Abstract
Background
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has impacted heavily on global health. Although real-time polymerase chain reaction (RT-PCR) is the current diagnostic method, challenges for low- and middle-income countries (LMICs) necessitate cheaper, higher-throughput, reliable rapid diagnostic tests (RDTs).
Objective
We reviewed the documented performance characteristics of available COVID-19 RDTs to understand their public health utility in the ongoing pandemic, especially in resource-scarce LMIC settings.
Methods
Using a scoping review methodology framework, common literature databases and documentary reports were searched up to 22 April 2020, irrespective of geographical location. The search terms included ‘SARS-CoV-2 AND serological testing’ and ‘COVID-19 AND serological testing’.
Results
A total of 18 RDTs produced in eight countries, namely China (6; 33.33%), the United States (4; 22.22%), Germany (2; 11.11%), Singapore (2; 11.11%), Canada, Kenya, Korea and Belgium (1 each; 5.56%), were evaluated. Reported sensitivity ranged from 18.4% to 100% (average = 84.7%), whereas specificity ranged from 90.6% to 100% (average = 95.6%). The testing time ranged from 2 min to 30 min. Of the 12 validated RDTs, the IgM/IgG duo kit with non-colloidal gold labelling system was reported to elicit the highest sensitivity (98% – 100%) and specificity (98% – 99% for IgG and 96% – 99% for IgM).
Conclusion
We found reports of high sensitivity and specificity among the developed RDTs that could complement RT-PCR for the detection of SARS-CoV-2 antibodies, especially for screening in LMICs. However, it is necessary to validate these kits locally.
Keywords: Coronavirus disease, COVID-19, SARS-CoV-2, rapid diagnostic test, low- and middle-income countries
Introduction
Coronavirus disease 2019 (COVID-19) is an emerging respiratory disease that was first reported to the World Health Organization (WHO) as a cluster of pneumonia of unknown origin from Wuhan, China, in December 2019.1 The unknown causative agent was found through deep sequencing to be severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) on 7 January 2020 and the disease COVID-19 was named on 11 February 2020. In response, WHO declared COVID-19 as a Public Health Emergency of International Concern on 30 January 2020 and a pandemic on 11 March 2020.1 As of 22 April 2020, an estimated 2 572 805 confirmed cases and 178 551 confirmed deaths from COVID-19 had been reported.2 The first 10 cases in Africa were reported in five countries (Nigeria, Algeria, Morocco, Egypt and Senegal).3 Although earlier cases of COVID-19 in many low- and middle-income countries (LMICs) were described as imported by travelers from China, Italy, the United Kingdom and Germany, community transmission has now become the major cause of new COVID-19 infections.3,4 Early, rapid, large-scale diagnosis and accurate diagnosis of COVID-19 is one of the key interventions for COVID-19 containment in both high-income and LMIC settings.3 The availability of the SARS-CoV-2 genome has led to the development and validation of various reverse transcriptase real-time polymerase chain reaction (RT-PCR) in vitro diagnostic test kits by different manufacturers for COVID-19 diagnosis.5,6 This diagnostic test is based on the detection of genes encoding the envelope (E), spike (S), nucleoplasid (N), RNA-dependent RNA polymerase and open reading frame 1a/b (e.g. orf1ab, orf1a, orf1b) polyproteins within the genomic RNA of SARS-CoV-2.5,6,7 Due to lack of culture facilities, the RT-PCR method is currently the reference standard method of confirming COVID-19 diagnosis in suspected cases globally. For epidemiological investigation, public health and clinical actions, RT-PCR has been shown to be very reliable at screening and confirming the diagnosis of COVID-19 using upper respiratory (e.g. nasopharyngeal swab, oropharyngeal swab, throat swab and nasal swab) and lower respiratory (e.g. sputum and bronchioalveolar lavage) samples.7,8 Real-time PCR has also been useful for monitoring viral RNA shedding dynamics during the acute phase of the disease and viral RNA decay and disappearance during the convalescence stage of the disease among survivors.9,10 However, in spite of its high analytical sensitivity its detection range is limited to 3.2 – < 10.0 RNA copies per reaction.6,7,8 The RT-PCR method has been reported from studies done inside and outside China to also be prone to giving false negative results under certain conditions, thereby missing some COVID-19 cases. These missed cases are therefore not isolated increasing community transmission.8,9,10 These conditions include insufficient or inappropriate sample for viral RNA isolation, poor sample transportation to the laboratory, poor storage of the isolated RNA samples, poor quality of the RT-PCR assay and poor timing for sample collection. The asymptomatic phase of SARS-CoV-2 infection – the first few days post infection onset and the convalescence phase ≥ 14 days post infection onset, especially in a missed infection, have been indicated as times when cases can be missed.7,8,9,10 Poor quality RT-PCR assay is characterised by an inconsistent cycle threshold value and/or lack of amplification signal for one or two targeted genes. These missed cases are therefore not isolated increasing for SAR-CoV-2 detection.6,7,8 Also, due to limited financial resources, the limited number of accredited molecular laboratories of biosafety level 2/3 and limited number of technical experts, the scaling up of RT-PCR for COVID-19 diagnosis is limited in LMICs.7,8,9,10
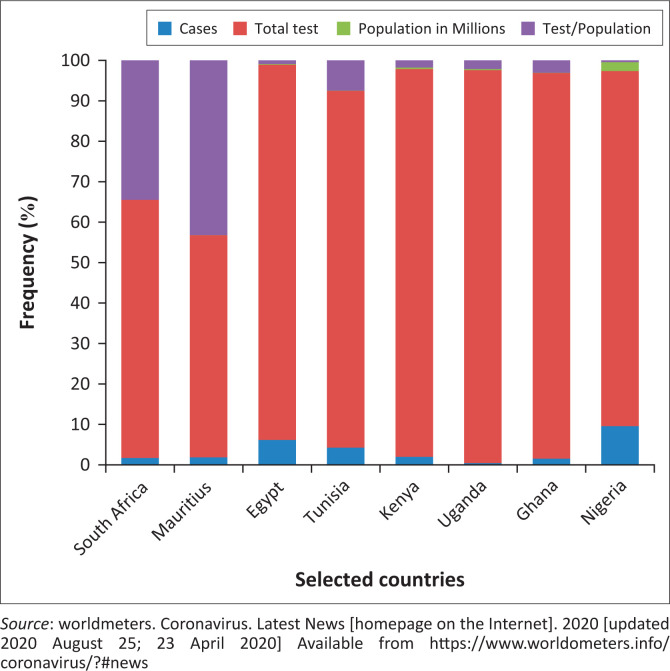
Taken together, the above challenges of RT-PCR have necessitated the deployment of serological rapid diagnostic tests (RDTs) for COVID-19 diagnosis, which could identify asymptomatic and convalescent COVID-19 cases undiagnosed by RT-PCR. COVID-19 serological RDTs are antigen-antibody based tests that detects SARS-CoV-2 IgM and/or IgG in human blood samples or SARS-CoV-2 viral antigen from respiratory samples within 15 min.8,11,12 Unlike the RT-PCR protocols, serological tests require less expensive equipment, no technical expertise or electricity to run and very minimal biosafety requirements. Also, unlike RDTs that use small amounts of biological sample (10 uL – 20 uL) and have an average run time of 15 minutes, the RT-PCR protocols use large amounts of samples (150 ul – 200 ul) and have an average run time of about 90 minutes.6,7,8 These advantages of serological RDTs have attracted serious attention for their use in large-scale COVID-19 serological RDTs are antigen-antibody based tests that detects SARS-CoV-2 IgM and/or IgG in human blood samples or SARS-CoV-2 viral antigen from respiratory samples within 15 min testing especially at the peripheral level of the health system and outside hospital settings in LMICs. Data from worldmeters show that African countries compared to other countries conducted fewer tests per population (Figure 1). This lower testing power means relatively fewer cases can be detected. Thus, the rollout of various RDT kits by different manufacturers could be a favourable development particularly for LMICs as RDTs can be easily scaled up for rapid COVID-19 diagnosis.11,12 Besides, RDTs can provide additional sero-epidemiological data that will be used to determine the magnitude of COVID-19 spread within a population. RDTs achieve this by identifying active and previous symptomatic or asymptomatic cases; these data are then used to calculate case-fatality rate and determine the anti-SARS-CoV-2 immunity level of a community.11,12 However, to harness the various epidemiological and clinical usefulness of currently available COVID-19 serological RDTs, it is important to determine and/or validate their performance levels. In the present scoping review, the following research questions will be answered: (1) what are the currently available serological RDTs for testing, (2) to what extent have these serological RDTs been validated by their manufacturers and (3) what is the level of performance characteristics of these serological RDTs? Presently, the level of accuracy of many serological RDTs available for use in LMICs remains unclear, coupled with insufficient information about their strengths and limitations. This review will provide insight into the performance characteristics of these kits and enable evidenced-based decisions for their possible use in large-scale COVID-19 testing and containment strategies in LMICs.
FIGURE 1.
Distribution of severe acute respiratory syndrome coronavirus-2 burden and test per population in selected African countries.
Methodology and data analysis
A scoping review was conducted using a methodology framework by Arksey13 with modification as described by Adhikari et al.14 This includes: (1) identifying a clear research objective and search strategies, (2) identifying relevant research articles, (3) selecting research articles, (4) extracting and charting of data, and (5) summarising, discussing, analysing and reporting the results. The online databases searched included Google Scholar, medRxiv, bioRxiv and PubMed, as well as documentary reports and white paper publications from relevant online websites including WHO, the United States Centers for Disease Control and Prevention (CDC) and the Nigeria Centre for Disease Control (NCDC) for information on new RDTs for COVID-19 published up to 22 April 2020. The search terms used include ‘SARS-CoV-2 AND testing’, ‘COVID-19 AND rapid test’ and ‘COVID-19 AND diagnostic kits’. Diagnostic kits published for the confirmation of other coronaviruses, such as the coronavirus associated with the 2003 SARS outbreak in Asia and Middle East respiratory syndrome-coronavirus, were excluded. All the members of the review teams were involved in paper search and selection and a consensus was reached through peer review. Duplicated publications and those with insufficient information were removed. The extracted data included the name of the diagnostic kit, manufacturer, test performance based on sensitivity, specificity, predictive positive and negative values, test principles and special characteristics and testing time. The data were entered into Excel (Microsoft, Redmond, Washington, United States) and exported to Statistical Product and Service Solution version 23 (IBM SPSS Inc., Chicago, Illinois, United States) for cleaning and analysis.
Review findings
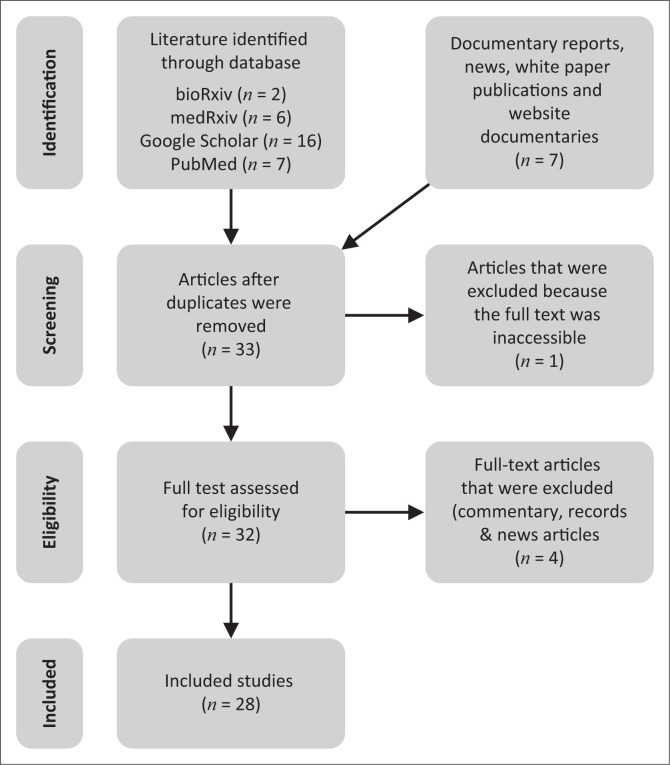
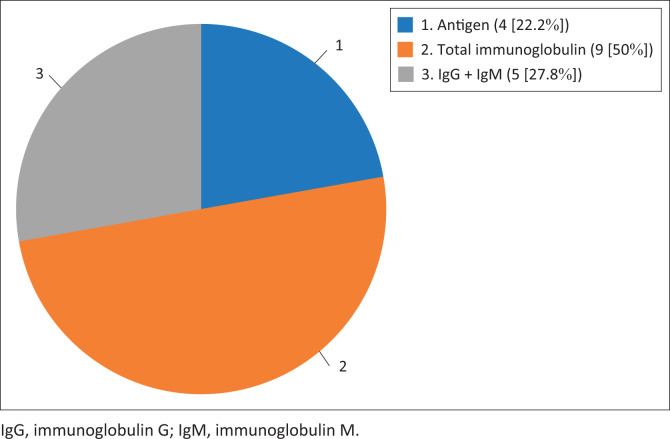
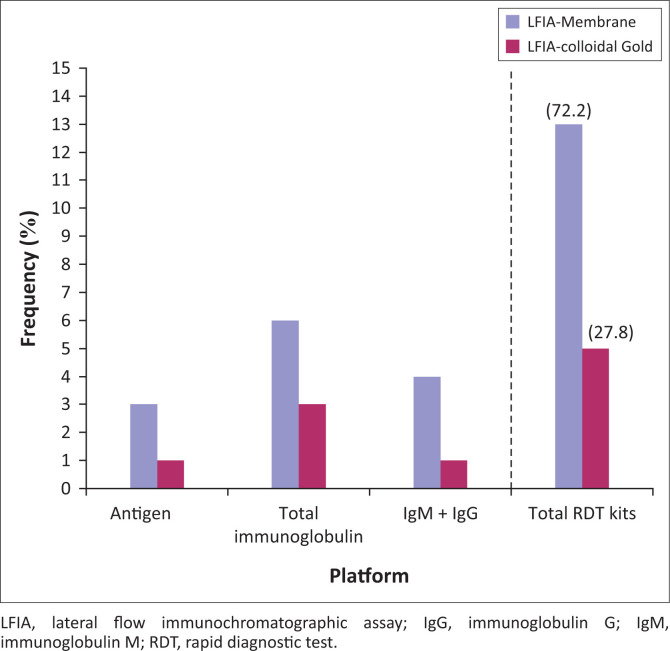
Overall, 28 publications on coronavirus-based diagnostic kits that matched the goal of this publication were included in this study (Figure 2). Articles were excluded based on duplication and lack of information on detection principle, type of kit, performance characteristics and manufacturers’ details. All eligible publications on COVID-19 diagnostic kits by country and performance as of 22 April 2020 were summarised in numbers and percentages using descriptive analysis. On the whole, a total of 18 serological RDT kits were included for analysis. Of these, four were antigen RDTs (22.2%), nine were total immunoglobulin RDTs (50%) and five were IgM/IgG serological RDTs (27.8%) (Figure 3). These kits were produced in eight countries, namely China (6; 33.33%), the United States (4; 22.22%), Germany (2; 11.11%), Singapore (2; 11.11%) and Kenya, Canada, Korea and Belgium (1 each; 6.56%) (Table 1). Fourteen of the RDT kits are antibody-detection kits for use with blood, plasma or serum (77.8%), and four were antigen-detection kits for use with swab, sputum or blood (22.2%). The majority of these kits (13; 72.22%) use lateral flow membrane technology, whereas the remaining five (27.78%) use colloidal gold (Figure 4).
FIGURE 2.
PRISMA flow diagram showing the scoping review process.
FIGURE 3.
Distribution of the serological rapid diagnostic tests by testing principle.
TABLE 1.
Performance characteristics of newly developed severe acute respiratory syndrome coronavirus rapid diagnostic kits analysed in this review, 22 April 2020.
| Serial no. | Name of kit | Manufacturer | Performance characteristics | Methods and specimen | Recorded comparison to real-time polymerase chain reaction | Time | References |
|---|---|---|---|---|---|---|---|
| Antigen detection based rapid diagnostic testing kits | |||||||
| 1. | Covid-19 Ag Resp-Strip | Coris BioConcept Belgium | Spe: 100.0% Sen: 96.0% PPV: 100.0% NPV: 96.2% |
LFIA membrane based – Nasopharyngeal | - | 15 min | 34 |
| 2. | COVID 19 Rapid test kit | KEMRI, Research Institute, Kenya | Not available | LFIA membrane based – Swab | Shorter test time | 15 min | 35 |
| 3. | Camtech Novel Coronavirus (COVID-19) Antigen Test | Camtech Diagnostics Pte Ltd, Singapore | Not available | Lateral Flow Colloidal Gold Immunochromatographic – blood/sputum | Include the use of reader (POCT device) | 30 min | 36 |
| 4. | Standard TM Q COVID-19 Ag Test |
SD Biosensor, Inc, Republic of Korea | Spe: 97.7% Sen: 84.4% |
LFIA membrane based – Nasopharyngeal | Cross reaction with SARS coronavirus and some chemicals | 30 min | 37 |
| Antibody based (total immunoglobulin) rapid diagnostic testing kits | |||||||
| 5. | Bodysphere IgM/IgG Rapid test |
Bodysphere Los Angeles, US | Spe: 91.0% Sen: 99.0% |
LFIA membrane based (IgG & IgM) – Blood, Plasma and Serum | Shorter test time | 2 min | 38 |
| 6. | COVID-19 total antibody test Assay | Ortho Clinical Diagnostics, Raritan, New Jersey, US | Not available | LFIA membrane based – blood | For use in immunodiagnostic & integrated systems | Not stated | 39 |
| 7. | SARS-CoV-2 rapid IgG-IgM antibody test kit | Jiangsu Medomics Medical Technologies, China | Spe: 90.6%, Sen: 88.7% |
LFIA membrane based (IgM & IgM) – blood | Ease of use and POCT with no additional device | 15 min | 40 |
| 8. | SARS-CoV-2 rapid IgG-IgM combined antibody test kit | Euroimmun Medical Laboratory Diagnostics & Epitope Diagnostics, Germany | Non available | LFIA membrane based (IgM & IgG) – blood | Not stated | Not stated | 41 |
| 9. | SARS-CoV-2 rapid IgG-IgM antibody test kit | Cold Spring Habour Laboratory, YHLO Biotech, Shenzhen, China | Spe: 90.6% Sen: 88.6 % |
LFIA for combined immunoglobulin (IgM & IgG) – blood | Shorter test time | 15 min | 42 |
| 10. | One Step Novel Coronavirus (COVID-19) IgM/IgG | Artron Laboratories Inc, Canada | Spe: 97.7% Sen: 93.4% |
LFIA membrane based (IgM & IgG) – blood | One step simple and easy to use cassette devises | Not stated | 43 |
| 11. | VivaDiag COVID-19 IgM-IgG VivaChek | VivaChek Biotech (Hangzhou) Co., Ltd, China | Spe: 91.7% Sen: 18. 4%, NPV: 26.3% PPV: 87.5% |
LFIA Colloidal Gold based IgM & IgG – serum or whole blood | Poorer Sen & NPV despite comparable Spe & PPV | 15 min | 20 |
| 12. | Colloidal Gold Immunochromatographic Assay combined (GICA) | Zhu Hai Liv Zon Diagnostics Inc, China | Spe: 100.0% Sen: 82.4% |
Lateral Flow Colloidal Gold Immunochromatographic based – blood | Shorter test time | 15 min | 44 |
| 13. | ThermoGenesis’ Rapid COVID-19 Serological Test Kit | ThermoGenesis Holdings, Inc. Wendy Samford, US | Spe: 100.0% Sen: 99.1% |
Lateral Flow Colloidal Gold Immunochromatographic –blood/ serum/ plasma | PCR-positive and negative patient blood samples indicate high reliability | 5 min | 45 |
| Antibody based (IgG) or/and (IgM) separated rapid diagnostic testing kit | |||||||
| 14. | All TestR 2019-nCOV IgG/IgM Rapid Test Cassette | Hangzhou AllTest Biotech Co. Ltd, China | IgG Spe: 98.0% Sen: 100.0% IgM Spe: 96.0% Sen: 85.0% |
LFIA membrane based (IgG/IgM) antibodies – serum, plasma and whole blood | Hematocrit level needs to be within 25–65%, cross reactivity with other virus & interferences with other substances | 10 min | 46 |
| 15. | BioMedomics COVID-19 test kit | Becton Dickinson New Jersey, US | Not available (Ongoing) | LFIA membrane based (IgM/IgG) – blood | Shorter test time | 15 min | 47 |
| 16. | Camtech COVID-19 IgM/IgG Cassette | Camtech Diagnostics Pte Ltd, Singapore | Reported as fast and simple but details not available | LFIA membrane based (IgM/IgG) – blood | Humidity affects the stability of the kit | 10 min | 36 |
| 17. | NADALR COVID-19 IgG/IgM Test Cassette | Nal von minden GmbH, Germany | IgG Spec: 99.0% Sen: 98.0% IgM Spec: 99.0% Sen: 94.0% |
LFIA membrane based (IgG/IgM) – whole blood, plasma or serum | Affected by temperature and cross-reaction with other viruses and interference with several chemicals. | 10 min | 48 |
| 18. | Colloidal Gold Immunochromatographic Assay (GICA) | Zhu Hai Liv Zon Diagnostics Inc, China | IgG Spe: 100.0% Sen: 81.3% IgM: Spec: 100.0% Sen: 57.1% |
Lateral flow Colloidal Gold Immunochromatographic based antigen – antibody in blood | Shorter test time | 15 min | 44 |
Spe, specificity; Sen, sensitivity; NPV, negative predictive value; PPV, positive predictive value; LFIA, lateral flow immunochromatographic assay; SARS, severe acute respiratory syndrome; CoV, coronavirus; POCT, point of care testing; IgG, immunoglobulin G; IgM, immunoglobulin M; US, United States.
FIGURE 4.
Distribution of the serological rapid diagnostic tests by testing platform.
In general, the sensitivity of the test kits irrespective of sample specification ranged from 18.4% to 100% and their specificity ranged from 90.6% to 100%. The pooled analysis revealed an average (range) sensitivity of 81.6% (72.9% – 88%) and specificity of 94.4% (88.2% – 97.5%). The sensitivity and specificity of lateral flow immunoassay membrane type RDT kits were in the range (average) of 84.4% – 100% (92.7%) and 90.6% – 100% (96%), respectively, and that of lateral flow immunoassay colloidal gold type were 18.4 – 99.1% (67.7%) and 91.7% – 100% (98.3%), respectively (Figure 4). Three of these kits, namely Bodysphere Rapid Test (Los Angeles, California, United States), Thermogenesis Rapid COVID-19 Test kit (Rancho Cordova, California, United States) and NADAL® COVID-19 Test kit (Regensburg, Germany), had a sensitivity of 99% – 100%. These three kits also had a specificity range of 91% – 100%. Asides their better sensitivity and specificity compared to other RDTs, these kits are for use with blood samples only, detect both IgG and IgM, and have shorter testing time of 2 – 10 min. The testing time for all the identified kits ranged from 2 to 30 min with an average testing time of 13.5 min (95% confidence interval = 10.8 min – 16.1 min). Only two of the kits provided information on positive predicted value and negative predictive value (range = 87.5% – 100.0% to 26.2% – 96.2%).
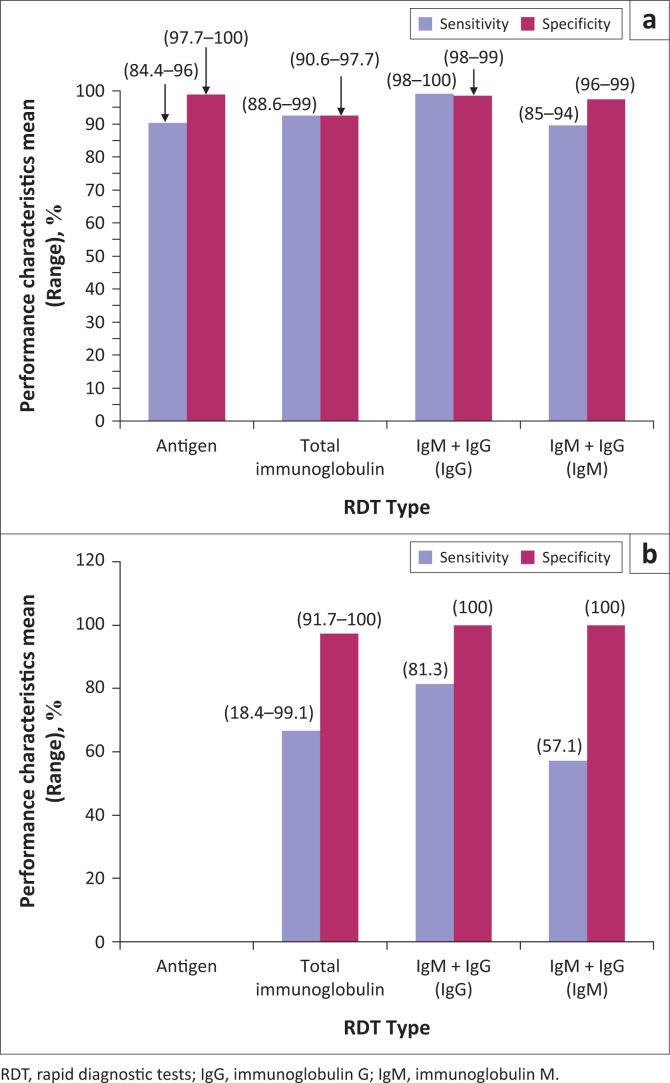
Out of the 18 RDTs identified, 6 (33%) were not subjected to performance validation by the manufacturers of the kits. Two of four antigen detection kits, seven of nine total immunoglobulin and three of five IgM + IgG serological kits were validated for sensitivity and specificity using RT-PCR assay as the reference method (Table 1).15 On the whole, eight of the 13 lateral flow immunoassay membrane type and four of the five lateral flow immunoassay colloidal gold type kits were validated. Of the 12 serological RDTs validated by RT-PCR, the IgM/IgG duo kit with non-colloidal gold labelling system was found to elicit the highest and acceptable sensitivity (98% – 100%) and specificity (98% – 99%) values for IgG and specificity of 96% – 99% for IgM compared to other RDT types and the counterpart colloidal gold system-based IgM/IgG duo kit (Figure 5).
FIGURE 5.
Performance characteristics of the different serological rapid diagnostic tests by testing platform; LFIA membrane (a) and colloidal gold devices (b).
Implications and recommendations
The need to expand diagnostic testing in order to cope with the current spread of COVID-19 infection in many settings in LMICs where resources for RT-PCR are limited and difficult to sustain has made RDT kits for SARS-CoV-2 an important tool in the global fight against the COVID-19 pandemic. For patients with suspected infection, RT-PCR is used to detect SARS-CoV-2 in sputum, throat and nasopharyngeal swab, and secretions of the lower respiratory tract samples such as bronchoalveolar lavage and bronchial washings.16,17,18 However, limited facilities and human resources for molecular testing using RT-PCR tends to slow down testing for COVID-19 in resource-limited countries. It has been argued that RDTs do not have sufficient evidence to support their use in the COVID-19 pandemic and hence should be used only in a research setting.19 Cassaniti et al. have earlier reported low sensitivity and specificity of serological assay which led to misdiagnosis of COVID-19 in the vast majority of the patients in their study population.20 The WHO has emphasised that tests with inadequate quality may miss patients with active infection or falsely categorise patients as having the disease, further hampering disease control efforts, hence the need for questioning the performance of SARS-CoV-2 RDT kits.19,21 Most manufacturers of the RDTs have performance characteristics of the kits validated using the RT-PCR technique as the reference method. However, several publications have reported the possibility of false-negative results using RT-PCR.22 Thus, the sensitivity and specificity data of reviewed kits should be understood in light of this bias.
The declaration of COVID-19 as a global pandemic and the huge concern of its transmission in LMICs where HIV, tuberculosis and malaria are currently endemic have necessitated the need to scale up diagnostic testing to mitigate further spread and the rising number of COVID-19 deaths outside China.23,24 In many settings in LMICs, such as small communities, riverine areas, health posts and primary health centres, resources for RT-PCR are absent.23,24 This has made the development of serological RDTs for the detection of specific SARS-CoV-2 antigens, anti-SARS-CoV-2 IgM and anti-SARS-CoV-2 IgG an attractive and very important tool in the global fight against the COVID-19 pandemic in LMICs. Findings from the 18 serological RDT kits analysed in this review imply that three different types of serological RDTs, antigen, total immunoglobulin, and combined IgM and IgG-based RDT with the ability to provide results between 2 min and 30 min are currently available for potential large-scale testing in LMICs using five types of biological samples (nasopharyngeal swab, throat swab, whole blood, plasma and serum). Due to challenges associated with more sensitive biological samples such as bronchoalveolar lavage and sputum, both nasopharyngeal and throat swabs are used for COVID-19 testing by RT-PCR in many settings.7,8,9 Also, whole blood, plasma or serum is often used as biological sample for RT-PCR for monitoring viremia to predict COVID-19 severity during the acute stage of infection and viral clearance during the convalescent stage.7,8 The latter is currently used to inform hospital discharge decisions in many countries; use of different samples for diagnosis and viral clearance determination can negatively impact on discharge decision-making.8,9,10,12 A potential way of circumventing discharge decision errors is to employ a diagnostic tool that uses the same type of sample for both diagnosis and viraemia monitoring such as the SARS-CoV-2 antigen and specific anti-SARS-CoV-2 IgM/IgG duo detection kit identified in this review. This can be integrated into the local COVID-19 management guidelines in LMICs. This guideline is currently being used in Malaysia and Europe.25,26 The primary weakness of RT-PCR for COVID-19 diagnosis lies in its inability to detect infection using nasopharyngeal samples collected outside the viral RNA shedding period. The shedding period is characterised by presence of low viral RNA, such as seen in asymptomatic, pre-symptom days (~2 days prior to symptom onset) and post-infection days (~14 post infection onset).26,27 Also, the RT-PCR, may also miss infections due to poor sample collection and preparation as well as poor storage of isolated RNA. These weaknesses can be addressed by serological RDTs, which detect the more stable viral immunogenic proteins such as the S and N proteins, which persist more than RNA or anti-SARS-CoV-2 IgM and IgG which have been reported to peak between 2 and 3 weeks and 17 days post infection onset.26,27 Guo et al.28 reported an improvement of COVID-19 identification by RT-PCR from 51.9% to 98.6% with the integration of an IgM-based immunoassay. However, the results of sensitivity (18.4% – 100%) and specificity (90.6% – 100.0%) reported for 12 of the 18 reviewed serological RDT kits by their manufacturers imply that the currently available COVID-19 RDTs are not equally accurate and only a few of them pass the sensitivity and specificity benchmark of 95%. Zainol et al.29 recently reported a sensitivity range of 72.7% – 100.0% and specificity range of 98.7% – 100.0% for IgM/IgG duo-based serological RDT kits for COVID-19 in their review in which nine serological kits were analysed. The authors also reported a sensitivity range of 86.4% – 90.6% and a specificity of 99% for total immunoglobulin-based RDTs. In Brazil, Castro et al.30 reported a mean (range) anti-SARS-CoV-2 IgM sensitivity of 82% (76% – 87%) and specificity of 97% (96% – 98%) and anti-SARS-CoV-2 IgG sensitivity of 97% (90% – 99%) and specificity of 98% (97% – 99%). Although in this review, only 5 of the 18 serological RDT kits offered combined IgM and IgG detection, we also found a better performance characteristic for this type of RDT kit compared to the antigen and total immunoglobulin kits using non-colloidal gold labelling system with acceptable sensitivity (98% – 100%) and a specificity (98% – 99%) values for IgG and specificity of 96% – 99% for IgM, suggesting the ability of these kits to detect past infections, confirm true negative results and rule out false positive COVID-19 testing results by RT-PCR. However, the performance of these kits to confirm recent infections seems to be below the benchmark of 95%, since they had a sensitivity range of 85% – 94%, which was even lower for colloidal gold labelling systems at 57.1%. Meanwhile, the improvement offered by the antigen-based RDT kits in this review can be said to be none or marginal at 84.4% – 96%. Another implication of these findings is that more than one serological RDT kit may be needed for a SARS-CoV-2 detection algorithm to improve confirmation and diagnosis of COVID-19 by RT-PCR, if deployed in LMICs. It is also important to note that 6 of the 18 reviewed serological RDT kits lacked reports on sensitivity and specificity, thus the accuracy in diagnosising COVID-19 is unknown as at the time of this review. This finding further reiterates the difficulty associated with SARS-CoV-2 serological RDT kit validation by manufacturers, since RT-PCR the reference method targets viral RNA instead of specific SARS-CoV-2 antibodies or antigens. A similar opinion has been shared by Castrol et al.,30 given the well-documented differences in the kinetics of the viral RNA (even between samples) and anti-SARS-CoV-2 antibodies in infected individuals.
As of 01 April 2020, the death toll for COVID-19 was over a million globally and the need for accurate intervention to stop transmission and re-infection of COVID-19 is now extremely necessary. The WHO advises countries to improve the rate of testing to identify an infected individual for appropriate isolation and treatment. The availability of efficient and rapid diagnostics for COVID-19 has been indicated as one of the mitigation strategies to control the pandemic. Rapid diagnostic tests are cheaper and more readily available; thus, they might be more useful stopping transmission by rapidly identifying positive and previous cases particularly in LMICs. These data will in turn be useful for both disease diagnosis and surveillance. The RDT will either detect the presence of viral proteins (antigens) expressed by the COVID-19 virus or the presence of antibodies in the blood of COVID-19-infected people.31,32 The performance of the kits has been shown to depend on several factors such as the onset of illness, the viral load in the specimen, the integrity of the specimen collected from suspected cases, processing, age, nutritional status, the severity of the disease, and certain medications or underlying disease condition, especially immune suppression diseases and the precise formulation of the reagents in the test kits.19
The LMICs reported the lowest rate of testing per population with corresponding lower numbers of cases compared with developed countries. This may be an indication of limited testing resources and facilities due to the challenges associated with RT-PCR. Therefore, there may be several cases in this population that are not detected with antecedent clinical implications. The use of RDTs will not only help to detect currently infected or previously exposed individuals who have developed immunity as well as identify asymptomatic carriers. These will inform decisions for public health measures, for example, cases among a more IgM-positive population may be an indication of a subclinical outbreak. The economic impact of movement restrictions and lockdowns in many of these countries is not well managed, adding unimaginable suffering in an already impoverished population. The use of RDTs for the screening of COVID-19 may help to determine individuals who are at lower risk and may be permitted to go back to work. When coupled with clinical symptoms and molecular testing, RDTs may serve as a first-line tool for diagnosis and help to better understand the spread of diseases.
Although the COVID-19 test kit market is in its infancy, the global COVID-19 outbreak and up-surging cases are driving the demand for RDTs, hence researchers throughout the world are striving to develop RDTs to track infected people. To date, very few countries have succeeded in developing SARS-CoV-2 testing kits, while some are still working on improving the performance of their products. With the dedicated global efforts on preventing the spread of COVID-19 and flattening the curve, significant improvement must have occurred in improving the performance of COVID-19 test kits. Increasing accessibility to testing among other interventions has improved the containment and transmission of the infection. While algorithms have been developed to limit testing to individuals that fulfil certain criteria, such as contact with the infected patients, clinical symptoms of COVID-19, travelling history to epidemic countries, etc., testing the entire population has been recommended.33 Resource limitations means most LMICs can not cope with the up-surge of infection and transmission. While testing per population is high in developed countries with over 3 million tested in the United States, testing per population is still very low in developing countries with less than 10 000 tested in Nigeria as of 22 April 2020. Therefore, the addition of validated serological-based RDT even with lower performance characteristics compared to RT-PCR may serve as complementary tools to increase the rate of testing per population, especially in LMICs where community transmission is now on the rise. Therefore, the use of RDTs operated as lateral flow immunochromatographic assays to detect both IgM and IgG on separate test lines using whole blood, plasma or serum samples is desirable for LMICs. Wang et al. reported that the combination of RT-PCR testing and clinical features for diagnosis of COVID-19 facilitated the management of the SARS-CoV-2 outbreak in China,22 and COVID-19 mass testing facilities have been strongly advocated to end the epidemic rapidly.34 The use of RDT will not only allow mass testing facilities in LMICs but coupled with clinical features in symptomatic patients and molecular testing (RT-PCR) in asymptomatic populations may help to contain transmission in LMICs.
Conclusion
Considering the peculiarity of LMICs, especially their economic situation, the standard RT-PCR may not be able to cope with the testing needs of these countries because of limited infrastructure and human resources. Generally, it is agreed that rapid testing techniques are useful for screening for early detection of symptomatic cases, which is crucial for averting community or hospital transmission and strengthening contact tracing and active surveillance. This review revealed considerable good performance of the RDT with manufacturer sensitivity and specificity using varieties of samples including blood samples. Hence, the use of RDT kits in LMICs may increase access to testing and better triaging of COVID-19 patients. We, however, identified that most of the proposed rapid kits have not been optimised and validated. It is important that the kits undergo further validation with samples from countries of proposed use in reference to RT-PCR before use.
Acknowledgements
Competing interests
The authors have declared that no competing interest exists.
Authors’ contributions
O.A. conceived the idea for the study. O.A., B.I., and A.O. designed the study. A.O., T.A.S., O.M.A. and O.P. searched for published work. O.A. and B.I. reviewed and made the selection of eligible studies. A.O., T.A.S., O.M.A. and O.P. extracted and compiled the data. O.A., B.I. and A.O. analysed the data while B.I. and A.O. prepared the first draft of the article. O.A. did the final editing of the article. All authors contributed to the writing of the article and have seen and approved the final version.
Ethical considerations
Ethical clearance was not required for this study.
Funding information
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
Footnotes
How to cite this article: Olalekan A, Iwalokun B, Akinloye OM, et al. COVID-19 rapid diagnostic test could contain transmission in low- and middle-income countries. Afr J Lab Med. 2020;9(1), a1255. https://doi.org/10.4102/ajlm.v9i1.1255
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nkengasong JN, Mankoula W. Looming threat of COVID-19 infection in Africa: Act collectively, and fast. Lancet (London, England). 2020;395(10227):841–842. 10.1016/S0140-6736(20)30464-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khadka S, Hashmi FK, Usman M. Preventing COVID-19 in low- and middle-income countries. Drugs Ther Perspect. 2020:1–3. 10.1007/s40267-020-00728-8 [DOI] [PMC free article] [PubMed]
- 5.Wang X, Yao H, Xu X, et al. Limits of detection of six approved RT-PCR kits for the novel SARS-coronavirus-2 (SARS-CoV-2). Clin Chem. 2020;66(7):977–979. 10.1093/clinchem/hvaa099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan JF, Yip CC, To KK, et al. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J Clin Microbiol. 2020;58(5):e00310–e00320. 10.1128/JCM.00310-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loeffelholz MJ, Tang Y-W. Laboratory diagnosis of emerging human coronavirus infections – The state of the art. Emerg Microb Infect. 2020;9(1):747–756. 10.1080/22221751.2020.1745095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, et al. False-negative results of initial RT-PCR assays for COVID-19: A systematic review. medRxiv. 2020:2020.04.16.20066787. 10.1101/2020.04 [DOI] [PMC free article] [PubMed]
- 9.Younes N, Al-Sadeq DW, Al-Jighefee H, et al. Challenges in laboratory diagnosis of the novel coronavirus SARS-CoV-2. Viruses. 2020;12(6):582 10.3390/v12060582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong L, Chuan J, Gong B, et al. Detection of serum IgM and IgG for COVID-19 diagnosis. Sci China Life Sci. 2020;63(5):777–780. 10.1007/s11427-020-1688-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haveri A, Smura T, Kuivanen S, et al. Serological and molecular findings during SARS-CoV-2 infection: The first case study in Finland, January to February 2020. Euro Surveill. 2020;25(11):2000266 10.2807/1560-7917.ES.2020.25.11.2000266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel R, Babady E, Theel ES, et al. Report from the American Society for Microbiology COVID-19 international summit, 23 March 2020: Value of diagnostic testing for SARS-CoV-2/COVID-19. mBio. 2020;11(2):e00722–20. 10.1128/mBio.00722-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arksey H, O’Malley L. Scoping studies: Towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 14.Adhikari SP, Meng S, Wu YJ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: A scoping review. Infect Dis Poverty. 2020;9(1):020–00646. 10.1186/s40249-020-00646-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tahamtan A, Ardebili A. Real-time RT-PCR in COVID-19 detection: Issues affecting the results. Expert Rev Mol Diagn. 2020;20(5):453–454. 10.1080/14737159.2020.1757437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020;ciaa344 https://doi.org/101093/cid/ciaa344 [DOI] [PMC free article] [PubMed]
- 17.Okba NMA, Muller MA, Li W, et al. SARS-CoV-2 specific antibody responses in COVID-19 patients. medRxiv. 2020:2020.03.18.20038059. [Google Scholar]
- 18.Gorse GJ, Donovan MM, Patel GB. Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in identifying coronavirus-associated illnesses. J Med Virol. 2020;92(5):512–517. 10.1002/jmv.25715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO Advice on the use of point-of-care immunodiagnostic tests for COVID-19 [homepage on the Internet]. Scientific Brief 8 April 2020 [cited 2020 Apr 08]. Available from: WHO/2019-nCoV/Sci_Brief/POC_immunodiagnostics/20201
- 20.Cassaniti I, Novazzi F, Giardina F, et al. Performance of VivaDiag COVID-19 IgM/IgG Rapid Test is inadequate for diagnosis of COVID-19 in acute patients referring to emergency room department. J Med Virol. 2020. March 30 10.1002/jmv.25800 [DOI] [PMC free article] [PubMed]
- 21.WHO Coronavirus disease (COVID-19) technical guidance: Laboratory testing for 2019-nCoV in humans [homepage on the Internet]. [cited 2020 Apr 08]. Available from: www.who.int; [cited 2020 Apr 06].
- 22.Wang Y, Kang H, Liu X, Tong Z. Combination of RT-qPCR testing and clinical features for diagnosis of COVID-19 facilitates management of SARS-CoV-2 outbreak. J Med Virol. 2020;92(6):538–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stackelberg O, Esmaeilzadeh M, Olsen B, Lundkvist Å. [Rapid point-of-care serology testing for sars-cov-2]. Lakartidningen, Stockholm. 2020;117. [PubMed] [Google Scholar]
- 24.Malaysia MoH Guidelines on COVID-19 management in Malaysia. 5th ed. The Ministry of Health Malaysia: Putrajaya; 2020. [Google Scholar]
- 25.Control ECfDPa. Novel coronavirus (SARS-CoV-2) discharge criteria for confirmed COVID-19 cases – When is it safe to discharge COVID-19 cases from the hospital or end home isolation? [homepage on the Internet]. [cited 2020 Apr 10]. Available from: https://www.ecdceuropaeu/sites/default/files/documents/COVID-19-Discharge-criteria.pdf
- 26.Chan PK, Ng KC, Chan RC, et al. Immunofluorescence assay for serologic diagnosis of SARS. Emerg Infect Dis. 2004;10(3):530–532. 10.3201/eid1003.030493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CY, Lin RTP, Renia L, Ng LFP. Serological approaches for COVID-19: Epidemiologic perspective on surveillance and control. Front Immunol. 2020;11:879 10.3389/fimmu.2020.00879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis. 2020;71(15):778–785. 10.1093/cid/ciaa310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zainol Rashid Z, Othman SN, Abdul Samat MN, Ali UK, Wong KK. Diagnostic performance of COVID-19 serology assays. Malaysian J Pathol. 2020;42(1):13–21. [PubMed] [Google Scholar]
- 30.Castro R, Luz PM, Wakimoto MD, Veloso VG, Grinsztejn B, Perazzo H. COVID-19: A meta-analysis of diagnostic test accuracy of commercial assays registered in Brazil. Braz J Infect Dis. 2020;24(2):180–187. 10.1016/j.bjid.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang P, Gao Q, Wang T, et al. Evaluation of recombinant nucleocapsid and spike proteins for serological diagnosis of novel coronavirus disease 2019 (COVID-19). medRxiv. 2020:2020.03.17.20036954. 10.1101/2020.03.17.20036954 [DOI]
- 32.Liu Y, Liu Y, Diao B, et al. Diagnostic indexes of a rapid IgG/IgM combined antibody test for SARS-CoV-2. medRxiv. 2020:2020.03.26.20044883. 10.1101/2020.03.26.20044883 [DOI]
- 33.Julian P. Covid-19 mass testing facilities could end the epidemic rapidly. BMJ. 2020;368:m1163 10.1136/bmj.m1163 [DOI] [PubMed] [Google Scholar]
- 34.Bioconcept C. Covid-19 Ag resp-strip [homepage on the Internet]. 2020. [cited 2020 Apr 29]. Available from: https://www.corisbiocom/Products/Human-Field/Covid-19.php
- 35.(KEMRI) KMRI Covid-19 results in 15 minutes: KEMRI starts manufacturing rapid test kits, Kenya Medical Research Institute (KEMRI) has started manufacturing Covid-19 rapid test kits to ease the testing burden at the State’s facilities [homepage on the Internet]. 2020. [cited 2020 Apr 07]. Available from: www.standardmediacoke/article/2001367222/kemri-starts-manufacturing-covid-19-rapid-test-kits
- 36.Cassette CC-II. Singapore’s Camtech, JN Medsys to increase production of COVID-19 test kits. 2020. [homepage on the Internet]. [cited 2020 Apr 16]. Available from: https://www.mobihealthnewscom/news/asia-pacific/singapore-s-camtech-jn-medsys-increase-production-covid-19-test-kits
- 37.Biosensor S. Standard Q COVID-19 Ag [homepage on the Internet]. 2020. [cited 2020 Apr 10]. Available from: http://www.sdbiosensorcom/xe/product/7672
- 38.EUA BEua Bodysphere touts 2-minute COVID-19 test [homepage on the Internet]. 2020. [cited 2020 Apr 02] Available from: https://www.massdevicecom/fda-clears-bodysphere-2-minute-covid-19-test/
- 39.Diagnostics OC. Ortho clinical diagnostics pick up EUA for COVID-19 total antibody assay [homepage on the Internet]. 2020. [cited 2020 Apr 15]. Available from: www.middionlinecom/ortho-clinical-diagnosics-picks-eva-covid-19-total-antibody-assay
- 40.Li Z, Yi Y, Luo X, et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020. 10.1002/jmv.25727 [DOI] [PMC free article] [PubMed]
- 41.Fellmann F. Jetzt beginnt die Suche nach den Genesenen. Tages Anzeiger. Euroimmun Medical Laboratory Diagnostics and Epitope Diagnostics; Germany: Lubeck; 2020. [Google Scholar]
- 42.Liu R, Liu X, Han H, et al. The comparative superiority of IgM-IgG antibody test to real-time reverse transcriptase PCR detection for SARS-CoV-2 infection diagnosis. preprints from medRxiv and bioRxiv. 2020. 10.1101/2020.03.28.20045765 [DOI]
- 43.Artronlab One step novel coronavirus (COVID-19) IgM/IgG antibody test kit [homepage on the Internet]. 2020. Available from: http://www.artronlabcom/products/CoVBrochure-ver2.pdf
- 44.Xiang J, Yan M, Li H, et al. Evaluation of enzyme-linked immunoassay and colloidal gold- immunochromatographic assay kit for detection of novel coronavirus (SARS-Cov-2) causing an outbreak of pneumonia (COVID-19). Pre print medRxiv preprint https://doi.org/101101/202002272002878
- 45.Thermogenesis A rapid covid-19 IgM/IgG serological test for point-of-care [homepage on the Internet]. [cited 2020 Apr 09]. Available from: https://www.thermogenesiscom/rapid-covid-19-point-of-care-diagnostic-test/
- 46.Cassette AT-nIIRT 2019-nCoV IgG/IgM rapid test cassette [homepge on the Internet]. 2020. [cited 2020 Feb 18]. Available from: https://www.assaygeniecom/content/Assay%20Genie%20Rapid%20COVID%20POC%20test.pdf
- 47.BD BTw BioMedomics teams with BD to launch rapid COVID-19 test [homepage on the Internet]. [cited 2020 Apr 06]. Available from: https://www.ncbiotechorg/news/biomedomics-teams-bd-launch-rapid-covid-19-test
- 48.kit TbTC- NADALR COVID-19 IgG/IgM test instruction manure [homepage on the Internet]. 2020. [cited 2020 Apr 20]. Available from: https://www.dailymailcouk/news/article-8128327/Test-test-Covid-kits-10-minute-finger-prick-tests-mask-diagnose-instantly.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.