Abstract
Purpose
In the MONALEESA-3 Phase III trial of patients with hormone receptor–positive human epidermal growth factor receptor–negative advanced breast cancer, ribociclib plus fulvestrant significantly improved progression-free survival (PFS) and overall survival (OS). Here, we present patient-reported outcomes from the trial, including health-related quality of life (HRQOL).
Methods
Patients were randomized (2:1) to receive ribociclib plus fulvestrant or placebo plus fulvestrant. Time to definitive 10% deterioration (TTD) from baseline in HRQOL (global health status [GHS] from the EORTC QLQ-C30 questionnaire) and pain (BPI-SF questionnaire) were assessed using Kaplan-Meier estimates; a stratified Cox regression model was used to estimate the hazard ratio (HR) and 95% CIs.
Results
Deterioration ≥10% in the EORTC-QLQ-C30 GHS was observed in 33% of patients in the ribociclib group vs 34% of patients in the placebo (reference) group (HR for TTD ≥ 10% = 0.81 [95% CI, 0.62–1.1]). Similar findings were noted for TTD ≥5% (HR = 0.79 [95% CI, 0.61–1.0]) and TTD ≥15% (HR = 0.81 [95% CI, 0.60–1.08]). TTD ≥10% in emotional functioning (HR = 0.76 [95% CI, 0.57–1.01]) trended in favor of the ribociclib group, whereas results for fatigue and pain were similar between arms. TTD ≥10% in BPI-SF pain severity index score (HR = 0.77 [95% CI, 0.57–1.05]) and worst pain item score (HR = 0.81 [95% CI, 0.58–1.12]) trended in favor of ribociclib vs placebo.
Conclusions
In addition to significantly prolonging PFS and OS compared with placebo plus fulvestrant, adding ribociclib to fulvestrant maintains HRQOL.
Keywords: Breast cancer, Metastatic, Patient-reported outcomes, Quality of life
Highlights
-
•
Ribociclib + fulvestrant allowed maintenance of global health status (GHS).
-
•
Time to deterioration (TTD) by 10% can convey duration until worsening of QOL.
-
•
TTD ≥10% was delayed with ribociclib in GHS and emotional functioning.
-
•
Ribociclib also demonstrated trends toward delayed TTD vs placebo in pain outcomes.
1. Introduction
Patients with breast cancer receiving early-line therapy typically report a relatively high health-related quality of life (HRQOL) that may deteriorate over time as the disease progresses [[1], [2], [3]]. Adding novel therapies to existing treatments can delay disease progression but can also result in additional toxicities, which may have an impact on HRQOL [4]. Consequently, advanced breast cancer is associated with reduced HRQOL scores, including role functioning, pain, and breast cancer-related symptoms [5]. Recent real-world evidence demonstrated that disease progression negatively affects the QOL of patients with metastatic breast cancer; therefore, maintaining QOL while improving progression-free survival (PFS) outcomes is a desirable goal in the clinical management of this disease [2,6]. The European Society for Medical Oncology guidelines recommend that the effect of new treatments on HRQOL be assessed in addition to impacts on efficacy and safety [7]. In recent trials, patient-reported outcomes (PROs) have emerged as crucial tools for the benefit-risk assessment of novel regimens [3,[8], [9], [10], [11], [12]].
Ribociclib, an oral CDK4/6 inhibitor, in combination with an aromatase inhibitor or fulvestrant is recommended by current guidelines across many countries as a treatment option in pre/perimenopausal and postmenopausal women with hormone receptor–positive (HR+)/human epidermal growth factor receptor–negative (HER2−) advanced breast cancer [7,13]. To date, all 3 randomized clinical trials (MONALEESA-2, MONALEESA-3, and MONALEESA-7) investigating combinations of ribociclib with endocrine therapy in patients with HR+/HER2− advanced breast cancer have shown a significantly longer PFS with ribociclib therapy vs placebo [[14], [15], [16]]. The MONALEESA-7 Phase III trial was the first study in which the addition of ribociclib to endocrine therapy demonstrated a statistically significant overall survival (OS) benefit compared with placebo (hazard ratio [HR] = 0.71 [95% CI, 0.54–0.95]; P = .00973) in premenopausal women with advanced breast cancer [17].
MONALEESA-3 is a Phase III trial of first- or second-line ribociclib plus fulvestrant vs placebo plus fulvestrant in postmenopausal HR+/HER2− patients with advanced breast cancer [16]. With a median follow-up of 20.4 months, ribociclib treatment demonstrated longer PFS vs placebo (median, 20.5 vs 12.8 months; HR = 0.593 [95% CI, 0.480–0.732]; P < .001) and a manageable safety profile, in line with previously published ribociclib studies. The PFS benefit was consistent across prespecified subgroups. Ribociclib plus fulvestrant reduced the risk of progression or death by 42% as first-line therapy (HR = 0.577 [95% CI, 0.415–0.802]) and by 44% as second-line or early-relapse therapy (HR = 0.565 [95% CI, 0.428–0.744]). OS was significantly improved with ribociclib treatment than with placebo (HR = 0.72 [95% CI, 0.57–0.92]; P < .01129) [18].
A growing body of evidence indicates that pain management may be associated with improved QOL [19]. This analysis evaluates PROs, including HRQOL and pain scores, in patients enrolled in the MONALEESA-3 trial.
2. Patients and methods
2.1. Study design and participants
MONALEESA-3 (NCT02422615) is a Phase III, randomized, double-blind, placebo-controlled trial evaluating ribociclib plus fulvestrant in patients with HR+/HER2− advanced breast cancer who are receiving treatment in the first- or second-line/early-relapse settings. Patients were randomized 2:1 to receive either ribociclib (600 mg/day; 3 weeks on/1 week off) or placebo in combination with fulvestrant (500 mg on day 1 of each 28-day cycle, with an additional dose on day 15 of cycle 1). Details of participants, study treatments, and procedures have been reported previously [16]. PROs were a secondary objective. The study was initiated in June 2015, and the data cutoff for this analysis was June 3, 2019. The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The study protocol was approved by an independent ethics committee and/or local institutional review boards for each study site. A steering committee comprising participating international investigators and Novartis representatives oversaw the study conduct. An independent data monitoring committee assessed the safety data. All patients provided written informed consent.
2.2. PRO assessments
PROs were analyzed using the European Organisation for Research and Treatment of Cancer (EORTC) quality-of-life questionnaire (QLQ)-C30 V3.0 along with the Brief Pain Inventory-Short form (BPI-SF) questionnaire and the EuroQoL 5-level instrument (EQ-5D-5L, Version 4.0) to evaluate HRQOL, functioning, treatment-related side effects, global health status, and disease symptoms, such as cancer-related pain. Patients were asked to complete the questionnaires at screening; every 8 weeks for the first 18 months, and then every 12 weeks until disease progression, death, withdrawal of consent, loss to follow-up, or patient/guardian decision; and at the end of treatment. No post-progression PRO assessments were performed.
Questionnaire responses were scored using a scale ranging from 0 to 100 for QLQ-C30 and from 0 to 10 for BPI-SF. For functional and global health status/QOL scales, a higher numerical score represents a better level of functioning/HRQOL/health states. For symptomatic scales, a higher numerical score represents greater symptom severity/higher pain intensity.
The primary variable of interest was global health status/global QOL score of the EORTC QLQ-C30. Secondary variables of interest included EORTC QLQ-C30 physical, emotional, and social functioning subscale scores and BPI-SF worst pain item, pain severity index, and pain interference indices.
2.3. Statistical analyses
Changes from baseline in all subscales obtained from EORTC QLQ-C30 were estimated using a linear mixed model that included treatment, stratification factors, baseline score, visit time, and treatment by visit time interactions as fixed effects and patient as a random effect in the model. Additionally, change from baseline in the domain scores, health states, overall health status, and index values at the time of each assessment were summarized. Descriptive statistics were used to summarize the changes in EORTC QLQ-C30 pain scores.
Time to definitive deterioration ≥ 10% (TTD; defined as ≥ 10% worsening of the scales score relative to baseline, with no later improvement above this threshold observed during the treatment period, or death due to any cause) in the global health status/QOL score was investigated. In addition, TTDs of 5% and 15% were assessed and presented. The survival distributions were presented using Kaplan-Meier curves. The median TTD along with 95% CIs was obtained from Kaplan-Meier estimates. HRs and 95% CIs were estimated using a stratified Cox regression model.
3. Results
3.1. Patient characteristics
Between June 2015 and June 2016, 726 patients were randomized at a 2:1 ratio to ribociclib plus fulvestrant (n = 484) or placebo plus fulvestrant (n = 242). Baseline demographics and disease characteristics have been published previously (Supplemental Table 1) [16].
Overall compliance rates of patients completing the EORTC QLQ-C30 questionnaire during the treatment period were high in both groups, with compliance rates at baseline of 92.1% in the ribociclib group and 93.4% in the placebo group. Compliance rates were >90% and ≥80% at the first postbaseline assessment and throughout the first year of treatment, respectively.
3.2. EORTC QLQ-C30 global health status/QOL score and symptom scales
The mean (standard deviation; SD) EORTC QLQ-C30 global health/QOL scores at baseline were well balanced between treatment groups: 65.5 (±19.1) in the ribociclib group and 68.4 (±18.5) in the placebo group (Table 1) and were in line with the EORTC QLQ-C30 reference values expected for recurrent/metastatic breast cancer [20] and those reported for a general population (ie, not cancer specific) of women aged 60–69 years in North American and Europe (mean, 65.6 years) [21].
Table 1.
Global and EORTC QLQ-C30 symptom scores.
| Score, Mean (SD) | Global HRQOL | Symptom Scores |
||||
|---|---|---|---|---|---|---|
| Fatigue | Diarrhea | Nausea/Vomiting | Pain | |||
| Ribociclib + fulvestrant | Baseline (n = 447) | 65.5 (19.1) | 32.2 (23.1) | 6.4 (15.7) | 6.4 (14.0) | 30.0 (25.5) |
| Cycle 3 day 1 (n = 362) | 69.9 (19.6) | 33.1 (23.9) | 6.7 (15.7) | 8.9 (15.4) | 25.1 (24.2) | |
| Cycle 15 day 1 (n = 219) | 71.0 (18.5) | 32.3 (22.6) | 6.7 (17.4) | 6.7 (12.3) | 25.3 (23.3) | |
| Placebo + fulvestrant | Baseline (n = 224) | 68.4 (18.5) | 30.5 (21.4) | 6.7 (14.5) | 7.0 (14.7) | 27.8 (25.9) |
| Cycle 3 day 1 (n = 169) | 70.9 (17.5) | 29.1 (21.5) | 6.7 (14.4) | 4.2 (9.8) | 24.1 (23.4) | |
| Cycle 15 day 1 (n = 96) | 73.5 (16.6) | 26.2 (18.9) | 6.9 (14.4) | 5.2 (11.7) | 23.1 (21.5) | |
| EORTC reference value n = 1147 [20] | 60.2 (25.5) | 36.3 (27.0) | 5.8 (15.2) | 10.3 (19.7) | 30.9 (29.6) | |
EORTC-QLQ-C30 European Organisation for Research and Treatment of Cancer core quality-of-life questionnaire, HRQOL health-related quality of life.
The mean (SD) EORTC QLQ-C30 symptom scores assessed at baseline included fatigue (32.2 [±23.1] in the ribociclib group vs 30.5 [±21.4] in the placebo group), diarrhea (6.4 [±15.7] vs 6.7 [±14.5]), nausea and vomiting (6.4 [±14.0] vs 7.0 [±14.7]), and pain (30.0 [±25.5] vs 27.8 [±25.9]), which were all similar to the EORTC QLQ-C30 reference values (Table 1) [20]. In general, at 8 weeks (day 1 of cycle 3) and later during treatment, the EORTC QLQ-C30 symptom scores were maintained or improved slightly, irrespective of treatment.
Throughout the study treatment, global health status/QOL scores were improved from baseline and generally similar between treatment groups (Table 1 and Fig. 1). In both groups, this improvement from baseline was consistently maintained until end of treatment, after which it declined.
Fig. 1.
Change from baseline in global health status/QOL scale score of EORTC QLQ-C30. C cycle, D day, EORTC-QLQ-C30 European Organisation for Research and Treatment of Cancer core quality-of-life questionnaire, EOT end of treatment, FUL fulvestrant, HRQOL health-related quality of life, LSM least squares mean, PBO placebo, QOL quality of life, RIB ribociclib, SEM standard error of the mean. The time profile provides the average estimates for the change from baseline to the respective cycle as derived using the linear effects model. Positive changes from baseline indicate improvement in HRQOL.
3.3. Time to definitive deterioration
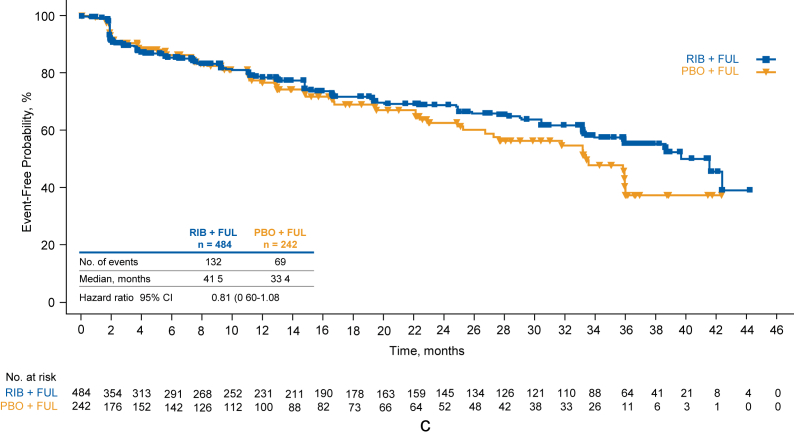
Deterioration ≥10% in global HRQOL was observed in 159/484 patients in the ribociclib group vs 83/242 patients in the placebo group (Fig. 2a). This resulted in a numerical trend in favor of ribociclib in TTD ≥10% in global HRQOL (HR = 0.81 [95% CI, 0.62–1.1]). Similar findings were noted for TTD ≥5% (HR = 0.79 [95% CI, 0.61–1.0]; Fig. 2b) and TTD ≥15% (HR = 0.81 [95% CI, 0.60–1.08]; Fig. 2c). A delay in TTD ≥10% in global HRQOL was observed for patients who did not experience a PFS event compared with those who experienced a PFS event in the ribociclib group (Supplemental Fig 1a), the placebo group (Supplemental Fig 1b), and for patients across both treatment groups (Supplemental Fig 1c).
Fig. 2.
Time to definitive deterioration of global health status/QOL scale score of EORTC QLQ-C3 from baseline by ≥10% (a), ≥5% (b), and ≥15% (c). EORTC-QLQ-C30 European Organisation for Research and Treatment of Cancer core quality-of-life questionnaire, FUL fulvestrant, PBO placebo, QOL quality of life, RIB ribociclib.
TTD ≥10% in EORTC QLQ-C30 functioning scores trended in favor of ribociclib. Mean TTD ≥10% with ribociclib vs placebo was 38.7 months (137 events) vs 34.9 months (69 events; HR = 0.82 [95% CI, 0.61–1.10]), respectively, for the physical functioning score; 38.6 months (137 events) vs 30.4 months (73 events; HR = 0.76 [95% CI, 0.57–1.01]), respectively, for the emotional functioning score; and 39.6 months (129 events) vs 38.8 months (53 events; HR = 1.03 [95% CI, 0.75–1.42]), respectively, for the social functioning score.
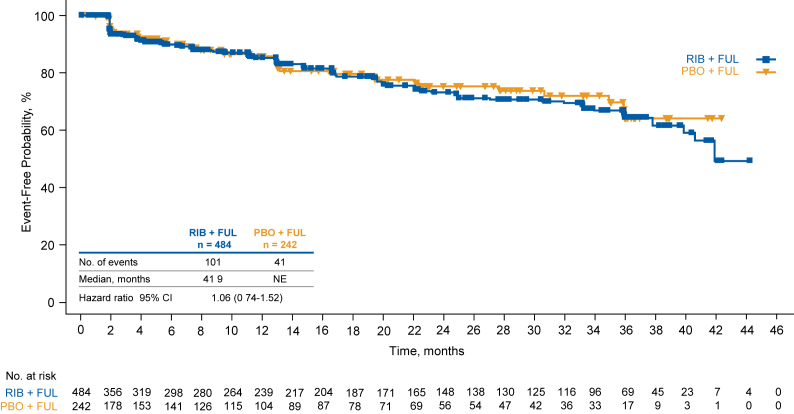
TTD ≥10% in EORTC QLQ-C30 fatigue score was similar between treatment groups: median, 38.7 months with ribociclib vs 36.0 months with placebo (HR = 0.91 [95% CI, 0.68–1.22]; Fig. 3). TTD ≥10% in EORTC QLQ-C30 pain score was also similar between treatment groups; median, 41.9 months with ribociclib vs not estimable with placebo (HR = 1.06 [95% CI, 0.74–1.52]; Fig. 4). There were few patients in this trial with a deterioration ≥10% in diarrhea or nausea/vomiting, and median values were not reached for either domain in either treatment arm (data not shown).
Fig. 3.
Time to definitive deterioration of fatigue score of EORTC QLQ-C3 from baseline by ≥10%. EORTC-QLQ-C30 European Organisation for Research and Treatment of Cancer core quality-of-life questionnaire, FUL fulvestrant, PBO placebo, RIB ribociclib.
Fig. 4.
Time to definitive deterioration of pain score of EORTC QLQ-C3 from baseline by ≥ 10%. EORTC-QLQ-C30 European Organisation for Research and Treatment of Cancer core quality-of-life questionnaire, FUL fulvestrant, NE not estimable, PBO placebo, RIB ribociclib.
3.4. Pain scores
At 8 weeks, the least squares mean change from baseline in EORTC QLQ-C30 pain scores was −4.0 in the ribociclib group (n = 362) vs −3.6 in the placebo group (n = 169), with a treatment difference of −0.3 (95% CI, −4.0 to 3.3). Through study treatment, pain scores were generally maintained until end of treatment (Supplemental Fig 2).
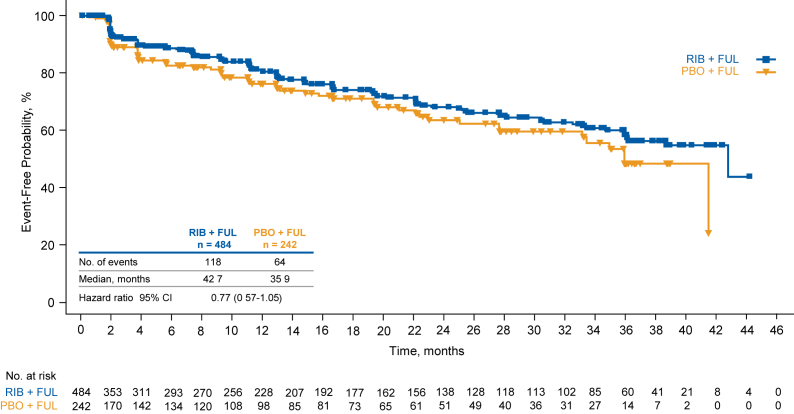
PROs based on the BPI-SF questionnaire indicated a trend in favor of ribociclib in TTD ≥10% in pain severity index score (HR = 0.77 [95% CI, 0.57–1.05]; Fig. 5). TTD ≥10% in BPI-SF worst pain item score (HR = 0.81 [95% CI, 0.58–1.12]) also favored ribociclib over placebo.
Fig. 5.
Time to definitive deterioration of pain severity index score of BPI-SF from baseline by ≥10%. FUL fulvestrant, PBO placebo, RIB ribociclib.
4. Discussion
This analysis of PROs in the MONALEESA-3 trial demonstrates that QOL was maintained until the end of treatment, and the previously demonstrated improvement in efficacy of ribociclib plus fulvestrant seemed to be accompanied by a lower risk for HRQOL deterioration compared with fulvestrant monotherapy, although the differences were not statistically significant. The EORTC QLQ-C30 global health/QOL score was improved from baseline and maintained during treatment but worsened when treatment was stopped in both treatment groups. Deterioration of HRQOL has been shown to coincide with disease progression [3]. The prolonged PFS reported in the ribociclib group (median, 20.5 months vs 12.8 months [16]) may have translated into the numerically longer median TTD ≥ 10% in global health score observed in this analysis.
Because of the established difficulty in interpreting longitudinal changes in individual symptom and functional subscales in HRQOL studies, only simple descriptive statistics were reported for changes from baseline in the PROs in this report. Comprehensive guidelines on the interpretation of EORTC QLC-C30 scores note that expected improvements over time are generally smaller than expected declines [22]. In this study, baseline EORTC QLQ-C30 global health/QOL and symptom subscale values were generally comparable to those in the general population across >10 countries worldwide and, most notably, with mean scores reported in women aged >60 years [21]. Therefore, an improvement in QOL during treatment would not be expected, and delaying deterioration in HRQOL in this trial, which was achieved with ribociclib treatment, should be considered an important result.
In addition to global health, the more specific EORTC QLQ-C30 symptom scores, including fatigue, diarrhea, nausea and vomiting, and pain, were maintained or slightly improved from baseline throughout treatment. It is important to note that patients in both groups reported a reduction in pain score as early as 8 weeks, which was maintained throughout treatment; this result is in line with the MONALEESA-2 and MONALEESA-7 studies of ribociclib-based combinations [23,24]. The ribociclib group also demonstrated a trend toward a longer time to deterioration of global HRQOL, which may be a result of having a longer PFS than patients in the placebo group. Furthermore, assessment of TTD of pain scores of the BPI-SF questionnaire, a particularly important QOL instrument, supported the positive trend observed in the ribociclib group. Altogether, these findings suggest that ribociclib treatment does not worsen specific EORTC QLQ-C30 symptoms and that the longer duration of PFS with ribociclib may result in a delay in deterioration of HRQOL in patients with HR+/HER2– advanced breast cancer.
In conclusion, these PRO findings indicate that overall HRQOL was maintained in postmenopausal HR+/HER2− patients with advanced breast cancer who received ribociclib plus fulvestrant as first- or second-line therapy. These results also reaffirm that, in MONALEESA-3, ribociclib prolonged PFS and OS while maintaining QOL. Generalizing to the entire MONALEESA trial program, these PROs from the MONALEESA-3 trial add to the growing body of evidence indicating that ribociclib in combination with endocrine therapy may maintain QOL while, at the same time, prolonging survival outcomes in patients with HR+/HER2− advanced breast cancer, irrespective of their menopausal status.
Declaration of competing interest
Dr. Fasching has received grants from Novartis, grants from Biontech, personal fees from Novartis, Roche, Pfizer, Celgene, Daiichi-Sankyo, TEVA, AstraZeneca, Merck Sharp & Dohme, Myelo Therapeutics, Macrogenics, Eisai, Puma, grants from Cepheid, outside the submitted work; Dr. Chan has nothing to disclose; Dr. Beck has received grants for his institution from Novartis, outside the submitted work; Dr. De Laurentiis has received personal fees from Pfizer, Novartis, Roche, Celgene, Astra Zeneca, Eisai, Eli Lilly, Amgen, MSD, outside the submitted work; Dr. Esteva has received grants and personal fees from Novartis, Pfizer, Genentech/Roche, personal fees from Celltrion Healthcare, Seattle Genetics, grants from GlaxoSmithKline, outside the submitted work; Dr. Jerusalem has received personal fees from Novartis; grants, personal fees and non-financial support from Novartis, Roche, Pfizer, personal fees and non-financial support from Lilly, Amgen, BMS, Astra-Zeneca, personal fees from Celgene, Puma Technology, Daiichi Sankyo, AbbVie, outside the submitted work; Dr. Neven has nothing to disclose; Dr. Pivot has nothing to disclose; Dr. Bianchi has received personal fees from Novartis, Eli Lilly, outside the submitted work; Dr. Martin has received personal fees from Lilly, Pfizer, AstraZeneca, GlaxoSmithKline, Pharmamar, Taiho Oncology, grants and personal fees from Novartis, Roche-Genentech, outside the submitted work; Dr. Chandiwana reports other from Novartis, during the conduct of the study; Dr. Lanoue reports other from Novartis, during the conduct of the study; Dr. Ridolfi reports other from Novartis, during the conduct of the study; Dr. Wang has received personal fees and other from Novartis, outside the submitted work; Dr. Rodriguez Lorenc has received personal fees and other from Novartis, outside the submitted work; Dr. Nusch reports other from Novartis and Amgen, outside the submitted work.
Acknowledgments
The authors thank the patients who participated in the study and their families. Medical writing assistance was provided by Mihaela Marina, PhD, of MediTech Media, Ltd., funded by Novartis Pharmaceutical Company.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2020.09.008.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Funding
This study was funded by Novartis Pharmaceutical Company.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.de Ligt K.M., Heins M., Verloop J., Ezendam N.P.M., Smorenburg C.H., Korevaar J.C., Siesling S. The impact of health symptoms on health-related quality of life in early-stage breast cancer survivors. Breast Canc Res Treat. 2019;178(3):703–711. doi: 10.1007/s10549-019-05433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller V., Nabieva N., Haberle L., Taran F.A., Hartkopf A.D., Volz B., Overkamp F., Brandl A.L., Kolberg H.C., Hadji P., Tesch H., Ettl J., Lux M.P., Luftner D., Belleville E., Fasching P.A., Janni W., Beckmann M.W., Wimberger P., Hielscher C., Fehm T.N., Brucker S.Y., Wallwiener D., Schneeweiss A., Wallwiener M. Impact of disease progression on health-related quality of life in patients with metastatic breast cancer in the PRAEGNANT breast cancer registry. Breast. 2018;37:154–160. doi: 10.1016/j.breast.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Rugo H.S., Dieras V., Gelmon K.A., Finn R.S., Slamon D.J., Martin M., Neven P., Shparyk Y., Mori A., Lu D.R., Bhattacharyya H., Bartlett C.H., Iyer S., Johnston S., Ettl J., Harbeck N. Impact of palbociclib plus letrozole on patient-reported health-related quality of life: results from the PALOMA-2 trial. Ann Oncol. 2018;29(4):888–894. doi: 10.1093/annonc/mdy012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miles D., von Minckwitz G., Seidman A.D. Combination versus sequential single-agent therapy in metastatic breast cancer. Oncologist. 2002;7(Suppl 6):13–19. [PubMed] [Google Scholar]
- 5.de Mello Ramirez Medina J., de Araujo Trugilho I., Mendes G.N.B., Silva J.G., da Silva Paiva M.A., de Aguiar S.S., Thuler L.C.S., Bergmann A. Advanced clinical stage at diagnosis of breast cancer is associated with poorer health-related quality of life: a cross-sectional study. Eur J Breast Health. 2019;15(1):26–31. doi: 10.5152/ejbh.2018.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kovic B., Guyatt G., Brundage M., Thabane L., Bhatnagar N., Xie F. Association between progression-free survival and health-related quality of life in oncology: a systematic review protocol. BMJ Open. 2016;6(9) doi: 10.1136/bmjopen-2016-012909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardoso F., Senkus E., Costa A., Papadopoulos E., Aapro M., Andre F., Harbeck N., Aguilar Lopez B., Barrios C.H., Bergh J., Biganzoli L., Boers-Doets C.B., Cardoso M.J., Carey L.A., Cortes J., Curigliano G., Dieras V., El Saghir N.S., Eniu A., Fallowfield L., Francis P.A., Gelmon K., Johnston S.R.D., Kaufman B., Koppikar S., Krop I.E., Mayer M., Nakigudde G., Offersen B.V., Ohno S., Pagani O., Paluch-Shimon S., Penault-Llorca F., Prat A., Rugo H.S., Sledge G.W., Spence D., Thomssen C., Vorobiof D.A., Xu B., Norton L., Winer E.P. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4) Ann Oncol. 2018;29(8):1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goetz M.P., Johnston S., Martin M., Tokunaga E., Park I.H., Huober J. SABCS annual meeting. 2018. Health-related quality of life in MONARCH 3: abemaciclib plus an aromatase inhibitor as initial therapy in women with HR+, HER2- advanced breast cancer. Abstract P6-16-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verma S., O’Shaughnessy J., Burris H.A., Campone M., Alba E., Chandiwana D. Health-related quality of life of postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer treated with ribociclib + letrozole: results from MONALEESA-2. Breast Cancer Res Treat. 2018;170(3):535–545. doi: 10.1007/s10549-018-4769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner N.C., Huang Bartlett C., Cristofanilli M. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373(17):1672–1673. doi: 10.1056/NEJMc1510345. [DOI] [PubMed] [Google Scholar]
- 11.Gaulin C., Osorio G. Patient reported outcomes in metastatic breast cancer studies: evaluating the impact of the FDA guidance for industry. J Sci Innov Med. 2019;2(1):4. doi: 10.29024/jsim.11. [DOI] [Google Scholar]
- 12.Krohe M., Hao Y., Lamoureaux R.E., Galipeau N., Globe D., Foley C., Mazar I., Solomon J., Sa L. Patient-reported outcomes in metastatic breast cancer: a review of industry-sponsored clinical trials. Breast Cancer. 2016;10:93–102. doi: 10.4137/BCBCR.S39385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NCCN Clinical Practice Guidelines in Oncology. Breast Cancer; 2020. Version 2.2020. [Google Scholar]
- 14.Hortobagyi G.N., Stemmer S.M., Burris H.A., Yap Y.S., Sonke G.S., Paluch-Shimon S., Campone M., Petrakova K., Blackwell K.L., Winer E.P., Janni W., Verma S., Conte P., Arteaga C.L., Cameron D.A., Mondal S., Su F., Miller M., Elmeliegy M., Germa C., O’Shaughnessy J. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29(7):1541–1547. doi: 10.1093/annonc/mdy155. [DOI] [PubMed] [Google Scholar]
- 15.Tripathy D., Im S.A., Colleoni M., Franke F., Bardia A., Harbeck N., Hurvitz S.A., Chow L., Sohn J., Lee K.S., Campos-Gomez S., Villanueva Vazquez R., Jung K.H., Babu K.G., Wheatley-Price P., De Laurentiis M., Im Y.H., Kuemmel S., El-Saghir N., Liu M.C., Carlson G., Hughes G., Diaz-Padilla I., Germa C., Hirawat S., Lu Y.S. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19(7):904–915. doi: 10.1016/S1470-2045(18)30292-4. [DOI] [PubMed] [Google Scholar]
- 16.Slamon D.J., Neven P., Chia S., Fasching P.A., De Laurentiis M., Im S.A., Petrakova K., Bianchi G.V., Esteva F.J., Martin M., Nusch A., Sonke G.S., De la Cruz-Merino L., Beck J.T., Pivot X., Vidam G., Wang Y., Rodriguez Lorenc K., Miller M., Taran T., Jerusalem G. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465–2472. doi: 10.1200/JCO.2018.78.9909. [DOI] [PubMed] [Google Scholar]
- 17.Im S.A., Lu Y.S., Bardia A., Harbeck N., Colleoni M., Franke F., Chow L., Sohn J., Lee K.S., Campos-Gomez S., Villanueva-Vazquez R., Jung K.H., Chakravartty A., Hughes G., Gounaris I., Rodriguez-Lorenc K., Taran T., Hurvitz S., Tripathy D. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307–316. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 18.Slamon D.J., Neven P., Chia S., Fasching P.A., De Laurentiis M., Im S.-A., Petrakova K., Val Bianchi G., Esteva F.J., Martin M., Nusch A., Sonke G.S., De la Cruz-Merino L., Beck J.T., Pivot X., Sondhi M., Wang Y., Chakravartty A., Rodriguez-Lorenc K., Taran T., Jerusalem G. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382(6):514–524. doi: 10.1056/NEJMoa1911149. [DOI] [PubMed] [Google Scholar]
- 19.Breivik H., Cherny N., Collett B., de Conno F., Filbet M., Foubert A.J., Cohen R., Dow L. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009;20(8):1420–1433. doi: 10.1093/annonc/mdp001. [DOI] [PubMed] [Google Scholar]
- 20.Scott N.W., Fayers P.M., Aaronson N.K., Bottomley A., de Graeff A., Groenvold M., Gundy C., Koller M., Petersen M.A., Sprangers M.A.G. 2008. EORTC QLQ-C30 reference values. Brussels, Belgium. [Google Scholar]
- 21.Nolte S., Liegl G., Peterson M.A., Aaronson N.K., Costantini A., Fayers P.M. General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the Unites States. Eur J Cancer. 2019;107:153–163. doi: 10.1016/j.ejca.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 22.Cocks K., King M.T., Velikova G., de Castro G., Jr., Martyn St-James M., Fayer P.M. Evidence-based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur J Cancer. 2012;48(11):1713–1721. doi: 10.1016/j.ejca.2012.02.059. [DOI] [PubMed] [Google Scholar]
- 23.Harbeck N., Villanueva Vázquez V., Tripathy D., Lu Y.-S., de Laurentiis M., Kümmel S., Taylor D., Bardia A., Hurvitz S., Chow L., Im S.A., Franke F., Hughes G., Miller M., Kong O., Chandiwana D., Colleoni M. European breast cancer conference (EBCC-11) 2018: abstract 1LBA. 2018. Ribociclib plus tamoxifen or a non-steroidal aromatase inhibitor in premenopausal women with hormone receptor-positive, HER2– advanced breast cancer: additional results from the MONALEESA-7 trial. [Google Scholar]
- 24.Janni W., Alba E., Bachelot T., Diab S., Gil-Gil M., Beck T.J. First-line ribociclib plus letrozole in postmenopausal women with HR+, HER2- advanced breast cancer: tumor response and pain reduction in the phase 3 MONALEESA-2 trial. Breast Canc Res Treat. 2018;169(3):469–479. doi: 10.1007/s10549-017-4658-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.