Abstract
Patients with advanced cancer suffer from psychosocial distress that may impair quality of life and that may be ameliorated by psychotherapeutic treatment. We describe here the methodology of a randomized controlled trial (RCT) to assess the effectiveness of a novel, brief, semi-structured psychotherapeutic intervention to reduce distress and increase well-being in patients with advanced or metastatic cancer. The intervention, called Managing Cancer and Living Meaningfully (CALM), was originally developed in Canada and we are now testing its Italian adaptation (CALM-IT).
The study is a single-blinded phase III RCT with assessment at baseline, 3 and 6 months with two conditions: CALM-IT versus a nonspecific supportive intervention (SPI). Eligibility criteria include: ≥ 18 years of age; fluency in the Italian language; no cognitive deficit, and diagnosis of advanced or metastatic cancer with an expected survival of 12–18 months. CALM-IT includes up to 12 sessions, delivered over 6 months and covers 4 domains: i) Symptom Management and Communication with Health Care Providers; ii) Changes in Self and Relations with Close Others; iii) Sense of Meaning and Purpose; and iv) the Future and Mortality. The primary outcome is difference in severity of depressive symptoms between treatment arm and the primary endpoint is 6 months. The secondary endpoint is 3 months and secondary outcomes are: generalized anxiety, distress about dying and death, demoralization, spiritual well-being, attachment security, posttraumatic growth, communication with partners, quality of life, and satisfaction with clinical care.
If shown to be effective, CALM-IT can be implemented nationally to relieve distress and to promote psychological well-being in patients with advanced cancer.
Keywords: CALM, End-of-life-intervention, Palliative care, Psychotherapy, Existential, Death anxiety, Clinical trial, Randomized controlled trial, Attachment security
List of abbreviations
- CALM
Managing Cancer and Living Meaningfully Psychotherapy
- CCS
Couple Communication Scale
- CEQ
Clinical Evaluation Questionnaire
- CA
Control arm
- DADDS
Death and Dying Distress Scale
- DS
Demoralization Scale
- ECR-M16
The Experience in Close Relationships Inventory Modified Short Term Version
- FACIT-Sp:
Functional Assessment of Chronic Illness Therapy- Spiritual Well-Being Scale
- GAD-7
Generalized Anxiety Disorder Questionnaire
- IA
Intervention arm
- MSAS-SF
Memorial Symptom Assessment Scale Short Form
- PHQ-9
Patient Health Questionnaire: Depression Module
- PTGI
Post-Traumatic Growth Inventory
- QUAL-EC
Quality of Life at the End of Life Cancer
- SPI
Supportive Psychosocial Intervention
1. Introduction
Engaging in life while facing impending mortality is a paradoxical phenomenon that has been termed “double awareness” [1]. The failure to accomplish this task in the context of advanced cancer, may lead to adverse psychological consequences including depression, severe death anxiety, demoralization, and existential distress [[2], [3], [4], [5], [6]]. In that regard, clinically significant depression has been reported in 16%–25% patients with advanced cancer, a three-fold higher prevalence than in the general population [7,8]. Depression in patients with cancer has been associated with reduced adherence to medication regimens, more prolonged hospitalizations, increased healthcare costs, and shorter survival [9,10].
Death-related anxiety is also frequent in advanced cancer and has been reported in 22%–55% of such patients, with fear of ‘running out of time’ [11] and dread of mortality [12] as central concerns. Distress of this kind has been understudied in palliative care [13,14], although it may affect treatment decisions, the capacity of patients to make sense of their situation, and be associated with depression [15]. It has been suggested that brief therapies incorporating spiritual well-being and evoking a sense of meaning may be most effective to relieve death anxiety in patients with advanced cancer [14].
Demoralization, a clinical state characterized by a sense of hopelessness and helplessness and a loss of meaning and purpose in life [16] is also common among patients with advanced cancer [17]. These effects may complicate adjustment at the end of life, in some cases resulting in a “psychological death” that occurs before the physical death [18]. Although a number of psychotherapeutic interventions have been developed for individuals and families facing advanced cancer [[19], [20], [21], [22]], none are yet routinely implemented in oncology settings as a standard of care.
A novel, brief, evidence-based intervention, called Managing Cancer and Living Meaningfully (CALM), has been recently developed by Rodin and collaborators in Toronto, Canada [[23], [24], [25]]. CALM is specifically tailored to the concerns and experiences of patients with advanced or metastatic cancer [26] and designed to reduce distress and increase well-being in this population. In preliminary pilot studies, CALM was found to decrease depression and anxiety and improve spiritual well-being and attachment security [23,25,27]. In a more recent, larger randomized controlled trial (RCT) with over 300 participants [28], CALM was shown to prevent and reduce depression, to decrease distress about dying and death, to increase preparation for the end of life, and to be associated with better communication with family and health care providers.
We believe that CALM offers the “common therapeutic language” [29] and flexibility required for a psychotherapeutic intervention to bridge cultural gaps and to maintain its value across human complexities and differences. However, it may also be that cultural differences in the doctor-patient relationship and communication, family, and social roles in the management of severe illness, and concepts of spirituality, faith and mortality should be taken into account in such interventions. [[30], [31], [32], [33], [34], [35], [36], [37], [38]]. We therefore conducted an unblinded phase II RCT in Ferrara, Italy to assess the feasibility, acceptability and preliminary efficacy of CALM in an Italian setting and to determine the sample size needed for a larger RCT [39]. The intervention consisted of 12 CALM sessions delivered twice monthly at the frequency of one session every second week. . Qualitative data from this study indicated that CALM-IT provides the experience of a secure relationship and reflective space in which illness-related issues and the end of life can be addressed. Quantitative findings demonstrated that CALM was associated with decreased depression, generalized anxiety, and death anxiety, and greater post-traumatic growth. This pilot study was an important starting point.
We now aim to test the effectiveness of CALM versus a non-manualized supportive psychosocial intervention (SPI) in two Italian centers in a single-blinded RCT. The primary outcome is difference in depressive symptoms in the two arms; secondary outcomes are demoralization, spiritual well-being, quality of life, posttraumatic growth, generalized anxiety, death anxiety, attachment security, communication with partners, and satisfaction with psychotherapy. Based on prior trial evidence [28] showing that effects strengthened over time and were strongest at 6 months, we chose the primary endpoint of six months (T2) and the secondary endpoint of three months (T1).
2. Methods
2.1. Study design
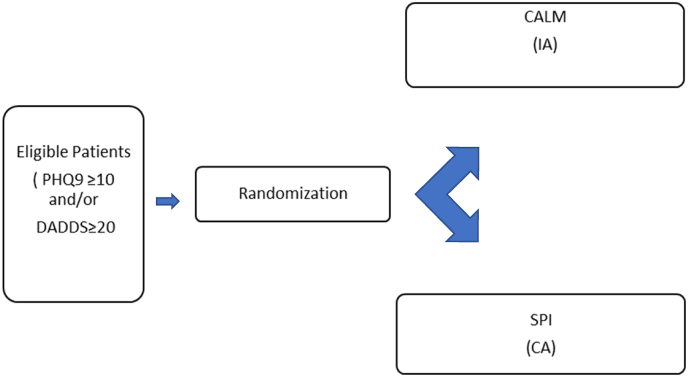
This study will be coordinated by the Psycho-Oncology and Psychiatry in Palliative Care Program, Institute of Psychiatry, University of Ferrara and the Integrated Department of Mental Health, S. Anna University Hospital/Health Trust, in Ferrara, Italy. Another Italian center from northern Italy will collaborate: the “Rita Levi Montalcini” Department of Neuroscience, University of Turin; The Clinical Psychology and Psycho-Oncology Unit, Department of Neuroscience, University of Turin, “Città della Salute e della Scienza” Hospital, Turin. This study substantially follows the methodology of a previously published German adaptation of CALM [40], the main differences are; 1) the use in the German study of the Distress Thermometer instead of the Death and Dying Distress Scale as a screening tool; 2) the number of sessions (8 for the German study and 12 for the Italian one), and 3) the use, in the German study, of the Brief Fatigue Inventory [41] as baseline and follow-up assessment tool. The present study is designed as a single-blinded phase III RCT with two arms, an intervention, and a control arm (Fig. 1), and has received approval from all relevant Ethics Committees. Participants in the intervention arm will receive the CALM-IT intervention, while those in the control arm will receive a non-specific supportive intervention (SPI). The latter consists of unstructured psychological support routinely delivered in our centers which will be delivered by psychosocial oncology clinicians who have not been trained in CALM. CALM-trained professionals in psychotherapy and psycho-oncology will deliver CALM-IT and, to avoid contamination, therapists in the control arm will not receive specific training in CALM. To ensure treatment fidelity, therapists will be supervised by RC, LG, GR, and CM and treatment integrity ratings will be completed using the CALM Treatment Integrity Measure utilized in the Canadian CALM RCT (28).
Fig. 1.
RCT design (CALM-IT RCT).
Legenda: IA: Intervention Arm; CA: Control Arm; CALM: Managing Cancer and Living Meaningfully Psychotherapy; SPI: Supportive Psychosocial Intervention.
2.2. Eligibility criteria
All patients who are referred for psychosocial support from any of the collaborating departments will be screened for eligibility. Inclusion criteria are: 1) 18 years of age or more; 2) fluency in Italian language; 3) absence of cognitive deficit as documented in the clinical records; 4) diagnosis of “wet” stage IIIB or IV lung cancer; any stage of pancreatic cancer, stage III or IV ovarian and fallopian tube cancers, or other stage IV gynecological cancer; and stage IV breast, genitourinary, gastrointestinal, melanoma, sarcoma, or endocrine cancers; 5) an expected survival of 12–18 months); 6) a score ≥10 on the Patient Health Questionnaire (PHQ9) and/or ≥ 20 on the Death and Dying Distress Scale (DADDS). Exclusion criteria are: 1) language barriers hindering psychotherapy; 2) inability to commit to the required 12 sessions; 3) concomitant psychotherapy.
2.3. Study procedures
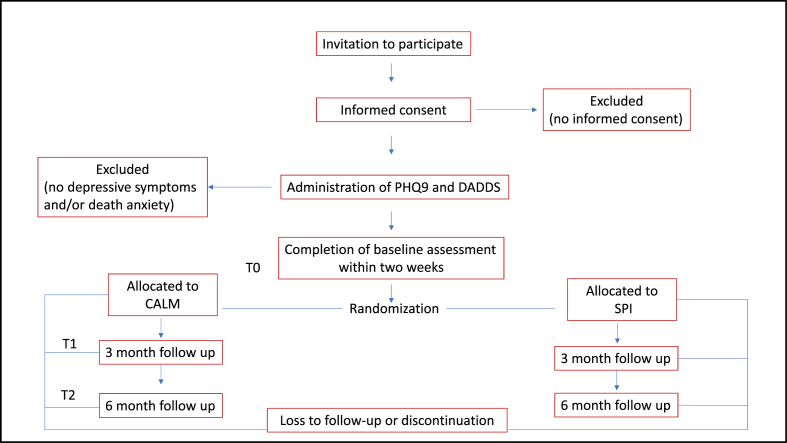
Patients with advanced cancer who are referred to the Psycho-Oncology Service will be identified and approached by research staff for study recruitment. As for all patients referred to the Psycho-Oncology Service, an unstructured interview will be conducted to determine whether any other intervention is needed. During the recruitment process, patients will be informed that they could be assigned on a random basis to one of two psychotherapeutic treatment arms, and the inclusion/exclusion criteria are reviewed. After providing informed consent, medical and demographic information is collected, baseline measures are administered, and eligible patients are randomized. The procedure will be the same for the two centers participating in the study. Fig. 2 shows the flow diagram of participants.
Fig. 2.
Flow diagram of participants.
All participants will complete all measures at baseline (T0), 3 months (T1) and 6 months (T2), except for the Clinical Evaluation Questionnaire (CEQ), which is administered T1 and T2 only.
At the enrollment phase, demographic (i.e. age, sex, education, marital and employment status) and medical data (i.e. site of disease, type and stage of cancer, past and current cancer and/or medical treatment) are collected using a standardized questionnaire and from the medical charts. The PHQ-9 and the DADDS are first administered to assess eligibility prior to study entry, followed by the remaining baseline measures.
2.4. Randomization
We will use simple randomization stratified by center with 1:1 allocation between arms. Computer-generated randomization for each center will be created, managed and protected by a password by L.Z., a researcher in the Institute of Psychiatry, Department of Biomedical and Specialty Surgical Sciences at University of Ferrara, who is independent from the research team. After having obtained written informed consent and administered baseline measures, research assistants will contact L.Z. by email to receive the subject's allocation, and inform patients about the therapist whom they will be seeing. No research team member will have access to the randomization list. Patients will be blinded to arm assignment. To check whether patients' blinding has been successful, we will ask participants during T2 assessment to which arm they believed they were allocated.
3. Trial conditions
3.1. CALM intervention
Twelve 45–60 minute sessions will be delivered twice monthly over a period of 6 months. Treatment fidelity will be ensured with regular supervision of therapists and assessed with the review of a random 10% of audio-recorded sessions and a treatment fidelity rating scale adapted from Spiegel and Spira [28,42].
According to the original CALM manual [43], the intervention will cover four domains:
-
1)
Symptom management and communication with health care providers, which addresses communication with health care providers and medical decision-making to ensure best care and control of symptoms.
-
2)
Changes in self and relations with close others, which targets self-esteem and identity and support needs in the face of cancer-related changes.
-
3)
Spirituality, sense of meaning and purpose, which facilitates the understanding of the personal meaning of suffering and dying, and the re-evaluation of priorities and goals while facing advanced cancer.
-
4)
Preparing for the future, sustaining hope and facing mortality, during which existential fears and concerns are acknowledged and balancing life and death, planning advanced treatment, and preparing the process of dying are addressed.
As in the German trial [40], patients decide in each CALM session which of these domains they want to discuss and to what extent each domain is covered. However, since the domains are conceptually and empirically intertwined, conversations about one of them typically evoke others. The amount of time dedicated to each domain varies, based on the most relevant concerns of each patient. Primary caregivers are invited to participate in one or more sessions as deemed appropriate by the patient and the therapist. Patients who show signs and/or symptoms of significant worsening of depression, or of suicidal risk or present other psychiatric co-morbidities which require treatment, are referred for psychiatric evaluation.
3.2. Unstructured supportive psycho-oncology intervention (SPI)
Patients randomized to the control arm will receive 12 sessions of SPI, at the same frequency as CALM. This therapeutic approach is based on an integrative approach consisting of psycho-oncological counseling, provision of information, crisis intervention as well as supportive individual therapy. As for the intervention arm, patients randomized to the control arm will receive up to twelve 50-min sessions delivered over 6 months.
3.3. Quality standards and therapists training
The CALM treatment manual [43] served as the framework for training in CALM. All study therapists are psychiatrists or clinical psychologists trained and certified in psychotherapy, with psycho-oncology expertise. CALM-IT therapists received comprehensive CALM training at the Ferrara coordinating center.
3.4. Primary outcome
The primary outcome measure is the PHQ-9 [44], a DSM-concordant, 9-item measure for
depression. A four-point Likert scale scores from 0 (not at all) to 3 (nearly every day), with a most consistently reported and recommended cut-off score of ≥10 suggesting depression [45]. For study purposes, two additional items assessing suicidal intent and interference of symptoms in one's life, were included. These additional items do not contribute to the total score of this measure and will not be included in our data analysis but will be used to trigger a risk assessment protocol. The Italian PHQ9 version [46] shows high internal consistency and discriminates well between individuals with and without depressive disorders for cut-offs of 9 and 10) [[46], [47], [48]].
3.5. Secondary outcomes
The secondary outcome measures are:
The Death and Dying Distress Scale (DADDS) [49] is a 15-item self-report measure assessing distress about death and dying, including distress about feeling like a burden to others and about wasted opportunities. It is scored on a six-point Likert scale from 0 (no distress) to 5 (very much distress), with higher scores indicating higher death anxiety and distress.
The Demoralization Scale (DS) [50] is a 24-item self-report tool assessing demoralization components of loss of meaning and purpose, dysphoria, disheartenment, and helplessness. Items are scored on a five-point Likert scale ranging from 0 (never) to 4 (all the time). Low levels of demoralization were indicated by a score 10, moderate demoralization by a score 11–36, and high demoralization by a score >37. The Italian version showed a good degree of stability and internal consistency [48, 51, 52].
The Generalized Anxiety Disorder Questionnaire (GAD-7) [53] is a 7-item self-report instrument screening the severity of GAD symptoms. Items are scored on a four-point Likert scale ranging from 0 (not at all) to 3 (nearly every day). Scores ≤4 indicate absence of anxiety, scores from 5 to 9 suggest that mild anxiety is present, and scores from 10 to 15 indicate moderate levels of anxiety. The Functional Assessment of Chronic Illness Therapy-Spiritual Well-Being Scale (FACIT-SP) [54] is a 12-item self-report tool, exploring spiritual well-being, in terms of sense of meaning, faith and inner peace. Items are scored on a five-point Likert scale from 0 (not at all) to 4 (very much), with higher scores indicating a greater spiritual wellbeing. The Italian validation study showed good internal consistency [55].
The Posttraumatic Growth Inventory (PTGI) [56] is a 21-item self-report measure of positive psychological changes after traumatic events. It consists of four subscales: New Possibilities, Appreciation of Life, Relating to Others and Spiritual Change. Items are scored on a three-point Likert scale ranging from 0 (not at all) to 2 (very much) with higher scores indicating greater post-traumatic growth. The Italian version of the GAD-7 was found to have good internal consistency [57].
The Quality of Life at the End of Life-Cancer Scale (QUAL-EC) [58] is a self-report measure of quality of life in patients near the end of life. It originally consists of four subscales: Symptom Control, Relationship with Health Care Providers, Preparation for End of Life and Life Completion. For this study purposes, the symptom control subscale will be not included in the data analyses, which will be based only on the remaining subscales (items 4–17). The summed score of the remaining subscales has been previously validated and referred to as the quality of life at the end of life [59]. Items are scored on a five-point Likert scale ranging from 1 (not at all) to 5 (completely), with higher scores indicating higher quality of life.
The Experiences in Close Relationships Inventory Modified Short Form Version (ECR-M16) [60], is a 16-item validated self-report measure of attachment security. The scale yields scores on two dimensions of attachment security: avoidance and anxiety. Items are scored on a seven-point Likert scale ranging from 1 (disagree) to 7 (agree), with a total sum score on each subscale ranging from 16 to 56. Higher scores on both subscales indicate less attachment security. The Italian version of ECR M16 has shown a high internal consistency [61].
Only patients who have a partner will complete the Couple Communication Scale (CCS) [62]. The CCS is a validated 10-item measure, taken from the PREPARE/ENRICH Inventory, that enquires about an individual's feelings, beliefs, and attitudes about the communication in his/her romantic relationship. Each item is scored from 1 (strongly disagree) to 5 (strongly agree).
Intervention and control participants will complete the CEQ [63] at 3 and 6-months. The CEQ is a seven-item self-report quantitative measure constructed for the Canadian CALM RCT [24], which assesses the amount of clinical benefit patients have experienced from therapy at the time of the assessment. Items are rated from 0 to 4, with 0 (no perceived benefit) and 4 (great perceived benefit). Satisfaction of patients will be qualitatively assessed by inviting them to write comments on a space under CEQ questionnaire.
Additional data collected are demographics, medical history, and the presence and severity of common cancer symptoms assessed by a shortened version of the original Memorial Symptom Assessment Scale (MSAS) [64]. symptom severity. The shortened version (MSAS- short form) [65] measures the presence and severity of 28 common physical symptoms of cancer. Items are scored on a five-point Likert scale ranging from 0 (not at all) to 4 (very much).
3.6. Confidentiality
All study-related information will be stored securely at the study site (Institute of Psychiatry, University of Ferrara, Ferrara coordinating center). All records that contain names or other personal identifiers, such as locator forms and informed consent forms, will be stored separately from study records identified by code number. All local databases will be secured with password-protected access systems. Data will be processed and treated according to General Data Protection Regulation, disciplining the protection of natural persons with regard to the processing of personal data. The General Data Protection Regulation is applicable to all European Union countries [66].
4. Statistical methods
4.1. Sample size calculation
To calculate the required sample size, we used a validated, manualized online power and sample size calculator that is ideal for longitudinal multilevel designs, GLIMMPSE [67]. A total sample size estimate for our primary hypothesis, that the treatment group (CALM) will demonstrate a greater improvement in depressive symptoms (PHQ-9) at follow-up periods, compared to the control group (SPI), was derived with a target power of 80% and alpha of .05 and with estimated mean scores, with variability, and cross-time correlations entered. For the primary hypothesis, the calculated total sample size is 124 patients (62 patients per treatment arm).
To account for anticipated attrition while maintaining the targeted power, we used the following equation to calculated an adjusted sample size: N = N0 * (1 + DRP), where N0 = original estimated sample size required at baseline; DRP = anticipated dropout rate across participants [68]. For an anticipated completion rate of 70% rate (30% dropout rate), the adjusted sample size for this attrition rate is N = 124 * (1 + .30) = 161.2. The adjusted sample size at baseline will therefore be 162, with 81 patients per treatment arm.
4.2. Data analysis
Statistical analyses will be carried out by using SPSS Statistics program [69]. For the final analyses we will use an intention-to-treat approach (ITT) and compare patients in the assigned treatment arms. To test the primary hypothesis that mean depression scores in the intervention arm will be lower than in the control arm at 3 and 6 months, we will use multilevel modeling (MLM) with maximum likelihood estimation to conduct the intent-to-treat analysis in testing hypotheses. MLM includes all participants, including those with missing data, in model estimation and also accounts for both inter-center and intra-center variability (i.e., hospital site from which patients were recruited and received assigned treatment; oncology clinic within hospital). Thus, this analytic approach has advantages over the ANCOVA analytic approach. To test for treatment-arm differences, we will compare the CALM and SPI groups on the primary outcome of PHQ-9 scores and on the secondary outcomes at the follow-up periods. This will entail the following set of MLM analyses:
-
i)
We will first test the level-1 predictor of Time, coded for the primary hypothesis as a categorical variable representing baseline and the 6-month follow-up period (primary endpoint). To code time for tests of secondary hypotheses, the categorical time variable will include baseline and the 3-month (secondary endpoint) and 6-month follow-up periods.
-
ii)
Next, we will add the level-2 treatment-group main effect and the cross-level treatment group × time interactions to test the hypothesized treatment-group differences at follow-up periods. Pairwise comparisons will evaluate the treatment-group difference at each follow-up period.
-
iii)
We will finally test whether the fixed effects in step ii change when any identified covariates are added to control for their effects.
As a sensitivity analysis, we will use multiple imputation to examine the influence of missing values. Lastly, prior trial results suggest that there may be arm differences in the processing of death-related distress such that individuals with moderate death anxiety tend to be more responsive to the intervention than either those with low death anxiety (approximately the lower third of the distribution) or those with high death anxiety (the upper third) [28]. We will conduct a sub-analysis to confirm this effect, by examining the effect of removing individuals with low and high death anxiety scores at baseline (i.e., DADDS < 15), following the Canadian protocol [24].
We will also qualitatively analyze the participants’ comments about CALM and SPI on the CEQ. NVivo Plus software package will be used for the qualitative analysis of data [70].
5. Discussion
The need to provide psychological care for individuals with advanced cancer care has been highlighted by a variety of international bodies, including the Worldwide Palliative Care Alliance and the World Health Organization [71], the European Association of Palliative Care [72] and the European Palliative Care Research Collaborative (EPCRC) [73], and the International Psycho-Oncology Society [74]. Tailored psychotherapeutic approaches for patients with advanced cancer may help to prevent and treat adverse psychological outcomes, to maintain optimal of quality of life and to infuse the end of life with meaning and purpose [75,76]. CALM has been developed to achieve these aims in this population.
CALM is built on a decade of theoretical and empirical work focusing on the impact of numerous factors including severity of physical symptoms, attachment style, and other interpersonal variables [7,[77], [78], [79]] affecting the risk of depression in patients with advanced cancer. This contrasts with Dignity Therapy, in which the focus is on enhancing the sense of legacy for individuals in the last months or weeks of life [80], CALM is intended for patients earlier in the course of disease to help them remain engaged with living, as they face the end of life. CALM addresses practical and relational issues related to cancer, including symptom management, role changes, and spiritual, existential, and mortality-related concerns. As detailed in the CALM treatment manual [43], the components of CALM that contribute to its therapeutic effects are the joint creation of meaning, the renegotiation of attachment security, the modulation of affects, and mentalization, all within an authentic supportive relationship.
Unique features of CALM include its foundation in relational, attachment and existential theory and its tailored focus on the specific problems of advanced cancer. The encouraging results in preliminary studies [25,27] and in a large randomized controlled trial in Toronto [28] suggest that CALM is a promising intervention for patients with advanced or metastatic cancer. Since culture molds the cognitive, emotional, and behavioural responses to cancer and cancer treatment, the awareness and knowledge of treatment options, and the acceptance of psychological interventions [[29], [30], [31], [32], [33], [34], [35], [36], [37], [38]] the application of a specific intervention such as CALM requires examination in specific cultural contexts. Currently, studies examining CALM are being carried out in Toronto, Canada, by Rodin and his research arm, and in collaboration with other investigators in Germany [40], United Kingdom, The Netherlands, China and Portugal.
6. Conclusion
This trial is being conducted to determine the effectiveness and benefit of CALM in an Italian cancer setting. If shown to be effective, a knowledge translation process will be undertaken to support the dissemination of CALM as a standard of care for advanced cancer patients in Italy.
Declarations
The work has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Authors' contributions
R.C., L.G., G.R., S.H. made substantial contributions to conception and design; R.C., L.G., G.R., C.M., M.G.N., S.S. L.Z, T.B., S.D. P., P.L., and M.B.M. have been involved in drafting the manuscript and revising it critically for important intellectual content. L.Z. and is following data curation; M.B.M will perform statistical analysis.
Each author has given final approval of the version to be published. Each author has agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
The study has received approval from the University Hospital and Health Authorities Ethics Committee of Ferrara, Italy. Any modifications to the protocol which may impact on the conduct of the study, potential benefit of the patient or may affect patient safety will be approved by the Ethics Committee prior to implementation.
Each participant subscribes an informed consent to take part in the study.
Consent for publication
All data from participants are provided in an anonymous way.
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
No funding was received.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
The Research Group in Ferrara (LG, RC, MGN) would like to express their gratitude to the University of Ferrara, the Global Institute of Psychosocial, Palliative and End-of-Life Care (GIPPEC), Unitalsi Triveneta and the Italian Medical Board/Association - Section of Ferrara (in the memory of Francesco Tomasi, M.D.), and the Associazione per il Supporto Psico-Oncologico (ASPO).
The Authors thank Dr. Kenneth Mah, from University Health Network, Toronto (UHN) for his precious contributions in the revision phase.
Contributor Information
Rosangela Caruso, Email: crsrng@unife.it, rosangela.caruso@unife.it.
Maria Giulia Nanni, Email: mariagiulia.nanni@unife.it.
Gary Rodin, Email: Gary.Rodin@uhn.ca.
Sarah Hales, Email: Sarah.Hales@uhn.ca.
Carmine Malfitano, Email: Carmine.Malfitano@uhnresearch.ca.
Silvia De Padova, Email: silvia.depadova@irst.emr.it.
Tatiana Bertelli, Email: tatiana.bertelli@irst.emr.it.
Martino Belvederi Murri, Email: martino.belvederimurri@unife.it.
Andrea Bovero, Email: abovero@cittadellasalute.to.it.
Marco Miniotti, Email: marco.miniotti@unito.it.
Paolo Leombruni, Email: paolo.leombruni@unito.it.
Luigi Zerbinati, Email: luigi.zerbinati@unife.it.
Silvana Sabato, Email: silvana.sabato@unife.it.
Luigi Grassi, Email: luigi.grassi@unife.it.
References
- 1.Rodin G., Zimmermann C. Psychoanalytic reflections on mortality: a reconsideration. J. Am. Acad. Psychoanal. Dyn. Psychiatr. 2008;36(1):181–196. doi: 10.1521/jaap.2008.36.1.181. [DOI] [PubMed] [Google Scholar]
- 2.Mystakidou K., Parpa E., Tsilika E., Athanasouli P., Pathiaki M., Galanos A., Pagoropoulou A., Vlahos L. Preparatory grief, psychological distress and hopelessness in advanced cancer patients. Eur. J. Canc. Care. 2008 Mar;17(2):145–151. doi: 10.1111/j.1365-2354.2007.00825.x. [DOI] [PubMed] [Google Scholar]
- 3.Bovero A., Botto R., Adriano B., Opezzo M., Tesio V., Torta R. Exploring demoralization in end-of-life cancer patients: prevalence, latent dimensions, and associations with other psychosocial variables Palliat Support Care. 2019 Oct;17(5):596–603. doi: 10.1017/S1478951519000191. [DOI] [PubMed] [Google Scholar]
- 4.Parpa E., Tsilika E., Galanos A., Nikoloudi M., Mystakidou K. Depression as mediator and or moderator on the relationship between hopelessness and patients' desire for hastened death. Support. Care Canc. 2019 Nov;27(11):4353–4358. doi: 10.1007/s00520-019-04715-2. Epub 2019 Mar 21. [DOI] [PubMed] [Google Scholar]
- 5.Bobevski I., Kissane D.W., Vehling S., McKenzie D.P., Glaesmer H., Mehnert A. Latent class analysis differentiation of adjustment disorder and demoralization, more severe depressive and anxiety disorders, and somatic symptoms in patients with cancer. Psycho Oncol. 2018 Nov;27(11):2623–2630. doi: 10.1002/pon.4761. Epub 2018 Jun 4. [DOI] [PubMed] [Google Scholar]
- 6.Diaz-Frutos D., Baca-Garcia E., García-Foncillas J. López-Castroman Predictors of psychological distress in advanced cancer patients under palliative treatments. J.Eur J Cancer Care (Engl). 2016 Jul;25(4):608–615. doi: 10.1111/ecc.12521. Epub 2016 Jun 8. [DOI] [PubMed] [Google Scholar]
- 7.Caruso R., Nanni M.G., Riba M., Sabato S., Mitchell A.J., Croce E., Grassi L. Depressive spectrum disorders in cancer: prevalence, risk factors and screening for depression: a critical review. Acta Oncol. 2017;56(2):146–155. doi: 10.1080/0284186X.2016.1266090. [DOI] [PubMed] [Google Scholar]
- 8.Caruso R., Nanni M.G., Riba M.B., Sabato S., Grassi L. Depressive spectrum disorders in cancer: diagnostic issues and intervention. A critical review. Curr. Psychiatr. Rep. 2017 Jun;19(6):33. doi: 10.1007/s11920-017-0785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd-Williams M., Shiels C., Taylor F., Dennis M. Depression--an independent predictor of early death in patients with advanced cancer. J. Affect. Disord. 2009;113:127–132. doi: 10.1016/j.jad.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Caruso R., Nanni M.G., Riba M.B., Sabato S., Grassi L. The burden of psychosocial morbidity related to cancer: patient and family issues. Int’l Rev Psychiatry. 2017;29(5):389–402. doi: 10.1080/09540261.2017.1288090. [DOI] [PubMed] [Google Scholar]
- 11.Vehling, Malfitano C., Shnall J., Watt S., Panday T., Chiu A., Rydall A., Zimmermann C.3, Hales S., Rodin G., Lo C. A concept map of death-related anxieties in patients with advanced cancer. BMJ Support. Palliat. Care. 2017 Dec;7(4):427–434. doi: 10.1136/bmjspcare-2016-001287. Epub 2017 Aug 2. [DOI] [PubMed] [Google Scholar]
- 12.Russac R.J., Gatliff G., Reece M. Death anxiety across the adult years: an examination of age and gender effects. Death Stud. 2007;31:549–561. doi: 10.1080/07481180701356936. [DOI] [PubMed] [Google Scholar]
- 13.Tong E., Deckert A., Gani N., Nissim R., Rydall A., Hales S., Rodin G., Lo C. Palliat Med. The meaning of self-reported death anxiety in advanced cancer. 2016 Sep;30(8):772–779. doi: 10.1177/0269216316628780. Epub 2016 Feb 8. [DOI] [PubMed] [Google Scholar]
- 14.Grossman C.H., Brooker J., Michael N., Kissane D. Death anxiety interventions in patients with advanced cancer: a systematic review. Palliat. Med. 2018 Jan;32(1):172–184. doi: 10.1177/0269216317722123. Epub 2017 Aug 8. [DOI] [PubMed] [Google Scholar]
- 15.Menzies R.E.1, Sharpe L.1, Dar-Nimrod I. The relationship between death anxiety and severity of mental illnesses. Br. J. Clin. Psychol. 2019 Nov;58(4):452–467. doi: 10.1111/bjc.12229. Epub 2019 Jul 18. [DOI] [PubMed] [Google Scholar]
- 16.Grassi L., Nanni M.G. Demoralization syndrome: new insights in psychosocial cancer care. Cancer. 2016;122(14):2130–2133. doi: 10.1002/cncr.30022. [DOI] [PubMed] [Google Scholar]
- 17.Tang P.L., Wang H.H., Chou F.H. A systematic review and meta-analysis of demoralization and depression in patients with cancer. Psychosomatics. 2015;56(6):634–643. doi: 10.1016/j.psym.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Rodin, G. Individual psychotherapy for the patient with advanced disease. In H. M. Chochinov& W. Breitbart (Eds.), Handbook of Psychiatry in Palliative Medicine 2009. (second ed., pp. 443–453). New York, NY: Oxford University Press.
- 19.Walker J., Sawhney A., Hansen C.H., Ahmed S., Martin P., Symeonides S., Murray G., Sharpe M. Review Treatment of depression in adults with cancer: a systematic review of randomized controlled trials. Psychol. Med. 2014;44(5):897–907. doi: 10.1017/S0033291713001372. [DOI] [PubMed] [Google Scholar]
- 20.Lemay K., Wilson K.G. Treatment of existential distress in life threatening illness: a review of manualized interventions. Clin. Psychol. Rev. 2008;28:472–493. doi: 10.1016/j.cpr.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Okuyama T., Akechi T., Mackenzie L., Furukawa T.A. Psychotherapy for depression among advanced, incurable cancer patients: a systematic reviewand meta-analysis. Canc. Treat Rev. 2017 May;56:16–27. doi: 10.1016/j.ctrv.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Teo I., Krishnan A., Lee G.L. Psychosocial interventions for advanced cancer patients: a systematic review. Psycho Oncol. May 2019 doi: 10.1002/pon.5103. [DOI] [PubMed] [Google Scholar]
- 23.Nissim R., Freeman E., Lo C., Zimmermann C., Gagliese L., Rydall A. Managing Cancer and Living Meaningfully (CALM): a qualitative study of a brief individual psychotherapy for individuals with advanced cancer. Palliat. Med. 2012;26(5):713–721. doi: 10.1177/0269216311425096. Epub 2011 Oct. [DOI] [PubMed] [Google Scholar]
- 24.Lo C., Hales S., Rydall A., Panday T., Chiu A., Malfitano C. Managing Cancer and Living Meaningfully: study protocol for a randomized controlled trial. Trials. 2015 doi: 10.1186/s13063-015-0811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo C., Hales S., Jung J., Chiu A., Panday T., Rydall A. Managing Cancer and Living Meaningfully (CALM): phase 2 trial of a brief individual psychotherapy for patients with advanced cancer. Palliat. Med. 2014;28(3):234–242. doi: 10.1177/0269216313507757. Epub 2013 Oct. [DOI] [PubMed] [Google Scholar]
- 26.Hales S., Lo C., Rodin G. Managing cancer and living meaningfully (CALM) therapy. In: Holland Jimmie C., Breitbart William S., Jacobsen Paul B., Loscalzo Matthew J., McCorkle Ruth, Butow Phyllis N., editors. Psycho-Oncology. Oxford University Press; New York: 2015. pp. 487–491. [Google Scholar]
- 27.Lo C., Hales S., Chiu A., Panday T., Malfitano C., Jung J. 2016 Jan 19. Managing Cancer and Living Meaningfully (CALM): Randomised Feasibility Trial in Patients with Advanced Cancer BMJ Support Palliat Care. pii: bmjspcare-2015-000866. [DOI] [PubMed] [Google Scholar]
- 28.Rodin G., Lo C., Rydall A., Shnall J., Malfitano C., Chiu A. Managing cancer and living meaningfully (CALM): a randomized controlled trial of a psychological intervention for patients with advanced cancer. J. Clin. Oncol. 2018;36(23):2422–2432. doi: 10.1200/JCO.2017.77.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacob K.S.1, Kuruvilla A. Psychotherapy across cultures: the form-content dichotomy. Clin. Psychol. Psychother. 2012 Jan-Feb;19(1):91–95. doi: 10.1002/cpp.736. Epub 2010 Dec 16. [DOI] [PubMed] [Google Scholar]
- 30.Wittkorwer E.D., Warms H. Cultural aspects of psychotherapy. Psychother. Psychosom. 1974;24:303–310. doi: 10.1159/000286749. [DOI] [PubMed] [Google Scholar]
- 31.Edge D., Lemetyinen H., Helman C.G. Psychology across cultures: challenges and opportunities. Psychol Psychother. 2019 Jun;92(2):261–276. doi: 10.1111/papt.12229. Culture, Health and Illness. 5th ed. Oxford, England: Oxford University Press; 2007. [DOI] [PubMed] [Google Scholar]
- 32.Travado L., Grassi L., Gil F., Ventura C., Martins C. Southern European Psycho-Oncology Study Arm. Physician-patient communication among Southern European cancer physicians: the influence of psychosocial orientation and burnout. Psycho Oncol. 2005 Aug;14(8):661–670. doi: 10.1002/pon.890. [DOI] [PubMed] [Google Scholar]
- 33.de Figueiredo J.M., Gostoli S. Culture and demoralization in psychotherapy. Adv. Psychosom. Med. 2013;33:75–87. doi: 10.1159/000348735. Epub 2013 Jun 25. [DOI] [PubMed] [Google Scholar]
- 34.Grassi L., Travado L., Moncayo F.L., Sabato S., Rossi E. SEPOS Arm. Psychosocial morbidity and its correlates in cancer patients of the Mediterranean area: findings from the Southern European Psycho-Oncology Study. J. Affect. Disord. 2004 Dec;83(2–3):243–248. doi: 10.1016/j.jad.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Nanni M.G., Caruso R., Travado L., Ventura C., Palma A., Berardi A.M., Meggiolaro E., Ruffilli F., Martins C., Kissane D., Grassi L. Relationship of demoralization with anxiety, depression, and quality of life: a Southern European study of Italian and Portuguese cancer patients. Psycho Oncol. 2018 Nov;27(11):2616–2622. doi: 10.1002/pon.4824. Epub 2018 Aug 6. [DOI] [PubMed] [Google Scholar]
- 36.Long C.O. Cultural and spiritual considerations in palliative care. J. Pediatr. Hematol. Oncol. 2011 Oct;33(2):S96–S101. doi: 10.1097/MPH.0b013e318230daf3. [DOI] [PubMed] [Google Scholar]
- 37.Travado L.1, Grassi L., Gil F., Martins C., Ventura C., Bairradas J. Southern European psycho-oncology study do spirituality and faith make a difference? Report from the southern European psycho-oncology study arm. ArmPalliat Support Care. 2010 Dec;8(4):405–413. doi: 10.1017/S147895151000026X.Epub.2010.Sep.28. [DOI] [PubMed] [Google Scholar]
- 38.Speck P. Culture and spirituality: essential components of palliative care. Postgrad. Med. 2016 Jun;92(1088):341–345. doi: 10.1136/postgradmedj-2015-133369. Epub 2016 Mar 1. [DOI] [PubMed] [Google Scholar]
- 39.Caruso R., Sabato S., Nanni M.G. Application of managing cancer and living meaningfully (CALM) in advanced cancer patients: an Italian pilot study [published online ahead of print, 2020 mar 19] Psychother. Psychosom. 2020:1–3. doi: 10.1159/000505875. [DOI] [PubMed] [Google Scholar]
- 40.Scheffold K., Philipp R., Engelmann D., Schulz-Kindermann F., Rosenberger C., Oechsle K., Härter M., Wegscheider K., Lordick F., Lo C., Hales S., Rodin G., Mehnert A. Efficacy of a brief manualized intervention Managing Cancer and Living Meaningfully (CALM) adapted to German cancer care settings: study protocol for a randomized controlled trial. BMC Canc. 2015 Aug 19;15:592. doi: 10.1186/s12885-015-1589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radbruch L., Sabatowski R., Elsner F., Everts J., Mendoza T., Cleeland C. Validation of the German version of the brief Fatigue inventory. J. Pain Symptom Manag. 2003;25:449–458. doi: 10.1016/s0885-3924(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 42.Spiegel D., Spira P. Psychosocial Treatment Laboratory, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine; Stanford, CA: 1991. Supportive-Expressive Arm Therapy: A Treatment Manual of Psychosocial Intervention for Women with Recurrent Breast Cancer. [Google Scholar]
- 43.Hales S., Lo C., Rodin G. Managing Cancer and Living Meaningfully (CALM): the Paradox of Advanced Disease. Oxford University Press (in press).
- 44.Spitzer R.L., Kroenke K., Williams J.B. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient Health Questionnaire. J. Am. Med. Assoc. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 45.Gilbody S., Richards D., Brealey S., Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J. Gen. Intern. Med. 2007;22(11):1596–1602. doi: 10.1007/s11606-007-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mazzotti E., Fassone G., Picardi A., Sagoni E., Ramieri L., Lega I., Camaioni D., Abeni D., Pasquini P. The patient health questionnaire (PHQ) for the screening of psychiatric disorders: a validation study versus the structured clinical interview for DSM-IV axis I (scid-I) Ital. J. Psychopathol. 2003;9:235–242. [Google Scholar]
- 47.Rizzo R., Piccinelli M., Mazzi M.A., Bellantuono C., Tansella M. The personal health questionnaire: a new screening instrument for detection of ICD-10 depressive disorders in primary care. Psychol. Med. 2000;30(Issue 4):831–840. doi: 10.1017/s0033291799002512. [DOI] [PubMed] [Google Scholar]
- 48.Belvederi Murri M., Zerbinati L., Ounalli H., Kissane D., Casoni B., Leoni M., Rossi G., Dall'Olio R., R Caruso R., Nanni M.G., Grassi L. Assessing demoralization in medically ill patients: factor structure of the Italian version of the demoralization scale and development of short versions with the item response theory framework. J. Psychosom. Res. 2020 Jan;128:109889. doi: 10.1016/j.jpsychores.2019.109889. Epub 2019 Nov 29. [DOI] [PubMed] [Google Scholar]
- 49.Lo C., Hales S., Zimmermann C., Gagliese L., Rydall A., Rodin G. Measuring death-related anxiety in advanced cancer: preliminary psychometrics of the death and dying distress scale. J Ped Hematol Oncol. 2011;33(Suppl 2):S140–S145. doi: 10.1097/MPH.0b013e318230e1fd. [DOI] [PubMed] [Google Scholar]
- 50.Kissane D.W., Wein S., Love A., Lee X.Q., Kee P.L., Clarke D.M. The Demoralization Scale: a report of its development and preliminary validation. J. Palliat. Care. 2004;20(4):269–276. [PubMed] [Google Scholar]
- 51.Grassi L., Costantini A., Kissane D., Brunetti S., Caruso R., Marchetti P., Sabato S., Nanni M.G. The factor structure and the use of the Demoralization Scale in Italian non-advanced cancer patients. Psycho Oncol. 2017;26(11):1965–1971. doi: 10.1002/pon.4413. [DOI] [PubMed] [Google Scholar]
- 52.Grassi L., Costantini A., Kissane D., Brunetti S., Caruso R., Piazza G., Marchetti P., Sabato S., Nanni The factor structure and use of the Demoralization Scale (DS‐IT) in Italian cancer patients. Psycho Oncol. 2017;26 doi: 10.1002/pon.4413. Italian version of Demoralization Scale: a validation study. Rivista di Psichiatria. 2013 May-Jun;48(3):234-239. DOI: 10.1708/1292.14291. [DOI] [PubMed] [Google Scholar]
- 53.Spitzer R.L., Kroenke K., Williams J.B., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 54.Peterman A.H., Fitchett G., Brady M.J., Hernandez L., Cella D. Measuring spiritual well-being in people with cancer: the functional assessment of chronic illness therapy - spiritual well-being scale (FACIT-Sp) Ann. Behav. Med. 2002;24(1):49–58. doi: 10.1207/S15324796ABM2401_06. [DOI] [PubMed] [Google Scholar]
- 55.Rabitti E., Cavuto S., Iani L., Ottonelli S., De Vincenzo F., Costantini M. The assessment of spiritual well-being in cancer patients with advanced disease: which are its meaningful dimensions? BMC Palliat. Care. 2020;19(1):26. doi: 10.1186/s12904-020-0534-2. Published 2020 Mar 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tedeschi R.G., Calhoun L.G. The posttraumatic growth inventory: measuring the positive legacy of trauma. J. Trauma Stress. 1996;9:455–471. doi: 10.1007/BF02103658. [DOI] [PubMed] [Google Scholar]
- 57.Prati G., Pietrantoni L. Italian adaptation and confirmatory factor Analysis of the full and the short form of the posttraumatic growth inventory. J. Loss Trauma. 2014;19 doi: 10.1080/15325024.2012.734203. [DOI] [Google Scholar]
- 58.Lo C., Burman D., Swami N., Gagliese L., Rodin G., Zimmermann C. Validation of the QUAL-EC for assessing quality of life in patients with advanced cancer. Eur. J. Canc. 2011;47(4):554–560. doi: 10.1016/j.ejca.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 59.Grünke B., Philipp R., Vehling S., Scheffold K., Härter M., Oechsle K. Measuring the psychosocial dimensions of quality of life in advanced cancer patients: psychometrics of the German Quality of Life at the End of Life–Cancer–Psychosocial (QUAL-EC-P) Questionnaire. J. Pain Symptom Manag. 2018;55(3):985–991.e1. doi: 10.1016/j.jpainsymman.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 60.Lo C., Walsh A., Mikulincer M., Gagliese L., Zimmermann C., Rodin G. Measuring attachment security in patients with advanced cancer: psychometric properties of a modified and brief Experiences in Close Relationships scale. Psycho Oncol. 2009;18:490–499. doi: 10.1002/pon.1417. [DOI] [PubMed] [Google Scholar]
- 61.Ghirardello D., Munari J., Testa S., Torta R., Veglia F., Civilotti C. Italian adaptation of the brief modified experiences in close relationships scale in a sample of cancer patients: factor analysis and clinical implications. Research in Psychotherapy: Psychopathology, Process and Outcome. 2018;21(3) doi: 10.4081/ripppo.2018.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olson D.H., Larson P.J. Life Innovations; Minneapolis: 2008. PREPARE/ENRICH: Customized Version. [Google Scholar]
- 63.Lo C., Hales S., Rydall A., Panday T., Chiu A., Malfitano C. Managing Cancer and Living Meaningfully: study protocol for a randomized controlled trial. Trials. 2015 doi: 10.1186/s13063-015-0811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Portenoy R.K., Thaler H.T., Kornblith A.B., Lepore J.M., Friedlander-Klar H., Kiyasu E. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur. J. Canc. 1994;30:1326–1336. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 65.Chang V.T., Hwang S.S., Feuerman M., Kasimis B. The memorial symptom assessment scale short form (MSAS-SF). Validity and reliability. Cancer. 2000;89:1162–1171. doi: 10.1002/1097-0142(20000901)89:5<1162::aid-cncr26>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 66.European Commission Data protection in the EU. EU. https://ec.europa.eu/info/law/law-topic/data-protection/data-protection-eu_en Website.
- 67.Kreidler S.M., Muller K.E., Grunwald G.K., Ringham B.M., Coker-Dukowitz Z.T., Sakhadeo U.R., Barón A.E., Glueck D.H. GLIMMPSE: online power computation for linear models with and without a baseline covariate. J. Stat. Software. 2013;54:i10. doi: 10.18637/jss.v054.i10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Overall J.E., Tonidandel S., Starbuck R.R. Rule-of-thumb adjustment of sample sizes to accommodate dropouts in a two-stage analysis of repeated measurements. Int. J. Methods Psychiatr. Res. 2006;15:1–11. doi: 10.1002/mpr.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.IBM Corp Released . IBM Corp; Armonk, NY: 2017. IBM SPSS Statistics for Windows, Version 25.0. [Google Scholar]
- 70.QSR International Pty Ltd . 2015. NVivo Qualitative Data Analysis Software Version 11.0. [Google Scholar]
- 71.Connor S.R., Sepulveda Bermedo M.C., editors. Global Atlas of Palliative Care at the End of Life. , Worldwide Palliative Care Alliance (WPCA) and World Health Orgnaization. WHO); 2014. [Google Scholar]
- 72.European Association of Palliative Care EAPC task force on education for psychologists. https://www.eapcnet.eu/eapc-arms/archives/task-forces-archives/psychology (accessed March 1, 2019)
- 73.Wasteson E., Brenne E., Higginson I.J., Hotopf M., Lloyd-Williams M., Kaasa S., Loge J.H. European Palliative Care Research Collaborative (EPCRC). Depression assessment and classification in palliative cancer patients: a systematic literature review. Palliat. Med. 2009;23(8):739–745. doi: 10.1177/0269216309106978. [DOI] [PubMed] [Google Scholar]
- 74.International Psycho-Oncology society (IPOS) IPOS international standard of quality cancer care. https://ipos-society.org/about/quality (accessed March 11, 2019)
- 75.Grassi L., Mezzich J.E., Nanni M.G., Riba M.B., Sabato S., Caruso R. A person-centered approach in medicine to reduce the psychosocial and existential burden of chronic and life-threatening medical illness. Int’l Rev Psychiatry. 2017;29(5):377–388. doi: 10.1080/09540261.2017.1294558. [DOI] [PubMed] [Google Scholar]
- 76.Rodin G. From evidence to implementation: the global challenge for psychosocial oncology. Psycho Oncol. 2018;27(10):2310–2323. doi: 10.1002/pon.4837. [DOI] [PubMed] [Google Scholar]
- 77.Gauthier L.R., Rodin G., Zimmermann C., Warr D., Librach S.L., Moore M., Shepherd F.A., Gagliese L. The communal coping model and cancer pain: the roles of catastrophizing and attachment style. J. Pain. 2012;13(12):1258–1268. doi: 10.1016/j.jpain.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 78.Diaz-Frutos D., Baca-Garcia E., García-Foncillas J., López-Castroman J. Predictors of psychological distress in advanced cancer patients under palliative treatments. Eur. J. Canc. Care. 2016;25(4):608–615. doi: 10.1111/ecc.12521. [DOI] [PubMed] [Google Scholar]
- 79.Potash M., Breitbart W. Affective disorders in advanced cancer. Hematol. Oncol. Clin. N. Am. 2002;16(3):671–700. doi: 10.1016/s0889-8588(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 80.Chochinov H.M., Hack T., Hassard T. Dignity therapy: a novel psychotherapeutic intervention for patients near the end of life. J. Clin. Oncol. 2005;23:5520–5525. doi: 10.1200/JCO.2005.08.391. [DOI] [PubMed] [Google Scholar]