Abstract
Cathaya argyrophylla is an ancient and threatened Pinaceae species endemic to China, but its eco-physiological traits are rarely reported. We hypothesized that Cathaya showed eco-physiological outliers to its Pinaceae relatives, which lead to its current endangered status. Here we collected the photosynthetic capacity (Pn, maximum photosynthesis rate) and branchlet hydraulic safety (P50, the water potential at which a 50% loss in conductivity occurs) of Pinaceae species globally, including our measurements on Cathaya. We applied the phylogenetic comparative methods to investigate: (i) the phylogenetic signal of the two key traits across Pinaceae species, and (ii) the trait–climate relationships and the photosynthesis–cavitation resistance relationship across Pinaceae species. We applied the polygenetic quantile regression (PQR) method to assess whether Cathaya showed eco-physiological outliers to its Pinaceae relatives in terms of cavitation resistance and photosynthetic capacity. It was found that P50, and to a less extent, Pn, had a strong phylogenetic signal consistent with niche conservation among Pinaceae species. Hydraulic safety largely determined non-threatened Pinaceae species’ distribution across moisture gradients at the global scale. There was also an adaptive trade-off relationship between Pn and P50. Cathaya is a high cavitation resistant, low photosynthetic capacity species. It showed eco-physiological outliers to its Pinaceae relatives because it had lower P50 and Pn below the 10% quantile boundaries along moisture and/or temperature gradients; also, it was above the 90% quantile boundary of the Pn and P50 relationship across non-endangered Pinaceae species. The PQR output demonstrated that in the subtropical area of China characterized by abundant rainfall, Cathaya has extra high hydraulic safety, suggesting inefficiency of carbon economy associated with either competition or other life history strategies, which lead to its current endangered status.
Keywords: Hydraulic safety, photosynthetic capacity, phylogenetic signal, species’ distribution, polygenetic quantile regression
Introduction
The ‘giant panda’ of the plant kingdom, Cathaya argyrophylla Chun et Kuang, was formally described in 1958 by Chun and Kuang (Chun, 1958; Ying et al., 1983). It is a monotypic genus of Pinaceae endemic to China and has been categorized as a paleoendemic species, with a fossil history dating at least to the Cretaceous (Ferguson et al., 1997; Liu et al., 2000). Cathaya is one of the most vulnerable conifers in the world with total number of mature individuals less than 5000 (Xie et al., 1999b). Having its first occurrence in North America and being a formerly widespread Asiatic species, Cathaya has now become severely restricted to only three sites in subtropical mountains of China as a consequence of Late Tertiary climatic deterioration and Quaternary glaciation (Sup. Fig. 1) (Liu and Basinger, 2000). Extant Cathaya is found in a few mesothermal, physiological arid (high water deficit), infertile and exposed slopes in this area (Taggart et al., 2009; Xie et al., 1999a). It was also found that the abilities of competition and recolonization of this species are very low and that the habitats suitable for this species are undergoing rapid deterioration and fragmentation (Fu, 1992; Xie and Chen, 1999); as such, identification of abiotic and biotic factors contributing to its endangered status is vital for its conservation purposes.
How has Cathaya been vulnerable to extinction? Probably, such limited adaptability to regional climates and environments are due to its unique eco-physiological traits (Hunter Jr et al., 2006; Lusk, 2008). A species’ uniqueness can be shown in three ways: (i) outlier to certain traits of its relatives (Hunter Jr and Gibbs, 2006); (ii) outlier to trait–environment relationships among its relatives (Newsome et al., 2020); and (iii) outlier to trait–trait relationships among its relatives (Brodribb et al., 2008). In the first case, blind, unpigmented, cave-dwelling invertebrates, fishes and amphibians are good examples of special characteristics in response to a rare type of habitat (Culver et al., 2000). In the second case, a good example is vertebrate species near the lower boundary of the relationship between habitat ranges and body sizes were more often in higher extinction risk categories (Newsome et al., 2020). In the third case, a good example is the tropic unique flat-leaved pine, Pinus krempfii, showing departure from the trade-off pattern between leaf hydraulic conductivity and mesophyll path length across pine species. This eco-physiological outlier probably is a key factor attributing to its limited adaptability and endangered status (Brodribb et al., 2008).
Although there is a large body of knowledge on the palaeoclimate, palaeogeology, anatomy, ecology, population genetics and evolutionary history of this species (Fu, 1992; Hu et al., 1984; Liu and Basinger, 2000; Qian et al., 2016; Ran et al., 2018; Xie et al., 1999b), the eco-physiological traits of Cathaya are rarely reported (Fan et al., 2005; Zhang et al., 2005). In the present study, we chose two key functional traits to test whether Cathaya shows eco-physiological outliers to its Pinaceae relatives. One is the drought tolerance assessed by the water potential at which a 50% loss in hydraulic conductivity occurs [P50, MPa (Choat et al., 2012)]; another is the competition capacity using the maximum photosynthetic capacity [Pn, μ mol CO2 m−2 s−1, (Augusto et al., 2014)] as a surrogate. Theoretically, low P50 (high drought tolerance) means high carbon investment on xylem (e.g. high wood density, small tracheid size). The synthesis of xylem tissue is costly [6.5 and 11.8 mmol glucose per g of cellulose and lignin, respectively (Lambers et al., 1992)]; as a result, less carbon investment to leaves and inefficiency of carbon economy (lower Pn) are expected (Augusto et al., 2014; Pittermann et al., 2012). We thereby hypothesize that there exists a trade-off between P50 and Pn among non-threatened Pinaceae species, which has already been observed for the Cupressaeae species (Pittermann et al., 2012).
It is well known that moisture gradient largely determines the distribution of species with different drought tolerances (Choat et al., 2018; Choat et al., 2006; Willson et al., 2006). In the subtropical area of China characterized by abundant rainfall, common species should evolve trait suits with low drought tolerance. Therefore, it is possible that the restricted range of Cathaya is due to its extra-high drought tolerance, which would deviate from the general relationship between species drought tolerance and regional rainfall/water deficit gradient. Further, the high drought tolerance of Cathaya possibly comes at a significant cost on competition capacity, if there exists a trade-off between P50 and Pn among non-threatened Pinaceae species. As such, low competition capacity would make Cathaya hard to overcome local species’ competitive advantages (ecological barrier) for its survival and dispersion. However, other possibilities cannot be excluded; for example, Cathaya could deviate from the P50–Pn trade-off. Such departure means the participation of some other life history trade-offs and/or the regulation of some unusual structure characteristics (Brodribb et al., 2008).
The supposed unusual suites of traits of Cathaya, either due to evolutionary conservatism (e.g. more ancient Pinaceae species have higher drought tolerance) or due to developmental constraints (e.g. the rarity of major mutations) (Brodribb et al., 2008; Hunter Jr and Gibbs, 2006; Lusk, 2008; Wiens et al., 2005), could be evaluated preliminarily by phylogenetic niche conservatism (PNC). PNC is the tendency of lineages to retain their niche-related traits through speciation events (Harvey et al., 1991; Ricklefs, 2010). Although it is a pattern, not a process, the initial general test can lead to specific tests addressing the hypothesized drivers (Crisp et al., 2012). Additionally, as the cross-species correlations may be biased by a lack of statistical independence among closely related species with shared evolutionary history, phylogenetic signals are also needed to be known a priori, in order to minimize the Type I error rates of non-phylogenetically corrected cross-species correlations (Ackerly, 2000; Blomberg et al., 2003).
In the present study, we addressed three questions: to detect (i) the phylogenetic signal of P50 and Pn across Pinaceae species, based on a global database and our measurements of Cathaya; (ii) which environmental variables drive the distribution of P50 and Pn across common Pinaceae species and if there exists a trade-off between P50 and Pn; and (iii) if Cathaya shows outliers to the general relationships among common Pinaceae species between P50/Pn and environmental variables and the P50 vs. Pn trade-off relationship.
Materials and methods
Field surveys were carried out at Jiaopengliao, Zixing City, Hunan Province, South China, with a geographic location of 25 ° 56 ′ 54 ″ N, 113 ° 29 ′ 24 ″E and an altitude of 1250 m, on the southwest part of Bamian Mountain Reserve. The forest was a natural mixed stand of conifer and broad-leaved trees. Besides Cathaya, there were also Castanopsis and Altingia tree species in the main canopy storey. The climate is a subtropical monsoon climate area with four distinct seasons and mild climate; the meteorological data could be seen in Supplementary Table 1. The main soil type is mountain yellow–brown earth, with a total nitrogen content of only 0.0061% (Zhang et al., 2005). A total of 26 sunlit branches from six mature trees were selected for cavitation resistance measurement, and leaves from one branch from the six mature trees were selected for photosynthesis measurement.
Light-response curve measurement
The light-response curve was measured from 9:00 to 12:00 AM in July with an infrared gas analyzer (LI-6400 Li-Cor, Lincoln, NE, USA) performed using the autoprogram function. One-year-old leaves were chosen for measurements. We chose the sequence of desired light settings of 1500; 1200; 800, 500, 300, 100, 50, 30 and 0 μmol (photon) m−2 s−1, a minimum wait time of 120 s and a maximum wait time of 200 s, and matching the infrared gas analyzers for the 50-μmol(CO2) mol(air)−1 difference in the CO2 concentration between the sample and the reference, which allowed them to be matched before every change in irradiance. Leaf temperature was kept between 24 and 26°C, and CO2 partial pressure of and relative humidity in the leaf cuvette was 60–80% during measurements. Leaf mass ratio (LMA) measurements were then taken on Pn-measured specimens in the laboratory. Ten to 15 fully expanded healthy leaves, not including petioles, were selected (Pérez-Harguindeguy et al., 2013). The stomatal conductance (gs) and transpiration rate (Tr) at saturating irradiance was also recorded.
The light-response curve can be fitted by a non-rectangular hyperbola (Lambers et al., 2008):
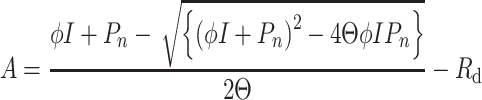 |
(1) |
where A is the net photosynthetic rate under specific irradiance; Pn is the light-saturated net photosynthetic rate; I is the irradiance;  is the quantum yield of assimilation, which is the initial slope for the light-response curve;
is the quantum yield of assimilation, which is the initial slope for the light-response curve; 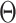 is the convexity of the curve; and Rd is the dark respiration rate.
is the convexity of the curve; and Rd is the dark respiration rate.  and Rd were calculated using linear regression analysis (A to irradiance <150 μmol m−2 s−1), then Pn and
and Rd were calculated using linear regression analysis (A to irradiance <150 μmol m−2 s−1), then Pn and  were derived empirically by fitting (1) to light response data. The light compensation point (LCP) was the irradiance when A = 0, below which there is insufficient light to compensate for respiratory carbon loss.
were derived empirically by fitting (1) to light response data. The light compensation point (LCP) was the irradiance when A = 0, below which there is insufficient light to compensate for respiratory carbon loss.
Cavitation vulnerability curve and tracheid diameter measurements
Three to five branchlets (40–70 cm long, 2–4 years old) near that used for the light-response measurement were cut from each sample tree before sunrise (before 8:00 AM) to measure the specific conductivity (ks; kg m−1 MPa−1 s−1), leaf specific conductivity (kl; kg m−1 MPa−1 s−1) and cavitation vulnerability curve (totally 26 samples). The branchlet was wrapped in moist paper towels immediately after being cut and transported to the laboratory. All the samples were re-cut to 22–24 cm underwater, and all the measurements were carried out in an air-conditioned laboratory (26°C). The maximum flow rate was measured under 8 kPa hydrostatic pressure after air emboli were flushed out by perfusion with 110 kPa distilled water (flowing through 0.2 μm filter) for 30 min. Measurements were initiated after ~ 2 min when the flow rate stabilized. The weight of the collected efflux was measured every 30 s with a precision balance (Sartorius, BP221S, Göttingen, Germany) to obtain the flow rate. kl was calculated by dividing the maximum flow rate by the total leaf area distal to the measured segment and by the pressure gradient. The leaf area was determined using a WinFOLIA system (Regent Instruments, Quebec City, Canada). ks was calculated by dividing the maximum flow rate by the segment’s cross-section sapwood area. The total acropetal-end cross-section area of the branch segment was determined from its maximum and minimum diameters. The area of the pith was determined from its dimensions measured under a dissecting microscope equipped with a stage micrometer and subtracted from the above acropetal-end cross-section area to determine the cross-section sapwood area. The Huber value was calculated as the total cross-section sapwood area per unit leaf area (Tyree et al., 1988).
The vulnerability of xylem to cavitation was characterized using a vulnerability curve, which was measured using a cavitation pressure chamber (PMS Instrument, Corvallis, Oregon, USA) according to Sperry and Saliendra (1994). A branch segment was inserted into a collar and sealed with both ends protruding. Air was injected into the collar at a set pressure, which was maintained for 15 min and then slowly decreased to 0.1 MPa. The hydraulic conductivity was then re-measured at a higher pressure. This procedure was repeated until at least 85% loss of hydraulic conductivity was reached. The percentage loss of conductivity (PLC) following each pressurization was calculated as PLC = 100 × (Kh–Khi)/Kh, where Khi is the hydraulic conductivity measured at pressure i. The vulnerability curve for each sample was fitted with an exponential sigmoidal equation (Pammenter et al., 1998):
 |
(2) |
where Ψ is the negative of the injection pressure and a and b are coefficients estimated using a non-linear regression model with gnls() function of R software (R Development Core Team, 2016). The coefficient b represents P50, a represents the steepness of vulnerability curve. Wood density (W) was measured on stem segments used in the measurement of vulnerability curves after the removal of pith and bark, and the fresh volume was measured by the Archimedes principle of water displacement. The dry mass was determined after drying at 104°C for 24 h. W is expressed as dry mass per unit of fresh volume (g cm−3).
Three to five samples out of those used for VC curve construction were used for the anatomical measurements. Micro thin sections (12 μm thick) were taken, following the procedure descried in Domec et al. (2010). Those sections were stained with Safranin O and mounted in resin for image analysis using ImageJ (Media Cybernetics, Silver Spring, MD). The mean hydraulic diameter [dh = Σd5/Σd4 where d = individual tracheid diameter (Kolb et al., 1999)] was estimated. The equivalent circle diameter was taken as tracheid diameter based on the lumen area measured (Wheeler et al., 2005). For each sample on pit-cambium transects separately, three radial files of tracheids were randomly selected, and all lumen areas were measured.
The global datasets, tree phylogenies and phylogenetic comparative analyses
We collected three datasets in the present study: P50 dataset, Pn dataset and P50 vs. dh dataset. The branchlet P50 dataset used in the present study can be accessed from the TRY Plant Traits Database (https://www.try-db.org/TryWeb/Home.php) (Choat et al., 2012). We found 35 Pinaceae species (in addition to Cathaya) from the database with definite locations on the maximally resolved seed plant tree (Zanne et al., 2014). Based on the P50 dataset, we selected the same Pinaceae species for the Pn dataset from the TRY Plant Traits Database. Pn with the same (or slightly different) latitude and longitude of the P50 dataset, measured under growth temperature, ambient CO2 and saturating irradiance, with VPD (vapour pressure deficit) ranging from 0.8 to 1.5 kPa and/or relative humidity from 55 to 80%, of sunlit leaves from mature trees, were chosen out of more than 14 000 records. Pn values of four Pinaceae species (Abies concolor, Cedrus deodara, Pinus caribaea, Pinus echinata) could not be found based on the selection criteria, and we added another two Pinaceae species’ data (Pinus krempfii, Pinus kesiya) from Brodribb et al. (2008), resulting in 34 Pinaceae species in the Pn dataset (including Cathaya). In the P50 dataset, there were 18 Pinaceae species with Pn measured at the same location (or a slightly different location with an elevation difference of <300 m); this sub-dataset was analyzed for the relationship between P50 and Pn. At last, we collected eight literatures (Davis et al., 1999; Domec et al., 2010; Hacke and Jansen 2009; Linton et al., 1998; Maherali et al., 2006; Mayr et al., 2006; Oliveras et al., 2003; Sterck et al., 2012), in which both dh and P50 of 14 Pinaceae species have been measured simultaneously, to construct the P50 vs. dh dataset. Six environmental variables [latitude, altitude, mean annual temperature (MAT), mean annual precipitation (MAP), mean temperature in the coldest month (MTCM) and water deficit (WD)] were selected for trait–environment analysis. Climate data (means over 1950–2000) were extracted from the WorldClim2 database with a spatial resolution of ca. 1 km (Fick et al., 2017) based on the geographic coordinates of sampling sites.
We then constructed the phylogenies for our species with the program Phylomatic (Webb et al., 2008), using a maximally resolved seed plant tree (Zanne et al., 2014). Phylogenies were constructed for the P50, Pn, P50 vs. Pn and P50 vs. dh dataset (Supplementary Figs. 2 and 3 for P50 and Pn). Afterwards, multiple approaches were applied to evaluate phylogenetic signals in P50 and Pn. The first approach is Pagel’s λ calculation (Freckleton et al., 2002) (using the ‘Phytools’ package in R). Secondly, the phylogenetic signal representation curve (PSR) approach has been adopted (Felizola Diniz Filho et al., 2012; Hawkins et al., 2014). The PSR area, expressing deviations from Brownian motion across the curve, is the same as Blomberg’s K statistic and can reveal whether traits are evolving at a slower (negative) or faster (positive) rate than expected under Brownian motion in different parts of the phylogeny (Felizola Diniz Filho et al., 2012). PSR curves for P50 and Pn were calculated by using the “PVR” package in R. The third approach (phylogenetic generalized least squares) followed Hawkins et al. (2014), Kozak et al. (2010) and Wiens et al. (2010). Three models of evolution for Pn and P50 were applied: a white noise (WN) model of random variation; a Brownian motion (BM) model of gradual and continuous drift in trait; and an Ornstein–Uhlenbeck (OU) model of constrained evolution. OU-constrained evolution (stabilizing selection) was regarded as the sign of phylogenetic signal to a more stringent sense (Crisp and Cook, 2012). The log-likelihood of phylogenetic generalized least squares fitting of the three models was calculated. The “GEIGER” package in R was used to calculate and compare the fit of each model using a likelihood test; the Akaike information criterion (AIC) derived from model likelihoods was used to determine whether each trait fits an OU model better than either a BM or WN model.
To investigate the relationship between P50/Pn and eight environmental variables (including the first two axes of principal component analysis, PC1 and PC2) among Pinaceae species, a phylogenetic generalized linear model (PGLS) with branch length transformations optimized for the maximum likelihood was applied, using the ‘caper’ package in R. In PGLS analysis, regression parameters are found by maximum likelihood (ML) and ‘weighted’ by the variance–covariance matrix that represents the phylogenetic relationships among the species. The relationship between Pn and P50, dh and P50 were also explored by PGLS. Assume that the pressure that causes the cavitation of water inside xylem tracheids is inversely proportional to the mean of the maximum (per tracheid) size of pit pores (dp) (air-seeding hypothesis (Zimmermann, 1983)) P50 ∝1/dp, and dh is linear to the size of its largest pore: dh∝dp (Martínez-Vilalta et al., 2002), resulting in P50 ∝1/dh. Therefore, for the P50 vs. dh relationship investigation, − P50 and dh were log-transformed beforehand.
To investigate whether Cathaya shows eco-physiological outliers to its Pinaceae relatives, the polygenetic quantile regression (PQR) approach was applied. Since P50/Pn is likely determined by multiple unmeasured factors, and species with trait values outside the quantile regression lines are more likely to be threatened with extinction, it is useful to define an approximate upper/lower boundary for P50/Pn at a given variable, rather than focusing on means (Hacke et al., 2017; Newsome et al., 2020). To conduct PQR across non-threatened species, we followed the method by Newsome et al. (2020). We firstly fitted the non-phylogenetic quantile regression model and obtained residuals; secondly, we constructed a phylogenetic “distance” matrix from the phylogenetic tree (one minus the correlation matrix); thirdly, we calculated the phylogenetic autocovariate using the inverse distance squared weighted mean of residuals; then, the phylogenetic autocovariate was included in the quantile regression model (we set the auto-covariate to its average value, thus the boundaries that we show are for species with average values of the residual auto-covariate). We fitted quantile regression models for the 90% and 10% quantiles to describe the upper and lower bounds, respectively. The PQR was carried out by using the ‘ape’ and ‘quantreg’ packages in R (Koenker et al., 2018).
Among the collected Pinaceae data, seven species were listed on the IUCN red list (IUCN, 2018) above the ‘Vulnerable’ category; they are Abies pinsapo (En, endangered), Cathaya argyrophylla (Vu, vulnerable), Cedrus libani (Vu), Cedrus brevifolia (Vu), Pinus albicaulis (En), Pinus caribaea (En) and Pinus krempfii (Vu).
Results
The Pn and branchlet P50 of Cathaya were 5.96 μmol m−2 s−1 (Table 1; Fig. 1A) and −5.64 MPa (Table 1; Fig. 1B), respectively. Pn was in the lowest 25% quartile range among compiled Pinaceae species [Sup. Table 1, compiled Pn data of Pinaceae species can also be seen in Brodribb and Feild (2008)]. Besides two Cedrus species (Cedrus libani, Cedrus brevifolia), Cathaya was the most cavitation resistant one among compiled Pinaceae species (Sup. Table 1) [compiled P50 data of Pinaceae species can also be seen in Delucia et al. (2000) and Martínez-Vilalta et al. (2004)]. High resistance to cavitation indicated low hydraulic conductance (ks and kl), as well as narrow tracheids (d and dh). In fact, the tracheid diameter of Cathaya was the narrowest one among the investigated Pinaceae species (Sup. Table 2; Sup. Fig. 3) [see also Hacke et al. (2017) and Martínez-Vilalta et al. (2012)]. The inefficiency of the carbon economy could also be indicated by low gs, Tr and Rd and high LMA (Table 1). The LCP (light compensation point) was 12.41 μmol m−2 s−1; however, we could not obtain a reliable LSP (light saturation point), as with the increase in irradiance, Pn increased slightly even at a very strong irradiance (>1500 μmol m−2 s−1); furthermore, there is no consensus on the accuracy of LSP estimation by a non-rectangular hyperbola (Li et al., 2019).
Table 1.
The branchlet’s hydraulic traits and leaf photosynthetic traits of Cathaya measured in the present study
| Branchlet’s hydraulic traits | Photosynthetic traits | ||
|---|---|---|---|
| P 50 (MPa) | −5.64 ± 0.39 | P n (μmol m−2 s−1) | 5.96 ± 0.41 |
| k s (kg m−1 MPa−1 s−1) | 0.45 ± 0.13 | g s (mmol m−2 s−1) | 72.48 ± 10.07 |
| k l (kg m−1 MPa−1 s−1) | 9.73 ± 4.05 E−5 | T r (mol m−2 s−1) | 0.67 ± 0.13 |
| Huber | 2.31 ± 0.35 E−4 | LCP (μmol m−2 s−1) | 12.41 ± 1.71 |
| d (μm) | 6.86 ± 0.23 | R d (μmol m−2 s−1) | 0.53 ± 0.07 |
| d h (μm) | 9.95 ± 0.45 | LMA (g m−2) | 204 ± 21 |
| W (g cm−3) | 0.55 ± 0.07 | ||
P 50: the water potential at which a 50% loss in conductivity occurs; ks: specific conductivity; kl: leaf-specific conductivity; Huber: Huber value; d: individual tracheid diameter; dh: mean hydraulic diameter; W: wood density; Pn: light-saturated net photosynthetic rate; gs: stomatal conductance; Tr: transpiration rate; LCP: light compensation point; Rd: dark respiration rate; LMA: leaf mass ratio.
Figure 1.

Light response curve (A) and percentage loss in hydraulic conductivity as a function of pressure for branchlets (B) of Cathaya. Curves were fitted using all data; only means (±1 SE) are presented. In subfigure A, the light-response curves were fitted by a nonrectangular hyperbola (n = 6). In subfigure B, the vulnerability curves were fitted with an exponential sigmoidal equation (n = 26)
We calculated the phylogenetic signal of P50 and Pn based on global datasets of Pinaceae species; Pagel’s λ of P50 and Pn were 0.99 and 0.53, respectively (Table 2). P50 was different from the null hypothesis (no phylogenetic dependence), while Pn was marginally different from the null hypothesis, suggesting P50 was more phylogenetically conservative than Pn. This could further be verified by the PSR area (Fig. 2); the PSR area for P50 was −0.16 while that for Pn was −0.11 (more negative area means more conservative). The fittings by phylogenetic generalized least squares showed that the Ornstein–Uhlenbeck (OU) model was the best model for comparative fits of P50 and Pn (Table 2), suggesting that both traits satisfied the most stringent definition of phylogenetic niche conservatism (Crisp and Cook, 2012; Hawkins et al., 2014; Losos, 2008).
Table 2.
Pagel’s λ and comparative fits of P50 and Pn to alternative evolutionary models of Pinaceae species, using phylogenetic generalized least squares
| Pagel’s λ | ΔAIC of model fit | ||||
|---|---|---|---|---|---|
| P | BM | OU | WN | ||
| P 50 | 0.992 | <0.001 | 0.051 | 0 | 29.937 |
| P n | 0.526 | 0.063 | 5.511 | 0 | 1.154 |
Three models were applied, a Brownian motion (BM) model, an Ornstein–Uhlenbeck (OU) model and a White noise (WN) model. Akaike information criterion difference between model fits was used to evaluate which model is the best among those compared. Model with ΔAIC = 0 is the best evolutionary model for data fit. P: significance test between λ and the maximum likelihood value at λ = 0 (no phylogenetic dependence)
Figure 2.

Phylogenetic signal representation curves (black squares) for (A) P50 and (B) Pn of Pinaceae species. The diagonal in the continuous traits is the relationship expected under a Brownian motion model of evolution
Table 3 shows the PGLS slopes of P50 and Pn with eight environmental variables (including the first two axes of principal component analysis on the six environmental variables, i.e. PC1 and PC2). For the non-threatened species, P50 had significant associations with altitude, MAP, WD and PC2. There were no associations between Pn and environmental variables across all the common species, and only PC1 showed a marginal association with Pn (P = 0.091). PC1 was mostly represented by temperature variables (MAT and MTCM), while PC2 was mostly represented by moisture variables (water deficit index, negatively with MAP and positively with WD) (Table 3).
Table 3.
Statistical significance [P value (determinant coefficient, t value)] of phylogenetic generalized linear model (PGLS) slopes between P50/Pn and eight environmental variables of non-threatened Pinaceae species
| Traits | Latitude | Altitude | MAT | MAP | MTCM | WD | PC1 | PC2 |
|---|---|---|---|---|---|---|---|---|
| P50 | 0.59 (0.01,0.54) |
0.010 (0.22,-2.78) | 0.561 (0.01,-0.59) |
0.013 (0.21,2.64) |
0.714 (0.01,-0.37) |
0.052 (0.13,-2.03) |
0.661 (0.01,-0.44) |
0.013 (0.21,-2.65) |
| Pn | 0.322 (0.04,-1.02) |
0.263 (0.05,-1.15) |
0.181 (0.07,1.39) |
0.189 (0.07,1.34) |
0.262 (0.05,1.15) |
0.661 (0.01,-0.44) |
0.091 (0.11,1.70) |
0.793 (0.01,-0.27) |
| P 50 | PC1 | PC2 | P n | PC1 | PC2 | |||
| Latitude | −0.773 | 0.418 | −0.762 | 0.077 | ||||
| Altitude | −0.352 | −0.446 | −0.533 | 0.286 | ||||
| MAT | 0.916 | 0.359 | 0.99 | 0.014 | ||||
| MAP | 0.566 | −0.776 | 0.185 | −0.946 | ||||
| MTCM | 0.926 | 0.206 | 0.947 | −0.047 | ||||
| WD | 0.080 | 0.863 | 0.491 | 0.85 |
The first two axes of principal component analysis, PC1 and PC2, across all investigated species in P50 and Pn datasets were also demonstrated.
MAT: mean annual temperature (°C); MAP: mean annual precipitation (mm); MTCM: mean temperature in the coldest month (°C); WD: water deficit (mm).
Principal component analysis was conducted after the data have been Z-score standardized.
Numbers in bold mean significant relationships between P50/Pn and environmental variables of non-threatened Pinaceae species revealed by PGLS.
Among non-threatened species, P50 was negatively related to altitude, WD and PC2 (water deficit index) and positively related to MAP (Fig. 3). If Cathaya and other five threatened species were positioned, Cathaya and two Cedrus (Cedrus libani, Cedrus brevifolia) showed significant deviations from the general trends: the three species had extraordinarily lower P50 than PGLS predictions and were below the 10% quantile boundaries. Further, the four PGLS models predicted that P50 of Cathaya should be −3.3~−4.2 MPa, while the measured value was −5.64 MPa. Pn was only related to PC1 (temperature index) (marginally, P = 0.091), indicating that higher photosynthetic capacity is associated with higher growth temperature (Fig. 4). Again Cathaya showed a sign of deviation from the general trend and was below the 10% quantile boundary. The PGLS model predicted that Pn should be around 11 μ mol CO2 m−2 s−1, while the measured value was 5.96 μmol m−2 s−1.
Figure 3.

P 50 as a function of (A) altitude; (B) MAP; (C) WD; and (D) PC2 (the second axis of principle component analysis on six environmental variables based on the P50 dataset, water deficit index) among non-threatened Pinaceae species globally. The black points showed the positions of endangered species. Api: Abies pinsapo. Car: Cathaya argyrophylla. Cbr: Cedrus brevifolia. Cli: Cedrus libani. Pal: Pinus albicaulis. Pca: Pinus caribaea. PC2 was largely represented by MAP (negatively) and WD (positively). The percentage in the bracket next to PC2 means how much of the variability in the data is accounted for by PC2. The phylogenetic generalized linear model (PGLS) with branch length transformations optimized for the maximum likelihood was applied. For PGLS models, Altitude: P50 = -4.05–0.17*Altitude (R2 = 0.22, t = −2.78, P = 0.011); MAP: P50 = -3.99 + 0.31*MAP (R2 = 0.21, t = 2.64, P = 0.013); WD: P50 = -4.05–0.22*WD (R2 = 0.13, t = −2.03, P = 0.052); PC2: P50 = -4.02–0.29*PC2 (R2 = 0.21, t = −2.65, P = 0.013). Principle component analysis (PCA) was conducted to extract the first two components (PC1 and PC2) from the six environmental Z-score standardized variables of all Pinaceae species investigated. Therefore, the PC1 and PC2 scores for endangered species have been obtained. The four PGLS models predicted the P50 of Cathaya should be −3.3~−4.2 MPa
Figure 4.

P n as a function of PC1 among non-endangered Pinaceae species globally. The black points showed the positions of endangered species. Api: Abies pinsapo. Car: Cathaya argyrophylla, Pkr: Pinus krempfii, Cbr: Cedrus brevifolia, Cli: Cedrus libani. Pal: Pinus albicaulis. PC1 was best represented by MAT (positively) and MTCM (positively). The percentage in the bracket next to PC1 means how much of the variability in the data is accounted for by PC1. Pkr deviated from the global trend, in linear with Broddrib et al.’s (2008) observation. Among all PGLS outputs, only PC1 has a marginally significant correlation with Pn (P = 0.09). For PGLS models, Pn = 8.13 + 1.72*PC1 (R2 = 0.11, t = 1.70, P = 0.091)
P 50 was positively related to Pn (trade-off) (P = 0.02) among the 13 non-threatened Pinaceae species, when PGLS was applied (Fig. 5A). If Cathaya and other threatened species were positioned, Cathaya and the two Cedrus species deviated from the general trend and were above the 90% quantile boundary, which means they maintained considerable Pn at such low P50 levels. There existed no significant relationship between dh and P50 among non-endangered species when PGLS was applied; despite that Cathaya had the narrowest tracheid, it was likely within the 10% and 90% quantile boundaries (Fig. 5B).
Figure 5.

The trade-off relationships between Pn and P50 (A), and the mean hydraulic diameter (dh) and P50 (B) among no-threatened Pinaceae species globally. Phylogenetic generalized linear model (PGLS) with branch length transformations optimized for the maximum likelihood was applied. Api: Abies pinsapo, Car: Cathaya argyrophylla, Cbr: Cedrus brevifolia, Cli: Cedrus libani, Pal: Pinus albicaulis. The black points showed the positions of endangered species. For the PGLS model, Pn = 19.47 + 3.48*P50 (R2 = 0.21, t = −2.68, P = 0.022). There was no significant relationship between dh and P50 (R2 = 0.03, t = −0.787, P = 0.44)
Discussion
Question 1: the phylogenetic signal of P50 and Pn across Pinaceae species
The hydraulic safety, which represents a realization of drought tolerance, had a strong phylogenetic signal consistent with niche conservation (Sup. Fig. 2A; Fig. 2A; Table 2). Moreover, to a less extent, the photosynthetic capacity, which represents a realization of carbon economy efficiency, also had a strong phylogenetic signal (Sup. Fig. 2B; Fig. 2B; Table 2). This could be validated by Pagel’s λ, PSR and model fitting comparison results. As such, not only morphological traits (Nobis et al., 2012) but also the key physiological functions of hydraulic safety and photosynthetic capacity showed phylogenetic niche conservation (PNC) signals among Pinaceae species. On the other hand, Wang et al. (2018) found that Pn showed more PNC than P50 among nine Picea species in mid-China, which is opposite to our finding. This is probably due to the ‘local scale effect’ (Forrestel et al., 2017) or statistical ‘funnel effect’ caused by the insufficient species amount investigated (Blomberg et al., 2003; Wright et al., 2005). The strong PNC of P50 and/or Pn as revealed by the present study is consistent with the observation on other gymnosperms (Brodribb et al., 2010; Pittermann et al., 2010; Willson et al., 2008). Ancient Pinaceae species tend to have high drought tolerance (low P50) and low photosynthetic capacity (low Pn), in accordance with their initial diversification in the Permian, which was characterized by physiological aridity, and consequently they have a number of traits that provide physiological drought tolerance, permitting them to survive on frozen soils, deep sand and steep slopes (Graham, 1999).
Question 2: the trait–environment relationship and the trade-off between P50 and Pn
There existed significant PGLS relationships (slope) between altitude (negative), WD (negative) and P50 among non-threatened species. The results demonstrated that moisture gradient (WD and/or MAP) largely drives the distribution of species with different cavitation resistance (Table 3; Fig. 3). This could be further demonstrated from the significant PGLS negative slope of the second component (PC2) vs. P50 relationship, while PC2 was largely determined by WD and MAP. High altitude, high WD and low MAP are conducive to high cavitation resistance (low P50) among non-threatened species. This output supports other studies that hydraulic safety largely determines species distribution across moisture gradients (Choat et al., 2018; Choat et al., 2012; Niinemets and Valladares, 2006; Willson and Jackson, 2006), although most of which did not pay attention to phylogenetic dependence of inter-specific data. On the contrary, there existed very weak or no relationships between environmental variables and photosynthetic capacity among non-threatened species (Table 3), suggesting that hydraulic safety is more adaptive than photosynthetic capacity across Pinaceae species. Only temperature (PC1) executed a marginal impact on Pn (Fig. 4), which indicated the temperature dependence of enzymes’ kinetics responsible for carbon assimilation (Brooks et al., 1985; Yamori et al., 2005).
It has been demonstrated that the hydraulic efficiency of branch has a tight association with leaf photosynthetic capacity (Brodribb et al., 2002; Scoffoni et al., 2016; Xiong et al., 2019). If there exists an adaptive trade-off relation between hydraulic efficiency and cavitation resistance among Pinaceae species (Choat et al., 2011; Gleason et al., 2016; Maherali et al., 2004), it means photosynthesis could have a negative association with cavitation resistance among the non-threatened species globally. As expected by either the carbon economy theory (Augusto et al., 2014) or kl–Pn coordination (Brodribb et al., 2002; Scoffoni et al., 2016; Xiong et al., 2019), the PGLS output of the 13 species did support the P50–Pn trade-off (Fig. 5A, P = 0.02), which was consistent with some other studies on gymnosperm species (Maherali et al., 2006; Pittermann et al., 2012; Sterck et al., 2012). On the contrary, the non-significant association between dh and P50 among the 14 Pinaceae species (Fig. 5B) did not support the theoretical model (Hacke et al., 2004, Martínez-Vilalta et al., 2002; Mayr et al., 2006), which means that the torus–margo structure of gymnosperms (Choat et al., 2009; Domec et al., 2009; Hacke et al., 2009) possibly masks the tracheid allometry deviated from the theoretical value based on relative simpler pit structure of angiosperms.
Question 3: does Cathaya deviate from the general relationship among non-threatened Pinaceae species between P50/Pn and environmental variables?
Cathaya did show eco-physiological outliers of P50/Pn to its Pinaceae relatives. Besides two Cedrus species (Cedrus libani, Cedrus brevifolia), Cathaya was the most cavitation-resistant Pinaceae species among the Pinaceae species reported. Pn was in the lowest 25% quartile range among compiled Pinaceae species (Sup. Table 1). Cathaya also had the narrowest tracheid among the investigated Pinaceae species (Sup. Table 2; Fig. 5B). More importantly, Cathaya had P50 under the low (10%) quantile boundaries of P50 vs. environmental variables (altitude, MAP, WD and moisture index PC2) (Fig. 3) and Pn under the 10% quantile boundary of Pn along the temperature gradient (temperature index PC1, Fig. 4). The extra high hydraulic safety of Cathaya and low photosynthetic capacity are probably due to its ancientness (Sup. Fig. 2 A, B, Table 2, Fig. 2), not developmental constraint. Interestingly, unlike Cathaya, the extra high hydraulic safety of Cedrus libani and Cedrus brevifolia could not be explained by their recent evolutionary history and may largely be attributed by developmental constraint (Sup. Fig. 2 A, B). High cavitation resistance is associated with inefficiency of carbon economy, leading to Cathaya’s lower growth competition ability with its coexisting angiosperm species (Augusto et al., 2014; Brodribb et al., 2007; Boyce et al., 2009; Körner, 1995; Lusk et al., 2003), and can only be confined to sites with arid (high water deficit), infertile and steep slopes in the subtropical mountains of South China.
However, it is worth noting that Cathaya was above the 90% quantile boundary of the Pn and P50 trade-off [although not as significant as the two Cedrus species (Fig. 5A)], indicating that it could maintain considerable Pn at such low P50 levels. The departure indicates the participation of some other life history trade-offs. For example, extra high cavitation resistance could come at a significant energy cost in terms of insufficient reproductive effort and does not necessarily compromise the competition capacity of mature individuals. Actually, it was found that the average seed weight of Cathaya was 0.0197 g [only 6% of the average seed weight of arbour species (Silvertown, 1982)], with over 60% of seeds without viability (Xie et al., 2000), indicating insufficient energy input, although other mechanism(s) could not be overlooked (Hunter Jr and Gibbs, 2006; Vaario et al., 2006; Wang et al., 2006). The other mechanism(s) include specific arbuscular mycorrhizal root interaction (Vaario et al., 2006) and low gene flow between isolated populations (Wang et al., 2006). Another possibility is that some unusual structure characteristics such as torus thickness and depth of the pit chamber (Hacke and Jansen, 2009; Pittermann et al., 2005) and tracheid wall reinforcement (Hacke et al., 2017), but not tracheid diameter (Fig. 5B), drive the deviation. It is worth noting that Cathaya does not have tubular sclereids in the leaf (Dayong Fan, unpublished data), unlike the tropic unique flat-leaved pine, Pinus krempfii (Brodribb et al., 2008). The torus-pit structure characteristics probably also led to the absence of the trade-off between P50 and ks within Cathaya (Sup. Fig. 4A), but not the P50–wood density relationship (Sup. Fig. 4B). As such, the mechanism(s) underlying the deviation of Cathaya (and the two Cedrus species) from the Pn and P50 trade-off merits future investigation.
Interestingly, in the relationships between hydraulic safety and environment, three threatened species (Pinus caribaea, Pinus albicaulis, Abies pinsapo) were between the 10% and 90% quantile boundaries (Fig. 3), which were quite different from Cathaya and the two Cedrus species. The result suggests different mechanisms of endangerment for the six threatened species. For example, Cathaya and the two Cedrus were restricted to narrow range and habitats (Vidaković, 1991; Xie et al., 1999b), since the site-specific environmental conditions might be quite different from the regional environmental conditions (the Climate data were extracted based on regional scale), and their deviations from trait–environment relationships could be expected, while for the other three species, they have broader distribution areas (Farjon, 1990; Thompson et al., 1999), so probably other mechanisms [e.g. inter-specific interaction, human disturbance (Hunter Jr and Gibbs, 2006)] but not the limited adaptability (hydraulic safety) underpinned their vulnerability to extinction. As such, the PQR method on functional traits and their relationships to the environment can offer a useful tool to reveal the mechanism of species endangerment, in support of the viewpoint by Newsome et al. (2020). It is also worth noting that although the phylogenetic comparative methods applied in the present study could minimize the Type I error rates of non-phylogenetically corrected cross-species correlations (Ackerly, 2000; Blomberg et al., 2003), other factors such as non-random sampling, intra-specific variation and measurement error could impact the output (Ives et al., 2007; Huey et al., 2019). As such, further investigation on the difference of eco-physiological traits between Cathaya argyrophylla and its Pinaceae relatives is warranted.
Conclusions
In the present study, we found that the hydraulic safety, and to a less extent, photosynthetic capacity, had a strong phylogenetic signal consistent with niche conservation among Pinaceae species. Hydraulic safety largely determined the distribution of non-threatened Pinaceae species across moisture gradients at the global scale. There was a phylogenetic trade-off between hydraulic safety and photosynthesis across non-threatened Pinaceae species. Cathaya is a shade-intolerant, high cavitation resistant, low photosynthetic capacity Pinaceae species. These traits were largely attributed by its ancientness. Cathaya also showed eco-physiological outliers to its Pinaceae relatives because it had lower Pn and P50 outside the 10% quantile boundaries along moisture and/or temperature gradients. The safety–photosynthesis trade-off could provide an explanation on how Cathaya is vulnerable to extinction, but other factors contributing to its endangered status could not be overlooked. The somewhat departure of Cathaya from the safety–photosynthesis trade-off merits future investigation. We suggested that the PQR method could be a useful tool to reveal the mechanism of species endangerment of Pinaceae species under the global climate change.
Supplementary Material
Acknowledgements
The authors thank Dr Thomas M. Newsome (from Global Ecology Lab, School of Life and Environmental Sciences, The University of Sydney, Sydney, New South Wales, Australia), for providing R code in terms of the polygenetic quantile regression method.
Funding
This work was supported by the National Key Research and Development Program of China (2019YFD1100403), National Natural Science Foundation of China (No. 31870430) and Hubei Shennongjia Forest Ecosystem National Field Scientific Observation and Research Station.
Supplementary material
Supplementary material is available at Conservation Physiology online.
References
- Ackerly DD. (2000) Taxon sampling, correlated evolution, and independent contrasts. Evolution 54: 1480–1492. [DOI] [PubMed] [Google Scholar]
- Augusto L, Davies T, Delzon S, De Schrijver A (2014) The enigma of the rise of angiosperms: can we untie the knot? Ecol Lett 17: 1326–1338. [DOI] [PubMed] [Google Scholar]
- Blomberg SP, Garland T Jr, Ives AR (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57: 717–745. [DOI] [PubMed] [Google Scholar]
- Boyce CK, Brodribb TJ, Feild TS, Zwieniecki MA (2009) Angiosperm leaf vein evolution was physiologically and environmentally transformative. Philos Trans R Soc B Biol Sci 276: 1771–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb T, Holbrook NM, Gutierrez M (2002) Hydraulic and photosynthetic co-ordination in seasonally dry tropical forest trees. Plant Cell Environ 25: 1435–1444. [Google Scholar]
- Brodribb TJ, Feild TS (2008) Evolutionary significance of a flat-leaved pinus in vietnamese rainforest. New Phytol 178: 201–209. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Feild TS (2010) Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification. Ecol Lett 13: 175–183. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Feild TS, Jordan GJ (2007) Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol 144: 1890–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A, Farquhar G (1985) Effect of temperature on the co 2/o 2 specificity of ribulose-1, 5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Planta 165: 397–406. [DOI] [PubMed] [Google Scholar]
- Choat B, Brodribb TJ, Brodersen CR, Duursma RA, Lopez R, Medlyn BE (2018) Triggers of tree mortality under drought. Nature 558: 531–539. [DOI] [PubMed] [Google Scholar]
- Choat B et al. (2012) Global convergence in the vulnerability of forests to drought. Nature 491: 752–755. [DOI] [PubMed] [Google Scholar]
- Choat B, Medek DE, Stuart SA, Pasquet-Kok J, Egerton JJ, Salari H, Sack L, Ball MC (2011) Xylem traits mediate a trade-off between resistance to freeze–thaw-induced embolism and photosynthetic capacity in overwintering evergreens. New Phytol 191: 996–1005. [DOI] [PubMed] [Google Scholar]
- Choat B, Pittermann J (2009) New insights into bordered pit structure and cavitation resistance in angiosperms and conifers. New Phytol 182: 557–560. [DOI] [PubMed] [Google Scholar]
- Chun W. (1958) A new genus of pinaceae-cathaya chun et kuang gen. Nov., from the southern and western China. Botanicheskii Zhurnal SSSR 43: 461–470. [Google Scholar]
- Crisp MD, Cook LG (2012) Phylogenetic niche conservatism: what are the underlying evolutionary and ecological causes? New Phytol 196: 681–694. [DOI] [PubMed] [Google Scholar]
- Culver DC, Master LL, Christman MC, Hobbs HH III (2000) Obligate cave fauna of the 48 contiguous United States. Conserv Biol 14: 386–401. [Google Scholar]
- Davis SD, Sperry JS, Hacke UG (1999) The relationship between xylem conduit diameter and cavitation caused by freezing. Am J Bot 86: 1367–1372. [PubMed] [Google Scholar]
- Delucia EH, Maherali H, Carey EV (2000) Climate-driven changes in biomass allocation in pines. Global Change Biol 6: 587–593. [Google Scholar]
- Domec J-C, Schäfer K, Oren R, Kim HS, McCarthy HR (2010) Variable conductivity and embolism in roots and branches of four contrasting tree species and their impacts on whole-plant hydraulic performance under future atmospheric co2 concentration. Tree Physiol 30: 1001–1015. [DOI] [PubMed] [Google Scholar]
- Domec J-C, Warren JM, Meinzer FC, Lachenbruch B (2009) Safety factors for xylem failure by implosion and air-seeding within roots, trunks and branches of young and old conifer trees. Iawa J 30: 101–120. [Google Scholar]
- Fan D, Zhang W, Chen Z, Xie Z (2005) Acclimation of Cathaya argyrophylla to light across a gradient of canopy openness. Acta Phytoecol Sinica 29: 713–723. [Google Scholar]
- Farjon A. (1990) Pinaceae. Drawings and descriptions of the genera abies, cedrus, pseudolarix, keteleeria, nothotsuga, tsuga, cathaya, pseudotsuga, larix and picea. Koeltz scientific books.
- Felizola Diniz Filho JA, Rangel TF, Santos T, Mauricio Bini L (2012) Exploring patterns of interspecific variation in quantitative traits using sequential phylogenetic eigenvector regressions. Evolution 66: 1079–1090. [DOI] [PubMed] [Google Scholar]
- Ferguson D, Liu Y, Zetter R (1997) The Paleoendemic Plants of East Asia: Evidence From the Fossil Record for Changing Distribution Patterns. University of Hong Kong. [Google Scholar]
- Fick SE, Hijmans RJ (2017) Worldclim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol 37: 4302–4315. [Google Scholar]
- Forrestel EJ, Donoghue MJ, Edwards EJ, Jetz W, Toit JC, Smith MD (2017) Different clades and traits yield similar grassland functional responses. Proc Natl Acad Sci USA 114: 705–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freckleton RP, Harvey PH, Pagel M (2002) Phylogenetic analysis and comparative data: a test and review of evidence. Am Nat 160: 712–726. [DOI] [PubMed] [Google Scholar]
- Fu L. (1992) The Red Book of Chinese Plants–Rare and Endangered Plants. Science Press, Beijing. [Google Scholar]
- Gleason SM et al. (2016) Weak tradeoff between xylem safety and xylem-specific hydraulic efficiency across the world’s woody plant species. New Phytol 209: 123–136. [DOI] [PubMed] [Google Scholar]
- Graham A. (1999) Late Cretaceous and Cenozoic History of North American Vegetation: North of Mexico. Oxford University Press on Demand. [Google Scholar]
- Hacke UG, Jansen S (2009) Embolism resistance of three boreal conifer species varies with pit structure. New Phytol 182: 675–686. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS, Pittermann J (2004) Analysis of circular bordered pit function ii. Gymnosperm tracheids with torus-margo pit membranes. Am J Bot 91: 386–400. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Spicer R, Schreiber SG, Plavcová L (2017) An ecophysiological and developmental perspective on variation in vessel diameter. Plant Cell Environ 40: 831–845. [DOI] [PubMed] [Google Scholar]
- Harvey PH, Pagel MD (1991) The Comparative Method in Evolutionary Biology. Oxford University Press Oxford. [Google Scholar]
- Hawkins BA, Rueda M, Rangel TF, Field R, Diniz-Filho JAF (2014) Community phylogenetics at the biogeographical scale: cold tolerance, niche conservatism and the structure of North American forests. J Biogeogr 41: 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Wang F (1984) Anatomical studies of Cathaya (Pinaceae). Am J Bot 71: 727–735. [Google Scholar]
- Huey RB, Garland T Jr, Turelli M (2019) Revisiting a key innovation in evolutionary biology: Felsenstein’s “phylogenies and the comparative method”. Am Nat 193: 755–772. [DOI] [PubMed] [Google Scholar]
- Hunter ML Jr, Gibbs JP (2006) Fundamentals of Conservation Biology. John Wiley & Sons. [Google Scholar]
- IUCN (2018) The iucn red list of threatened species In Version 2018–1. Union for Conservation of Nature and Natural Resources, International. [Google Scholar]
- Ives AR, Midford PE, Garland T Jr (2007) Within-species variation and measurement error in phylogenetic comparative methods. Syst Biol 56: 252–270. [DOI] [PubMed] [Google Scholar]
- Koenker R, Portnoy S, Ng P, Zeileis A, Grosjean P, Ripley B (2018) Quantreg: Quantile regression, version 5.3. 4. R package.
- Kolb KJ, Sperry JS (1999) Differences in drought adaptation between subspecies of sagebrush (Artemisia tridentata). Ecology 80: 2373–2384. [Google Scholar]
- Körner C. (1995) Leaf diffusive conductances in the major vegetation types of the globe. Ecophysiology of Photosynthesis. Springer; pp 463–490. [Google Scholar]
- Kozak KH, Wiens JJ (2010) Niche conservatism drives elevational diversity patterns in appalachian salamanders. Am Nat 176: 40–54. [DOI] [PubMed] [Google Scholar]
- Lambers H, Chapin FS III, Pons TL (2008) Plant Physiological Ecology, Springer Science & Business Media.
- Lambers H, Poorter H (1992) Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences. Advances in Ecological Research, Vol. 23. Elsevier, pp 187–261.
- Li YL, Liu XG, Hao K, Yang QL, Yang XQ, Zhang WH, Cong Y (2019) Light-response curve of photosynthesis and model fitting in leaves of Mangifera indica under different soil water conditions. Photosynthetica 57: 796–803. [Google Scholar]
- Linton M, Sperry J, Williams D (1998) Limits to water transport in Juniperus osteosperma and Pinus edulis: implications for drought tolerance and regulation of transpiration. Funct Ecol 12: 906–911. [Google Scholar]
- Liu Y, Basinger J (2000) Fossil Cathaya (Pinaceae) pollen from the Canadian High Arctic. Int J Plant Sci 161: 829–847. [Google Scholar]
- Losos JB. (2008) Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol Lett 11: 995–1003. [DOI] [PubMed] [Google Scholar]
- Lusk C. (2008) Constraints on the evolution and geographical range of pinus. New Phytol 178: 1–3. [DOI] [PubMed] [Google Scholar]
- Lusk CH, Wright I, Reich PB (2003) Photosynthetic differences contribute to competitive advantage of evergreen angiosperm trees over evergreen conifers in productive habitats. New Phytol 160: 329–336. [DOI] [PubMed] [Google Scholar]
- Maherali H, Moura CF, Caldeira MC, Willson CJ, Jackson RB (2006) Functional coordination between leaf gas exchange and vulnerability to xylem cavitation in temperate forest trees. Plant Cell Environ 29: 571–583. [DOI] [PubMed] [Google Scholar]
- Maherali H, Pockman WT, Jackson RB (2004) Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology 85: 2184–2199. [Google Scholar]
- Martínez-Vilalta J, Prat E, Oliveras I, Piñol J (2002) Xylem hydraulic properties of roots and stems of nine mediterranean woody species. Oecologia 133: 19–29. [DOI] [PubMed] [Google Scholar]
- Martínez-Vilalta J, Sala A, Piñol J (2004) The hydraulic architecture of pinaceae–a review. Plant Ecol 171: 3–13. [Google Scholar]
- Martínez-Vilalta J, Mencuccini M, Álvarez X, Camacho J, Loepfe L, Piñol J (2012) Spatial distribution and packing of xylem conduits. Am J Bot 99: 1189–1196. [DOI] [PubMed] [Google Scholar]
- Mayr S, Hacke U, Schmid P, Schwienbacher F, Gruber A (2006) Frost drought in conifers at the alpine timberline: xylem dysfunction and adaptations. Ecology 87: 3175–3185. [DOI] [PubMed] [Google Scholar]
- Newsome TM, Wolf C, Nimmo DG, Kopf RK, Ritchie EG, Smith FA, Ripple WJ (2020) Constraints on vertebrate range size predict extinction risk. Glob Ecol Biogeogr 29: 76–86. [Google Scholar]
- Niinemets Ü, Valladares F (2006) Tolerance to shade, drought, and waterlogging of temperate northern hemisphere trees and shrubs. Ecol Monogr 76: 521–547. [Google Scholar]
- Nobis MP, Traiser C, Roth-Nebelsick A (2012) Latitudinal variation in morphological traits of the genus pinus and its relation to environmental and phylogenetic signals. Plant Ecol Divers 5: 1–11. [Google Scholar]
- Oliveras I, Martínez-Vilalta J, Jimenez-Ortiz T, Lledó MJ, Escarré A, Piñol J (2003) Hydraulic properties of Pinus halepensis, Pinus pinea and Tetraclinis articulata in a dune ecosystem of Eastern Spain. Plant Ecol 169: 131. [Google Scholar]
- Pammenter NW, Vander Willigen C (1998) A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiol 18: 589–593. [DOI] [PubMed] [Google Scholar]
- Pérez-Harguindeguy N, Díaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, Bret-Harte MS, Cornwell WK, Craine JM (2013) Gurvich DE et al. New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 61: 167–234. [Google Scholar]
- Pittermann J, Choat B, Jansen S, Stuart SA, Lynn L, Dawson TE (2010) The relationships between xylem safety and hydraulic efficiency in the cupressaceae: the evolution of pit membrane form and function. Plant Physiol 153: 1919–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittermann J, Sperry JS, Hacke UG, Wheeler JK, Sikkema EH (2005) Torus-margo pits help conifers compete with angiosperms. Science 310: 1924–1924. [DOI] [PubMed] [Google Scholar]
- Pittermann J, Stuart SA, Dawson TE, Moreau A (2012) Cenozoic climate change shaped the evolutionary ecophysiology of the Cupressaceae conifers. Proc Natl Acad Sci USA 109: 9647–9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S, Yang Y, Tang CQ, Momohara A, Yi S, Ohsawa M (2016) Effective conservation measures are needed for wild Cathaya argyrophylla populations in China: insights from the population structure and regeneration characteristics. Forest Ecol Manag 361: 358–367. [Google Scholar]
- Ran J, Shen T, Wu H, Gong X, Wang X (2018) Phylogeny and evolutionary history of pinaceae updated by transcriptomic analysis. Mol Phylogenet Evol 129: 106–116. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE. (2010) Evolutionary diversification, coevolution between populations and their antagonists, and the filling of niche space. Proc Natl Acad Sci USA 107: 1265–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoffoni C, Chatelet DS, Pasquet-kok J, Rawls M, Donoghue MJ, Edwards EJ, Sack L (2016) Hydraulic basis for the evolution of photosynthetic productivity. Nat Plants 2: 1–8. [DOI] [PubMed] [Google Scholar]
- Silvertown J. (1982) Introduction to Plant Population, Longman Higher Education.
- Sperry J, Saliendra N (1994) Intra-and inter-plant variation in xylem cavitation in Betula occidentalis. Plant Cell Environ 17: 1233–1241. [Google Scholar]
- Sterck FJ, Martínez-Vilalta J, Mencuccini M, Cochard H, Gerrits P, Zweifel R, Herrero A, Korhonen JF, Llorens P, Nikinmaa E (2012) Understanding trait interactions and their impacts on growth in Scots pine branches across europe. Funct Ecol 26: 541–549. [Google Scholar]
- Taggart RE, Cross AT (2009) Global greenhouse to icehouse and back again: the origin and future of the boreal forest biome. Glob Planet Change 65: 115–121. [Google Scholar]
- Thompson RS, Anderson KH, Bartlein PJ (1999) Atlas of Relations Between Climatic Parameters and Distributions of Important Trees and Shrubs in North America. US Department of the Interior, US Geological Survey. [Google Scholar]
- Tyree MT, Sperry JS (1988) Do woody plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress?: Answers from a model. Plant Physiol 88: 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaario L-M, Xing S-T, Xie Z-Q, Lun Z-M, Sun X, Li YH (2006) In situ and in vitro colonization of cathaya argyrophylla (pinaceae) by ectomycorrhizal fungi. Mycorrhiza 16: 137–142. [DOI] [PubMed] [Google Scholar]
- Vidaković M. (1991) Conifers: Morphology and Variation, Grafičko Zavod Hrvatske.
- Wang H, Ge S (2006) Phylogeography of the endangered Cathaya argyrophylla (Pinaceae) inferred from sequence variation of mitochondrial and nuclear DNA. Mol Ecol 15: 4109–4122. [DOI] [PubMed] [Google Scholar]
- Wang M, Wang J, Zhang A, Zhang X, Sun S, Zhao C (2018) Functional traits related to environmental divergence in combination with phylogenetic relationship of picea species. Dendrobiology 80: 131–142. [Google Scholar]
- Webb CO, Ackerly DD, Kembel SW (2008) Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics 24: 2098–2100. [DOI] [PubMed] [Google Scholar]
- Wheeler JK, Sperry JS, Hacke UG, Hoang N (2005) Inter-vessel pitting and cavitation in woody Rosaceae and other vesselled plants: a basis for a safety versus efficiency trade-off in xylem transport. Plant Cell Environ 28: 800–812. [Google Scholar]
- Wiens JJ, Ackerly DD, Allen AP, Anacker BL, Buckley LB, Cornell HV, Damschen EI, Jonathan Davies T, Grytnes JA, Harrison SP (2010) Niche conservatism as an emerging principle in ecology and conservation biology. Ecol Lett 13: 1310–1324. [DOI] [PubMed] [Google Scholar]
- Wiens JJ, Graham CH (2005) Niche conservatism: integrating evolution, ecology, and conservation biology. Annu Rev Ecol Evol Syst 36: 519–539. [Google Scholar]
- Willson CJ, Jackson RB (2006) Xylem cavitation caused by drought and freezing stress in four co-occurring Juniperus species. Physiol Plant 127: 374–382. [Google Scholar]
- Willson CJ, Manos PS, Jackson RB (2008) Hydraulic traits are influenced by phylogenetic history in the drought-resistant, invasive genus Juniperus (Cupressaceae). Am J Bot 95: 299–314. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Cornelissen JH, Falster DS, Garnier E, Hikosaka K, Lamont BB, Lee W, Oleksyn J, Osada N (2005) Assessing the generality of global leaf trait relationships. New Phytol 166: 485–496. [DOI] [PubMed] [Google Scholar]
- Xie Z, Chen W (1999. a) The endangering causes and preserving strategies for cathaya argyrophylla, a plant endemic to China. Acta Ecologica Sinica 23: 1–7. [Google Scholar]
- Xie Z, Chen W, Lu P, Hu D (1999. b) The demography and age structure of the endangered plant population of Cathaya argyrophylla. Acta Ecologica Sinica 19: 523–528. [Google Scholar]
- Xie Z, Li Q (2000) Seed characteristics of endangered plant Cathaya argyrophylla. Acta Phytoecol Sinica 24: 82–86. [Google Scholar]
- Xiong D, Nadal M (2019) Linking water relations and hydraulics with photosynthesis. Plant J 101: 800–815. [DOI] [PubMed] [Google Scholar]
- Yamori W, Noguchi K, Terashima I (2005) Temperature acclimation of photosynthesis in spinach leaves: analyses of photosynthetic components and temperature dependencies of photosynthetic partial reactions. Plant Cell Environ 28: 536–547. [Google Scholar]
- Ying J, Ma C, Li L, Zhang Z, Zhang W (1983) Studies on the Cathaya communities. Acta Bot Sinica 25: 157–169. [Google Scholar]
- Zanne AE, Tank DC, Cornwell WK, Eastman JM, Smith SA, FitzJohn RG, McGlinn DJ, O’Meara BC, Moles AT, Reich PB (2014) Three keys to the radiation of angiosperms into freezing environments. Nature 506: 89–92. [DOI] [PubMed] [Google Scholar]
- Zhang W, Fan D, Xie Z, Jiang X (2005) The seasonal photosynthetic responses of seedlings of the endangered plant Cathaya argyrophylla to different growth light environments. Biodiversity Sci 13: 387–397. [Google Scholar]
- Zimmermann M. (1983) Xylem Structure and the Ascent of Sap. Springer-Verlag, Berlin. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


