Abstract
Infection rates, severity, and fatalities due to COVID‐19, the pandemic mediated by SARS‐CoV‐2, vary greatly between countries. With few exceptions, these are lower in East and Southeast Asian and Sub‐Saharan African countries compared with other regions. Epidemiological differences may reflect differences in border closures, lockdowns, and social distancing measures taken by each county, and by cultural differences, such as common use of face masks in East and Southeast Asian countries. The plasma serine protease inhibitor alpha‐1 antitrypsin was suggested to protect from COVID‐19 by inhibiting TMPRSS2, a cell surface serine protease essential for the SARS‐CoV‐2 cell entry. Here, we present evidence that population differences in alpha‐1 antitrypsin deficiency allele frequencies may partially explain national differences in the COVID‐19 epidemiology. Our study compared reported national estimates for the major alpha‐1 antitrypsin deficiency alleles PiZ and PiS (SERPINA1 rs28929474 and rs17580, respectively) with the Johns Hopkins University Coronavirus Resource Center dataset. We found a significant positive correlation (R = .54, P = 1.98e−6) between the combined frequencies of the alpha‐1 antitrypsin PiZ and PiS deficiency alleles in 67 countries and their reported COVID‐19 mortality rates. Our observations suggest that alpha‐1 antitrypsin deficiency alleles may contribute to national differences in COVID‐19 infection, severity, and mortality rates. Population‐wide screening for carriers of alpha‐1 antitrypsin deficiency alleles should be considered for prioritizing individuals for stricter social distancing measures and for receiving a SARS‐CoV‐2 vaccine once it becomes available.
Keywords: alpha‐1 antitrypsin, COVID‐19, rs17580, rs28929474, SERPINA1
Abbreviations
- AAT
alpha‐1 antitrypsin
- ANCOVA
analysis of covariance
- COPD
chronic obstructive pulmonary diseases
- COVID‐19
coronavirus disease‐2019
- HDI
Human Development Index
- PiS
alpha‐1 antitrypsin deficiency allele S (SERPINEA1 rs17580)
- PiZ
alpha‐1 antitrypsin deficiency allele Z (SERPINEA1 rs28929474)
- SARS
severe acute respiratory syndrome
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SERPINA1
Serpin Family A Member 1 (the gene coding for alpha‐1 antitrypsin)
- TMPRSS2
Transmembrane Serine Protease 2
1. INTRODUCTION
Differences between countries in state‐mandated border closures, lockdowns, and social distancing regulations provide obvious reasons for the great variation between countries in COVID‐19 infection rates and morbidity. Differences in cultural customs have also been attributed a role. For example, wearing face masks was common in some Asian countries prior to the current pandemic, and this may have contributed to lower COVID‐19 infection and fatality rates in these countries. 1 Genetic variations between ethnic groups have also been proposed as a factor that contributes to differences in COVID‐19 epidemiology. The relatively low rates of COVID‐19 infection and mortality in East and Southeast Asian countries, including Japan, China, South Korea, Thailand, Vietnam, Cambodia, and Malaysia, are notable, and remain little understood. 2 Relatively low COVID‐19 infection and mortality rates are also notable for several Sub‐Saharan African countries. While these rates may be due to lower testing for SARS‐CoV‐2 (severe acute respiratory syndrome coronavirus 2; the virus causing COVID‐19), and fewer international arrivals, they may also reflect genetic variations between the populations. However, at the time of this writing, no studies have reported associations of genetic variations between populations with COVID‐19 infection and fatality rates.
The serine protease TMPRSS2 is well established as essential for priming SARS‐CoV‐2 cell entry, following binding to its ACE2 cell membrane receptor. 3 Accordingly, the synthetic serine protease inhibitors nafamostat mesylate and camostat mesylate were shown to inhibit TMPRSS2 4 and are undergoing clinical trials in COVID‐19 patients. The major human blood serine protease inhibitor (serpin) is alpha‐1 antitrypsin, which is encoded by the SERPINA1 gene and produced primarily in the liver, similar to many blood proteins. Comparing regions in Italy, differential alpha‐1 antitrypsin genetic deficiency rates were proposed to correlate with COVID‐19 infection. This was based on higher rates of both alpha‐1 antitrypsin deficiency (carriers of SERPINA1 rs28929474, termed PiZ) and COVID‐19 infection rates in Lombardy, Italy than in central and southern Italian regions. 5 Based on these observations, as well as on its antiviral and anti‐inflammatory properties, alpha‐1‐antitrypsin was proposed as a tentative host protective factor against COVID‐19. 6 We therefore examined a possible association between the distributions of common SERPINA1 single nucleotide polymorphisms (SNPs) underlying alpha‐1 antitrypsin deficiency and between COVID‐19 epidemiology on a global scale.
2. METHODS
National allele frequency estimates for the alpha‐1 antitrypsin PiS (SERPINA1 rs17580) and PiZ (SERPINA1 rs28929474) alleles were obtained from Blanco et al. 7 National parameters were obtained from the United Nations database and The World Bank Open Data (data.worldbank.org). For combining the effects of the PiS and PiZ alleles on alpha‐1 antitrypsin serum concentration, their frequencies were summed, and weighted by their approximate effect size (50% and 90% respectively, according to Mitchell et al 8 ). Confounder correction and assessment of individual factor effect sizes were done using analysis of covariance (ANCOVA). All statistical analyses were made in the R programming environment. Country‐specific epidemiological parameters of the ongoing COVID‐19 pandemic were compared between all countries using data from the Johns Hopkins University Coronavirus Resource Center 9 (accessed September 7, 2020). We focused on countries with a population of over one million in order to reduce noise, for example, from small island nations.
3. RESULTS
We examined if population variations in human serine protease inhibitor (serpin) genes may help explain the lower COVID‐19 infection rates and fatalities in East and Southeast Asia and Sub‐Saharan Africa, compared to other regions. Among the 43 human serpin genes, SERPINA1, coding for alpha‐1 antitrypsin, a major blood serine protease inhibitor, showed the most striking variations in SNP frequencies between Europeans and non‐Europeans. We examined in particular variations in SERPINA1 rs28929474 (the PiZ allele). This allele is recognized as the major allele responsible for antitrypsin deficiency in humans, and was recently suggested to account for the higher COVID‐19 infection rates in Lombardy compared with other Italian regions. 5 SERPINA1 rs28929474 was about eight‐fold less frequent in East and Southeast Asian than in South European populations (17 compared to 2 alleles per 1000 individuals). For the milder deficiency PiS allele, SERPINA1 rs17580 disparity was even greater (86 compared to 5 alleles per 1000 individuals, over a 17‐fold reduction). The combined analysis of the global PiZ and PiS allele frequencies is shown in Figure 1A.
FIGURE 1.
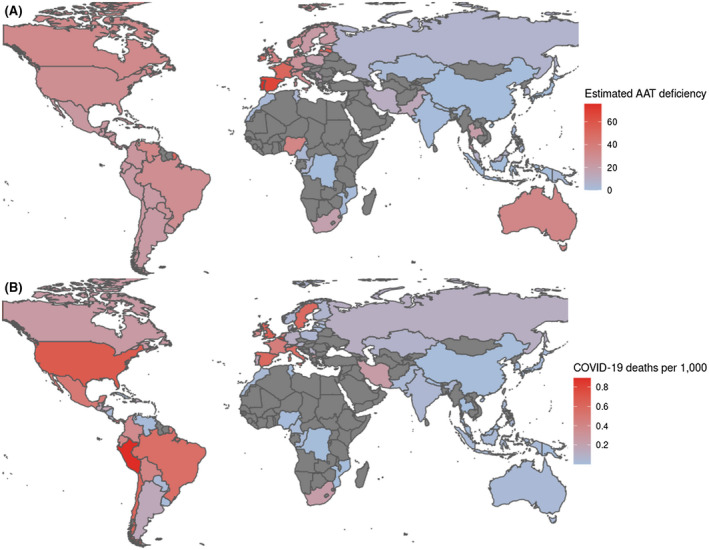
Demographics of national alpha‐1 antitrypsin (AAT) deficiency and COVID‐19 fatality rates per 1,000 population. A, Estimated population‐wide alpha‐1 antitrypsin (AAT) deficiency, based on frequencies of alleles PiZ and PiS (SERPINA1 rs28929474 and rs17580, respectively) aggregated by Blanco et al, 7 and summed proportionally to their respective effect sizes on alpha‐1 antitrypsin serum concentration. B, COVID‐19 fatality rates per 1,000 population, from the Johns Hopkins University Coronavirus Resource Center 9 (September 7, 2020)
Our comparison of epidemiological data showed that nearly all the countries reporting >100 COVID‐19 mortalities per million population (as of September 7, 2020) were in Europe or the Americas. In contrast, East and Southeast Asian, and also Sub‐Saharan African countries, were the predominant countries with low mortality rates per million population (Figure 1B; Tables S2 and S3). The COVID‐19 population‐adjusted fatality rates for Thailand, Vietnam, and Cambodia were 0.8, 0.2, and <0.1 per million, respectively 9 ; low COVID‐19 fatality rates were also notable for several Sub‐Saharan African nations, such as Congo, and Mozambique (2 and 0.7 per million, respectively). Thus, the difference is up to ~100‐fold lower compared with some European and American countries. The far lower population‐adjusted burden of COVID‐19 in many Asian and Sub‐Saharan African countries remains notable when comparing total infections per one million people (Tables S2 and S3).
Lower COVID‐19 infection and fatality rates in many Asian and Sub‐Saharan countries could stem from confounding factors, such as Human Development Index (HDI), urbanization level, and volume of international travel, as well as lower proportions of elderly people. However, our analysis of covariance resulted in a significant association between estimated alpha‐1 antitrypsin deficiency and COVID‐19 mortality after controlling for confounders (Figures S1, S2, and Table S1; a full analysis workflow is available at: https://gitlab.com/shep/serpina1_covid_supplementary). Moreover, infection and fatality rates remain among the highest globally in several South‐American countries with low HDI and relatively low proportions of elderly persons. The striking difference in the COVID‐19 burden between South‐American countries and between East and Southeast Asian countries, therefore, seems to be best explained by ethnic genetic variation. Until additional candidate alleles are found to better explain such national differences, the population variations in the allele frequencies of alpha‐1 antitrypsin deficiency present a possible genetic explanation for the varying national COVID‐19 burden.
We applied estimated country‐specific frequencies of alpha‐1 antitrypsin deficiency 8 to analyze COVID‐19 fatalities per thousand inhabitants vs. estimated alpha‐1 antitrypsin deficiency in 67 countries (Figure 2). The Pearson correlation of R = .54 (P = 1.98e−6) suggests an association of alpha‐1 antitrypsin deficiency with COVID‐19 fatalities. To eliminate the confounding effects of such factors as urbanization and age distribution, we used a one‐way analysis of covariance, resulting in significant association also after adjusting for confounders (Figures S1, S2, and Table S1). Considering the above, we propose that the low carrier frequency of alpha‐1 antitrypsin deficiency PiZ and PiS alleles in East and Southeast Asian and Sub‐Saharan African countries may in‐part explain the lower COVID‐19 infection and mortality rates in these regions.
FIGURE 2.
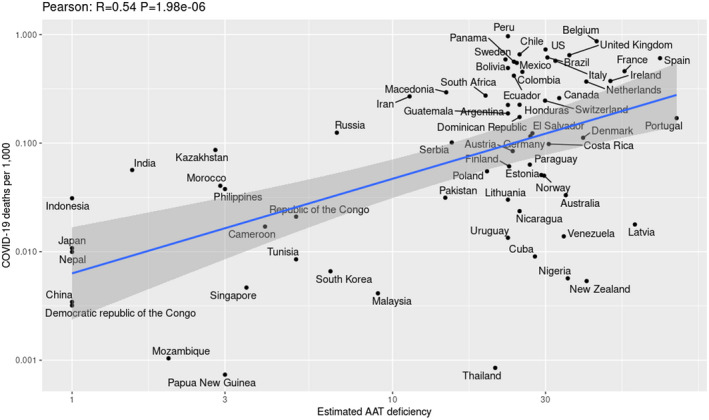
Positive correlations between estimated national alpha‐1 antitrypsin deficiency allele frequencies and COVID‐19 fatality rates per 1000 population. The Pearson correlation of R = 0.54 (P = 1.98e‐6) suggests an association of national rates of alpha‐1 antitrypsin deficiency with the national rates of COVID‐19 fatalities. Only countries with a population larger than a million are included. Highlighted region represents the 95% confidence interval
4. DISCUSSION
In addition to being a major blood serpin, alpha‐1 antitrypsin is known as a potent anti‐inflammatory protein and a key regulator of the human acute phase immune response. McElvaney et al 10 recently reported elevated plasma alpha‐1 antitrypsin levels in COVID‐19 patients. Chronic obstructive pulmonary disease (COPD) and liver illnesses such as fibrosis are common manifestations of alpha‐1 antitrypsin deficiency, which are also prominent comorbidities characterizing severe COVID‐19 cases. Moreover, COPD patients carrying one or two copies of the alpha‐1 antitrypsin PiZ allele were reported to have higher interleukin‐17 (IL‐17) and decreased interferon‐γ (IFN‐γ) serum levels compared with those having wild‐type (PiMM) alpha‐1 antitrypsin 11 ; while IL‐17 blockers were recently proposed as safe COVID‐19 therapeutics deserving clinical trials. 12
Notably, alpha‐1 antitrypsin was shown to have distinct endogenous anti‐inflammatory properties, superior to those of corticosteroids, that may improve tissue well‐being during inflammation while preventing undesired corticosteroid‐mediated side effects. 13 Additionally, alpha‐1 antitrypsin deficiency was associated with vitamin D deficiency among patients of type‐2 diabetes, 14 while vitamin D deficiency was reported as a COVID‐19 infection risk factor. 15 Lastly, a 2004 proteomics study reported that sera samples of SARS patients had dramatically elevated levels of truncated forms of alpha‐1 antitrypsin, which correlated with SARS severity, and suggested that these truncated AAT forms could serve as SARS biomarkers with a sensitivity of 100%. 16 Based on their findings, the authors suggested that AAT plays a key role in the protection of lung function against respiratory infections, and its degradation in SARS patients plays a key role in SARS pathogenesis. 16 However, to the best of our knowledge, no studies (including in preprint form) explored levels of truncated AAT forms in sera samples from COVID‐19 patients. Together, these observations implicate alpha‐1 antitrypsin deficiency in insufficient immune response to SARS‐CoV‐2 infection, and thereby in reduced protection against COVID‐19.
Indeed, the bacillus Calmette‐Guérin (BCG) vaccination was proposed as protective from severe COVID‐19, 17 while it was shown to increase alpha‐1 antitrypsin blood levels in BCG vaccinated individuals together with trained immunity. 18 Notably, a recent study (posted as preprint and not yet published at submission of this manuscript) demonstrated in vitro inhibition of human TMPRSS2 by alpha‐1 antitrypsin, thus suggesting that it might be effective as a COVID‐19 treatment. 19
Another recent preprint showed that alpha‐1 antitrypsin may inhibit in vitro SARS‐CoV‐2 infection in human airway epithelium at physiological concentrations. 20 A clinical trial of inhaled alpha‐1 antitrypsin in COVID‐19 patients is currently recruiting participants (NCT04385836). If alpha‐1 antitrypsin deficiency will be established as a genetic risk factor for severe COVID‐19, and keeping in mind that vaccine availability would be scarce for at least one year following its approval, 21 population‐wide screening for alpha‐1 antitrypsin deficient individuals would be desirable for identifying deficient individuals and prioritizing them for vaccination.
CONFLICT OF INTEREST
The authors declare that no conflicts of interest exist.
AUTHOR CONTRIBUTIONS
D. Gurwitz and N. Shomron conceived the study; G. Shapira analyzed the data and statistics and prepared the figures and Supporting Informations. All authors took part in writing the article.
Supporting information
Supplementary Material
Dataset S1
Dataset S2
ACKNOWLEDGMENTS
D. Gurwitz is supported by the Yoran Institute for Human Genome Research at Tel Aviv University. The N. Shomron lab is partially supported by the Adelis Foundation. The authors thank Cindy Cohen for professional editorial assistance.
Shapira G, Shomron N, Gurwitz D. Ethnic differences in alpha‐1 antitrypsin deficiency allele frequencies may partially explain national differences in COVID‐19 fatality rates. The FASEB Journal. 2020;34:14160–14165. 10.1096/fj.202002097
Noam Shomron and David Gurwitz are equal last authors
This article was fast‐tracked under a recently instituted interim policy in which editors may, at their discretion, accept coronavirus‐related manuscripts submitted for the Review, Perspectives, and Hypotheses categories without additional review.
Contributor Information
Noam Shomron, Email: nshomron@tauex.tau.ac.il.
David Gurwitz, Email: gurwitz@tauex.tau.ac.il.
REFERENCES
- 1. Iwasaki A, Grubaugh ND. Why does Japan have so few cases of COVID‐19? EMBO Mol Med. 2020;12(5):e12481 10.15252/emmm.202012481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gaye B, Khoury S, Cene CW, et al. Socio‐demographic and epidemiological consideration of Africa's COVID‐19 response: what is the possible pandemic course? Nat Med. 2020;26(7):996‐999. 10.1038/s41591-020-0960-y [DOI] [PubMed] [Google Scholar]
- 3. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yamamoto M, Kiso M, Sakai‐Tagawa Y, et al. The anticoagulant nafamostat potently inhibits SARS‐CoV‐2 S protein‐mediated fusion in a cell fusion assay system and viral infection in vitro in a cell‐type‐dependent manner. Viruses. 2020;12(6):629 10.3390/v12060629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vianello A, Braccioni F. Geographical overlap between alpha‐1 antitrypsin deficiency and COVID‐19 infection in Italy: casual or causal? Arch Bronconeumol. 2020;56:609‐610. S0300–2896(20)30169–1. 10.1016/j.arbres.2020.05.015. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 6. de Loyola MB, Dos Reis TTA, de Oliveira GXLM, da Fonseca PJ, Argañaraz GA, Argañaraz ER. Alpha‐1‐antitrypsin: a possible host protective factor against Covid‐19. Rev Med Virol. 2020:e2157. 10.1002/rmv.2157. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blanco I, Bueno P, Diego I, et al. Alpha‐1 antitrypsin Pi*SZ genotype: estimated prevalence and number of SZ subjects worldwide. Int J Chron Obstruct Pulmon Dis. 2017;12:1683‐1694. 10.2147/COPD.S137852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mitchell EL, Khan Z. Liver disease in alpha‐1 antitrypsin deficiency: current approaches and future directions [published correction appears in Curr Pathobiol Rep. 2018;6(1):97]. Curr Pathobiol Rep. 2017;5(3):243‐252. 10.1007/s40139-017-0147-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20(5):533‐534. 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McElvaney OJ, McEvoy N, McElvaney OF, et al. Characterization of the inflammatory response to severe COVID‐19 illness. Am J Respir Crit Care Med. 2020;202:812‐821. 10.1164/rccm.202005-1583OC. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pervakova MY, Mazing AV, Lapin SV, et al. High serum level of IL‐17 in patients with chronic obstructive pulmonary disease and the alpha‐1 antitrypsin PiZ Allele. Pulm Med. 2020;2020:9738032 10.1155/2020/9738032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pacha O, Sallman MA, Evans SE. COVID‐19: a case for inhibiting IL‐17? Nat Rev Immunol. 2020;20(6):345‐346. 10.1038/s41577-020-0328-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schuster R, Motola‐Kalay N, Baranovski BM, et al. Distinct anti‐inflammatory properties of alpha1‐antitrypsin and corticosteroids reveal unique underlying mechanisms of action. Cell Immunol. 2020;356:104177 10.1016/j.cellimm.2020.104177 [DOI] [PubMed] [Google Scholar]
- 14. Lindley VM, Bhusal K, Huning L, Levine SN, Jain SK. Reduced 25(OH) vitamin D association with lower alpha‐1‐antitrypsin blood levels in type 2 diabetic patients. J Am Coll Nutr. 2020:1‐6. 10.1080/07315724.2020.1740629. Online ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of vitamin D status and other clinical characteristics with COVID‐19 test results. JAMA Netw Open. 2020;3(9):e2019722 10.1001/jamanetworkopen.2020.19722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ren YI, He Q‐Y, Fan J, et al. The use of proteomics in the discovery of serum biomarkers from patients with severe acute respiratory syndrome. Proteomics. 2004;4(11):3477‐3484. 10.1002/pmic.200400897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yitbarek K, Abraham G, Girma T, Tilahun T, Woldie M. The effect of Bacillus Calmette‐Guérin (BCG) vaccination in preventing sever infectious respiratory diseases other than TB: implications for the COVID‐19 pandemic. Vaccine. 2020:S0264–410X(20)31049–5 10.1016/j.vaccine.2020.08.018. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cirovic B, de Bree LCJ, Groh L, et al. BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe. 2020;28(2):322‐334.e5. 10.1016/j.chom.2020.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Azouz NP, Klingler AM, Rothenberg ME. Alpha 1 antitrypsin is an inhibitor of the SARS‐CoV2–priming protease TMPRSS2. bioRxiv. 10.1101/2020.05.04.077826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wettstein L, Conzelmann C, Müller JA, et al. Alpha‐1 antitrypsin inhibits SARS‐CoV‐2 infection. bioRxiv. 10.1101/2020.07.02.183764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vaidyanathan G. India will supply coronavirus vaccines to the world—will its people benefit? Nature. 2020;585:167‐168. 10.1038/d41586-020-02507-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Dataset S1
Dataset S2


