Abstract
Research has shown that getting to glycemic targets early on leads to better outcomes in people with type 2 diabetes; yet, there has been no improvement in the attainment of A1C targets in the past decade. One reason is therapeutic inertia: the lack of timely adjustment to the treatment regimen when a person’s therapeutic targets are not met. This article describes the scope and priorities of the American Diabetes Association’s 3-year Overcoming Therapeutic Inertia Initiative. Its planned activities include publishing a systematic review and meta-analysis of approaches to reducing therapeutic inertia, developing a registry of effective strategies, launching clinician awareness and education campaigns, leveraging electronic health record and clinical decision-support tools, influencing payer policies, and potentially executing pragmatic research to test promising interventions.
Diabetes affects 463 million adults worldwide, including more than 34 million Americans, the vast majority of whom have type 2 diabetes (1–3). The disease greatly increases risks of microvascular and macrovascular complications; an estimated 32% of people with diabetes have cardiovascular disease (CVD), 35% have diabetic eye disease, 20–40% have chronic kidney disease (CKD), and about 50% have peripheral neuropathy (1,4,5). Diabetes is also the seventh leading cause of death, both in the United States and worldwide (6,7). Direct and indirect costs of diagnosed diabetes were estimated to be $327 billion in the United States in 2017 (8), and, globally, medical expenditures on diabetes are estimated to be $760 billion and expected to increase to $845 billion by 2045 (1).
Landmark trials have demonstrated that getting to glycemic targets sooner leads to better outcomes and reduces risks of microvascular and macrovascular complications in people with type 2 diabetes (9–16). Achieving glycemic targets early in the disease trajectory is associated with maintaining lower A1C levels for longer periods (17,18). As the evidence base supporting early attainment of glycemic targets has expanded, so too has the therapeutic armamentarium, which now includes 12 different classes of glucose-lowering medications, as well as advanced diabetes-related technologies (19,20).
These developments have led to the publication of detailed clinical practice guidelines and consensus recommendations outlining individualized treatment algorithms and calling for shared decision-making by clinicians and people with diabetes (19,21–23). Despite implementation of evidence-based guidelines, however, there has been no corresponding improvement in reaching glycemic targets (24,25). Indeed, the achievement of individualized A1C targets declined from 69.8 to 63.8% between 2007 and 2014, and the proportion of people with an A1C >9% increased from 12.6 to 15.5% (Figure 1) (26).
FIGURE 1.
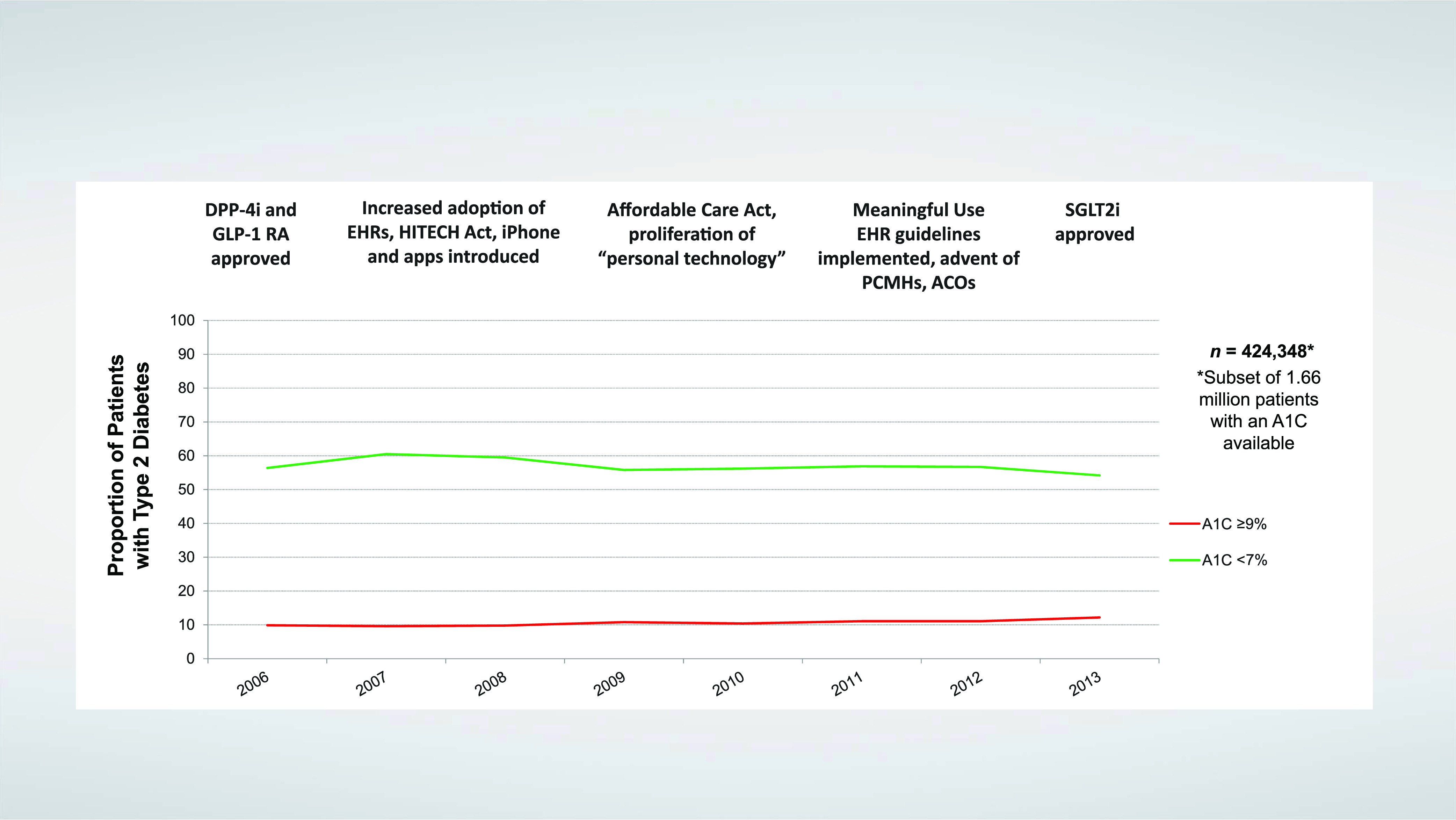
Type 2 diabetes trends in the United States, 2006–2013. Advances in health technologies, drug therapies, and public policy have not translated to improvements in diabetes care quality. ACO, accountable care organization; DPP-4i, dipeptidyl peptidase 4 inhibitor; GLP-1RA, glucagon-like peptide 1 receptor agonist; HITECH, Health Information Technology for Economic and Clinical Health; PCMH, patient-centered medical home; SGLT2i, sodium–glucose cotransporter 2 inhibitor. Adapted from ref. 24.
One of the underlying causes of this stagnation in progress toward improved diabetes outcomes is therapeutic inertia: the failure to advance therapy or to deintensify therapy when it is appropriate to do so (27). The term “therapeutic inertia” specifically refers to decisions regarding pharmacologic treatment (27), whereas the broader concept of “clinical inertia” refers to the underuse of interventions known to prevent negative outcomes and thus also encompasses care deficits such as lack of screening, risk assessment, preventive measures, attention to adherence barriers, and referrals (27,28). Both therapeutic inertia and clinical inertia result in a lack of timely adjustment to the treatment regimen when therapeutic targets are not met for people with diabetes and may impede their ability to attain optimal glycemia and achieve other relevant clinical goals.
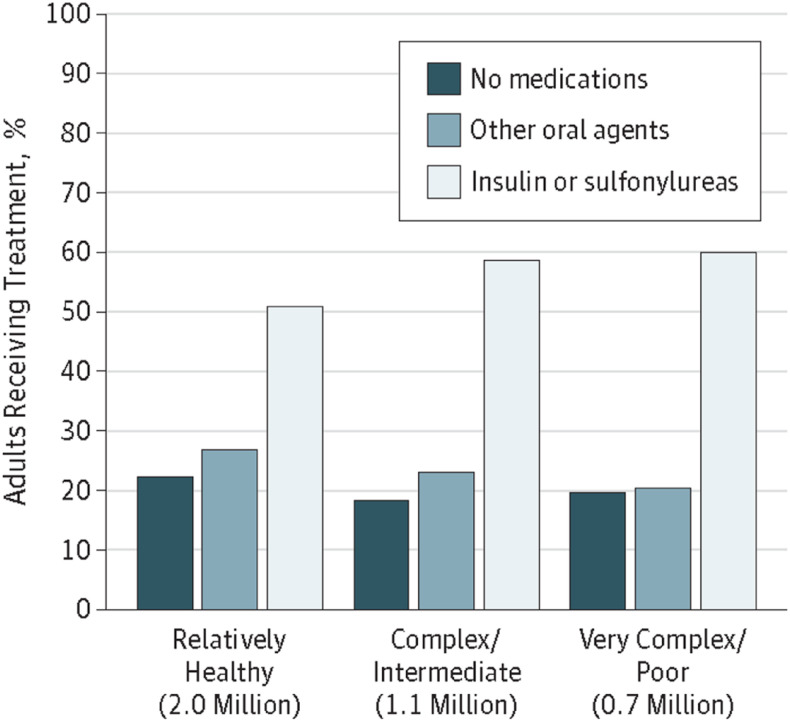
Therapeutic inertia is evident at all stages of type 2 diabetes treatment, from prescription of an initial antihyperglycemic agent to intensification with insulin (29). Numerous studies have documented delays in therapy intensification for people not meeting their glycemic targets (17,30–38). A 2016 systematic review found a median time to treatment intensification after an above-target A1C measurement of >1 year (range 0.3 to >7.2 years) (39). Delays in needed deintensification of therapy—another important aspect of diabetes therapeutic inertia—have also been well documented (Figure 2) (40,41). Furthermore, diabetes self-management education and support (DSMES), which is crucial to better equip people with diabetes to participate in the shared decision-making process and more successfully carry out their diabetes self-management plan (42,43), is underutilized (44,45).
FIGURE 2.
Treatment of older U.S. adults (≥65 years of age) with diabetes with an A1C <7% across health status categories. There was no statistical difference in type of treatment across health status categories among these adults (P = 0.43). The number of U.S. adults corresponding to older adults with diabetes with an A1C <7% in each health status category is indicated in millions of people. Reprinted with permission from Lipska et al. (40). ©2015 American Medical Association. All rights reserved.
Early therapeutic inertia reduces the likelihood of achieving glycemic targets later in the disease process (17). It also deprives people of the documented legacy effect through which early attainment of glycemic targets continues to reduce the development and progression of complications decades later (10,15,16). Retrospective cohort studies have found that delays in intensification of treatment to reduce hyperglycemia early on hasten diabetic retinopathy (46), cardiovascular events (47), and mortality (48).
Therapeutic inertia is a multifactorial problem arising from complex barriers encountered at the clinician, patient, and health system levels (49,50). Overcoming it will require the concerted effort of multiple stakeholder groups—including multidisciplinary diabetes care clinicians, people with diabetes, advocacy and research organizations, policymakers, payers, health systems, and the pharmaceutical and technology industries—to effect change at all levels of the diabetes care ecosystem (51,52).
Numerous efforts ranging in scope from modest to ambitious have been undertaken to overcome therapeutic inertia at all levels, many of which have been described recently (29). What is lacking is an overarching organizational structure to identify the most effective approaches and solutions and promote their use on a wider scale. To address this need, the American Diabetes Association (ADA) recently launched a 3-year initiative called Overcoming Therapeutic Inertia (OTI), the details of which are described below.
Taking Aim at Diabetes Therapeutic Inertia
The OTI Initiative was conceived to assist the greater community of diabetes stakeholders in developing practical, real-world solutions to this complex problem that is adversely affecting outcomes for people living with diabetes. Its overall goal is to promote the adoption of evidence-based practices, strategies, programs, and tools that address key determinants of therapeutic inertia in diabetes care, leading to more timely treatment modification and improved outcomes among adults with type 2 diabetes. More specific objectives and priorities include:
Improving understanding of therapeutic inertia and its impact on the health of people with diabetes (i.e., creating a sense of urgency with regard to achieving glycemic targets and other important clinical outcomes), particularly among primary care clinicians;
Helping clinicians recognize likely therapeutic inertia using existing systems and tools at their disposal;
Conducting research to identify and promote activities, skills, and methodologies that are associated with achieving clinical targets throughout the journey of a person living with diabetes;
Improving clinicians’ understanding of and adherence to ADA’s Standards of Medical Care in Diabetes (53), with a focus on appropriate and timely treatment intensification;
Developing and disseminating user-friendly decision-support tools for use by clinicians and people with diabetes;
Promoting the adoption and expansion of person-centered diabetes care and the development of individualized diabetes management plans; and
Identifying crucial systems-level barriers contributing to therapeutic inertia and facilitating long-term strategies to promote change through consensus-building and engagement with key stakeholders.
Although therapeutic inertia also occurs in specialty care settings, primary care clinicians provide care to the vast majority of people with diabetes. Therefore, the OTI Initiative seeks to address the barriers faced by the primary care clinicians on the front lines of most type 2 diabetes care, including physicians, nurse practitioners, physician assistants, diabetes care and education specialists, nurses, registered dietitian nutritionists, pharmacists, and medical assistants. The initiative will also include efforts to increase awareness of the concept and consequences of therapeutic inertia among medical and other health care professionals still engaged in their formal training programs. Although people with diabetes will certainly benefit from these efforts, we acknowledge that improving clinician competence at effective, timely treatment intensification is not sufficient to address the multifactorial challenges of therapeutic and clinical inertia. Clinicians must also be armed with skills, strategies, and resources to support improved engagement with people with diabetes, promote DSMES, and address patient-level barriers to meeting treatment recommendations. Payers and electronic health record (EHR) companies are also key stakeholders, and therapeutic inertia cannot be solved without understanding and addressing the contributors related to payer policies, EHR workflows, and data-sharing.
To meet these multiple objectives within the time frame of the initiative and thereby achieve its ambitious overarching goal of reducing therapeutic inertia in diabetes, the ADA has formed partnerships with several other leading medical professional groups. Representatives from the American Medical Group Association, the American Association of Nurse Practitioners, the American Pharmacists Association, and the Association of Diabetes Care & Education Specialists serve on the OTI Steering Committee or on one of its action teams. The initiative is also seeking strategic alliances with other medical professional societies; nonprofit organizations; and corporations with an interest in diabetes, including medical technology and digital health companies and medical insurance payers, as well as sponsorship and engagement from the diabetes pharmaceutical and device industries.
Three-Phase Action Plan
Development of the OTI Initiative was envisioned as a three-phase process designed first to align a wide spectrum of stakeholders, then to collect and assess existing information and set priorities, and finally to implement and evaluate practical solutions to accelerate diabetes management and improve long-term outcomes for people with type 2 diabetes.
Phase 1: Convening Stakeholders
The ADA began this process by hosting a full-day summit titled “Overcoming Therapeutic Inertia: Accelerating Diabetes Care FOR_LIFE” in November 2018. More than 100 professionals, including physician and nonphysician primary care clinicians, endocrinologists, diabetes care and education specialists, and representatives from professional organizations, health systems, government agencies, payer groups, the pharmaceutical and medical device industries, and patient advocacy groups participated, demonstrating a high level of interest in addressing this pervasive problem. Representatives from various stakeholder groups presented evidence and perspectives on the impact of, barriers leading to, and possible solutions for therapeutic inertia, with moderated discussions after each presentation. The OTI Initiative Steering Committee met the next day to review the presentations and feedback received during the event (Figure 3). From this event, key themes and recommendations for future directions emerged. These proceedings have been summarized elsewhere (54).
FIGURE 3.
Word cloud representation of feedback from the 2018 therapeutic inertia summit. Participants were asked to list the top three words that describe potential solutions to therapeutic inertia. The size of the words indicates the relative frequency with which they were mentioned (54).
Phase 2: Charting a Course
After the 2018 summit, the OTI Steering Committee and ADA staff commenced setting priorities, brainstorming possible solutions, and strengthening partnerships. The Steering Committee appointed four working groups to delve deeper into issues surrounding access for people with diabetes, practice optimization, research, and policy and partnerships (the latter of which was later incorporated into ADA’s existing advocacy and governmental affairs structures).
At a day-long meeting in October 2019, each of the three remaining working groups presented and led discussions on the priorities and proposed solutions it had identified to address therapeutic inertia within its respective topic area (i.e., access, practice optimization, or research). Committee members then reached consensus on a short but impactful list of priorities for the 3-year campaign. These included:
Identifying the most effective approaches for overcoming therapeutic inertia in clinical practice;
Increasing awareness of the value of achieving glycemic and other clinical targets early in the course of diabetes;
Improving understanding among diabetes care clinicians of ways they can reduce therapeutic inertia in their clinics;
Increasing primary care clinicians’ proficiency at adjusting therapy appropriately as needed throughout a person’s lifetime with diabetes;
Compiling and disseminating practical approaches and strategies to help primary care practices identify, using existing resources (e.g., EHRs), people who are likely to be experiencing therapeutic inertia and in need of attention; and
Reducing systemic barriers to timely diabetes therapy adjustment, including but not limited to restrictions on a person’s access to medications, devices, and services; a lack of physician access to current payer formularies at the point of care; and low utilization of DSMES services.
Phase 3: Implementing Solutions
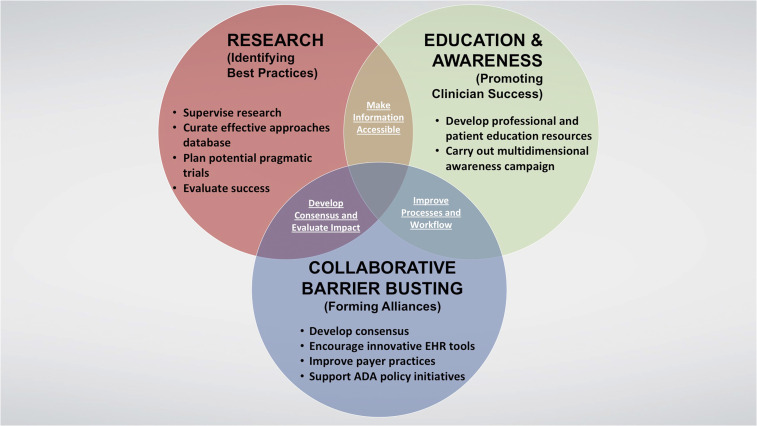
The final phase of the initiative, now underway, will involve implementing and continuously evaluating a multi-layered campaign to increase awareness of therapeutic inertia, provide critical resources and information for both clinicians and people with diabetes to promote more timely attainment of glycemic and other relevant clinical targets, and ultimately improve the lives of people with type 2 diabetes, as illustrated in Figure 4. The campaign will encompass numerous complementary activities with short-, intermediate-, and long-range impact. While some of these activities are still being refined, many are nearing roll-out and are described in the remainder of this article and summarized in Table 1.
FIGURE 4.
Framework for the ADA’s 3-year OTI campaign.
TABLE 1.
Summary of the 3-Year OTI Campaign
| Year | Education & Awareness | Research | Collaborative Barrier Busting |
|---|---|---|---|
| 2020: Foundation |
|
|
|
| 2021: Expansion |
|
|
|
| 2022: Depth |
|
||
OTI: A Multifaceted 3-Year Campaign
Gathering Evidence
With stakeholders aligned and a plan of action in hand, the OTI Initiative launched its implementation phase at the start of 2020. A key first step was to commission an independent research firm to conduct a comprehensive review of both evidence-based and practice-tested strategies to reduce therapeutic inertia directly or to ameliorate factors known to contribute to it. This crucial project, which will inform all subsequent efforts within the initiative, encompassed both a systematic review and meta-analysis of peer-reviewed literature and a more flexible and iterative landscape scan. The systematic review identified research-tested interventions aimed at or designed to influence the practices of primary care clinicians and endocrinologists who care for people with type 2 diabetes and the health care systems in which they work. The aim of the landscape scan was to glean insights from 1) pragmatic practice-tested diabetes-related interventions, including some that may not have been reported in the scientific literature; 2) programs focusing on other disease states that might be applicable to diabetes; 3) “gray literature” sources (i.e., publication entities such as government agencies, nonprofit organizations, academic centers, and private companies); and 4) relevant publicly available, Internet-based resources. The systematic review and landscape scan were nearing completion at the time of this writing, and their results were expected to be summarized in a white paper and submitted for publication in a peer-reviewed research journal later in 2020.
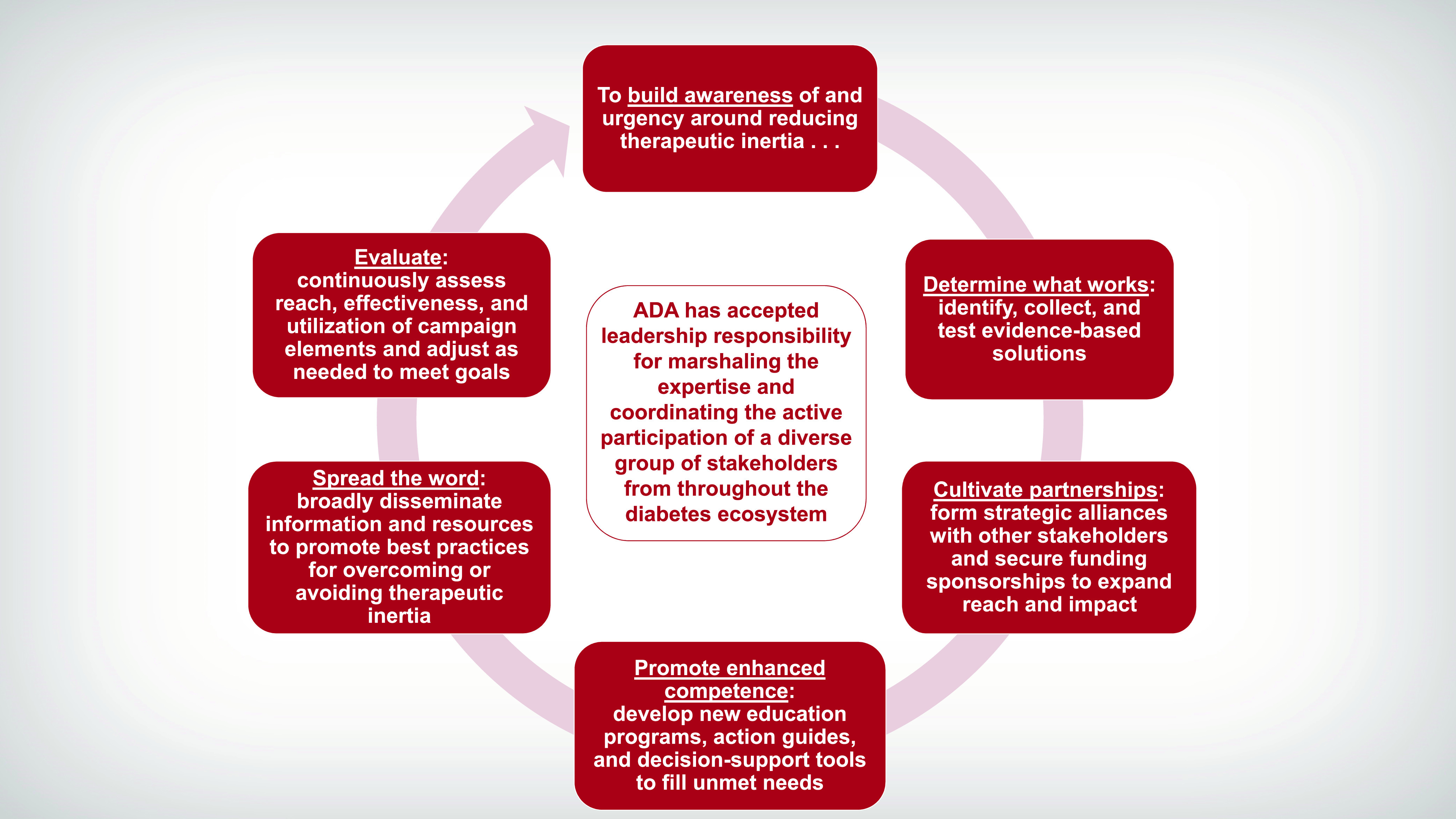
The OTI Steering Committee also reorganized its previous working groups into new teams focusing on three broad action categories: Education & Awareness, Research, and Collaborative Barrier Busting (Figure 5).
FIGURE 5.
The OTI Initiative’s action teams and their priorities.
Education & Awareness: Promoting Success in Primary Care
The campaign’s overall goals within this focus area are 1) to increase awareness of diabetes therapeutic inertia and build a sense of urgency around the need to reach glycemic targets earlier and improvement in other relevant clinical outcomes and 2) to provide primary care clinicians and other diabetes care professionals with strategies and tools to identify, assess, and combat therapeutic inertia in their own practices. To achieve these goals, the Education & Awareness Team is developing numerous resources, which will be freely available on an OTI Internet presence within the ADA’s DiabetesPro website, along with links to additional resources, educational programs, research summaries, and practice tools from other sources.
Among the new resources in development is a primary care–focused curriculum on overcoming diabetes therapeutic inertia. A series of professional education modules will include case-based presentations on topics such as 1) why clinicians should care about this vexing problem; 2) how to identify people with diabetes at risk for experiencing it; 3) tips for optimizing the medical office workflow to accelerate the pace of clinical target attainment; 4) guidance on the use of newer pharmacologic interventions (e.g., glucagon-like peptide 1 receptor agonists and sodium–glucose cotransporter 2 inhibitors) to positively affect clinical outcomes such as CVD, CKD, and heart failure (HF), and on the use of technologies such as continuous glucose monitoring to better assess regimen effectiveness; and 5) information on accessing DSMES services for people living with diabetes. A printable clinical action guide will accompany each module.
As the campaign progresses, the OTI website will also offer:
A customizable diabetes care plan to facilitate shared decision-making between clinicians and people with diabetes;
A series of short “practice pearls” videos from diabetes experts;
A succinct handout for diabetes care clinicians explaining what therapeutic inertia is, its causes, and why, if left unchecked, it will lead to long-term adverse outcomes for people with diabetes;
A brief self-assessment tool that clinicians can use to determine their own understanding of therapeutic inertia;
Access to additional assessment tools focused on patient-level barriers to achieving important clinical goals, including social determinants of health, diabetes distress, depression, and health literacy;
An engagement toolkit aimed at helping primary care clinicians equip their patients who have diabetes with the knowledge and skills they need to fully participate in their own diabetes treatment decisions and keep their clinical management efforts on track; and
If feasible with available resources, a free, online consumer guide tool that will allow people with diabetes and clinicians to search and compare hundreds of diabetes devices, supplies, and medications all in one place.
In 2021 and 2022, these materials will coalesce into a full awareness and professional education campaign involving the ADA’s OTI staff and volunteer leadership team, as well as partner organizations that are well positioned to expand its reach. The Education & Awareness Team will develop a series of continuing education webinars, tentatively planned to launch during the second half of 2020. The OTI volunteer leadership will also seek speaking and writing opportunities to deliver educational content on the topic and to present the findings of the systematic review, meta-analysis, and landscape scan. Additionally, OTI staff will set up exhibits at diabetes-related professional meetings to further awareness of and encourage participation in this effort.
To inform the awareness campaign, ADA has also commissioned a science-focused communications and marketing firm to define audiences, develop a messaging framework, and outline a strategy for spreading the word. Although components of the awareness campaign are still being refined and tested, its key messages likely will be similar to the following:
Getting to goal is possible—act now! Too many people with type 2 diabetes struggle to achieve and maintain glycemic targets in a timely manner, despite improved knowledge of the disease and a wealth of available treatment strategies and pharmacotherapies.
We can overcome diabetes therapeutic inertia. Primary care clinicians are uniquely positioned to break down barriers and help people with diabetes achieve glycemic and other clinical targets and lower their A1C early in the course of diabetes by following the latest ADA clinical practice guidelines and initiating appropriate pharmacotherapies and other management strategies in a timely manner.
The better we treat, the more we save. Replacing therapeutic inertia with timely action can spare people with diabetes from severe life-threatening complications and reduce the burden and financial costs of diabetes to individuals, health care systems, and society.
We’re all in this together. Making a lasting impact will require a concerted effort from people living with diabetes, clinicians, diabetes educators, social workers, mental health professionals, and other stakeholders. A multidisciplinary approach is the best model of care for people with diabetes, and the same approach will be needed to overcome therapeutic inertia.
Research: Identifying Best Practices
The OTI Initiative’s top research-related priorities are 1) to aid clinicians in identifying people with diabetes who may be experiencing therapeutic inertia and understanding the potential reasons why and 2) to help them more efficiently overcome barriers to attaining clinical goals, to the extent they can, through the use of existing resources to adjust therapeutic regimens in a timely and appropriate manner whenever needed. Although some formidable barriers such as financial insecurity, homelessness, or family dysfunction are intractable, others can be more easily overcome if given adequate attention. The OTI Research Team is charged with carrying out activities to aid clinicians in identifying and addressing these issues. Supervising the previously mentioned systematic review, meta-analysis, and landscape scan fell within the remit of this team, as does overseeing a commissioned market research survey of primary care clinician perceptions of the issue. This survey will assess baseline knowledge and attitudes, focusing not only on clinicians’ general understanding of therapeutic inertia as a concept, but also on whether and how they understand the problem to be manifested in their own practice.
The OTI is also laying the groundwork for the eventual launch of an online database of effective approaches for targeting therapeutic inertia. Members of the Research Team are developing inclusion criteria and a protocol for reviewing quality improvement projects, interventions, digital health solutions, products, and tools for possible inclusion in this searchable database, as well as a plan for expanding and curating it over time. The aim is to create a clearinghouse of successful ideas and programs, both from within the diabetes ecosystem and from other chronic disease care communities, that can be replicated or adapted for various clinical settings and populations.
Later in the 3-year initiative, this team will design and seek funding for at least one pragmatic clinical trial to test approaches and interventions for overcoming therapeutic inertia, possibly within a large health system or in a network of federally qualified health centers.
Finally, the Research Team is taking the lead in developing progress milestones and metrics for evaluating the success and overall impact of the 3-year OTI effort.
Collaborative Barrier Busting: Forming Alliances
At the systems level, the OTI Initiative is targeting problems of access, including 1) inadequate access to medications, devices, and services that have been shown to improve clinical outcomes for people with diabetes; 2) poor clinician access to up-to-date insurance formularies and inadequate decision-support tools at the point of care; and 3) suboptimal referral to and availability of DSMES services. Additionally, there is a need to optimize clinic processes and workflows to facilitate the timely achievement of glycemic targets and other relevant clinical goals in all people with type 2 diabetes.
To address these issues, the Collaborative Barrier Busting Team has been charged with exploring opportunities to leverage EHR functionality and influence payer policies to help clinicians make better and more efficient point-of-care treatment decisions and to remove barriers to access and facilitate the timely adoption of treatments by people with diabetes.
This effort will involve forming alliances with payer groups and demonstrating to them the potential financial benefits of encouraging more timely application of evidence-based treatments within the populations they serve. Working with representatives from payer organizations, this team will evaluate current practices with regard to copayment requirements, reimbursement limitations, preapproval policies, and formulary changes. The team and its payer partners will then work toward the development of consensus guidelines designed to encourage changes to any policies or procedures found to impede early and sustained disease control and prevention. The goals of this effort will be to improve access to medications, devices, and services that have been shown to improve clinical outcomes for people with diabetes; to ease administrative burdens on clinicians and their clinic staff; and to ensure that diabetes medical and education services are adequately reimbursed.
As part of this initiative, the OTI Initiative will seek opportunities to work with other consumer and professional organizations to eliminate short- and long-term barriers for people with diabetes and clinicians seeking access to diabetes technologies and devices. It hopes to create policy recommendations that will improve device access for individuals covered by Medicare or Medicaid, with the longer-term plan of leveraging the same proposals with private payers.
The Collaborative Barrier Busting Team will also strive to make a similar business case to EHR companies and to develop consensus recommendations that encourage innovations such improved deployment of real-time decision-support tools within existing EHR workflows and prompts for referral for DSMES services. Plans are underway to partner with one large EHR company to pilot-test integration of a decision-support and treatment intensification tool based on the treatment algorithm for type 2 diabetes depicted in the 2020 ADA Standards of Care. That algorithm advocates a person-centered, individualized approach and is designed, in part, to reduce therapeutic inertia (19).
Throughout the initiative’s 3-year span, the OTI Steering Committee, and particularly its Collaborative Barrier Busting Team, will also support ADA’s existing public policy and legislative programs aimed at accelerating the pace of optimizing diabetes care. These efforts include, among other strategies, advocating for expanded coverage for diabetes technologies and DSMES services; addressing high medication costs and other barriers to appropriate adoption and use by people with diabetes; advancing legislation to ensure that people with diabetes will have coverage for the most appropriate medications for their needs; and promoting the use of alternative care and education delivery formats such as telehealth, shared medical appointments, and online or digital coaching and therapeutics.
Conclusion
The OTI Initiative will serve as a catalyst for meaningful change throughout the diabetes ecosystem. Individually, each priority identified within the initiative’s three action categories aims to either accelerate the pace at which clinicians advance diabetes therapy or improve the ability of a person with diabetes to adopt and maintain an appropriate therapeutic regimen. Taken together, these strategies should increase the number of people achieving and maintaining an A1C <7% and decrease the percentage of those with an A1C >9%, while also reducing complications such as CVD, CKD, HF, and diabetic eye and nerve diseases. The ultimate goal is to reduce the incidence of chronic diabetes complications and mortality, lower their associated personal and societal costs, and improve long-term outcomes and quality of life for people living with type 2 diabetes.
Article Information
Acknowledgments
K.K. is supported by the National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands and the NIHR Leicester Biomedical Research Centre.The authors thank Sacha Uelmen, Director of Diabetes Education and Prevention Programs for the Association of Diabetes Care & Education Specialists, for her contributions to the OTI Initiative and her review of this manuscript.
Funding
The development of this article was supported by OTI Initiative Strategic Sponsors AstraZeneca and Sanofi and Supporting Sponsors Merck and Novo Nordisk
Dualities of Interest
R.A.G. is an employee of the American Diabetes Association and has served on advisory boards for Form Health, Health Reveal, Lark, Onduo, and Vida Health. C.B. is a consultant for and stock shareholder in Xeris Pharmaceuticals. T.H. is an employee of and stock shareholder in Novo Nordisk. N.D.K. is an employee of and stock shareholder in AstraZeneca. K.K. has received personal fees from Amgen, Bayer, Berlin-Chemie AG/Menarini Group, Napp Pharmaceuticals, Roche, and Sanofi and grants and personal fees from AstraZeneca, Boehringer Ingelheim, Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, Sanofi, and Servier. L.M. is a consultant for Applied Pharmaceuticals and Sanofi, serves on advisory boards for Novo Nordisk and Sanofi, and is a speaker for Novo Nordisk and Sanofi. E.M. serves on advisory boards for Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk, and Sanofi; is a speaker for Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, and Novo Nordisk; and has received research grants from Abbott and Pendulum. L.M.N. is a speaker for Abbott Diabetes Care, AstraZeneca, Janssen, Novo Nordisk, and Xeris Pharmaceuticals; consultant for Abbott Diabetes Care, Novo Nordisk, Sanofi, and Xeris Pharmaceuticals; and serves on advisory boards for Abbott Diabetes Care, Janssen, Novo Nordisk, Sanofi, and Xeris Pharmaceuticals. S.N.R. is an employee of and stock shareholder in Merck. P.S. is employed by the American Diabetes Association. No other potential conflicts of interest relevant to this work were reported.
Author Contributions
P.S. supervised the writing of the manuscript. All authors contributed to discussion, reviewed and edited the manuscript, and approved the final version for submission. This manuscript contains no data or data analysis, and, therefore, there is no guarantor of these.
References
- 1.Karuranga S, Malanda B, Saeedi P, Salpea P, Eds. IDF Diabetes Atlas. 9th ed. Brussels, Belgium, International Diabetes Federation, 2019. Available from https://www.diabetesatlas.org/en/. Accessed 26 February 2020 [Google Scholar]
- 2.Centers for Disease Control and Prevention National Diabetes Statistics Report 2020: Estimates of Diabetes and Its Burden in the United States. Available from https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed 25 February 2020
- 3.Centers for Disease Control and Prevention Type 2 diabetes. Available from https://www.cdc.gov/diabetes/basics/type2.html. Accessed 26 February 2020
- 4.Gheith O, Farouk N, Nampoory N, Halim MA, Al-Otaibi T. Diabetic kidney disease: world wide difference of prevalence and risk factors. J Nephropharmacol 2015;5:49–56 [PMC free article] [PubMed] [Google Scholar]
- 5.Hicks CW, Selvin E. Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr Diab Rep 2019;19:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Deaths and mortality. Available from https://www.cdc.gov/nchs/fastats/deaths.htm. Accessed 16 April 2020
- 7.World Health Organization Top 10 causes of death. Available from https://www.who.int/gho/mortality_burden_disease/causes_death/top_10/en. Accessed 16 April 2020
- 8.American Diabetes Association Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018;41:917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 10.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 11.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 12.Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 14.Duckworth W, Abraira C, Moritz T, et al.; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 15.Agrawal L, Azad N, Bahn GD, et al.; VADT Study Group . Intensive glycemic control improves long-term renal outcomes in type 2 diabetes in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care 2019;42:e181–e182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayward RA, Reaven PD, Wiitala WL, et al.; VADT Investigators . Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–2206 [DOI] [PubMed] [Google Scholar]
- 17.Mauricio D, Meneghini L, Seufert J, et al. . Glycaemic control and hypoglycaemia burden in patients with type 2 diabetes initiating basal insulin in Europe and the USA. Diabetes Obes Metab 2017;19:1155–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdul-Ghani MA, Puckett C, Triplitt C, et al. . Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes: results from the Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes (EDICT): a randomized trial. Diabetes Obes Metab 2015;17:268–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Diabetes Association 9. Pharmacological approaches: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S98–S110 [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association 7. Diabetes technology: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S77–S88 [DOI] [PubMed] [Google Scholar]
- 21.Davies MJ, D’Alessio DA, Fradkin J, et al. . Management of hyperglycemia in type 2 diabetes, 2018: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41:2669–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buse JB, Wexler DJ, Tsapas A, et al. . 2019 Update to: management of hyperglycemia in type 2 diabetes, 2018: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020;43:487–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garber AJ, Abrahamson MJ, Barzilay JI, et al. . AACE/ACE comprehensive diabetes management algorithm 2015. Endocr Pract 2015;21:438–447 [DOI] [PubMed] [Google Scholar]
- 24.Lipska KJ, Yao X, Herrin J, et al. . Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006–2013. Diabetes Care 2017;40:468–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khunti K, Ceriello A, Cos X, De Block C. Achievement of guideline targets for blood pressure, lipid, and glycaemic control in type 2 diabetes: a meta-analysis. Diabetes Res Clin Pract 2018;137:137–148 [DOI] [PubMed] [Google Scholar]
- 26.Carls G, Huynh J, Tuttle E, Yee J, Edelman SV. Achievement of glycated hemoglobin goals in the US remains unchanged through 2014. Diabetes Ther 2017;8:863–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khunti K, Davies MJ. Clinical inertia: time to reappraise the terminology? Prim Care Diabetes 2017;11:105–106 [DOI] [PubMed] [Google Scholar]
- 28.Allen JD, Curtiss FR, Fairman KA. Nonadherence, clinical inertia, or therapeutic inertia? J Manag Care Pharm 2009;15:690–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khunti S, Khunti K, Seidu S. Therapeutic inertia in type 2 diabetes: prevalence, causes, consequences and methods to overcome inertia. Ther Adv Endocrinol Metab 2019. Epub (doi: 10.1177/2042018819844694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah BR, Hux JE, Laupacis A, Zinman B, van Walraven C. Clinical inertia in response to inadequate glycemic control: do specialists differ from primary care physicians? Diabetes Care 2005;28:600–606 [DOI] [PubMed] [Google Scholar]
- 31.Khunti K, Godec TR, Medina J, et al. . Patterns of glycaemic control in patients with type 2 diabetes mellitus initiating second-line therapy after metformin monotherapy: retrospective data for 10,256 individuals from the United Kingdom and Germany. Diabetes Obes Metab 2018;20:389–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu AZ, Qiu Y, Davies MJ, Radican L, Engel SS. Treatment intensification in patients with type 2 diabetes who failed metformin monotherapy. Diabetes Obes Metab 2011;13:765–769 [DOI] [PubMed] [Google Scholar]
- 33.Mata-Cases M, Franch-Nadal J, Real J, et al. . Therapeutic inertia in patients treated with two or more antidiabetics in primary care: factors predicting intensification of treatment. Diabetes Obes Metab 2018;20:103–112 [DOI] [PubMed] [Google Scholar]
- 34.Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care 2013;36:3411–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meneghini LF, Mauricio D, Orsi E, et al.; DUNE Investigators . The Diabetes Unmet Need with Basal Insulin Evaluation (DUNE) study in type 2 diabetes: achieving HbA1c targets with basal insulin in a real-world setting. Diabetes Obes Metab 2019;21:1429–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stone MA, Charpentier G, Doggen K, et al.; GUIDANCE Study Group . Quality of care of people with type 2 diabetes in eight European countries: findings from the Guideline Adherence to Enhance Care (GUIDANCE) study. Diabetes Care 2013;36:2628–2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khunti K, Damci T, Meneghini L, Pan CY, Yale JF; SOLVE Study Group . Study of Once Daily Levemir (SOLVE™): insights into the timing of insulin initiation in people with poorly controlled type 2 diabetes in routine clinical practice. Diabetes Obes Metab 2012;14:654–661 [DOI] [PubMed] [Google Scholar]
- 38.Khunti K, Nikolajsen A, Thorsted BL, Andersen M, Davies MJ, Paul SK. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab 2016;18:401–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khunti K, Gomes MB, Pocock S, et al. . Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab 2018;20:427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med 2015;175:356–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hambling CE, Seidu SI, Davies MJ, Khunti K. Older people with type 2 diabetes, including those with chronic kidney disease or dementia, are commonly overtreated with sulfonylurea or insulin therapies. Diabet Med 2017;34:1219–1227 [DOI] [PubMed] [Google Scholar]
- 42.American Diabetes Association Facilitating behavior change and well-being to improve health outcomes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S48–S65 [DOI] [PubMed] [Google Scholar]
- 43.Powers MA, Bardsley J, Cypress M, et al. . Diabetes self-management education and support in type 2 diabetes: a joint position statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics. Diabetes Care 2015;38:1372–1382 [DOI] [PubMed] [Google Scholar]
- 44.Strawbridge LM, Lloyd JT, Meadow A, Riley GF, Howell BL. Use of Medicare’s diabetes self-management training benefit. Health Educ Behav 2015;42:530–538 [DOI] [PubMed] [Google Scholar]
- 45.Li R, Shrestha SS, Lipman R, Burrows NR, Kolb LE, Rutledge S; Centers for Disease Control and Prevention (CDC) . Diabetes self-management education and training among privately insured persons with newly diagnosed diabetes: United States, 2011–2012. MMWR Morb Mortal Wkly Rep 2014;63:1045–1049 [PMC free article] [PubMed] [Google Scholar]
- 46.Osataphan S, Chalermchai T, Ngaosuwan K. Clinical inertia causing new or progression of diabetic retinopathy in type 2 diabetes: a retrospective cohort study. J Diabetes 2017;9:267–274 [DOI] [PubMed] [Google Scholar]
- 47.Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol 2015;14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laiteerapong N, Ham SA, Gao Y, et al. . The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (the Diabetes & Aging Study). Diabetes Care 2019;42:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross SA. Breaking down patient and physician barriers to optimize glycemic control in type 2 diabetes. Am J Med 2013;126(Suppl. 1):S38–S48 [DOI] [PubMed] [Google Scholar]
- 50.Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med 2012;29:682–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zafar A, Stone MA, Davies MJ, Khunti K. Acknowledging and allocating responsibility for clinical inertia in the management of type 2 diabetes in primary care: a qualitative study. Diabet Med 2015;32:407–413 [DOI] [PubMed] [Google Scholar]
- 52.Okemah J, Peng J, Quiñones M. Addressing clinical inertia in type 2 diabetes mellitus: a review. Adv Ther 2018;35:1735–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.American Diabetes Association Introduction: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S1–S2 [DOI] [PubMed] [Google Scholar]
- 54.American Diabetes Association Summary of Proceedings of the American Diabetes Association Summit “Overcoming Therapeutic Inertia: Accelerating Diabetes Care FOR_LIFE.” Arlington, VA, American Diabetes Association, 2019 [Google Scholar]