Abstract
Introduction: Tocilizumab (TCZ) is an anti-interleukin-6 antibody that has been used for the treatment of severe coronavirus disease 2019 (COVID-19). However, concrete evidence of its benefit in reducing mortality in severe COVID-19 is lacking. Therefore, we performed a systematic review and meta-analysis of relevant studies that compared the efficacy of TCZ in severe COVID-19 vs. standard of care (SOC) alone.
Methods: A literature search for studies that compared “tocilizumab” and “standard of care” in the treatment of COVID-19 was done using major online databases from December 2019 to June 14, 2020. Search words “Tocilizumab,” “anti-interleukin-6 antibody,” and “COVID-19” or “coronavirus 2019” in various combinations were used. Articles in the form of abstracts, letters without original data, case reports, and reviews were excluded. Data were gathered on an Excel sheet, and statistical analysis was performed using Review Manager 5.3.
Results: Sixteen studies were eligible from 693 initial studies, including 3,641 patients (64% males). There were 13 retrospective studies and three prospective studies. There were 2,488 patients in the SOC group (61.7%) and 1,153 patients (68.7%) in the TCZ group. The death rate in the TCZ group, 22.4% (258/1,153), was lower than in the SOC group, 26.21% (652/2,488) [pooled odds ratio 0.57 (95% CI 0.36–0.92), p = 0.02]. There was a significant heterogeneity (inconsistency index = 80%) among the included studies.
Conclusion: The addition of TCZ to the SOC might reduce mortality in severe COVID-19. More extensive randomized clinical trials are needed to validate these findings.
Keywords: tocilizumab (IL-6 inhibitor), COVID-19, coronavirus (2019-nCoV), SARS-C0V-2 infection, tocilizumab, tocilizumab (TCZ)
Introduction
Coronavirus disease 2019 (COVID-19) is a viral disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that originated from Wuhan city of Hubei province in China in December 2019 (1, 2). COVID-19 has spread around the world, affecting more than 9 million people, with more than 473,000 deaths globally (3). About 6–10% of COVID-19 patients develop acute respiratory distress syndrome (ARDS) with a high mortality rate (4, 5). The virus commonly spreads through droplets. However, the virus has also been found in gastrointestinal secretions (6–8). Therefore, potentially, the virus transmission could happen through the fecal–oral route as well. Common clinical symptoms of COVID-19 include fever, cough, malaise, shortness of breath, and fatigue. (9) Gastrointestinal manifestations such as diarrhea and abnormal liver chemistries have also been observed (10, 11). Laboratory abnormalities in COVID-19 are increased inflammatory markers such as C-reactive protein, ferritin, erythrocyte sedimentation rate, interleukin-6 (IL-6), and lymphocytopenia, among others (12). COVID-19 has posed challenges for healthcare workers because of the absence of effective and proven treatment. COVID-19 has a high case fatality rate of 4.5% among patients older than 60 years (13). As a result, various antiviral and anti-inflammatory medications such as hydroxychloroquine, remdesivir, monoclonal antibodies, and convalescent serum are being used on a compassionate basis in patients with severe COVID-19 (14–16). Currently, treatment of severe COVID-19 with antiviral therapy such as remdesivir, hydroxychloroquine, lopinavir, ritonavir, or any other antiviral agents along with supportive treatment is considered as the standard of care (SOC). Recently, steroids such methylprednisolone has been included as a standard of treatment (17).
ARDS is characterized by inflammatory cytokine release syndrome, among which IL-6 has a pivotal role (18). IL-6 is one of the significant inflammatory markers released during the cytokine storm due to COVID-19 (19). Tocilizumab (TCZ) has been proposed as one of the potential treatments in patients with severe COVID-19 due to its ability to block IL-6-mediated inflammatory response. TCZ is an anti-IL-6 monoclonal antibody previously used in the treatment of rheumatoid arthritis and giant cell arteritis (20). Various single-arm non-randomized studies have evaluated the effect of TCZ in severe COVID-19 and claimed a notable improvement in the clinical condition of these patients (16, 21–29). Table 1 lists currently published single-arm studies that evaluated TCZ in the treatment of severe COVID-19. Several randomized controlled trials (RCTs) are currently being conducted to evaluate the efficacy of TCZ in COVID-19 (30, 31). In this article, we performed a systematic review and meta-analysis of studies that compared the role of TCZ on mortality in severe COVID-19 as compared to the SOC.
Table 1.
Characteristics of single-arm studies evaluating Tocilizumab in COVID 19 patients.
| Author, month, year | Country | Type of study | Patients n (males) | Age in years (Mean ± SD or median with range) | Comorbidities | Severity | Treatment | Mortality in % |
|---|---|---|---|---|---|---|---|---|
| Alattar, April 2020 (21) | Qatar | Retrospective | 25 (23) | 58 (50–63) | Diabetes- 48% CKD 16% Cardiac 12% |
Critical 100% | TCZ±H, L/R, R* | 12% |
| Alberici, April 2020 (22) | Italy | Retrospective | 20 (16) | 59 (51–64) | Renal transplant patients | Severe 13% Critical -none |
TCZ +/- H | 25% |
| Uysal June 2020 (23) | Turkey | Retrospective | 12 (6) | 65 ± 11.3 | Diabetes 58% Hypertension 58% |
Severe 83% Critical 17% |
TCZ+H, O | 0% |
| Hassoun July 2020 (24) | USA | Retrospective | 9 (6) | 60 (37–88) | Diabetes 11% Hypertension 44% Cardiac 11% Obesity 33% |
Severe 55% Critical 44% |
TCZ + corticosteroids | 22% |
| Luo, March 2020 (16) | China | Retrospective | 15 (12) | 73 (62–80) | Diabetes 26% Hypertension 60% |
Severe 40% Critical 46.7% |
TCZ+methyl prednisolone | 20% |
| Marfella, May 2020 (25) | Italy | Retrospective | 78 (55) | 65.7 ± 13.4 | Hyperglycemia (39.7%) | NA | TCZ | NA |
| Morena, May 2020 (26) | Italy | Prospective | 51 (40) | 60 (50–70) | Cardiovascular diseases 49% Hypertension 29.4% Diabetes 11.9% |
Severe 88% Critical – 11.7% |
TCZ ±H, L/R, R | 27% |
| Sciascia, May, 2020 (27) | Italy | Prospective | 63 (56) | 62.6 ± 12.5 | NA | Severe 100% | TCZ | 11% |
| Toniati, July 2020 (28) | Italy | Prospective | 100 (88) | 62 (57–71) | Diabetes 17% Hypertension 46% CKD 11% COPD 9% |
Severe 57% Critical 43% |
TCZ | 20% |
| Xu, April 2020 (29) | China | Retrospective | 21 (18) | 56.8 ± 16.5 (25–88) | Hypertension 43% Diabetes 23.8% Cardiac 9.5% COPD 4.8% |
Severe 81% Critical 19% |
TCZ | 0% |
TCZ, Tocilizumab; H, Hydroxychloroquine; L/R, Lopinavir/Ritonavir; R*, Ribavirin; R, Remdesivir; O, Oseltamivir; SD, Standard Deviation; CKD, Chronic Kidney Disease; NA, Not applicable; COPD, Chronic Obstructive Pulmonary Disease.
Materials and Methods
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) statement (32).
Definitions
Severe COVID-19: Patients with respiratory rate ≥30 breaths/min, or peripheral capillary oxygen saturation ≤ 93% or PaO2/FiO2 ≤ 300 mmHg, or combination of these findings.
Critical COVID-19: Patients with confirmed COVID-19 who required intensive care unit (ICU) management due to mechanical ventilation or sepsis management.
SOC (Control group): Patients with confirmed COIVD-19 were treated with any combination of the following treatment options: supplementary oxygen, antiviral agents (remdesivir, lopinavir/ritonavir, darunavir/cobicistat), antimalarial agents (hydroxychloroquine), anti-inflammatory agents such as steroids, and antibiotics (azithromycin).
TCZ group: Patients were treated with TCZ in addition to SOC.
Patients, Intervention, Comparison, and Outcomes (PICO) Questions
Patients: Patients admitted to hospitals with a confirmed diagnosis of COVID-19.
Intervention: Evaluation of TCZ in the treatment of severe COVID-19.
Comparison: TCZ and SOC in the treatment of severe COVID-19.
Outcome: Reduction in all-cause mortality.
Search Strategy
An electronic database literature search for studies that compared “TCZ and SOC” for the treatment of COVID-19 was performed on PubMed, Embase, Cochrane library, Web of Science, and MedRxiv for articles published from December 2019 until June 14, 2020. Search words “Tocilizumab,” “anti-interleukin-6 antibody,” and “COVID-19” or “coronavirus 2019” in various combinations.
Study Selection Criteria
We included clinical studies that reported mortality data in severe COVID-19 patients who were treated with TCZ. The primary outcome of interest was mortality in severe COVID-19. Only studies that compared mortality rates of patients who received TCZ and SOC were included in the meta-analysis. The articles, which were in the form of only abstracts, letters without original data, case reports, reviews, meta-analysis, animal studies, or studies that did not report original data on both TCZ and SOC groups, were excluded. Further attempts were made to identify relevant studies through references to find eligible studies. Studies that are published in the English language only or English translation is available for studies published in other languages were considered for the meta-analysis.
Data Extraction
Initial data collection was done by two independent investigators (UB and KN) who imported the data onto a standardized Excel sheet. When there was no consensus on the eligibility of the studies, a third author (HG) reviewed the study independently, and the final decision was made regarding inclusion or exclusion of the study. The following data were extracted from each study: authors' names, country, type of study, number of patients in SOC and TCZ groups, patients' gender, age, drugs used in SOC, and mortality in both groups.
Quality Assessment
The quality of the included studies was assessed using the Cochrane risk-of-bias assessment tool. Quality assessment and scoring of each included studies that were performed based on the selection of study groups, compatibility, and assessment of outcomes.
Outcomes
The primary outcome of interest was mortality in severe COVID-19 patients in those who received SOC vs. TCZ. Sensitivity analysis was performed to evaluate the effect of each study on the pooled estimates by the exclusion of one study at a time. Any significant change in pooled estimates was reported. We also performed a sensitivity analysis for studies from Europe and the USA, retrospective vs. prospective studies, and studies that used antiviral medications vs. those that did not. Sensitivity analysis was also done to evaluate the difference in primary end points when steroid was used in the SOC.
Statistical Analysis
The data extracted from studies found on online databases, including PubMed, Embase, Cochrane library, and Web of Science, were peer-reviewed. Studies that were retrieved from medRxiv were not peer-reviewed, and analysis performed on these studies was considered exploratory. The primary outcome of interest was pooled all-cause mortality in severe COVID-19. Categorical variables were reported in percentage and continuous variable in mean with standard deviation. The pooled estimates with a 95% confidence interval (CI) were synthesized by meta-analysis using the “DerSimonian–Laird random-effects model.” Heterogeneity across the included studies was performed using inconsistency index I2. The heterogeneity was classified as low, moderate, and substantial heterogeneity when the inconsistency index was 25, 50, and 75%, respectively. The publication bias was assessed using funnel plot analysis and Egger's test. Statistical analysis was done using Review Manager (Version 5.3; The Nordic Cochrane Center, Copenhagen, Denmark) and STATA 14.2 (StataCorp., 4905 Lakeway Drive, College Station, Texas 77845, USA).
Results
Study Characteristics
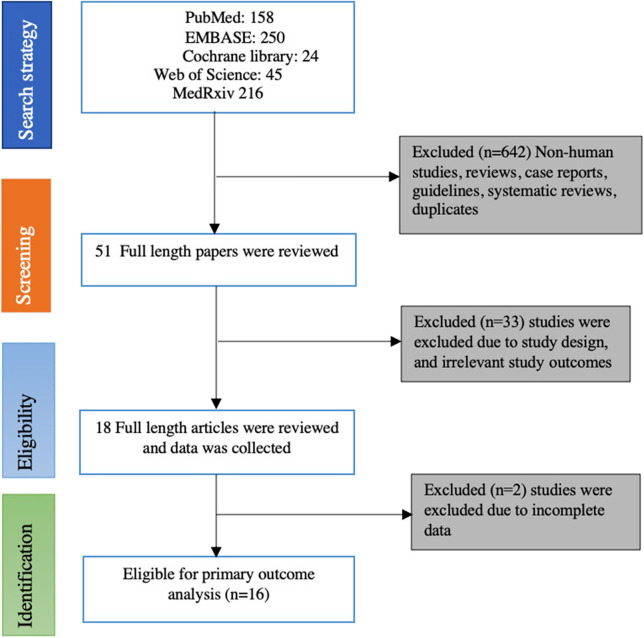
A total of 693 studies were identified through an initial literature search. After exclusion of duplicates and screening of articles based on the title and abstract, 51 articles remained. These articles were further reviewed thoroughly, and finally, 16 studies were eligible for the primary outcome. There were 13 retrospective studies (17, 33–44) and three prospective studies (45–47). Figure 1 provides details of the literature search and study selection process.
Figure 1.
Flowchart of selecting eligible studies for the meta-analysis.
There were a total of 3,641 patients, with 64% of males from 16 included studies. There were 2,488 patients (61.7% males) in the SOC group and 1,153 patients (68.7%) in the TCZ group. All patients included in the meta-analysis received SOC, and the TCZ group received TCZ in addition to SOC. Hydroxychloroquine was used in all the included studies, azithromycin was used in six studies, lopinavir/ritonavir combination was used in six studies, steroids were used in 12 studies, darunavir and cobicistat combination was used in three studies, and remdesivir was used in two studies. Table 2 lists the studies included in the meta-analysis and study characteristics.
Table 2.
Characteristics of studies included in the meta-analysis.
| Author | Country | Type of study | SOC treatment | Subjects total | TCZ n | TCZ males | TCZ age median (range) or mean ± SD | TCZ mortality | SOC total n | SOC males n | SOC age | SOC mortality n |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Campochiaro et al. (33) | Italy | Retrospective study | H, L/R | 65 | 32 | 29 | 64 (53–75) | 5 | 33 | 27 | 60 (55–75.5) | 11 |
| Capra et al. (34) | Italy | Retrospective study | H, L/R | 85 | 62 | 45 | 63 (54–73) | 2 | 23 | 19 | 70 (55–80) | 11 |
| Colaneri et al. (37) | Italy | Prospective study | H, Heparin, S | 112 | 21 | 19 | NA | 5 | 91 | 63 | NA | 19 |
| Klopfenstein et al. (35) | France | Retrospective study | H, L/R | 45 | 20 | NA | 76.8 ± 11 | 5 | 25 | NA | 70.7 ± 15 | 12 |
| Quartuccio et al. (36) | Italy | Retrospective study | H, L/R, D/C, S | 111 | 42 | 33 | 62.4 ± 11.8 | 4 | 69 | 44 | 56.2 ± 14.2 | 0 |
| Roumier et al. (17) | France | Retrospective study case control | S | 59 | 30 | 24 | 58.8 (12.4) | 3 | 29 | 24 | 71.2 (15.4) | 9 |
| Wadud et al. (44) | USA | Retrospective study case control | H,A, S | 94 | 44 | NA | 55.5 | 17 | 50 | NA | 66 | 26 |
| Ramaswamy et al. (42) | USA | Retrospective study | H,A, S | 86 | 21 | 13 | 63.2 (15.6) | 3 | 65 | 36 | 63.8 (15.9) | 8 |
| Ip et al. (37) | USA | Retrospective cohort study | H,A, S | 547 | 134 | 99 | 62 (53–70) | 62 | 413 | 257 | 69 (58–77) | 231 |
| Somers et al. (47) | USA | Prospective study cohort study | H, R*, S | 154 | 78 | 42 | 55 ± 14.9 | 18 | 76 | 40 | 58 ± 14.9 | 36 |
| Kewan et al. (38) | USA | Retrospective cohort study | H,A, S | 51 | 28 | 20 | 62 (53–71) | 3 | 23 | 11 | 70 (55–75) | 2 |
| Martinez-Sanz et al. (40) | Spain | Retrospective cohort study | H,A, S, L/R | 1,229 | 260 | 191 | 65 (55–76) | 61 | 969 | 574 | 68 (57–80) | 120 |
| Guaraldi et al. (36) | Italy | Retrospective cohort study | H, L/R, D/C, | 544 | 179 | 127 | 64 (54–72) | 13 | 365 | 232 | 69 (57–78) | 73 |
| Mikulska et al. (46) | Italy | Prospective study cohort study | H, L/R, D/C, S | 95 | 29 | 24 | 65.9 (10.2); | 6 | 66 | 41 | 73.5 (14.4) | 22 |
| Moreno Garcia et al. (35) | Spain | Retrospective study | H,A, S, L/R | 171 | 77 | 53 | 61.5 (12.4) | 8 | 94 | 59 | 61.4 (16) | 17 |
| Rojas-Marte et al. (43) | USA | Retrospective case control study | H,A, S, R* | 193 | 96 | 74 | 58.8 6 (13.6) | 43 | 97 | 63 | 62.0 6 (14) | 55 |
H, Hydroxychloroquine; L/R, Lopinavir/Ritonavir; D/C, Darunavir/Covicistat; S, Steroid; A, Azithromycin; R*, Remdesivir; NA, Not available; SOC, Standard of care; TCZ, Tocilizumab.
Primary Outcome and Sensitivity Analysis
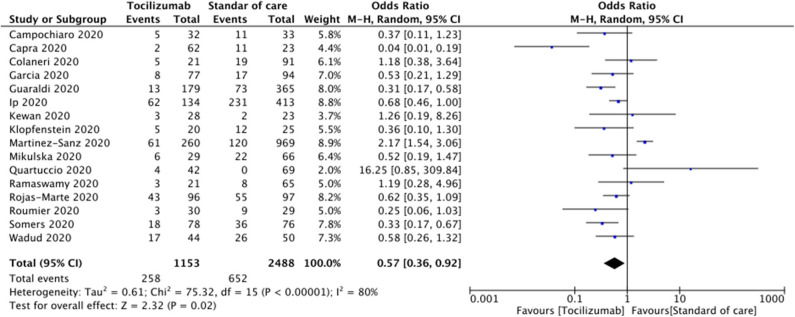
The mortality rate of COVID-19 patients in the TCZ group was 22.4% (258/1,153), and the mortality rate in the SOC group was 26.21% (652/2,488). The pooled odds ratio (OR) was 0.57 (95% CI 0.36–0.92; p = 0.02). Forest plot analysis of the primary outcome is shown in Figure 2. There was substantial heterogeneity among the included studies (I2 = 80%).
Figure 2.
Forest plot comparing patients treated with tocilizumab vs. standard of care alone.
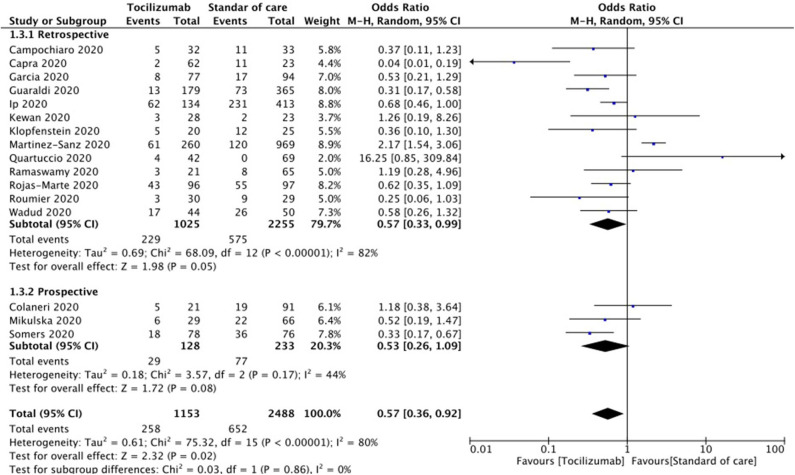
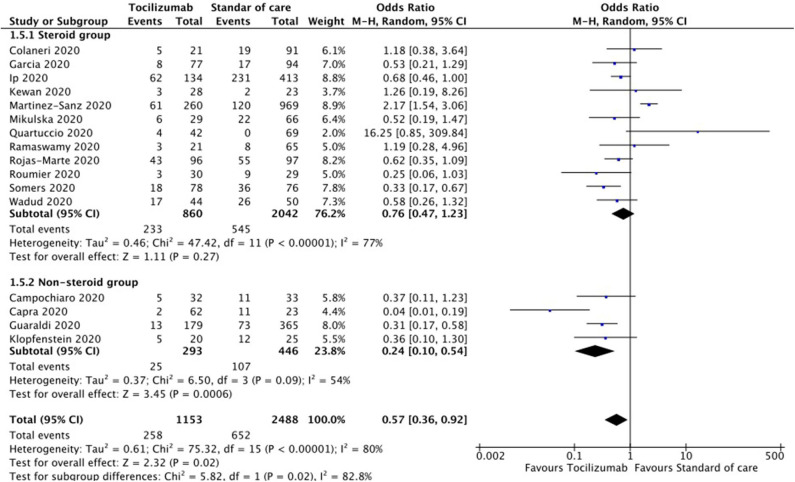
Sensitivity analysis by the exclusion of each study at a time showed no significant difference except when Capra et al. (34) was excluded; loss of statistical significance was observed [pooled OR 0.65 (95% CI 0.42–1.01), p = 0.060]. Sensitivity analysis of retrospective and prospective studies showed pooled OR 0.57 (95% CI 0.33–0.99; p = 0.05) and pooled OR 0.53 (95% CI 0.26–01.09; p = 0.08), respectively. Figure 3 shows forest plot analysis for the pooled estimations of retrospective and prospective studies. Sensitivity analysis of studies that used steroid as SOC and those that did not showed a pooled OR of 0.76 (95% CI 0.47–1.23, p = 0.27) and pooled OR of 0.24 (95% CI 0.10–0.54, p < 0.01), respectively. Figure 4 shows the forest plot analysis of sensitivity analysis for the use of steroids in the included studies. Further, sensitivity analysis of studies from Europe and USA showed a pooled OR of 0.52 (95% CI 0.23–1.17, p = 0.12) and a pooled OR of 0.61 (95% CI 0.46–0.79, p < 0.01), respectively. Supplementary Figure 1 shows a forest plot for sensitivity analysis of American and European studies. Quality assessment of included studies was done using the Cochrane risk-of-bias tool with parameters for each study and is shown in Supplementary Table 1.
Figure 3.
Forest plot for sensitivity analysis of retrospective and prospective studies.
Figure 4.
Forest plot for sensitivity analysis of steroid use in standard of care (SOC) (random-effects model).
Publication Bias
Analysis of publication bias using the funnel plot showed the clustering of studies toward the peak and median (Supplementary Figure 2). There were four studies that were outside of the funnel. Further analysis using Egger's test (p = 0.087) and Begg's test (Z = 0.09) suggested no significant publication bias.
Discussion
In this meta-analysis including 3,641 patients, the TCZ group had 3.81% less number of deaths when compared to SOC [n = 16; pooled OR 0.57 (95% CI 0.36–0.92), p = 0.02]. The analysis also showed that when steroids are used in the treatment of severe COVID-19, there was no statistical difference in mortality between TCZ and SOC [n = 12; pooled OR 0.76 (95% CI 0.47–1.23), p = 0.27]. However, when steroid was not used, the TCZ group had significantly low mortality when compared to the SOC group [n = 4; pooled OR 0.24 (95% CI 0.10–0.54), p < 0.01]. Sensitivity analysis showed that mortality trends in prospective studies suggest that the TCZ group did not show a significant difference when compared to SOC [n = 3; pooled OR 0.53 (95% CI 0.26–01.09), p = 0.08]. This is likely due to a lack of power since there were only three studies with the small study population. Possible reasons for substantial heterogeneity among the studies included in the meta-analysis (I2 = 80%) are the difference in the age and comorbidities, variability in the follow-up period, definition of death due to COVID-19, and response to treatment.
A recent meta-analysis by Lan et al. including 592 patients from seven retrospective studies, showed that the number of deaths in the TCZ group (16.3%) was lower than that in the SOC (24.1%) without statistical significance. The authors concluded that there was no conclusive evidence to suggest that TCZ provides an additional benefit in the treatment of severe COVID-19 (48). However, the study was limited by a small number of patients and did not compare TCZ and the use of steroids in the SOC. Our study included both prospective and retrospective studies with a significantly large number of studies and 3,641 patients. Sensitivity analysis allowed us to evaluate the potential beneficial effects of steroids.
Our meta-analysis shows that the addition of TCZ might significantly reduce mortality in severe COVID-19. However, when steroids are used in the SOC, the absence of a significant difference in mortality suggests the possible role of anti-inflammatory properties of steroids in the treatment of severe COVID-19. However, data available in the included studies were insufficient to deduce the effectiveness of steroids in the treatment of severe COVID-19. A recent clinical trial showed that dexamethasone reduced 28-day mortality in COVID-19 patients who are receiving respiratory support (49). Larger RCTs are needed to ascertain the benefit of steroids in the treatment of COVID-19. It should also be noted that the timing of TCZ administration in the TCZ group can make a significant difference. Administration of TCZ at the beginning of the cytokine storm would be beneficial than starting it when the cytokine storm is uncontrollable. We do not know the timing of TCZ and steroids administration. Moreover, in clinical practice, expensive treatment such as TCZ may be delayed over more economical options such as steroids. Therefore, larger RCTs are needed to evaluate the benefit of TCZ over steroids and their timing of administration.
It was also observed that studies from Europe showed no significant difference in mortality between the TCZ group and the SOC group [n = 10; pooled OR 0.52 (95% CI 0.23–1.17), p = 0.12]. However, studies from the USA did show lower mortality in the TCZ group when compared to SOC [n = 6; pooled OR 0.61 (95% CI 0.46–0.79), p < 0.01]. This could be related to an acute shortage of intensive care unit (ICU) beds in Italy and a rapid rise in COVID-19 cases in Italy, since most of the European studies were from Italy. This also highlights that the availability of resources can significantly affect the outcomes.
SARS-CoV-2, the causative organism of COVID-19, commonly enters the human body through inhalation of droplets (50). Once the virus comes in contact with respiratory mucosa, the spike (S) protein on the viral cell membrane fuses with the angiotensin-converting enzyme 2 (ACE2) receptors expressed on the mucosal cells, and the viral RNA enters the cell (51, 52). These infected cells undergo apoptotic changes and phagocytosed by macrophages and presented to antigen-presenting T cells, which in turn leads to CD4 T cell-dependent immune response and results in increased antibody production from B lymphocytes. Ultimately, the unrestricted release of inflammatory cytokines from the immune response leads to “cytokine release syndrome” (CRS). Patients with severe COVID-19 have been shown to have lymphocytopenia and increased plasma concentration of pro-inflammatory cytokines such as IL-6, IL-10, granulocyte colony-stimulating factor (G-CSF), monocyte chemoattractant protein 1 (MCP-1), and tumor necrosis factor (TNF) (53).
The role of TCZ in the treatment of COVID-19 is hypothesized based on its ability to block IL-6 activity, a pro-inflammatory cytokine that plays a significant role in the development of ARDS. In a prospective, single-arm, observational study by Xu et al. 21 patients were treated with TCZ. At the end of 5 days, there was a significant improvement in the inflammatory level of markers, lymphocytopenia, and interstitial lung changes—oxygen requirement reduced in all of the patients. None of the patients died (29). In another prospective, single-arm study by Toniati et al. 100 critically ill COVID-19 patients requiring mechanical ventilation were treated with TCZ. At follow-up at the end of 10 days, 77% of patients experienced significant improvement in respiratory condition and clearing of bilateral interstitial opacities on chest X-ray. The study showed overall mortality of 20%. In another single-arm, observational study, 63 hospitalized patients with COVID-19 treated with TCZ also had improvement in oxygen requirement, with an increased likelihood of survival (hazard ratio 2.2, 95% CI 1.3–6.7, p < 0.05) and 11% overall mortality. Several other studies showed that TCZ might have a beneficial effect in the treatment of COVID-19 in organ transplant recipients (54–56). Based on these encouraging results, several RCTs are underway to evaluate the efficacy of TCZ in COVID-19.
Although our study shows that there is no clear evidence to show the superiority of TCZ over SOC, nearly 45% less death in the TCZ group suggests a possible benefit in reducing the mortality in severe COVID 19. Future studies should consider evaluating TCZ against SOC and convalescent serum in the management of severe COVID-19. It is also important to note that TCZ may have a supporting role in combination with SOC, and this needs to be explored as well.
Our study has a few limitations that need to be noted; most of the included studies are retrospective, observational studies with only three prospective studies. Most of the included studies were from Italy and the United States. Therefore, the results may not be generalizable. The risk of selection bias is high since most studies did not report whether patients included in the study were consecutive. We were unable to estimate the effect of resource shortage on mortality since there was a reported shortage of ICUs to provide appropriate care for severe COVID-19 patients in Italy. Some of the severe COVID-19 patients were treated in the general ward. Mortality can be significantly affected by comorbidities, which is not evaluated in our meta-analysis. This may have played a role in patient mortality and, therefore, might have affected the outcomes. It should be noted that the administration of antiviral agents was not uniform, and not all patients received the same antiviral agents in either the TCZ group or SOC group. Therefore, the findings of this meta-analysis need to be validated in well-designed randomized clinical trials. Also, we were unable to determine which patients would benefit from TCZ and which patients do not. Lastly, a pooled analysis of studies with substantial heterogeneity is debatable. Large RCTs with sufficient power to evaluate the effectiveness of TCZ in the treatment of COVID-19 are needed. Also, there appears to be a role for steroids in the treatment of severe COVID-19. Future studies should evaluate TCZ vs. steroids in treating severe COVID patients in randomized clinical trials.
In conclusion, this systematic review and meta-analysis summarize that the addition of TCZ to SOC might reduce mortality in severe COVID-19. With no definitive treatment available, TCZ might be a choice in the treatment of severe COVID-19. Extensive, high-quality prospective studies and RCTs are urgently needed to evaluate the role of TCZ in the treatment of severe COVID-19.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
UB and HG contributed to the conception and design. UB and AN contributed to the literature search. UB, AN, and MG contributed to the first draft. All authors contributed to critical revision, editing, and gave final approval.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This manuscript has been released as a pre-print at medRxiv, Boregowda et al. (57).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.586221/full#supplementary-material
Forest plot for sensitivity analysis for the American and European studies.
Funnel plot analysis of publication bias.
Risk of Bias Assessment Tool for Non-randomized Studies (RoBANS) summary of risk of bias assessment.
References
- 1.Bogoch II, Watts A, Thomas-Bachli A, Huber C, Kraemer MUG, Khan K. Pneumonia of unknown aetiology in Wuhan, China: potential for international spread via commercial air travel. J Travel Med. (2020) 27:taaa008. 10.1093/jtm/taaa008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao S, Lin Q, Ran J, Musa SS, Yang G, Wang W, et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: A data-driven analysis in the early phase of the outbreak. Int J Infect Dis. (2020) 92:214–7. 10.1101/2020.01.23.916395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World health Organization WHO Coronavirus Disease (COVID-19). Dashboard: WHO; (2020). Available online at: https://covid19.who.int/ (accessed June 24, 2020). [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Zai J, Wang X, Li Y. Potential of large “first generation” human-to-human transmission of 2019-nCoV. J Med Virol. (2020) 92:448–54. 10.1002/jmv.25693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. (2020) 5:335–7. 10.1016/S2468-1253(20)30048-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perisetti A, Gajendran M, Boregowda U, Bansal P, Goyal H. COVID-19 and gastrointestinal endoscopies: Current insights and emergent strategies. Dig Endosc. (2020) 32:715–22. 10.1111/den.13693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. (2020) 130:2620–9. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, et al. COVID-19: abnormal liver function tests. J Hepatol. (2020) 73:566–74. 10.1016/j.jhep.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boregowda U, Aloysius MM, Perisetti A, Gajendran M, Bansal P, Goyal H. Serum activity of liver enzymes is associated with higher mortality in COVID-19: a systematic review and meta-analysis. Front Med. (2020) 7:431. 10.3389/fmed.2020.00431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. (2020) 8:420–2. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. (2020) 20:669–77. 10.1016/S1473-3099(20)30243-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molina JM, Delaugerre C, Le Goff J, Mela-Lima B, Ponscarme D, Goldwirt L, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. (2020) 50:384 10.1016/j.medmal.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. (2020) 395:1569–78. 10.1016/S0140-6736(20)31022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. (2020) 92:814–8. 10.1002/jmv.25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roumier M, Paule R, Groh M, Vallee A, Ackermann F. Interleukin-6 blockade for severe COVID-19. medRxiv [Preprint]. (2020). 10.1101/2020.04.20.20061861 [DOI] [Google Scholar]
- 18.Park WY, Goodman RB, Steinberg KP, Ruzinski JT, Frank Radella I, Park DR, et al. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. (2001) 164:1896–903. 10.1164/ajrccm.164.10.2104013 [DOI] [PubMed] [Google Scholar]
- 19.Gao Y, Li T, Han M, Li X, Wu D, Xu Y, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. (2020) 92:791–6. 10.1002/jmv.25770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venkiteshwaran A. Tocilizumab. MAbs. (2009) 1:432–8. 10.4161/mabs.1.5.9497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alattar R, Ibrahim TBH, Shaar SH, Abdalla S, Shukri K, Daghfal JN, et al. Tocilizumab for the treatment of severe coronavirus disease 2019. J Med Virol. (2020) 92:2042–49. 10.1002/jmv.25964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alberici F, Delbarba E, Manenti C, Econimo L, Valerio F, Pola A, et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. (2020) 97:1083–8. 10.1016/j.kint.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borku Uysal B, Ikitimur H, Yavuzer S, Ikitimur B, Uysal H, Islamoglu MS, et al. Tociluzumab challenge: a series of cytokine storm therapy experience in hospitalized Covid-19 pneumonia patients. J Med Virol. (2020). 10.1002/jmv.26111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassoun A, Thottacherry ED, Muklewicz J, Aziz QU, Edwards J. Utilizing tocilizumab for the treatment of cytokine release syndrome in COVID-19. J Clin Virol. (2020) 128:104443. 10.1016/j.jcv.2020.104443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marfella R, Paolisso P, Sardu C, Bergamaschi L, D'Angelo EC, Barbieri M, et al. Negative impact of hyperglycaemia on tocilizumab therapy in Covid-19 patients. Diab Metab. (2020). 10.1016/j.diabet.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morena V, Milazzo L, Oreni L, Bestetti G, Fossali T, Bassoli C, et al. Off-label use of tocilizumab for the treatment of SARS-CoV-2 pneumonia in Milan, Italy. Eur J Intern Med. (2020) 76:36–42. 10.1016/j.ejim.2020.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sciascia S, Aprá F, Baffa A, Baldovino S, Boaro D, Boero R, et al. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol. (2020) 38:529–32. [PubMed] [Google Scholar]
- 28.Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun Rev. (2020) 19:102568. 10.1016/j.autrev.2020.102568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. (2020) 117:10970–5. 10.1073/pnas.2005615117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rilinger J, Kern WV, Duerschmied D, Supady A, Bode C, Staudacher DL, et al. A prospective, randomised, double blind placebo-controlled trial to evaluate the efficacy and safety of tocilizumab in patients with severe COVID-19 pneumonia (TOC-COVID): a structured summary of a study protocol for a randomised controlled trial. Trials. (2020) 21:470. 10.1186/s13063-020-04447-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maes B, Bosteels C, De Leeuw E, Declercq J, van Damme K, Delporte A, et al. Treatment of severely ill COVID-19 patients with anti-interleukin drugs (COV-AID): a structured summary of a study protocol for a randomised controlled trial. Trials. (2020) 21:468 10.1186/s13063-020-04453-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campochiaro C, Della-Torre E, Cavalli G, De Luca G, Ripa M, Boffini N, et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. (2020) 76:43–9. 10.1016/j.ejim.2020.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capra R, De Rossi N, Mattioli F, Romanelli G, Scarpazza C, Sormani MP, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Intern Med. (2020) 76:31–5. 10.1016/j.ejim.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreno Garcia E, Rico Caballero V, Albiach L, Aguero D, Ambrosioni J, Bodro M, et al. Tocilizumab is associated with reduction of the risk of ICU admission and mortality in patients with SARS-CoV-2 infection. medRxiv [Preprint]. (2020). 10.1101/2020.06.05.20113738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. (2020) 2:e474–84. 10.1016/S2665-9913(20)30173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ip A, Berry DA, Hansen E, Goy AH, Pecora AL, Sinclaire BA, et al. Hydroxychloroquine and tocilizumab therapy in COVID-19 patients - an observational study. PLoS ONE. (2020) 15:e0237693. 10.1371/journal.pone.0237693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kewan T, Covut F, Al-Jaghbeer MJ, Rose L, Gopalakrishna KV, Akbik B. Tocilizumab for treatment of patients with severe COVID-19: a retrospective cohort study. EClinicalMedicine. (2020) 24:100418. 10.1016/j.eclinm.2020.100418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klopfenstein T, Zayet S, Lohse A, Balblanc JC, Badie J, Royer PY, et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med Mal Infect. (2020) 50:397–400. 10.1016/j.medmal.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez-Sanz J, Muriel A, Ron R, Herrera S, Ron R, Perez-Molina JA, et al. Effects of tocilizumab on mortality in hospitalized patients with COVID-19: a multicenter cohort study. medRxiv [Preprint]. (2020). 10.1101/2020.06.08.20125245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quartuccio L, Sonaglia A, McGonagle D, Fabris M, Peghin M, Pecori D, et al. Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: results from a single Italian Centre study on tocilizumab versus standard of care. J Clin Virol. (2020) 129:104444. 10.1016/j.jcv.2020.104444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramaswamy M, Mannam P, Comer R, Sinclair E, McQuaid DB, Schmidt ML. Off-label real world experience using tocilizumab for patients hospitalized with COVID-19 disease in a regional community health system: a case-control study. medRxiv. (2020). 10.1101/2020.05.14.20099234 [DOI] [Google Scholar]
- 43.Rojas-Marte G, Khalid M, Mukhtar O, Hashmi AT, Waheed MA, Ehrlich S, et al. Outcomes in patients with severe COVID-19 disease treated with tocilizumab: a case-controlled study. QJM. (2020) 113:546–50. 10.1093/qjmed/hcaa206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wadud N, Ahmed N, Mannu Shergil M, Khan M, Krishna MG, Gilani A, et al. Improved survival outcome in SARs-CoV-2 (COVID-19) acute respiratory distress syndrome patients with Tocilizumab administration. medRxiv. (2020) 8:695 10.1101/2020.05.13.20100081 [DOI] [Google Scholar]
- 45.Colaneri M, Bogliolo L, Valsecchi P, Sacchi P, Zuccaro V, Brandolino F, et al. Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo Covid19 registry (SMACORE). Microorganisms. (2020) 8:695. 10.3390/microorganisms8050695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mikulska M, Nicolini LA, Signori A, Di Biagio A, Sepulcri C, Russo C, et al. Tocilizumab and steroid treatment in patients with severe Covid-19 pneumonia. medRxiv [Preprint]. (2020). 10.1101/2020.06.22.20133413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Somers EC, Eschenauer GA, Troost JP, Golob JL, Gandhi TN, Wang L, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. medRxiv [Preprint]. (2020). 10.1101/2020.05.29.20117358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lan SH, Lai CC, Huang HT, Chang SP, Lu LC, Hsueh PR. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents.(2020) 2020:106103. 10.1016/j.ijantimicag.2020.106103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. (2020). NEJMoa2021436. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kutti-Sridharan G, Vegunta R, Vegunta R, Mohan B, Rokkam PV. SARS-CoV2 in different body fluids, risks of transmission, and preventing COVID-19: a comprehensive evidence-based review. Int J Prev Med.. (2020) 11:97 10.4103/2008-7802.289268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. (2020) 395:565–74. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kopel J, Perisetti A, Gajendran M, Boregowda U, Goyal H. Clinical insights into the gastrointestinal manifestations of COVID-19. Dig Dis Sci. (2020) 65:1932–9. 10.1007/s10620-020-06362-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. (2020) 215:108427. 10.1016/j.clim.2020.108427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pereira MR, Mohan S, Cohen DJ, Husain SA, Dube GK, Ratner LE, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. (2020) 20:1800–8. 10.1111/ajt.15941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fontana F, Alfano G, Mori G, Amurri A, Lorenzo T, Ballestri M, et al. Covid-19 pneumonia in a kidney transplant recipient successfully treated with tocilizumab and hydroxychloroquine. Am J Transplant. (2020) 20:1902–6. 10.1111/ajt.15935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lauterio A, Valsecchi M, Santambrogio S, De Carlis R, Merli M, Calini A, et al. Successful recovery from severe COVID-19 pneumonia after kidney transplantation: the interplay between immunosuppression and novel therapy including tocilizumab. Transpl Infect Dis. (2020) 2020:e13334 10.1111/tid.13334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boregowda U, Perisetti A, Nanjappa A, Gajendran M, Goyal H. Addition of tocilizumab to the standard of care reduces mortality in severe COVID-19: a systematic review and meta-analysis. medRxiv [Preprint]. (2020). 10.1101/2020.07.10.20150680 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forest plot for sensitivity analysis for the American and European studies.
Funnel plot analysis of publication bias.
Risk of Bias Assessment Tool for Non-randomized Studies (RoBANS) summary of risk of bias assessment.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.