Abstract
All gibbon species (Primates: Hylobatidae) are facing high extinction risk due to habitat loss and hunting. The Hainan gibbon Nomascus hainanus is the world’s most critically endangered primate, and one of the priority conservation actions identified is to establish artificial canopy corridors to reconnect fragmented forests. The effectiveness of artificial canopy bridge as a conservation tool for wild gibbons has not been widely tested, and the results are rarely published. We constructed the first canopy bridge for Hainan gibbon in 2015 to facilitate passage at a natural landslide; mountaineering-grade ropes were tied to sturdy trees with the help of professional tree climbers and a camera trap was installed to monitor wildlife usage. Hainan gibbon started using the rope bridge after 176 days, and usage frequency increased with time. All members in the gibbon group crossed the 15.8 m rope bridge except adult male. Climbing was the predominant locomotor mode followed by brachiation. This study highlights the use and value of rope bridges to connect forest gaps for wild gibbons living in fragmented forests. While restoring natural forest corridors should be a priority conservation intervention, artificial canopy bridges may be a useful short-term solution.
Subject terms: Ecology, Zoology
Introduction
Habitat fragmentation is one of the major causes of biodiversity loss1–5. The impact is particularly detrimental to arboreal wildlife, as forest gaps affect dispersal, foraging and energy efficiency, and breeding opportunity6–9, increase vulnerability to predation, diseases and parasites, accidental fatalities such as roadkill and electrocution10–16, resulting in an increased risk of extirpation in isolated populations17–19. To mitigate fragmentation impacts, artificial aerial wildlife crossing structures, commonly known as artificial canopy bridges, are being increasingly built worldwide to re-establish canopy connectivity20, which is more prevalent in South America and Australia12,20–25.
The great expanse of Asian tall forests supports a diverse assemblage of arboreal wildlife26,27, which are subjected to multiple threats including some of the highest forest fragmentation rates in the world5. Asia is the second richest continent for primate diversity after the Neotropics26, but the use of artificial canopy bridges as a conservation intervention is limited, and the effectiveness of these attempts are rarely examined and documented16. Artificial canopy bridges have been constructed for orangutan (Pongo pygmaeus)28,29, western hoolock gibbon (Hoolock hoolock)7,30, Javan slow loris (Nycticebus javanicus)16and dusky langur (Trachypithecus obscurus)31, and in forests bisected by roads in South Asia32,33.
Gibbons (Primates: Hylobatidae) are a group of highly specialised Asian apes which live in social groups and form well-established territories34. Canopy connectivity is critical for gibbons as they are strictly arboreal travel mainly through continuous tree canopy, forest fragmentation thus presents a major conservation challenge for gibbons7,14,34–36. The construction of artificial canopy bridge for gibbon conservation has not been widely experimented and its success rate rarely documented. Only two successful cases have been reported: lar gibbon in Thailand used rope bridges to cross a road37, and Western hoolock gibbon in India used bamboo and rope bridges to travel between isolated forest patches7,30. The HUTAN Kinabatangan Orang-utan Conservation Programme (KOCP) in Sabah constructed various types of artificial canopy bridges for primates in fragmented forest, which have been used by the Müller’s gibbon (Hylobates muelleri) (Boonratana, R., pers. comm. 2020). The current IUCN Red List of Threatened Species categorized all 20 gibbon species as threatened with extinction, and the crested gibbons (genus Nomascus) of southern China and Indochina are particularly imperiled, with five of the seven assessed species listed as Critically Endangered, and the remaining two Endangered.
The Hainan gibbon (Nomascus hainanus) is endemic to China’s Hainan Island. It is the rarest primate species on Earth with around 30 individuals remaining for the entire species, all living in Hainan Bawangling National Nature Reserve (hereafter BWL)38–40. With its precarious status many conservation recommendations have been suggested by concerned scientists, one of the priorities identified is to build artificial canopy crossings to connect the gibbons’ fragmented habitat41. Since 2005, we have been monitoring the Hainan gibbon population in a long-term conservation programme40.
In July 2014, Super Typhoon Rammasun, the strongest typhoon making landfall in Mainland China since 1949, hit Hainan Island and BWL received the highest rainfall in modern history (> 500 mm/24 h)42. Landslides scarred the forest-clad slopes throughout BWL, creating forest gaps up to 30 m wide (Fig. 1).
Figure 1.
One of numerous landslides in the gibbon forest of Bawangling three months after Super Typhoon Rammasun, taken on 24 October 2014. Photograph taken by L.G.
One of the main ‘arboreal highways’43 of our study gibbon group, Group C, was damaged by a landslide, creating an open gully measuring 15 m wide on average (806–895 m asl, midpoint at 19° 05′ 47.28″ N, 109° 14′ 43.36″ E) (Fig. 2). The damaged arboreal highway is situated in the core home range of Group C, and was used on average every 2 days before the typhoon damage (KFBG unpubl. data). Subsequent to the typhoon, gibbons continued to use the broken ‘arboreal highway’ by leaping across the landslide with difficulty using fronds and leaves of Arenga pinnata along the open gully, or an alternative and more hazardous pathway by leaping over two widely-spaced surviving trees (Elaeocarpus dubius and Castanopsis hystrix, respectively) followed by a drop of 10 m to the sub-canopy (Supplementary Video S1 online). We observed gibbons showing signs of hesitation when attempting to cross the forest gap, especially the adult females and small juveniles. To avoid accidental injuries or deaths, we constructed a two-pronged canopy rope bridge across the damaged arboreal highway on 06 December 2015. Here, we contribute to understanding the value of canopy bridge as a conservation intervention for gibbons living in fragmented forests.
Figure 2.
Forest gap of the canopy bridge site 10 months after Typhoon Rammasun, taken on 4 May 2015. Photograph taken by A.L.
Results
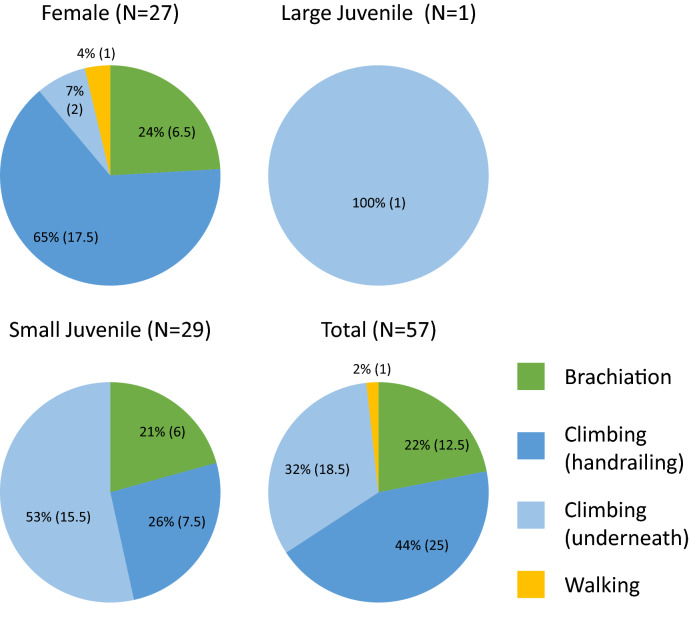
The gibbons were first photographed crossing the rope bridge 176 days after the bridge was placed. Data recording of the camera trap was intermittent due to camera malfunction and adverse weather preventing site access, and data were collected between 06 December 2015–2019 October 2016, 18 August–27 November 2018 and 06 January–25 February 2019. Throughout the study period of 470 days, we logged 208 photographs and 53 video footages of gibbons using the rope bridge (detailed information of all 52 crossing events can be found in Supplementary Table S1 online). During the 2015–2016 data collecting period, the camera trap angle failed to capture the crossing sequence, therefore only 48 out of the 52 crossing events, recorded during 2018 and 2019, could be used for analysis of locomotor behaviour. In the 2018–2019 study period, the gibbon group consisted of one breeding male, two breeding females, three large juveniles, two small juveniles and one infant. For analysis of locomotor behaviour, we did not consider the dependent infant attached to the mothers.
Age–sex difference in locomotor mode of gibbon crossings
Gibbons used all five locomotor modes for crossing the artificial crossing, but bridging was only employed when mounting and descending the rope bridges via connecting trees and Arenga pinnata, and therefore was not counted in the locomotor behaviour analysis. Climbing was the most common locomotor mode (76% of all modes employed), followed by brachiation and rarely walking. Two types of climbing were used: ‘Handrailing’ is walking on one rope with hands holding the second rope as handrails, or climbing underneath the ropes legs first with all limbs. Most climbing involved handrailing (57% of all climbing recorded). Walking was rare and only successfully employed by adult females, with many failed attempts by juveniles. Small juveniles frequently employed a combination of climbing and brachiation, with climbing overwhelmingly used (79% of all data); underneath climbing was the most used locomotion (Fig. 3). All gibbon group members were recorded using the canopy bridge except the adult male, but large juveniles rarely used the canopy bridges and was recorded only once crossing the forest gap by rope. Instead the breeding male and large juveniles were repeatedly observed leaping across the forest gap by our monitoring team. See Fig. 4a–d for examples of different locomotor modes employed by Hainan gibbon on the canopy bridge, and Supplementary Video S2 online provides a video footage of different age–sex of Group C crossing the forest gap, using the canopy bridges as well as leaping.
Figure 3.
Age–sex difference in dominant form of locomotor mode on canopy bridge by the Hainan gibbon group. N is total number of individual crossing recorded by camera trap. Bridging was only used for entering and leaving the canopy bridge, and is excluded for analysis.
Figure 4.
Locomotor modes of Hainan gibbons using a canopy bridge. (a) climbing (handrailing); (b) climbing (underneath); (c) walking; (d) brachiation. Camera trap photographs taken by Kadoorie Farm and Botanic Garden.
Frequency of gibbon crossings
For the three data collecting periods, the usage frequency increased as the gibbon group become habituated to the canopy bridge (Table 1).
Table 1.
Usage frequency of artificial canopy bridge by the Hainan gibbon group during the three study periods in 2015–2019.
| Recording period | No. of day | Number of crossing event | Frequency (event per day) |
|---|---|---|---|
| 06 Dec 2015–19 Oct 2016 | 318 | 4 | 0.0126 |
| 18 Aug 2018–27 Nov 2018 | 102 | 27 | 0.2647 |
| 06 Jan 2019 –25 Feb 2019 | 50 | 21 | 0.4200 |
Crossing sequence in a gibbon group
From our limited dataset, there was no well-defined age-sex selection in initiation of gap crossing. Half of the events were initiated by adult females, while the others were led by juveniles (see Supplementary Table S1 online).
Usage by other mammals
Our camera traps also recorded the use of rope bridge by Pallas’s squirrel (Callosciurus erythraeus), a small flying squirrel (Hylopetes sp.) and a small rodent (Subfamily Murinae).
Discussion
Forest fragmentation is one of the biggest threats to primate conservation44, and is becoming a primary concern in the study of primate conservation biology45. This study demonstrated the world’s rarest primate uses a simple artificial canopy bridge, and showed that artificial canopy bridge is a valuable conservation tool to reconnect fragmented gibbon habitats. The 15.8 m canopy bridge monitored was used for crossings in both directions, and both females and juveniles could initiate crossing.
A locomotor behaviour study of the cao-vit gibbon (Nomascus nasutus), the closest relative of Hainan gibbon, reported that adult females appear to employ safer locomotor modes. In comparison adult males choose riskier modes such as leaping46, which is in concordance with our observations as the adult male Hainan gibbon never, and near full-grown large juveniles rarely, used the canopy bridge over the study period. It is probable adults and near full-grown gibbons are more muscular and skilful to make big ricochetal brachiation or leap to overcome the forest gap. Simultaneously the forest gap presents too much an obstacle for the weaker and less skilful immatures. Adult females have similar physique to adult males, but it is likely leaping is too risky for pregnant and infant-carrying females with the extra weight. An alternative explanation is that innate maternal care behaviour resulted in the females always travel with their younger offspring using the rope bridges.
In areas where the target species are habituated to human presence, habituation to artificial crossings could be rapid: the western hoolock gibbon living in a village environs used a bamboo bridge in 15 days7, Javan slow loris in an agroforestry landscape used single-lined bridges after 20 days16, the western ringtail possum (Pseudocheirus occidentalis) around a major road with daily traffic volume up to 15,000 cars used the artificial bridge in 36 days25. It took the Hainan Gibbon 176 days to start using the canopy bridge, with a gradual increase in bridge use frequency from 2016 to 2019.
Different designs of artificial crossings for canopy connectivity have been constructed for wildlife: horizontal ‘ship’ ladder and pole bridge in Latin American24, box-tunnel bridge, rope ladder and netted flat bridge in Australia22,25, and simple rope and bamboo bridges in Asia7,37. It has been reported that target species could be selective on bridge type16,47, and bridges designed for gibbons are not always used, as canopy bridges erected for eastern hoolock gibbon (Hoolock leuconedys) in India for a translocation operation were never used36 , and the isolated population of western hoolock gibbons in northeast India never cross a metal canopy bridge until a natural tree canopy bridge was established after 13 years48. Rope bridges are inexpensive, material readily available, easy to transport in remote hilly regions, technically simple to make and install, and have been used by three of the four extant gibbon genera (Nomascus, Hoolock and Hylobates). We suggest it may be the most cost-effective artificial canopy bridge type for Hylobatidae, and should be constructed and tested in areas where gibbon habitat is fragmented.
Brachiation is the predominant locomotion mode during travel by gibbons49. It is possible the rope diameter for building our canopy bridge is not the most desirable for Hainan gibbon locomotion as climbing instead of brachiation was the most employed mode in our study. Gibbon locomotion studies in Malaysia show that Siamang Symphalangus syndactylus, Lar Gibbon Hylobates lar and Agile Gibbon H. agilis tend to brachiate on larger support and climb on smaller ones49, and support between 20–100 mm diameter was most frequently used (> 60%) for a similar study on cao-vit gibbon46. Our flexible canopy bridge was constructed using 13 mm diameter ropes and may not be providing the most desirable support for brachiation in Hainan gibbon. However, one must consider the logistic and financial implications, as well as the sustenance of supporting trees for choosing thicker ropes or more rigid materials for building canopy bridges.
We considered the canopy bridge as a temporary measure in the conservation management of Hainan gibbon, and launched a reforestation project at the landslide after completion of the canopy bridge construction as a long-term solution to restore forest connectivity. Twenty-five large saplings (> 150 cm tall) of a fast-growing native tree, Bishchofia javanicus, a favourite gibbon food tree with large crown, were planted underneath the canopy bridge. Two years after the landslide, dense growth of pioneering trees, e.g. Ficus esquiroliana, Broussonetia papyrifera, Trema orientalis and Macaranga denticulate also regenerated underneath the rope bridges, and together with the planted saplings, they have been providing an alternative crossing route for the gibbons since November 2018. The lifetime of climbing ropes varies depending on use frequency and manufacture specifications, but a general replacement guideline for human usage is 3–5 years (Cho Wing Chung, pers. comm., 2020). Although gibbons weigh a lot less than human and the ropes should last longer than the safety standard for human usage, it was our plan to replace the ropes in 2020. However, the COVID-19 pandemic precluded traveling thus replacement. The condition of the ropes has been visually monitored regularly by our gibbon monitoring team for signs of external wear and tear. Although we cannot assess fatigue in the ropes, the dense growth of regenerated vegetation underneath the rope bridge is believed to provide a safe landing platform for the gibbons should an accidental fall happened. We recommend building canopy bridges at the early stage of a forest gap restoration project to provide temporary connectivity, but reforestation with fast-growing native trees should also be simultaneously carried out as a long-term solution where it is socioeconomically feasible.
Methods
Study area
Xiaofutouling is a peak (1030 m asl) on the eastern border of BWL, and the zonal vegetation is seasonal tropical rainforest. The forest of Xiaofutouling was selectively logged in the 1970s and subjected to intense human use due to its proximity to villages, but enhanced patrolling effort in the last nine years has substantially curbed human disturbance. Despite these sustained disturbances, the mountain is still covered by contiguous old-growth forest with a canopy height of 25 m above ca. 700 m asl (Fig. 5). Common tree species include Castanopsis spp., Elaeocarpus spp., Endospermum chinense, Ficus spp., Liquidambar formosana, Lithocarpus spp., Nephelium topengii and Pinus latteri, some of which are important gibbon food tree species.
Figure 5.
Landscape of the study area (Xiaofutouling is in the foreground), taken on 21 June 2018. Photograph taken by Z.M.
The study gibbon group
The study group, Group C, was formed in 2011 and started breeding in 2013, gradually becomes the largest gibbon group in BWL. At the onset of the study, Group C consisted of eight gibbons including one breeding male, two breeding females, one large juvenile and four small juveniles. With three births and two individuals dispersed by late 2018, Group C had nine gibbons (one breeding male, two breeding females, three large juveniles, two small juveniles and one infant) at the end of the study. Survivorship of immature gibbons had been 100% according to our long-term monitoring data. The group has been habituated by a gibbon monitoring team we trained since 2011, allowing detailed observation during daily tracking.
The canopy bridges
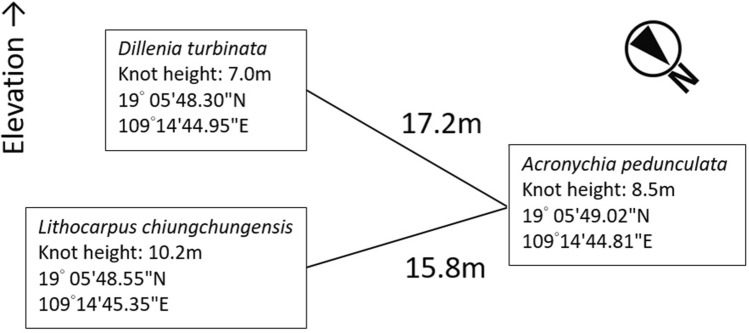
With consideration to the unique brachiation locomotion of gibbons, we designed a simple rope bridge, which is technically and economically low-cost, and minimize the risk of entanglement during brachiation. Since canopy bridge construction at the study site involved much logistical challenges, including 3 h of uphill hiking, camping and technical tree climbing on steep terrain, we designed a two-pronged, double-roped canopy bridge network, making sure a back-up rope is always available at the damaged arboreal highway. On 06 December 2015, three professional tree climbers constructed the canopy bridge at 824 m asl (19 °05′ 48.3″ N, 109° 14′ 44.9″ E) (Fig. 6). Each bridge consisted of two mountaineering-grade 13 mm diameter ropes tied at the same knot on sturdy trees at the natural gibbon crossing. One bridge measures 15.8 m in length and the other 17.2 m (Fig. 7). Distances and tree heights were measured by a Nikon Forest Pro laser rangefinder. The total cost of constructing the canopy bridge was ca. USD $5000, including raw materials, service fee of professional tree climbers and logistical cost of reaching the remote site.
Figure 6.
Installation of the canopy bridge by professional tree climbers, taken on 06 December 2015. Photograph taken by C.F.M.
Figure 7.
A schematic diagram of the canopy bridge for Hainan gibbon showing GPS coordinates, rope length, knot height and species of supporting trees.
Crossing monitoring
In order to evaluate the effectiveness of the artificial canopy bridge for Hainan gibbon, we monitored the rope bridge by installing a camera trap from 06 December 2015 (Loreda L710 [December 2015 to August 2018] and ScoutGuard SG-990V [August 2018 to February 2019]). Once triggered, the camera trap recorded three still images followed by a 10-s video. We identified separate crossing events by all crossings recorded within a 5-min period based on the time and date of photographs/videos taken by the camera trap; thus it could be a single gibbon crossing or multiple individuals crossing in quick succession. As our long-term ground monitoring data show that members within the same gibbon group always travel together, our study provided data on the age–sex difference in locomotor behaviour of Hainan gibbon using artificial canopy bridge.
To avoid any potential problem induced by temporal autocorrelation, we used camera trap video footages to study the dominant form of locomotion of each individual in crossing the rope bridge to analyse the age-sex difference in locomotor mode of Hainan gibbon. We categorized five different locomotion modes in the present study following Fan et al.46: (i) brachiation, which is arm-over-arm suspensory progression, which can be rapid and ricochetal; (ii) climbing, which is any continuous progression involving three or more limbs; (iii) bridging, which is a special form of climbing in which the animal crosses a small discontinuity in the canopy; (iv) walking, bipedal locomotion along a support with arms held out for balance; and (v) leaping, where the animals launches themselves by pulling with the arms, and usually lands on all four limbs, and dropping was included in leaping. We also recorded sequence of crossing by different age and sex in the gibbon group; we followed the same publication for age-sex categorizations, large juveniles were distinguished from small juveniles based on body size, being approaching the size of but smaller than full-grown adults.
Informed consents have been obtained from the persons in Figs. 2 and 6 to publish the images in an online open-access publication.
Supplementary information
Acknowledgements
We thank the Forestry Department of Hainan Province and the Hainan Bawangling National Nature Reserve for permission to conduct our study. We are grateful to the three professional tree climbers, Mr. Cho Wing Chung, Mr. Lai Pak Ki and Mr. Ling Kai Ho, who constructed the canopy bridge under adverse weather condition. We are in debt to the gibbon monitoring team for their diligent work in protecting and monitoring the Hainan gibbon and its habitat. Ellis Li, Michael Hui, Colin Yeung, Lu Gang and Anny Li of KFBG contributed in various ways to this work. This study was funded by Kadoorie Farm & Botanic Garden.
Author contributions
B.P.L.C. and X.J.H. conceived and supervised the project. B.P.L.C. and C.F.M. designed the canopy bridge. B.P.L.C., Y.F.P.L., C.F.M. and Z.M. collected field data. Y.F.P.L. and Z.M. analysed the data and prepared figures. B.P.L.C. wrote the main manuscript text. All authors reviewed and accepted the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-72641-z.
References
- 1.Saunders DA, Hobbs RJ, Margules CR. Biological consequences of ecosystem fragmentation: a review. Conserv. Biol. 1991;5:18–32. doi: 10.1002/ajp.23076. [DOI] [Google Scholar]
- 2.Fahrig L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003;34:487–515. doi: 10.1146/annurev.ecolsys.34.011802.132419. [DOI] [Google Scholar]
- 3.Haddad NM, et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015;1:1–10. doi: 10.1126/sciadv.1500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alamgir M, et al. High-risk infrastructure projects pose imminent threats to forests in Indonesian Borneo. Sci. Rep. 2019;9(140):1–10. doi: 10.1038/s41598-018-36594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen MC, et al. The fate of tropical forest fragments. Sci. Adv. 2020;6:1–10. doi: 10.1126/sciadv.aax8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onderdonk DA, Chapman CA. Coping with forest fragmentation: the primates of Kibale National Park, Uganda. Int. J. Primatol. 2000;21:587–611. doi: 10.1023/A:1005509119693. [DOI] [Google Scholar]
- 7.Das J, Biswas J, Bhattacherjee PC, Rao SS. Canopy bridges: an effective conservation tactic for supporting gibbon populations in forest fragments. Gibbons. 2009;1:467–475. doi: 10.1007/978-0-387-88604-6. [DOI] [Google Scholar]
- 8.Taylor AC, Walker FM, Goldingay RL, Ball T, van der Ree R. Degree of landscape fragmentation influences genetic isolation among populations of a gliding mammal. PLoS ONE. 2011;6:1–9. doi: 10.1371/journal.pone.0026651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarma K, Kumar A, MuraliKrishna C, Tripathi OP, Gajurel PR. Ground feeding observations on corn (Zea mays) by eastern hoolock gibbon (Hoolock leuconedys) Curr. Sci. 2013;104:587–589. [Google Scholar]
- 10.Chapman CA, et al. Do food availability, parasitism, and stress have synergistic effects on red colobus populations living in forest fragments? Am. J. Phys. Anthropol. 2006;131:525–534. doi: 10.1002/ajpa.20477. [DOI] [PubMed] [Google Scholar]
- 11.Donaldson A, Cunneyworth P. Case study: canopy bridges for primate conservation. Handb. Road Ecol. 2015;1:341–343. doi: 10.1002/9781118568170.ch41. [DOI] [Google Scholar]
- 12.Hernández-pérez E. Rope bridges: a strategy for enhancing habitat connectivity of the Black Howler Monkey (Alouatta pigra) Neotrop. Primates. 2015;22:94–96. [Google Scholar]
- 13.Gregory T, Carrasco-Rueda F, Alonso A, Kolowski J, Deichmann JL. Natural canopy bridges effectively mitigate tropical forest fragmentation for arboreal mammals. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-04112-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni Q, et al. Microhabitat use of the western black-crested gibbon inhabiting an isolated forest fragment in southern Yunnan, China: implications for conservation of an endangered species. Primates. 2018;59:45–54. doi: 10.1007/s10329-017-0634-7. [DOI] [PubMed] [Google Scholar]
- 15.Al-Razi H, Maria M, Muzaffar S. Mortality of primates due to roads and power lines in two forest patches in Bangladesh. Zoologia. 2019;36:1–6. doi: 10.3897/zoologia.36.e33540. [DOI] [Google Scholar]
- 16.Birot H, Campera M, Imron MA, Nekaris KAI. Artificial canopy bridges improve connectivity in fragmented landscapes: the case of Javan slow lorises in an agroforest environment. Am. J. Primatol. 2020;82:1–10. doi: 10.1002/ajp.23076. [DOI] [PubMed] [Google Scholar]
- 17.Forman RTT, Alexander LE. Roads and their major ecological effects. Annu. Rev. Ecol. Syst. 1998;29:207–231. doi: 10.1146/annurev.ecolsys.29.1.207. [DOI] [Google Scholar]
- 18.Sarma K, Kumar A. The day range and home range of the Eastern Hoolock Gibbon Hoolock leuconedys (Mammalia: Primates: Hylobatidae) in lower dibang valley district in Arunachal Pradesh India. J. Threat. Taxa. 2016;8:8641–8651. doi: 10.11609/jott.2739.8.4.8641-8651. [DOI] [Google Scholar]
- 19.Estrada A, et al. Impending extinction crisis of the world’s primates: why primates matter. Sci. Adv. 2017 doi: 10.1126/sciadv.1600946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balbuena D, Alonso A, Panta M, Garcia A, Gregory T. Mitigating tropical forest fragmentation with natural and semi-artificial canopy bridges. Diversity. 2019 doi: 10.3390/d11040066. [DOI] [Google Scholar]
- 21.Valladares-Padua CB, Junior LC, Padua S. A pole bridge to avoid primates road kils. Neotrop. Primates. 1995;3:13. [Google Scholar]
- 22.Weston N, Goosem M, Marsh H, Cohen M, Wilson R. Using canopy bridges to link habitat for arboreal mammals: successful trials in the Wet Tropics of Queensland. Aust. Mammal. 2011;33:93–105. doi: 10.1071/AM11003. [DOI] [Google Scholar]
- 23.Gregory T, et al. Methods to establish canopy bridges to increase natural connectivity in linear infrastructure development. Soc. Pet. Eng. J. 2013 doi: 10.2118/165598-MS. [DOI] [Google Scholar]
- 24.Teixeira FZ, Printes RC, Fagundes JCG, Alonso AC, Kindel A. Canopy bridges as road overpasses for wildlife in urban fragmented landscapes. Biota Neotrop. 2013;13:117–123. doi: 10.1590/S1676-06032013000100013. [DOI] [Google Scholar]
- 25.Yokochi K, Bencini R. A remarkably quick habituation and high use of a rope bridge by an endangered marsupial, the western ringtail possum. Nat. Conserv. 2015;11:79–94. doi: 10.3897/natureconservation.11.4385. [DOI] [Google Scholar]
- 26.Mittermeier RA, Rylands AB, Wilson DE. Handbook of the Mammals of the World. Barcelona: Lynx Edicions; 2013. [Google Scholar]
- 27.Wilson DE, Lacher TE. Handbook of the Mammals of the World. Barcelona: Lynx Edicions; 2016. [Google Scholar]
- 28.Ancrenaz, M. Orang-utan Bridges in Lower Kinabatangan: Field surveys between Abai and Batu Puteh. https://www.arcusfoundation.org/wp%E2%80%90content/uploads/2010/01/Kinabatangan%E2%80%90Orangutan%E2%80%90Rope%E2%80%90Bridges%E2%80%90Ancrenaz%E2%80%902010.pdf (2010).
- 29.Goossens B, Ambu LN. Sabah wildlife department and 10 years of research: Towards a better conservation of Sabah’s wildlife. J. Oil Palm Environ. 2012;3:38–51. doi: 10.5366/jope.2012.05. [DOI] [Google Scholar]
- 30.Kumar A, Devi A, Gupta AK, Sarma K. Population, behavioural ecology and conservation of Hoolock Gibbon in Northeast India. Rare Anim. India. 2013;1:242–266. [Google Scholar]
- 31.Yap JL, Ruppert N, Rosely NFN. Activities, habitat use and diet of wild Dusky Langurs, Trachypithecus obscurus in different habitat types in Penang, Malaysia. J. Sustain. Sci. Manag. 2019;14:71–85. [Google Scholar]
- 32.Arjun Oli. Banke National Park builds canopy bridge to reduce wild animal road accidents. myRepublicahttps://myrepublica.nagariknetwork.com/news/banke-national-park-builds-canopy-bridge-to-reduce-wild-animal-road-accidents (2019).
- 33.Lekshmi Priya S. Kerala Sanctuary builds ‘canopy bridges’, saves animals from road hits!. The Better Indiahttps://www.thebetterindia.com/185838/kerala-forest-department-chinnar-wildlife-sanctuary-canopy-bridges-india (2019).
- 34.Chivers DJ. Malayan Forest Primates: Ten Years’ Study in Tropical Rain Forest. New York: Plenum Press; 1982. [Google Scholar]
- 35.Cheyne SM, Thompson CJH, Chivers DJ. Travel adaptations of Bornean Agile Gibbons Hylobates albibarbis (Primates: Hylobatidae) in a degraded secondary forest, Indonesia. J. Threat. Taxa. 2013;5:3963–3968. doi: 10.11609/JoTT.o3361.3963-8. [DOI] [Google Scholar]
- 36.Campbell CO, Cheyne SM, Rawson BM. Best practice guidelines for the rehabilitation and translocation of gibbons. IUCN SSC Primate Spec. Group. 2015 doi: 10.2305/IUCN.CH.2015.SSC-OP.51.en. [DOI] [Google Scholar]
- 37.Saralamba, C. & Menpreeda, W. Increasing connectivity through artificial canopy bridge for the gibbons: a case study on the activity budget. Abstract for The 87th Annual Meeting of the American Association of Physical Anthropologists, Austin (Texas) (2018).
- 38.Chan, B. P. L., Fellowes, J. R., Geissmann, T., & Jianfeng, Z. Hainan Gibbon Status Survey and Conservation Action Plan: VERSION I (Last Updated November 2005). Kadoorie Farm & Botanic Garden Technical Report No.3 (2005).
- 39.Chan, B. P. L. Hainan gibbon Nomascus hainanus (Thomas, 1892). In Primates in Peril: The World’s 25 Most Endangered Primates 2014–2016. (eds Schwitzer, C., et al.). IUCN SSC Primate Spec. Gr. (PSG), Int. Primatol. Soc. (IPS), Conserv. Int. (CI), Bristol Zool. Soc. Arlington, VA. 67–69 (2015).
- 40.Chan BPL, Lo YFP, Mo Y. New hope for the Hainan gibbon: formation of a new group outside its known range. Oryx. 2020;54:296. doi: 10.1017/S0030605320000083. [DOI] [Google Scholar]
- 41.Zeng, X. et al. Hainan Gibbon Conservation Action Plan 2016–2020. https://www.gibbons.asia/wp-content/uploads/2017/03/Hainan-Gibbon-Action-Plan-2016-2020.pdf (2016).
- 42.Zheng Y, Cai Q, Cheng S, Li X. Characteristics on intensity and precipitation of super typhoon Rammasun (1409) and reason why it rapidly intensified offshore. Torrential Rain Disast. 2014;33:333–341. [Google Scholar]
- 43.Chivers, D. J. The Siamang in Malaya. A Field Study of a Primate in Tropical Rainforest. Karger (1974). [PubMed]
- 44.Cristóbal-Azkarate J, Arroyo-Rodríguez V. Diet and Activity Pattern of Howler Monkeys (Alouatta palliata) in Los Tuxtlas, Mexico: effects of Habitat Fragmentation and Implications for Conservation. Am. J. Primatol. 2007;69:1013–1029. doi: 10.1002/ajp.20420. [DOI] [PubMed] [Google Scholar]
- 45.Arroyo-Rodríguez V, Mandujano S. Conceptualization and measurement of habitat fragmentation from the primates’ perspective. Int. J. Primatol. 2009;30:497–514. doi: 10.1007/s10764-009-9355-0. [DOI] [Google Scholar]
- 46.Fan P, Scott MB, Fei H, Ma C. Locomotion behavior of cao vit gibbon (Nomascus nasutus) living in karst forest in Bangliang Nature Reserve, Guangxi, China. Integr. Zool. 2013;8:356–364. doi: 10.1111/j.1749-4877.2012.00300.x. [DOI] [PubMed] [Google Scholar]
- 47.Mass V, et al. Lemur bridges provide crossing structures over roads within a forested mining concession near moramanga, toamasina province, Madagascar. Conserv. Evid. 2011;8:11–18. [Google Scholar]
- 48.Naresh Mitra. Guwahati: Natural bridge reunites hoolock gibbons after 100 years. Times of Indiahttps://timesofindia.indiatimes.com/city/guwahati/natural-bridge-reunites-hoolock-gibbons-after-100-years/articleshow/69998213.cms (2019).
- 49.Fleagle JG. Locomotion and posture. In: Chivers DJ, editor. Malayan Forest Primates: Ten Years’ Study in Tropical Rain Forest. New York: Plenum Press; 1980. pp. 191–207. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.