target cells allowing viral replication are infected and subsequently exposed to serial concentrations of the test compounds. Historically, compounds were analyzed for their ability to reduce the amount of virus PFU on cellular layers (39). Although this method has the advantage of addressing the complete viral life cycle, it has been widely replaced by molecular methods that allow automated quantification. The antiviral effects of test compounds can be assessed by monitoring viral replication by either quantification of viral RNA using real-time PCR (rtPCR) or measurement of fluorescent reporter gene expression (RGE) from genetically modified virus strains. A special type of reporter gene assay is viral replicon (REPL) assays using genetically modified virus genomes that undergo transcriptional and translational processes inside the target cell but do not yield infectious progeny. Often, genes encoding structural proteins are exchanged by reporter genes that enable the monitoring of viral replication. This approach has been used to study the inhibition of HCV replication. Alternatively, viral antigens (AGs) can be quantified by fluorescence- or chemiluminescence-based immunostaining. Antiviral effects can also be measured indirectly by assessing virus-induced cytopathic effects (CPE) in the presence of test compounds. Assays measuring CPE can display not only direct antiviral effects but also beneficial effects of compounds with antivirulence properties. This is an advantage for high-throughput screening due to a gain of signal (40). In addition, CPE can be used to measure antiviral activity when no robust RGE- or rtPCR-based assay is available. It is unclear which type of assay provides the best information that can predict in vivo efficacy. However, the comparability of results from direct methods measuring viral replication and from CPE-based assays is limited by the fact that viral loads and related CPE do not automatically have a linear association.
TABLE 1.
In vitro antiviral spectrum of remdesivir in cell-based assaysd
| Order | Family | RNA virus | Strain | Target cell type(s) | Assay type | EC50 (μM) | Reference |
|---|---|---|---|---|---|---|---|
| Amarillovirales | Flaviviridae | AHFV | 200300001 | A549 | CPE | 4.2 | 21 |
| HCV | GT 1b-Con1 | Huh-7 | REPL | 0.057 | 20 | ||
| KFDV | P9605 | A549 | CPE | 1.8 | 21 | ||
| OHFV | Bogoluvovska | A549 | CPE | 1.2 | 21 | ||
| TBEV | Hypr | A549 | CPE | 2.1 | 21 | ||
| Bunyavirales | Arenaviridae | JUNV | Romero | HeLa | AG | 0.47 | 24 |
| LASV | Josiah | HeLa | AG | 1.48 | 24 | ||
| LASV | Josiah | Huh-7 | AG | 4.5 | 21 | ||
| Hantaviridae | ANDV | Chile 9717869 | Huh-7 | AG | 7.0 | 21 | |
| Nairoviridae | CCHV | IbAr 10200 | Huh-7 | AG | NIHC | 21 | |
| Phenuiviridae | RVFV | ZH501-GFP | Huh-7 | RGE | NIHC | 21 | |
| Martellivirales | Togaviridae | CHIV | AF15561 | U2OS | AG | NIHC | 24 |
| VEEV | SH3 | HeLa | AG | NIHC | 24 | ||
| Mononegavirales | Filoviridae | EBOV | EBOV-GFP | HMVEC-TERT | RGE | 0.06 | 24 |
| EBOV-GFP | Huh-7 | RGE | 0.07 | 24 | |||
| EBOV-GFP | HMVEC-TERT | RGE | 0.053 | 20 | |||
| Makona (WT) | hPM | AG | 0.09 | 24 | |||
| Makona (WT) | HFF-1 | AG | 0.13 | 24 | |||
| Makona (WT) | Huh-7 or hPM,c Vero | VY | 0.003 | 21 | |||
| Makona-ZsG | Huh-7 | RPE | 0.014 | 21 | |||
| Mayinga-GFP | Huh-7 | RGE | 0.066 | 21 | |||
| Mayinga-Gluc | Huh-7 | RGE | 0.021 | 21 | |||
| Kiwit | HeLa | AG | 0.10 | 20 | |||
| Kiwit | hPM | AG | 0.086 | 20 | |||
| Zaire (WT) | HeLa | AG | 0.14 | 24 | |||
| MARV | Bat371-Gluc | Huh-7 | RGE | 0.019 | 21 | ||
| Bat371-GFP | Huh-7 | RGE | 0.014 | 21 | |||
| Paramyxoviridae | NiV | M-Luc2AM | HeLa or 293T/17 | RGE | 0.045 | 21 | |
| M-GFP2AM | HeLa or 293T/17 | RGE | 0.029 | 21 | |||
| M-1999 | HeLa or HMVEC-L,c Vero | VY | 0.047 | 21 | |||
| B-2004 | HeLa,c Vero/H358 (CPE) | VY/CPE | 0.032 | 21 | |||
| HeV | 1996 | HeLa,c Vero/HeLa (CPE) | VY/CPE | 0.055 | 21 | ||
| hPIV-3 | JS-GFP | HeLa | RGE | 0.018 | 21 | ||
| MV | rMVEZGFP(3) | HeLa | RGE | 0.037 | 21 | ||
| MuV | IA 2006 | HeLa | AG | 0.79 | 21 | ||
| Pneumoviridae | RSV | A2 | HepG2 | CPE | 0.02 | 24 | |
| A2 | HepG2 | CPE | 0.053 | 20 | |||
| rgRSV224 (A2) | HeLa | RGE | 0.021 | 21 | |||
| Rhabdoviridae | VSV | New Jersey | HeLa, H358 | CPE | NIHC | 21 | |
| Nidovirales | Coronaviridae | HCoV-OC43a | VR-1558 | Huh-7 | AG | 0.15 | 46 |
| HCoV-229Ea | VR-740 | Huh-7 | CPE | 0.024 | 46 | ||
| VR-740 | LLC-PK1 | CPE | 3.8 | 46 | |||
| MHV | MHV-A59 | DBT | PA | 0.03 | 26 | ||
| MERS-CoV | Jordan N3 | Vero E6 | AG | 0.34 | 24 | ||
| MERS-GFP | HAE | RGE | 0.074 | 25 | |||
| MERS CoV nLUC | Calu-3 | RGE | 0.025 | 25 | |||
| MERS-RFP | HAE | rtPCR | 0.07 | 26 | |||
| EMC 2012 | Calu-3 | RGE | 0.12 | 28 | |||
| MERS CoV nLUC | Calu-3 | RGE | 0.09 | 28 | |||
| PDCoV | OH-FD22 LLCPK P5 | LLC-PK1 | CPE | NIHC | 46 | ||
| OH-FD22 LLCPK P5 | Huh-7 | CPE | 0.02 | 46 | |||
| SARS-CoV | SARS-RFP | HAE | RGE | 0.069 | 25 | ||
| SARS-GFP | HAE | rtPCR | 0.07 | 26 | |||
| Frankfurt 1b | Vero E6 | CPE | 4.3 | 4 | |||
| SARS-CoV-2 | Wuhan/WIV04/2019 | Vero E6 | rtPCR/AG | 0.77 | 3 | ||
| Hong Kong/VM20001061/2020 | Vero E6 | CPE | [25] | 47 | |||
| Hong Kong/VM20001061/2020 | Vero E6 | rtPCR | [26.9] | 47 | |||
| Hong Kong/VM20001061/2020 | Vero E6 | VY | [23.15] | 47 | |||
| Australia/VIC01/2020b | Vero E6 | CPE | 4.9 | 4 | |||
| Ortervirales | Retroviridae | HIV-1 | NA | NA | NA | NIHC | 24 |
Endemic CoVs.
Clinical isolates.
Primary infection before transferring viral progeny to Vero cell cultures.
Virus abbreviations: HFV, Alkhurma hemorrhagic fever virus; ANDV, Andes virus; CCHV, Crimean-Congo hemorrhagic fever virus; CHIV, chikungunya virus; EBOV, ebolavirus; HCV, hepatitis C virus; HCoV, human coronavirus; HeV, Hendra virus; hPIV-3, human parainfluenza virus type 3; HIV-1, human immunodeficiency virus type 1; JUNV, Junín virus; KFDV, Kyasanur Forest disease virus; LASV, Lassa fever virus; MARV, Marburg virus; MERS-CoV, Middle East respiratory syndrome-related coronavirus; MHV, murine hepatitis virus; MuV, mumps virus; MV, measles virus; NiV, Nipah virus; OHFV, Omsk hemorrhagic fever virus; PDCoV, porcine deltacoronavirus; RSV, respiratory syncytial virus; RVFV, Rift Valley fever virus; SARS-CoV, severe acute respiratory syndrome coronavirus; TBEV, tick-borne encephalitis virus; VEEV, Venezuelan equine encephalitis virus; VSV, vesicular stomatitis virus. Abbreviations of cell types: Calu-3, human lung epithelial cell line; DBT, murine astrocytoma delayed brain tumor cells; HAE, primary human airway epithelial cells; LLC-PK1, porcine kidney cells; HMVEC-TERT, TERT-immortalized human foreskin microvascular endothelial cells (ATCC 4025); hPM, human primary macrophages; Huh-7, epithelial-like tumorigenic hepatocyte cell line; Vero E6, African green monkey kidney cells. Assay type abbreviations: AG, antigen reduction assay; CPE, cytopathic effect inhibitory assay; PA, plaque assay; rtPCR, real-time PCR; RGE, reporter gene expression assay; REPL, viral replicon assay; VY, virus yield reduction assay. Abbreviations of efficacy measures: EC50, half-maximal effective drug concentration; NIHC, no inhibition at the highest concentration tested. Values in brackets indicate EC50 values calculated based on log10 viral load fitting. WT, wild type; NA, not applicable.
The choice of target cell displays another important factor. Activity against filoviruses is classically assessed in Vero E6 cells that were derived through the immortalization of African green monkey kidney cells. This cell line is known to highly express the angiotensin-converting enzyme 2 (ACE-2) receptor, which is required for viral entry of both SARS-CoV and SARS-CoV-2 into the target cell (41). In addition, Vero E6 cells support the replication of SARS-CoVs to high titers, which made them a standard cell model to study related pathogens (42–45). Besides common target cells, the antiviral activity of remdesivir was evaluated in human cell lines and primary cells that represent more clinically oriented in vitro systems. Activity against filoviruses was tested in a human liver cancer cell line (Huh-7) and human primary macrophages (hPMs). Anti-CoV activity was evaluated in a human lung epithelial cell line (Calu-3), primary HAE cells, and immortalized human foreskin microvascular endothelial cells (HMVECs). In contrast to SARS-CoV experiments that were conducted in HAE cells, assays against SARS-CoV-2 were conducted in Vero E6 cells, which might explain the 1-log-lower antiviral efficacy measured for SARS-CoV-2 (3, 25, 26). A recent comparison of replication kinetics and CPE of SARS-CoV and SARS-CoV-2 in Vero E6 cells concluded that there were no significant differences in drug sensitivities to remdesivir, thereby supporting this hypothesis (4).
In Vitro Efficacy of Remdesivir
In 2016, Warren et al. (24) tested the small-molecule nucleoside analog remdesivir (GS-5734) against EBOV. The half-maximum effective concentrations (EC50s) for EBOV inhibition were between 0.06 and 0.14 μM in different cell types, including human macrophages and endothelial cells. It was also shown that remdesivir inhibits the replication of other pathogenic RNA viruses such as RSV (EC50, 0.019 μM) and MERS-CoV (EC50, 0.34 μM) while having low cytotoxicity in a wide range of human primary cells and cell lines (24). The characterization of the antiviral spectrum of remdesivir in vitro was subsequently reevaluated and expanded across multiple virus families, including representatives of the filo-, paramyxo-, pneumo-, arena-, rhabdo-, flavi-, and coronavirus families, including zoonotic and epidemic CoVs (20, 21, 25, 26, 28). Remdesivir effectively inhibited EBOV (EC50, 0.003 to 0.1 μM) and RSV (EC50, 0.021), with results being comparable to those reported by Warren et al. (24). In addition, efficacy against Marburg virus (EC50, 0.014 to 0.19 μM) and several paramyxoviruses (EC50, 0.018 to 0.79 μM) was demonstrated in cell-based assays (21). The EC50s for MERS-CoV and SARS-CoV in reporter gene-based assays conducted by Sheahan et al. (25) were 0.025 to 0.12 μM and 0.069 μM, respectively, in HAE or Calu-3 cells. These results were later confirmed by Agostini et al. (26) using rtPCR (both EC50s, 0.07). Brown et al. (46) demonstrated that the antiviral spectrum of remdesivir also includes porcine CoVs and endemic human CoVs (HCoVs) that are associated with the common cold (HCoV-OC43 and -229E).
After the outbreak of SARS-CoV-2 in January 2020, remdesivir was rapidly tested in a Vero E6 cell-based model that made use of direct viral quantification by rtPCR along with the antimalaria and immune-modulating drug chloroquine and known antivirals such as ribavirin and penciclovir. Remdesivir was active against SARS-CoV-2, with an EC50 of 0.77 μM, which was slightly lower than that of chloroquine (EC50, 1.13 μM) and remarkably lower than those of other tested antivirals (EC50, 2.12 to 109.50 μM). More recently, a comprehensive comparison of the antiviral effects of remdesivir on the replication kinetics and cytopathology of clinical isolates of SARS-CoV and SARS-CoV-2 was made in Vero E6 cells. Here, both viruses showed similar sensitivities to remdesivir, with EC50s of 4.3 and 4.9 μM, respectively, when measuring the 50% cytopathologic effect (4). These values are substantially higher than those reported in previous studies that made use of reporter gene or rtPCR assays, which seems to have a methodological background. However, the higher values in this study may also reflect significantly lower susceptibilities of SARS-CoV and SARS-CoV-2 clinical isolates to remdesivir. Choy et al. (47) recently determined the EC50 (23.15 to 25 μM) for another SARS-CoV-2 clinical isolate (Hong Kong/VM20001061/2020) using three different Vero E6 cell-based assays. However, the comparability of these results to those of previous studies is limited by viral load calculations fitted to logarithmic scales (log10 RNA copies per milliliter) in order to estimate the effect of increasing drug concentrations. Finally, efficacy data were also reported by the manufacturer. Gilead’s fact sheet on remdesivir reports an EC50 of 0.137 μM (preliminary data), but no information is given on the specific strain that was tested by the China CDC in collaboration with Gilead Sciences (48). A complete overview of (peer-reviewed) published in vitro efficacy data is given in Table 1.
Antiviral Resistance
Nucleoside analogs such as remdesivir are generally expected to have a higher barrier to antiviral resistance than other antivirals such as neuraminidase inhibitors due to their well-conserved and vulnerable drug target (19). However, a general obstacle in the development of antiviral nucleoside analogs against CoVs is the presence of a potent exoribonuclease (ExoN)-mediated proofreading function of the RdRp subdomain nsp12, which causes resistance against ribavirin and 5-fluorouracil (26). ExoN is able to identify and remove incorporated nucleoside analogs. An important observation was that virus mutants lacking ExoN are 4-fold more sensitive to remdesivir (EC50, 0.019 μM) than the wild type with intact proofreading, implying that remdesivir is prone to ExoN-mediated proofreading. Nevertheless, this effect was shown to be concentration dependent, and even with intact ExoN proofreading, remdesivir inhibits viral replication still at submicromolar concentrations in direct antiviral assays (26). The relatively modest effect of ExoN on remdesivir susceptibility was attributed to the fact that the incorporation of remdesivir into replicating RNA occurs efficiently in comparison to natural nucleotides and to the mechanism of delayed chain termination that causes nsp12 inhibition by remdesivir (37, 49, 50). The additional natural nucleotides that are subsequently incorporated may have protective effects against ExoN activity (49).
Although nsp12 RdRp is highly conserved among CoVs, the amino acid identity still varies between 67 and 100% when including human and zoonotic CoVs (25), and variations in the amino acid sequence could have effects on viral susceptibility to remdesivir. Therefore, Brown et al. (46) tested the antiviral activity of remdesivir in strains with the most divergent RdRp compared to SARS- and MERS-CoV. They included endemic human CoVs (229E and OC43) and a porcine CoV known to harbor a native residue in the nsp12 subunit that confers antiviral resistance in betacoronaviruses. They found that the activity of remdesivir includes both contemporary human and highly divergent zoonotic CoVs and that natural variations in wild-type RdRp do not confer remdesivir resistance.
Another important factor regarding the remdesivir drug target is that mutations leading to changes in neighboring amino acids of the nsp12 subunit will most probably result in a substantial loss of viral fitness. Agostini et al. (26) demonstrated that mutations in nsp12 can be induced in wild-type murine hepatitis virus (MHV) by serial passage in the presence of increasing concentrations of GS-441524. These mutations (F476L and V553L) led to decreased remdesivir susceptibility, with a 2.4-fold or 5-fold shift in the EC50 (0.057 to 0.13 μM). The introduction of these substitutions into the SARS-CoV genome had a similar effect on remdesivir susceptibility, but mutants were unable to compete against the wild-type strain in coinfection passages without selective pressure. Furthermore, variants carrying F476L or V553L were attenuated in vivo, as shown by decreased lung titers in a mouse model of SARS-CoV infection.
In summary, remdesivir has a high genetic barrier to resistance development, and known resistant variants suffer from a loss of competitive fitness. This may suggest that these mutations will most likely not be maintained in nature and do not favor an uncontrolled spread of remdesivir-resistant variants. Compounds that are able to block ExoN-mediated proofreading would be of interest for combination therapy as they significantly increase virus susceptibility to remdesivir in vitro.
Efficacy in Animal Models
Ebolavirus disease.
The pro moiety of remdesivir can be degraded by serum esterase, which negatively affects efficacy and the pharmacokinetic profile. Because of high serum carboxylesterase activity in most rodents primarily used for animal models, initially, there was no suitable rodent model to study the efficacy of remdesivir in vivo (24). The first in vivo studies were therefore performed in NHPs that have lower or no serum esterase activities and are therefore comparable to humans (51). In an NHP model of fatal EVD, rhesus monkeys were inoculated with EBOV by intramuscular injection and treated for 12 days with an intravenous (i.v.) vehicle (n = 3), 3 mg/kg of body weight on day 0 or day 2 (n = 6 per group), 3 mg/kg after a 10-mg/kg loading dose on day 2 or day 3 (n = 6 per group), or 10 mg/kg of GS-5734 once daily starting 3 days after inoculation. Of 12 animals treated 3 days after virus exposure (10 mg/kg daily or 3 mg/kg with a 10-mg/kg loading dose), 100% of NHPs survived the 28-day in-life phase. In contrast, only 8 out of 18 subjects treated on day 0 or 2 survived the infection (all at 3 mg/kg daily; 6 received a 10-mg/kg loading dose). Antiviral effects (reduction in plasma viral RNA) and reductions in clinical signs were more pronounced in the 10-mg/kg treatment group. Warren et al. concluded that there was substantive postexposure protection with daily dosing of 10 mg/kg remdesivir (24).
MERS-CoV.
For MERS-CoV, a head-to-head assessment of remdesivir versus combinations of lopinavir, ritonavir, and interferon beta was performed in esterase-deficient mice with a humanized dipeptidylpeptidase 4 (DDP4) receptor. Humanization of the DDP4 receptor is required for MERS-CoV infection in mouse models because the wild-type DDP4 receptor does not enable MERS-CoV spike protein binding. Wild-type mice are therefore not susceptible to MERS-CoV infections (52). In this study, the therapeutic and prophylactic properties of remdesivir could be reproduced, and in the overall view of the authors, remdesivir was superior to other tested antivirals and combination treatments (28).
Remdesivir was also evaluated in a rhesus macaque model of MERS-CoV infection. The prophylactic administration of 5 mg/kg 24 h before inoculation with MERS-CoV prevented all six tested animals from developing active disease. Therapeutic administration by 12 h postinoculation reduced clinical signs of the disease, viral replication in the lungs, and pathological lung tissue processes in all six animals of the treatment group. Those authors therefore considered remdesivir to be a potential candidate against the novel SARS-CoV-2 (29).
SARS-CoV and SARS-CoV-2.
Given the limited plasma stability of remdesivir in rodents that is caused by high serum esterase activity, its antiviral activity against CoVs was evaluated in a mouse model of SARS-CoV infection using carboxylesterase 1c knockout (Ces1c−/−) mice. Here, prophylactic and early therapeutic administration of remdesivir (24 h before or 24 h after inoculation) reduced viral loads in the lungs and improved respiratory functions and clinical signs of the disease when given subcutaneously at doses of 25 mg/kg twice daily (25). In comparison to studies in NHPs or humans, high doses of remdesivir are necessary in mouse models, as tissue levels of the active remdesivir metabolite are approximately 10 times lower than those in NHPs, even in esterase-deficient mice. In addition, remdesivir-TP is more rapidly degraded in rodent lung tissues than in NHP lungs and primary human airway cells (25, 28).
Recently, the efficacy of remdesivir treatment was finally tested in a rhesus macaque model of SARS-CoV-2 infection (53). Animals were infected with SARS-CoV-2 (n = 12) by combined intranasal, oral, ocular, and intratracheal inoculations and subsequently treated with intravenous placebo or remdesivir (10-mg/kg loading dose followed by 5 mg/kg daily) for 6 days starting at 12 h postinfection. Remdesivir-treated animals did not show signs of respiratory disease and had lower lung virus titers and less lung tissue damage than the placebo group. According to the manufacturer’s information on the pharmacokinetic bridge from rhesus monkeys to humans, these doses approximate serum drug exposures that are equivalent to 200 mg and 100 mg, respectively (48). It is also important to note that the dynamics of acute SARS-CoV-2 infection progress more rapidly in animal models than in humans and that optimal treatment time points that are calculated based on expected viral load peaks cannot be directly translated to humans (54). Although the results of this study have to be interpreted with caution until final publication, they support early treatment initiation with remdesivir for SARS-CoV-2 infections.
CLINICAL STUDIES
Compassionate-Use Experience
More than 1,200 adult patients, 76 pediatric patients, and 96 pregnant women with COVID-19 were treated with remdesivir through the compassionate-use program according to the manufacturer Gilead Sciences. Liver function test abnormalities were reported in 19 of 163 evaluated cases (55). Once the COVID-19 epidemic started in China, at a time when there was no clinical trial in preparation, the first observational data for remdesivir arose from patients treated under compassionate use. In a prospective cohort study funded by Gilead Sciences, 61 patients with COVID-19 were treated with remdesivir for a 10-day course (200 mg on day 1, followed by 100 mg daily). Clinical improvement was observed in 36 (68%) of 53 evaluable patients (56). Another study from Italy reported on 35 patients treated with remdesivir in a general infectious disease ward. Interpretations of data from this study are very limited due to the low sample size, as only 22 patients completed a 10-day treatment course. The most frequent adverse events (AEs) were elevations of liver transaminase levels (15/35 patients) and acute kidney injury (8/35 patients) (57). As there were no control groups, no efficacy statements can be made based on these studies. One approach with a simulated control group is currently under peer review and suggests reductions in mortality with remdesivir (58). Several clinical trials were conducted to evaluate its efficacy against EVD and COVID-19.
Phase 2/3 Clinical Trials
Ebolavirus disease.
Two studies on remdesivir were conducted in the context of ebolavirus disease, PREVAIL IV (ClinicalTrials.gov identifier NCT02818582) and the Pamoja Tulinde Maisha (PALM) trial (ClinicalTrials.gov identifier NCT03719586). PREVAIL IV was a small phase 2 study in 38 men with evidence of ebolavirus persistence in their semen, who were formerly included in the observational EVD survivor study PREVAIL III. However, no reliable safety or efficacy data on remdesivir were derived from this study. The first randomized controlled phase 2/3 clinical trial evaluating the efficacy of remdesivir (PALM) started in 2018 in the Democratic Republic of Congo during an outbreak of EVD. Within 9 months, a total of 681 patients were enrolled in the study assessing the efficacies of four different therapeutic strategies in an open-label parallel 1:1:1:1 design. Patients received either remdesivir, the single monoclonal antibody MAb114, a coformulated composition of 3 human IgG1 monoclonal antibodies called REGN-EB3, or the triple monoclonal neutralizing antibody complex ZMapp (control group). The primary endpoint was death at day 28 of enrollment. An interim analysis on 9 August 2019 that included data from 499 patients showed that the mortality rates with both ZMapp (84/169; 49.7%) and remdesivir (93/175; 53.1%) were higher than those with MAb114 (61/174; 35.1%) and REGN-EB3 (52/155; 33.5%). Therefore, randomization into these groups was subsequently stopped. As this study did not include a placebo control arm, no definite conclusions on the clinical efficacy of remdesivir against EVD can be made. However, the observed mortality rate of approximately 50% is comparable to that of the natural course of the disease and thus does not suggest a substantial clinical benefit of remdesivir (59).
COVID-19.
The first phase 3 randomized, double-blind, placebo-controlled trial evaluating the efficacy of remdesivir against COVID-19 (ClinicalTrials.gov identifier NCT04257656) started in February 2020 in Wuhan. Hospitalized patients with severe COVID-19 were enrolled (defined as having hypoxia and radiological signs of lung involvement) and treated for 10 days with standard doses of remdesivir (200 mg on day 1 and 100 mg on days 2 to 10; n = 158) or a placebo (n = 79). Due to the rapidly changing dynamics of the outbreak in China, with a local decrease in new includable cases during March 2020, the trial was stopped preterm with only 237 patients enrolled and could not reach the calculated target enrollment size. In the final analysis of the present data set, treatment with remdesivir was not associated with significant clinical improvement in the treatment arm compared to the placebo (hazard ratio [HR], 1.23 [95% confidence interval {CI}, 0.87 to 1.75]). Furthermore, there was no significant difference between groups regarding mortality and time to viral clearance. In a subgroup of patients who were treated early within 10 days of symptom onset, remdesivir was associated with a numerical median reduction of 5 days in the time to clinical improvement, but this finding was not statistically significant (HR, 1.52 [95% CI, 0.95 to 2.43]). Those authors therefore proposed to evaluate remdesivir earlier in the course of COVID-19 (60).
The adaptive COVID-19 treatment trial (ACTT) started at the end of February 2020 and included a total of 60 study sites globally. A total of 1,063 hospitalized patients with all stages of COVID-19 that included signs of lower respiratory tract involvement (hypoxia or radiological evidence) were enrolled until 19 April 2020. Patients received either standard doses of remdesivir (n = 541) or a placebo (n = 522) for 10 days in a double-blind design. The primary outcome was time to recovery, defined as discharge from the hospital or continued hospitalization for infection control purposes only (no further medical treatment needed) (5). On 27 April 2020, the Data and Safety Monitoring Board (DSMB) concluded that there was a significant effect of remdesivir after reviewing an interim data analysis. Based on the available data, the DSMB recommended enabling patients of the placebo arm to benefit from a switch to remdesivir, which required early unblinding for a limited number of patients (61). The preliminary data have recently been published and show that treatment with remdesivir was associated with a reduction in the time to recovery from a median of 15 to 11 days (recovery rate ratio [RRR], 1.32 [95% CI, 1.12 to 1.55] [P < 0.001]). This effect was independent of symptom duration prior to randomization. But subgroup analyses showed that patients with the need for oxygen therapy (ordinal score of 5) benefit most from the treatment (RRR, 1.47 [95% CI, 1.17 to 1.84] [n = 421]), while no effect could be demonstrated for patients on invasive ventilation and/or extracorporeal membrane oxygenation (ECMO). The mortality rate by 14 days was 7.1% (remdesivir) versus 11.9% (placebo), which was not statistically significant (HR, 0.7 [95% CI, 0.47 to 1.03] [P = 0.06]) (5). Based on the preliminary results of this trial, the FDA issued an emergency-use authorization for remdesivir only 2 days after the initial press release from the NIAID. While complete results have not yet been published, another clinical trial was conducted, which evaluated the optimal treatment duration with remdesivir. The randomized open-label trial GS-US-540-5773 (previously SIMPLE) started in March 2020 and evaluated the efficacy of a 10-day treatment regimen (n = 197) versus a 5-day regimen (n = 200) of remdesivir in severe COVID-19. The results were recently published and suggest similar effects of 5-day and 10-day treatments when adjusting for baseline clinical status. Clinical improvement, clinical recovery, and mortality by day 14 were assessed. Due to the absence of a control group, these results do not permit an overall assessment of the efficacy of remdesivir (62). In addition to the study of severe COVID-19 cases, another open-label trial in patients with moderate COVID-19 is ongoing (Table 2).
TABLE 2.
Phase 2/3 clinical trials of remdesivir against viral diseases
| Trial identifier (short name) | Official title | Start date (day mo yr) | No. of participants | Status | Key efficacy finding(s) (remdesivir) | Source or reference |
|---|---|---|---|---|---|---|
| NCT02818582 (PREVAIL IV) | Double-blind, randomized, two-phase, placebo-controlled phase II trial of GS-5734 to assess antiviral activity, longer-term clearance of ebolavirus, and safety in male Ebola survivors with evidence of ebolavirus persistence in semen | 1 July 2016 | 38 | Completed 7 Oct 2019 | NA | https://clinicaltrials.gov/ct2/show/study/NCT02818582 |
| NCT03719586 (PALM) | Multicenter, multioutbreak, randomized controlled safety and efficacy study of investigational therapeutics for the treatment of patients with ebolavirus disease | 21 Nov 2018 | 681 | Completed 9 Sept 2019 | Interim analysis showed that it was inferior to other investigational drugs in preventing death at 28 days (primary endpoint) in patients with EVD | 27 |
| NCT04257656 | Phase 3 randomized, double-blind, placebo-controlled multicenter study to evaluate the efficacy and safety of remdesivir in hospitalized adult patients with severe COVID-19 | 6 Feb 2020 | 237 | Completed preterm, 30 Mar 2020 | No significant clinical improvement (HR, 1.23 [95% CI, 0.87–1.75]) | 60 |
| NCT04252664 CO-US-540-5764 | Phase 3 randomized, double-blind, placebo-controlled multicenter study to evaluate the efficacy and safety of remdesivir in hospitalized adult patients with mild and moderate COVID-19 | 12 Feb 2020 | 308 | Suspended | NA | https://clinicaltrials.gov/ct2/show/NCT04252664 |
| NCT04280705 CO-US-540-5776 (ACTT) | Multicenter, adaptive, randomized blinded controlled trial of the safety and efficacy of investigational therapeutics for the treatment of COVID-19 in hospitalized adults | 21 Feb 2020 | 1,063 | Completed 21 May 2020 | Preliminary results: a 10-day course of remdesivir reduced time to recovery in hospitalized adults (rate ratio for recovery: 1.32 [95% CI, 1.12–1.55]) and was associated with a numerical reduction in mortality rate on day 14 (7.1% vs 11.9%) | 5 |
| NCT04292899 GS-US-540-5773 (SIMPLE) | Phase 3 randomized study to evaluate the safety and antiviral activity of remdesivir (GS-5734) in participants with severe COVID-19 | 6 Mar 2020 | 397 | Completed 9 April 2020 | Similar improvements in clinical status, recovery, mortality, and adverse events in 5-day vs 10-day regimens of remdesivir | 62 |
| NCT04292730 GS-US-540-5774 (SIMPLE) | Phase 3 randomized study to evaluate the safety and antiviral activity of remdesivir (GS-5734) in participants with moderate COVID-19 compared to standard-of-care treatment | 15 Mar 2020 | Aim, 1,600 | Ongoing | NA | https://clinicaltrials.gov/ct2/show/NCT04292730 |
| NCT04330690 (SOLIDARITY) | Multicenter, adaptive, randomized, open-label, controlled clinical trial of the safety and efficacy of investigational therapeutics for the treatment of COVID-19 in hospitalized patients (CATCO [Canadian Treatments for COVID-19]), in conjunction with the Public Health Emergency SOLIDARITY trial (World Health Organization) | 18 Mar 2020 | Aim, 2,900 | Ongoing | NA | https://clinicaltrials.gov/ct2/show/NCT04330690 |
| NCT04321616 (SOLIDARITY) | NOR (Norwegian) SOLIDARITY multicenter trial on the efficacy of different antiviral drugs in SARS-CoV-2-infected patients | 28 Mar 2020 | Aim, 700 | Ongoing | NA | https://clinicaltrials.gov/ct2/show/NCT04321616 |
| IRCT20200405046953N1 (SOLIDARITY) | Randomized trial of additional treatments for COVID-19 in hospitalized patients who are all receiving the local standard of care—Iranian SOLIDARITY multicenter trial | 8 Apr 2020 | Aim, 3,000 | Ongoing | NA | https://www.irct.ir/trial/46930 |
| NCT04349410 | The Fleming (FMTVDM) directed COVID-19 treatment protocol | 11 Apr 2020 | Aim, 500 | Completed 14 September 2020 | NA | https://clinicaltrials.gov/ct2/show/NCT04349410 |
| CO-US-540-5758 | Phase 3 randomized, double-blind, placebo-controlled, multicenter study to evaluate the efficacy and safety of remdesivir combined with standard of care in hospitalized adult patients with severe 2019-nCoV respiratory disease | NA | NA | Planned | NA | NA |
| NCT04315948 CO-US-540-5804 (DisCoVeRy) | Multicenter, adaptive, randomized trial of the safety and efficacy of treatments of COVID-19 in hospitalized adults | NA | Aim, 3,100 | Ongoing | NA | https://clinicaltrials.gov/ct2/show/record/NCT04315948 |
| CO-US-540-5824 (WHO) | Multicenter, adaptive, randomized trial of the safety and efficacy of treatments of COVID-19 in hospitalized adults | NA | NA | Planned | NA | NA |
SAFETY AND DRUG TOLERABILITY
Gilead Sciences, the manufacturer of remdesivir, conducted four phase 1 clinical trials to evaluate the safety, tolerability, and pharmacokinetics of remdesivir (GS-US-399-1812, -1954, -4231, and -5505) in a total of 138 patients, of whom 131 received remdesivir and 7 received a placebo. Overall, the drug is generally well tolerated. Adverse events (pooled data) occurred in only a few cases and included phlebitis (8 subjects), constipation (7), headache (6), ecchymosis (5), nausea (5), and pain in extremities (5). Only a few grade 1 and 2 laboratory abnormalities were detected: transient elevations of alanine aminotransferase (ALT)/aspartate aminotransferase (AST) levels from day 5 until day 25 (12 subjects), mild reversible prolongation of the prothrombin time without changes in international normalized ratio (INR) (7 subjects), and mild hyperglycemia (4 subjects). There were no signs of nephrotoxicity in healthy subjects and no patterns of clinically relevant changes in vital signs or electrocardiograms (63). The available safety data from phase 1/2 studies are provided in a fact sheet for health care providers that was published in the context of the emergency-use authorization issued by the FDA and can be downloaded (55). In the first phase 3 trial of remdesivir that was conducted in the context of EVD, one event of hypotension occurred in the remdesivir arm that was judged as not being related to underlying EVD by the site investigators. The event occurred during the administration of the loading dose and led to a fatal cardiac arrest. An independent pharmacovigilance committee concluded that the death could not be readily distinguished from underlying fulminant EVD (27). Safety data from the compassionate-use study are not conclusive as there was no control group. However, the most frequently reported adverse events in patients treated with remdesivir were increases in hepatic enzyme levels (32 patients; 60%) and diarrhea (5 patients; 9%) (56). In the Chinese phase 3 trial where 155 patients with COVD-19 received remdesivir, no deaths occurred that were judged as being possibly related or related to the study drug. The frequencies of adverse events (most frequently constipation, hypoalbuminemia, hypokalemia, and anemia) were virtually identical in the treatment and placebo groups (60). Preliminary data from the ACTT study do not change this picture. The incidences of most adverse events were not found to be significantly different among the treatment and placebo groups. Grade 3 to 4 adverse events in general and some adverse events such as anemia or increased transaminase levels occurred slightly more often in the placebo group (grade 3 to 4 AEs in 172 versus 156 with remdesivir). Other adverse events occurred slightly more often in the remdesivir group (increased creatinine levels, pyrexia, and hyperglycemia) (5). In the open-label trial on patients with severe COVID-19, the most common adverse events were nausea (9%), worsening of respiratory failure (8%), elevated AST levels (77%), and constipation (7%) (62).
Taken together, at this time, there is no evidence for grade 3 to 4 or even severe adverse events resulting from once-daily doses of remdesivir (75 mg up to 225 mg i.v.) for treatment durations of up to 14 days. The drug seems to be well tolerated. Grade 1 and 2 adverse events have been described in healthy volunteers and patients with COVID-19 treated with remdesivir and mainly refer to transient elevations of ALT or AST levels. There are not sufficient data on the safety of remdesivir in patients younger than 18 years of age and pregnant women. Long-term toxicities are known from other nucleoside analogs used for sustained antiviral treatments of chronic infections with HIV or HBV but should not be of relevance for the relatively short-term treatments with remdesivir.
DOSING AND ADMINISTRATION
Prodrug Design
Nucleoside analogs require active cellular uptake by nucleoside transporters and intracellular activation by cellular and viral kinases to become their active NTP metabolite. This activation process requires three phosphorylation steps, of which the first step is most often inefficient and rate limiting (64). A common problem of nucleoside analogs is that they yield suboptimal levels of NTP at the site of infection (65). Remdesivir is a monophosphoramidate prodrug of a 1′-cyano-substituted adenosine nucleoside analog that is able to bypass the rate-limiting first phosphorylation step to effectively deliver intracellular NTP (66). The prodrug component is necessary to mask the negatively charged phosphonate group, which allows faster entry into target cells independently of membrane transporters. Phosphonate-containing pronucleotides have the disadvantage that their diacids are deprotonated at a physiological pH (67). Remdesivir has suboptimal oral bioavailability and therefore can be administered only by intravenous infusion in the actual formulation. However, there might be pharmacological approaches to solve this problem. One example of a clinically approved nucleoside phosphonate with an oral formulation is the nucleoside reverse transcriptase inhibitor (NRTI) tenofovir (67), which is one of the drugs most frequently used for therapy of HIV infections.
Drug Exposure
The prodrug remdesivir (GS-5734) has a relatively short systemic half-life (∼0.9 h) and is rapidly converted intracellularly into several intermediate metabolites (GS-704277 and GS-4471524) before being converted into the more stable and active TP metabolite (GS-443902) (24, 54). Plasma concentrations of remdesivir that are reached by the administration of therapeutic doses are several times higher than the concentrations required to inhibit SARS-CoV-2 replication in vitro (48, 54, 68). A dose of 200 mg remdesivir yields a maximum concentration of drug in serum (Cmax) of 9.03 μM (area under the concentration-time curve [AUC] of 4.85 μM), and subsequent dosing of 100 mg daily reaches a Cmax of 4.33 μM (AUC of 2.59 μM) on day 5 (54). Data on tissue distributions are available from studies with cynomolgus monkeys, where remdesivir and its metabolites were detectable in testes, eyes, and brain 4 h after a 10-mg/kg dose, which is comparable to 200 mg in humans. Unfortunately, no data on pulmonary drug delivery were reported in this publication (24). Distribution into lung tissues was recently studied in six rhesus macaques, where the intermediate metabolite GS-441524, which was used as a surrogate for tissue loading, could be detected in all samples 24 h after injection of remdesivir. The intermediate metabolite GS-704277 was not detectable in lung tissue samples (53). It remains unclear how plasma concentrations of remdesivir and its metabolites correlate with pulmonary drug delivery in humans, and there are speculations of suboptimal exposure in respiratory target cells of SARS-CoV-2 (69). Thus, therapeutic strategies that improve pulmonary drug exposure might be helpful to further improve the clinical efficacy of remdesivir.
Dosing Recommendations
Remdesivir is administered by intravenous infusion over 30 to 120 min. The standard dose for adults and pediatric patients weighing 40 kg and higher is a loading dose of 200 mg followed by once-daily doses of 100 mg. Dose adjustments are necessary for pediatric patients weighing less than 40 kg. It is not known if dose adjustments based on kidney or liver function are necessary. Administration in patients with a glomerular filtration rate (GFR) below 30 ml/min is not recommended based on the potential accumulation of sulfobutylether-β-cyclodextrin sodium salt present in both formulations of remdesivir (55). The optimal treatment duration for COVID-19 is still unknown. In phase 3 trials, a treatment course of 5 or 10 days was investigated. Based on these data, the actual recommendations in the context of emergency authorizations are 5 days for patients who do not require mechanical ventilation, which can be extended up to 10 days if patients do not demonstrate clinical improvement. For patients on mechanical ventilation, the actual recommended treatment duration is 10 days (55).
CONCLUSIONS
Remdesivir against Ebolavirus Disease
The in vitro activity of remdesivir against EBOV has been demonstrated in various cell-based models and for many different EBOV strains (20, 21, 24). In addition, it showed therapeutic and prophylactic effects in a rhesus monkey model of lethal EVD (24). However, in the PALM study, remdesivir was less efficacious than other investigational drugs. As this study did not include a placebo control group, no definite conclusions on the clinical efficacy of remdesivir in EVD can be made. The mortality rate among patients treated with remdesivir was approximately 50%, which was similar to that of the control group treated with the triple monoclonal antibody ZMapp but significantly higher than those with MAb114 (35.1%) and REGN-EB3 (33.5%) (27). In a previous trial on EVD, the mortality rates were 22% among ZMapp-treated patients and about 37% among patients who received the standard of care only (59). The reason for these differences in mortality rates is unclear and may result from differences in the virulence of EBOV, sample size, patient population, or standard-of-care practices. However, a final interpretation of the clinical effects of remdesivir remains elusive, and based on the results of the PALM trial, it is unlikely that remdesivir will be clinically reevaluated for the treatment of EVD.
Remdesivir against COVID-19
Remdesivir is active in vitro against various CoVs, including SARS-CoV-2 (3, 4, 47, 55), and its mechanism of action has been studied extensively. Animal studies that included nonhuman primate models of MERS-CoV and, recently, SARS-CoV-2 support its efficacy, especially when administered early in the course of the disease (29, 70). Finally, a phase 3 trial showed beneficial clinical effects of remdesivir in patients who require supplemental oxygen, while clinical efficacy for critically ill patients who require mechanical ventilation could not be demonstrated (5). Remdesivir reduces the time to recovery by 31%, which is a relatively modest but clearly therapeutic effect (5). Besides these beneficial effects on patients, this may help to reduce the number of inpatient days, with positive effects on hospital costs and capacity issues that have emerged during the COVID-19 pandemic in several countries. With regard to mortality, a lower 14-day mortality rate of patients treated with remdesivir was reported from the ACTT study, which may indicate a beneficial but not statistically significant effect. Taking into account that the study was not powered to evaluate mortality, this is still a positive signal that must be further evaluated in large-scale studies. A meta-analysis with pooled data from the two available RCTs that is still under peer review concludes a statistically significant reduction in mortality (risk ratio [RR], 0.69 [95% CI, 0.49 to 0.99]) (71). Although the clinical data on remdesivir are not fully published yet, the emergency authorizations and recent approval by the EMA are encouraging, as most other investigational drugs have failed until now (72, 73), leaving remdesivir the only antiviral with clinically proven efficacy against COVID-19 to date. Complete results of the ACTT study, including impacts on viral loads and mortality by day 28, as well as results from ongoing trials and meta-analyses will provide more information on the clinical efficacy of remdesivir.
Future Perspective
After decades of research on direct-acting antiviral drugs, remdesivir is the first nucleoside analog that can be used to treat infections caused by a respiratory virus. In the light of its beneficial clinical effects, its favorable safety profile, and the absence of alternatives to treat COVID-19, remdesivir will increasingly be used outside the context of clinical trials or compassionate-use programs. The drug is already available in the United States and Japan based on emergency-use authorizations and was recently approved in Europe. However, treatment with the antiviral drug remdesivir alone will not be sufficient to reliably save the lives of patients suffering from COVID-19 or to solve the hazardous public health issues caused by the ongoing COVID-19 pandemic. Antiviral therapy in hospitalized patients cannot prevent the virus from being transmitted among communities and cannot reverse pathophysiological processes that have occurred already at the time of diagnosis. In general, prophylactic measures would be much more efficient in reducing COVID-19-associated morbidity and mortality as well as economic implications (1). The prophylactic use of remdesivir might be effective, as it completely protected exposed macaques from MERS-CoV-induced clinical disease (29). Prophylactic effects are also known from other virostatic-acting drugs like neuraminidase inhibitors that may prevent influenza virus infections and can also be used as postexposition prophylaxis (74). However, the prophylactic use of remdesivir is generally hampered by its poor oral bioavailability and the absence of an oral formulation. Further pharmacological efforts are needed to make the drug accessible to an outpatient population. Recently, the manufacturer announced in an open letter that a phase 1 trial with remdesivir inhalation is being planned and already accepted by the FDA (75). Interestingly, another SARS-CoV-2 active nucleoside analog called EIDD-1931, which is orally bioavailable, is currently in preclinical evaluation (76). Finally, it should be mentioned that drug pricing will also have significant implications for the possibility of applying remdesivir with a broader scope.
The therapeutic efficacy of remdesivir might be improved by the addition of other antivirals or immunomodulatory agents. It has recently been shown that glucocorticoids are able to improve clinical outcomes in cases of severe and critical COVID-19 (77). Based on these data, it can be expected that physicians will use both remdesivir and glucocorticoids to treat patients with severe or critical COVID-19. However, combination therapy should be used with caution, as drug interactions may occur. In vitro, remdesivir acts as the substrate or inhibitor of several drug-metabolizing enzymes (e.g., CYP3A4), which could influence the exposure levels of other therapeutic agents. In addition, these agents may interfere with the pharmacokinetics of remdesivir. The immunomodulatory drug hydroxychloroquine, for example, seems to reduce the antiviral activity of remdesivir by impairing its intracellular metabolic activation (55). Another approach that may improve clinical outcomes could be combination therapy with direct antiviral drugs that target several processes within the viral life cycle. Although this strategy is highly effective in the therapy of chronic infections with HIV and HCV, it is unclear if this is true for acute infections with SARS-CoV-2. Clinical trials evaluating combination therapy are needed to estimate their role in COVID-19.
ACKNOWLEDGMENTS
This research received no specific financial support. J.J.M. and I.S. receive funding from German Center for Infection Research (DZIF) stipends TI 07.001_Malin_00 and TI 07.001_SUAREZ_00. J.R. receives funding from the DZIF Thematic Translational Unit Tuberculosis (TTU TB) (grant numbers TTU 02.806 and 02.905) and the German Research Foundation (DFG RY 159). The funders had no role in data collection, interpretation, or the decision to submit the work for publication.
We acknowledge Patrick Lane, ScEYEnce Studios, for graphical enhancement of the presented figures.
G.F. has served as an advisor to Gilead Sciences and has conducted clinical research supported by Gilead Sciences. J.J.M., V.P., I.S., and J.R. declare no potential conflicts of interest.
All authors critically discussed the present study results and their clinical significance. J.J.M. wrote the original draft and was responsible for conceptualization, investigation, and visualization. I.S. and V.P. were involved in reviewing and editing the manuscript. G.F. and J.R. supervised the manuscript preparation process, were involved in reviewing, and were responsible for manuscript validation.
Biographies

Jakob J. Malin (M.D.) obtained an M.Sc. in Medicine at Maastricht University and a doctoral degree in the field of viral infectious diseases at the University of Bonn. He works as a medical doctor and clinician-scientist at the University Hospital of Cologne, completing a clinical residency in internal medicine and infectious diseases. He is involved in coordinating and conducting clinical trials on anti-infectives, including investigational drugs against COVID-19. Moreover, he contributes to the development of clinical guidelines on COVID-19 pharmacotherapy released by the German Society of Infectious Diseases (DGI). Dr. Malin has a strong translational research focus on antimicrobial drug discovery. Since 2012, he has been working on antimicrobial compounds against several pathogens, including opportunistic mycobacteria. He is affiliated with the Translational Research Unit for Infectious Diseases (TRU-ID) at the Center for Molecular Medicine Cologne (CMMC), where he works as a postdoctoral researcher and is currently expanding his translational working field for SARS-CoV-2.

Isabelle Suárez (M.D.) has been a consultant at Department I of Internal Medicine of the University of Cologne, Germany, since 2009. She has gained broad clinical experience in general internal medicine with a focus on Infectious Diseases (ID). She was board certified in Internal Medicine in 2016 and certified as an ID specialist in 2017. In recent years, she has been working in the ID outpatient clinic taking care of a large variety of infectious diseases such as HIV infections and tuberculosis (TB). In 2017, she joined the Translational Research Unit—Infectious Diseases (Head, Dr. Rybniker) at the University of Cologne as a postdoc. Her research focuses on TB, especially the management of extrapulmonary TB and the identification of biomarkers. Receiving stipends from the German Center for Infection Research (DZIF) and the DFG German Research Foundation allowed her to conduct studies as a clinician-scientist in this field.

Vanessa Priesner (M.D.) is an attending physician and clinician-scientist at Department I of Internal Medicine of the University of Cologne. Trained and certified in the field of travel medicine, she currently works in the outpatient clinic for infectiology. Dr. Priesner did her doctorate on protection from Clostridium difficile toxin B-catalyzed Rac1/Cdc42 glucosylation by tauroursodeoxycholic acid-induced Rac1/Cdc42 phosphorylation at the Institute for Toxicology of the University of Hannover. Since 2014, she continued and completed her residency for internal medicine at the University Hospital of Cologne, where she has been involved as a research physician in various clinical (non)interventional multicenter studies in the field of infectiology and oncology. Her main research interest focuses on ambulant parenteral antibiotic therapy (APAT) in Germany.

Gerd Fätkenheuer (M.D.) is a Professor of Internal Medicine and Infectious Diseases at the University of Cologne and head of the Division of Infectious Diseases. As a principal investigator in translational studies and clinical trials, he has done extensive research in the field of HIV infection. He has also published on a broad range of infectious diseases, including bacterial, virological, and fungal infections. Between 2012 and 2019, he was coleader of the Translational Thematic Unit (TTI) HIV Infection of the German Research Center of Infectious Diseases (DZIF). From 2013 to 2019, he served as the president of the Infectious Diseases Society of Germany.

Jan Rybniker (M.D., Ph.D.) is an attending physician and clinician-scientist at Department I of Internal Medicine of the University of Cologne. He did his Ph.D. on transposon mutagenesis of mycobacteria and host shutoff proteins of mycobacteriophages at the Institute for Medical Microbiology Cologne and the University of Tennessee (Prof. Pamela Small). In 2011, he joined Professor Stewart Cole’s group at the Ecole Polytechnique Fédérale de Lausanne (EPFL) as a postdoc, where he worked on antibiotic drug discovery targeting Mycobacterium tuberculosis. Since 2016, Dr. Rybniker has been head of the Translational Research Unit—Infectious Diseases, the main research laboratory of the Infectious Diseases Department of the University Hospital Cologne. In the past 10 years, his research focus was on the identification of novel antimicrobial substances with activity against multidrug-resistant pathogens.
REFERENCES
- 1.Dhama K, Khan S, Tiwari R, Sircar S, Bhat S, Malik YS, Singh KP, Chaicumpa W, Bonilla-Aldana DK, Rodriguez-Morales AJ. 2020. Coronavirus disease 2019—COVID-19. Clin Microbiol Rev 33:e00028-20. doi: 10.1128/CMR.00028-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins University. 2020. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE). Johns Hopkins University, Baltimore, MD. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6. Accessed 6 July 2020. [Google Scholar]
- 3.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. 2020. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogando NS, Dalebout TJ, Zevenhoven-Dobbe JC, Limpens RWAL, van der Meer Y, Caly L, Druce J, de Vries JJC, Kikkert M, Bárcena M, Sidorov I, Snijder EJ. 22 June 2020. SARS-coronavirus-2 replication in Vero E6 cells: replication kinetics, rapid adaptation and cytopathology. J Gen Virol doi: 10.1099/jgv.0.001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC, ACTT-1 Study Group Members . 22 May 2020. Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute of Allergy and Infectious Diseases. 2020. NIH clinical trial shows remdesivir accelerates recovery from advanced COVID-19. National Institute of Allergy and Infectious Diseases, Rockville, MD. www.nih.gov/news-events/news-releases/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19. [Google Scholar]
- 7.European Medicines Agency. 2020. First COVID-19 treatment authorised for use in the EU. European Medicines Agency, Amsterdam, Netherlands. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines-covid-19#remdesivir-section. Accessed 3 July 2020. [Google Scholar]
- 8.Elion GB. 1993. Acyclovir: discovery, mechanism of action, and selectivity. J Med Virol 1993(Suppl 1):2–6. doi: 10.1002/jmv.1890410503. [DOI] [PubMed] [Google Scholar]
- 9.De Clercq E. 2011. A 40-year journey in search of selective antiviral chemotherapy. Annu Rev Pharmacol Toxicol 51:1–24. doi: 10.1146/annurev-pharmtox-010510-100228. [DOI] [PubMed] [Google Scholar]
- 10.Holý A. 2006. Antiviral acyclic nucleoside phosphonates structure activity studies. Antiviral Res 71:248–253. doi: 10.1016/j.antiviral.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR, Jacobson IM, Kowdley KV, Nyberg L, Subramanian GM, Hyland RH, Arterburn S, Jiang D, McNally J, Brainard D, Symonds WT, McHutchison JG, Sheikh AM, Younossi Z, Gane EJ. 2013. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 12.te Velthuis AJW. 2014. Common and unique features of viral RNA-dependent polymerases. Cell Mol Life Sci 71:4403–4420. doi: 10.1007/s00018-014-1695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koonin EV. 1991. The phylogeny of RNA-dependent RNA polymerases of positive-strand RNA viruses. J Gen Virol 72(Part 9):2197–2206. doi: 10.1099/0022-1317-72-9-2197. [DOI] [PubMed] [Google Scholar]
- 14.Cho A, Saunders OL, Butler T, Zhang L, Xu J, Vela JE, Feng JY, Ray AS, Kim CU. 2012. Synthesis and antiviral activity of a series of 1′-substituted 4-aza-7,9-dideazaadenosine C-nucleosides. Bioorg Med Chem Lett 22:2705–2707. doi: 10.1016/j.bmcl.2012.02.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng JY, Cheng G, Perry J, Barauskas O, Xu Y, Fenaux M, Eng S, Tirunagari N, Peng B, Yu M, Tian Y, Lee YJ, Stepan G, Lagpacan LL, Jin D, Hung M, Ku KS, Han B, Kitrinos K, Perron M, Birkus G, Wong KA, Zhong W, Kim CU, Carey A, Cho A, Ray AS. 2014. Inhibition of hepatitis C virus replication by GS-6620, a potent C-nucleoside monophosphate prodrug. Antimicrob Agents Chemother 58:1930–1942. doi: 10.1128/AAC.02351-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho A, Zhang L, Xu J, Lee R, Butler T, Metobo S, Aktoudianakis V, Lew W, Ye H, Clarke M, Doerffler E, Byun D, Wang T, Babusis D, Carey AC, German P, Sauer D, Zhong W, Rossi S, Fenaux M, McHutchison JG, Perry J, Feng J, Ray AS, Kim CU. 2014. Discovery of the first C-nucleoside HCV polymerase inhibitor (GS-6620) with demonstrated antiviral response in HCV infected patients. J Med Chem 57:1812–1825. doi: 10.1021/jm400201a. [DOI] [PubMed] [Google Scholar]
- 17.Yates MK, Seley-Radtke KL. 2019. The evolution of antiviral nucleoside analogues: a review for chemists and non-chemists. Part II: complex modifications to the nucleoside scaffold. Antiviral Res 162:5–21. doi: 10.1016/j.antiviral.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seley-Radtke KL, Yates MK. 2018. The evolution of nucleoside analogue antivirals: a review for chemists and non-chemists. Part 1: early structural modifications to the nucleoside scaffold. Antiviral Res 154:66–86. doi: 10.1016/j.antiviral.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordan PC, Stevens SK, Deval J. 2018. Nucleosides for the treatment of respiratory RNA virus infections. Antivir Chem Chemother 26:2040206618764483. doi: 10.1177/2040206618764483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegel D, Hui HC, Doerffler E, Clarke MO, Chun K, Zhang L, Neville S, Carra E, Lew W, Ross B, Wang Q, Wolfe L, Jordan R, Soloveva V, Knox J, Perry J, Perron M, Stray KM, Barauskas O, Feng JY, Xu Y, Lee G, Rheingold AL, Ray AS, Bannister R, Strickley R, Swaminathan S, Lee WA, Bavari S, Cihlar T, Lo MK, Warren TK, Mackman RL. 2017. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of Ebola and emerging viruses. J Med Chem 60:1648–1661. doi: 10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
- 21.Lo MK, Jordan R, Arvey A, Sudhamsu J, Shrivastava-Ranjan P, Hotard AL, Flint M, McMullan LK, Siegel D, Clarke MO, Mackman RL, Hui HC, Perron M, Ray AS, Cihlar T, Nichol ST, Spiropoulou CF. 2017. GS-5734 and its parent nucleoside analog inhibit filo-, pneumo-, and paramyxoviruses. Sci Rep 7:43395. doi: 10.1038/srep43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGuigan C, Cahard D, Sheeka HM, De Clercq E, Balzarini J. 1996. Aryl phosphoramidate derivatives of d4T have improved anti-HIV efficacy in tissue culture and may act by the generation of a novel intracellular metabolite. J Med Chem 39:1748–1753. doi: 10.1021/jm950605j. [DOI] [PubMed] [Google Scholar]
- 23.McGuigan C, Hassan-Abdallah A, Srinivasan S, Wang Y, Siddiqui A, Daluge SM, Gudmundsson KS, Zhou H, McLean EW, Peckham JP, Burnette TC, Marr H, Hazen R, Condreay LD, Johnson L, Balzarini J. 2006. Application of phosphoramidate ProTide technology significantly improves antiviral potency of carbocyclic adenosine derivatives. J Med Chem 49:7215–7226. doi: 10.1021/jm060776w. [DOI] [PubMed] [Google Scholar]
- 24.Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, Siegel D, Perron M, Bannister R, Hui HC, Larson N, Strickley R, Wells J, Stuthman KS, Van Tongeren SA, Garza NL, Donnelly G, Shurtleff AC, Retterer CJ, Gharaibeh D, Zamani R, Kenny T, Eaton BP, Grimes E, Welch LS, Gomba L, Wilhelmsen CL, Nichols DK, Nuss JE, Nagle ER, Kugelman JR, Palacios G, Doerffler E, Neville S, Carra E, Clarke MO, Zhang L, Lew W, Ross B, Wang Q, Chun K, Wolfe L, Babusis D, Park Y, Stray KM, Trancheva I, Feng JY, Barauskas O, Xu Y, Wong P, Braun MR, Flint M, et al. 2016. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, Leist SR, Pyrc K, Feng JY, Trantcheva I, Bannister R, Park Y, Babusis D, Clarke MO, Mackman RL, Spahn JE, Palmiotti CA, Siegel D, Ray AS, Cihlar T, Jordan R, Denison MR, Baric RS. 2017. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med 9:eaal3653. doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, Smith EC, Case JB, Feng JY, Jordan R, Ray AS, Cihlar T, Siegel D, Mackman RL, Clarke MO, Baric RS, Denison MR. 2018. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio 9(2):e00221-18. doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulangu S, Dodd LE, Davey RT, Jr, Tshiani Mbaya O, Proschan M, Mukadi D, Lusakibanza Manzo M, Nzolo D, Tshomba Oloma A, Ibanda A, Ali R, Coulibaly S, Levine AC, Grais R, Diaz J, Lane HC, Muyembe-Tamfum J-J, PALM Writing Group, Sivahera B, Camara M, Kojan R, Walker R, Dighero-Kemp B, Cao H, Mukumbayi P, Mbala-Kingebeni P, Ahuka S, Albert S, Bonnett T, Crozier I, Duvenhage M, Proffitt C, Teitelbaum M, Moench T, Aboulhab J, Barrett K, Cahill K, Cone K, Eckes R, Hensley L, Herpin B, Higgs E, Ledgerwood J, Pierson J, Smolskis M, Sow Y, Tierney J, Sivapalasingam S, Holman W, Gettinger N, Vallee D, Nordwall J, PALM Consortium Study Team . 2019. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med 381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheahan TP, Sims AC, Leist SR, Schafer A, Won J, Brown AJ, Montgomery SA, Hogg A, Babusis D, Clarke MO, Spahn JE, Bauer L, Sellers S, Porter D, Feng JY, Cihlar T, Jordan R, Denison MR, Baric RS. 2020. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun 11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, Scott D, Cihlar T, Feldmann H. 2020. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A 117:6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tchesnokov EP, Feng JY, Porter DP, Gotte M. 2019. Mechanism of inhibition of Ebola virus RNA-dependent RNA polymerase by remdesivir. Viruses 11:326. doi: 10.3390/v11040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tchesnokov EP, Obikhod A, Schinazi RF, Gotte M. 2008. Delayed chain termination protects the anti-hepatitis B virus drug entecavir from excision by HIV-1 reverse transcriptase. J Biol Chem 283:34218–34228. doi: 10.1074/jbc.M806797200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Domaoal RA, McMahon M, Thio CL, Bailey CM, Tirado-Rives J, Obikhod A, Detorio M, Rapp KL, Siliciano RF, Schinazi RF, Anderson KS. 2008. Pre-steady-state kinetic studies establish entecavir 5′-triphosphate as a substrate for HIV-1 reverse transcriptase. J Biol Chem 283:5452–5459. doi: 10.1074/jbc.M707834200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seifer M, Hamatake RK, Colonno RJ, Standring DN. 1998. In vitro inhibition of hepadnavirus polymerases by the triphosphates of BMS-200475 and lobucavir. Antimicrob Agents Chemother 42:3200–3208. doi: 10.1128/AAC.42.12.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Innaimo SF, Seifer M, Bisacchi GS, Standring DN, Zahler R, Colonno RJ. 1997. Identification of BMS-200475 as a potent and selective inhibitor of hepatitis B virus. Antimicrob Agents Chemother 41:1444–1448. doi: 10.1128/AAC.41.7.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao Y, Yan L, Huang Y, Liu F, Zhao Y, Cao L, Wang T, Sun Q, Ming Z, Zhang L, Ge J, Zheng L, Zhang Y, Wang H, Zhu Y, Zhu C, Hu T, Hua T, Zhang B, Yang X, Li J, Yang H, Liu Z, Xu W, Guddat LW, Wang Q, Lou Z, Rao Z. 2020. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 368:779–782. doi: 10.1126/science.abb7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirchdoerfer RN, Ward AB. 2019. Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nat Commun 10:2342. doi: 10.1038/s41467-019-10280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordon CJ, Tchesnokov EP, Woolner E, Perry JK, Feng JY, Porter DP, Götte M. 2020. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem 295:6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Postnikova E, Cong Y, DeWald LE, Dyall J, Yu S, Hart BJ, Zhou H, Gross R, Logue J, Cai Y, Deiuliis N, Michelotti J, Honko AN, Bennett RS, Holbrook MR, Olinger GG, Hensley LE, Jahrling PB. 2018. Testing therapeutics in cell-based assays: factors that influence the apparent potency of drugs. PLoS One 13:e0194880. doi: 10.1371/journal.pone.0194880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirano N, Fujiwara K, Matumoto M. 1976. Mouse hepatitis virus (MHV-2). Plaque assay and propagation in mouse cell line DBT cells. Jpn J Microbiol 20:219–225. doi: 10.1111/j.1348-0421.1976.tb00978.x. [DOI] [PubMed] [Google Scholar]
- 40.Green N, Ott RD, Isaacs RJ, Fang H. 2008. Cell-based assays to identify inhibitors of viral disease. Expert Opin Drug Discov 3:671–676. doi: 10.1517/17460441.3.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banerjee A, Nasir JA, Budylowski P, Yip L, Aftanas P, Christie N, Ghalami A, Baid K, Raphenya AR, Hirota JA, Miller MS, McGeer AJ, Ostrowski M, Kozak RA, McArthur AG, Mossman K, Mubareka S. 2020. Isolation, sequence, infectivity, and replication kinetics of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis 26:2054–2063. doi: 10.3201/eid2609.201495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsuyama S, Nao N, Shirato K, Kawase M, Saito S, Takayama I, Nagata N, Sekizuka T, Katoh H, Kato F, Sakata M, Tahara M, Kutsuna S, Ohmagari N, Kuroda M, Suzuki T, Kageyama T, Takeda M. 2020. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci U S A 117:7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mossel EC, Huang C, Narayanan K, Makino S, Tesh RB, Peters CJ. 2005. Exogenous ACE2 expression allows refractory cell lines to support severe acute respiratory syndrome coronavirus replication. J Virol 79:3846–3850. doi: 10.1128/JVI.79.6.3846-3850.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaye M. 2006. SARS-associated coronavirus replication in cell lines. Emerg Infect Dis 12:128–133. doi: 10.3201/eid1201.050496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown AJ, Won JJ, Graham RL, Dinnon KH, III, Sims AC, Feng JY, Cihlar T, Denison MR, Baric RS, Sheahan TP. 2019. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res 169:104541. doi: 10.1016/j.antiviral.2019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choy KT, Wong AY, Kaewpreedee P, Sia SF, Chen D, Hui KPY, Chu DKW, Chan MCW, Cheung PP, Huang X, Peiris M, Yen HL. 2020. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res 178:104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.US Food and Drug Administration. 2020. Fact sheet for health care providers: emergency use authorization (EUA) of Veklury (remdesivir)—revision 1. US Food and Drug Administration, Silver Spring, MD. https://www.fda.gov/media/137566/download. Accessed 2 July 2020. [Google Scholar]
- 49.Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Götte M. 2020. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem 295:4773–4779. doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shannon A, Le NT-T, Selisko B, Eydoux C, Alvarez K, Guillemot J-C, Decroly E, Peersen O, Ferron F, Canard B. 2020. Remdesivir and SARS-CoV-2: structural requirements at both nsp12 RdRp and nsp14 exonuclease active-sites. Antiviral Res 178:104793. doi: 10.1016/j.antiviral.2020.104793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bahar FG, Ohura K, Ogihara T, Imai T. 2012. Species difference of esterase expression and hydrolase activity in plasma. J Pharm Sci 101:3979–3988. doi: 10.1002/jps.23258. [DOI] [PubMed] [Google Scholar]
- 52.Cockrell AS, Yount BL, Scobey T, Jensen K, Douglas M, Beall A, Tang XC, Marasco WA, Heise MT, Baric RS. 2016. A mouse model for MERS coronavirus-induced acute respiratory distress syndrome. Nat Microbiol 2:16226. doi: 10.1038/nmicrobiol.2016.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williamson BN, Feldmann F, Schwarz B, Meade-White K, Porter DP, Schulz J, van Doremalen N, Leighton I, Yinda CK, Pérez-Pérez L, Okumura A, Lovaglio J, Hanley PW, Saturday G, Bosio CM, Anzick S, Barbian K, Cihlar T, Martens C, Scott DP, Munster VJ, de Wit E. 2020. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature 585:273–276. doi: 10.1038/s41586-020-2423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jorgensen SC, Kebriaei R, Dresser LD. 2020. Remdesivir: review of pharmacology, pre-clinical data and emerging clinical experience for COVID-19. Pharmacotherapy 40:659–671. doi: 10.1002/phar.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.US Food and Drug Administration. 2020. Fact sheet for health care providers: emergency use authorization (EUA) of Veklury (remdesivir). Revised 08/2020. US Food and Drug Administration, Silver Spring, MD. www.fda.gov/media/137566/download. Accessed 12 August 2020. [Google Scholar]
- 56.Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure F-X, Nicastri E, Oda R, Yo K, Quiros-Roldan E, Studemeister A, Redinski J, Ahmed S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, D’Arminio Monforte A, Ismail S, Kato H, Lapadula G, L’Her E, Maeno T, Majumder S, Massari M, Mora-Rillo M, Mutoh Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, DeZure A, Zhao Y, Zhong L, Chokkalingam A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK, Winterbourne L, Desai P, et al. 2020. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med 382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Antinori S, Cossu MV, Ridolfo AL, Rech R, Bonazzetti C, Pagani G, Gubertini G, Coen M, Magni C, Castelli A, Borghi B, Colombo R, Giorgi R, Angeli E, Mileto D, Milazzo L, Vimercati S, Pellicciotta M, Corbellino M, Torre A, Rusconi S, Oreni L, Gismondo MR, Giacomelli A, Meroni L, Rizzardini G, Galli M. 2020. Compassionate remdesivir treatment of severe Covid-19 pneumonia in intensive care unit (ICU) and non-ICU patients: clinical outcome and differences in post-treatment hospitalisation status. Pharmacol Res 158:104899. doi: 10.1016/j.phrs.2020.104899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hsu C-Y, Lai C-C, Yen AM-F, Chen SL-S, Chen H-H. 2020. Efficacy of remdesivir in COVID-19 patients with a simulated two-arm controlled study. medRxiv 10.1101/2020.05.02.20088559. [DOI]
- 59.PREVAIL II Writing Group, Multi-National PREVAIL II Study Team, Davey RT, Jr, Dodd L, Proschan MA, Neaton J, Neuhaus Nordwall J, Koopmeiners JS, Beigel J, Tierney J, Lane HC, Fauci AS, Massaquoi MBF, Sahr F, Malvy D. 2016. A randomized, controlled trial of ZMapp for Ebola virus infection. N Engl J Med 375:1448–1456. doi: 10.1056/NEJMoa1604330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. 2020. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mozersky J, Mann DL, DuBois JM. 2020. The National Institute of Allergy and Infectious Diseases decision to stop the adaptive COVID-19 trial: on solid ethical and scientific grounds. JACC Basic Transl Sci 5:645–647. doi: 10.1016/j.jacbts.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, Spinner CD, Galli M, Ahn M-Y, Nahass RG, Chen Y-S, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wei X, Gaggar A, Brainard DM, Towner WJ, Muñoz J, Mullane KM, Marty FM, Tashima KT, Diaz G, Subramanian A, GS-US-540-5773 Investigators . 27 May 2020. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gilead Sciences. 2020. Remdesivir (GS-5734) investigator’s brochure version 5.0, p 115–116. Gilead Sciences, Foster City, CA. [Google Scholar]
- 64.Jordheim LP, Durantel D, Zoulim F, Dumontet C. 2013. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat Rev Drug Discov 12:447–464. doi: 10.1038/nrd4010. [DOI] [PubMed] [Google Scholar]
- 65.Deval J, Symons JA, Beigelman L. 2014. Inhibition of viral RNA polymerases by nucleoside and nucleotide analogs: therapeutic applications against positive-strand RNA viruses beyond hepatitis C virus. Curr Opin Virol 9:1–7. doi: 10.1016/j.coviro.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murakami E, Niu C, Bao H, Micolochick Steuer HM, Whitaker T, Nachman T, Sofia MA, Wang P, Otto MJ, Furman PA. 2008. The mechanism of action of β-d-2′-deoxy-2′-fluoro-2′-C-methylcytidine involves a second metabolic pathway leading to β-d-2′-deoxy-2′-fluoro-2′-C-methyluridine 5′-triphosphate, a potent inhibitor of the hepatitis C virus RNA-dependent RNA polymerase. Antimicrob Agents Chemother 52:458–464. doi: 10.1128/AAC.01184-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pertusati F, Serpi M, McGuigan C. 2012. Medicinal chemistry of nucleoside phosphonate prodrugs for antiviral therapy. Antivir Chem Chemother 22:181–203. doi: 10.3851/IMP2012. [DOI] [PubMed] [Google Scholar]
- 68.Humeniuk R, Mathias A, Cao H, Osinusi A, Shen G, Chng E, Ling J, Vu A, German P. 2020. Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID-19, in healthy subjects. Clin Transl Sci 13:896–906. doi: 10.1111/cts.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun D. 2020. Remdesivir for treatment of COVID-19: combination of pulmonary and IV administration may offer aditional [sic] benefit. AAPS J 22:77. doi: 10.1208/s12248-020-00459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Williamson BN, Feldmann F, Schwarz B, Meade-White K, Porter DP, Schulz J, van Doremalen N, Leighton I, Kwe Yinda C, Pérez-Pérez L, Okumura A, Lovaglio J, Hanley PW, Saturday G, Bosio CM, Anzick S, Barbian K, Cihlar T, Martens C, Scott DP, Munster VJ, de Wit E. 2020. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. bioRxiv 10.1101/2020.04.15.043166. [DOI] [PMC free article] [PubMed]
- 71.Alexander PE, Piticaru J, Lewis K, Aryal K, Thomas P, Szczeklik W, Fronczek J, Polok K, Alhazzani W, Mammen M. 2020. Remdesivir use in patients with coronavirus COVID-19 disease: a systematic review and meta-analysis. medRxiv 10.1101/2020.05.23.20110932. [DOI]
- 72.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, et al. 2020. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, Mourão MPG, Brito-Sousa JD, Baía-da-Silva D, Guerra MVF, Hajjar LA, Pinto RC, Balieiro AAS, Pacheco AGF, Santos JDO, Jr, Naveca FG, Xavier MS, Siqueira AM, Schwarzbold A, Croda J, Nogueira ML, Romero GAS, Bassat Q, Fontes CJ, Albuquerque BC, Daniel-Ribeiro C-T, Monteiro WM, Lacerda MVG, CloroCovid-19 Team . 2020. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open 3:e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jefferson T, Jones MA, Doshi P, Del Mar CB, Hama R, Thompson MJ, Spencer EA, Onakpoya I, Mahtani KR, Nunan D, Howick J, Heneghan CJ. 2014. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev 2014:CD008965. doi: 10.1002/14651858.CD008965.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O’Day D. 2020. An open letter from Daniel O’Day, chairman & CEO, Gilead Sciences. Gilead Sciences, Foster City, CA. https://stories.gilead.com//articles/an-open-letter-from-daniel-oday-june-22. Accessed 25 June 2020. [Google Scholar]
- 76.Sheahan TP, Sims AC, Zhou S, Graham RL, Pruijssers AJ, Agostini ML, Leist SR, Schäfer A, Dinnon KH, III, Stevens LJ, Chappell JD, Lu X, Hughes TM, George AS, Hill CS, Montgomery SA, Brown AJ, Bluemling GR, Natchus MG, Saindane M, Kolykhalov AA, Painter G, Harcourt J, Tamin A, Thornburg NJ, Swanstrom R, Denison MR, Baric RS. 2020. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med 12:eabb5883. doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. 17 July 2020. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]