Abstract
Introduction:
Leprosy in pediatric population continues to remain as one of the major public health problems in India. BCG vaccination has been implicated in producing some protection against leprosy.
Objectives:
The present study intended to find out the patterns of leprosy in the pediatric age group (<12 years) and to determine the proportion of paucibacillary (PB) and multibacillary (MB) leprosy cases among the BCG-vaccinated and nonvaccinated groups.
Methodology:
It is a cross-sectional study among patients with leprosy up to the age of 12 years attending a tertiary care hospital in Eastern India by comprehensive history taking and through clinical examination. The history of BCG vaccination was enquired and the BCG scar was looked for to determine the BCG-vaccinated and nonvaccinated group. After collecting the data in a predesigned case sheet, the data were analyzed.
Result:
Of the 137 patients included in this study, 71.53% belonged to the 11–12 years age, 27.74% the 5–10 years age, and 1 patient the <5 year age group. The sex ratio was 1:1.63. PB leprosy was more common than MB leprosy. Smear positivity, deformity, and reaction were not very frequent. Among the vaccinated patients, the ratio of PB and MB leprosy was 5.3:1, while in the nonvaccinated group the ratio was 1.2:1. Data analysis showed that the proportion of MB leprosy was statistically significant in the nonvaccinated group than in the vaccinated group (p = 0.0352).
Conclusion:
Our study pointed that BCG vaccination might have a role in enhancing the cell-mediated immunity (CMI).
Keywords: BCG vaccine, cell-mediated immunity, childhood, leprosy
Introduction
Leprosy is a chronic granulomatous disease caused by Mycobacterium leprae. It is essentially a disease of the peripheral nerves but can also affect the skin and other organs. This is one of the oldest diseases known to mankind. Leprosy, at this moment, remains a public health problem only in a few countries and India accounts for almost 80% of the cases of worldwide.[1] This disease can affect all age groups ranging from infancy to old age. Males are seen to be more frequently affected than the females.[2] Multidrug therapy (MDT) has treated the disease effectively and after the introduction of MDT, the prevalence of leprosy has decreased world-wide.[3] However, it still remains a major public health problem in India. Myriad manifestations can occur in this condition depending on the status of the cell-mediated immunity of the individual. Childhood leprosy cases is an important indicator of continued transmission of leprosy in the society and one of the important strategies of the World Health Organization is “to reduce transmission of the disease and reduction of grade-2 disability among new child cases.”[4] BCG vaccination has been implicated in producing some protection against leprosy.[5] The present study aimed to find the patterns of leprosy among the pediatric population and to determine the difference in the patterns of leprosy among the BCG-vaccinated and nonvaccinated groups.
Methodology
In this cross-sectional study, consecutive pediatric leprosy patients (up to age of 12 years), whose parents consented to take part in the study, attending the outpatient department of a tertiary care hospital in Eastern India from January 2005 to December 2015 were included. Ethical approval was obtained from the competent authority prior to the initiation of the study. Comprehensive history taking, thorough clinical examination, and necessary laboratory investigation like slit-skin smear examination were done. The pediatric leprosy patients were categorized according to the WHO classification into paucibacillary (PB) and multibacillary (MB).[6] During history taking, the parents were asked about the status of the BCG vaccination of the child and also examined for the BCG vaccination scar. Those who had no vaccination scar and no convincing history of vaccination were considered as BCG nonvaccinated patients. After collecting the data in a predesigned case data sheet, the data was analyzed by using the MecCalc software version 10.2.0.0≵ by Acacialaan, B-8400, Ostend, Belgium. Chi square test was used as a test of significance.
Results
Of the 137 patients included in this study, 98 patients (71.53%) belonged to the 11–12 years age group, 38 (27.74%) to the 5–10 years age group, and 1 (0.03%) to the <5 year age group. Eighty five patients (62.04%) were male with a female to male ratio 1:1.63. In the study group, 97 patients (70.8%) were Hindus and the rest were Muslims. Most of the patients were from rural areas (78.83%), 22 patients (16.05%) were slum dwellers, and only 17 patients (12.4%) belonged to the above poverty line.
The single patient in the <5 age group had PB leprosy, [Figure 1] that was more frequent among the 5–10 years age group (71.05%) than the 11–12 years age group (59.14%). On the contrary, MB leprosy [Figure 2] was frequent in the 11–12 years age group (40.81%) than in the 5–10 years age group (28.94%) [Table 1].
Figure 1.
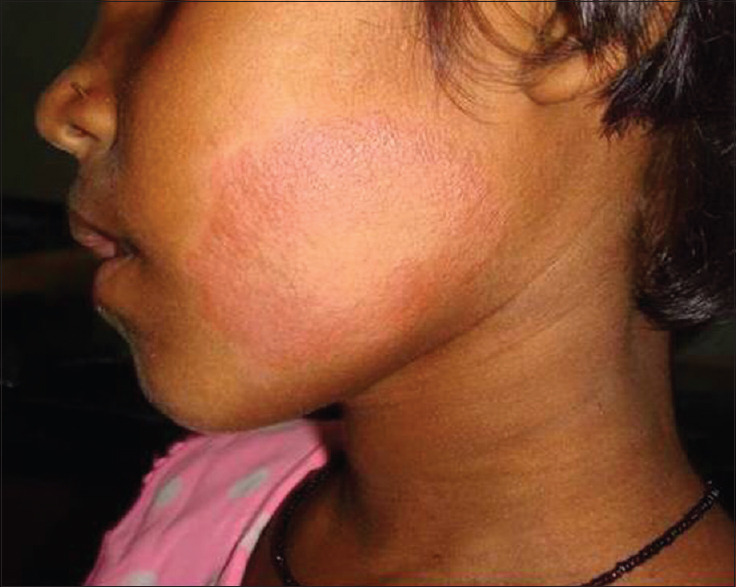
PB leprosy in a 7 year old girl
Figure 2.
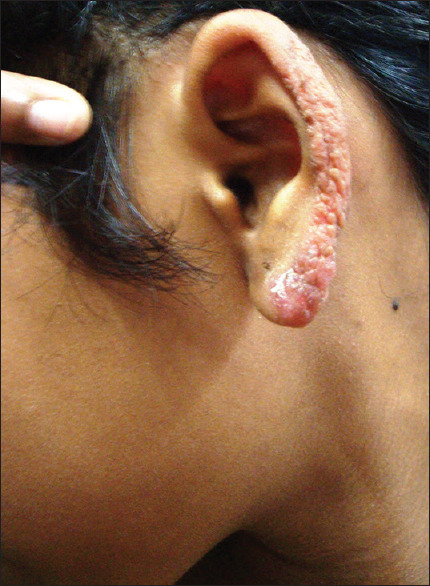
MB leprosy in a 11 yr old girl
Table 1.
Proportion of PB Leprosy and MB Leprosy in pediatric population
| Paucibacillary leprosy (%) | Multibacillary leprosy (%) | |
|---|---|---|
| >5 years age (n=1) | 1 (100%) | - |
| 5-10 years (n=38) | 71.05 | 28.94 |
| 11-12 years (n=98) | 59.14 | 40.81 |
We observed that in the study population, 14.6% patients were positive for slit skin smear. The signs of reaction were present in 13.41% cases and, among them, 15 patients showed type 1 reaction and only two patients showed type 2 reaction. Deformities were found in 20 patients (14.6%). Among the deformities, claw hand [Figure 3] was the commonest (70%; 14 patients out of 20) followed by foot drop (10%), lagophthalmos (10%), and facial palsy (10%).
Figure 3.
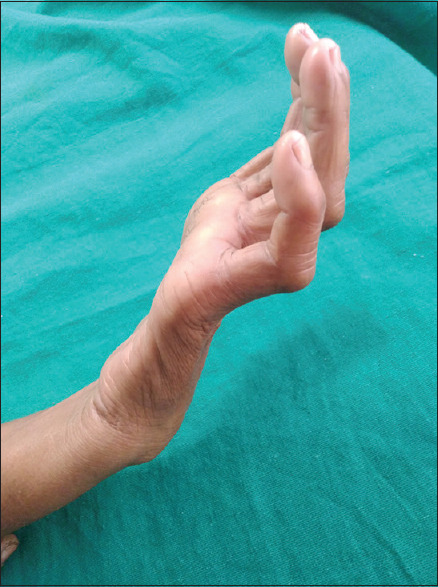
Claw hand in a 11 yr old child
Ninety nine patients (72.26%) were not vaccinated with BCG in the study group. Among the different age groups, 21 patients (55.26%) in the 5–10 years age group and 78 patients (79.51%)) in the 11–12 years age group were not vaccinated with BCG. The comparison of PB and MB leprosy among the vaccinated and nonvaccinated groups showed that in the vaccinated group the ratio of PB and MB leprosy was 5.3:1 (PB leprosy 32 patients and MB leprosy 6 patients) while in the nonvaccinated group the ratio of PB and MB leprosy was 1.2:1 (PB 54 patient and MB 45 patients) [Figure 4]. Statistical analysis showed that the proportion of MB leprosy was significantly higher in the nonvaccinated group than in the vaccinated group (p = 0.035).
Figure 4.
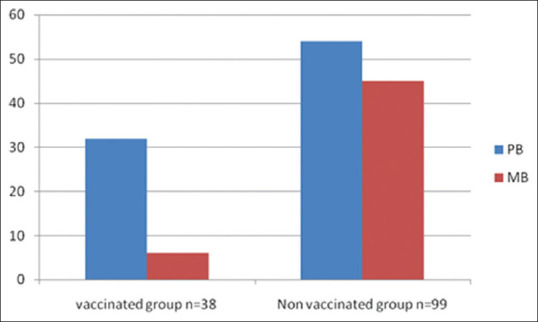
Comparison of PB and MB leprosy among BCG vaccinated and nonvaccinated group
Discussion
Childhood leprosy continues to remain as one of the major public health problems in India.[1] Childhood leprosy is important because this age group is more vulnerable to acquire the infection from household contacts than the adults, thus reflecting the active transmission of the disease in the community. This is corroborated at a global level by a study conducted in Brazil which showed that the transmission of leprosy in children less than 15 years of age occurred mainly through intradomiciliary contacts.[7] Monitoring childhood leprosy in terms of incidence and occurrence of deformities are crucial for better control and understanding of the disease transmission. Even in the era of wide acceptance of MDT, childhood leprosy is still a public health problem as has been shown in school surveys and a house-to-house search for new cases in the African country of Cameroon.[8] In this background, we carried out our study to find out the demographic profile of childhood leprosy in Eastern India. Our patients belonged to the 4–12 years age group which correlates with the previous studies.[1]
Higher occurrence of the disease among the male population correlates with a previous Indian study.[9] This is probably attributed to higher exposure of male child to active cases.
The prevalence of the various forms of the disease in children is mostly derived from hospital-based studies. Borderline tuberculoid (BT) is the commonest with prevalence ranging from 55% to 90%. Conversely, the prevalence of Lepromatous leprosy (LL) was relatively low ranging from 1.6% to 4.9%.[10,11,12,13,14] A study from Delhi showed that the occurrence of BT leprosy was little higher (around 90%) in the pediatric population.[15] Another study from Mangalore, Karnataka demonstrated the proportion of TT Hansen was around 46% and BT Hansen was around 44%.[16] Our study showed a higher occurrence of PB leprosy than MB leprosy. Interestingly, the prevalence of leparomatous and multibacillary leprosy was significantly higher than the previous studies (5.26% to 17.24% in different age group) indicating that though the overall incidence is declining, cases with MB are on a rising trend. These findings corroborate with the study done in Rajashtan, where the proportion of LL leprosy in the childhood population was more than 18%.[17] However, a broad spectrum population-based study is required in this field before reaching any conclusion.
The prevalence of reaction (13.41%) was lower than the other hospital-based studies in India (approximately 25% of the cases).[10,11,12] The prevalence of deformity in the study population is also higher (14.28%) than the other Indian studies (2.5% to 10.5%)[1] which correlates with the higher occurrence of MB spectrum among our study populations. However, more affection of upper extremities (claw hand) correlates with the other Indian studies.[18,19]
Leprosy is a disease associated with defective cell-mediated immunity (CMI) specific to this disease. CMI decreases from PB to MB leprosy. Although the multidrug therapy kills the bacilli, it has no role in enhancing the CMI. It cannot prevent the susceptibility to acquired infection nor effectively clear dead bacilli from the body rendering the individual to dead bacilli–related complications like reaction. To enhance the CMI of the host, various vaccines have been tried. Vaccine studies have utilized live or killed whole mycobacterium, such as Bacille Calmette-Guérin (BCG), Indian cancer research center (ICRC) bacilli, and Mycobacterium w either alone or in combination with killed Mycobacterium leprae.[20] Twelve case-control studies show a median vaccine efficacy of BCG for the prevention of transmission of leprosy 63% (range 20–90%). Two prospective studies and two randomized community trials showed a median efficacy of 70% (range 42-80%). The duration of this partial protection is at least 10–15 years.[21,22] We found a lower number of MB cases who are vaccinated than who are not vaccinated indicating that BCG seems to have some definitive role to modify the CMI.
BCG vaccination is given to the children in our country as a part of the national immunization programme. It is usually recommended to be given at birth for institutional delivery or as soon as possible after the birth in case of home delivery. Despite being implemented for more than 30 years, immunization coverage among children aged 12–23 months in the country has increased at a slow pace, from 35% in 1992–1993 to 62% in 2015–2016.[23] A study done by Singh et al. showed that even in 2019, the complete immunization coverage among 12–23 month children in Bihar is near to 90%.[24] In many instances, primary care physicians and rural physicians may be the first contact of the children to the health care professionals. So, all the primary care physicians must encourage the parents for BCG vaccination in the children as it seems to influence the CMI and not only prevents childhood tuberculosis but also seems the occurrence of severe form of leprosy.
Key Message
PB leprosy was the commonest in the <12 years age group. The prevalence of MB leprosy increased as the child grows. Smear positivity, deformity, and reaction were not very frequent in this age group. The proportion of MB leprosy was more among the BCG nonvaccinated group than among the BCG-vaccinated groups. These observations point toward the fact that BCG may have some influence on childhood leprosy.
Limitations
As it was not a community-based study in which the BCG-vaccinated persons are followed up for the occurrence of leprosy later in the life and compared with proper control, it was not possible to calculate a predictive value regarding the protective ability and influence of BCG vaccine on leprosy from this study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the parents of the patients given their consent for the clinical information of their children to be reported in the journal. The parents understand that the name and initials will not be disclosed and due efforts will be made to conceal the identity of themselves and their children, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Dayal R, Sanghi S. Leprosy in childhood. In: Kar HK, Kumar B, editors. ILA Textbook of Leprosy. 1st ed. Joypee Brothers Medical Publishers (p) Ltd; 2010. pp. 325–31. [Google Scholar]
- 2.Thangaraj RH. 3rd ed. New Delhi: South Asia, The leprosy Mission (pub); 1983. A Manual of Leprosy; pp. 3–9. [Google Scholar]
- 3.Sundar Rao PS, Jesudasan K, Mani K, Christian M. Impact of MDT on incidence rates of leprosy among household contacts. Part 1. Baseline data. Int J Lepr Other Mycobact Dis. 1989;57:647–51. [PubMed] [Google Scholar]
- 4.Narang T, Kumar B. Leprosy in children. Indian J Paediatr Dermatol. 2019;20:12–20. [Google Scholar]
- 5.Merle CS, Cunha SS, Rodrigues LC. BCG vaccination and leprosy protection: Review of current evidence and status of BCG in leprosy control. Expert Rev Vaccines. 2010;9:209–22. doi: 10.1586/erv.09.161. [DOI] [PubMed] [Google Scholar]
- 6.WHO Expert Committee on Leprosy. Seventh report. Geneva: World Health Organization; 1998. Tech Rep Ser 874. [Google Scholar]
- 7.Vieira MCA, Nery JS, Paixão ES, Freitas de Andrade KV, Oliveira Penna G, Teixeira MG. Leprosy in children under 15 years of age in Brazil: A systematic review of the literature. PLoS Negl Trop Dis. 2018;12:e0006788. doi: 10.1371/journal.pntd.0006788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nsagha DS, Bamgboye EA, Oyediran AB. Childhood leprosy in essimbiland of Cameroon: Results of chart review and school survey. Nig Q J Hosp Med. 2009;19:214–9. [PubMed] [Google Scholar]
- 9.Horo I, Rao PS, Nanda NK, Abraham S. Childhood leprosy: Profiles from a leprosy referral hospital in West Bengal, India. Indian J Lepr. 2010;82:33–7. [PubMed] [Google Scholar]
- 10.Vara N. Profile of new cases of childhood leprosy in a hospital setting. Indian J Lepr. 2006;78:231–6. [PubMed] [Google Scholar]
- 11.Kumar B, Rani R, Kaur I. Childhood leprosy in Chandigarh: A clinico histopathological correlation. Int J Lep Other Mycobact Dis. 2000;68:330–1. [PubMed] [Google Scholar]
- 12.Burman KD, Rijall A, Agrawal S, Agarwalla A, Verma KK. Childhood leprosy in eastern Nepal: A hospital based study. Indian J Lepr. 2003;75:53–8. [PubMed] [Google Scholar]
- 13.Palit A, Inamadar AC. Childhood leprosy in India over the past two decades. Lepr Rev. 2014;85:93–9. [PubMed] [Google Scholar]
- 14.Prasad PV. Childhood leprosy in a rural hospital. Indian J Pediatr. 1998;65:751–4. doi: 10.1007/BF02731059. [DOI] [PubMed] [Google Scholar]
- 15.Ghunawat S, Relhan V, Mittal S, Sandhu J, Garg VK. Childhood leprosy: A retrospective study Delhi. Indian J Dermatol. 2018;63:455–8. doi: 10.4103/ijd.IJD_99_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babu A, Bhatt MR, Jayaraman J. Childhood leprosy in post elimination era: A vision achieved or a concern growing at large. Indian J Paediatr Drmatol. 2018;19:26–30. [Google Scholar]
- 17.Balai M, Agarwal C, Gupta LK, Khare AK, Mittal A. Current scenario of childhood leprody at a tertiary care hospital of Southern Rajasthan. Indian Online Dermatol J. 2017;8:494–5. doi: 10.4103/idoj.IDOJ_8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kar BR, Job CK. Visible deformity in childhood leprosy: A 10 year study. Int J Lep Other Mycobact Dis. 2005;73:243–48. [PubMed] [Google Scholar]
- 19.Sardana K. A study of leprosy in children, from a tertiary pediatric hospital in India. Lepr Rev. 2006;77:160–2. [PubMed] [Google Scholar]
- 20.Gormus BJ, Meyers WM. Under-explored experimental topics related to integral mycobacterial vaccines for leprosy. Expert Rev Vaccines. 2003;2:791–804. doi: 10.1586/14760584.2.6.791. [DOI] [PubMed] [Google Scholar]
- 21.Velema JP, Ogbeiwi OI. ILEP organisations should strive for high BCG coverage in communities at risk of leprosy. Lepr Rev. 2007;78:88–101. [PubMed] [Google Scholar]
- 22.Zodpey SP, Ambadekar NN, Thakur A. Effectiveness of bacillus calmette guerin (BCG) vaccination in the prevention of leprosy: A population-based case-control study in Yavatmal District, India. Public Health. 2005;119:209–16. doi: 10.1016/j.puhe.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Ministry of Health and Family Welfare, Govt. of India. Road map for achieving 90% full immunization coverage in India – A guidance document to the states. New Delhi: 2019. [Last accessed on 2019 Jan 23]. Available from: https://nhm.gov.in/New_Updates_2018/NHM_Components/Immunization/Guildelines_for_immunization/Roadmap_document_for_90%25_FIC.pdf . [Google Scholar]
- 24.Singh CM, Mishra A, Agarwal N, Mishra S, Lohani P, Ayub A. Immunization coverage among the children aged 12-23 months: A cross sectional study in low performing blocks in Bihar. Indian J Family Med Prim Care. 2019;8:3949–55. doi: 10.4103/jfmpc.jfmpc_619_19. [DOI] [PMC free article] [PubMed] [Google Scholar]


