Abstract
Background
Myosteatosis has been reported to be a novel biomarker that could predict survival outcomes in patients with colorectal cancer. However, results have been conflicting. This systematic review and meta‐analysis aimed to evaluate the long‐term impact of myosteatosis on the survival of these patients.
Methods
A systematic search of PubMed, Embase, and Cochrane up to 27 November 2019 generated 7022 records. Studies that reported hazard ratio (HR) for overall survival, cancer‐specific survival, or disease‐free survival based on myosteatosis or radiodensity were included. A total of 110 full‐text articles were considered for inclusion, and 14 were selected for qualitative analysis. Inverse variance method was used with random effects model for data analysis.
Results
The total number of enrolled patients included in the meta‐analysis was 6518 for univariate and 8572 for multivariate HR analysis, from 12 and 10 studies, respectively. Patients with myosteatosis had a significant increase in overall mortality compared with non‐myosteatosis patients by both univariate analysis [HR 1.38, 95% confidence interval (CI) 1.21 to 1.58, P < 0.00001] and multivariate analysis (HR 1.55, 95% CI 1.23 to 1.96, P < 0.00001). In subgroup analysis based on studies that reported HRs of both sarcopenia and myosteatosis, the negative effect of myosteatosis on overall survival was independent of sarcopenia using univariate values (sarcopenia HR 1.48, 95% CI 1.14 to 1.91, P = 0.003 vs. myosteatosis HR 1.51, 95% CI 1.17 to 1.96, P = 0.002) and multivariate values (sarcopenia HR 1.28, 95% CI 1.09 to 1.49, P = 0.002 vs. myosteatosis HR 1.38, 95% CI 1.07 to 1.80, P = 0.001).
Conclusions
This meta‐analysis demonstrates that myosteatosis is associated with worse overall survival in patients with colorectal cancer. More investigation is needed to standardize the measurement protocol for myosteatosis and to further optimize its prognostic power for colorectal cancer patients.
Keywords: Myosteatosis, Skeletal muscle density, Survival, Colorectal cancer
Introduction
Post‐operative tumour stage or pre‐operative disease dissemination status for unresectable patients has traditionally been used to determine the prognosis of the cancer patients. 1 However, there has been an increasing interest on the extent of host tumour response as an additional indicator of cancer prognosis, such as host systemic inflammation and body composition of macromolecules. 2 , 3 , 4 Over the last decade, the concept of sarcopenia has gained grounds in the oncology field, where sarcopenia identified pre‐operatively was found to be associated with adverse oncological outcomes and increased morbidity for surgical patients. 5 , 6 , 7 Similarly, evaluation of qualitative measures of skeletal muscles in computed tomography (CT), which is expressed in various terms such as myosteatosis, skeletal muscle radiodensity (SMD), or skeletal muscle radiation attenuation, has also been increasingly studied, particularly in patients with colorectal cancer (CRC). 8 , 9 , 10 Because these terms refer to the same physiological changes of skeletal muscle, we have chosen to use the term ‘myosteatosis’ throughout this manuscript for consistency.
Martin et al. 11 defined myosteatosis as a mean value less than 41 Hounsfield unit (HU) for patients with body mass index (BMI) less than 25 and a mean value less than 33 for a BMI greater than 25, using CT‐defined cross‐sectional skeletal muscle measurements at the third lumbar vertebra. Using these cut‐offs, many investigators identified that patients with low SMD were associated with higher overall and CRC‐specific mortality when compared with those with normal SMD levels. 4 , 8 , 12 A recent study has shown that myosteatosis is associated with shorter survival in multiple cancer types. 13 However, there were other studies not showing clear association between myosteatosis and survival for patients with CRC. 8 , 14 To reconcile these findings and to consolidate the role of myosteatosis as a possible prognostic factor in CRC, a review of the existing evidence thus far seemed timely and appropriate.
Thus, we performed an in‐depth systematic review and meta‐analysis to investigate the long‐term impact of myosteatosis or SMD on survival in patients with CRC.
Methods
All procedures used in this study were performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines. 15
Data sources and searches
We included articles that reported on human studies published in the English language up to 27 November 2019 from PubMed, Embase, and Cochrane Central. The full list of search terms by category is included in Supporting Information, Appendix S1, and the search strategy with the number of search results is provided in Appendix S2.
Study selection
The list of retrieved studies was initially screened by titles, abstracts, and availability of full‐text article. J. K. and C. M. L. screened full‐text articles of relevant studies independently, and discrepancies were resolved by discussion. Studies were selected on several inclusion criteria. First, the patient population consisted of CRC patients. Second, the primary outcomes were measured and reported as hazard ratios (HRs) of overall survival (OS) and/or disease‐free survival (DFS) or cancer‐specific survival (CSS) with myosteatosis or radiodensity as one of the variables. Third, previously published definitions of myosteatosis were used and identified based on decreased mean HU on radiodensity, instead of assessing changes in radiodensity pre‐operative or post‐operative stages. Studies that did not contain primary data, such as those only available as conference abstracts, editorials, or commentaries, were excluded. When the same patient cohort was used in multiple publications, the study that included more appropriate data for our study was included.
Data extraction
We extracted all mean HRs with 95% confidence interval (CI) for OS, DFS, and/or CSS separately, along with measurement method, location of the CT scan, and the definition of myosteatosis including references. Other clinical data collected from full‐text articles included study design, study site, number of patients enrolled, basic patient demographics (age and gender), software used for muscle density measurement, and the time point of CT exam. Cohen's inter‐rater κ statistics for inclusion agreement was 0.620 (95% CI 0.320 to 0.919), with strength of agreement considered ‘good’. 16
Definition of myosteatosis
Myosteatosis in most studies was defined as SMD <41 HU in patients with BMI <25 kg/m2 and <33 in patients with BMI ≥25 mg/m2, which was suggested by Martin et al. 11 For study by Dolan et al. 17 myosteatosis was defined by Xiao et al. 10 as <35.5 HU in men and <32.5 HU in women. Similarly, most studies included in our meta‐analysis assessed the area of total skeletal muscle from a single image taken at the third lumbar vertebra, except Okugawa et al. 14 which used intramuscular adipose tissue content (IMAC) calculated from mean CT value of region of interest (ROI) of multifidus muscle (HU) divided by mean CT value of ROI of subcutaneous fat in HU and assessed the superior aspect of fourth lumbar vertebra and the psoas muscle. For the particular study, high IMAC using sex‐specific median values was used to define myosteatosis. 14 The variations in the definition of myosteatosis used in the included study are summarized in Table 1. In this study, we included both search terms of ‘low radiodensity’ and ‘myosteatosis’ to identify the patient cohorts, and they refer to the same methods of HU measurements within CT images. To avoid confusion, we have used ‘myosteatosis’ throughout the manuscript to describe findings that were identified as ‘myosteatosis’ or ‘low SMD’.
Table 1.
Study characteristics
| Author | Year | Country | Study design | N | Myosteatosis, n (%) | Age | CRC stage | Measurement location | Measurement method | Myosteatosis definition | Software used | Outcomes (univariate) | Outcomes (multivariate) | Total length of follow‐up | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blauwhoff‐Buskermolen et al. | 2016 | The Netherlands | Prospective | 67 | 47 (64.2) | 66.4 ± 10.6 | Metastatic | CT L3, single | Mean HU | <41 HU (BMI < 25) | sliceOmatic v5.0 | OS | OS | Total 3.5 years | Blauwhoff‐Buskermolen et al. 27 |
| <33 HU (BMI ≥ 25) | |||||||||||||||
| Charette et al. | 2019 | Belgium | Post hoc analysis of two non‐randomized Phase II trials | 217 | 42 (19.3) | 63.0 ± 11.0 | Chemorefractory metastatic | CT L3, two adjacent CT slices | Mean HU | <22.5 | PLANET Onco software (DOSIsoft, France) | OS | OS | NA | Charette et al. 30 |
| Deng et al. | 2018 | Taiwan | Retrospective | 101 | NA | 63.7 ± 13.7 | Stages I–IV | CT L4, three consecutive slides | Mean HU | NA | MATLAB v8.3 | OS, PFS | None | >5 years | Deng et al. 32 |
| Dolan et al. | 2019 | UK | Retrospective—from a prospective database | 650 | 341 (52.5) | Divided to <65 (234), 65–74 (251), >74 (165) | Stages I–III | CT L3, single | Mean HU | <35.5 HU (men) | NIH ImageJ 1.47 | None | OS | 9.25 years | Dolan et al. 17 |
| <32.5 HU (women) | |||||||||||||||
| Hopkins et al. | 2019 | Canada | Retrospective—from a prospective database | 968 | 537 (55.5) | 65.8 ± 11.8 | Stages I–III | CT L3, single | Mean HU | <38.2 (men) and <35.7 (women) HU for BMI < 25 | MATLAB | CSS, OS, DFS | CSS, OS | Median 5.2 years (range 0.01–10.25) | Hopkins et al. 31 |
| <31.9 (men) and <33.6 (women) HU for BMI ≥ 25 | |||||||||||||||
| Kroenke et al. | 2018 | USA | Prospective | 3262 | 966 (29.6) | Divided to <50 (432), 50–60 (806), 60–70 (941), >70 (1083) | Stages I–III | CT L3, single | Mean HU | <35.5 HU (men) | sliceOmatic v5.0 | None | CSS, OS | Median 6.9 years (range 0–10.9) | Kroenke et al. 9 |
| <32.5 HU (women) | |||||||||||||||
| Looijaard et al. | 2019 | The Netherlands | Retrospective | 378 | NA | 73.4 (IQR 69.5–78.4) | Stages I–IV | CT L3, single | Mean HU, IMAT | NA | sliceOmatic v5.0 | OS | OS | Median 5.3 years (IQR 3.7–6.6) | Looijaard et al. 33 |
| Malietzis et al. | 2016 | UK | Retrospective—from a prospective database | 805 | 625 (77.6) | Median 69 (IQR 61–77) | Stages I–IV | CT L3, single | Mean HU | <41 HU (BMI < 25) | sliceOmatic v4.3 | OS, DFS | None | Median 47 months (IQR 24.9–65.6) | Malietzis et al. 8 |
| <33 HU (BMI ≥ 25) | |||||||||||||||
| McSorley et al. | 2018 | UK | Retrospective—from a prospective database | 322 | 186 (57.8) | Divided to <65 (106), 65–74 (127), >74 (89) | Stages 0–III | CT L3, single | Mean HU | <41 HU (BMI < 25) | NIH ImageJ 1.47 | CSS, OS | CSS, OS | Median 56 months (range 35–96) | McSorley et al. 4 |
| <33 HU (BMI ≥ 25) | |||||||||||||||
| Okugawa et al. | 2018 | Japan | Retrospective—from a prospective database | 308 | 153 (49.7) | Divided to <67 (159) or >67 (149) | Stages I–IV | CT L4 (superior aspect), psoas muscle index | IMAC (intramuscular adipose tissue content) | male −0.36, female −0.24 (sex‐specific median value) | AquarisNET server (TeraRecon) | CSS, DFS | None | Median 35.9 months (mean: 39.2 ± 28.6) | Okugawa et al. 14 |
| Sabel et al. | 2013 | USA | Retrospective | 302 | NA | 67.9 ± 12.4 | Stages I–IV | CT L4, single | Mean HU | No cut point indicated | MATLAB v13.0 | OS, DFS | None | Median 2.81 years (mean 3.23 years) | Sabel et al. 34 |
| Sueda et al. | 2018 | Japan | Retrospective | 211 | 110 (52.1) | Divided to >65 (53) or <65 (53) | Stages I–III | CT L3, single | Mean HU | <41 HU (BMI < 25) | SYNAPSE VINCENT analyser (Fujifilm Co., Ltd., Tokyo, Japan) | CSS, OS, DFS | CSS, OS, DFS | Median 57.6 months | Sueda et al. 12 |
| <33 HU (BMI ≥ 25) | |||||||||||||||
| Van Baar et al. | 2018 | The Netherlands | Prospective | 1681 | 648 (39) | 67.7 ± 10.3 | Stages I–III | CT L3, single | Mean HU | <36.4 (men) and <31.1 (women) HU for BMI < 25 | sliceOmatic v5.0 | None | CSS, OS, DFS | Median 48 months (range 0–119) | Van Baar et al. 28 |
| <31.6 (men) and <29.3 (women) HU for BMI ≥ 25 | |||||||||||||||
| Van Vugt et al. | 2018 | The Netherlands | Prospective | 816 | 523 (64.1) | Median 70 | Stages I–III | CT L3, single | Mean HU | <41 HU for BMI < 25 | FatSeg (in‐house) | OS, DFS | OS | Median 76.5 | Van Vugt et al. 29 |
| <33 HU for BMI ≥ 25 |
NA, not applicable.
Data synthesis and statistical analysis
Results were grouped separately according to the final outcomes by HR for myosteatosis with 95% CI of OS, CSS, or DFS. A meta‐analysis was performed using a random effects model because of assumed heterogeneity between studies. 18 Random effects model allows the true effect size to differ from study to study, as it assumes that studies included in the analysis are random samples of all possible studies that meet the inclusion criteria. 19 This may be more reflective of the current meta‐analysis as different studies recruited patients of varying cancer stage, ethnicity, gender proportions, and co‐morbidities. We compared HR values available for OS, CSS, and DFS by univariate vs. multivariate analysis, and because most HR values were available for OS (12 studies for univariate values and 10 studies for multivariate values, respectively, as outlined in Table S1), we focused our meta‐analysis of myosteatosis on OS.
For the data analysis, inverse variance method was used to obtain pooled HRs and 95% CIs. Statistical analysis was performed using the Review Manager software (RevMan, Version 5.3 for Windows, Oxford, UK; the Cochrane Collaboration, 2014), to calculate the summary effect size, 95% CI, and P‐values of random and fixed effect models. Forest plots were used to visualize the results, and heterogeneity between studies was assessed using the I 2 statistic and the P‐value from the χ 2‐based Cochran's Q test. I 2 values reflect the percentage of variation among studies attributed to heterogeneity rather than to chance. Thus, I 2 values higher than 25%, 50%, or 75% were considered to describe low, moderate, or high heterogeneity, respectively, 20 and χ 2 < 0.10 was used to define statistically significant heterogeneity. 21
Assessment of publication bias
To check for publication bias, we generated funnel plots of log[HR] against its standard error and used Egger's regression asymmetry test. Where the asymmetry was found, the potential impact of the publication bias was assessed by the Duval and Tweedie non‐parametric ‘trim‐and‐fill’ method.22 Meta‐Essentials (Version 1.4; Rotterdam, The Netherlands: Erasmus Research Institute of Management) was used to perform the Egger's regression asymmetry test. 23 All tests of significance were two sided, and P‐values <0.05 were considered to be statistically significant.
Quality assessment
The Newcastle–Ottawa scale scoring for cohort studies for the meta‐analysis of myosteatosis in CRC has already been published. 13 , 24 The Quality in Prognosis Studies (QUIPS) tool was used to assess the quality of the methodology of included studies, by considering each of the domains outlined by Hayden et al. 25 and rating for whether the study was conducted in a way to limit the potential bias (yes, no, partly, or unclear). In this systematic review, studies that were identified as having an overall high risk of bias were those that did not have clear criteria for myosteatosis and did not perform statistical analyses such as multivariate analyses to account for potential confounding factors. The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) 26 was performed to assess the quality of evidence for the effect of myosteatosis on the OS of CRC patients using the GRADEPro GDT software (McMaster University, 2015, developed by Evidence Prime, Inc).
Results
Identification of studies and study characteristics
Our search on 27 November 2019 retrieved 7751 publications (3732 from Embase, 666 from PubMed, and 3353 from Cochrane) (Figure 1). After removal of duplicates, 7022 records were screened independently by the two authors (J. K. and C. M. L.), which led to exclusion of 6847 by title review, 65 abstracts, 11 review articles, editorials, and comments, 72 studies with no data on myosteatosis, and 13 with no data on the association between myosteatosis and patient survival. A total of 110 full‐text articles were considered for inclusion. After full‐text review of each article, 14 were selected for qualitative analysis. Among 14 included studies, nine studies were retrospective, while four studies were prospective, 9 , 27 , 28 , 29 and one study was a post hoc analysis of two non‐randomized Phase II trials. 30
Figure 1.
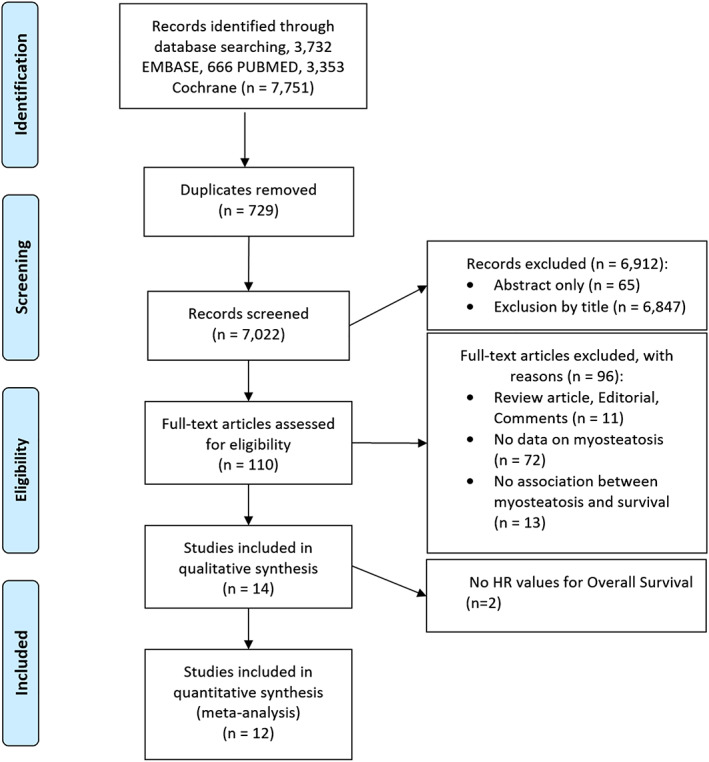
Flow diagram depicting the selection process for studies. HR, hazard ratio.
Included studies and patient characteristics
All patients included in the study had been diagnosed with CRC, mostly between Stages I–III (seven studies) and Stages I–IV (five studies), and also at metastatic (one study) or chemorefractory status (one study). The number of patients categorized as having myosteatosis ranged from 42 to 966 or 19–78% of the study cohort, with total number of patients included in the meta‐analysis being 6518 for univariate and 8572 for multivariate HR analysis for OS. Patients with myosteatosis, excluding studies that did not provide a clear definition or cut‐offs for myosteatosis to determine the precise size of the myosteatosis cohort, totalled to be 3059 for univariate and 3401 for multivariate HR analysis. Time at which the CT was analysed was mostly at pre‐operative evaluation, but where specified, varied between 21 days before surgery 31 to within 3 months of surgery 17 or within 4 months of chemotherapy or radiotherapy. 9
Myosteatosis and overall survival in colorectal cancer
Patients with myosteatosis had a significant increase in the overall mortality compared with non‐myosteatosis patients by both univariate analysis (HR 1.38, 95% CI 1.21 to 1.58, P < 0.00001) (Figure 2) and multivariate analysis (HR 1.55, 95% CI 1.23 to 1.96, P < 0.00001) (Figure 3A). This indicates that myosteatosis has an independent prognostic significant effect on OS. However, a fairly large dispersion was observed in both plots (I 2 = 92% and 80%, respectively). By funnel plot, publication bias was detected for univariate analysis (Egger P = 0.004) (Figure S2A) but not for multivariate analysis (Egger P = 0.125) (Figure S2B). Studies that did not show significance in the forest plot 32 , 33 , 34 in Figure 2 were largely those with no clear cut‐off or definition for myosteatosis and which scored very poorly in the QUIPS assessment. For example, in Looijaard et al., 33 muscle density measured in HU without any cut‐off for myosteatosis was used for the HR calculation, which yielded an insignificant HR value.
Figure 2.
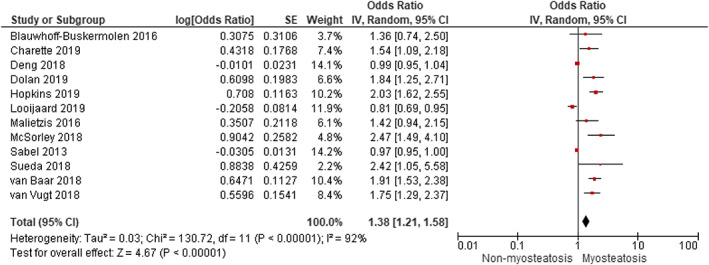
Meta‐analysis of univariate results reporting impact of myosteatosis on overall survival in patients with colorectal cancer using the random effects model. CI, confidence interval; df, degrees of freedom; IV, inverse variance; SE, standard error.
Figure 3.
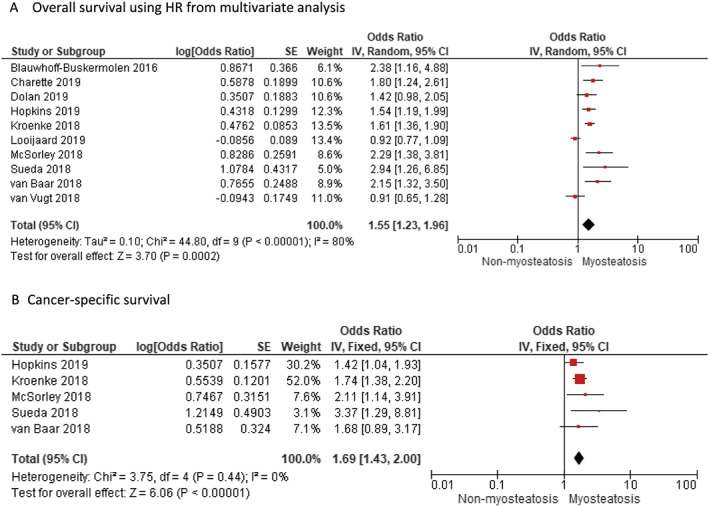
Meta‐analysis of multivariate results reporting impact of myosteatosis on (A) overall survival and (B) cancer‐specific survival in patients with colorectal cancer using the random effects model. CI, confidence interval; df, degrees of freedom; IV, inverse variance; SE, standard error.
Myosteatosis and cancer‐specific survival and disease‐free survival in colorectal cancer
We identified five studies that reported adjusted data on the effect of myosteatosis on CSS. Meta‐analysis of the five adjusted studies showed a significant increase in CRC‐specific mortality with the presence of myosteatosis for random effects model (HR 1.69, 95% CI 1.43 to 2.00, P < 0.00001) (Figure 3B). Heterogeneity was low among these studies (I 2 = 20%). We also performed a meta‐analysis of DFS HR values, but interestingly, there was no effect of myosteatosis on DFS (HR 1.00, 95% CI 0.95 to 1.05, P = 0.88) (Figure S1).
Comparison of myosteatosis and sarcopenia in colorectal cancer
Using nine and seven studies that reported the impact of both sarcopenia and myosteatosis in their study cohort by univariate and multivariate analysis of HR for OS, respectively (Table 2), we found that sarcopenia and myosteatosis had independent negative effects on OS. Figures 4 and 5 show the negative effect of sarcopenia and myosteatosis using univariate values (sarcopenia HR 1.48, 95% CI 1.14 to 1.91, P = 0.003 vs. myosteatosis HR 1.51, 95% CI 1.17 to 1.96, P = 0.002) and multivariate values (sarcopenia HR 1.28, 95% CI 1.09 to 1.49, P = 0.002 vs. myosteatosis HR 1.38, 95% CI 1.07 to 1.80, P = 0.001) (Figures 4 and 5). Two studies 9 , 31 reported the effect of having both sarcopenia and myosteatosis on patient survival (HR 2.02, 95% CI 1.65 to 2.47 and HR 2.24, 95% CI 1.63 to 3.09, respectively).
Table 2.
Studies that report both sarcopenia and myosteatosis and the respective HR for OS
| For OS | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| Study | Sarcopenia (HR, 95% CI) | Myosteatosis (HR, 95% CI) | Sarcopenia (HR, 95% CI) | Myosteatosis (HR, 95% CI) | Combined sarcopenia and myosteatosis (HR, 95% CI) |
| Charette et al. 30 | 2.06 (1.45 to 2.93) | 1.54 (1.09 to 2.18) | 1.49 (1.04 to 2.15) | 1.80 (1.24 to 2.61) | |
| Deng et al. 32 | 0.93 (0.84 to 1.02) | 0.99 (0.95 to 1.04) | NA | NA | |
| Dolan et al. 17 | 1.74 (1.21 to 2.49) | 1.84 (1.25 to 2.72) | 1.50 (1.04 to 2.18) | 1.42 (0.98 to 2.05) | |
| Hopkins et al. 31 | 2.01 (1.67 to 2.50) | 2.03 (1.61 to 2.54) | 1.45 (1.15 to 1.84) | 1.54 (1.19 to 1.98) | 2.24 (1.63 to 3.09) |
| Kroenke et al. 9 | NA | NA | 1.30 (1.07 to 1.57) | 1.63 (1.30 to 2.05) | 2.02 (1.65 to 2.47) |
| Looijaard et al. 33 | 0.953 (0.812 to 1.119) | 0.814 (0.694 to 0.955) | 0.998 (0.840 to 1.187) | 0.918 (0.771 to 1.093) | |
| Malietzis et al. 8 | 1.77 (1.32 to 2.38) | 1.42 (1.09 to 2.50) | 1.70 (1.25 to 2.31) | NA | |
| McSorley et al. 4 | 1.40 (0.88 to 2.24) | 2.47 (1.49 to 4.10) | NA | 2.29 (1.38 to 3.81) | |
| Okugawa et al. 14 | NA | NA | NA | NA | |
| Sabel et al. 34 | NA | 0.97 (0.95 to 1.00) | NA | NA | |
| Sueda et al. 12 | 2.14 (0.99 to 4.97) | 2.42 (1.10 to 5.84) | 2.29 (1.04 to 5.41) | 2.94 (1.32 to 7.17) | |
| Van Baar et al. 28 | NA | NA | NA | 1.91 (1.53 to 2.38) | |
| Van Vugt et al. 29 | 1.35 (1.03 to 1.76) | 1.75 (1.29 to 2.36) | 1.06 (0.80 to 1.42) | 0.91 (0.65 to 1.29) | |
CI, confidence interval; HR, hazard ratio; OS, overall survival; NA, not applicable.
Figure 4.
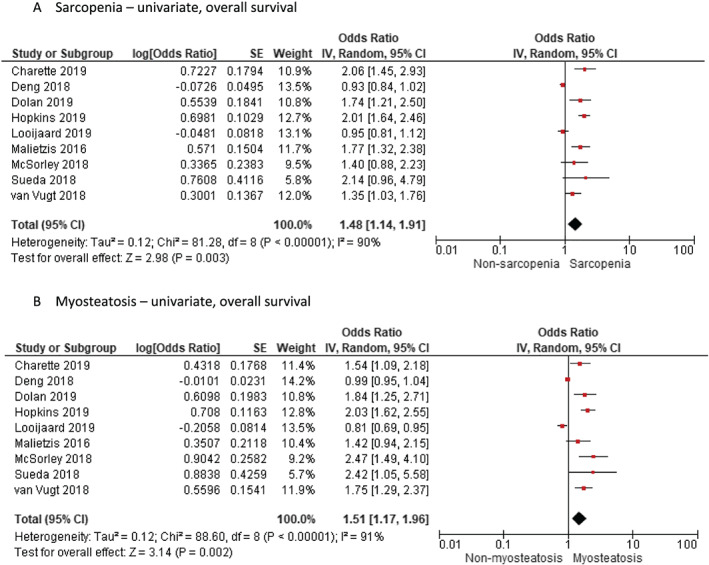
Meta‐analysis of univariate results reporting impact of (A) sarcopenia and (B) myosteatosis by random effects model in studies that report both findings in the same study cohort. CI, confidence interval; df, degrees of freedom; IV, inverse variance; SE, standard error.
Figure 5.
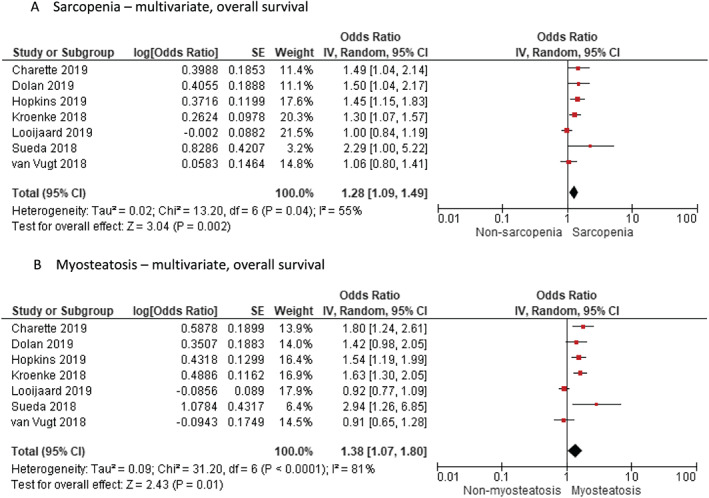
Meta‐analysis of multivariate results reporting impact of (A) sarcopenia and (B) myosteatosis by random effects model in studies that report both findings in the same study cohort. CI, confidence interval; df, degrees of freedom; IV, inverse variance; SE, standard error.
Quality assessment
The 14 studies were assessed for each of the six domains of the QUIPS tool, where a rating of ‘yes’ means that the study was designed and conducted to sufficiently limit the potential biases within that domain. ‘Unclear’ denotes that the answer to the item was not reported clearly, and ‘partly’ similarly indicates that the item was not fully addressed. Most studies were retrospective cohort studies from prospectively maintained databases, with well‐defined collection period and detailed description of the patient populations. However, one study 32 did not provide sufficient baseline characteristics of the cohort, and four studies either did not provide clear definition of myosteatosis 32 , 33 , 34 or had very small proportion of the study cohort with myosteatosis, 30 which affected both study attrition and prognostic factor measurement. Outcome measurement was judged to be clearly defined by all studies for overall mortality. For confounding measurement, minimum requirements were set as that the results were adjusted for age, gender, and disease stage. Studies adjusted for less or different factors were denoted as ‘partly’. 14 , 27 , 33 , 34 Regarding statistical approach, four studies did not include multivariate analyses and were judged as with high potential for this bias. 8 , 14 , 32 , 34 One study was noted as ‘partly’ for the statistical approach domain 33 as muscle density without cut‐off was used in the analysis. Three studies were judged to be at an overall high risk of bias, 32 , 33 , 34 given inadequate definition or measurement of myosteatosis and ‘partly/unclear’ or high risk of bias for the confounding measurement and statistical approach (Table S2). The GRADE assessment of studies included in the meta‐analysis is provided in Table 3, which gave ‘Low’ score on the overall certainty of evidence as there were no randomized controlled trial studies and mostly retrospective studies.
Table 3.
GRADE assessment of studies included in the meta‐analysis for overall survival
| Certainty assessment | No. of patients | Effect | Certainty | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Myosteatosis | Non‐myosteatosis | Relative (95% CI) | Absolute (95% CI) | ||
| Univariate analysis | ||||||||||||
| 12 | Observational studies | Not serious | Not serious | Not serious | Not serious |
Publication bias strongly suspected All plausible residual confounding would reduce the demonstrated effect a |
3059/6518 (46.9%) | 3459/6518 (53.1%) | HR 1.38 (1.21 to 1.58) | 117 more per 1000 (from 69 more to 167 more) |
⨁⨁◯◯ Low |
|
| Multivariate analysis | ||||||||||||
| 10 | Observational studies | Not serious | Not serious | Not serious | Not serious | None | 3401/8572 (39.7%) | 5171/8572 (60.3%) | HR 1.55 (1.23 to 1.96) | 158 more per 1000 (from 76 more to 233 more) |
⨁⨁◯◯ Low |
|
CI, confidence interval; GRADE, Grading of Recommendations, Assessment, Development and Evaluation; HR, hazard ratio.
Funnel plot is not symmetrical, and Egger regression has a significant P‐value of 0.004.
Discussion
This systematic review and meta‐analysis demonstrate that myosteatosis measured in pre‐treatment periods could be used as an independent predictor of worse survival outcomes in patients with CRC.
Sarcopenia has been increasingly recognized not only as a prognosticator of adverse outcomes but also as a predictor of post‐operative morbidity. 7 , 31 , 32 , 35 , 36 , 37 Even in CRC, the negative impact of sarcopenia on survival has been increasingly investigated. 38 , 39 , 40 Intramuscular fat accumulation, referred to as ‘myosteatosis’, is an early change within the muscle architecture, associated with significantly decreased muscle quality. 41 In addition to the loss of muscle mass, however, the concept of sarcopenia has been extended to include muscle quality and function. 42 Recent studies looking at the effect of skeletal muscle mass and/or composition on the survival of CRC patients have found that patients with both sarcopenia and myosteatosis have worse OS than those patients with sarcopenia or myosteatosis alone. 9 , 31 However, there are also studies reporting no prognostic impact of myosteatosis in patients with CRC, 27 , 34 which prompted us to look into whether myosteatosis was an independent risk factor as in the case for sarcopenia. Although there was a large meta‐analysis looking at myosteatosis and prognosis in multiple cancer types, 13 detailed analysis is still required for CRC because of its prevalence and controversies of the outcomes.
The exact pathogenesis of myosteatosis or its relationship to sarcopenia is not clearly understood, though some clinical data suggest that there may be a common mechanism to sarcopenia and myosteatosis. Systemic inflammatory response in patients with CRC, for example, has been associated with both sarcopenia and myosteatosis, with high neutrophil‐to‐lymphocyte ratio being an independent predictor of reduced muscle mass (odds ratio 1.78, 95% CI 1.29 to 2.45) and myosteatosis (odds ratio 1.60, 95% CI 1.03 to 2.45). 8 It is thought that anti‐tumour proteins and the pro‐inflammatory cytokines such as interleukin‐1β 43 against cancer contribute to systemic inflammation and muscle wasting, cachexia, and sarcopenia. 44 The basic mechanism of myosteatosis and its relation to survival outcome might be important to identify the most appropriate intervention at the time of diagnosis such as anti‐inflammatory medications, which may improve long‐term survival outcomes, 31 and future interventional studies such as means of increasing muscle mass before surgery may be helpful. The exact pathogenesis of myosteatosis and how it differs in different cancer types are also subjected to further investigation.
Although our analysis revealed the association of myosteatosis with worse survival, there are several issues on the use of myosteatosis as a significant and standardized prognostic factor in patients with CRC. Firstly, there is currently no unified or validated consensus on the definition of myosteatosis. Most of the included studies used <41 HU in patients with BMI <25 kg/m2 and <33 in patients with BMI ≥25 mg/m2 as a cut‐off value of radiodensity, 11 but others have also used <35.5 HU in men and <32.5 HU in women 10 or IMAC calculation, 14 or their own criteria (22.5 HU). 30 The clinical significance of myosteatosis may vary depending on which criteria are used, and future studies should correlate myosteatosis with measurements of muscle function. For instance, in a study using their own cut‐off of 22.5 HU, muscle density was found to be an important prognostic factor in the multivariate analysis (HR 1.8, 95% CI 1.24 to 2.61), but when using the cut‐off values originated from Martin et al. 11 which were generated from mixed group of cancers and mostly from poor prognosis, low muscle density was not associated with OS (HR 1.25, 95% CI 0.89 to 1.77). 30 Even in our study, for univariate analysis (Figure 2), the three studies with HR values close to 1 or on the side of favouring myosteatosis were those without clear criteria for myosteatosis. Secondly, the HU may vary depending on the phase of the CT used for measurement. One study reported that mean SMD in the unenhanced phase is significantly lower than that measured in the arterial and portal venous phase [unenhanced phase (30.9 ± 8.0 HU) vs. arterial (38.0 ± 9.9 HU) or portal venous (38.7 ± 9.2 HU) phase (both P < 0.001)]. 45 In this meta‐analysis, comparison of the CT protocol or phase could not be performed because of lack of such information. These limitations may render cross‐comparison between different studies challenging.
There are also several limitations to our study. Not every study looking at the OS of CRC patient had measured SMD as one of their variables, for example, which led to their exclusion for the purpose of our study. Similarly, myosteatosis was often not the main focus of many of the studies included, which means that the patient demographics were often not sufficiently stratified within the study population to identify potential confounding variables, other than a ‘yes/no’ on the presence of myosteatosis and a report on the final survival outcome in a form of meta‐analysis. Other sources of variations among studies may include different cancer stage, race, and gender ratio of the patient population.
In this meta‐analysis, studies including OS were more represented than those studies reporting CSS or DFS. Meta‐analysis of studies that report the impact of both sarcopenia and myosteatosis in their study, myosteatosis had negative effects on OS independent from sarcopenia, by both univariate and multivariate analyses. Interestingly, however, myosteatosis did not have an effect on DFS, unlike OS or CSS. This could suggest that myosteatosis does not reflect the aggressiveness of CRC but acts mainly as an indicator of overall fragility of the host. Because myosteatosis increases with age and is known to be associated with obesity and diabetes, 46 , 47 it would be important to ensure matched analysis of patients in future studies. It still remains uncertain whether the worse survival outcomes for patients with myosteatosis are associated with aggressive tumour behaviour, impaired host immune defence, or a combination of both as its possible mechanism. Although an investigation of this question was beyond the scope of our study, this phenomenon might be useful in elucidating the association of myosteatosis with worse outcomes in patients with CRC.
We hereby report the association between myosteatosis and OS in CRC and that myosteatosis is an independent predictor of worse survival. More investigation is needed to standardize the measurement protocol for myosteatosis and to further optimize its prognostic power by cancer stage and patient demographics for CRC patients.
Conflict of interest
None declared.
Supporting information
Table S1. Number of studies with available hazard ratios of univariate and multivariate analysis with respect to different survival outcomes including overall survival, cancer‐specific survival and disease‐free survival.
Table S2. Risk of Bias Assessment Using the Quality in Prognosis Studies (QUIPS) Tool
Figure S1. Meta‐analysis of multivariate results reporting impact of myosteatosis on disease‐free survival (DFS) in patients with colorectal cancer by random effect model. CI, confidence interval; df, degrees of freedom; IV, inverse variance; SE, standard error
Figure S2. Funnel plot of the meta‐analysis of univariate and multivariate HR of included studies indicating presence of publication bias.
Appendix 1: Search strategy
Appendix S2. Search query and number of items found
Acknowledgement
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 48 We would like to thank Na Won Kim, PhD (Librarian, Yonsei University Medical Library, Seoul, Korea), for her generous help in the searching process.
Lee C. M., and Kang J. (2020) Prognostic impact of myosteatosis in patients with colorectal cancer: a systematic review and meta‐analysis, Journal of Cachexia, Sarcopenia and Muscle, 11, 1270–1282, doi: 10.1002/jcsm.12575.
References
- 1. NCCN clinical practice guidelines in oncology, NCCN Guidelines for Treatment of Cancer by Site (2019). https://www.nccn.org/professionals/physician_gls/default.aspx (accessed September 2, 2019).
- 2. Dolan RD, Lim J, McSorley ST, Horgan PG, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: systematic review and meta‐analysis. Sci Rep 2017;7:16717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dolan RD, McSorley ST, Horgan PG, Laird B, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: systematic review and meta‐analysis. Crit Rev Oncol Hematol 2017;116:134–146. [DOI] [PubMed] [Google Scholar]
- 4. McSorley ST, Black DH, Horgan PG, McMillan DC. The relationship between tumour stage, systemic inflammation, body composition and survival in patients with colorectal cancer. Clin Nutr Edinb Scotl 2018;37:1279–1285. [DOI] [PubMed] [Google Scholar]
- 5. Brown JC, Cespedes Feliciano EM, Caan BJ. The evolution of body composition in oncology—epidemiology, clinical trials, and the future of patient care: facts and numbers. J Cachexia Sarcopenia Muscle 2018;9:1200–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta‐analysis and systematic review. Eur J Cancer Oxf Engl 2016;1990:58–67. [DOI] [PubMed] [Google Scholar]
- 7. Simonsen C, de Heer P, Bjerre ED, Suetta C, Hojman P, Pedersen BK, et al. Sarcopenia and postoperative complication risk in gastrointestinal surgical oncology: a meta‐analysis. Ann Surg 2018;268:58–69. [DOI] [PubMed] [Google Scholar]
- 8. Malietzis G, Currie AC, Athanasiou T, Johns N, Anyamene N, Glynne‐Jones R, et al. Influence of body composition profile on outcomes following colorectal cancer surgery. BJS 2016;103:572–580. [DOI] [PubMed] [Google Scholar]
- 9. Kroenke CH, Prado CM, Meyerhardt JA, Weltzien EK, Xiao J, Cespedes Feliciano EM, et al. Muscle radiodensity and mortality in patients with colorectal cancer. Cancer 2018;124:3008–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xiao J, Caan BJ, Weltzien E, Cespedes Feliciano EM, Kroenke CH, Meyerhardt JA, et al. Associations of pre‐existing co‐morbidities with skeletal muscle mass and radiodensity in patients with non‐metastatic colorectal cancer. J Cachexia Sarcopenia Muscle 2018;9:654–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol Off J Am Soc Clin Oncol 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 12. Sueda T, Takahasi H, Nishimura J, Hata T, Matsuda C, Mizushima T, et al. Impact of low muscularity and myosteatosis on long‐term outcome after curative colorectal cancer surgery: a propensity score‐matched analysis. Dis Colon rectum 2018;61:364–374. [DOI] [PubMed] [Google Scholar]
- 13. Aleixo GFP, Shachar SS, Nyrop KA, Muss HB, Malpica L, Williams GR. Myosteatosis and prognosis in cancer: systematic review and meta‐analysis. Crit Rev Oncol Hematol 2019;145:102839. [DOI] [PubMed] [Google Scholar]
- 14. Okugawa Y, Toiyama Y, Yamamoto A, Shigemori T, Yin C, Narumi A, et al., Clinical impact of muscle quantity and quality in colorectal cancer patients: a propensity score matching analysis, JPEN J Parenter Enteral Nutr 2018;42:1322–1333. [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–174. [PubMed] [Google Scholar]
- 17. Dolan RD, Almasaudi AS, Dieu LB, Horgan PG, McSorley ST, McMillan DC. The relationship between computed tomography‐derived body composition, systemic inflammatory response, and survival in patients undergoing surgery for colorectal cancer. J Cachexia Sarcopenia Muscle 2019;10:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 19. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed‐effect and random‐effects models for meta‐analysis. Res Synth Methods 2010;1:97–111. [DOI] [PubMed] [Google Scholar]
- 20. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deeks JJ, Higgins JP, Altman DG. Analysing data and undertaking meta‐analyses In Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons, Ltd; 2008; p 243–296. [Google Scholar]
- 22. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 23. Suurmond R, van Rhee H, Hak T. Introduction, comparison, and validation of Meta‐Essentials: a free and simple tool for meta‐analysis. Res Synth Methods 2017;8:537–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wells GA, Tugwell P, O'Connell D, Welch V, Peterson J, Shea B, & Losos, M . The Newcastle–Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses, http://www.Ohri.ca/Programs/Clinical_epidemiology/Oxford.Asp (2001). https://ci.nii.ac.jp/naid/20000796643/ (accessed August 11, 2019).
- 25. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158:280–286. [DOI] [PubMed] [Google Scholar]
- 26. Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Syst Rev 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blauwhoff‐Buskermolen S, Versteeg KS, de van der Schueren MAE, den Braver NR, Berkhof J, Langius JAE, et al. Loss of muscle mass during chemotherapy is predictive for poor survivalof patients with metastatic colorectal cancer. J Clin Oncol Off J Am Soc Clin Oncol 2016;34:1339–1344. [DOI] [PubMed] [Google Scholar]
- 28. van Baar H, Beijer S, Bours MJL, Weijenberg MP, van Zutphen, M , van Duijnhoven, FJB , et al. Low radiographic muscle density is associated with lower overall and disease‐free survival in early‐stage colorectal cancer patients. J Cancer Res Clin Oncol 2018;144:2139–2147. [DOI] [PubMed] [Google Scholar]
- 29. van Vugt JLA, Coebergh van den Braak RRJ, Lalmahomed ZS, Vrijland WW, Dekker JWT, Zimmerman DDE, et al. Impact of low skeletal muscle mass and density on short and long‐term outcome after resection of stage I–III colorectal cancer. Eur J Surg Oncol 2018;44:1354–1360. [DOI] [PubMed] [Google Scholar]
- 30. Charette N, Vandeputte C, Ameye L, Bogaert CV, Krygier J, Guiot T, et al. Prognostic value of adipose tissue and muscle mass in advanced colorectal cancer: a post hoc analysis of two non‐randomized phase II trials. BMC Cancer 2019;19:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hopkins JJ, Reif RL, Bigam DL, Baracos VE, Eurich DT, Sawyer MB. The impact of muscle and adipose tissue on long‐term survival in patients with stage I to III colorectal cancer. Dis Colon rectum 2019;62:549–560. [DOI] [PubMed] [Google Scholar]
- 32. Deng C‐Y, Lin Y‐C, Wu JS, Cheung Y‐C, Fan C‐W, Yeh K‐Y, et al. Progressive sarcopenia in patients with colorectal cancer predicts survival. Am J Roentgenol 2018;210:526–532. [DOI] [PubMed] [Google Scholar]
- 33. Looijaard SMLM, Meskers CGM, Slee‐Valentijn MS, Bouman DE, Wymenga ANM, Klaase JM, et al. Computed tomography-based body composition is not consistently associated with outcome in older patients with colorectal cancer. Oncologist 2019;25:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sabel MS, Terjimanian M, Conlon ASC, Griffith KA, Morris AM, Mulholland MW, et al. Analytic morphometric assessment of patients undergoing colectomy for colon cancer. J Surg Oncol 2013;108:169–175. [DOI] [PubMed] [Google Scholar]
- 35. da Cunha LP, Silveira MN, Mendes MCS, Costa FO, Macedo LT, de Siqueira NS, et al. Sarcopenia as an independent prognostic factor in patients with metastatic colorectal cancer: a retrospective evaluation. Clin Nutr ESPEN 2019;32:107–112. [DOI] [PubMed] [Google Scholar]
- 36. Feliciano EC, Chen WY. Clinical implications of low skeletal muscle mass in early stage breast and colorectal cancer. Proc Nutr Soc 2018;77:382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thuijs DJ, Head SJ, Stone GW, Puskas JD, Taggart DP, Serruys PW, et al. Outcomes following surgical revascularization with single versus bilateral internal thoracic arterial grafts in patients with left main coronary artery disease undergoing coronary artery bypass grafting: insights from the EXCEL trial. Eur. J. Cardio‐Thorac. Surg. Off. J. Eur. Assoc. Cardio‐Thorac. Surg. 2019;55:501–510. [DOI] [PubMed] [Google Scholar]
- 38. Choi MH, Oh SN, Lee IK, Oh ST, Won DD. Sarcopenia is negatively associated with long‐term outcomes in locally advanced rectal cancer. J Cachexia Sarcopenia Muscle 2018;9:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chung E, Lee HS, Cho E‐S, Park EJ, Baik SH, Lee KY, et al. Prognostic significance of sarcopenia and skeletal muscle mass change during preoperative chemoradiotherapy in locally advanced rectal cancer, Clin. Nutr. Edinb. Scotl 2020;39:820–828. [DOI] [PubMed] [Google Scholar]
- 40. Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol 2017;3:e172319–e172319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Perkisas S, Lamers S, Degerickx R, Van Mieghem E, Vandewoude M, Verhoeven V, et al. The relation between mortality, intramuscular adipose tissue and sarcopenia in hospitalized geriatric patients. Eur Geriatr Med 2018;9:801–807. [DOI] [PubMed] [Google Scholar]
- 42. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Deans DA, Wigmore SJ, Gilmour H, Paterson‐Brown S, Ross JA, Fearon KC. Elevated tumour interleukin‐1β is associated with systemic inflammation: a marker of reduced survival in gastro‐oesophageal cancer. Br. J. Cancer. 2006;95:1568–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abdel‐Rahman O, Cheung WY. Revisiting the prognostic relevance of muscle mass among non‐metastatic colorectal cancer. Transl. Gastroenterol. Hepatol. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Vugt JLA, Coebergh van den Braak RRJ, Schippers HJW, Veen KM, Levolger S, de Bruin RWF, et al. Contrast‐enhancement influences skeletal muscle density, but not skeletal muscle mass, measurements on computed tomography. Clin. Nutr. Edinb. Scotl. 2018;37:1707–1714. [DOI] [PubMed] [Google Scholar]
- 46. Miljkovic I, Kuipers A, Cvejkus R, Bunker C, Patrick A, Gordon C, et al. Myosteatosis increases with aging and is associated with incident diabetes in African ancestry men. Obes Silver Spring Md 2016;24:476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hausman GJ, Basu U, Du M, Fernyhough‐Culver M, Dodson MV. Intermuscular and intramuscular adipose tissues: bad vs. good adipose tissues. Adipocyte 2014;3:242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143‐1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Number of studies with available hazard ratios of univariate and multivariate analysis with respect to different survival outcomes including overall survival, cancer‐specific survival and disease‐free survival.
Table S2. Risk of Bias Assessment Using the Quality in Prognosis Studies (QUIPS) Tool
Figure S1. Meta‐analysis of multivariate results reporting impact of myosteatosis on disease‐free survival (DFS) in patients with colorectal cancer by random effect model. CI, confidence interval; df, degrees of freedom; IV, inverse variance; SE, standard error
Figure S2. Funnel plot of the meta‐analysis of univariate and multivariate HR of included studies indicating presence of publication bias.
Appendix 1: Search strategy
Appendix S2. Search query and number of items found


