Abstract
Introduction
Sarcoidosis is a systemic granulomatous disease of unknown cause afflicting young to middle-aged adults. The majority of patients with active pulmonary sarcoidosis complain of overwhelming fatigue, which often persists despite administration of immune-modulating drugs typically used to treat sarcoidosis. Nicotine offers an alternative to conventional treatments, which are associated with a spectrum of serious untoward effects, including diabetes mellitus, osteoporosis, bone marrow suppression, severe infections, cirrhosis. The described pilot randomized trial aims to provide preliminary data required to design subsequent Phase II/III trials to formally evaluate nicotine as a novel low-cost and highly-effective, safe treatment option for patients with active pulmonary sarcoidosis.
Methods
and Design: This is a randomized double-blind controlled trial of adults with confirmed pulmonary sarcoidosis, allocated in equal proportion to sustained release transdermal nicotine or placebo patch. The primary objective outcome is the improvement in forced vital capacity at study week 26 from baseline measurement. Secondary measures include lung texture score, and self-reported outcomes including the Fatigue Assessment Scale, the St George's Respiratory Questionnaire, and the Sarcoidosis Assessment Tool.
Discussion
Current therapies for active pulmonary sarcoidosis, remain either expensive and often with numerous side-effects, as with novel industry developed therapies, or with reduced quality of life, as with corticosteroids. Nicotine therapy provides promise as a safe, available, and cost-effective intervention strategy, which we expect to be acceptable to patients.
ClinicalTrials.gov
Keywords: Randomized controlled trial, Pulmonary sarcoidosis, Nicotine, Forced vital capacity, Quality of Life
1. Introduction
Sarcoidosis is a systemic granulomatous disease of unknown cause afflicting young to middle-aged adults. Sarcoidosis is characterized by the development of non-necrotizing granulomatous inflammation in the absence of identifiable infectious, autoimmune or environmental causes. The disease typically involves the lungs, frequently leading to impaired exercise tolerance and associated dyspnea. The majority of patients with active pulmonary sarcoidosis complain of overwhelming fatigue, which often persists despite administration of immune-modulating drugs typically used to treat sarcoidosis [1,2]. First-line therapies for sarcoidosis are often ineffective, poorly tolerated, and promote long-term health complications. Industry-sponsored clinical trials have tested proprietary, expensive, and potentially toxic therapies that are typically reserved for refractory cases of sarcoidosis [3]. Therefore, investigation of alternative therapies, particularly those already approved for other uses, is of particular interest. Repurposing of well tolerated and widely available therapies is efficient in terms of time and dollars spent for the discovery of effective treatments for patients with sarcoidosis.
Nicotine may be beneficial for the treatment of pulmonary sarcoidosis. At least three independent epidemiological studies have indicated that smokers and chewing tobacco users, who are chronically exposed to nicotine, have nearly a 2-fold lower risk of developing sarcoidosis [[4], [5], [6]]. Nicotine reprograms inflammatory pathways through the actions of α7 nicotinic receptors, thereby inhibiting various pro-inflammatory immune responses [[7], [8], [9], [10]]. Reprogramming of Th1 immunity could explain the reported benefits of nicotine treatment for Crohn's disease (e.g., nicotine enemas) and hypersensitivity pneumonitis, granulomatous disorders of the intestines and lungs, respectively [11,12]. Our pilot observational work has shown that nicotine is well-tolerated and normalizes Th1 type immune responses in patients with active pulmonary sarcoidosis [13].
Nicotine offers an alternative to conventional treatments, which are associated with a spectrum of serious untoward effects, including diabetes mellitus, osteoporosis, bone marrow suppression, severe infections, cirrhosis [14,15]. For instance, the use of corticosteroids, the mainstay of pulmonary sarcoidosis treatment, promotes all of the aforementioned detrimental health risks and despite being efficacious for suppressing sarcoidosis disease activity is independently associated with a reduced quality-of-life [16]. Potent anti-TNFα agents are typically reserved for the treatment of refractory disease, given they are expensive, are not formally approved for use in sarcoidosis and they may cause serious complications [14,15,17]. Nicotine has anti-inflammatory properties that could explain the lower risk of sarcoidosis among smokers [[4], [5], [6]], and nicotine my offer improvement in quality of life relating to improved mental focus and attentiveness, which are common symptoms of sarcoidosis [18]. Based upon these observations and our supportive preliminary data, we hypothesized that nicotine would be safe and well-tolerated therapy in patients with active pulmonary sarcoidosis and that it would be an effective therapy for sarcoidosis. If shown to be effective, the contribution has the potential to be significant given nicotine is readily available to patients, has been shown to be well-tolerated in other patient groups, and represents a low-cost, low-risk alternative to currently available treatments. Our pilot randomized trial aims to provide preliminary data required to design subsequent Phase II/III trials aimed at the formal evaluation of nicotine as a novel low-cost and highly-effective safe treatment option for patients with active pulmonary sarcoidosis.
2. Methods
2.1. Overview of study design, rationale and objectives
This is a randomized, parallel 2-arm trial for adults with confirmed pulmonary sarcoidosis. It is double-blinded and placebo-controlled. We note that this pilot randomized trial is primarily designed to provide clinical efficacy and safety data to inform a decision to move onto a larger and more comprehensive multi-site phase III randomized clinical trial. The current objective standard for assessing disease status in patients with pulmonary sarcoidosis are the clinical endpoints of serial pulmonary function testing, particularly forced vital capacity (FVC). Therefore, our first aim is to measure the improvement over baseline at 24 weeks of therapy in FVC in patients assigned to transdermal nicotine therapy compared to those assigned to placebo.
However, serial FVC measurements can vary by up to 10%, and expected changes in FVC with effective therapies is typically less than 5%, necessitating larger clinical trials to demonstrate definitive efficacy. In contrast, changes in radiographic pulmonary disease manifestations in the context of effective therapies for sarcoidosis are less variable than FVC [19], and more directly represents changes in disease burden in lung tissues. Changes in pulmonary radiographic disease burden are also an accepted surrogate for FVC and other clinical endpoints [[20], [21], [22], [23]]. Therefore, we have developed an objective computerized CT image analysis tool that can detect the common manifestations of pulmonary sarcoidosis and correlates strongly with FVC [24]. Of note, our data demonstrates strong correlations between the Lung Texture Score (LTS) derived from computerized CT image analysis and lung disease severity (as reflected by FVC, total lung capacity (TLC), and lung diffusing capacity) in other interstitial lung diseases, which further validates this novel lung CT image analysis approach. Our second aim is to explore changes in LTS at 24 weeks of therapy between randomized groups.
A further aim is to assess subjective clinical endpoints relating to disease-specific symptoms. For instance, fatigue is among the most common and disabling symptoms associated with sarcoidosis, which is often refractory to conventional sarcoidosis treatments [16,25], and there is reason to believe that nicotine treatment will attenuate these symptoms [18,26,27]. Finally, we will determine the safety and tolerability of nicotine therapy for patients with pulmonary sarcoidosis.
2.2. Ethics approval and consent to participate
The trial protocol and consent documents were approved under a common IRB application by The Ohio State University Biomedical Institutional Review Board (IRB), as the primary site, and the Cleveland Clinic Foundation IRB. Prior to study specific activities, consent was obtained from all potential participants.
2.3. Recruitment, enrollment, and retention
Patients were approached, consented, and enrolled as they were sequentially identified at two recruitment sites (The Ohio State University Wexner Medical Center (OSUMC) and the Cleveland Clinic Foundation) based on study eligibility criteria (Fig. 1). Diagnosis of pulmonary sarcoidosis was determined based on established clinical criteria [28]. If during screening the potential participant had clinically significant worsening of sarcoidosis that required adjustment of medication, this potential participant was considered a screen failure and not eligible at that screening visit. Eligible participants were required to be on no treatment or have stable treatment for at least one month prior to enrollment. Eligible and consented patients were randomized in a 1:1 ratio to receive transdermal nicotine up to 21 mg/daily or a similar-appearing placebo patch. Baseline measurements, including demographics, medical history, vital signs, current medications, symptoms, and pulmonary function tests (PFTs) were made at the screening and baseline visits on all patients prior to receiving their randomized assigned treatment. A chest x-ray was performed at screening if one had not been done within the past year. Prior to all radiation procedures, a urine pregnancy test was performed on female patients.
Fig. 1.
Inclusion & Exclusion criteria.
To cover travel, parking, and time expenses related to participation, participants were provided $50 stipend at each of the six in person study visits, and when necessary, more support based on the distance needed to travel. Finally, the study team arranged travel to and from study visits by taxi.
2.4. Study treatment
Sustained release transdermal nicotine (7 mg, 14 mg, and 21 mg daily dose patches) and matching placebo patches were provided to all randomized participants. Participants were instructed to apply the nicotine patches or matching control patches daily according to the manufacturer's recommendations and in compliance with an FDA approved dosing regimen.
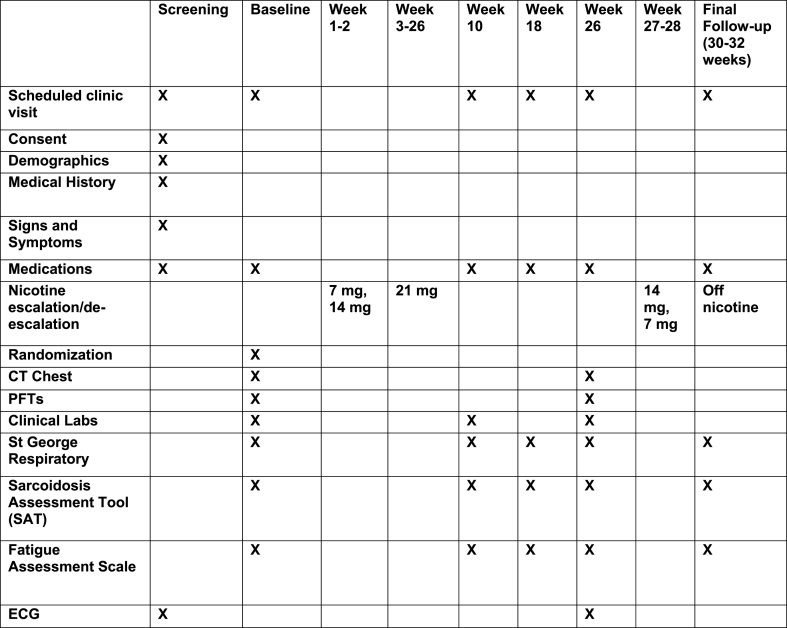
Treatment Regimen: To optimize patient compliance and minimize side effects, nicotine (or matching placebo patch) treatment began with a 2-week phase-in period, during which the dose of treatment was increased weekly towards the highest tolerated dose, via transdermal patch (7 mg patch, 14 mg patch, 21 mg patch). Patients were maintained on the highest tolerated dose (7–21 mg patch) through study week 26. Nicotine treatment was then deescalated weekly (i.e., 14 mg, 7 mg), and discontinued (Fig. 2). When intolerance developed, the dose was reduced to the highest previously tolerated dose. Intolerance to nicotine was determined as any side effect, minor or serious, which caused the patient to choose to or be recommended by their clinical team to reduce dose.
Fig. 2.
Study Timeline and Follow up.
Randomization and Blinding: Patients were randomized to nicotine or placebo patch in a 1:1 ratio through the electronic data capture platform REDCap [29]. The randomization scheme was developed and implemented by the study statisticians; it was stratified by study site in permuted blocks of varying size of two and four. The allocation scheme was labeled “A” or “B”, with the allocation key held with one of the study statisticians and one non-clinical study staff member who labeled study drug to ensure clinical staff who recruited and followed participants were blinded to treatment group.
Preparation, Administration, and Monitoring of Study Drug: Study drug was pre-packaged with an assigned study randomization number corresponding to the allocated randomized treatment. At each clinical center, identically labeled nicotine and placebo patches were distributed to participants as per their study randomization number. Participants were instructed to apply one new patch every day to clean and dry skin. Participants were advised to apply patches to a new site on the body each day to avoid local skin irritation. Study drug was distributed on the day of randomization and at week 10, or as needed to modify the study drug dose. Subject compliance with study therapy and abstinence from nicotine use external to the trial was monitored objectively at regular intervals, by measuring serum cotinine levels, a stable nicotine metabolite (baseline, study week 10, study week 26).
Prior and Concomitant Therapy: Study subjects remained on prior and concomitant medications, as tolerated, for the duration of the study. The addition of new medications or changes in current medication doses were not advised, and noted as a protocol violation; however patients were not withdrawn from study therapy in these instances, unless otherwise necessary due to adverse reactions or by the choice of the study subject.
2.5. Outcomes
Primary outcome: The primary endpoint of the study will be improvement in FVC over baseline at study week 26. Post-bronchodilator FVC, forced expiratory volume in 1 s (FEV1), peak expiratory flow rate (PEFR), and forced expiratory flow at 25%–75% of vital capacity (FEF25-75) were measured according to the body temperature, pressure, and saturation (BTPS) standard convention. PFTs will be repeated up to 8 times to obtain 3 acceptable readings according to American Thoracic Society (ATS) guidelines. Acceptable repeatability is achieved when the difference between the largest and the next largest FVC is ≤ 0.150 L and the difference between the largest and the next largest FEV1 is ≤ 0.150 L. However, if a subject was too tired to meet this consistency requirement, the PFT values from the best effort were recorded as long as this best effort meets the other ATS criteria. Subjects were advised to refrain from using short acting bronchodilators for at least 4 h and long-acting bronchodilators for at least 12 h prior to the screening visit. Percent predicted FVC and FEV1 were calculated according to the Crapo equation, with correction for race [30].
Secondary outcomes: The secondary clinical endpoint of major interest was the improvement in total burden of lung disease over baseline, as reflected by the computer-generated CT image analysis or changes in lung function and measured by LTS [24]. CT scans were performed at the baseline assessment or within 3 months prior to enrollment so long as the following criteria were also met: 1) the CT scan was obtained for clinical reasons and on the same scanner used for the research study, 2) the patient's sarcoidosis treatment regimen was unchanged within the past 3 months, and 3) respiratory symptoms (cough, dyspnea) were stable. The imaging protocol was standardized across the clinical centers: Non-contrast CT scans were obtained by using a helical technique with a 16- or 64- detector row CT scanner. Images were obtained from lung apices to the lung bases in a single breath hold with the following parameters: collimation, 1.25 or 0.625 mm; field of view, 36 cm; beam pitch, 1.35 or 1.375; gantry speed, 0.5 or 0.6 s/rotation; 120 kVp; 150–200 mA. Other outcomes include serum cotinine, as a measure of nicotine metabolism, and patient reported surveys including the Fatigue Assessment Scale, the St. George Respiratory Questionnaire, and the Sarcoidosis Assessment tool [[31], [32], [33], [34]].
Study participants underwent scheduled venipuncture under sterile technique for the purpose of obtaining 10 ml of blood to measure nicotine and nicotine metabolites (at baseline, 10, and 28 weeks). The blood samples were stored on site at OSU and CCF at −80 °F.
Standardized surveys were administered at scheduled study-related visits or by mail (self-addressed first class mail through the US postal service). The Fatigue Assessment Scale (FAS) is a 10-item patient self-report instrument with five items reflecting physical fatigue and five assessing mental fatigue. The response options range from never (1) to always (5) for a total score from 10 to 50 [34]. The St George's Respiratory Questionnaire (SGRQ) is a disease-specific survey instrument designed for use with adult subjects to assess the impact of respiratory disease and its treatment on the subject's health outcomes [31,32]. The SGRQ is self-administered and is usually completed within 10–20 min. The instrument contains 76 items in three domains: symptoms (frequency and severity), activity limitations, and impacts on social and psychological functioning. The Sarcoidosis Assessment Tool (SAT) is a self-administered health-related quality-of-life (HRQL) instrument that is validated against other standardized HRQL instruments for patients with active sarcoidosis and is further validated to specifically detect changes in the severity of lung involvement [33].
2.6. Sample size and analysis methods
For this pilot randomized trial, we planned to recruit 50 patients (25 per treatment group) within the study timeframe given patient volume and estimated study participation rates. Based on the clinical improvement in FVC observed in comparable immune modulating agents, such as infliximab, we expected to see up to 3% average improvement in FVC in the nicotine treatment group [19]. At the design stage, we acknowledged that 25 patients per treatment group would be enough to gather data for future planning, but would be too few to achieve significance unless the effect of nicotine on FVC was large, over 5%, assuming 5–10% variance between consecutive FVC measurements. Further, this pilot study was not designed to detect radiographic changes after nicotine treatment [13]. However, based upon the results of a recent Phase II sarcoidosis clinical trial that noted significant improvements in the “radiology score” with low dose anti-TNFα drug compared to controls, and which was structured almost identically to the study proposed herein, we would require 55 patients in each group (nicotine treatment and placebo) to detect a 15% difference in LTS score change before and after treatment with 90% power and type I error at 0.05 assuming a standard deviation of LTS score change at 20%. Thus, it is expected that this pilot study will be underpowered to detect a significant change in the LTS following nicotine treatment, but will be useful for estimating the next study size.
Descriptive summaries of all patient characteristics, including demographics, medical history, and clinical baseline measures will be used to assess data quality and completeness, as well as to characterize the cohort overall and by randomized group. Analysis of the primary endpoint, FVC, and secondary measures such as the LTS score, the FAS, SGRQ, and SAT, will employ a longitudinal model, with baseline and post intervention measurements as dependent variables to estimate the change between study week 26 and baseline [35]. These models will contain fixed effects for randomized treatment group, time point, and the interaction between treatment group and time. All patients will be included in these longitudinal mixed models, whether or not baseline or post intervention measurements are missing. With just two measures, we will use an unstructured covariance structure, which allows the variances of the baseline and post measures to differ. We will consider adjustment for race and sex, however interaction effects will only be explored in sensitivity analyses. We will report p-values for these as secondary outcomes, and derive meaning only if our primary outcome (FVC) shows significance. Bivariate plots will be used to describe the relationship between changes in FVC and LTS. Assuming a 10% dropout before follow-up, complete data will be obtained from approximately 22 subjects in each arm of the study. The missing at random (MAR) assumption will be our primary adjustment for potential missing data bias [36]. Sensitivity analyses will confirm the main findings as recommended by the special NAS panel on missing data in clinical trials [36].
2.7. Safety reporting
Study reporting of safety data will include all unexpected adverse events occurring between the time of consent and final follow up (study week 32). Adverse events will be classified as: related, possibly related, or unrelated to study treatment, along with the severity of each event (mild, moderate, or severe). All adverse events were discussed with the Data Safety Monitoring Committee and monitored in cooperation with the study Steering Committee.
2.8. Recruitment, enrollment, and baseline characteristics
Trial recruitment began in October 2015 and continued through January 2019 across two sites. During this time 343 patients were assessed for eligibility, of which 50 were consented and randomized. The majority of those screened did not meet eligibility criteria (n = 260), with the most not having active disease (n = 87), followed by exclusion due to a recent modification in their medication or were recently actively taking anti-TNF-alpha therapy (n = 34), or due to cardiac disease (n = 33) (Fig. 3). Only 8% (n = 28) declined to participate. One randomized patient was subsequently determined not to have active disease, and never began treatment. Two-thirds of patients were recruited at OSUMC and 57% (n = 28) identified themselves as White non-Hispanic race and ethnicity; 61% (n = 30) were female. Patients were on average 54 (sd: 10.0) years of age and more than two-thirds were overweight or obese (Table 1). Nine (18%) patients were former smokers.
Fig. 3.
Study recruitment.
Table 1.
Characteristics of randomized participants.
| Baseline Characteristic | N = 49 (%) |
|---|---|
| Recruitment Site | |
| Cleveland Clinic | 16 (32.7%) |
| The Ohio State University | 33 (67.3%) |
| Race and ethnicitya | |
| White Non-Hispanic | 28 (57.1%) |
| Black Non-Hispanic | 16 (32.7%) |
| Hispanic | 2 (4.1%) |
| Sex | |
| Female | 30 (61.2%) |
| Male | 19 (38.8%) |
| Baseline BMI Categoryb | |
| Under/Normal weight | 7 (14.3%) |
| Overweight | 17 (34.7%) |
| Obese | 22 (44.9%) |
| MRC grade | |
| 1 - Shortness of breath when hurrying on the level or walking up a slight hill | 27 (56.3%) |
| 2 - Walks slower than people of the same age on the level because of breathlessness or as to stop for breath when walking at own pace on the level | 15 (31.3%) |
| 3 - Stops for breath after walking about 100 m or after a few minutes on the level | 4 (8.3%) |
| 4 - Too breathless to leave the house or breathless when dressing or undressing | 2 (4.2%) |
| Smoking | |
| Former Smoker | 9 (18.4) |
| Never Smoker |
40 (81.6) |
|
Mean (SD) |
|
| Age at enrollment | 53.9 (10.0) |
| Baseline BMI [n = 46]b | 31.9 (7.6) |
| Baseline average FVC | 3.1 (1.1) |
| Baseline average FEV | 2.3 (0.8) |
| Pack years of tobacco cigarette smoking [n = 9]: | 14.4 (14.4) |
Three participants are missing race/ethnicity.
Three participants are missing BMI.
3. Discussion
The overarching goal of this pilot randomized trial is to evaluate the feasibility, safety, tolerability, and preliminary efficacy of treatment with nicotine versus placebo for the treatment of pulmonary sarcoidosis. Current therapies for active pulmonary sarcoidosis, remain either expensive and often with numerous side-effects, as with novel industry developed therapies, or with reduced quality of life, as with corticosteroids [3,16]. Nicotine therapy provides promise as a safe, available, and cost-effective intervention strategy, which we expect to be acceptable to patients.
At higher concentrations (μM), nicotine suppresses antigen-mediated TNFα production, induces regulatory T cells, suppresses Th1 type immune responses, particularly in the lung, and is therefore expected to suppress Th1-dependent granuloma formation [[7], [8], [9], [10],37,38]. Moreover, cigarette smokers and smokeless tobacco users have approximately a 2-fold reduction in risk of developing sarcoidosis [[4], [5], [6]]. Transdermal nicotine patch delivery systems are designed to attain in vivo drug levels approximating that of regular cigarette smokers, which is in the 10–40 nM range [39,40]. Nicotine in this concentration, when achieved in animals, is shown to suppress T-cell mediated immune responses [41]. The 21 mg/day nicotine patch achieves drug levels within this range in humans and is shown to be well tolerated in terms of serious adverse events in large clinical studies [39,40,42]. Furthermore, the transdermal nicotine delivery approach reduces the risk of developing dependency by providing steady nicotine levels in vivo [43]. To the extent that nicotine levels attained in smokers prevents the development and progression of sarcoidosis, the 21 mg/day transdermal nicotine dose achieves our goal of attaining a biologically relevant dose of nicotine that will be well-tolerated in most subjects.
While our trial will provide initial objective and patient reported outcome data about the efficacy and acceptability of nicotine therapy, it will also provide the opportunity to explore associations between nicotine blood levels and objective and patient reported responses. Taken together these data will move further safe and effective therapies for an under-studied rare disease.
Funding
This study was supported by the National Institutes of Health [R34HL123586]; and by award number UL1TR001070 from the National Center for Advancing Translational Sciences. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the national Center for Advancing Translational Sciences or the National Institutes of Health.
Declaration of competing interest
The authors report no competing interests.
Contributor Information
Erinn M. Hade, Email: hade.2@osu.edu.
Rachel M. Smith, Email: Rachel.Smith@osumc.edu.
Daniel A. Culver, Email: CULVERD@ccf.org.
Elliott D. Crouser, Email: Elliott.Crouser@osumc.edu.
References
- 1.Hinz A. Fatigue in patients with sarcoidosis, compared with the general population. Gen. Hosp. Psychiatr. 2011;33(5):462–468. doi: 10.1016/j.genhosppsych.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 2.de Kleijn W.P. Fatigue in sarcoidosis: American versus Dutch patients. Sarcoidosis Vasc. Diffuse Lung Dis. 2009;26(2):92–97. [PubMed] [Google Scholar]
- 3.Denys B.G. Steroid-resistant sarcoidosis: is antagonism of TNF-alpha the answer? Clin. Sci. (Lond.) 2007;112(5):281–289. doi: 10.1042/CS20060094. [DOI] [PubMed] [Google Scholar]
- 4.Newman L.S. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am. J. Respir. Crit. Care Med. 2004;170(12):1324–1330. doi: 10.1164/rccm.200402-249OC. [DOI] [PubMed] [Google Scholar]
- 5.Carlens C. Smoking, use of moist snuff, and risk of chronic inflammatory diseases. Am. J. Respir. Crit. Care Med. 2010;181(11):1217–1222. doi: 10.1164/rccm.200909-1338OC. [DOI] [PubMed] [Google Scholar]
- 6.Valeyre D. Smoking and pulmonary sarcoidosis: effect of cigarette smoking on prevalence, clinical manifestations, alveolitis, and evolution of the disease. Thorax. 1988;43(7):516–524. doi: 10.1136/thx.43.7.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jonge W.J., Ulloa L. The alpha 7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br. J. Pharmacol. 2007;151(7):915–929. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kox M. GTS-21 inhibits pro-inflammatory cytokine release independent of the Toll-like receptor stimulated via a transcriptional mechanism involving JAK2 activation. Biochem. Pharmacol. 2009;78(7):863–872. doi: 10.1016/j.bcp.2009.06.096. [DOI] [PubMed] [Google Scholar]
- 9.Yanagita M. Nicotine modulates the immunological function of dendritic cells through peroxisome proliferator-activated receptor-gamma upregulation. Cell. Immunol. 2012;274(1–2):26–33. doi: 10.1016/j.cellimm.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S., Petro T.M. The effect of nicotine on murine CD4 T cell responses. Int. J. Immunopharm. 1996;18(8–9):467–478. doi: 10.1016/s0192-0561(96)00054-9. [DOI] [PubMed] [Google Scholar]
- 11.Ingram J.R. Vol. 2008. Gastroenterol Res Pract; 2008. Nicotine enemas for active Crohn's colitis: an open pilot study; p. 237185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanchet M.R., Israel-Assayag E., Cormier Y. Inhibitory effect of nicotine on experimental hypersensitivity pneumonitis in vivo and in vitro. Am. J. Respir. Crit. Care Med. 2004;169(8):903–909. doi: 10.1164/rccm.200210-1154OC. [DOI] [PubMed] [Google Scholar]
- 13.Julian M.W. Nicotine treatment improves Toll-like receptor 2 and Toll-like receptor 9 responsiveness in active pulmonary sarcoidosis. Chest. 2013;143(2):461–470. doi: 10.1378/chest.12-0383. [DOI] [PubMed] [Google Scholar]
- 14.Chen E.S., Moller D.R. Sarcoidosis-scientific progress and clinical challenges. Nat. Rev. Rheumatol. 2011;7(8):457–467. doi: 10.1038/nrrheum.2011.93. [DOI] [PubMed] [Google Scholar]
- 15.Morgenthau A.S., Iannuzzi M.C. Recent advances in sarcoidosis. Chest. 2011;139(1):174–182. doi: 10.1378/chest.10-0188. [DOI] [PubMed] [Google Scholar]
- 16.Cox C.E. Health-related quality of life of persons with sarcoidosis. Chest. 2004;125(3):997–1004. doi: 10.1378/chest.125.3.997. [DOI] [PubMed] [Google Scholar]
- 17.Vermeire S., Van Assche G., Rutgeerts P. Serum sickness, encephalitis and other complications of anti-cytokine therapy. Best Pract. Res. Clin. Gastroenterol. 2009;23(1):101–112. doi: 10.1016/j.bpg.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Poltavski D.V., Petros T. Effects of transdermal nicotine on prose memory and attention in smokers and nonsmokers. Physiol. Behav. 2005;83(5):833–843. doi: 10.1016/j.physbeh.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Baughman R.P. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am. J. Respir. Crit. Care Med. 2006;174(7):795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]
- 20.Behr J. A small change in FVC but a big change for IPF: defining the minimal clinically important difference. Am. J. Respir. Crit. Care Med. 2011;184(12):1329–1330. doi: 10.1164/rccm.201110-1794ED. [DOI] [PubMed] [Google Scholar]
- 21.Baughman R.P. Changes in chest roentgenogram of sarcoidosis patients during a clinical trial of infliximab therapy: comparison of different methods of evaluation. Chest. 2009;136(2):526–535. doi: 10.1378/chest.08-1876. [DOI] [PubMed] [Google Scholar]
- 22.Bergin C.J. Sarcoidosis: correlation of pulmonary parenchymal pattern at CT with results of pulmonary function tests. Radiology. 1989;171(3):619–624. doi: 10.1148/radiology.171.3.2717731. [DOI] [PubMed] [Google Scholar]
- 23.Muller N.L. Sarcoidosis: correlation of extent of disease at CT with clinical, functional, and radiographic findings. Radiology. 1989;171(3):613–618. doi: 10.1148/radiology.171.3.2717730. [DOI] [PubMed] [Google Scholar]
- 24.Erdal B.S. Quantitative computerized two-point correlation analysis of lung CT scans correlates with pulmonary function in pulmonary sarcoidosis. Chest. 2012;142(6):1589–1597. doi: 10.1378/chest.11-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lower E.E., Harman S., Baughman R.P. Double-blind, randomized trial of dexmethylphenidate hydrochloride for the treatment of sarcoidosis-associated fatigue. Chest. 2008;133(5):1189–1195. doi: 10.1378/chest.07-2952. [DOI] [PubMed] [Google Scholar]
- 26.Picciotto M.R., Brunzell D.H., Caldarone B.J. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13(9):1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 27.Salin-Pascual R.J. Antidepressant effect of transdermal nicotine patches in nonsmoking patients with major depression. J. Clin. Psychiatr. 1996;57(9):387–389. [PubMed] [Google Scholar]
- 28.Crouser E.D. Diagnosis and detection of sarcoidosis. An official American thoracic society clinical practice guideline. Am. J. Respir. Crit. Care Med. 2020;201(8):e26–e51. doi: 10.1164/rccm.202002-0251ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris P.A. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crapo R.O., Morris A.H., Gardner R.M. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am. Rev. Respir. Dis. 1981;123(6):659–664. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 31.Barr J.T. American translation, modification, and validation of the St. George's Respiratory Questionnaire. Clin. Therapeut. 2000;22(9):1121–1145. doi: 10.1016/S0149-2918(00)80089-2. [DOI] [PubMed] [Google Scholar]
- 32.Jones P.W., Quirk F.H., Baveystock C.M. The St george's respiratory Questionnaire. Respir. Med. 1991;85(Suppl B):25–31. doi: 10.1016/s0954-6111(06)80166-6. discussion 33-7., P. [DOI] [PubMed] [Google Scholar]
- 33.Judson M.A. Validation and important differences for the Sarcoidosis Assessment Tool. A new patient-reported outcome measure. Am. J. Respir. Crit. Care Med. 2015;191(7):786–795. doi: 10.1164/rccm.201410-1785OC. [DOI] [PubMed] [Google Scholar]
- 34.Michielsen H.J., De Vries J., Van Heck G.L. Psychometric qualities of a brief self-rated fatigue measure: the Fatigue Assessment Scale. J. Psychosom. Res. 2003;54(4):345–352. doi: 10.1016/s0022-3999(02)00392-6. [DOI] [PubMed] [Google Scholar]
- 35.Liu G.F. Should baseline be a covariate or dependent variable in analyses of change from baseline in clinical trials? Stat. Med. 2009;28(20):2509–2530. doi: 10.1002/sim.3639. [DOI] [PubMed] [Google Scholar]
- 36.The Prevention and Treatment of Missing Data in Clinical Trials. 2010. Washington (DC) [Google Scholar]
- 37.Nouri-Shirazi M., Guinet E. A possible mechanism linking cigarette smoke to higher incidence of respiratory infection and asthma. Immunol. Lett. 2006;103(2):167–176. doi: 10.1016/j.imlet.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 38.Matsunaga K. Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages to Legionella pneumophila infection by nicotine. J. Immunol. 2001;167(11):6518–6524. doi: 10.4049/jimmunol.167.11.6518. [DOI] [PubMed] [Google Scholar]
- 39.Johnstone E. Determinants of the rate of nicotine metabolism and effects on smoking behavior. Clin. Pharmacol. Ther. 2006;80(4):319–330. doi: 10.1016/j.clpt.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Hukkanen J., Jacob P., 3rd, Benowitz N.L. Metabolism and disposition kinetics of nicotine. Pharmacol. Rev. 2005;57(1):79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 41.Nizri E. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J. Immunol. 2009;183(10):6681–6688. doi: 10.4049/jimmunol.0902212. [DOI] [PubMed] [Google Scholar]
- 42.Pickworth W.B., Bunker E.B., Henningfield J.E. Transdermal nicotine: reduction of smoking with minimal abuse liability. Psychopharmacology (Berlin) 1994;115(1–2):9–14. doi: 10.1007/BF02244745. [DOI] [PubMed] [Google Scholar]
- 43.Henningfield J.E. Tobacco dependence and withdrawal: science base, challenges and opportunities for pharmacotherapy. Pharmacol. Ther. 2009;123(1):1–16. doi: 10.1016/j.pharmthera.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]