Abstract
Background
The Global Leadership Initiative on Malnutrition (GLIM) criteria have been recently launched by consensus of the major nutrition societies. GLIM criteria are partly constructed on the previous definition of malnutrition developed by the European Society of Clinical Nutrition and Metabolism (ESPEN). We aimed to assess malnutrition according to the ESPEN and GLIM criteria at baseline and to determine the corresponding risk of mortality during a 4‐year follow‐up in community‐dwelling older adults from the SarcoPhAge (Sarcopenia and Physical Impairment with advancing Age) study. The relationship between malnutrition and incidence of 4‐year adverse health consequences (institutionalization, hospitalization, falls, and fractures) was assessed.
Methods
This prospective population‐based cohort was part of SarcoPhAge, which included 534 older adults in Belgium, followed up from 2013 to 2019. Community‐dwelling healthy volunteers ≥65 years old were recruited. Mortality and adverse health consequences were collected annually by interview or phone call. Baseline malnutrition was defined according to the GLIM and ESPEN criteria. Agreement between the two definitions was reported by Cohen's kappa coefficient. Adjusted Cox regression and Kaplan–Meier survival curves were performed for malnutrition. Logistic regression was used for the other outcomes.
Results
From 534 subjects in SarcoPhAge, the records for 411 participants (73.2 ± 6.05 years old; 55.7% women) had all the variables needed to apply the GLIM criteria. Prevalence of baseline malnutrition was 23.4% for GLIM and 7% for ESPEN criteria (k = 0.30, low agreement). The adjusted Cox regression showed a significant increased mortality risk according to malnutrition status as defined by the GLIM [adjusted hazard ratio = 4.41 (95% confidence interval: 2.17–8.97)] and ESPEN [adjusted hazard ratio = 2.76 (95% confidence interval: 1.16–6.58)] criteria. Survival curves differed significantly between malnourished and non‐malnourished groups, regardless of the definition used (log rank P < 0.001 for both). No association was found between baseline malnutrition according to these two criteria and 4‐year risk of institutionalization, hospitalization, falls, or fractures (all P > 0.05).
Conclusions
Malnutrition according to the GLIM criteria was associated with a 4.4‐fold higher mortality risk, double that of the ESPEN criteria, during a 4‐year follow‐up. No association was found between malnutrition according to these two criteria and incidence of other health adverse consequences. GLIM criteria anticipate mortality and might guide interventions, with important implications for clinical practice and research.
Keywords: Diagnosis, GLIM, malnutrition, malnutrition, prospective study, SarcoPhAge
Introduction
Malnutritionis a major cause of adverse health consequences in community‐dwelling older people: excess mortality, disability, reduced physical performance, falls, institutionalization, and hospitalization. 1 , 2 , 3 Despite the severe adverse consequences of the disease and its reversibility when early targeted therapeutic approaches are applied, a unified, acknowledged malnutrition definition had been lacking until recent times, 1 , 4 , 5 with three consequences. First, the prevalence of malnutrition varied widely depending on the definition applied 6 ; a recent systematic review showed that there was no evidence to recommend any of the existing previous definitions of malnutrition. 7 Second, differences between the definitions hindered the development of effective therapeutic interventions for malnutrition and nutrition‐related diseases. 8 , 9 Finally, the latest findings in malnutrition and nutrition‐related diseases, the International Classification of Disease (ICD‐10), and the approaches used in clinical practice are not well‐aligned. 5
The World Health Organization Global strategy and action plan towards a decade of optimal ageing, 2020–2030 (https://www.who.int/ageing/en/), includes among its goals to provide better nutritional care worldwide. Aligned with this objective, the Society of Sarcopenia, Cachexia, and Wasting Disorders has joined its efforts with the American Society for Parenteral and Enteral Nutrition, European Society of Clinical Nutrition and Metabolism (ESPEN), the Latin American Federation of Nutritional Therapy, Clinical Nutrition and Metabolism (FELANPE), and the Parenteral and Enteral Nutrition Society of Asia (PENSA), to develop the Global Leadership Initiative on Malnutrition (GLIM) in 2016.
As a first step to meet World Health Organization goals on improving nutritional care, GLIM focused on providing a common definition of the disease that should be linked and coherent with the nutrition‐related conditions; as a second step, the definition should be acknowledged by the ICD and implemented in clinical practice. This process is underway. GLIM has launched the first consensus‐based universal definition of malnutrition, suitable for every adult, healthcare setting, and medical specialty, which is intended to be cost‐effective, feasible worldwide for coding and reimbursement purposes, and incorporated into the ICD revision process. 10
The GLIM criteria have a three‐step diagnostic structure: (i) screening by any validated screening tool; (ii) diagnosis, which requires at least one phenotypic criterion [unintentional weight loss, low body mass index (BMI), and reduced muscle mass] AND at least one aetiologic criterion (reduced food intake or assimilation and disease burden or inflammatory conditions); and (iii) severity grading. The phenotypic criteria are the three anthropometric measures that composed the previous internationally acknowledged definition of malnutrition, which was the ESPEN consensus, 1 launched in 2015. 11 , 12 , 13 The ESPEN consensus was the first effort of the largest societies on clinical nutrition and metabolism to obtain an international consensus, and it was found to be related with a 4.4‐fold higher mortality risk in community‐dwelling older people 11 and 2.7‐fold higher risk in the hospitalized diabetic older population 14 ; however, the GLIM criteria are recent and have not yet been tested in a lot of studies.
We propose to assess the relationship between adverse health consequences and malnutrition according to the GLIM and ESPEN criteria in the SarcoPhAge (Sarcopenia and Physical Impairment with Advancing Age) cohort, a population of older adults followed up during 4 years. Our primary hypothesis is that malnutrition according to the GLIM and ESPEN criteria at baseline increases the 4‐year risk of death, and the risk is higher when the disease is identified by the GLIM criteria. Our secondary hypothesis is that malnutrition according to these two definitions at baseline increases the number of institutionalizations, hospitalizations, falls, and fractures in community‐dwelling older people during this follow‐up period.
Our primary objective is to assess the association between GLIM and ESPEN criteria at baseline and the risk of mortality in community‐dwelling older adults from the SarcoPhAge study during a 4‐year follow‐up. As secondary objectives, we will assess the relationships between institutionalization, hospitalization, falls, and fractures in this population.
Methods
Population
Prospective cohort study of community‐dwelling older adults followed up for 4 years in the SarcoPhAge study, which initially aimed to assess the health and functional consequences of sarcopenia. SarcoPhAge was conducted in Belgium and included 534 older volunteers at baseline, with annual follow‐up from June 2013. The 5‐year follow‐up is still in process. The blood samples needed to assess inflammation in our study were available for only 411 (77%) of SarcoPhAge participants. Data collection and procedures have been described in detail elsewhere. 15 The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) 16 statement was followed.
Volunteers were recruited from press advertisements and general, geriatrics, osteoporosis, rehabilitation, and rheumatology outpatient clinics led by the clinicians involved in SarcoPhAge. 15 The assessment was conducted in a university research unit in Liège (Belgium) that has the interdisciplinary team, facilities, and technical devices needed to ensure the proper assessments and the administration of proposed tests (precision weighbridge, measuring tape, dual X‐ray absorptiometry (DXA), outpatient clinics, laboratories, centrifuges, −80° freezers, etc.), as previously described. 15 Blood samples were collected at recruitment and analysed in standardized conditions.
Community‐dwelling healthy volunteers ≥65 years old were included, with no selection criteria related to health or demographic characteristics, except the exclusion of subjects with an amputated limb or with a BMI above 50 kg/m2.
Main outcome measure
Main outcome measure: Deaths during a 4‐year follow‐up in the SarcoPhAge study. Secondary outcome measures: Falls (yes/no), fractures (yes/no), institutionalization (yes/no), and hospitalization (yes/no) during the 4‐year follow‐up period. Data were collected annually by direct interview or assessed by a phone call if the participant did not attend the annual visit.
Diagnosis of malnutrition according to the GLIM criteria
Diagnosis of malnutrition according to the GLIM criteria 10 is composed of a three‐step diagnostic structure:
Screening: The Mini‐Nutritional Assessment–Short Form (MNA‐SF) was used. Scores between 12 and 14 points were considered ‘normal nutritional status’; from 8 to 11, ‘at risk of malnutrition’; and 7 to 0, ‘malnutrition’. 17 The GLIM criteria were later applied for purpose of analysis.
-
Diagnosis: requires at least one phenotypic criterion and at least one aetiologic criterion
-
Phenotypic criteria.
Weight loss (%): Body weight (kg) was measured to the nearest 0.1 kg on a precision weighbridge. Unintentional weight loss was obtained by clinical interview at baseline. A weight loss >4.5 kg in the past year was reported and used as a threshold. 18
Reduced body mass index (kg/m2): Height was measured in meters (m), and BMI was calculated (kg/m2). BMI was considered reduced if <20 or <22 kg/m2 in subjects younger and older than 70 years, respectively. 10
Reduced muscle mass: DXA (Hologic Discovery A, USA), daily calibrated, was used to determine the sum of muscle mass (fat‐free mass) and muscle mass of the four limbs [appendicular lean mass (ALM)]. Fat‐free mass and ALM were divided by squared height (kg/m2) to obtain fat‐free mass index (FFMI) and appendicular lean mass index (ALMI) values, respectively. Thresholds for reduced muscle mass recommended by GLIM were used: FFMI < 17 in men and <15 kg/m2 in women or ALMI < 7 kg/m2 in men and <5.5 kg/m2 in women. 10 , 19 For purpose of analysis, either a reduced ALMI or FFMI was used to construct the GLIM criteria.
-
Etiologic criteria
Reduced food intake or assimilation: The first MNA‐SF 17 item was used to determine reduced food intake: "Has food intake declined over the past 3 months due to loss of appetite, digestive problems, chewing or swallowing difficulties?" Severe and moderate decrease was considered positive answers. 17 Chronic gastrointestinal conditions that adversely impact food assimilation or absorption of nutrients were also considered.
Disease burden and inflammation: Interleukin‐6 (IL‐6) and insulin‐like growth factor 1 (IGF‐1) were selected as biomarkers to assess inflammation, following recommendations by the Targeting Aging with MEtformin Biomarkers Workgroup for the selection of blood‐based biomarkers for geroscience‐guided clinical trials. 20 Quartiles for IGF‐1 and IL‐6 in our own data were calculated in both sexes, and the lowest quartile was considered as a sex‐specific threshold (IGF‐1: ≤88 ng/mL in men and ≤82 ng/mL in women and IL‐6: >3.84 pg/mL in men and >2.99 pg/mL in women). 21 , 22 These thresholds were very close to previously published criteria for community‐dwelling older people. 21 , 22 Disease burden was not assessed, and the number of diseases was recorded.
-
Severity grading: It was not calculated in our study. Diagnosis of malnutrition was considered as a dichotomous variable for purpose of analysis (yes/no): e.g. individuals that met GLIM criteria were diagnosed of malnutrition and those who did not meet them were considered as not malnourished.
Diagnosis of malnutrition according to the ESPEN criteria
Diagnosis of malnutrition according to the ESPEN criteria 1 has a two‐step diagnostic structure:
Screening: MNA‐SF was administered (see previous discussion). 17 The ESPEN criteria were then applied for purpose of analysis.
Diagnosis: The ESPEN definition proposes two ways to diagnose malnutrition. Alternative 1: BMI < 18.5 kg/m2. Alternative 2: Unintentional weight loss combined with age‐related BMI (<20 kg/m2 in <70 years or <22 kg/m2 in ≥ 70 years) or FFMI < 17 kg/m2 in men and <15 kg/m2 in women.
Covariate data collection
Demographic and clinical data were collected during interviews and phone calls and used as covariates. The number of concomitant diseases and drugs taken was recorded. Cognitive status was assessed by the Mini‐Mental State Examination (maximum score: 30 points) and considered decreased if <24 points. 23 Instrumental activities of daily living (maximum score: 8 points) were recorded and considered decreased if <5 points in men and <8 points in women. 24 Level of physical activity was not assessed.
Diagnosis of sarcopenia according to the revised European consensus on definition and diagnosis
Muscle strength, expressed in kilograms, was measured by a handgrip with a hand‐held dynamometer (Saehan Corporation, MSD Europe Bvba, Belgium) following standardized methods: Southampton protocol 26 and the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases position paper. 25 The highest value of three reproducible voluntary isometric contractions of finger flexor muscles was selected. Values were considered decreased if <26 kg in men and <16 kg in women, as recommended by the EWGSOP2. 19
Muscle mass was assessed by DXA to calculate ALM (see previous discussion). ALM was divided by height2 to calculate ALMI, and the cut‐off points recommended by the EWGSOP2 for ALMI were followed (<7 kg/m2 in men and <5.5 kg/m2 in women). 19
Physical performance was assessed by the Short Physical Performance Battery (SPPB) test through assessment of balance, walking speed, and chair‐and‐stand test. A score ≤8 points (max. 12) was considered a decreased performance. 19 , 27 Gait speed was part of the SSPB and was measured by the 4 m walking test. A threshold at 0.8 m/s was set. 19
Quality of life was assessed by the Short Form 36 Physical Component Summary score, the Short Form 36 Mental Health Component Summary score (maximum: 100 points), 28 and EuroQol 5D that ranged from 0 (the worst possible health status) to 1 (the best possible health status). 29
Statistical method
The data were processed using the SPSS Statistics 24 (IBM Corporation, Armonk, NY, USA) software package. The normality of the variables was checked by examining the histogram, the quantile–quantile plot, the Shapiro–Wilk test, and the difference between the mean and the median values. Quantitative variables following a Gaussian distribution were expressed as mean ± standard deviation; quantitative variables not following a Gaussian distribution were expressed as median (25th percentile–75th percentile); qualitative variables were described by absolute and relative (%) frequencies.
The number of participants diagnosed with malnutrition according to either GLIM or ESPEN criteria was measured. To assess agreement between the criteria, we reported the Cohen kappa coefficient (overall concordance rate). A coefficient less than 0 indicates disagreement, a coefficient between 0 and 0.20 reflects very slight agreement, 0.20–0.4 reflects low agreement, 0.4–0.6 reflects moderate agreement, 0.6–0.8 reflects strong agreement, and the agreement is almost perfect when the value is between 0.81 and 1. 30
A global evaluation of all subjects' baseline characteristics was then performed. Characteristics of subjects diagnosed with malnutrition with either ESPEN criteria or GLIM criteria were compared against subjects with no malnutrition through a logistic regression. For well‐known sex‐specific variables (gait speed, muscle strength, fat‐free mass, FFMI, and ALMI), sex was also introduced as a covariate in the regression.
Lastly, the links between malnutrition and outcomes were investigated. If survival data were available (death), we applied the Cox proportional hazards model, giving the hazard ratio (HR) and 95% confidence interval (CI). Survival curves were evaluated using the Kaplan–Meier method to explore the influence of malnutrition on survival. Log‐rank tests were performed. If survival data were not available (i.e. falls, fracture, hospitalization, and institutionalization), we used a logistic regression model, giving the odds ratio (OR). Crude and adjusted HR/OR was computed. The first multivariable model took into account age and sex as confounding factors, while a second model included also the number of concomitant diseases, number of drugs, and cognitive status as covariates. The results were considered statistically significant at the 5% critical level.
Results
Participants
The total baseline SarcoPhAge population is composed of 534 subjects. Among these participants, blood tests were only available for 411 participants (77% of the total sample). Because GLIM criteria required the assessment of inflammation as one of the aetiologic criteria, the total population for this manuscript is composed with those 411 subjects with blood test available. Analyses on 4‐year follow‐up incidence of deaths were performed on 402 subjects because nine of the participants were lost to follow‐up. This means that it was impossible to contact them during the last year of follow‐up period and, therefore, impossible to be sure about their dead/alive status. Analyses on the other outcomes (hospitalizations, institutionalizations, falls, and fractures) after 4‐year of follow‐up included 366 participants. Indeed, it was impossible to collect these data either by phone or by postal survey for 45 of the participants (19 died, 9 were lost to follow‐up, and 17 refused to participate to the follow‐up assessments and/or refused to reply to phone calls or postal survey) (Figure 1 ). The 45 participants not included in the analyses were significantly older (P = 0.002), presented significantly more co‐morbidities (P = 0.009), had a worst cognitive status (P < 0.001), lower SPPB score (P < 0.001), and lower gait speed (P < 0.001). Moreover, among those 45 participants not included in the analyses of outcomes, we observed a higher prevalence of deaths (19/45 (42.2%) vs. 16/366 (4.37%), P < 0.001) and malnutrition according to the GLIM criteria (20/45 (44.4%) vs. 76/366 (20.8%), P < 0.001) compared with the 366 others analysed.
FIGURE 1.
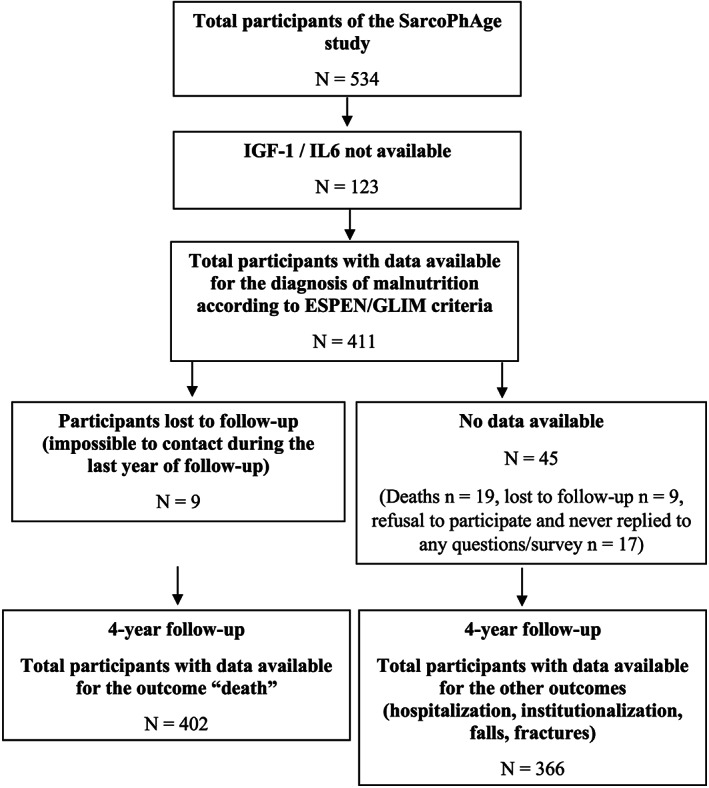
Flow chart of the SarcoPhAge follow‐up. ESPEN, European Society of Clinical Nutrition and Metabolism; GLIM, Global Leadership Initiative on Malnutrition; IGF‐1, insulin‐like growth factor 1; IL‐6, interleukin‐6.
A total of 411 individuals (55.7% women) with a median age of 73.2 ± 6.05 years were assessed. Malnutrition according to the ESPEN criteria was present in 30 (7.3%) individuals and in 96 (23.4%) if the GLIM criteria were applied. Baseline clinical characteristics of the sample are detailed in Table 1 . Malnourished individuals, regardless of the criteria, presented significantly lower BMI, lower fat‐free mass, lower FFMI, lower ALMI, lower muscle strength (all sex‐adjusted P values < 0.001), more co‐morbidities (P = 0.008, ESPEN; P < 0.001, GLIM), worse cognitive status (P = 0.027, ESPEN; P = 0.015, GLIM), and worse quality of life (EuroQol: P = 0.001, ESPEN; P = 0.02, GLIM).
TABLE 1.
Baseline characteristics
| Studied sample (n = 411) | Malnutrition according to the ESPEN consensus | Malnutrition according to the GLIM criteria | |||||
|---|---|---|---|---|---|---|---|
| Yes (n = 30) | No (n = 381) | P value | Yes (n = 96) | No (n = 315) | P value | ||
| Age, years | 73.2 ± 6.05 | 74.3 ± 8.08 | 73.1 ± 5.87 | 0.29 | 73.9 ± 6.83 | 72.9 ± 5.80 | 0.19 |
| Sex | |||||||
| Women | 229 (55.7) | 21 (70) | 208 (54.6) | 0.11 | 62 (64.5) | 167 (53) | 0.046 |
| Body mass index, kg/m2 | 26.8 ± 4.68 | 20.9 ± 2.72 | 27.2 ± 4.48 | <0.001 | 23.9 ± 3.96 | 27.7 ± 4.53 | <0.001 |
| Fat‐free mass, kg | 46.9 ± 11.7 | 36.7 ± 9.06 | 47.7 ± 11.5 | <0.001 * | 40.3 ± 9.81 | 48.9 ± 11.5 | <0.001 * |
| FFMI, kg/m2 | 17.1 ± 2.93 | 13.9 ± 2.95 | 17.4 ± 2.78 | <0.001 * | 15.2 ± 2.64 | 17.7 ± 2.77 | <0.001 * |
| ALMI, kg/m2 | 6.92 ± 1.39 | 5.69 ± 0.83 | 7.01 ± 1.38 | <0.001 * | 6.03 ± 1.18 | 7.18 ± 1.36 | <0.001 * |
| Number of concomitant diseases per subject | 4 (2–6) | 5 (3–7.25) | 4 (2–6) | 0.008 | 4 (3–7) | 4 (2–5) | <0.001 |
| Number of drugs per subject | 5 (3–8) | 5.5 (4.0–8.25) | 5 (3–8) | 0.57 | 6 (4–8) | 5 (3–8) | 0.06 |
| MNA (/14) | |||||||
| Well‐nourished | 357 (86.9) | 10 (33.3) | 347 (91.1) | <0.001 | 58 (60.4) | 299 (94.9) | <0.001 |
| Risk of malnutrition | 47 (11.4) | 15 (50) | 32 (8.4) | <0.001 | 31 (32.3) | 16 (5.1) | <0.001 |
| Malnutrition | 7 (1.7) | 5 (16.7) | 2 (0.5) | <0.001 | 7 (7.3) | 0 (0) | <0.001 |
| MMSE (/30) | 29 (28–29) | 28 (26.75–29) | 29 (28–29) | 0.027 | 28 (27–29) | 29 (28–29) | 0.015 |
| IADL Lawton | |||||||
| /8 for women | 8 (8–8) | 8 (6–8) | 8 (8–8) | 0.015 | 8 (7–8) | 8 (8–8) | 0.02 |
| /5 for men | 5 (5–5) | 5 (4–5) | 5 (5–5) | 0.42 | 5 (4–5) | 5 (5–5) | 0.002 |
| Gait speed, m/s | 0.99 ± 0.28 | 0.96 ± 0.34 | 0.99 ± 0.28 | 0.77 * | 0.90 ± 0.31 | 1.02 ± 0.27 | <0.001 * |
| SPPB (/12) | 10 (8–11) | 9.5 (6–12) | 10 (9–11) | 0.23 | 9 (6.25–11) | 10 (9–11) | <0.001 |
| Muscle strength (kg) | 29.5 ± 11.6 | 22.1 ± 6.73 | 30.1 ± 11.7 | <0.001 * | 24.3 ± 9.52 | 31.1 ± 11.7 | <0.001 * |
| Sarcopenia EWGSOP2 (n, %) | 16 (3.9) | 7 (23.3) | 9 (2.4) | <0.001 | 14 (14.6) | 2 (0.6) | <0.001 |
| Insulin‐like growth factor 1 (ng/mL) a | 104.9 (84.5–131.6) | 101.5 (86.2–133.3) | 105.3 (84.4–131.5) | 0.70 | 89.1 (73.8–119.1) | 109.2 (89.6–134.1) | <0.001 |
| Interleukin‐6 (pg/mL) b | 1.78 (0.72–3.38) | 1.39 (0.7–4.9) | 1.77 (0.74–3.26) | 0.85 | 3.16 (1.28–6.63) | 1.46 (0.7–2.67) | 0.01 |
| Quality of life | |||||||
| SF‐36 MCS score (/100) | 45.2 (35.4–53.3) | 38.4 (32.7–50.1) | 45.4 (36.2–53.4) | 0.056 | 41.2 (33.7–51.9) | 46 (36.5–53.5) | 0.064 |
| SF‐36 PCS score (/100) | 44.9 (37.1–51.4) | 42.3 (32.0–51.9) | 45.2 (37.4–51.3) | 0.18 | 40.7 (34.9–47.3) | 46.1 (38.8–52.1) | <0.001 |
| EuroQol 5D | 0.81 (0.71–0.82) | 0.54 (0.46–0.81) | 0.83 (0.76–0.83) | 0.001 | 0.80 (0.50–0.83) | 0.83 (0.76–0.83) | 0.02 |
ALMI, appendicular lean mass index; ESPEN, European Society of Clinical Nutrition and Metabolism; EWGSOP2, European Working Group on Sarcopenia in Older People 2; FFMI, fat‐free mass index; GLIM, Global Leadership Initiative on Malnutrition; IADL, instrumental activities of daily living (Lawton); MMSE, Mini‐Mental State Examination; MNA, Mini‐Nutritional Assessment; MCS, Mental Component Summary; PCS, Physical Component Summary; SF, Short Form; SPPB, Short Physical Performance Battery
Age, BMI, fat‐free mass, fat‐free mass index, ALMI, muscle strength (sex specific), and gait speed (sex specific) had a normal distribution and were expressed by mean and standard deviation. All the other ones were not normal and expressed by median + (P25–P75).
IGF‐1 levels were divided into sex‐specific quartiles (C1–C4) calculated in our sample, and the lowest quartile was selected as a cut‐off point for our study. Men: C1: ≤88 ng/mL, C2: 89–106 ng/mL, C3: 107–134 ng/mL, C4: ≥135 ng/mL; women: C1: ≤82 ng/mL, C2: 83–103 ng/mL, C3: 104–127 ng/mL, C4: ≥128 ng/mL.
IL‐6 levels were divided into sex‐specific quartiles (C1–C4) calculated in our sample, and the lowest quartile was selected as a cut‐off point for our study: >3.84 pg/mL in men and >2.99 pg/mL in women.
A logistic regression with sex as covariate in the model was run in those variables marked with an asterisk, because these variables are well known to be sex‐dependent and therefore need a multivariate logistic regression.
Malnourished women had significantly worse instrumental activities of daily living than women with good nutritional status (P = 0.015, ESPEN; P = 0.02, GLIM); in men, this difference was only observed for those who met the GLIM criteria (P < 0.001).
A Cohen kappa coefficient of 0.30 (asymptote standard error: 0.054; P < 0.001) between both definitions was found, meaning low concordance between ESPEN and GLIM criteria, meaning that the two definitions do not identify the same subjects; that is, from the 30 subjects who met the ESPEN criteria, 20% were not malnourished according to the GLIM. Supporting Information, Figure S3 showed the overlap of the malnourished subjects according to the two criteria. The specific components of the ESPEN (Table 2 ) and the GLIM criteria (Table 3 ) have been determined.
TABLE 2.
Prevalence of malnutrition according to the ESPEN criteria in community‐dwelling older population from the SarcoPhAge study at baseline (n = 411)
| Diagnosis | First option: Body mass index < 18.5 kg/m2 | 7 (1.7) |
| OR | ||
| Second option: Unintentional weight loss + low body mass index (<20 kg/m2 if <70 years, or <22 kg/m2 if ≥70 years) | 13 (3.2) | |
| or | ||
| Unintentional weight loss + low fat‐free mass index (<17 kg/m2 in men and 15 kg/m2 in women) | 24 (5.8) | |
| Total number of participants meeting the ESPEN criteria | 30 (7.3) | |
ESPEN, European Society of Clinical Nutrition and Metabolism
TABLE 3.
Prevalence of malnutrition according to the GLIM criteria in community‐dwelling older adults from the SarcoPhAge study at baseline (n = 411)
| Diagnostic (at least one phenotypic criterion AND one aetiologic criterion) | Phenotypic criterion a | |
| Non‐volitional weight loss | 55 (13.4) | |
| Low body mass index | 46 (11.2) | |
| Reduced muscle mass | 141 (34.3) | |
| Aetiologic criterion b | ||
| Reduced food intake or assimilation | 52 (12.7) | |
| Disease burden/inflammatory condition | 102 (24.8) | |
| Total number of participants meeting the GLIM criteria | 96 (23.4) | |
GLIM, Global Leadership Initiative on Malnutrition
Phenotypic criterion: Non‐volitional weight loss: ≥4.5 kg last year (Fried phenotype); low body mass index: <20 kg/m2 if <70 years or <22 kg/m2 if ≥70 years; reduced muscle mass: FFMI < 17 in men and <15 kg/m2 in women; ALM/height2 < 7 in men and <5.5 kg/m2 in women (EWGSOP2).
Aetiologic criterion: Reduced food intake or assimilation: that is, as recorded in MNA; disease burden or inflammation: Insulin‐like growth factor 1 levels were divided into sex‐specific quartiles (C1–C4) calculated in our sample, and the lowest quartile was selected as a cut‐off point for our study. Men: C1: ≤88 ng/mL, C2: 89–106 ng/mL, C3: 107–134 ng/mL, C4: ≥135 ng/mL; women: C1: ≤82 ng/mL, C2: 83–103 ng/mL, C3: 104–127 ng/mL, C4: ≥128 ng/mL. Interleukin‐6 levels were divided into sex‐specific quartiles (C1–C4) calculated in our sample, and the lowest quartile was selected as a cut‐off point for our study: >3.84 pg/mL in men and >2.99 pg/mL in women.
Figure 2 depicts the 4‐year survival analysis for subjects with baseline malnutrition according to the (A) GLIM and (B) ESPEN criteria, which differed significantly according to malnutrition status, regardless of the definition used (log rank P < 0.001 for both). Table 4 shows a more detailed analysis by proportional HRs. Subjects who met the ESPEN criteria had a significant increase in mortality risk, HR: 4.16 (95% CI: 1.89–9.16), and that increase was consistent in the adjusted model for age, sex, number of concomitant diseases, number of drugs, and cognitive status as covariates: HR 2.76 (95% CI: 1.16–6.58). For GLIM criteria, the crude analysis showed a significant increase in mortality risk: HR 5.14 (95% CI: 2.61–10.1), and mortality differences were also consistent in the adjusted model for covariates: HR 4.41 (95% CI: 2.17–8.97).
FIGURE 2.
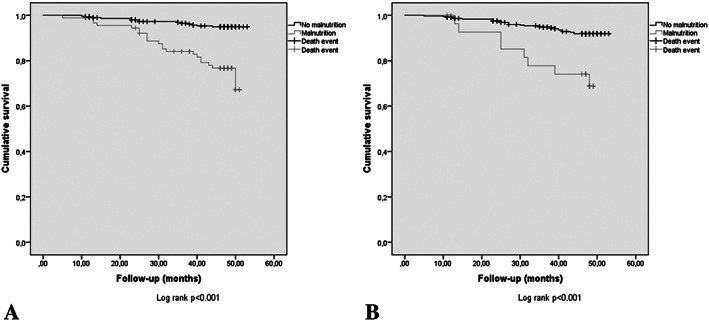
Four‐year survival analysis for subjects with baseline malnutrition according to the (A) Global Leadership Initiative on Malnutrition criteria and not malnourished subjects and according to the (B) European Society of Clinical Nutrition and Metabolism criteria and not malnourished subjects.
TABLE 4.
Four‐year incidence of deaths and its association with malnutrition at baseline according to the ESPEN consensus and GLIM criteria
| Studied sample | Malnutrition according to the ESPEN consensus | Malnutrition according to the GLIM criteria | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 402 | |||||||||||
| Yes (n = 29) | No (n = 373) | Crude HR (95% CI) | Model 1 HR (95% CI) | Model 2 HR (95% CI) | Yes (n = 92) | No (n = 310) | Crude HR (95% CI) | Model 1 HR (95% CI) | Model 2 HR (95% CI) | ||
| Four‐year incidence of deaths (n, %) | 35 (8.7) | 8 (27.6) | 27 (7.2) | 4.16 (1.89–9.16) | 3.39 (1.45–7.95) | 2.76 (1.16–6.58) | 21 (22.8) | 14 (4.5) | 5.14 (2.61–10.1) | 5.59 (2.80–11.1) | 4.41 (2.17–8.97) |
CI, confidence interval; ESPEN, European Society of Clinical Nutrition and Metabolism; GLIM, Global Leadership Initiative on Malnutrition; HR, hazard ratio
Because survival data were available (death), we applied the COX proportion hazards model giving the HR and 95% CI. Model 1: Age and sex as covariates. Model 2: Age, sex, number of concomitant diseases, number of drugs, and cognitive status as covariates.
Other outcomes associated with malnutrition were assessed (Supporting Information, Table S2 ) in the 366 subjects with available data about adverse health outcomes during the 4‐year follow‐up (Figure 1 ). Eleven subjects (3%) were institutionalized, 189 (51.6%) were hospitalized at least once, 149 (40.7%) fell at least once, and 35 (9.6%) had at least one fracture during the 4‐year follow‐up. However, no significant differences between subjects with and without malnutrition according to none of the criteria in the analysis by proportional ORs were found.
Additionally, to the study objective, two additional new exploratory aetiology‐based approaches of GLIM criteria were calculated: Option 1 (at least one phenotypic criterion AND reduced food intake) and GLIM criteria Option 2 (at least one phenotypic criterion AND reduced food intake and inflammation) (Supporting Information, Table S3 ). The prevalence of malnutrition with GLIM Option 1 was n = 36 (8.8%) and with GLIM Option 2 was n = 22 (5.4%). For GLIM criteria Option 1, the crude analysis showed a significant increase in mortality risk: HR 3.13 (95% CI: 1.42–6.9), and mortality differences were also consistent in the adjusted model for covariates: HR 3.33 (95% CI: 1.44–7.73). For GLIM criteria Option 2, the crude analysis showed a significant increase in mortality risk: HR 3.96 (95% CI: 1.64–9.56), and mortality differences were also consistent in the adjusted model for covariates: HR 3.20 (95% CI: 1.27–8.01). The combination of food intake and inflammation as aetiologic criteria reduced the prevalence of malnutrition, of course, but did not seem to impact the results in a different way as compared with intake only. Based on these results, the original version of GLIM criteria had better predictive validity for the prediction of mortality than two exploratory options based on combinations of the aetiologic criteria.
Discussion
Our study showed that malnutrition according to both ESPEN and GLIM criteria was related to mortality in community‐dwelling older people in a 4‐year follow‐up. The 4.4‐fold mortality risk associated to GLIM criteria almost doubled the risk obtained by assessing malnutrition with the ESPEN criteria, independently of the socio‐demographic and clinical characteristics of participants. Results of the present study showed a 4‐times higher prevalence of malnutrition when using GLIM instead of ESPEN criteria. This prevalence is similar to the 18% for adults older than 40 and 25.7% in the individuals older than 70 years reported in a cross‐sectional study in community‐dwelling Japanese older people, 31 which is, to authors' knowledge, the only study where GLIM criteria have been applied; therefore, further research would be needed to compare our findings. The authors consider that the close relationship found between malnutrition and mortality might be explained by the evidence‐based close relationship between any of the five criteria that compose the new definition, which were selected due to their strong relationship with mortality, as described by Cederholm et al. 32 The nature of the link between malnutrition and mortality is mediated by oxidative stress, altered micronutrient balance, catabolic changes, and inflammation (present in the definition as aetiologic criteria), and this damage in intracellular homeostasis leads to tissue dysfunction, organ failure, altered body composition, and weight loss (phenotypic criteria). In the absence of adequate therapeutic approaches, the overall process leads to the onset of nutrition‐related diseases and the end of individual's physiological ageing. 33 , 34
Low concordance between GLIM and ESPEN criteria was observed; a possible hypothesis is that all criteria considered in ESPEN are included in GLIM, and, in addition, GLIM also considers the aetiologic criteria. This low concordance and the lower prevalence obtained by applying ESPEN are in consonance with previously reported rates: The prevalence of malnutrition identified by the ESPEN definition (20.2%) was much lower than that reported using the American Society for Parenteral and Enteral Nutrition and Academy of Nutrition and Dietetics definition (63.2%) in older patients in post‐acute care. 6 Although it is possible that GLIM criteria were extremely sensitive and might over diagnose malnutrition in our study, this cannot be deduced from our observations, as GLIM criteria identified the individuals at higher risk of mortality. The GLIM proposed different measurements (ALMI, FFMI, etc.), technical devices (DXA, bioimpedanciometry, etc.), and cut‐off points for muscle mass. Further studies about GLIM criteria that compare the predictive validity for health adverse consequences of the options proposed by GLIM and include pragmatic approaches should be a priority.
The low concordance between the two criteria found and the higher prevalence found for GLIM has several implications in clinical practice. First, more patients would be suitable to be treated for a reversible disease 34 ; second, meeting their needs would not necessarily involve an increase in the prescription of artificial supplements. As far as we know, there is not a systematic review or meta‐analysis that provides evidence to recommend commercial formulas in comparison with home‐made enriched diets in community‐dwelling otherwise healthy older adults. Finally, in those patients whose clinical characteristics indicate the prescription of nutritional therapies, they have been shown to be cost‐effective in terms of efficacy and safety. 34 Implementation of the GLIM criteria in clinical practice might provide overall benefits from an individual point of view and from a public health perspective. 9
Sarcopenia was present in 16 (3.9%) individuals in SarcoPhAge, and this nutrition‐related disease was present in 14 of the 96 (14.6%) subjects who met the GLIM criteria. The relationship between malnutrition and mortality in our study was found to be closer than the three‐fold higher mortality for sarcopenia previously reported in SarcoPhAge. 35 Moreover, the relationship among sarcopenia and a dietary intake below the reference values from the Nutritional Belgian Recommendations of 2016 (https://www.health.belgium.be/sites/default/files/uploads/fields/fpshealth_theme_file/css_9285_avis_rec_nutr.pdf) with lower intake of micronutrients (potassium, magnesium, phosphorus, iron, and vitamin K) and macronutrients (lipids and proteins) has been recently published in SarcoPhAge. 36
The coherency between malnutrition according to GLIM and sarcopenia by the EWGSOP has been ensured by sharing a reduced muscle mass as a criterion for both. Moreover, different diagnostic cut‐off points for muscle mass can produce variations in the prevalence of sarcopenia from 9.25% to 18% within the same population 37 ; this issue has been avoided for GLIM by the recommendation of the same thresholds as the EWGSOP2. This coherence might be an advantage for the development of therapeutic approaches and design of trials that could benefit both diseases. 38 , 39 Data from our research group indicate that GLIM criteria are also strong predictors of the onset of sarcopenia, as they were associated with an approximately four‐fold higher risk of developing sarcopenia and severe sarcopenia during a 4‐year follow‐up. 40
Surprisingly, no association was found between malnutrition and institutionalization, hospitalization, falls, and fractures. This lack of association might be a bias due to the 45 subjects lost to follow‐up, who were not included in the analyses; those individuals who were more likely to present adverse health consequences might have been lost for the analysis. A negative effect of sarcopenia on physical disabilities and institutionalization has been reported in a prior analysis of SarcoPhAge data. 35 Further studies are needed in order to shed light about these outcomes.
Strengths and limitations
The challenge of accurate weight assessment in older people has been widely reported 11 ; the objective assessment of subjects' weight could be considered a strength of our study. Weight loss, as defined by one of the Fried phenotype criterion in our study, has been shown to be a strong predictor for mortality and disability in the elderly in the Cardiovascular Health Study, among others. 18 However, this is not exactly the amount of weight loss over time recommended by GLIM (def. >5% in 6 months or >10% beyond), it did not allow the calculation of the severity grading, and this could be considered a limitation of our study. The other two phenotypic criteria were applied succinctly, and the authors do not consider this issue to be a source of important differences in prevalence of malnutrition at baseline in our study.
The DXA has been claimed to be the most accurate assessment of body composition 41 ; its use is a strength of our study. The ALM/height2 cut‐off point that appears in the EWGSOP2 and the original paper with the GLIM criteria published in Journal of Cachexia, Sarcopenia and Muscle 10 was updated 5.5 kg/m2 and published as a corrigendum, 19 following values obtained by Studenski et al. 42 The use of the updated cut‐off point could be considered a strength of our study. However, muscle mass is closely related to physical activity, exercise, and sedentary behaviour, 43 and they were not reported in our study, which could be considered a limitation.
The food intake measurement we used as the first phenotypic criterion of our study involves a slightly longer time frame than the one recommended by GLIM (3 months vs. 1–2 weeks); this difference might be considered more of a strength than a limitation in our study. MNA is the specific tool recommended by experts to better assess food intake and anorexia of ageing in older people, 44 as it is effective to detect the onset of nutrition‐related diseases (i.e. frailty). 45 Moreover, MNA has been recently validated as a tool to detect the onset of nutrition‐related diseases (frailty) in a similar population of community‐dwelling older people. 46 The use of MNA, a validated tool widely in use, 47 , 48 might be a strength of our study.
Other strengths of our study are the use of the recommended biochemical markers for geroscience‐guided clinical trials, 20 the sex‐specific cut‐off points calculated specifically for our own population data, and values close to those previously published in a similar population of community‐dwelling older adults from the BELFRAIL study for IL‐6 21 and IGF‐1. 22 IGF‐1 has neurotrophic action and is involved in the maintenance of cognitive status and musculoskeletal health. Lower levels IGF‐1 are related to cognitive and physical impairment, disability, and frailty. 22 Additionally, to the study objective, two new exploratory aetiology‐based approaches of GLIM criteria were calculated and included in the Supporting Information; the original version of GLIM criteria had better predictive validity for the 5‐year prediction of mortality than the two exploratory options. These additional findings might be interesting for further discussion and to gain knowledge about GLIM criteria.
Our study participants were recruited volunteers, which mean they were already aware and concerned about nutritional‐related syndromes, and might involve a better health status than those individuals who refused joining a research cohort. This could be a selection bias, as it has been previously pointed out in SarcoPhAge. 35 Moreover, voluntary subjects were relatively young, able to walk, and free of cognitive impairments. They are probably healthier than the Belgian representative population of the same age. Food assimilation and disease burden were not assessed in the study, and it could be a limitation of our study. These possible biases could have affected the observed prevalence of malnutrition, which could be higher in the general older population.
Conclusions
Malnutrition according to the GLIM criteria was first applied in a longitudinal study and associated with a 4.4‐fold higher mortality risk, double that of the ESPEN criteria, during a 4‐year follow‐up. Further research will shed light on the relationships between malnutrition according to the GLIM criteria and other adverse health consequences. Applying GLIM criteria might be helpful to better identify malnutrition, apply targeted early therapeutic approaches, and provide better care for community‐dwelling older adults.
Conflict of interest
D.S‐R., M.L., J‐Y. R., E.C., O.B., and C.B. declare that they have no conflict of interest.
Funding
No funding has been received to develop this research.
Authors' contributions
O.B., J‐Y.R., D.S‐R., and C.B. conceived the study; D.S‐R. and C.B. wrote the manuscript; D.S‐R. did literature review; M.L. and C.B. participated in data collection; D.S‐R. and C.B. analysed and interpreted the data; J‐Y.R., E.C., O.B., and C.B. corrected the manuscript. All co‐authors read and approved the final version of the manuscript.
Ethical statements
Authors certify that the ethical guidelines for publishing of the Journal of Cachexia, Sarcopenia and Muscle: update 2019 has been followed. 49 National and international research ethics guidelines were followed, including the Deontological Code of Ethics and the 1964 Declaration of Helsinki and its later amendments. Data were entered and are treated in accordance with the provisions of the applicable data protection law in Belgium and the General Data Protection Regulation (EU) no. 2016/679 of the European Parliament and Council, dated 27 April 2016, which entered into force last 25 May 2018. Liège University Ethics Committee's approval was obtained (ref. 2012/277), and all subjects signed the informed consent.
Supporting information
Table S2. 4‐year incidence of institutionalization, hospitalization, falls, fractures and their association with malnutrition at baseline according to ESPEN consensus and GLIM criteria
Table S3. Four‐year incidence of deaths and its association with malnutrition at baseline according to two different etiology‐based approaches of GLIM criteria: Option 1 (at least 1 phenotypic criterion AND reduced food intake) and option 2 (at least 1 phenotypic criterion AND reduced food intake AND inflammation)
Data S1. Sample distribution, malnutrition according to GLIM and ESPEN criteria in the SarcoPhAge study
Data S2. Four‐year risk of mortality in malnourished older adults diagnosed by the European Society for Clinical Nutrition (ESPEN) and the Global Leadership Initiative on Malnutrition (GLIM) criteria: data from the SarcoPhAge study
Acknowledgement
The authors acknowledge Fabienne Damblon for her excellent support to researchers.
Sanchez‐Rodriguez D., Locquet M., Reginster J.‐Y., Cavalier E., Bruyère O., and Beaudart C. (2020) Mortality in malnourished older adults diagnosed by ESPEN and GLIM criteria in the SarcoPhAge study, Journal of Cachexia, Sarcopenia and Muscle, 11, 1200–1211, doi: 10.1002/jcsm.12574
References
- 1. Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S, et al. Diagnostic criteria for malnutrition–an ESPEN consensus statement. Clin Nutr 2015;34:335–340. [DOI] [PubMed] [Google Scholar]
- 2. Söderström L, Rosenblad A, Thors Adolfsson E, Bergkvist L. Malnutrition is associated with increased mortality in older adults regardless of the cause of death. Br J Nutr 2017;117:532–540. [DOI] [PubMed] [Google Scholar]
- 3. Malafarina V, Reginster JY, Cabrerizo S, Bruyère O, Kanis JA, Martinez JA, et al. Nutritional status and nutritional treatment are related to outcomes and mortality in older adults with hip fracture. Nutrients 2018;10:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. White JV, Guenter P, Jensen G, Malone A, Schofield M, Academy Malnutrition Work Group, ASPEN Malnutrition Task Force, ASPEN Board of Directors . Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Parenter Enteral Nutr 2012;36:275–283. [DOI] [PubMed] [Google Scholar]
- 5. Cederholm T, Jensen GL. To create a consensus on malnutrition diagnostic criteria. JPEN J Parenter Enteral Nutr 2017;41:311–314. [DOI] [PubMed] [Google Scholar]
- 6. Sánchez‐Rodríguez D, Marco E, Ronquillo‐Moreno N, Maciel‐Bravo L, Gonzales‐Carhuancho A, Duran X, et al. ASPEN‐AND‐ESPEN: a postacute‐care comparison of the basic definition of malnutrition from the American Society of Parenteral and Enteral Nutrition and Academy of Nutrition and Dietetics with the European Society for Clinical Nutrition and Metabolism definition. Clin Nutr 2019;38:297–302. [DOI] [PubMed] [Google Scholar]
- 7. Marshall S, Craven D, Kelly J, Isenring E. A systematic review and meta‐analysis of the criterion validity of nutrition assessment tools for diagnosing protein‐energy malnutrition in the older community setting (the MACRo study). Clin Nutr 2017;37:1902–1912. [DOI] [PubMed] [Google Scholar]
- 8. Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr 2017;36:49–64. [DOI] [PubMed] [Google Scholar]
- 9. Sieber CC. Malnutrition and sarcopenia. Aging Clin Exp Res 2019;31:793–798. [DOI] [PubMed] [Google Scholar]
- 10. Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition–a consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle 2019;10:207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sánchez‐Rodríguez D, Marco E, Schott A‐M, Rolland Y, Blain H, Vázquez‐Ibar O, et al. Malnutrition according to ESPEN definition predicts long‐term mortality in general older population: findings from the EPIDOS study–Toulouse cohort. Clin Nutr 2018;38:2652–2658 Published Online First: 1 December. [DOI] [PubMed] [Google Scholar]
- 12. Sánchez‐Rodríguez D, Annweiler C, Ronquillo‐Moreno N, Vázquez‐Ibar O, Escalada F, Duran X, et al. Prognostic value of the ESPEN consensus and guidelines for malnutrition: prediction of post‐discharge clinical outcomes in older inpatients. Nutr Clin Pract 2019;34:304–312. [DOI] [PubMed] [Google Scholar]
- 13. Sánchez‐Rodríguez D, Marco E, Ronquillo‐Moreno N, Miralles R, Vázquez‐Ibar O, Escalada F, et al. Prevalence of malnutrition and sarcopenia in a post‐acute care geriatric unit: applying the new ESPEN definition and EWGSOP criteria. Clin Nutr 2017;36:1339–1344. [DOI] [PubMed] [Google Scholar]
- 14. Sanz‐París A, Gómez‐Candela C, Martín‐Palmero Á, García‐Almeida JM, Burgos‐Pelaez R, Matía‐Martin P, et al. Application of the new ESPEN definition of malnutrition in geriatric diabetic patients during hospitalization: a multicentric study. Clin Nutr 2016;35:1564–1567. [DOI] [PubMed] [Google Scholar]
- 15. Beaudart C, Reginster JYY, Petermans J, Gillain S, Quabron A, Locquet M, et al. Quality of life and physical components linked to sarcopenia: the SarcoPhAge study. Exp Gerontol 2015;69:103–110. [DOI] [PubMed] [Google Scholar]
- 16. von Elm E, Altman DG, Egger M. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. The Lancet (London, England) 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 17. Guigoz Y, Lauque S, Vellas BJ. Identifying the elderly at risk for malnutrition. The Mini Nutritional Assessment. Clin Geriatr Med 2002;18:737–757. [DOI] [PubMed] [Google Scholar]
- 18. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 19. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Justice JN, Ferrucci L, Newman AB, Aroda VR, Bahnson JL, Divers J, et al. A framework for selection of blood‐based biomarkers for geroscience‐guided clinical trials: report from the TAME Biomarkers Workgroup. Gero Sci 2018;40:419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adriaensen W, Matheï C, Vaes B, Van Pottelbergh G, Wallemacq P, Degryse JM. Interleukin‐6 predicts short‐term global functional decline in the oldest old: results from the BELFRAIL study. Age (Omaha) 2014;36:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Doi T, Makizako H, Tsutsumimoto K, Hotta R, Nakakubo S, Makino K, et al. Association between insulin‐like growth factor‐1 and frailty among older adults. J Nutr Health Aging 2018;22:68–72. [DOI] [PubMed] [Google Scholar]
- 23. Folstein MF, Folstein SE, McHugh PR. ‘Mini‐mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 24. Lawton MP, Brody EM. Assessment of older people: self‐maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–186. [PubMed] [Google Scholar]
- 25. Beaudart C, Rolland Y, Cruz‐Jentoft AJ, Bauer JM, Sieber C, Cooper C, et al. Assessment of muscle function and physical performance in daily clinical practice. Calcif Tissue Int 2019;105:1–14. [DOI] [PubMed] [Google Scholar]
- 26. Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 2011;40:423–429. [DOI] [PubMed] [Google Scholar]
- 27. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower‐extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332:556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jenkinson C. The SF‐36 physical and mental health summary measures: an example of how to interpret scores. J Health Serv Res Policy 1998;3:92–96. [DOI] [PubMed] [Google Scholar]
- 29. Hurst NP, Kind P, Ruta D, Hunter M, Stubbings A. Measuring health‐related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ‐5D). Br J Rheumatol 1997;36:551–559. [DOI] [PubMed] [Google Scholar]
- 30. Landis JR, Koch GG. An application of hierarchical kappa‐type statistics in the assessment of majority agreement among multiple observers. Biometrics 1977;33:363–374. [PubMed] [Google Scholar]
- 31. Maeda K, Ishida Y, Nonogaki T, Mori N. Reference body mass index values and the prevalence of malnutrition according to the Global Leadership Initiative on Malnutrition criteria. Clin Nutr 2019;39:180–184. [DOI] [PubMed] [Google Scholar]
- 32. Cederholm T, Compher C, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al. Response to the letter: comment on “GLIM criteria for the diagnosis of malnutrition–a consensus report from the global clinical nutrition community”. Some considerations about the GLIM criteria–a consensus report for the diagnosis of malnutrition by Drs. LB da Silva Passos and DA De‐Souza. Clin Nutr 2019;38:1480–1481. [DOI] [PubMed] [Google Scholar]
- 33. Simpson SJ, Le Couteur DG, James DE, George J, Gunton JE, Solon‐Biet SM, et al. The geometric framework for nutrition as a tool in precision medicine. Nutr Heal Aging 2017;4:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Muscaritoli M, Krznarić Z, Singer P, Barazzoni R, Cederholm T, Golay A, et al. Effectiveness and efficacy of nutritional therapy: a systematic review following Cochrane methodology. Clin Nutr 2017;36:939–957. [DOI] [PubMed] [Google Scholar]
- 35. Locquet M, Beaudart C, Hajaoui M, Petermans J, Reginster JY, Bruyère O. Three‐year adverse health consequences of sarcopenia in community‐dwelling older adults according to 5 diagnosis definitions. J Am Med Dir Assoc 2019;20:43–46.e2. [DOI] [PubMed] [Google Scholar]
- 36. Beaudart C, Locquet M, Touvier M, Reginster JY, Bruyère O. Association between dietary nutrient intake and sarcopenia in the SarcoPhAge study. Aging Clin Exp Res 2019;31:815–824. [DOI] [PubMed] [Google Scholar]
- 37. Beaudart C, Reginster J‐Y, Slomian J, Buckinx F, Locquet M, Bruyère O. Prevalence of sarcopenia: the impact of different diagnostic cut‐off limits. J Musculoskelet Neuronal Interact 2014;14:425–431. [PubMed] [Google Scholar]
- 38. Sánchez‐Rodríguez D, Annweiler C, Cederholm T. A translational approach for the clinical application of recently updated definitions of malnutrition (GLIM) and sarcopenia (EWGSOP2). Maturitas 2019;122:89–90. [DOI] [PubMed] [Google Scholar]
- 39. Morley JE. Treatment of sarcopenia: the road to the future. J Cachexia Sarcopenia Muscle 2018;9:1196–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Beaudart C, Sanchez‐Rodriguez D, Locquet M, Reginster JY, Lengelé L, Bruyère O. Malnutrition as a strong predictor of the onset of sarcopenia. Nutrients 2019;11:2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beaudart C, McCloskey E, Bruyère O, Cesari M, Rolland Y, Rizzoli R, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr 2016;16:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol ‐ Ser A Biol Sci Med Sci 2014;69:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sánchez‐Sánchez JL, Mañas A, García‐García FJ, Ara I, Carnicero JA, Walter S, et al. Sedentary behaviour, physical activity, and sarcopenia among older adults in the TSHA: isotemporal substitution model. J Cachexia Sarcopenia Muscle 2019;10:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fougère B, Morley JE. Editorial: weight loss is a major cause of frailty. J Nutr Health Aging 2017;21:933–935. [DOI] [PubMed] [Google Scholar]
- 45. Lorenzo‐López L, Maseda A, De Labra C, Regueiro‐Folgueira L, Rodríguez‐Villamil JL, Millán‐Calenti JC. Nutritional determinants of frailty in older adults: a systematic review. BMC Geriatr 2017;17:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lilamand M, Kelaiditi E, Cesari M, Raynaud‐Simon A, Ghisolfi A, Guyonnet S, et al. Validation of the Mini Nutritional Assessment‐Short Form in a population of frail elders without disability. Analysis of the Toulouse Frailty Platform population in 2013. J Nutr Health Aging 2015;19:570–574. [DOI] [PubMed] [Google Scholar]
- 47. Sanchez‐Rodriguez D, Marco E, Meza‐Valderrama D, Dávalos‐Yerovi V, Duarte E. Taking a step toward implementation of Global Leadership Initiative on Malnutrition (GLIM) criteria in geriatric rehabilitation. European Geriatric Medicine. 2020. 10.1007/s41999-020-00325-y [DOI] [PubMed] [Google Scholar]
- 48. Kalan U, Arik F, Isik AT, Soysal P. Nutritional profiles of older adults according the Mini‐Nutritional Assessment. Aging Clin Exp Res Published Online First: 7 June 2019. [DOI] [PubMed] [Google Scholar]
- 49. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S2. 4‐year incidence of institutionalization, hospitalization, falls, fractures and their association with malnutrition at baseline according to ESPEN consensus and GLIM criteria
Table S3. Four‐year incidence of deaths and its association with malnutrition at baseline according to two different etiology‐based approaches of GLIM criteria: Option 1 (at least 1 phenotypic criterion AND reduced food intake) and option 2 (at least 1 phenotypic criterion AND reduced food intake AND inflammation)
Data S1. Sample distribution, malnutrition according to GLIM and ESPEN criteria in the SarcoPhAge study
Data S2. Four‐year risk of mortality in malnourished older adults diagnosed by the European Society for Clinical Nutrition (ESPEN) and the Global Leadership Initiative on Malnutrition (GLIM) criteria: data from the SarcoPhAge study


