Abstract
Background
Skeletal muscle wasting is an extremely common feature in patients with heart failure, affecting approximately 20% of ambulatory patients with even higher values during acute decompensation. Its occurrence is associated with reduced exercise capacity, muscle strength, and quality of life. We sought to investigate if the presence of muscle wasting carries prognostic information.
Methods
Two hundred sixty‐eight ambulatory patients with heart failure (age 67.1 ± 10.9 years, New York Heart Association class 2.3 ± 0.6, left ventricular ejection fraction 39 ± 13.3%, and 21% female) were prospectively enrolled as part of the Studies Investigating Co‐morbidities Aggravating Heart Failure. Muscle wasting as assessed using dual‐energy X‐ray absorptiometry was present in 47 patients (17.5%).
Results
During a mean follow‐up of 67.2 ± 28.02 months, 95 patients (35.4%) died from any cause. After adjusting for age, New York Heart Association class, left ventricular ejection fraction, creatinine, N‐terminal pro‐B‐type natriuretic peptide, and iron deficiency, muscle wasting remained an independent predictor of death (hazard ratio 1.80, 95% confidence interval 1.01–3.19, P = 0.04). This effect was more pronounced in patients with heart failure with reduced than in heart failure with preserved ejection fraction.
Conclusions
Muscle wasting is an independent predictor of death in ambulatory patients with heart failure. Clinical trials are needed to identify treatment approaches to this co‐morbidity.
Keywords: Wasting, Sarcopenia, Cachexia, Heart failure, Survival
Introduction
Heart failure (HF) has huge socio‐economic impact and is associated with high morbidity and mortality. Likewise, patients often present with reduced quality of life and low exercise capacity as can be directly measured or extrapolated from breathlessness and fatigability. In addition, most patients present with co‐morbidities that directly or indirectly worsen exercise tolerance, morbidity, and/or mortality. Among these, atrial fibrillation, diabetes mellitus, chronic obstructive pulmonary disease, sleep‐disordered breathing, iron deficiency, and chronic kidney disease play important roles. Recently, skeletal muscle wasting has emerged as an important co‐morbidity of HF 1 that affects both patients with HF with preserved ejection fraction (HFpEF) and those with HF with reduced ejection fraction (HFrEF) in that it further reduces low peak oxygen consumption, 6 min walk distance, and handgrip as well as quadriceps strength. 2 , 3 , 4 The prevalence of skeletal muscle wasting has been reported to be 19–52% among all patients with HF. The large range mirrors the association with the cohort under investigation. For example, stable ambulatory patients seem to have lower prevalence values than patients with acute or advanced HF, 5 , 6 , 7 and patients with dilated cardiomyopathy may have higher prevalence values than patients with ischaemic heart disease. 2 , 3 , 8
It is important to note that skeletal muscle wasting, in geriatric populations commonly called sarcopenia, is different from cachexia, because only cachexia is by definition associated with weight loss. 4 Recent data have shown that cachexia in patients with HF, usually termed cardiac cachexia, can be associated with skeletal muscle wasting; however, this is not a prerequisite, because also fat tissue and bone mineral density can be diminished during these catabolic wasting processes. 9 Reduced exercise performance, however, is primarily present in those patients who have lost skeletal muscle. 10 This finding coincides with reduced peripheral blood flow in patients with muscle wasting. 11 Interestingly, mitochondrial energy production can be improved in the skeletal muscle by intravenous iron therapy in patients with iron deficiency, 12 underlining the importance of iron provision for energy production in this system. Treatment of muscle wasting and iron deficiency may provide an interesting means to improve exercise capacity and quality of life, both of which are difficult to increase using guideline ‐recommended HF treatments. 12
It has long been established that cardiac cachexia is associated with reduced survival, and this finding is independent of other prognostic factors such as age, reduced peak oxygen consumption, left ventricular ejection fraction (LVEF), or New York Heart Association (NYHA) class. 13 The aim of the present analysis from the Studies Investigating Co‐morbidities Aggravating Heart Failure (SICA‐HF) was to test whether skeletal muscle wasting itself carries prognostic information in patients with HF, irrespective of whether HFrEF or HFpEF is present.
Methods
Study population
Patients were prospectively enrolled as part of SICA‐HF at the Department of Cardiology, Charité Medical School, Campus Virchow‐Klinikum, Berlin, Germany, between February 2010 and March 2014. All subjects provided written informed consent before being enrolled. The local ethics committee approved the study, which was funded by the European Commission's Seventh Framework Programme (FP7/2007‐2013) under Grant Agreement Number 241558. All principles of the Declaration of Helsinki were fulfilled. Clinical data from this dataset have been published before and have served to characterize body composition as well as clinical features of patients with wasting disorders in HF. 2 , 3 , 11
The inclusion criteria of SICA‐HF were broad and have been published before. 14 In brief, patients were eligible if the following criteria were met: age >18 years, clinical signs and symptoms of chronic HF with LVEF ≤ 40% (HFrEF) or with an LVEF > 40%, and a left atrial dimension ≥4.0 cm (HFpEF). Exclusion criteria embraced previous heart transplantation, cardiac or embolic events within 6 weeks prior to the baseline examination, and patients on haemodialysis or with known pregnancy. Transthoracic two‐dimensional echocardiography was performed before entering the study to assess standard cardiac parameters including LVEF using Simpson's biplane technique. Blood was drawn for serum and plasma sampling early in the morning after an overnight fast and after at least 15 min of supine rest. In addition, standard parameters were assessed including a full blood count and routine clinical biochemistry parameters.
Definition of wasting
All patients underwent standardized procedures to assess muscle mass and body weight. Dual‐energy X‐ray absorptiometry (DEXA) was used to assess body composition. Like in previous publications from this dataset, muscle wasting was defined according to previously published criteria suggested to diagnose sarcopenia, 15 that is, an appendicular skeletal muscle mass 2 SDs below the mean of a healthy young reference group aged 18–40 years. This translates into an appendicular skeletal muscle mass below 7.26 kg in men or 5.45 kg in women. 16 Appendicular lean mass was defined as the lean mass of both arms and legs combined. Lean mass data from DEXA scan were evaluated, and the patients' muscle mass index was calculated. 17 This index assesses appendicular skeletal muscle mass (ASM in kg), calculated as the lean muscle mass of both arms and legs divided by height (in m) squared. 18 A DEXA scanner model ‘lunar prodigy’ was used with ‘lunar en Core 2002’ software (both from GE Medical Systems, Madison, WI, USA). Body weight was assessed after an overnight fast wearing light clothing without shoes on standardized weighing scales.
Clinical biochemistry
Iron deficiency was defined as recommended in the guidelines of the European Society of Cardiology as serum ferritin <100 ng/mL or serum ferritin 100–299 ng/mL with transferrin saturation <20%. N‐terminal pro B‐type natriuretic peptide was assessed on the Elecsys system using Roche assays (Roche, Basel, Switzerland). The glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration formula.
Survival
Patients were followed until August 2018 when the database was censored and for a mean of 67.2 ± 28.0 months or until death. All survivors were followed for at least 100 months.
Statistical analysis
Baseline characteristics are expressed as mean ± standard deviation or as number of patients with percentage. Unpaired Student's t‐test and Fisher's exact test were used to analyse the difference between groups. N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) was not normally distributed and therefore transformed by logarithm of 10 in order to achieve normal distribution. To estimate the influence of risk factors, Cox proportional hazard analysis was performed, firstly as single‐predictor (or unadjusted) model und subsequently as multivariate (or adjusted) model. All statistical analyses were performed using the Statistical Package for the Social Sciences for Windows (IBM SPSS Statistics Version 25, IBM Corporation, Armonk, NY, USA). We considered a two‐tailed P‐value <0.05 as statistically significant.
Results
Study population
A total of 268 patients were enrolled, 87 of whom had HFpEF and 181 had HFrEF. The majority of patients were male; however, the proportion of female vs. male was higher in patients with HFpEF than in HFrEF (Table 1). Most patients had ischaemic heart disease and were in NYHA Classes II and III. Co‐morbidities were highly prevalent; in particular, patients had a high prevalence of diabetes mellitus (41%), hypertension (81%), and atrial fibrillation (38%); 61.3% of patients with HFrEF had received a device implantation as detailed in Table 1; as expected, this proportion was significantly lower among patients with HFpEF.
TABLE 1.
Baseline characteristics of the study population
| All patients (n = 268) | HFpEF (n = 87) | HFrEF (n = 181) | P‐value | |
|---|---|---|---|---|
| Baseline demographics | ||||
| Age (years) | 67.14 ± 10.86 | 68.48 ± 11.33 | 66.49 ± 10.61 | 0.16 |
| Sex (% female) | 57 (21.3%) | 29 (33.3%) | 28 (15.5%) | 0.001 |
| Aetiology (% ischaemic) | 162 (60.4%) | 40 (46%) | 122 (67.4%) | <0.001 |
| BMI (kg/m2) | 28.95 ± 5.03 | 30.35 ± 4.94 | 28.27 ± 4.94 | 0.001 |
| Systolic blood pressure (mmHg) | 127.43 ± 22.94 | 138.86 ± 22.88 | 121.87 ± 20.86 | <0.001 |
| Diastolic blood pressure (mmHg) | 75.04 ± 12.55 | 78.34 ± 13.49 | 73.43 ± 11.78 | 0.003 |
| Heart rate (b.p.m.) | 65.12 ± 10.76 | 63.69 ± 11.30 | 65.82 ± 10.45 | 0.14 |
| LVEF (%) | 39.00 ± 13.25 | 55.29 ± 5.96 | 31.17 ± 7.31 | <0.001 |
| NYHA class | 2.32 ± 0.63 | 2.17 ± 0.66 | 2.39 ± 0.60 | 0.007 |
| Medical history and co‐morbidities | ||||
| Diabetes mellitus (%) | 109 (41.3%) | 40 (47.6%) | 69 (38.3%) | 0.18 |
| Hypertension (%) | 214 (80.8%) | 77 (90.6%) | 137 (76.1%) | 0.005 |
| Previous myocardial infarction (%) | 129 (49.6%) | 23 (26.7%) | 106 (61%) | <0.001 |
| Previous percutaneous intervention (%) | 126 (48.8%) | 32 (36.8%) | 94 (55%) | 0.008 |
| Previous CABG procedure (%) | 54 (21.1%) | 12 (14.1%) | 42 (24.6%) | 0.07 |
| Atrial fibrillation (%) | 101 (38.1%) | 26 (30.2%) | 75 (41.9%) | 0.08 |
| Muscle wasting (%) | 47 (17.5%) | 8 (9.2%) | 39 (21.5%) | 0.02 |
| Iron deficiency (%) | 137 (51.3%) | 42 (48.8%) | 95 (52.5%) | 0.6 |
| Anaemia (%) | 81 (30.2%) | 22 (25.3%) | 59 (32.6%) | 0.26 |
| Cachexia (%) | 52 (20.2%) | 10 (11.8%) | 42 (24.3%) | 0.021 |
| Device implantation (%) | 125 (46.6%) | 14 (16.1%) | 111 (61.3%) | <0.001 |
| ICD (%) | 56 (44.8%, n = 125) | 2 (14.3%, n = 14) | 54 (48.6%, n = 111) | 0.02 |
| CRT‐P or CRT‐D (%) | 50 (40%, n = 125) | 5 (35.7%, n = 14) | 45 (40.5%, n = 111) | 0.78 |
| Pacemaker (%) | 19 (15.2%, n = 125) | 7 (50%, n = 14) | 12 (10.8%, n = 111) | 0.001 |
| Medication | ||||
| ACE inhibitor or ARB (%) | 251 (93.7%) | 78 (89.7%) | 173 (95.6%) | 0.11 |
| Beta‐blocker (%) | 239 (89.2%) | 67 (77%) | 172 (95%) | <0.001 |
| Mineralocorticoid receptor antagonist (%) | 123 (45.9%) | 18 (20.7%) | 105 (58%) | <0.001 |
| Loop diuretic (%) | 147 (55.1%) | 30 (34.5%) | 117 (65%) | <0.001 |
| Thiazide (%) | 80 (29.9%) | 34 (39.1%) | 46 (25.4%) | 0.03 |
| Oral anticoagulant (%) | 90 (33.8%) | 19 (22.1%) | 71 (39.4%) | 0.006 |
| Aspirin or clopidogrel (%) | 192 (71.6%) | 53 (60.9%) | 139 (76.8%) | 0.009 |
| Statin (%) | 184 (68.7%) | 53 (60.9%) | 131 (72.4%) | 0.07 |
| Digitalis (%) | 29 (10.8%) | 5 (5.7%) | 24 (13.3%) | 0.09 |
| Laboratory parameters | ||||
| Haemoglobin (g/dL) | 13.42 ± 1.51 | 13.41 ± 1.42 | 13.42 ± 1.55 | 0.96 |
| Leucocytes (/nL) | 6.94 ± 2.03 | 6.63 ± 1.76 | 7.09 ± 2.13 | 0.1 |
| Platelets (/nL) | 222.62 ± 68.77 | 223.12 ± 52.38 | 222.38 ± 75.52 | 0.9 |
| Sodium (mmol/L) | 141.39 ± 4.04 | 142.37 ± 3.81 | 140.92 ± 4.07 | 0.007 |
| Potassium (mmol/L) | 4.49 ± 0.57 | 4.44 ± 0.51 | 4.51 ± 0.60 | 0.6 |
| Creatinine (mg/dL) | 1.186 ± 0.43 | 1.08 ± 0.34 | 1.24 ± 0.46 | 0.02 |
| GFR (CKD‐EPI, mL/min) | 59.66 ± 14.84 | 62.09 ± 13.59 | 58.51 ± 15.3 | 0.1 |
| Serum ferritin (μg/L) | 86.54 ± 32.0 | 90.11 ± 32.86 | 84.85 ± 31.53 | 0.2 |
| TSAT (%) | 23.53 ± 9.92 | 25.57 ± 10.58 | 22.57 ± 9.46 | 0.02 |
| Transferrin (mg/dL) | 270 ± 49.3 | 257.93 ± 46.14 | 275.73 ± 49.84 | 0.006 |
| NT‐proBNP (ng/L) | 1478 ± 2696 | 403 ± 498 | 2113 ± 3219 | <0.001 |
| Albumin (g/L) | 37.05 ± 3.76 | 37.26 ± 3.43 | 36.95 ± 3.91 | 0.535 |
ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; CRT‐D, cardiac resynchronization therapy‐ defibrillator; CRT‐P, cardiac resynchronization therapy‐ pacemaker; GFR, glomerular filtration rate; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; TSAT, transferrin saturation.
Muscle wasting was present in 47 patients (17.5%), 39 of whom had HFrEF and 8 of whom had HFpEF (P = 0.016). Overall, patients with muscle wasting were older, were more likely to be male, had a higher NT‐proBNP value, were more likely to be iron deficient, and were more likely to be anaemic (Table 2). No major difference was detected for kidney function between patients with vs. without muscle wasting, but patients with muscle wasting tended to be more symptomatic as highlighted by a trend towards higher NYHA class (Table 2). More than 50% of the overall population were found to be iron deficient being present in 95 of 181 patients with HFrEF (52.5%) and in 42 of 87 patients with HFpEF (48.8%, P = 0.6).
TABLE 2.
Patients' characteristics by muscle status
| Without muscle wasting (n = 221) | With muscle wasting (n = 47) | P‐value | |
|---|---|---|---|
| Age (years) | 66.12 ± 11.04 | 71.94 ± 8.60 | 0.001 |
| Sex (% female) | 55 (24.9%) | 2 (4.3%) | 0.001 |
| Iron deficiency (%) | 103 (46.8%) | 34 (72.3%) | 0.002 |
| Anaemia (%) | 59 (26.7%) | 22 (46.8%) | 0.009 |
| NYHA class | 2.29 ± 0.60 | 2.47 ± 0.72 | 0.1 |
| GFR (CKD‐EPI, mL/min) | 59.80 ± 14.93 | 59.05 ± 14.53 | 0.8 |
| NT‐proBNP (ng/L) | 1272 ± 2469 | 2459 ± 3467 | <0.001 |
| Albumin (g/L) | 37.26 ± 3.62 | 36.09 ± 4.27 | 0.055 |
| Transferrin (mg/dL) | 267.81 ± 45.64 | 280.23 ± 63.29 | 0.207 |
CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; GFR, glomerular filtration rate; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association.
Survival analysis
A total of 95 patients (35.5%) died during a mean follow‐up of 67.2 ± 28.02 months, 25 (9.3%) of whom had muscle wasting. Kaplan–Meier analyses for mortality revealed a 12 month mortality rate of 4.9% [95% confidence interval (CI) 2.16–7.64%] and a 24 month mortality rate of 12.2% (95% CI 8.08–16.32%). This proportion was significantly higher in patients with muscle wasting: Kaplan–Meier analyses for mortality revealed a 12 month mortality rate of 11.11% (95% CI 1.89–20.3%) in patients with muscle wasting compared with 3.5% (95% CI 0.95–6.05%) and a 24 month mortality rate of 22.22% (95% CI 10.05–34.35%) in comparison with 10% (95% CI 5.89–14.12%) in those without (P = 0.003, Figure 1). Not surprisingly, survivors had a lower mean NT‐proBNP of 885.5 ± 1630 ng/L as compared with 2374.6 ± 3389.1 ng/L in non‐survivors (P = 0.001).
FIGURE 1.
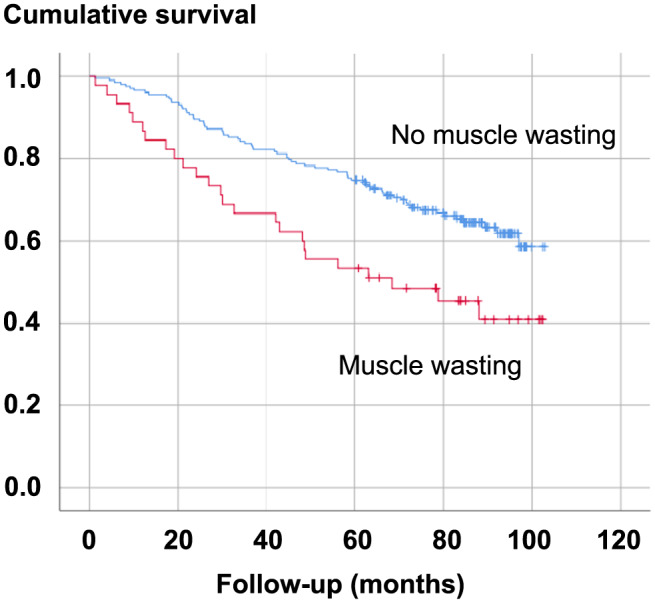
Kaplan–Meier survival curves by status of muscle wasting in the overall cohort.
Several baseline variables were investigated with regard to their impact on all‐cause death using single‐predictor Cox proportional hazard analysis: age, NYHA class, LVEF, serum creatinine, NT‐proBNP, the presence of iron deficiency, and the presence of muscle wasting all predicted survival (Table 3 and Figure 1) and were entered into a multivariable model. After adjusting for all the aforementioned variables, LVEF, serum creatinine, and the presence of muscle wasting remained independent predictors of death. Splitting the models according to the presence of HFpEF or HFrEF showed that in patients with HFpEF, serum creatinine remained the only independent predictor of death, whereas in patients with HFrEF, LVEF and muscle wasting remained independent predictors of death; however, in this model, also a trend existed towards significance for advancing age (Table 3). Kaplan–Meier survival curves were constructed for illustrative purposes and showed an early separation of the survival lines (Figure 1).
TABLE 3.
Single and multivariable Cox proportional hazard models for death from any cause
| All patients | All patients | HFpEF | HFrEF | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Single‐predictor model | Multivariate model | Multivariate model | Multivariate model | |||||||||||||
| HR | 95% CI | χ 2 | P | HR | 95% CI | Wald | P | HR | 95% CI | Wald | P | HR | 95% CI | Wald | P | |
| Age (per year) | 1.03 | 1.01–1.06 | 9.43 | 0.002 | 1.02 | 0.99–1.05 | 1.71 | 0.192 | 0.98 | 0.93–1.03 | 0.94 | 0.331 | 1.03 | 0.99–1.07 | 3.65 | 0.056 |
| NYHA (per 1 unit increase) | 2.24 | 1.58–3.18 | 20.74 | <0.001 | 1.36 | 0.86–2.16 | 1.72 | 0.191 | 2.03 | 0.63–6.51 | 1.40 | 0.236 | 1.10 | 0.63–1.92 | 0.12 | 0.729 |
| LVEF (per 1 unit increase) | 0.96 | 0.94–0.97 | 27.83 | <0.001 | 0.96 | 0.92–0.99 | 5.87 | 0.015 | 0.91 | 0.81–1.02 | 2.84 | 0.092 | 0.95 | 0.91–0.99 | 5.54 | 0.019 |
| Creatinine (per 1 unit increase) | 2.23 | 1.60–3.09 | 23.43 | <0.001 | 1.94 | 1.12–3.38 | 5.51 | 0.019 | 27.57 | 4.19–181.54 | 11.89 | 0.001 | 1.34 | 0.73–2.48 | 0.89 | 0.345 |
| Log NT‐proBNP (per 1 SD increase) | 2.04 | 1.58–2.62 | 31.32 | <0.001 | 1.22 | 0.85–1.75 | 1.15 | 0.284 | 0.88 | 0.37–2.11 | 0.08 | 0.780 | 1.45 | 0.94–2.23 | 2.88 | 0.090 |
| Iron deficiency (present) | 1.62 | 1.07–2.45 | 5.27 | 0.023 | 1.08 | 0.64–1.81 | 0.08 | 0.772 | 1.99 | 0.58–6.82 | 1.19 | 0.276 | 0.91 | 0.50–1.65 | 0.09 | 0.759 |
| Muscle wasting (present) | 1.98 | 1.25–3.13 | 8.88 | 0.003 | 1.80 | 1.01–3.19 | 4.04 | 0.044 | 1.86 | 0.32–10.70 | 0.48 | 0.489 | 1.97 | 1.05–3.71 | 4.45 | 0.035 |
| HFpEF vs. HFrEF | 0.37 | 0.22–0.63 | 14.60 | <0.001 | 0.56 | 0.20–1.60 | 1.164 | 0.281 | ||||||||
| χ 2 model = 60.55 | χ 2 model = 29.17 | χ 2 model = 32.56 | ||||||||||||||
CI, confidence interval; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; SD, standard deviation.
Discussion
We show here that skeletal muscle wasting, as defined using the cut‐off values for sarcopenia, is an independent predictor of death in a mixed cohort of patients with HF. This effect was predominantly driven by patients with HFrEF who represented the majority of patients under study in the present analysis. In patients with HFpEF, the minority of patients with lower event rate, muscle wasting did not remain an independent predictor of death after multivariable adjustment, most probably a reflection of the overall low event rate in this cohort.
Data are accumulating to suggest that catabolic wasting processes are present in advanced HF. These probably include activation of pro‐inflammatory cytokines, low anabolic drive, 19 growth hormone resistance, and overactivity of the renin–angiotensin–aldosterone system. The net result is loss of functioning skeletal muscle via the ubiquitin–proteasome system, autophagy, and increased myocyte apoptosis. 4 It is assumed that skeletal muscle as one of the main energy depots of the human muscle is lost earlier than other tissues like adipose tissue or even bone mineral density. 9 However, the latter two are also affected in patients with manifest cachexia in advanced HF in whom tissue loss is so advanced that affected patients are losing body weight. One of the main challenges remains therefore to identify patients early and before weight loss becomes apparent. Our present study underscores the assumption that the loss of skeletal muscle without manifest weight loss already identifies patients at increased risk of death. Indeed, cardiac cachexia had already been described as an independent predictor of death more than 20 years ago, but it is now becoming clear that skeletal muscle plays a pivotal role in this setting, because patients who lose muscle are unable to exercise to a sufficient degree as highlighted by low quality of life. Apart from those named earlier, mechanisms involve reduced peripheral blood flow, 11 abnormal ergoreflex physiology, 20 and altered nutritional intake, 21 but probably also iron deficiency and anaemia. 22 , 23 In particular, iron deficiency has been shown to have effects in skeletal muscle, because enzymes of the mitochondrial electron transport chain are iron dependent, and recent data show that iron administration can help to improve phosphocreatine recovery in the muscle. 24
Previous studies have aimed to use skeletal muscle mass as a predictor of death in patients with HF but were hampered by methodological problems, sample size, or both. For example, Lopez et al. 6 have tried to extrapolate skeletal muscle mass using abdominopelvic computed tomography scans performed during an acute hospitalization in order to diagnose sarcopenia in 160 patients with HF, and they observed a 4.5‐fold increase in the risk of death in those patients labelled as sarcopenic. However, it is difficult, if not impossible, to use abdominopelvic imaging to understand appendicular skeletal muscle mass. Recently, this approach has been discouraged, as it may be a ‘flawed premise’. 25 Apart from this, the area of interest, psoas muscle, can be assessed by the volume of the entire muscle, its unidimensional thickness, its cross‐sectional area either unadjusted in cm2, normalized to patient height, normalized to body surface area, or normalized to the area of the adjacent vertebral body. 25 Thus, even though these authors identified low psoas muscle area in patients with chronic HF after an acute hospitalization as an independent predictor of death, these findings should not be extrapolated to the skeletal muscle mass of the extremities. Very similarly, Tsuji et al. 5 examined psoas muscle mass in 78 patients undergoing left ventricular assist device implantation. They found that perioperative muscle wasting as extrapolated from the psoas measurement approach was associated with higher mortality in their patient cohort. Nichols et al. used DEXA scanning in 60 male patients with coronary heart disease and found that 13 of these (21.7%) had low skeletal muscle mass, as defined using a skeletal mass index <7.26 kg/m2 or appendicular skeletal mass <25.72%. In this comparatively small cohort of patients without HF, lower skeletal muscle mass was associated with a higher risk of all‐cause mortality. Tsuchida and colleagues found a sarcopenia prevalence of 52.6% among 38 patients with acutely decompensated HF but mainly used sarcopenia as a predictor of high BNP levels. 7
Our findings from patients with HF call for closer scrutiny of skeletal muscle and also for a wider availability of DEXA scanning. As mentioned, previous studies have been hampered by methodological problems, because DEXA scanning is not routinely available in many hospitals. Computed tomography and magnetic resonance imaging are alternatives, but the assessment of appendicular skeletal muscle with these imaging techniques is not common for reasons of radiation exposure, time restrictions, or cost/reimbursement issues. DEXA scanning, on the other hand, is a very quick alternative (scanning time less than 2 min) that provides crucial information about a patient's body composition in the limbs as well as in the whole body. It is comparatively cheap and simple to handle. It remains a matter of speculation if screening tools such as the SARF‐F questionnaire or skinfold measurements can identify patients with HF in need of DEXA scanning or other imaging techniques, which would make patient selection even easier. 26 , 27 Patients with low skeletal muscle, that is, sarcopenia or at risk of muscle loss (pre‐sarcopenia), 28 may benefit from more aggressive HF therapies, because retrospective analyses have highlighted the importance of using standard HF medications like beta‐blockers or angiotensin‐converting enzyme inhibitors in these patients. 29 , 30 Additional treatment approaches include exercise training (endurance exercise, muscle strength training, and inspiratory muscle training), nutritional support (e.g. high‐calorie nutritional supplements and branched‐chain amino acids), 31 , 32 , 33 and possibly anabolic substances. 4 , 34 , 35 Unfortunately, none of these interventions have been tested in prospective randomized controlled trials of adequate size so far, but smaller trials suggest that exercise training improves quality of life and the distance covered during the 6 min walk test. 12
Taken together, muscle wasting beyond the cut‐offs defined to identify sarcopenia identifies a large proportion of patients with HF who have low muscle strength, quality of life, and exercise capacity and who are likely to become frail in that they may be at increased risk of falling, risk of fractures, and hospitalizations. Our data show that these patients are also at two‐fold increased risk of death, an effect primarily driven by events in patients with HFrEF. Future studies are needed to better understand this effect in patients with HFpEF, because the event rate in this subgroup of our study population remained small.
Funding
Preparation of this manuscript was partly funded by a grant from the Innovative Medicines Initiative – Joint Undertaking (IMI‐JU 115621) and the German Center for Cardiovascular Research (DZHK).
Conflict of interest
S.v.H. has been a paid consultant for and/or received honoraria payments from AstraZeneca, Bayer, Boehringer Ingelheim, BRAHMS, Chugai, Grünenthal, Helsinn, Hexal, Novartis, Respicardia, Roche, Sorin, and Vifor. S.v.H. owns shares in Actimed. S.v.H. reports research support from IMI and the German Centre for Cardiovascular Research (DZHK). M.S.A. has received personal fees from Servier and research support from the DZHK (German Centre for Cardiovascular Research) and BMBF (German Ministry of Education and Research). The Department of Intensive Care Medicine (full departmental disclosure, J.C.S.) has/had research and development/consulting contracts (full disclosure) with Orion Corporation, Abbott Nutrition International, B. Braun Medical AG, CSEM SA, Edwards Lifesciences Services GmbH/SA, Kenta Biotech Ltd, Maquet Critical Care AB, Omnicare Clinical Research AG, and Nestlé. Educational grants have been received from Fresenius Kabi, GSK, MSD, Lilly, Baxter, Astellas, AstraZeneca, B. Braun Medical AG, CSL Behring, Maquet, Novartis, Covidien, Nycomed, Pierre Fabre Pharma (Roba Pharma), Pfizer, and Orion Pharma. No personal financial gain resulted from respective development/consulting contracts and/or grants. W.D. reports speaker fees and advisory honoraria from Aimediq, Bayer, Boehringer Ingelheim, Medtronic, Pfizer, Sanofi‐Aventis, Sphingotec, and Vifor Pharma. W.D. also reports research support from EU (Horizon 2020), the German Ministry of Education and Research, German Centre for Cardiovascular Research, Vifor Pharma, and ZS Pharma. G.H. reports lecture fees and/or consultancy honoraria from AstraZeneca, Corvia, Impulse Dynamics, Novartis, Servier, and Vifor as well as fees for editorial board activities from Springer. S.D.A. reports personal fees from Bayer, Boehringer Ingelheim, Cardiac Dimension, Impulse Dynamics, Novartis, Servier, St. Jude Medical, and Vifor Pharma and grant support from Abbott Vascular and Vifor Pharma, outside the submitted work. All other authors do not have a conflict of interest to disclose.
Acknowledgements
The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. 36
von Haehling S., Garfias Macedo T., Valentova M., Anker M. S., Ebner N., Bekfani T., Haarmann H., Schefold J. C., Lainscak M., Cleland J., Doehner W., Hasenfuss G., and Anker S. D. (2020) Muscle wasting as an independent predictor of survival in patients with chronic heart failure, Journal of Cachexia, Sarcopenia and Muscle, 11, 1242–1249, doi: 10.1002/jcsm.12603
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 2. Bekfani T, Pellicori P, Morris DA, Ebner N, Valentova M, Steinbeck L, et al. Sarcopenia in patients with heart failure with preserved ejection fraction: impact on muscle strength, exercise capacity and quality of life. Int J Cardiol 2016. Nov 1;222:41–46. [DOI] [PubMed] [Google Scholar]
- 3. Fülster S, Tacke M, Sandek A, Ebner N, Tschöpe C, Doehner W, et al. Muscle wasting in patients with chronic heart failure: results from the Studies Investigating Co‐morbidities Aggravating Heart Failure (SICA‐HF). Eur Heart J 2013;34:512–519. [DOI] [PubMed] [Google Scholar]
- 4. von Haehling S, Ebner N, Dos Santos MR, Springer J, Anker SD. Muscle wasting and cachexia in heart failure: mechanisms and therapies. Nat Rev Cardiol 2017;14:323–341. [DOI] [PubMed] [Google Scholar]
- 5. Tsuji M, Amiya E, Hatano M, Nitta D, Maki H, Bujo C, et al. Abdominal skeletal muscle mass as a predictor of mortality in Japanese patients undergoing left ventricular assist device implantation. ESC Heart Fail 2019;6:526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lopez PD, Nepal P, Akinlonu A, Nekkalapudi D, Kim K, Cativo EH, et al. Low skeletal muscle mass independently predicts mortality in patients with chronic heart failure after an acute hospitalization. Cardiology 2019;142:28–36. [DOI] [PubMed] [Google Scholar]
- 7. Tsuchida K, Fujihara Y, Hiroki J, Hakamata T, Sakai R, Nishida K, et al. Significance of sarcopenia evaluation in acute decompensated heart failure. Int Heart J 2018;59:143–148. [DOI] [PubMed] [Google Scholar]
- 8. Hajahmadi M, Shemshadi S, Khalilipur E, Amin A, Taghavi S, Maleki M, et al. Muscle wasting in young patients with dilated cardiomyopathy. J Cachexia Sarcopenia Muscle 2017;8:542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. von Haehling S. The wasting continuum in heart failure: from sarcopenia to cachexia. Proc Nutr Soc 2015;74:367–377. [DOI] [PubMed] [Google Scholar]
- 10. Emami A, Saitoh M, Valentova M, Sandek A, Evertz R, Ebner N, et al. Comparison of sarcopenia and cachexia in men with chronic heart failure: results from the Studies Investigating Co‐morbidities Aggravating Heart Failure (SICA‐HF). Eur J Heart Fail 2018;20:1580–1587. [DOI] [PubMed] [Google Scholar]
- 11. Dos Santos MR, Saitoh M, Ebner N, Valentova M, Konishi M, Ishida J, et al. Sarcopenia and endothelial function in patients with chronic heart failure: results from the Studies Investigating Comorbidities Aggravating Heart Failure (SICA‐HF). J Am Med Dir Assoc 2017;18:240–245. [DOI] [PubMed] [Google Scholar]
- 12. von Haehling S, Arzt M, Doehner W, Edelmann F, Evertz R, Ebner N, et al. Improving exercise capacity and quality of life using non‐invasive heart failure treatments: evidence from clinical trials. Eur J Heart Fail 2020; 10.1002/ejhf.1838 [DOI] [PubMed] [Google Scholar]
- 13. Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb‐Peploe KM, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 1997;349:1050–1053. [DOI] [PubMed] [Google Scholar]
- 14. von Haehling S, Lainscak M, Doehner W, Ponikowski P, Rosano G, Jordan J, et al. Diabetes mellitus, cachexia and obesity in heart failure: rationale and design of the Studies Investigating Co‐morbidities Aggravating Heart Failure (SICA‐HF). J Cachexia Sarcopenia Muscle 2010;1:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc 2011;12:403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med 2001;137:231–243. [DOI] [PubMed] [Google Scholar]
- 17. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 18. Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, et al. Appendicular skeletal muscle mass: measurement by dual‐photon absorptiometry. Am J Clin Nutr 1990;52:214–218. [DOI] [PubMed] [Google Scholar]
- 19. Jankowska EA, Biel B, Majda J, Szklarska A, Lopuszanska M, Medras M, et al. Anabolic deficiency in men with chronic heart failure: prevalence and detrimental impact on survival. Circulation 2006. Oct 24;114:1829–1837. [DOI] [PubMed] [Google Scholar]
- 20. Piepoli MF, Kaczmarek A, Francis DP, Davies LC, Rauchhaus M, Jankowska EA, et al. Reduced peripheral skeletal muscle mass and abnormal reflex physiology in chronic heart failure. Circulation 2006;114:126–134. [DOI] [PubMed] [Google Scholar]
- 21. Saitoh M, Dos Santos MR, Emami A, Ishida J, Ebner N, Valentova M, et al. Anorexia, functional capacity, and clinical outcome in patients with chronic heart failure: results from the Studies Investigating Co‐morbidities Aggravating Heart Failure (SICA‐HF). ESC Heart Fail 2017;4:448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dziegala M, Josiak K, Kasztura M, Kobak K, von Haehling S, Banasiak W, et al. Iron deficiency as energetic insult to skeletal muscle in chronic diseases. J Cachexia Sarcopenia Muscle 2018;9:802–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tkaczyszyn M, Drozd M, Węgrzynowska‐Teodorczyk K, Flinta I, Kobak K, Banasiak W, et al. Depleted iron stores are associated with inspiratory muscle weakness independently of skeletal muscle mass in men with systolic chronic heart failure. J Cachexia Sarcopenia Muscle 2018;9:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Charles‐Edwards G, Amaral N, Sleigh A, Ayis S, Catibog N, McDonagh T, et al. Effect of iron isomaltoside on skeletal muscle energetics in patients with chronic heart failure and iron deficiency: FERRIC‐HF II randomized mechanistic trial. Circulation 2019;139:2386–2398. [DOI] [PubMed] [Google Scholar]
- 25. Baracos VE. Psoas as a sentinel muscle for sarcopenia: a flawed premise. J Cachexia Sarcopenia Muscle 2017;8:527–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC‐F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle 2016;7:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bauer J, Morley JE, Schols AMWJ, Ferrucci L, Cruz‐Jentoft AJ, Dent E, et al. Sarcopenia: a time for action. An SCWD position paper. J Cachexia Sarcopenia Muscle 2019;10:956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clark AL, Coats AJS, Krum H, Katus HA, Mohacsi P, Salekin D, et al. Effect of beta‐adrenergic blockade with carvedilol on cachexia in severe chronic heart failure: results from the COPERNICUS trial. J Cachexia Sarcopenia Muscle 2017;8:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anker SD, Negassa A, Coats AJ, Afzal R, Poole‐Wilson PA, Cohn JN, et al. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin‐converting‐enzyme inhibitors: an observational study. Lancet 2003;361:1077–1083. [DOI] [PubMed] [Google Scholar]
- 31. Rozentryt P, von Haehling S, Lainscak M, Nowak JU, Kalantar‐Zadeh K, Polonski L, et al. The effects of a high‐caloric protein‐rich oral nutritional supplement in patients with chronic heart failure and cachexia on quality of life, body composition, and inflammation markers: a randomized, double‐blind pilot study. J Cachexia Sarcopenia Muscle 2010;1:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aquilani R, Viglio S, Iadarola P, Opasich C, Testa A, Dioguardi FS, et al. Oral amino acid supplements improve exercise capacities in elderly patients with chronic heart failure. Am J Cardiol 2008;101:104E–110E. [DOI] [PubMed] [Google Scholar]
- 33. Kim IY, Park S, Smeets ETHC, Schutzler S, Azhar G, Wei JY, et al. Consumption of a specially‐formulated mixture of essential amino acids promotes gain in whole‐body protein to a greater extent than a complete meal replacement in older women with heart failure. Nutrients 2019;11:pii: E1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saitoh M, Ishida J, Ebner N, Anker SD, Springer J, von Haehling S. Myostatin inhibitors as pharmacological treatment for muscle wasting and muscular dystrophy. JCSM Clin Rep 2017;2: 10.17987/jcsm-cr.v2i1.37 [DOI] [Google Scholar]
- 35. Ishida J, Saitoh M, Ebner N, Springer J, Anker SD, von Haehling S. Growth hormone secretagogues: history, mechanism of action, and clinical development. JCSM Rapid Commun 2020;3:25–37. [Google Scholar]
- 36. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]


