Background
Before the discovery of insulin in 1922, treatment of diabetes was limited to palliative methods along with carbohydrate restriction. Bouchardat observed resolution of glycosuria with calorie-controlled diets as well as intermittent fasts (1). From 1914 to 1919, Dr. Frederick Allen placed patients on severely restricted caloric diets (≤500 kcal/day), and that approach was adopted to some extent by Dr. Elliott Joslin until the advent of insulin therapy (1). The biggest issue then was patient compliance, which continues to be important in diabetes management today (1).
Around the time insulin was discovered, the ketogenic diet was promoted to treat epilepsy in children (2). The ketogenic diet can be defined as an eating pattern that includes <50 g carbohydrate per day or in which carbohydrates comprise <10% of calories consumed. In contrast, a low-carbohydrate diet includes 51–130 g carbohydrate per day or ≤25% of calories consumed (3). This case report describes the incorporation of the ketogenic diet in a youth with type 1 diabetes.
Case Presentation
A 14-year-old girl presented to our hospital in February 2018 for follow-up of type 1 diabetes after disagreement with her diabetes care provider regarding her choice of the ketogenic diet. She was diagnosed with type 1 diabetes in February 2014, presenting with polyuria, polydipsia, and weight loss. She was admitted to a teaching hospital with an A1C of 13.3% and sent home on basal-bolus multiple daily injection insulin therapy. Her medical history was unremarkable, and there was no history of other autoimmune-associated disorders. Since March 2015, her diabetes had been managed with an Omnipod pump. At her initial visit, she was on 0.6 units/kg/day of insulin, with a 66% basal rate. Her most recent visit in December 2019 revealed a total daily dose of 0.75 units/kg/day and a basal rate of 77.5%. Over the course of 2 years, the patient’s A1C levels had ranged from 5.5 to 7.7%; her cholesterol and other laboratory values were normal (Table 1).
TABLE 1.
Laboratory Test Values
| Test | Date | Value | Normal Range |
|---|---|---|---|
| A1C, % | 12/18/2019 | 6.4 | 3.4–6.1 |
| 08/26/2019 | 6.3 | ||
| 05/15/2019 | 5.5 | ||
| 02/13/2019 | 6.8 | ||
| 10/03/2018 | 7.3 | ||
| 05/14/2018 | 7.1 | ||
| 02/14/2018 | 7.7 | ||
| Total cholesterol, mg/dL | 05/15/2019 | 176 | 125–211 |
| 05/14/2018 | 213 | ||
| HDL cholesterol, mg/dL | 05/15/2019 | 75 | 28–79 |
| 05/14/2018 | 84 | ||
| LDL cholesterol, mg/dL | 05/15/2019 | 81 | 50–130 |
| 05/14/2018 | 108 | ||
| VLDL cholesterol, mg/dL | 05/15/2019 | 15 | 2–23 |
| 05/14/2018 | 31 | ||
| Endomysial antibody screen | 05/15/2019 | Negative | NA |
| 05/14/2018 | Negative | ||
| Tissue transglutaminase IgA, units/mL | 05/14/2018 | 0.4 | 0–10 |
| 05/15/2019 | 0.3 | ||
| Triglycerides, mg/dL | 05/15/2019 | 74 | 36–129 |
| 05/14/2018 | 153 | ||
| Thyroid-stimulating hormone, µU/mL | 05/15/2019 | 2.21 | 0.51–4.91 |
| 05/14/2018 | 2.31 |
NA, not applicable.
The patient began the ketogenic diet in July 2017 after extensive research. Her diet included 35 g carbohydrate per day (10–12 g at breakfast, 10 g at lunch, and 7–14 g at dinner). She noted vast subjective improvements on this diet, with statements such as: “I feel more in control and less discouraged when I had fluctuating blood sugars,” “I do not have to stuff myself with rescue carbs when my blood sugars are low and only need 7 g when my blood sugar is 50,” and “Exercise affects me much less, which makes me feel safer.” She summarized by explaining how the diet had helped her blood glucose levels, resulting in her feeling “more steady, more in control, empowered, safe, [and having] less brain fog with highs and lows, less worry, and more predictable.”
Questions
Is the ketogenic diet appropriate for patients with type 1 diabetes?
If so, is there a certain age-group in which the diet would be most successful?
Are there present or future physiologic/laboratory abnormalities secondary to the ketogenic diet?
Are there psychosocial barriers to applying the ketogenic diet?
Commentary
In a meta-analysis on the effect of low-carbohydrate diets on type 1 diabetes management, Turton et al. (3) concluded that no overall effect could be determined because of the heterogeneity of the studies. Most studies relating to the ketogenic diet have involved adults. Ranjan et al. (4) noted that a low-carbohydrate diet (<50 g/day) resulted in more euglycemia, less time in hypoglycemia, and less glucose variability compared with a high-carbohydrate diet. In their study, Lennerz et al. (5) noted that “exceptional” glycemic control of type 1 diabetes (mean A1C 5.67%) with low rates of adverse events (2% diabetes-related hospitalizations: 1% for diabetic ketoacidosis and 1% for hypoglycemia) were reported by a community of children and adults who consumed a very-low-carbohydrate diet (36 ± 5 g/day), but they but could not clearly generalize to a wider spectrum of patients with type 1 diabetes.
However, there are limitations and concerns regarding implementation of the ketogenic diet in adults, and even more so in children. Adherence to the diet is challenging. In a report by Nielsen et al. (6), even after adults with type 1 diabetes attended an educational class on dietary restriction, only 50% adhered to the program after 4 years (6). In addition, a case report in an adult with type 1 diabetes noted the development of dyslipidemia and increased episodes of hypoglycemia (7). de Bock et al. (8) noted that low carbohydrate consumption in growing children can lead to growth deficits, fatigue, and a higher lipid risk profile. Finally, McClean et al. (9) also did not recommend low-carbohydrate or ketogenic diets because of clinical risks of endocrine, metabolic, and—most importantly—psychological issues, as well as a lack of consensus regarding acceptable levels of ketosis in patients with type 1 diabetes, “making it challenging to discern diet-induced ketosis from early diabetic ketoacidosis.”
An additional issue of concern is that patients with type 1 diabetes who follow the ketogenic diet do not always tell their provider because of these controversial issues. Indeed, 27% of participants in the study by Lennerz et al. (5) did not discuss their adherence to the ketogenic diet with their diabetes care providers; of those who did, only 49% agreed or strongly agreed that their diabetes providers were supportive. Thus, improved communication is essential regarding diet and diabetes management.
Our patient came to us after having issues with her previous diabetes care providers. We agreed to assume care after discussing the various issues described above, including patient satisfaction and quality of life on the ketogenic diet and concerns regarding dyslipidemia and hypoglycemia. Growth and development were less of an issue because the patient was 14 years of age at her first visit. Her most recent A1C was 6.4%.
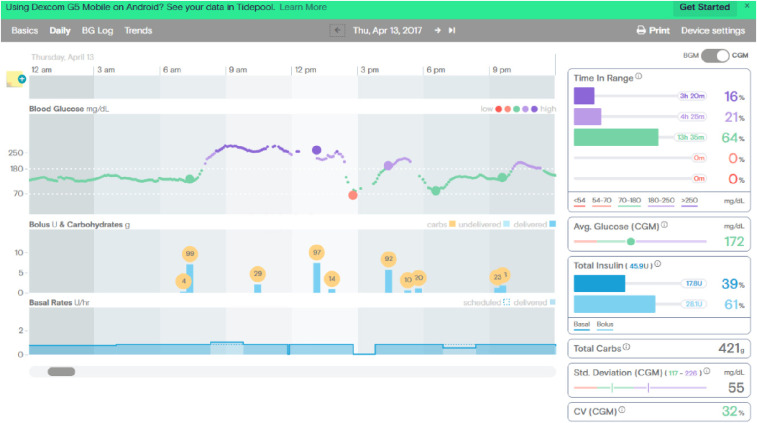
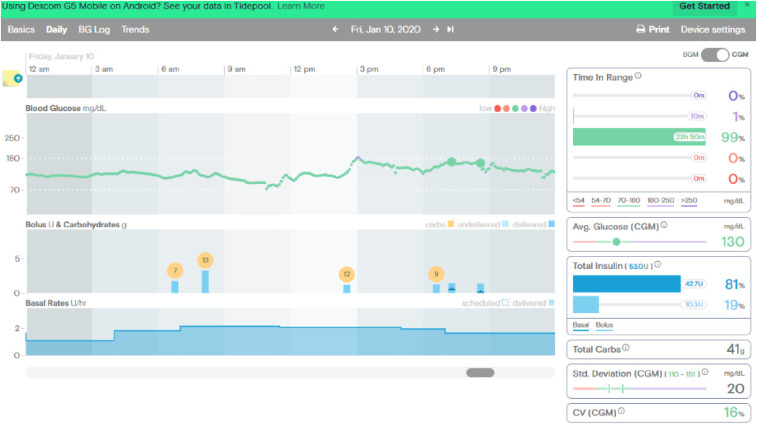
Before initiating the ketogenic diet, her continuous glucose monitoring (CGM) tracings showed time in range of 64% with 0% lows (Figure 1), whereas on the ketogenic diet, she was in range 99% of the time with 0% lows (Figure 2).
FIGURE 1.
CGM tracings before beginning the ketogenic diet.
FIGURE 2.
CGM tracings after beginning the ketogenic diet.
Her lipid profile had normalized as of her last visit, and her height and weight at that time were >97%, with a BMI of 21.48 kg/m2. There have been no episodes of diabetic ketoacidosis or significant hypoglycemia. Finally, the patient has consistently indicated great satisfaction and satiety on the ketogenic diet.
Clinical Pearls
The ketogenic diet should be considered as an option in the management of type 1 diabetes, even in youth. The diabetes team must at least be open to individualization and discussion of a family’s dietary preferences.
Both subjective and objective data must be evaluated by the diabetes team.
Communication is essential between the diabetes provider and the family.
Close follow-up is required to monitor patients’ growth, lipid profile, and psychosocial status. Most importantly, the patient must be willing to adhere to the diet.
Article Information
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Guarantor Author
As the sole author, F.R.C. is the guarantor of this work and, as such, had full access to all data reported and takes responsibility for the integrity of the data and the accuracy of the case presentation.
References
- 1.Schade DS, Santiago JV, Skyler JS, Rizza RA, Eds. Intensive Insulin Therapy. Princeton, NJ, Excerpta Medica, 1983, p. 2–3 [Google Scholar]
- 2.Mobbs CV, Mastaitis J, Isoda F, Poplawski M. Treatment of diabetes and diabetic complications with a ketogenic diet. J Child Neurol 2013;28:1009–1014 [DOI] [PubMed] [Google Scholar]
- 3.Turton JL, Raab R, Rooney KB. Low-carbohydrate diets for type 1 diabetes mellitus: a systematic review. PLoS One 2018;13:e0194987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranjan A, Schmidt S, Damm-Frydenberg C, Holst JJ, Madsbad S, Nørgaard K. Short-term effects of a low carbohydrate diet on glycaemic variables and cardiovascular risk markers in patients with type 1 diabetes: a randomized open-label crossover trial. Diabetes Obes Metab 2017;19:1479–1484 [DOI] [PubMed] [Google Scholar]
- 5.Lennerz BS, Barton A, Bernstein RK, et al. Management of type 1 diabetes with a very low-carbohydrate diet. Pediatrics 2018;141:e20173349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen JV, Gando C, Joensson E, Paulsson C. Low carbohydrate diet in type 1 diabetes, long-term improvement and adherence: a clinical audit. Diabetol Metab Syndr 2012;4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leow ZZX, Guelfi KJ, Davis EA, Jones TW, Fournier PA. The glycaemic benefits of a very-low-carbohydrate ketogenic diet in adults with type 1 diabetes mellitus may be opposed by increased hypoglycaemia risk and dyslipidaemia. Diabet Med 2018. Epub (DOI: 10.1111/dme.13663) [DOI] [PubMed] [Google Scholar]
- 8.de Bock M, Lobley K, Anderson D, et al. Endocrine and metabolic consequences due to restrictive carbohydrate diets in children with type 1 diabetes: an illustrative case series. Pediatr Diabetes 2018;19:129–137 [DOI] [PubMed] [Google Scholar]
- 9.McClean AM, Montorio L, McLaughlin D, McGovern S, Flanagan N. Can a ketogenic diet be safely used to improve glycaemic control in a child with type 1 diabetes? Arch Dis Child 2019;104:501–504 [DOI] [PubMed] [Google Scholar]