Abstract
Background
Malnutrition plays an essential role in the mechanism of pathogenesis for sarcopenia. In late life, both food consumption and energy intakes decline. One of key factors for reduced energy intakes is anorexia of ageing. The aim of this study is to examine the association between anorexia of ageing and sarcopenia among community‐dwelling elderly Japanese individuals.
Methods
This uses population‐based, cross‐sectional cohort study of elderly Japanese individuals. Anorexia of ageing was assessed via a simplified nutritional appetite questionnaire. Handgrip strength and walking speed were tested, and skeletal muscle mass was assessed using a bio‐impedance analysis device. Subjects with sarcopenia were defined as those who met the criteria of the Asian Working Group for Sarcopenia. The association between anorexia of ageing and sarcopenia was then analysed via multiple regression analysis.
Results
In total, 9,496 elderly Japanese individuals were evaluated (mean age 74.1 ± 5.4 years; male, 47.0%). The prevalence of anorexia of ageing was 9.8% (n = 927) in the present study. The prevalence of sarcopenia in men was 1.1%, 1.8%, 6.1%, 10.1%, and 21.2% and was 1.6%, 3.3%, 3.6%, 4.8%, and 7.4% in women aged 65–69, 70–74, 75–79, 80–84, and 85 years and older, respectively. The prevalence of anorexia also showed an age‐dependent increase in both sexes (P < 0.001, respectively). The prevalence of anorexia in men was 8.3%, 6.3%, 9.8%, 13.6%, and 12.9% and was 7.9%, 9.4%, 10.5%, 17.6%, and 17.1% in women aged 65–69, 70–74, 75–79, 80–84, and 85 years and older, respectively. In multivariable logistic regression model adjusted for the covariates except for albumin, anorexia of ageing was independently associated with sarcopenia (OR: 1.45, 95% CI: 1.07 to 1.95; P = 0.015). This significant association remained even after adjusting for all covariates including nutritional status (OR: 1.42, 95% CI: 1.06 to 1.92, P = 0.020).
Conclusions
Anorexia of ageing is associated with sarcopenia among Japanese elderly individuals. Further studies are needed to determine whether a causal association exists between anorexia and sarcopenia.
Keywords: Anorexia of ageing, Sarcopenia, Older people
Introduction
Sarcopenia has been described as an age‐related decline in skeletal muscle mass as well as muscle function1 and leads to serious health problems, including disability.2, 3, 4 It is important for the elderly to shorten the period of having a disability and prolong the period of being healthy.5 Thus, the modifiable risk factors were particularly focused on that likely to contribute to develop intervention from sarcopenia.
Malnutrition plays an essential role in the mechanism of pathogenesis for sarcopenia.6 In late life, the people has lower levels of physical activity and a reduction in energy needs, then both food consumption and energy intakes decline. In fact, a meta‐analysis showed that calculated reduction in energy intakes fell into quite a narrow range at 16–20% in older groups (aged ~70 years) compared with younger groups (aged ~26 years).7 Although there are various pathway for the reduced energy intakes, one of key factors is anorexia of ageing.8
Anorexia of ageing is supposed as the beginning of various geriatric syndromes. Fried proposed the frailty cycle, in which, the decrease in dietary intake coupled with a decrease in physical exercise leads to a decline in muscle mass that in turn makes older individuals more vulnerable and develop secondary complications such as sarcopenia.9 Anorexia of ageing is considered to be the precursor of sarcopenia, which means that anorexia of ageing may lead to sarcopenia. However, there have been few studies on the association between anorexia of ageing and sarcopenia, and the association between anorexia of ageing and sarcopenia remains unclear. Thus, this study aimed to elucidate whether anorexia of ageing is associated with sarcopenia.
Materials and methods
Participants
This prospective cohort study evaluated community‐dwelling older adults enrolled in the National Center for Geriatrics and Gerontology–Study of Geriatric Syndromes,10 who were recruited from either Nagoya City or Obu City. The inclusion criteria were residence in either Nagoya City (July 2013–December 2013) or Obu City (February 2015–August 2016) and age 65 years or older at the time of examination. A thousand five hundred fourteen participants were excluded according to exclusion criteria: (i) disability in basic activities of daily living (n = 23); (ii) severe neurological disease [dementia (n = 45), cerebrovascular disease (n = 652), Parkinson's disease (n = 27)]; (iii) general cognitive impairment [Mini‐Mental State Examination (MMSE) score ≤ 18] (n = 105), and (iv) missing data [sarcopenia status (n = 416), simplified nutrition assessment questionnaire (n = 91), education history (n = 3), medication (n = 41), hypertension (n = 2), diabetes (n = 5), hyperlipidaemia (n = 4), physical activity (n = 9), Geriatric Depression Scale (n = 31), blood nutrition data (n = 60)].
This study was approved by the ethics committee of the National Center for Geriatrics and Gerontology. All participants voluntarily provided informed consent before inclusion in the study.
Assessment of sarcopenia
Sarcopenia was assessed based on muscle mass, muscle strength, and physical performance according to the recommendations of the Asian Working Group for Sarcopenia (AWGS).1 According to the AWGS, individuals had sarcopenia if they were aged >60 years and had a low handgrip strength (<26 kg in men and < 18 kg in women) and/or a lower walking speed (<0.8 m/s) with a low skeletal muscle mass index (<7.0 kg/m2 in men and 5.7 kg/m2 in women).
Skeletal muscle mass was examined using a multi‐frequency bioelectrical impedance analyser (MC‐980A; TANITA, Tokyo, Japan), which is a tool for assessing whole and segmental body composition. The surface of the hand electrode was placed in contact with each of the participants' five fingers, and their heels and forefeet were placed on the circular‐shaped foot electrode. The participants held out their arms and legs so that they would not contact any other body parts during the measurements. Measurements were carried out by well‐trained staff and completed within 30 s. The appendicular skeletal muscle mass was then calculated using the following formula developed by the National Center for Geriatrics and Gerontology: appendicular skeletal muscle mass (men) = (0.197 × height2/50 kHz resistance) + (0.179 × weight) − 0.019, appendicular skeletal muscle mass (female) = (0.221 × height2/50 kHz resistance) + (0.117 × weight) + 0.881. Then, the skeletal muscle mass was converted into the skeletal muscle mass index by dividing the muscle mass by the height of the subject squared (kg/m2).
Participants walked on a flat and straight surface at a comfortable walking speed. Two markers were used to indicate the start and end of a 2.4 m walking path, with a 2 m section to be traversed before passing the start marker, such that participants were walking at a comfortable pace by the time they reached the timed path. Participants were asked to continue walking for an additional 2 m past the end of the path to ensure a consistent walking pace while on the timed path. Walking time was measured over a 2.4 m distance in seconds using a stopwatch, and the participants' walking speed (m/s) was calculated. Handgrip strength was measured using a Smedley‐type handheld dynamometer (Takei Ltd., Niigata, Japan).
Anorexia of ageing
All participants completed the Japanese version of the simplified nutritional appetite questionnaire (SNAQ). SNAQ has been evaluated to be sufficiently reliable and valid for examining appetite among community‐dwelling older adults in Japan.11 The original SNAQ is a 4‐item single‐domain questionnaire; responses were made using a 5‐point, verbally labelled Likert‐type scale: (i) My appetite is (A. very poor, B. poor, C. average, D. good, E. very good); (ii) When I eat, (A. I feel full after eating only a few mouthfuls; B. I feel full after eating about a third of a meal; C. I feel full after eating over half a meal; D. I feel full after eating most of the meal; E. I hardly ever feel full); (iii) I feel hungry (A. rarely, B. occasionally, C. some of the time, D. most of the time, E. all the time); and (iv) Food tastes (A. very bad, B. bad, C. average, D. good, E. very good). The total SNAQ score is the sum of each item score, with lower scores indicating more appetite deterioration. Nakatsu et al. showed that the Cronbach's α coefficient for the SNAQ was 0.55.11 Also, analysis of construct validity showed the SNAQ correlated moderately Mini Nutritional Assessment–Short Form (r = 0.30, P < 0.05) and 15‐item Geriatric Depression Scale (GDS) (r = −0.43, P < 0.001).11 The total score range from 4 (worst) to 20 (best), with 13 score and lower considered to indicate anorexia of ageing.12
Sociodemographic variables and covariates
The participants' sociodemographic characteristics (i.e. age, sex, and educational level), medical history [i.e. number of medications and chronic diseases (hypertension, diabetes, and hyperlipidaemia)], smoking habit (i.e. current, stopped, or never smoked), and drinking habit (i.e. current, stopped, or never drank) were assessed via face‐to‐face interviews. The examined covariates were as follows: depressive symptoms, muscle strength, physical activity, blood nutritional data (i.e. total protein and albumin), and history of falls.
Physical activity was evaluated using the following questions: (i) “Do you engage in moderate levels of physical exercise or sports aimed at health?” (ii) “Do you engage in low levels of physical exercise aimed at health?” Participants who answered no to both of these questions were classified as being physically inactive.13 Cognitive function was assessed using the MMSE, which is a 30‐point scale. Higher scores indicated a greater ability to maintain cognitive function.14 Depressive symptoms were measured using the 15‐item GDS,15 which contains 15 yes/no question items and gives a score between 0 and 15.16 Higher score means having more depressive symptoms. Venous blood was collected by well‐trained nurses, and serum was prepared from blood samples within 24 h. To obtain serum, whole blood samples were allowed to coagulate at room temperature for 30 min and then centrifuged at room temperature for 5 min at 3000 revolutions per minute. Total protein and albumin were measured by trained staff using an automatic analyser (JCA‐BM6070. JEOL Ltd.) in a laboratory at Good Life Design Co., Ltd., Aichi‐ken, Japan.
Statistical analysis
Statistical analyses were performed using SPSS (version 20.0). Significant level was set at P < 0.05 in all analysis. First, Student's t‐test and χ2 test were used to compare the variables between the participants with/without anorexia of ageing. The previous studies showed that the prevalence of sarcopenia was significantly difference between sex or age group,17, 18 thus the prevalence of sarcopenia and anorexia was examined stratified by sex and age group using the Cochran–Armitage test. Multiple logistic regression models were then used to examine the association between anorexia of ageing and sarcopenia, with sarcopenia as the dependent variable. Three models, namely, cure model, Adjusted Model 1, and Adjusted Model 2, were made. Adjusted Model 1 was adjusted for the covariates (age, sex, education, body mass index, polypharmacy, chronic disease, drinking and smoking habits, depressive symptoms, weakness in muscle strength, and decline in cognitive function) except for blood nutritional status, while Adjusted Model 2 was adjusted for the covariates including blood nutritional status. The odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Total protein and albumin levels were categorized into approximate quartiles (albumin, Q1: ≤4.2 g/dL vs. Q2–Q4: >4.2 g/dL; total protein, Q1: ≤7.0 g/dL vs. Q2–Q4: >7.0 g/dL). Polypharmacy, cognitive decline, and depression symptoms were defined using cut‐offs drawn from previous studies (polypharmacy, five or more medications19; cognitive decline, a maximum MMSE score of 2314; depression symptoms, six or higher score on GDS15).
Results
Patient characteristics
Of the 11 010 participants, 1514 were excluded and thus data from 9496 older adults [mean age 74.1 ± 5.4 years (range, 65–96 years); 4465 men, 5031 women] were analysed in the current study. The prevalence of anorexia of ageing was 9.8% (n = 927) in the present study, then the participants were divided into two groups (without anorexia of ageing or with anorexia of ageing group). Table 1 presents each group's demographic characteristics and scale scores. The participants with anorexia of ageing was likely to be sarcopenia [6.6% (n = 61)], compared with those without anorexia of ageing [3.7% (n = 318)] (P < 0.001). The mean age, education level, number of medications, MMSE score, GDS score, and albumin levels varied significantly between groups. The proportion of male participants and those with hyperlipidaemia was lower among participants with anorexia of ageing. Meanwhile, the proportion of participants who had hypertension was higher in the participants with anorexia of ageing. There were significant differences in smoking habit and physical inactivity between the groups, while total protein did not vary significantly between the groups. Further, the proportion of participants who had hyperlipidaemia, diabetes, or drinking habit did not vary significantly between the groups.
Table 1.
Participant characteristics
| Overall cohort | Without anorexia of ageing group | With anorexia of ageing group | P value | |
|---|---|---|---|---|
| N = 9496 | N = 8569 (90.2%) | N = 927 (9.8%) | ||
| Age, years ± SD | 74.1±5.4 | 73.9±5.3 | 75.3±5.8 | <0.001 |
| Male, number (%) | 4465 (47.0) | 4070 (47.5) | 395 (42.6) | 0.005 |
| Education, years ± SD | 11.9±2.5 | 12.0±2.5 | 11.2±2.5 | <0.001 |
| Medication, number ± SD | 3.0±2.8 | 3.0±2.7 | 3.8±3.2 | <0.001 |
| Chronic disease, number (%) | ||||
| Hypertension | 4245 (44.7) | 3792 (44.3) | 453 (48.9) | 0.007 |
| Diabetes | 1244 (13.1) | 1118 (13.0) | 126 (13.6) | 0.64 |
| Hyperlipidaemia | 3620 (38.1) | 3274 (38.2) | 346 (37.3) | 0.599 |
| Drinking habit, number (%) | ||||
| Never drinkers | 4995 (52.6) | 4482 (52.3) | 513 (55.3) | 0.213 |
| Stopped drinking | 2328 (24.5) | 2114 (24.7) | 214 (23.1) | |
| Current drinkers | 2173 (22.9) | 1973 (23) | 200 (21.6) | |
| Smoking habit, number (%) | ||||
| Never smokers | 5824 (61.3) | 5252 (61.3) | 572 (61.7) | < 0.001 |
| Stopped smoking | 2959 (31.2) | 2709 (31.6) | 250 (27) | |
| Current smokers | 713 (7.5) | 608 (7.1) | 105 (11.3) | |
| Physical inactivity | 2028 (21.4) | 1771 (20.7) | 257 (27.7) | < 0.001 |
| MMSE, score ± SD | 26.6 ± 2.5 | 26.7 ± 2.5 | 25.8 ± 2.7 | <0.001 |
| GDS, score ± SD | 2.6 ± 2.6 | 2.5 ± 2.5 | 4.1 ± 3.3 | <0.001 |
| Blood nutritional data, g/dL ± SD | ||||
| Total protein | 7.31 ± 0.42 | 7.31 ± 0.42 | 7.3 ± 0.44 | 0.438 |
| Albumin | 4.37 ± 0.29 | 4.37 ± 0.28 | 4.32 ± 0.32 | <0.001 |
| SNAQ, score ± SD | 15.3 ± 1.4 | 15.6 ± 1.2 | 12.5 ± 0.8 | <0.001 |
| Sarcopenia, number (%) | 379 (4.0) | 318 (3.7) | 61 (6.6) | <0.001 |
GDS, Geriatric Depression Scale; MMSE, Mini‐Mental State Examination; SD, standard deviation.
Prevalence of anorexia of ageing and sarcopenia
According to the suggested algorithm of the AWGS, the prevalence of sarcopenia and anorexia of ageing in older adults aged 65 to 96 years was 4.0% and 9.8% in this cohort, respectively. The proportion of participants with sarcopenia was significantly higher in the anorexia of ageing group (Table 1). The prevalence of sarcopenia showed an age‐dependent increase in both sexes (P < 0.001, respectively). The prevalence of sarcopenia in men was 1.1%, 1.8%, 6.1%, 10.1%, and 21.2% and was 1.6%, 3.3%, 3.6%, 4.8%, and 7.4% in women aged 65–69, 70–74, 75–79, 80–84, and 85 years and older, respectively (Figure 1A). The prevalence of anorexia also showed an age‐dependent increase in both sexes (P < 0.001, respectively). The prevalence of anorexia in men was 8.3%, 6.3%, 9.8%, 13.6%, and 12.9% and was 7.9%, 9.4%, 10.5%, 17.6%, and 17.1% in women aged 65–69, 70–74, 75–79, 80–84, and 85 years and older, respectively (Figure 1B).
Figure 1.
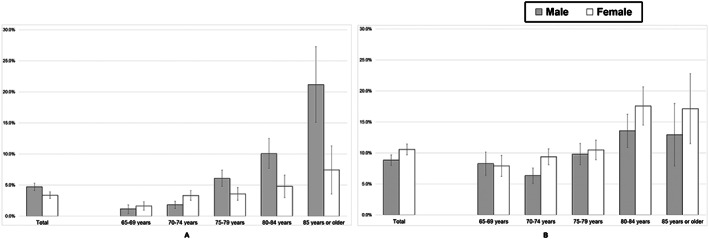
Prevalence of sarcopenia (A) and anorexia of ageing (B) among Japanese older adults according to age group.
Association between anorexia of ageing and sarcopenia
Table 2 shows the association between anorexia of ageing and sarcopenia using the multiple logistic regression models. The crude model indicated that anorexia of ageing had significantly independent association with sarcopenia (OR: 1.83, 95% CI: 1.38 to 2.42, P < 0.001). In the model adjusted with the covariates except for nutritional status (total protein and albumin) (Table 2, Adjusted Model 1), anorexia of ageing was independently associated with sarcopenia (OR: 1.45, 95% CI: 1.07 to 1.95, P = 0.015). Similar results were obtained in the model adjusted for all covariates including nutritional status (OR: 1.42, 95% CI: 1.06 to 1.92, P = 0.020).
Table 2.
Association between anorexia of ageing and sarcopenia on multiple logistic regression models
| Crude model | Adjusted Model 1 | Adjusted Model 2 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Anorexia of ageing | 1.83 (1.38 —2.42) | <0.001 | 1.45 (1.07 — 1.95) | 0.015 | 1.42 (1.06 —1.92) | 0.020 |
| Sociodemographic data | ||||||
| Age (vs. 74 years or younger) | 2.80 (2.23 —3.53) | <0.001 | 2.68 (2.13 — 3.38) | <0.001 | ||
| Male (vs. female) | 1.62 (1.19 — 2.20) | 0.002 | 1.62 (1.19 — 2.20) | 0.002 | ||
| Education (vs. 10 years or longer) | 1.01 (0.80 — 1.27) | 0.948 | 1.01 (0.80 — 1.27) | 0.945 | ||
| Medical information | ||||||
|
Polypharmacy (vs. four medications or less) |
1.56 (1.22 — 1.99) | <0.001 | 1.52 (1.19 — 1.95) | 0.001 | ||
| Hypertension | 0.44 (0.34 — 0.55) | <0.001 | 0.45 (0.35 — 0.56) | <0.001 | ||
| Diabetes | 0.91 (0.67 — 1.25) | 0.571 | 0.92 (0.67 — 1.25) | 0.581 | ||
| Hyperlipidaemia | 0.72 (0.56 — 0.91) | 0.006 | 0.74 (0.59 — 0.95) | 0.016 | ||
| Lifestyle factor | ||||||
| Drinking habit | ||||||
| Never drinker | Reference | Reference | ||||
| Stopped drinking | 0.69 (0.52 — 0.90) | 0.007 | 0.69 (0.52 — 0.91) | 0.008 | ||
| Current drinker | 0.49 (0.36 — 0.67) | <0.001 | 0.49 (0.36 —0.67) | <0.001 | ||
| Smoking habit | ||||||
| Never smoker | Reference | Reference | ||||
| Stopped smoking | 0.93 (0.68 — 1.26) | 0.632 | 0.91 (0.67 — 1.24) | 0.558 | ||
| Current smoker | 1.02 (0.66 — 1.58) | 0.921 | 1.00 (0.65 — 1.56) | 0.990 | ||
| Physical inactivity | 1.63 (1.29 — 2.05) | <0.001 | 1.62 (1.28 — 2.04) | <0.001 | ||
| Neuropsychological factor | ||||||
| Cognitive decline (vs. MMSE score of 24 or higher) | 1.31 (1.00 — 1.73) | 0.053 | 1.30 (0.98 — 1.71) | 0.064 | ||
| Depressive symptoms (vs. GDS score of 5 or lower) | 1.44 (1.10 — 1.88) | 0.009 | 1.41 (1.07 — 1.84) | 0.014 | ||
| Nutritional status | ||||||
| Lower total protein (vs. 7.0 g/dL or higher) | 0.98 (0.75 — 1.27) | 0.854 | ||||
| Lower albumin (vs. 4.2 g/dL or higher) | 1.57 (1.24 — 2.00) | <0.001 | ||||
Note. Adjusted Model 1: Adjusted for sociodemographic factors (age, sex, and education), medical conditions (polypharmacy, hypertension, diabetes, and hyperlipidaemia), lifestyle factors (drinking habit, smoking habit, and physical activity), and neuropsychological factors (cognitive decline and depressive symptoms). Adjusted Model 2: Adjusted for the factors used in Model 1 plus nutritional status (lower total protein and lower albumin).
CI, confidence interval; GDS; Geriatric Depression Scale; MMSE, Mini‐Mental State Examination; OR, odds ratio.
Discussion
The present study investigated the association between anorexia of ageing and sarcopenia. The findings show that the prevalence of sarcopenia and anorexia increased with age in both sexes. Anorexia of ageing was independently associated with sarcopenia among older people. Stratified analysis by sex showed that anorexia of ageing was independently associated with sarcopenia in older women, but not in men.
A systematic review reported that the prevalence of anorexia of ageing ranges between 13.0% and 21.2% in community‐dwelling older adults.20 Meanwhile, a lower prevalence (9.8%) was observed in this study. One potential explanation is that our participants were relatively younger compared with those in the previous study.21 In fact, our findings showed that prevalence of anorexia of ageing was affected by ageing. In addition, there was a methodological difference on assessment for anorexia of ageing between the present study (SNAQ) and the previous study (Minimum Data Set for Home Care).21 Therefore, future research should need to compare SNAQ to the other assessments for identifying anorexia of ageing and to verify which method is appropriate.
The prevalence of sarcopenia was higher in women than that in men among 74 years or younger groups. In contrast, the prevalence of sarcopenia was higher in men than that in women in 75 years or older groups. This finding was line with the previous studies.17, 18 However, the potential mechanism has remained unclear. One potential explanation is that insulin‐like growth factor 1 (IGF‐1) has an essential key in this phenomenon. IGF‐1 is one of the important mediators for muscle growth and repairing. Albani reported that a sex‐specific pattern for IGF‐1 attenuation with ageing was found.22 The IGF‐1 level in men was significantly reduced in 85 year‐over group compared to 70–85 year‐old group, which was considered to be affected by ageing. On the other hand, there was no significant change between 70–85 year group and 85 year‐old among women. In other words, IGF‐1 level of older women might be less affected by ageing. This finding supports the prevalence of sarcopenia in our results. Thus, the age–sex‐specific prevalence of sarcopenia might be dependent on the IGF‐1 level.
The current study indicated that anorexia of ageing was independently associated with sarcopenia among community‐dwelling Japanese older people. This result is in line with that of a previous study by Landi et al. who used the European Consensus to assess sarcopenia and reported that anorexia is common among community‐dwelling old–old people (over 80 years) and that the presence of anorexia is associated with sarcopenia.23 Anorexia of ageing may be correlated with a high risk of qualitative low intake of single nutrients, in particular, protein and vitamins.24 In turn, elderlies with an acceptable nutritional status may have been shown to have poor protein intake, which leads to frailty that is comprehensive concept including sarcopenia.25 In fact, poor nutritional quality leads to frailty.26, 27 The anorexia of ageing decreases food intake, which leads to a decline in physical function, muscle mass, and strength as in sarcopenia 24. It could be hypothesized that this selective malnutrition is directly correlated with the onset of sarcopenia. Our results, showing a correlation between anorexia and sarcopenia in the subjects without clinical evidence of malnutrition, support this hypothesis.
Sarcopenia is a major geriatric syndrome,1 and thus, identifying predictive factors may help prevent older people from sarcopenia. Blood nutritional data can identify risk of sarcopenia among older adults,28 however there are many environments where blood collection cannot be performed. In contrast, SNAQ can be conveniently implemented in clinical community setting. In addition, in the present study, albumin and total protein as the blood nutrition data did not significantly associated with sarcopenia among our female participants, whereas SNAQ did: SNAQ cut‐off point indicating anorexia of ageing had 1.6 times greater OR of sarcopenia. These findings indicate that SNAQ is also important tool as an assessment of nutrition in the community.
Fried proposed anorexia of ageing contribute wasting or reduced muscle quantity/quality subsequent to frailty.9 With ageing, there is a decrease in food intake known as the anorexia of ageing coupled with a decline in muscle mass and an increase in fat mass.29 In fact, the amount of protein intake, the distribution, and the source of protein intake are all important to maximally stimulate postprandial muscle protein synthetic response and muscle mass/quality accretion in the elderly.30 Bourgeois et al. showed that combining measures of skeletal muscle mass and quality will significantly improve prediction of skeletal muscle function.31 In other words, both skeletal muscle mass and quality are important for skeletal muscle function in the elderlies. Moreover, the measurement of jump force is more strongly related to bone outcomes.32 Namely, maintain in skeletal muscle function leads to maintain in bone quality. According to these studies, in late life, maintain in appetite (prevention from anorexia of ageing) is important for elderlies to maintain skeletal muscle function or bone quality.
The major strength of the present study was that the analysis was conducted using a relatively large sample of Japanese older individuals. Nevertheless, there were several limitations in the present study. First, this study was a cross‐sectional design; therefore, the results do not show any causal associations between anorexia of ageing and sarcopenia. Second, there was selection bias in the present study. Our participants were healthy elderlies who were have the ability to access health check‐ups, which might have led to the underestimation of the prevalence of sarcopenia. Third, sarcopenia was related to many factors (i.e. tumour necrosis factor‐α and chronic obstructive pulmonary disease), but we had not measured this information. These covariates should be considered in future studies on the association between anorexia of ageing and sarcopenia. Fourth, some variables were self‐reported including anorexia of ageing (e.g. education history, medication, and chronic disease history). These factors and others should be examined in diverse methods in future studies. Fifth, we evaluated skeletal muscle mass using bio‐impedance analyser, not dual‐energy X‐ray absorptiometry (DXA). Although further standardization is needed to ensure that the assessment and cut points are used accurately on all makes and models of DXA systems, DXA provides a precise measure of lean mass.33, 34, 35 Finally, we could not exclude the participants with short‐term or chronic disorders (cancer, heart disease, kidney disease, etc.) which are known to effect appetite. It remains unclear what is the best way to assess and normalize the skeletal muscle mass among overweight or obese patients, particularly chronic kidney disease or end‐stage renal disease.36 Because of the invitation‐type survey, our participants were the elderly who could visit the health check‐ups' site without any problems because there were few participants who have end‐stage chronic disease. However, this should be considered in future studies.
In conclusion, the present study showed that anorexia is common among community‐dwelling older adults in Japanese. Anorexia is not an inevitable side effect of ageing, but many changes associated with the process of ageing can promote it. Optimizing the nutritional status may decrease the risk of functional decline and sarcopenia in the elderly population. Prospective studies investigating the effect of specific intervention for anorexia on the risk of sarcopenia in larger populations are needed to provide definitive evidence for clinical guidelines.
Funding
This work was supported by the Japanese Ministry of Health, Labour and Welfare (Project for Optimizing Long‐Term Care) [Grant B‐3]; the National Center for Geriatrics and Gerontology (Research Funding for Longevity Sciences) [Grants 22‐16 and 26‐33]; and the Strategic Basic Research Programs (RISTEX Redesigning Communities for Aged Society), Japan Science and Technology Agency.
Author contributions
Tsutsumimoto planned the study, wrote the first draft of the manuscript, and coordinated the review and editing process leading to the final manuscript. Doi participated in the design of the study and wrote the paper. Nakakubo, Kim, Kurita, and Ishi corrected data and contributed to the editorial process and review of the manuscript. Shimada supervised the study, suggested many ideas that have been pursued in this research, and participated in the planning, editorial, and review processes that led to the final manuscript.
Conflict of interest
None declared.
Data availability statement
The authors do not have permission to share data.
Acknowledgements
The authors are deeply grateful to the Obu City Office and Nagoya City Office for the help with participant recruitment. MEXT‐Supported Program for the Strategic Research Foundation at Private Universities, 2015‐2019 from the Ministry of Education, Culture, Sports, Science and Technology (S1511017). The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017.37
Tsutsumimoto K., Doi T., Nakakubo S., Kim M., Kurita S., Ishii H., and Shimada H. (2020) Association between anorexia of ageing and sarcopenia among Japanese older adults, Journal of Cachexia, Sarcopenia and Muscle, 11, 1250–1257, doi: 10.1002/jcsm.12571.
References
- 1. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- 2. Janssen I. Influence of sarcopenia on the development of physical disability: the Cardiovascular Health Study. J Am Geriatr Soc 2006;54:56–62. [DOI] [PubMed] [Google Scholar]
- 3. Phillips A, Strobl R, Vogt S, Ladwig KH, Thorand B, Grill E. Sarcopenia is associated with disability status‐results from the KORA‐Age study. Osteoporos Int 2017;28:2069–2079. [DOI] [PubMed] [Google Scholar]
- 4. Tang TC, Hwang AC, Liu LK, Lee WJ, Chen LY, Wu YH, et al. FNIH‐defined sarcopenia predicts adverse outcomes among community‐dwelling older people in Taiwan: results from I‐Lan longitudinal aging study. J Gerontol A Biol Sci Med Sci 2018;73:828–834. [DOI] [PubMed] [Google Scholar]
- 5. Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability‐adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–2223. [DOI] [PubMed] [Google Scholar]
- 6. Walston JD. Sarcopenia in older adults. Curr Opin Rheumatol 2012;24:623–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giezenaar C, Chapman I, Luscombe‐Marsh N, Feinle‐Bisset C, Horowitz M, Soenen S. Ageing is associated with decreases in appetite and energy intake—a meta‐analysis in healthy adults. Nutrients 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morley JE. Pathophysiology of the anorexia of aging. Curr Opin Clin Nutr Metab Care 2013;16:27–32. [DOI] [PubMed] [Google Scholar]
- 9. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 10. Shimada H, Tsutsumimoto K, Lee S, Doi T, Makizako H, Lee S, et al. Driving continuity in cognitively impaired older drivers. Geriatr Gerontol Int 2015;16:508–514. [DOI] [PubMed] [Google Scholar]
- 11. Nakatsu N, Sawa R, Misu S, Ueda Y, Ono R. Reliability and validity of the Japanese version of the simplified nutritional appetite questionnaire in community‐dwelling older adults. Geriatr Gerontol Int 2015;15:1264–1269. [DOI] [PubMed] [Google Scholar]
- 12. Wilson MM, Thomas DR, Rubenstein LZ, Chibnall JT, Anderson S, Baxi A, et al. Appetite assessment: simple appetite questionnaire predicts weight loss in community‐dwelling adults and nursing home residents. Am J Clin Nutr 2005;82:1074–1081. [DOI] [PubMed] [Google Scholar]
- 13. Shimada H, Makizako H, Doi T, Yoshida D, Tsutsumimoto K, Anan Y, et al. Combined prevalence of frailty and mild cognitive impairment in a population of elderly Japanese people. J Am Med Dir Assoc 2013;14:518–524. [DOI] [PubMed] [Google Scholar]
- 14. Folstein MF, Robins LN, Helzer JE. The Mini‐Mental State Examination. Arch Gen Psychiatry 1983;40:812. [DOI] [PubMed] [Google Scholar]
- 15. Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull 1988;24:709–711. [PubMed] [Google Scholar]
- 16. Friedman B, Heisel MJ, Delavan RL. Psychometric properties of the 15‐item Geriatric Depression Scale in functionally impaired, cognitively intact, community‐dwelling elderly primary care patients. J Am Geriatr Soc 2005;53:1570–1576. [DOI] [PubMed] [Google Scholar]
- 17. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 18. Lin CC, Lin WY, Meng NH, Li CI, Liu CS, Lin CH, et al. Sarcopenia prevalence and associated factors in an elderly Taiwanese metropolitan population. J Am Geriatr Soc 2013;61:459–462. [DOI] [PubMed] [Google Scholar]
- 19. Gnjidic D, Hilmer SN, Blyth FM, Naganathan V, Waite L, Seibel MJ, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community‐dwelling older men at risk of different adverse outcomes. J Clin Epidemiol 2012;65:989–995. [DOI] [PubMed] [Google Scholar]
- 20. Malafarina V, Uriz‐Otano F, Gil‐Guerrero L, Iniesta R. The anorexia of ageing: physiopathology, prevalence, associated comorbidity and mortality. A systematic review. Maturitas 2013;74:293–302. [DOI] [PubMed] [Google Scholar]
- 21. Landi F, Liperoti R, Lattanzio F, Russo A, Tosato M, Barillaro C, et al. Effects of anorexia on mortality among older adults receiving home care: an observation study. J Nutr Health Aging 2012;16:79–83. [DOI] [PubMed] [Google Scholar]
- 22. Albani D, Batelli S, Polito L, Vittori A, Pesaresi M, Gajo GB, et al. A polymorphic variant of the insulin‐like growth factor 1 (IGF‐1) receptor correlates with male longevity in the Italian population: a genetic study and evaluation of circulating IGF‐1 from the "Treviso Longeva (TRELONG)" study. BMC Geriatr 2009;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Barillaro C, et al. Association of anorexia with sarcopenia in a community‐dwelling elderly population: results from the ilSIRENTE study. Eur J Nutr 2013;52:1261–1268. [DOI] [PubMed] [Google Scholar]
- 24. Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre‐cachexia: joint document elaborated by Special Interest Groups (SIG) "cachexia‐anorexia in chronic wasting diseases" and "nutrition in geriatrics". Clin Nutr 2010;29:154–159. [DOI] [PubMed] [Google Scholar]
- 25. Jyvakorpi SK, Pitkala KH, Puranen TM, Björkman MP, Kautiainen H, Strandberg TE, et al. High proportions of older people with normal nutritional status have poor protein intake and low diet quality. Arch Gerontol Geriatr 2016;67:40–45. [DOI] [PubMed] [Google Scholar]
- 26. Bonnefoy M, Berrut G, Lesourd B, Ferry M, Gilbert T, Guerin O, et al. Frailty and nutrition: searching for evidence. J Nutr Health Aging 2015;19:250–257. [DOI] [PubMed] [Google Scholar]
- 27. Leon‐Munoz LM, Guallar‐Castillon P, Lopez‐Garcia E, Rodriguez‐Artalejo F. Mediterranean diet and risk of frailty in community‐dwelling older adults. J Am Med Dir Assoc 2014;15:899–903. [DOI] [PubMed] [Google Scholar]
- 28. Gariballa S, Alessa A. Association between nutritional blood‐based biomarkers and clinical outcome in sarcopenia patients. Clin Nutr ESPEN 2018;25:145–148. [DOI] [PubMed] [Google Scholar]
- 29. Morley JE. Anorexia of ageing: a key component in the pathogenesis of both sarcopenia and cachexia. J Cachexia Sarcopenia Muscle 2017;8:523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tieland M, Trouwborst I, Clark BC. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle 2018;9:3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bourgeois B, Fan B, Johannsen N, Gonzalez MC, Ng BK, Sommer MJ, et al. Improved strength prediction combining clinically available measures of skeletal muscle mass and quality. J Cachexia Sarcopenia Muscle 2019;10:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zengin A, Pye SR, Cook MJ, Adams JE, Rawer R, Wu FC, et al. Associations of muscle force, power, cross‐sectional muscle area and bone geometry in older UK men. J Cachexia Sarcopenia Muscle 2017;8:598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buckinx F, Landi F, Cesari M, Fielding RA, Visser M, Engelke K, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle 2018;9:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clark BC, Tavoian D, Goodpaster BH, Cawthon PM, Hansen RD, Manini TM. Comment on: “Pitfalls in the measurement of muscle mass: a need for a reference standard” by Buckinx et al. J Cachexia Sarcopenia Muscle 2018;9:1269–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Buckinx F, Landi F, Cesari M, Fielding RA, Visser M, Engelke K, et al. The authors reply: Letter on: “Pitfalls in the measurement of muscle mass: a need for a reference standard” by Clark et al. J Cachexia Sarcopenia Muscle 2018;9:1272–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saitoh M, Ishida J, Springer J. Considering technique of assessment and method for normalizing skeletal muscle mass. J Cachexia Sarcopenia Muscle 2017;8:853–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.


