Abstract
Despite thromboprophylaxis, patients with coronavirus disease 2019 (COVID‐19) exhibit hypercoagulability and higher venous thromboembolic risk, although its real incidence is still unknown. The aim of this study was to evaluate the incidence of venous thromboembolism (VTE) in patients with COVID‐19 admitted to both intensive care units (ICUs) and medical wards (MWs). Consecutive patients admitted for COVID‐19 to the MW and the ICU at Padua University Hospital, all receiving thromboprophylaxis, underwent systematic ultrasonography of the internal jugular, and the upper and lower limbs veins every 7 days (± 1 day) after the admission; and, if negative, once‐weekly until discharge or death. In case of suspected pulmonary embolism, a multidetector computed tomographic angiography was performed. The primary outcome was the proportion of any deep‐vein thrombosis (DVT) and symptomatic pulmonary embolism in both groups. An extended blood coagulative test was performed as well. From March 4 to April 30, 2020, a total of 85 patients were investigated, 44 (52%) in MWs and 41 (48%) in the ICU. Despite thromboprophylaxis, VTE occurred in 12 patients in the MWs (27.3%) and 31 patients in the ICU (75.6%) with an odds ratio of 9.3 (95% confidence interval (CI) 3.5–24.5; P < 0.001). Multiple‐site DVT occurred in 55.6% of patients (95% CI 39.6–70.5). Increased D‐dimer levels significantly correlated with VTE (P = 0.001) and death (P = 0.015). Summarizing, patients with COVID‐19 admitted to the MW or ICU showed a high frequency of venous thromboembolism, despite standard‐dose or high‐dose thromboprophylaxis. Whether thrombosis, particularly asymptomatic events, may play a role in the morbidity and mortality of patients with COVID‐19 remain to be clarified.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Patients with coronavirus disease 2019 (COVID‐19) display both hypercoagulability and higher thromboembolic risk. The actual incidence of the latter is ill‐defined.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ What is the actual incidence of venous thromboembolism (VTE) in these patients? Does the incidence differ in diverse settings, such as an intensive care unit (ICU) or a medical ward (MW)?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Patients with COVID‐19 admitted to the ICU or a MW display a 5‐fold higher risk of VTE than would be expected from the literature. Patients in the ICU experience VTE nine times more frequently than those in an MW. This occurs despite pharmacological thromboprophylaxis with standard or high‐dose prophylaxis with enoxaparin or fondaparinux. Patients with COVID‐19 also exhibit an unexpectedly high incidence of multiple‐site deep‐vein thrombosis. Hypercoagulability, as expressed by increased D‐dimer levels, significantly correlates with VTE and death.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ Our results suggest that thromboprophylaxis does not adequately prevent VTE in these patients. A randomized trial comparing anticoagulant treatment with prophylaxis seems warranted.
The severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) has spread throughout the world starting from December 2019 and has not stopped yet, causing the occurrence of a new coronavirus disease 2019 (COVID‐19) and becoming a global health threat. Most patients infected with SARS‐CoV‐2 are asymptomatic or experience a mild disease course. Nonetheless, about 14% of the patients with COVID‐19 are admitted to medical wards (MWs), whereas about 5% develop a severe respiratory failure, thus requiring admission to intensive care units (ICUs). 1
Venous thromboembolism (VTE)—including deep‐vein thrombosis (DVT) and pulmonary embolism (PE)—is a common disease affecting 1 to 2 in 1,000 persons per year in the general population, and most often arises as a complication of another pathological condition. 2 The VTE risk, in patients hospitalized in MWs for an infectious respiratory disease, is as high as 5% and 17%, with and without thromboprophylaxis, respectively. The corresponding figures for patients admitted to the ICU, are 16% and 31%, with and without thromboprophylaxis, respectively. 3 , 4 , 5 , 6 , 7 A number of specific factors, let alone respiratory failure per se, account for the higher VTE‐prevalence observed in patients in the ICU, including central venous catheters, mechanical ventilation, and neuromuscular‐blockade. 8 , 9 , 10 , 11
Patients with COVID‐19 may express coagulation activation at presentation, as shown by elevated D‐dimer, which is associated with a worse clinical evolution and higher in‐hospital mortality. 12 , 13 , 14 , 15 , 16 , 17 , 18 Furthermore, patients with COVID‐19 who are admitted to an ICU may also experience a severe hypercoagulable state fostered by high serum levels of prothrombotic cytokines—which might place these patients at an even higher risk of developing VTE. 19 , 20
In this sense, it is noteworthy that a number of recent studies, mostly retrospective, reported a similarly high incidence of VTE in patients with COVID‐19 to that observed in patients hospitalized for a non‐COVID infectious respiratory disease, being as high as 20%, and tending to be more frequent in patients admitted to the ICU than in those admitted to the MWs. 21 , 22 , 23 , 24 , 25 , 26
Based on these findings, we designed a prospective study, using systematic ultrasonographic and laboratory screening to assess the actual incidence of VTE and hypercoagulable state in patients with COVID‐19, admitted to the ICU or MW (Infectious Diseases Units) of Padua University Hospital.
METHODS
Study design
This was an observational cohort study. Our Institutional Review Board approved the study protocol, prior to data collection. Informed consent for noninvasive diagnostic procedures was managed according to internal hospital policy. The study was conducted in compliance with the principles of the Declaration of Helsinki.
Patients
Consecutive patients aged ≥ 18 years with confirmed COVID‐19 who were admitted to an MW for a mild (slight clinical symptoms, no signs of pneumonia on imaging) to moderate (fever and respiratory symptoms, with pneumonia signs on imaging) disease; or to the ICU for a severe (patients with any of the following conditions: respiratory distress with respiratory rate ≥ 30 breaths/minute; SPO2 ≤ 93% at rest; PaO2/FiO2 ≤ 300 mm Hg (1 mm Hg = 0.133 kPa)) to critical (patients with any of the following conditions: respiratory failure requiring mechanical ventilation; shock; or other organ failure) disease, were enrolled. 27 COVID‐19 was confirmed by a reverse transcription polymerase chain reaction on nasopharyngeal swabs or sputum samples. On admission, all patients were prescribed thromboprophylaxis with low‐molecular‐weight heparin (LMWH; enoxaparin 40 mg once‐daily) or fondaparinux (2.5 mg once‐daily), according to current recommendations for medical patients.
Outcomes
The primary outcome of the study was the incidence of symptomatic PE, as well as symptomatic or asymptomatic DVT in both groups.
The secondary outcome was the incidence of superficial vein thrombosis (SVT) and hypercoagulable state in both groups.
An independent committee, blinded on patients’ allocation group, adjudicated the thromboembolic events based on all relevant documentation and footage.
Outcomes assessment
All patients underwent systematic bilateral color‐coded Doppler ultrasonography (CCDU) both for superficial‐vein and deep‐vein systems of the upper and lower limbs, and of the internal jugular veins at 7 days (± 1 day) from the hospital admission. Patients with normal baseline ultrasound results were scheduled for further scans once weekly for the entire duration of their hospital stay.
CCDU was performed according to standardized protocols. 28 , 29 Diagnostic methodology is described in the Supplementary Material. Vein incompressibility was the only diagnostic criterion used to rule‐in DVT and SVT. 30 All CCDU examinations were performed by certified and skilled vascular physicians, with long‐standing experience in vascular ultrasonography.
Nonfatal symptomatic PE (e.g., unexplained hypotension or hypoxia disproportionate to the severity of pneumonia) was confirmed by multidetector computed tomographic pulmonary angiography (CTPA). Fatal PE was adjudicated by autopsy, or on clinical grounds in case of sudden and otherwise inexplicable death, according to an independent physician.
Laboratory measurements
Traditional coagulation parameters, such as hemoglobin, platelet count, prothrombin time/international normalized ratio, activated partial thromboplastin time (aPTT), fibrinogen, antithrombin, and D‐dimer, as well as serum creatinine, alanine aminotransferase, and aspartate aminotransferase were measured in all enrolled patients. Whole blood thromboelastometry profiles were obtained using a ROTEM Sigma apparatus (Instrumentation Laboratory – Werfen, Barcelona, Spain), as previously described. 19 INTEM and EXTEM assays (evaluation of intrinsic and extrinsic coagulation pathways) and FIBTEM test (evaluation of fibrinogen contribution to blood clot) were performed in all patients. The following ROTEM parameters were analyzed: (1) clotting time, corresponding to the initiation phase of the clotting process; (2) clot formation time, measuring the propagation phase of whole blood clot formation; (3) maximum clot firmness (MCF), indicating the maximum amplitude in millimeters reached in the thromboelastogram. Samples were routinely obtained as close as possible to the first CCDU scan.
Statistical analysis
According to the literature, 5% and 15% of patients admitted to MWs or ICUs will experience VTE despite thromboprophylaxis. 3 , 4 , 5 , 6 , 7 Preliminary studies in patients with COVID‐19 report a remarkably high incidence of VTE, up to 3‐fold that expected, despite thromboprophylaxis. 20 , 21 , 22 , 23 , 24 , 25 Thus, we hypothesized that the incidence of VTE in our patients with COVID‐19 receiving thromboprophylaxis would be about 15% in patients admitted to MWs, and about 45% in patients admitted to ICUs, respectively.
Based on these assumptions, we estimated that, including at least 42 subjects per group, the 2‐sided 95% confidence interval (CI) around the point estimate proportion for VTE would extend from 9.5% to 20.5%, and from 38% to 52%, in patients admitted to the MW and to the ICU, respectively (nQuery Advisor version 5; Statistical Solutions, Cork, Ireland).
Variables were first visually inspected, as usual: categorical variables with frequency tables, and continuous variables with sample statistics (e.g., average, median, SD, minimum and maximum value, and 25th and 75th quartiles). The selected baseline covariates were then compared for “like‐to‐like” in the two groups, with appropriate statistical tests for discrete and continuous variables.
Primary and secondary outcomes analyses were performed on an intention‐to‐treat population, including all enrolled subjects. Raw cumulative proportions of subjects with VTE in the two groups were tested by Fisher’s exact test.
Cox’s proportional hazard model was used to estimate hazard ratios for VTE occurrence, adjusted for various potential confounders, including group membership, age, sex, thromboprophylaxis dose, and all additional factors significantly associated with VTE at the univariate analysis (i.e., obesity, chronic heart failure, hypertension, and several laboratory findings). Proportional hazards assumptions were first visually checked by log minus log survival plots for selected variables. Covariates significantly associated with the model were selected in a “forward stepwise” fashion, by the Wald method.
The cumulative proportion of patients experiencing VTE was described by the Kaplan–Meier method, and differences were tested by the log‐rank test. All statistical tests were 2‐tailed, at a significance level of 0.05, unless otherwise specified. All analyses were performed with IBM‐SPSS Statistics version 26 package (IBM‐SPSS, Chicago, IL).
RESULTS
Among 85 patients hospitalized for COVID‐19 at Padua University Hospital between March 4 and April 30, 2020, 44 (52%) were admitted to the MW and 41 (48%) to the ICU.
Baseline characteristics of the study population are summarized in Table 1 . The demographics and medical history details, including comorbidities and concomitant medications, were similar in both groups; except for weight and body mass index, that were significantly higher in the ICU population. The mean time period from admission to discharge or death was 16 days (SD ± 6) in MWs and 27 days (SD ± 12) in the ICU. At the end of the study (April 30, 2020), 64 patients (75.2%) had been discharged, 13 (15.3%) were still hospitalized, and 8 (9.4%) had died.
Table 1.
Baseline characteristics of the study population, according to setting
| Patients characteristics | Setting | P value | |
|---|---|---|---|
| MW (n = 44) | ICU (n = 41) | ||
| Male sex, n (%) | 28 (64) | 33 (80) | 0.10 |
| Age, years | 67 (±14) | 67 (±11) | 0.94 |
| Weight, kg | 76 (±15) | 86 (±19) | 0.02 |
| BMI, kg/m2 | 26 (±4) | 28 (±5) | 0.04 |
| Length of stay in hospital, days | 16 (±6) | 27 (±12) | 0.001 |
| Concomitant disease | |||
| Hypertension, n (%) | 21 (48) | 18 (44) | 0.18 |
| Diabetes, n (%) | 13 (30) | 9 (22) | 0.20 |
| Stroke, n (%) | 1 (2) | 0 | |
| COPD, n (%) | 4 (9) | 5 (12) | 0.73 |
| Previous VTE, n (%) | 1 (2) | 1 (2) | 1.00 |
| Cancer, n (%) | 5 (11) | 5 (12) | 1.00 |
| Immobilization >3 days, n (%) | 35 (80) | 32 (78) | 1.00 |
| Known thrombophilia, n (%) | 1 (2) | 1 (2) | 1.00 |
| Concomitant medications | |||
| Antiplatelet therapy, n (%) | 5 (11) | 6 (15) | 1.00 |
| ACE‐inhibitors/AT‐1, n (%) | 13 (30) | 14 (34) | 0.82 |
| Antiviral drugs, n (%) | 0 | 7 (17) | 0.005 |
Data are expressed as mean (± SD) or number and frequency, as appropriate.
ACE, angiotensin converting enzyme; AT‐1, angiotensin receptor type 1 blockers; BMI, body mass index; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; MW, medical ward; VTE, venous thromboembolism.
All patients received thromboprophylaxis, as prescribed on admission. Enoxaparin 40 mg once‐daily, or fondaparinux 2.5 mg once‐daily were administered to 59 patients (69.4%), whereas 26 patients (30.6%) displaying increased coagulability as assessed by ROTEM, received enoxaparin 60 mg or 80 mg once‐daily if they weighted less or more than 80 Kg, respectively, or fondaparinux 5.0 mg once‐daily, or 60 mg twice‐daily, if they weighted > 100 kg. Three patients needed extra‐corporeal‐membrane‐oxygenation; all of them received intravenous high‐dose heparin, in order to maintain an aPTT of 60 seconds and an activated clotting time of 180–200 seconds.
High doses were more often used in the ICU (41%), than in the MW (26%); to the contrary, standard doses were more frequently given in the MW (74%) than in the ICU (59%). There were no differences in terms of VTE between patients administered standard or high‐dose prophylaxis (P = 0.48), as reported in Table 2 . Standard or high‐dose prophylaxis did not account for different rates of VTE, when considering ICU and MW separately (Figures S1 a–c). Only one minor bleeding event was observed in an ICU patient on high‐dose (60 mg once‐daily) enoxaparin.
Table 2.
Incidence of VTE according to prophylactic dose and setting
| Dose | MW c | ICU c | Overall c | OR | P value |
|---|---|---|---|---|---|
| Standard a |
9/35 25.7% (14.2–42.1) |
19/24 79.2% (59.5–90.8) |
28/59 47.5% (35.3–60.0) |
0.6 (0.3–1.7) |
0.48 |
| High b |
3/9 33.3% (12.1–64.6) |
12/17 70.6% (46.9–86.7) |
15/26 57.7% (38.9–74.5) |
ICU, intensive care unit; MW, medical ward; OR, odds ratio; VTE, venous thromboembolism.
Enoxaparin 40 mg once‐daily, or fondaparinux 2.5 mg, once‐daily.
Enoxaparin 60 mg or 80 mg once‐daily, or fondaparinux 5.0 mg once‐daily (< 100 kg); 60 mg twice‐daily (> 100 kg).
Expressed as number with VTE/ number at risk, % (95% confidence interval).
Outcomes
Overall, 43 patients (50.6%; 95% CI 40.2–61.0) experienced VTE; 12 (27.3%; 95% CI 16.3–41.8) in the MW, and 31 (75.6%; 95% CI 60.7–86.2) in the ICU (P < 0.001; odds ratio 9.3; 95% CI 3.5–24.5). A detailed description of VTE events is reported in Table 3 .
Table 3.
Type and distribution of VTE events by setting
|
VTE events % (95% CI) |
MW (n = 44) |
ICU (n = 41) | Total (n = 85) | OR | P value |
|---|---|---|---|---|---|
| Overall VTE |
12 27.3% (16.3–41.8) |
31 75.6% (60.7–86.2) |
43 50.6% (40.2–61.0) |
9.3 (3.5–24.5) |
< 0.001 |
| PE | 0 |
4 9.8% (3.9–22.5 |
4 9.8% (3.9–22.5 |
||
| Overall DVT |
10 22.7% (12.8–37.0) |
26 63.4% (48.1–76.4) |
36 42.4% (32.4–53.0) |
5.8 (2.2–15.2) |
< 0.001 |
| IJV | 4 | 3 | 7 | ||
| Upper limbs | 0 | 9 | 9 | ||
| Lower limbs | 6 | 14 | 20 | ||
| Proximal | 2 | 8 | 10 | ||
| Distal | 4 | 6 | 10 | ||
| SVT |
2 4.5% (1.3–15.1) |
1 2.4% (0.4–12.6) |
3 3.5% (1.2–9.9) |
0.5 (0.05–6.0) | 0.99 |
| Upper limbs | 2 | 1 | 3 | ||
| Lower limbs | 0 | 0 | 0 | ||
| Overall death |
2 4.5% (1.3–15.1) |
6 14.6% (6.9–28.4) |
8 9.4% (4.8–17.5) |
3.6 (07–19.0) | 0.15 |
| Death with VTE | 2/2 | 6/6 | 8/8 |
CI, confidence interval; DVT, deep‐vein thrombosis; ICU, intensive care unit; IJV, internal jugular vein; MW, medical ward; OR, odds ratio; PE, pulmonary embolism; SVT, superficial vein thrombosis; VTE, venous thromboembolism.
Time‐to‐event analysis of VTE is shown in Figure 1 . Namely, the cumulative proportion of VTE‐free patients in the MW at 7, 14, 21, and >21 days was 91% (95% CI 86.7–95.3), 80.2% (95% CI 73.9–86.5), 62.4% (95% CI 53.1–71.7), and 62.4% (95% CI 53.1–71.7), respectively. The corresponding figures in the ICU were 70.7% (95% CI 63.6–77.8), 63.2% (95% CI 55.6–70.8), 31.6% (95% CI 24.1–39.1), and 10.5% (95% CI 3.9–17.1), at 7, 14, 21, and >21 days, respectively (P = 0.034).
Figure 1.
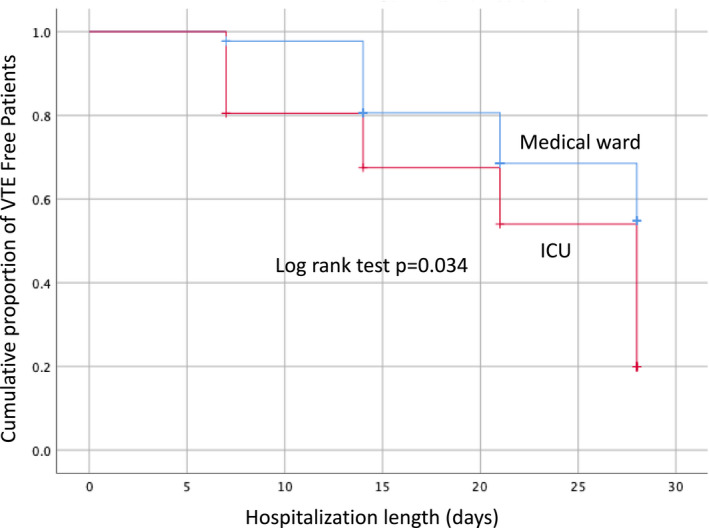
Cumulative proportion of venous thromboembolism (VTE)‐free patients by setting. ICU, intensive care unit.
Overall, four patients (4.7%; 95% CI 1.8–11.5) had PEs. All PE events occurred in the ICU patients (9.8%; 95% CI 3.9–22.5), and they were all fatal. PE was objectively confirmed by CTPA in two patients, whereas it was diagnosed on clinical grounds in the other two. Other 10 patients (8 in the MW, and 2 in the ICU) underwent CTPA that ruled‐out PE.
DVT occurred in 36 patients (42.4%; 95% CI 32.4–53.0), distributed as follows: internal jugular veins in 7 patients (19.4%), proximal deep‐veins of the upper limbs in 9 patients (25%), and deep‐veins of the lower limbs in 20 subjects (55.6%; 27.8% proximal, and 27.8% distal; respectively). The incidence of DVT was significantly higher in the ICU than in the MW (63.4%; 95% CI 48.1–76.4 vs. 22.7%; 95% CI 12.8–37.0, respectively; P < 0.001), with an odds ratio of 5.8 (95% CI 2.2–15.2).
SVT occurred in three patients (3.5%; 95% CI 1.2–9.9) and all of them occurred in the upper limbs. The incidence of SVT did not differ between patients in the MW and the ICU (P = 1).
Notably, 55.6% of the patients (95% CI 39.6–70.5) had multiple‐site venous thrombosis (upper and lower limbs; internal jugular and lower or upper limbs, or both; unilateral or bilateral proximal and/or distal lower limbs; both superficial and deep), with a slightly higher prevalence in the patients in the ICU than in the MW (57.7%; 95% CI 38.9–74.5 vs. 50.0%; 95% CI 23.7–76.3, respectively; P = 0.72). A detailed distribution of all aforementioned events is reported in Supplementary Material Table S1 .
DVT and SVT were symptomatic in 66% of the patients, without significant difference between the two groups.
Catheter‐related thrombosis occurred in 11 patients (13%): 2 patients in the MW with peripherally inserted central venous‐catheters (cephalic vein), and 9 patients in the ICU with central venous catheters (common femoral or internal jugular veins).
We recorded eight fatalities (2 in the MW and 6 in the ICU). Death was PE‐related in four ICU patients; whereas of the other four, all with confirmed DVT, only one had PE excluded by autopsy. The cause of death could not be clearly established in the other three patients (1 in the MW and 2 in the ICU).
Of the many covariates evaluated with the Cox’s proportional hazard model, only D‐dimer plasma levels equal to or higher than 1,000 ng/dL were significantly associated with VTE (hazard ratio, 2.2; 95% CI 1.1–4.4; P = 0.034; Table S2 ). The cumulative proportion of VTE‐free patients by basal D‐dimer levels, overall and by setting, are reported in Figure S2 a–c.
Laboratory findings
Patients in the ICU developed more severe anemia (P = 0.003) and significantly increased alanine aminotransferase (P = 0.011; Table S3 ). No difference in creatinine levels was observed. All enrolled patients showed platelet count and coagulation times (prothrombin time/aPTT) within normal range, and elevated D‐dimer levels—significantly higher in the ICU than in the MW (P = 0.002). Patients in the ICU also showed significantly increased fibrinogen levels (P = 0.001), whereas antithrombin levels were similar and within normal range in both groups. As regard to the ROTEM parameters, the entire cohort showed higher MCF in FIBTEM (normal values 6–21 mm), indicating increased clot strength. Patients in the ICU showed higher MCF in FIBTEM compared with MW (30.2 ± 7.6 mm vs. 35.6 ± 9.3 mm, P = 0.066; Table S3 ). All patients who developed VTE showed significantly elevated D‐dimer levels (P = 0.001) and a trend toward MCF in FIBTEM increase (31.1 ± 9.9 mm vs. 35 ± 7.9 mm, P = 0.19; Table 4 ). Notably, patients who died had significantly higher D‐dimer levels (median 2,196 ng/mL, 25/75 quartile, 1,268–3,233) than survivors (547 ng/mL, 25/75 quartile, 196–1,624; P = 0.015).
Table 4.
Laboratory and coagulative parameters in patients with and without VTE
| Parameters | No VTE | VTE | P value |
|---|---|---|---|
| Hemoglobin, g/L | 117.8 ± 15.8 | 112.6 ± 16.5 | 0.16 |
| Platelets, ×109/L | 250.9 ± 83.2 | 262.1 ± 129.2 | 0.66 |
| Hematocrit, % | 35.6 ± 4.6 | 34.9 ± 4.7 | 0.53 |
| AST, U/L | 39.0 [18–58] | 41.0 [25–59.5] | 0.73 |
| ALT, U/L | 31.0 [25–51] | 38.0 [28–67.5] | 0.27 |
| Creatinine, µmol/L | 67.4 ± 43.6 | 86.8 ± 94.7 | 0.27 |
| PT, % | 92.7 ± 18.3 | 87.1 ± 15.7 | 0.19 |
| INR | 1.2 ± 0.3 | 1.1 ± 0.1 | 0.49 |
| aPTT, seconds | 23.7 ± 7.4 | 23.0 ± 7.5 | 0.69 |
| D‐dimer, μg/L | 259 [150–934] | 1310 [577–2485] | 0.001 |
| Fibrinogen, g/L | 5.0 [3.5–5.6] | 4.9 [3.9–5.7] | 0.73 |
| Antithrombin, % | 95.7 ± 21.3 | 92.6 ± 21.0 | 0.58 |
| MCF INTEM, mm | 68.9 ± 5.8 | 71.9 ± 5.8 | 0.13 |
| MCF EXTEM, mm | 72.3 ± 6.3 | 72.1 ± 11.1 | 0.97 |
| MCF FIBTEM, mm | 31.1 ± 9.9 | 35.0 ± 7.9 | 0.19 |
Data are expressed as mean ± SD or median and [range interquartile] as appropriate.
ALT, alanine aminotransferase; aPTT, activated partial thromboplastin time; AST, aspartate aminotransferase; INR, international normalized ratio; MCF, maximum clot firmness; PT, prothrombin time; VTE, venous thromboembolism.
DISCUSSION
The aim of this study was to prospectively investigate the incidence of VTE in consecutive patients with COVID‐19‐related pneumonia, admitted to the ICU or MW. Of note, all patients received either prophylactic‐dose or high‐dose LMWH or fondaparinux.
We found an astounding 5‐fold higher VTE incidence than that expected in similar patients without COVID‐19 (Table 3 ). According to the literature, the prevalence of VTE in patients with COVID‐19 is increased up to 30%. 22 , 23 , 24 , 25 , 26 , 27 Indeed, previous studies 22 , 23 , 24 , 25 , 26 , 27 presented some diagnostic limitations, implying that the reported VTE incidence might have been underestimated. Noteworthy, we performed serial, once‐weekly, CCDU throughout the whole hospitalization period in all patients, and this could explain the higher incidence of VTE recorded in our cohort.
We also observed a significant ninefold higher cumulative VTE risk in the ICU than in the MW (P = 0.034; Figure 1 ), that is again in agreement with published data, although on a different scale of magnitude.
PE was confirmed by CTPA or on clinical grounds in four of eight patients who died. This is in line with a recent autopsy study on patients with COVID‐19, in which the prevalence of fatal PE was 33%; notably 58% also had asymptomatic DVT of the lower limbs or of the pelvic veins. 31
Interestingly, 56% of our patients had multiple‐site DVT, an infrequent circumstance in similar COVID‐19‐free patients admitted to the ICU or the MW, that is a unique feature of our study. Such a frequent occurrence of multiple‐site DVT fairly correlates with the hypercoagulable state observed in our patients, as inferred from the remarkably high D‐Dimer and fibrinogen levels. Indeed, hypercoagulability appears to be a typical feature of patients with COVID‐19. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20
Furthermore, we observed a significant direct correlation between high D‐dimer levels and both VTE (P = 0.002) and mortality (P = 0.015).
Similarly, several recent retrospective studies reported a strong direct correlation among D‐dimer levels, VTE, and mortality, in patients with COVID‐19. 15 , 16 , 20 , 32
Overall, 49% of the study patients, irrespective of both LMWH or fondaparinux doses (prophylactic vs. high) and of setting (ICU vs. MW), developed VTE. Recently published expert opinions suggested therapeutic doses of LMWH or fondaparinux should reasonably be used in patients with COVID‐19, mainly those admitted to the ICU. 25 , 33 In this respect, a properly conducted trial comparing therapeutic with standard or high prophylactic doses would be desirable, especially in order to also ascertain the risk of bleeding complications, particularly in patients in the ICU. Of note, we registered only one minor bleeding episode in a patient in the ICU given high‐dose LMWH.
Our study presented some limitations. First, we included a small sample of patients, consequently the 95% CI around the point estimate of thromboembolic events is not acceptably narrow; however, even in the worst hypothesis, the estimated prevalence of VTE would be 16.3% and 60.7%, in the MW and in the ICU, respectively; that is 3‐fold to 4‐fold the expected figure, in non‐COVID‐19 patients.
Second, we do not have follow‐up data on DVT‐free patients who were discharged from the MW without thromboprophylaxis. However, according to the emergency department and the anagraphic databases of our institution, there were no hospital re‐admissions or deaths of these patients, in the period from discharge up to May 15, 2020.
Third, of the eight patients who died, only one underwent autopsy, and we can hypothesize that PE incidence could be underestimated. This is because autopsy was strongly discouraged by the Italian Ministry of Health, due to the risk of COVID‐19 transmission.
Fourth, one may argue about the clinical relevance of distal DVT and about the sensitivity and specificity of CCDU for the diagnosis; however, whole‐leg CCDU is currently the reference test worldwide for diagnosing distal DVT. 34 , 35 Furthermore, no patients in our study had distal DVT involving a single venous segment; instead, we only recorded multiple‐site distal DVTs.
CONCLUSION
Patients with COVID‐19 admitted to the MW or the ICU showed an unexpectedly high incidence of objectively documented VTE, despite thromboprophylaxis with anticoagulants given at standard or intermediate doses. In these patients, high D‐dimer levels strictly correlate with VTE occurrence and death. Whether thrombosis, particularly asymptomatic events, may play a role in the mortality of patients with COVID‐19 still remains to be demonstrated.
Funding
No funding was received for this work.
Conflict of Interest
The authors declared no competing interests for this work.
Author Contributions
G.C., E.C., and E.B. wrote the manuscript. G.A., G.C., and E.C. designed the research. G.A., G.C., E.C., E.B., P.P., C.P., M.C., A.C., I.T., P.N., A.B. L.S., and P.S. performed the research. G.C., E.B., F.N., A.C., I.T., P.N., and P.S. analyzed the data.
Supporting information
Supplementary Material
Acknowledgments
The authors would like to dedicate their study to all the Italian patients, doctors, nurses, paramedics, and other volunteers who are fighting or lost their lives to COVID‐19.
G.A., G.C., and E.C. contributed equally to this work.
Trial Registration: ClinicalTrials.gov Identifier: NCT04359212
References
- 1. Wu, Z. & McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 323, 1239–1242 (2020). [DOI] [PubMed] [Google Scholar]
- 2. Naess, I.A. , Christiansen, S.C. , Romundstad, P. , Cannegieter, S.C. , Rosendaal, F.R. & Hammerstrom, J. Incidence and mortality of venous thrombosis: a population‐based study. J. Thromb. Haemost. 5, 692–699 (2007). [DOI] [PubMed] [Google Scholar]
- 3. Clayton, T.C. , Gaskin, M. & Meade, T. Recent respiratory infection and risk of venous thromboembolism: case‐control study through a general practice database. Int. J. Epidemiol. 40, 819–827 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rogers, M.A.M. , Levine, D.A. , Blumberg, N. , Flanders, S.A. , Chopra, V. & Langa, K.M. Triggers for hospitalization for venous thromboembolism. Circulation 125, 2092–2099 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alikhan, R. et al Prevention of venous thromboembolism in medical patients with enoxaparin: a subgroup analysis of the MEDENOX study. Blood Coagul. Fibrinol. 14, 341–346 (2003). [DOI] [PubMed] [Google Scholar]
- 6. Boonyawat, K. & Crowther, M. Venous thromboembolism prophylaxis in critically ill patients. Semin. Thromb. Haemost. 41, 68–74 (2015). [DOI] [PubMed] [Google Scholar]
- 7. Geerts, W. , Cook, D. , Selby, R. & Etchells, E. Venous thromboembolism and its prevention in critical care. J. Crit. Care 17, 95–104 (2002). [DOI] [PubMed] [Google Scholar]
- 8. Castellucci, L.A. , Wells, P.A. & Duffet, L. Non‐leg venous thrombosis in critically ill patients. JAMA 313, 411 (2015). [DOI] [PubMed] [Google Scholar]
- 9. Cook, D. , Attia, J. , Weaver, B. , McDonald, E. , Meade, M. & Crowther, M. Venous thromboembolic disease: an observational study in medical‐surgical intensive care unit patients. J. Crit. Care 15, 127–132 (2000). [DOI] [PubMed] [Google Scholar]
- 10. Ejaz, A. et al Thromboprophylaxis in intensive care unit patients: a literature review. Cureus 10, e3341 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaplan, D. et al VTE incidence and risk factors in patients with severe sepsis and septic shock. Chest 148, 1224–1230 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hezi‐Yamit, A. et al Synergistic induction of tissue factor by coagulation factor Xa and TNF: evidence for involvement of negative regulatory signaling cascades. Proc. Natl. Acad. Sci. USA 102, 12077–12082 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Esmon, C.T. The impact of the inflammatory response on coagulation. Thromb. Res. 114, 321–327 (2004). [DOI] [PubMed] [Google Scholar]
- 14. Lorente, L. et al Sustained high plasma plasminogen activator inhibitor‐1 levels are associated with severity and mortality in septic patients. Thromb. Res. 134, 182–186 (2014). [DOI] [PubMed] [Google Scholar]
- 15. Zhang, L. et al D‐dimer levels on admission to predict in‐hospital mortality in patients with COVID‐19. J. Thromb. Haemost. 18, 1324–1329 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lippi, G. & Favaloro, E. D‐dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb. Haemost. 120, 876–878 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang, C. et al Clinical features of patients infected with novel 2019 coronavirus in Wuhan, China. Lancet 395, 497–506 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen, N. et al Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395, 507–513 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spiezia, L. et al COVID‐19 ‐related severe hypercoagulability in patients admitted to Intensive Care Unit for acute respiratory failure. Thromb. Haemost. 120, 998–1000 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang, C. , Wu, Z. , Li, J.W. , Zhao, H. & Wang, G.Q. The cytokine release syndrome (CRS) of severe COVID‐19 and interleukin‐6 receptor (IL‐6R) antagonist tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents 55, 105954 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Llitjos, J.F. et al High incidence of venous thromboembolic events in anticoagulated severe COVID‐19 patients. J. Thromb. Haemost. 18, 1743–1746 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Middeldorp, S. et al Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J. Thromb. Haemost. 18, 1995–2002 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cui, S. , Chen, S. , Li, X. , Liu, S. & Wang, F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 18, 1421–1424 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lodigiani, C. et al Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 191, 9–14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klok, F.A. et al Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb. Res. 191, 145–147 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raj Aryal, M. et al Venous thromboembolism in COVID‐19: towards an ideal approach to thromboprophylaxis, screening, and treatment. Curr. Cardiol. Rep. 22, 52 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhai, Z. et al Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb. Haemost. 120, 937–948 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bernardi, E. et al Serial 2‐point ultrasonography plus D‐dimer vs whole‐leg color‐coded doppler ultrasonography for diagnosing suspected symptomatic deep vein thrombosis: a randomized controlled trial. JAMA 300, 1653–1659 (2008). [DOI] [PubMed] [Google Scholar]
- 29. Kleinjan, A. et al Safety and feasibility of a diagnostic algorithm combining clinical probability, D‐dimer testing, and ultrasonography for suspected upper extremity deep venous thrombosis. a prospective management study. Ann. Intern. Med. 160, 451–457 (2014). [DOI] [PubMed] [Google Scholar]
- 30. Cogo, A. et al Compression ultrasonography for diagnostic management of patients with clinically suspected deep vein thrombosis: prospective cohort study. Br. J. Med. 316, 17–20 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wichmann, D. et al Autopsy findings and venous thromboembolism in patients with COVID‐19: a prospective cohort study. Ann. Intern. Med. 173, 268–277 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tang, N. , Bai, H. , Chen, X. , Gong, J. , Li, D. & Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 18, 1094–1099 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bikdeli, B. et al COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up. J. Am. Coll. Cardiol. 75, 2950–2973 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kearon, C. et al A randomized trial of diagnostic strategies after normal proximal vein ultrasonography for suspected deep venous thrombosis: D‐dimer testing compared with repeated ultrasonography. Ann. Intern. Med. 142, 490–496 (2005). [DOI] [PubMed] [Google Scholar]
- 35. Kearon, C. et al Management of suspected deep venous thrombosis in outpatients by using clinical assessment and D‐dimer testing. Ann. Intern. Med. 135, 108–111 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


