Brucellosis is a bacterial disease of domestic animals and humans. The pathogenic ability of Brucella organisms relies on their stealthy strategy and their capacity to replicate within host cells and to induce long-lasting infections. Brucella organisms barely induce neutrophil activation and survive within these leukocytes by resisting microbicidal mechanisms. Very few Brucella-infected neutrophils are found in the target organs, except for the bone marrow, early in infection.
KEYWORDS: Brucella, brucellosis, neutrophils, neutropenia, native immunity, adaptive immunity, Trojan horse
SUMMARY
Brucellosis is a bacterial disease of domestic animals and humans. The pathogenic ability of Brucella organisms relies on their stealthy strategy and their capacity to replicate within host cells and to induce long-lasting infections. Brucella organisms barely induce neutrophil activation and survive within these leukocytes by resisting microbicidal mechanisms. Very few Brucella-infected neutrophils are found in the target organs, except for the bone marrow, early in infection. Still, Brucella induces a mild reactive oxygen species formation and, through its lipopolysaccharide, promotes the premature death of neutrophils, which release chemokines and express “eat me” signals. This effect drives the phagocytosis of infected neutrophils by mononuclear cells that become thoroughly susceptible to Brucella replication and vehicles for bacterial dispersion. The premature death of the infected neutrophils proceeds without NETosis, necrosis/oncosis, or classical apoptosis morphology. In the absence of neutrophils, the Th1 response exacerbates and promotes bacterial removal, indicating that Brucella-infected neutrophils dampen adaptive immunity. This modulatory effect opens a window for bacterial dispersion in host tissues before adaptive immunity becomes fully activated. However, the hyperactivation of immunity is not without a price, since neutropenic Brucella-infected animals develop cachexia in the early phases of the disease. The delay in the immunological response seems a sine qua non requirement for the development of long-lasting brucellosis. This property may be shared with other pathogenic alphaproteobacteria closely related to Brucella. We propose a model in which Brucella-infected polymorphonuclear neutrophils (PMNs) function as “Trojan horse” vehicles for bacterial dispersal and as modulators of the Th1 adaptive immunity in infection.
INTRODUCTION
In 1902, the British bacteriologist and surgeon Sir Percy William Bassett-Smith (1861–1927) of the Royal Naval Hospital studied the plasma-opsonizing properties in Mediterranean fever patients infected with “Microccocus melitensis” (Brucella melitensis) (1). This bacterium was the etiological agent of Mediterranean fever, or Malta fever, later known as brucellosis, discovered in 1887 by a team of investigators under the leadership of Surgeon Captain David Bruce on the island of Malta. According to Bassett-Smith's experience, one of the most prominent and constant features of human brucellosis was the “relative reduction of the number, as well as the phagocytic activity, of the polymorphonuclear white cells.” He related this phenomenon to the “causation of the prolonged course and recurrent nature of the disease that can last for decades” (2), as suffered by Alice C. Evans (1881–1975), the brilliant bacteriologist who championed the procedure of milk pasteurization and found that M. melitensis and the bacillus of Bang's disease (Brucella abortus) were related. She acquired the infection in 1922 and experienced this long-lasting and recurrent disease for 22 years (3). Surgeon Bassett-Smith, who knew the methods described by the Nobel Prize laureate Élie Metchnikoff (1845–1916), noted that polymorphonuclear neutrophils (PMNs) were capable of phagocytizing a large number of M. melitensis organisms without lysis of these leukocytes (4). Moreover, the process could proceed in the absence of specific opsonins (antibodies), as then proposed by Metchnikoff (5). Later, the American bacteriologist Irvin Forest Huddleson (1893–1965) extended and illustrated these observations in 1933 (6).
The first comprehensive hematological profiles, carried out in 300 human brucellosis patients in 1939, demonstrated that 40 to 50% of patients with active brucellosis displayed absolute and relative neutropenia (7). The correlation between bacteremia and neutropenia was established a few years later (8). Based on clinical and experimental data, some investigators suggested in 1941 that through a bacterium-extractable substance, Brucella organisms were “toxic” to PMNs (9). While some supported this proposal, others denied it, raising a significant controversy among leading brucellologists of the time (8, 10). Finally, in 1943, investigators observed that in contrast to other Gram-negative bacteria, Brucella organisms did not prime for the so-called Shwartzman reaction, a response that recruits large numbers of PMNs in the skin after the second injection of endotoxin (11).
Amid the 20th century, brucellosis became a global and relevant zoonotic problem. Due to its high prevalence in humans, a significant number of clinicians studied the disease. One of the best known American medical brucellologists of the epoch was Wesley W. Spink (1904–1988), from the University of Minnesota. He had ample experience with hundreds of human brucellosis cases and with experimental models (8). In 1951, one of his students, Abraham I. Braude (1917–1984), who later became a renowned scientist in his own right, observed that soon after invasion, Brucella-infected PMNs bound Kupffer cells gathering around them in the liver sinusoids. After a few hours, the Kupffer cells were remarkably full of bacteria, while the liver sinusoids were devoid of Brucella-infected PMNs (12). These events suggested that the mononuclear phagocytic cells in the affected organs phagocytized the circulating, infected PMNs. Spink claimed that PMNs played a significant role in the pathogenesis of the disease and reported that “phagocytosis of brucellae by these leukocytes may be detrimental to the host since the bacteria may not be killed but protected by this intracellular localization” (8). Other studies revealed that Brucella organisms caused mononuclear granulomas, transient pancytopenia, and hemophagocytosis after the invasion of the bone marrow (BM) (8, 10). These results suggested that infected cells in the hematopoietic tissues (including PMNs) were removed, mainly after long-lasting infections. In his classic book The Nature of Brucellosis, Wesley W. Spink proposed that “systemic dissemination of the infection may be dependent, in part, upon the circulation within the bloodstream of these phagocytes [PMNs], containing viable brucellae” (8).
In the last 3 decades of the 20th century, various groups unequivocally demonstrated that Brucella organisms were naturally resistant to the killing action of PMNs (13–15) and that together with resident macrophages (Mϕs) and dendritic cells (DCs), PMNs were the first cells to encounter and internalize Brucella organisms after mucosal invasion (16, 17). All of these phenomena agreed with the low proinflammatory response at early times in the infection, the lack of endotoxic symptoms, and the absence of coagulopathies in brucellosis patients and animals (18, 19).
Despite these remarkable clinical and experimental observations and the significant role that PMNs play against other bacterial diseases, the function of these leukocytes in brucellosis remained unexplored for several decades. On the one hand, cellular microbiologists focused on unveiling the intracellular life cycle of Brucella organisms in Mϕs, DCs, and epithelial cells but not in PMNs (20, 21). As expected, the absence of Brucella replication in PMNs precluded these cells from studies in cellular microbiology. On the other hand, most studies in brucellosis concentrated on cells of the acquired immune system rather than on PMNs participating in the early proinflammatory responses (22, 23). In recent years, however, renewed interest in the role that PMNs have in Brucella infections has increased. Here, we critically review most of the work done in clinical and experimental brucellosis concerning PMNs and establish a model for the function of these leukocytes in the disease.
THE DISEASE NAMED BRUCELLOSIS
Members of the genus Brucella, mainly Brucella melitensis, Brucella suis, Brucella abortus, and Brucella canis, are pathogens responsible for a worldwide disease known as brucellosis, which affects domestic animals and humans (24). The high prevalence of the infection in low- and middle-income nations has a detrimental impact on public health and causes significant economic losses (25). In high-income countries, where the disease has been eradicated from livestock, brucellosis exists in wildlife and dogs (26) and is an “exotic” human infection, often overlooked or confused with other chronic illnesses (27). This matter is not trivial, since a large proportion of immigrants and refugees in high-income countries arrive from areas where brucellosis is endemic. Other Brucella organisms, such as Brucella ovis, a pathogen of rams, Brucella ceti and Brucella pinnipedialis, infecting marine mammals, and Brucella neotomae and Brucella microti, parasites of Cricetidae rodents, are of less zoonotic relevance and therefore not considered dangerous human pathogens (24).
As suggested by their names, the zoonotic Brucella species have different host preferences: B. melitensis for goats and sheep, B. abortus for cattle, B. suis for pigs, and B. canis for dogs. The various Brucella organisms are capable of cross-infecting different animal species, but such infections are, with some exceptions, mostly sporadic and not epizootic. The zoonotic Brucella species display some differences in virulence. The most dangerous is B. melitensis, and the least dangerous is B. canis, with B. suis and B. abortus standing somewhere in the middle (24, 28, 29). Still, the genetic relatedness of the species is very close, with a DNA similarity near 98 to 99%. Consequently, they show similar phenotypic characteristics and possess the same virulence factors required for infection, intracellular life, and dispersion (24). Regardless of the bacterial species causing brucellosis, the clinical symptoms observed in infected humans are the same, and the same antibiotic regimen is used to treat the disease, namely, a combination of doxycycline and streptomycin or a combination of doxycycline and rifampin for at least 7 weeks (8, 10, 19).
Brucella organisms generally invade through the mucosal membranes by direct contact with infected animals or their secretions. Although several studies have shown that Brucella organisms are capable of trespassing the respiratory and oral tract, the conjunctiva, lachrymal ducts, and vaginal and preputial mucosa, the exact mechanisms of epithelial invasion in these tissues remain unknown (17). It has been shown that epithelial cells readily phagocytize Brucella organisms (21). In the ligated ileal loop model, lymphoepithelial M-like cells phagocytize and transfer bacteria to the submucosal plexus when infected with large numbers of brucellae (16). However, this effect may be the consequence of the model, and it is doubtful that invasion occurs through the intestinal route, since it is rather inefficient compared with other routes (30). Moreover, Brucella is markedly sensitive to gastric juices, and no association between achlorhydria and brucellosis has been observed (8).
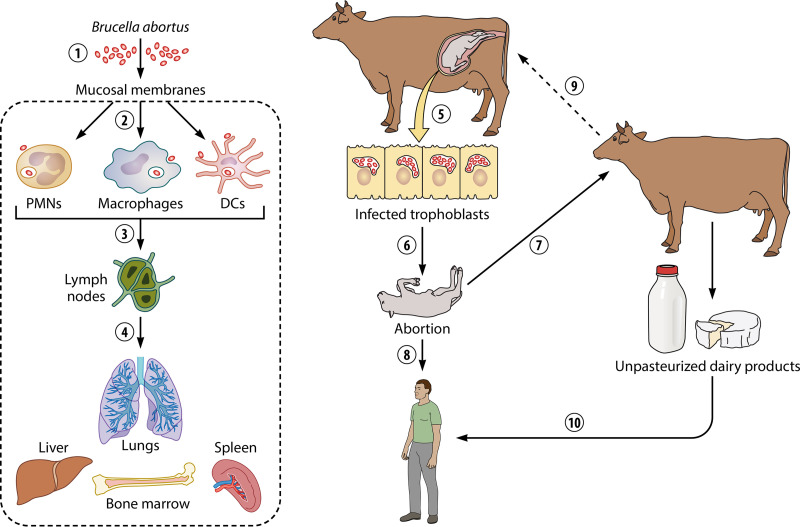
Brucellosis is a disease that progresses with a long incubation period that may last weeks, months, or even years (8, 10). Experimental eye infection in bovines induces a delay of 6 to 8 days, before the appearance of a local mild inflammatory response. The inflamed submucosa contains mainly mononuclear phagocytes, plasma cells, metachromatic mastocytes, eosinophilic leukocytes, and just a few PMNs (17). After initial replication at this site, the bacteria drain into the regional lymph nodes. During this period, the infected animals do not show signs of disease (31), and in pregnant animals, the bacteria replicate extensively in placental trophoblasts, causing placentitis and abortion in the last trimester. In males, the bacteria invade the testes and cause orchiepididymitis. The aborted fetus is the primary source of infection for other animals (31). Humans commonly acquire the bacterium from infected animals or through the ingestion of unpasteurized, contaminated dairy products (Fig. 1) (32).
FIG 1.
Cycle of Brucella abortus in the bovine host and humans. Brucella organisms infect through the mucosal membranes (1); once inside, local professional phagocytes such as Mϕs, DCs, and PMNs internalize the invading bacterium (2). From here, the bacterium moves to regional lymph nodes (3), and then it spreads to different organs of the reticuloendothelial system, such as the lungs, spleen, liver, and BM (4). In the pregnant animal, Brucella invades the placental trophoblasts, replicating extensively within these cells, causing placentitis (5) and abortion in the last trimester of pregnancy (6). The fetus becomes a source of infection for other animals (7) and humans (8). If the newly infected animal is pregnant, the abortive bacterial cycle continues (9). Humans may become infected by direct contact with secretions of animals with brucellosis or through ingestion of unpasteurized, contaminated dairy products (10).
In contrast to infection in the preferred natural host, brucellosis in humans is a grave debilitating disease (8, 10). The Mexican brucellologist Maximiliano Ruiz-Castañeda (1892–1992) described 34 different symptoms and signs (10), and Spink described about the same number (8). The disease progresses without the characteristic endotoxic symptoms of other bacterial infections and is seldom associated with distinctive clinical or laboratory markers. In its mild form, the disease displays a nonpathognomonic collection of symptoms, such as fever, joint pain, myalgia, headache, and neurasthenia, among others, and, therefore, is difficult to diagnose. If not properly treated, the bacterium may invade and replicate in vital organs, such as the bone marrow (BM), the heart, and the brain, causing a severe syndrome, even death (8, 10, 33).
The natural replicative niche of Brucella organisms corresponds to the intracellular environment of animal cells. Alternatively, they are better defined as facultatively extracellular intracellular pathogens rather than facultative intracellular parasites (32). It is therefore not surprising that Brucella species are closely related and share functional and structural properties with other animal and plant cell-associated alphaproteobacterial parasites, such as Bartonella, Rickettsia, Anaplasma, Afipia, Wolbachia, Ochrobactrum, Sinorhizobium, and Agrobacterium (24, 34). The primary host cells in which Brucella organisms replicate are monocytes (Mo), Mϕs, DCs, and placental trophoblasts (21). Infected at much lower rates are B lymphocytes, fibroblasts, osteoblasts, granulocyte-progenitor cells, hepatocytes, and erythrocytes (35–40). In the course of brucellosis, mononuclear phagocytic leukocytes are recruited at the site of infection and are the main effectors engaged by adaptive immunity (41–43). As already mentioned, PMNs also ingest Brucella organisms in high numbers, but their function in brucellosis departs from that of mononuclear phagocytic cells.
As with other chronic bacterial infections, the resistance to pathogenic Brucella organisms relies on strong cell-mediated immunity. This response involves the activation of the bactericidal mechanisms and antigen-presenting functions of Mϕs and DCs and the concomitant activation and expansion of CD4+ and CD8+ T cells, which are the principal immune effectors in brucellosis (22). An adequate Th1 immunity, with significant production of gamma interferon (IFN-γ), interleukin-12 (IL-12), IL-6, and other cytokines, is critical for the clearance of Brucella infections. Studies in animals and humans have demonstrated that IFN-γ is the central cytokine in the adaptive immune response against brucellosis. Animals deficient in this cytokine or its receptor are highly susceptible to infection (44). Although antibodies play some role against brucellosis, clinical observations and experimentation in animals have shown that they may be dispensable, together with B cells (45). Like other cell-associated alphaproteobacterial pathogens, in order to establish long-lasting infections, Brucella has evolved different strategies to evade innate and adaptive immune responses (23, 46). Vaccination with B. melitensis Rev1 and B. abortus S19 live vaccines effectively protect against ovine/caprine brucellosis and bovine brucellosis, respectively (47). Brucella extracts or attenuated strains have been indiscriminately and dangerously used to vaccinate humans. Despite these efforts, no suitable vaccines are available for humans (47).
THE ROLE OF PMNs IN BACTERIAL INFECTIONS
In this section, we summarize some of the most relevant information on the function of PMNs within the context of bacterial diseases. For further details on the role of PMNs, we recommend comprehensive reviews published elsewhere (48–52).
PMNs are essential components of the innate immune system devoted to the control of microbial infections and are the first leukocytes to be recruited at the invasion sites (53). PMNs are also the primary cell effectors in the innate immune response and play a role in regulating adaptive immunity (48, 54). PMN homeostasis maintains a delicate balance between granulopoiesis in the bone marrow (BM), storage, release, intravascular margination, clearance, and destruction (48). In the BM, the production of PMNs corresponds to 5 × 1010 to 10 × 1010 cells/day, following a maturation process that includes invagination of the nucleus and lysosomal granule formation (55). Mature PMNs migrate from the BM to peripheral blood and the mononuclear phagocytic system and beneath the mucous membranes (56). The half-life span of PMNs is open to dispute, ranging from close to 6 h to 6 days and subject to various circumstances (51, 57), such as their location in tissues, which include the BM, reticuloendothelial system, lymphatic organs, and blood. Chronic diseases may prolong or shorten the life span of PMNs, a phenomenon that depends on the course of the illness and the type of pathogen.
After the bacterial invasion of tissues, PMNs become rapidly activated by cytokines and chemotactic signals, migrating through endothelial membranes of the small blood vessels to exert their microbicidal properties (53). Recruitment of PMNs at a site of infection is a crucial phenomenon of the innate immune response. Chemoattractants, such as chemokines, cytokines, complement-derived fragments, leukotrienes, and microbial components such as N-formylated peptides, influence leukocyte migration through the activation of G protein-coupled receptors on the PMN membrane (50).
PMNs are powerful professional phagocytes capable of ingesting and killing microbes intracellularly by activating hydrolytic enzymes, cationic microbicidal peptides, and reactive oxygen species (ROS). On their surface, PMNs express receptors for complement proteins, immunoglobulins (Fc), integrins, selectins, cell adhesion molecules (CAMs), and mannose, as well as scavenger receptors, which function as adherence molecules or for phagocytosis. Activated PMNs may degranulate and discharge ectosomes, microbicidal substances in the surroundings, or discharge PMN-extracellular traps (NETs). These last elements are composed of sticky DNA, histones, and other proteins that entrap and kill microorganisms through their cationic or enzymatic action (58). PMNs also produce chemokines, cytokines, prostaglandins, lipoxins, and other lipid mediators to attract, activate, and regulate cells of the immune system required for defense (49).
In many bacterial diseases, the primed, activated PMNs extend their life span and enhance their microbicidal activity (53, 58, 59). Cytokines such as IFN-γ can activate and prolong the life of these leukocytes in infections (60). After the bacterial invaders are destroyed, and the inflammatory response slowly resolves, a careful elimination of the recruited PMNs occurs. This process, which avoids further harm and favors tissue repair, initiates when the recruited PMNs undergo apoptosis at the resolution site. The dying PMNs express “eat me” signals such as phosphatidylserine on the cell surface, engaging Mϕs and DCs to phagocytize these apoptotic leukocytes in manners that do not promote proinflammatory responses, generally known as nonphlogistic processes (61). Indeed, phagocytic cells with internalized apoptotic PMNs are not fully activated and hamper the release of proinflammatory agonistic signals but promote the production of regulatory cytokines such as IL-10 and transforming growth factor beta (TGF-β) (56, 62). In other cases, PMNs become fully proinflammatory: they enhance their microbicidal activities, release cytokines and chemokines, degranulate, undergo NETosis, or die by necrosis or oncosis (52).
Several bacteria have evolved strategies to influence the function of PMNs (63, 64). The canine and zoonotic pathogen Anaplasma phagocytophilum, a close phylogenetic relative of Brucella, evades the host immune response by furtive strategies (65, 66) and actively invades PMNs by a process in which the bacterium takes control of the lipid raft domain-containing glycosylphosphatidylinositol-anchored protein. Once inside PMNs, Anaplasma modifies the vesicular biogenesis to create a unique intracellular membrane-bound compartment that allows their replication in seclusion from lysosomal destruction (65). To survive within PMNs, Anaplasma hampers the activation of NADPH oxidase and the subsequent degranulation of these cells. Furthermore, A. phagocytophilum lacks genes for the synthesis of lipopolysaccharide (LPS), cyclic β-glucans (CβG), peptidoglycan, flagella, and fimbria. Therefore, this bacterium lacks some of the essential pathogen-associated molecular patterns (PAMPs) required to trigger the innate immune response through the action of pattern recognition receptors, known as PRRs (65).
Bartonella henselae and Afipia felis, pathogens that are close relatives of Brucella organisms, inhibit human PMN oxidative function and survive within these leukocytes (67). In cats (the preferred mammal host), bacteremia progresses without neutrophilia or endotoxicity (68). Likewise, a significant characteristic of trench fever bacteremia caused by Bartonella quintana is the absence of septic shock symptoms such as disseminated intravascular coagulation, neutrophilia, or organ failure (69). This phenomenon is related to the absence of agonistic effects of its nonendotoxic LPS on Toll-like receptor 4 (TLR4). It is noteworthy that Bartonella LPS shares many structural features with Brucella LPS (Br-LPS) (70–73), which departs from the classical endotoxic LPSs (74). It seems, therefore, that these closely related intracellular alphaproteobacteria share several features associated with a stealthy strategy to invade host cells.
Gram-negative bacteria from other class subdivisions follow different strategies. For instance, Shigella flexneri kills PMNs by necrosis, a process characterized by the release of tissue-injurious granular proteins (75). This event allows the bacterium to disrupt the epithelial barrier and enter its colonic host cells and cause dysentery. Likewise, Pseudomonas aeruginosa strains are toxic and cause lysis and oncosis of PMNs. The depletion of PMNs by P. aeruginosa contributes to the pathophysiology of the disease by facilitating bacterial extracellular replication and persistent infections (76, 77).
Although PMNs efficiently internalize chlamydiae, a significant proportion of the ingested bacteria survive inside these cells (78) and use these leukocytes as “Trojan horse” vehicles for bacterial dispersion into different organs (79). Chlamydia trachomatis prevents the activation of PMNs by releasing a protease-like activating factor that targets and releases a formyl peptide receptor-2 on the surface of PMNs. The cleavage of this protein dampens the G protein-coupled receptor signal and prevents the downstream activation of PMNs. The protease-like activating factor suppresses the oxidative burst, interferes with chemical-mediated activation of PMNs and NET formation, and enables the pathogen to survive inside PMNs for extended periods (80).
Even Gram-positive bacteria such as Neisseria gonorrhoeae, which promotes the intense recruitment of PMNs at the infection site, display some evasion strategies. The fact that a proportion of N. gonorrhoeae organisms can be cultured from PMN-rich exudates is an indication that at least some bacteria resist the microbicidal mechanisms of PMNs. It seems, therefore, that some of the ingested gonococci are able to express significant amounts of virulence factors, such as pili, fimbria, Opa proteins, lipooligosaccharide (LOS), and other unknown components, which protect them from oxidative and nonoxidative antimicrobial activities and modulate PMN phagocytosis as well as the release of antimicrobial components (81).
Besides the primary function of PMNs in innate immunity, these cells also regulate inflammation and adaptive immunity in various diseases. These effects may be stimulatory or inhibitory, and they may promote or dampen the adaptive immune response (48, 82–86). As expected, the stimulatory or inhibitory effects exerted on T and B lymphocytes, antigen-presenting cells (87–94), or NK cells (95, 96) are reliant on the disease and the infection model. Moreover, there are significant variations in the immune profiles between acute and chronic processes (97–99). The regulatory mechanisms exerted by PMNs on the immune response are discussed below in the context of brucellosis.
BRUCELLA VIRULENCE STRATEGIES
The overall virulence of Brucella is expressed by three different but related properties (Fig. 2): (i) the bacterial ability to control its intracellular life to replicate in host cells (20, 21), (ii) the bacterial capacity to behave as a stealthy pathogen at the onset of infection to avoid early immune activation (46, 100, 101), and (iii) the resistance of Brucella organisms to bactericidal substances (102, 103). These properties probably evolved from ancestral characteristics of free-living alphaproteobacteria.
FIG 2.
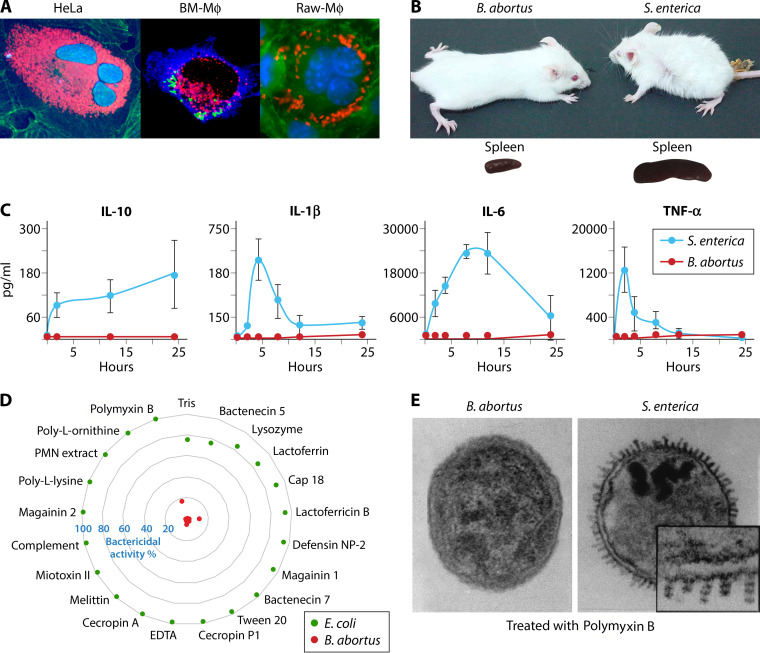
Three main virulent properties expressed by Brucella organisms. (A) B. abortus replicates extensively in dividing epithelial HeLa cells (left panel, bacteria in red, nuclei in blue, actin cytoskeleton in green) and in BM Mϕ (middle panel, bacteria in green, calnexin-positive compartments in blue, Cop-II compartments in red) or in dividing Raw Mϕ (right panel, bacteria in red, nuclei in blue, cytoskeleton in green). (Photos courtesy of Esteban Chaves [left], Jean Pierre Gorvel [middle], and Esteban Chaves and Pamela Altamirano-Silva [right], reproduced with permission.) (B and C) The capacity of B. abortus to behave as a stealthy pathogen is revealed by the absence of endotoxic symptoms and low spleen inflammation at the onset of infection (24 to 48 h) in B. abortus-infected mice in comparison with Salmonella enterica serovar Typhimurium-infected mice (B) and the negligible amounts of proinflammatory cytokines produced in comparison to S. enterica Typhimurium infection in mice at the onset of infection (C). (Panel C adapted from reference 100, published under the terms of the Creative Commons Attribution license [http://creativecommons.org/licenses/by/2.0].) (D) B. abortus resists the attack of an extensive collection of bactericidal substances, several of them expressed by cells. (E) While the outer membrane of B. abortus remains intact upon treatment with cationic peptides (polymyxin B), S. enterica Typhimurium demonstrates “blebbing,” chromatin condensation, and death (adapted from reference 102). Note in panel A that Brucella replicates extensively without inducing noticeable toxic effects in cells. Note in panel B that while the Salmonella-infected mouse (multiplicity of infection [MOI], 103) shows the characteristic endotoxic cachexia symptoms, the Brucella-infected mouse (MOI, 106) looks unaffected.
Control of Intracellular Life by Brucella Organisms
The ability of Brucella organisms to replicate inside Mϕs, Mo, DCs, and epithelial cells is directly related to the pathogenicity of these bacteria (Fig. 2A). Once inside permissive host cells, the Brucella-containing vacuole interacts with components of the endocytic and secretory pathways, such as early endosomes and vacuoles that intersect with the endoplasmic reticulum, where the bacterium replicates. In this process, the bacterium avoids fusion with lysosomes in a nontoxic encounter, extending the life of Mϕs and Mo and promoting the maturation of DCs (100, 104–106). Brucella ends its intracellular cycle within vacuoles of the autophagocytic apparatus, followed by the release of competent bacteria ready to infect PMNs and other cells (107). Once in PMNs, brucellae survive and persist within the phagosomes, resisting the bactericidal activities of these leukocytes for a protracted period (13–15).
The bacterial factors involved in the entry of Brucella organisms into cells are just partially known. PMNs and other professional phagocytes readily ingest opsonized Brucella with antibodies and complement through Fc and complement receptors (21). Brucella lacks classical surface virulence factors such as pili, fimbria, capsules, endotoxic moieties, toxins, or motile flagella (32). Still, several mutations in genes coding for various proteins (e.g., BvrS/BvrR, Omp25/Omp31 family, Omp2b, CGH, Efp, CcmC, BmaC, BtaE) and Br-LPS determinants (O chain, core, and lipid A) affect the binding of and penetration by Brucella to host cells (108–115). For most of these proteins, however, there is no demonstration of specific attachment to a cellular receptor. Furthermore, several of these mutations induce pleiotropic defects in the cell envelope, complicating matters even more.
Three exceptions are the following: (i) the O-chain of the Br-LPS that binds scavenger lectin-like receptors in Mϕs, (ii) the BmaC membrane protein that binds to fibronectin and the cognate receptor in epithelial cells, and (iii) BtaE, a polar protein that recognizes hyaluronic acid (41, 113, 115, 116). Br-LPS binds to C-type lectins on the surface of Mϕs, DCs, and PMNs (41, 117), an anticipated event, as mannose residues and sugars with mannose configuration are present in the Br-LPS core oligosaccharide and the perosamine O-chain homopolymer (Fig. 3A). Both BmaC and BtaE localize at the new pole of the bacterial surface, suggesting that this region may be functionally involved in adhesion, consistent with the inherent polarization of Brucella organisms (113, 115). The entrance of Brucella into cells involves the recruitment of several molecular determinants. Mϕs commonly ingest Brucella organisms through a zipper-like phagocytosis mechanism, with moderate recruitment of actin filaments and activation of the cyclic AMP/kinase pathway, followed by phosphorylation of the transcription factor CREB. In epithelial cells, Brucella organisms penetrate by phagocytosis via moderate recruitment of actin filaments, activation of small GTPases of the Rho subfamily, such as Cdc42, Rac, and Rho, and signals mediated by second messengers that include tyrosine kinase (Tyr-K), mitogen-activated protein kinase (MAPK), and phosphatidylinositol 3-kinase (PI3-K) (21).
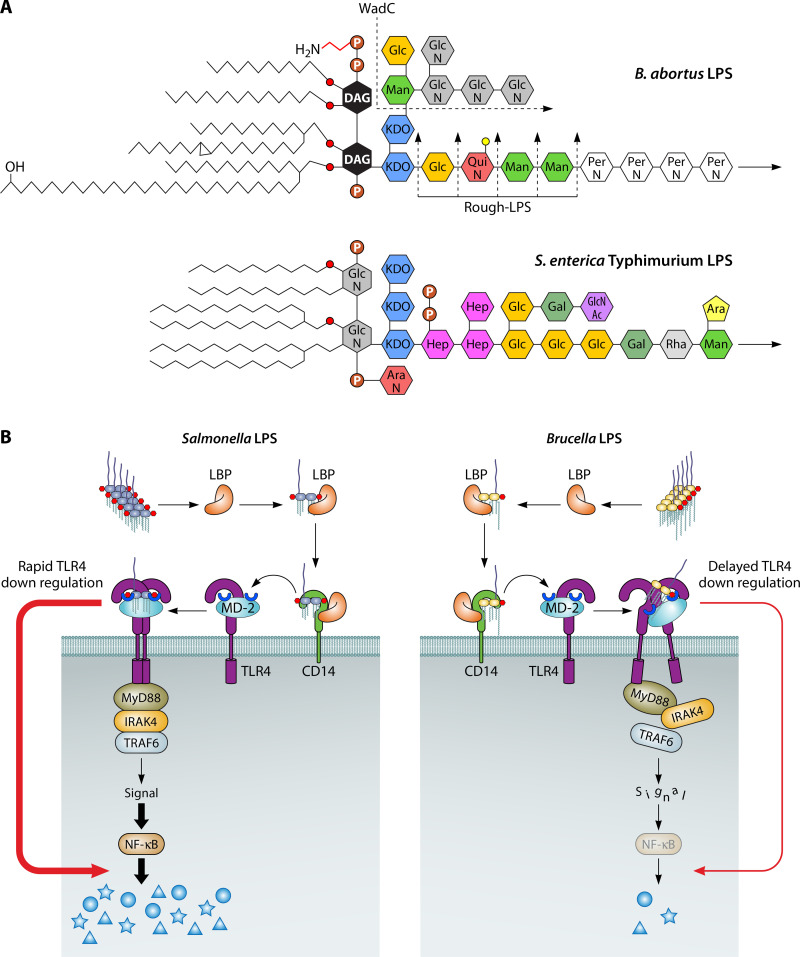
FIG 3.
Noncanonical Br-LPS determines several virulence properties of Brucella species. (A) Comparison of the schematic structures of the LPSs from B. abortus and S. enterica Typhimurium. Br-LPS displays a nonendotoxic lipid A composed of a diaminoglucose (DAG) backbone replaced by long-chain fatty acids up to 30 C long and a core oligosaccharide with low-density negative charges and an O-chain polysaccharide composed of a homopolymer of N-formyl-perosamine sugars (70, 71, 150, 151). In contrast, S. enterica LPS has an endotoxic lipid A composed of a glucosamine (GlcN) backbone replaced by shorter-chain fatty acids with a core oligosaccharide with high-density negative charges and an O chain with ramifications (74). The B. abortus WadC mutant lacks positively charged GlcN sugars, exposing the negative charges of 2-keto-3-deoxyoctulosonic acid (KDO) and phosphates, while the B. abortus rough-LPS mutants, which may occur at different levels, lack the protective O-chain polysaccharide. Man, mannose; Glc, glucose; QuiN, quinovosamine; PerN, N-formylperosamine; AraN, arabinosamine; Hep, heptose; Gal, galactose; GlcNAc, acetyl-glucosamine; Rha, rhamnose; Ara, arabinose; P, phosphate; P-H2N, ethanolamine phosphate. (B) The classical endotoxic Salmonella LPS readily binds to the lipid-binding protein (LBP) and CD14 receptor that transfer the LPS to the MD-2 coreceptor required for activation of TLR4 and downstream events that result in the induction of NF-κB and production of proinflammatory cytokines. In contrast, Br-LPS barely promotes the synthesis of proinflammatory cytokines due to its defective MD-2 binding, lack of TLR4 activation, and subsequent triggering of downstream events. Similar nonagonistic effects have been found for other Brucella molecules, such as flagellin, lipoproteins, and ornithine-containing lipids (135, 143, 144).
Brucella organisms have adapted their physiology for intracellular life (118). Therefore, it is understandable that the vast majority of the so-called virulence factors described are related to cell cycle and metabolism (119, 120). However, some molecules are strictly required for transit from extracellular to intracellular environments, trafficking, and replication within cells. Br-LPS is one of the main factors involved in virulence. Brucella rough mutants display disruptive intracellular biogenesis, do not evade lysosomal fusion in Mϕs and DCs, are readily destroyed by PMNs, and are attenuated in animal models (13, 21, 121). The mode by which Br-LPS modulates the biogenesis of phagosomes in Mϕs and epithelial is barely known. In Mϕs, Br-LPS is released within phagocytic compartments. Once inside, Br-LPS binds to the vacuolar membrane and hampers several functions, including antigen presentation and activation (122).
A significant Brucella constituent involved in virulence corresponds to the two-component regulatory BvrR/BvrS system, which works as a master regulator of other systems (114, 123–126). The bvrR and bvrS gene mutants are attenuated and readily destroyed by PMNs and other cells (Fig. 4A). Coordinated functions between the BvrR and BvrS proteins are required to modulate the bacterial physiology and the shift from extracellular to intracellular life. This system controls the homeostasis of components of the cell envelope, such as outer membrane proteins and Br-LPS. Since BvrR/BvrS mutants display cell envelope defects, they are also sensitive to the microbicidal action of PMNs. The phosphorylated regulatory protein BvrR interacts with the genes coding for quorum-sensing VjbR and the type IV secretion system VirB, essential for intracellular life. The BvrS/BvrR system also controls pathways related to carbon and nitrogen metabolism.
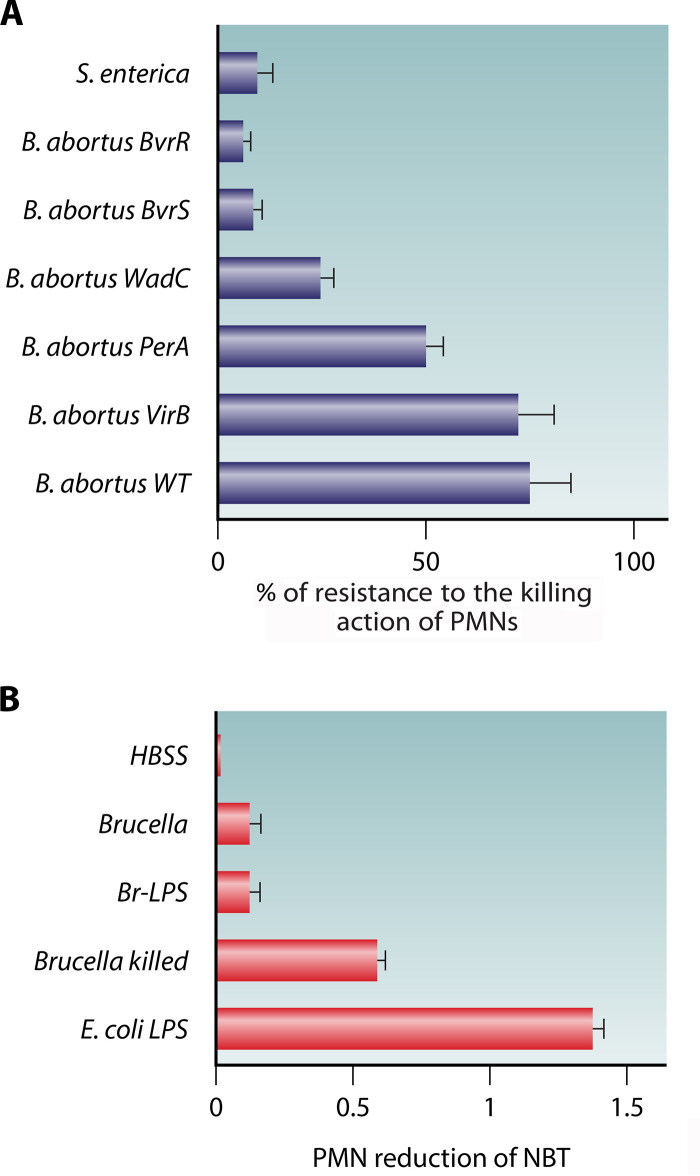
FIG 4.
Brucella is resistant to the killing action of PMNs and barely induces activation of these leukocytes. (A) Sensitivity to the bactericidal action of human PMNs (after 1.5 h) against S. enterica and B. abortus mutants. The B. abortus BvrR and B. abortus BvrS mutants have membrane and lipid A-fatty acid defects; the B. abortus WadC mutant has a core defect; the B. abortus PerA mutant lacks O-chain polysaccharide. The B. abortus VirB mutant is a smooth bacterium defective in the type IV secretion apparatus. Note that resistance to the bactericidal action of human PMNs is similar in the virulent B. abortus wild type (WT) and the attenuated B. abortus VirB mutant. (B) In contrast to E. coli LPS and killed Brucella, live B. melitensis and Br-LPS promote a meager reduction of nitroblue tetrazolium (NBT) of goat’s PMNs (Hanks' balanced salt solution [HBSS]). The NBT reduction indirectly measures ROS activity as well as the activity of oxidoreductases such as NADPH. (Based on data from reference 204.)
Like other cell-associated alphaproteobacteria (127), Brucella requires the VirB type IV secretion system for intracellular survival. Attenuated Brucella VirB mutants are readily killed by Mϕs, DCs, and epithelial cells, since they do not avoid the fusion of Brucella-containing vacuoles with lysosomes (106, 128). The function of the VirB system is connected with the BvrS/BvrR and the VjbR complexes (123, 125), all essential for virulence (129). There are several putative effector molecules tentatively associated with the VirB apparatus. The mechanisms of transfection and the function of most of these molecules are still unclear (130), and very probably, they do not play a role in PMN parasitism, since VirB mutants survive for a protected period in these leukocytes (Fig. 4A).
Although the flagellum-like apparatus is not conspicuous on the bacterial cell, it is required at later stages of Brucella infection (131). Finally, Brucella periplasmic cyclic β-glucan (CβG) prevents fusion of the Brucella-containing vacuole with lysosomes by modifying cholesterol-rich lipid rafts present on the vacuolar membrane and is in control of the endosomal maturation process (132).
Brucella Organisms Behave as Stealth Pathogens
Brucella organisms barely induce proinflammatory responses at the onset of infection (Fig. 2B and C) (100), a characteristic related to the structure of their putative PAMPs. Various types of PRRs, such as TLRs and NODs, barely recognize Brucella PAMPs, since these bacterial molecules display molecular modifications (46, 100, 133–135). Despite some initial controversies on the role of TLR4 in murine brucellosis (136), evidence for low agonistic activity of Br-LPS is robust and extensive (100, 106, 135, 137, 138). As in other alphaproteobacterial pathogens (71, 73), this phenomenon is linked to the nonconventional core-lipid A moiety of Br-LPS (Fig. 3A). Br-LPS does not fit and barely binds to the MD-2 coreceptor of TLR4. This event hampers the recycling and activation of TLR4 and the subsequent induction of NF-κB that promotes the synthesis of a collection of proinflammatory cytokines (Fig. 3B) (137). Moreover, Br-LPS induces just low quantities of ROS mediators (Fig. 4B), and the blocking of TLR4 does not hamper the interaction of Br-LPS with PMNs (139).
A similar controversy has been observed for TLR2 and TLR9 (100, 137, 140–142). While some authors do not find any effect on bacterial replication when one of these TLRs is absent, others find a slight difference in the bacterial loads. It seems evident that the different Myd88-dependent TLRs cooperate in the control of brucellosis at later stages. It has been shown that TLR2 and TLR4 cooperate in Mϕ survival at later stages of B. abortus infections (100) and in the survival of B. microti-infected mice. This event is significant, since in contrast to other Brucella species, B. microti causes bacteremia and displays strong pathogenicity for mice (142).
Likewise, the Brucella nonmotile flagellum-like apparatus (131) is not agonistic for TLR5, a property shared with various alphaproteobacterial pathogens (143, 144). Brucella-derived outer membrane fragments containing large amounts of lipoproteins induce low proinflammatory responses compared to conventional PAMPs (100). Ornithine-containing lipids that are potent PAMPs in other bacteria (145) do not have a detectable agonistic function during Brucella infections (135). Other molecules, such as β-cyclic glucans (CβG), have both pro- and anti-inflammatory properties (146).
The Brucella outer membrane works as a shield for PAMPs such as CpG, peptidoglycan, and CβG (138, 147). This property is related to the high hydrophobicity of the Brucella outer membrane, which results from the long hydrocarbon aliphatic chains (up to 30 C atoms) replacing phospholipids, ornithine lipids, lipoproteins, and the lipid A moiety (135, 148). Some of these long-chain fatty acids may span the Brucella outer membrane, making the bacterium strongly resistant to disruption (102).
In any case, the overall mild agonistic effect that Brucella organisms exert over PPRs is reflected in the low activation of infected Mϕs, DCs, and PMNs and the deficient production of proinflammatory cytokines at the onset of infection (Fig. 2C) (46, 100). These experimental observations correlate with the often-prolonged incubation time, the nonendotoxic clinical manifestations, and the absence of distinctive proinflammatory markers in brucellosis patients (8, 10, 149). Consequently, live Brucella organisms and their putative PAMPs barely activate PMNs and other immune cells (Fig. 4B), giving these furtive pathogens time to invade, replicate, and spread through different tissues before the activation of adaptive immunity.
Brucella Organisms Are Resistant to Bactericidal Substances
The resistance of Brucella organisms to bactericidal molecules of physiological fluids and cells is due, in part, to the structure of its cell envelope. The positively charged Br-LPS core oligosaccharide, composed of amino sugars, and the O-chain and NH homopolymers, built of nonreducing N-formyl-perosamine residues (Fig. 3A) (150, 151), form a shield, masking the available negative charges on the smooth brucella surface (152). This property makes the invading bacterium “invisible” to various PRRs and resistant to the microbicidal action of cellular cationic microbicidal molecules of PMNs and other cells (102, 103, 148, 153, 154). Indeed, the outer membrane of virulent brucellae is resistant to complement, oxidative substances, defensins, bactenecins, cathelicidins, lysozyme, phospholipases, lactoferrin, and PMN extracts, among others (Fig. 2D and E). The sensitivity of cell envelope Brucella mutants to these substances is linked, directly or indirectly, to modifications of Br-LPS (114, 133, 138).
One attractive characteristic not directly linked to the structure of the cell envelope is the resistance of Brucella organisms to DNA alkylating stress mediated by phagocytic cells. Genes involved in repair and base excision repair pathways are required by the bacterium to confront alkylation stress within phagosomes (155). This event is commensurate with the absence of bacterial replication within PMNs, a characteristic that favors resistance to the killing action.
Evolution of Brucella Virulence Mechanisms
The close phylogenetic relatedness of Brucella organisms with other cell-associated alphaproteobacteria has allowed comparison of the structure and function of several systems (138). Ochrobactrum spp. are the closest phylogenetic relatives of Brucella spp. These bacteria share with Brucella pathogens structural and functional features, such as lipid A, ornithine-containing lipids, flagellin, lipoproteins, CβG, and orthologous systems such as VirB, BvrS/BvrS, and VjbR, among others (34, 138, 143, 156). Still, the PAMPs of this opportunistic pathogen keep some structural properties that induce proinflammatory responses (138). Upon infection, Ochrobactrum kills neutropenic mice, promotes the release of much higher cytokine secretion, and induces neutrophilia and recruitment of PMNs. Ochrobactrum LPS has more negative charges in the core oligosaccharide than Br-LPS, a property that makes the outer membrane of this opportunistic bacterium more susceptible to the recognition of microbicidal cationic substances. In comparison to Br-LPS, which barely binds MD-2/TLR4 molecules, Ochrobactrum anthropi LPS binds with a high affinity to the MD-2 coreceptor of TLR4. Consequently, O. anthropi LPS triggers NF-κB (138) and induces a more robust cytokine response and activation of Mϕs and PMNs than Br-LPS. This behavior reveals that in Brucella, the putative PAMPs have been eliminated, modified, or hidden compared to free-living alphaproteobacteria.
The B. abortus WadC core mutant directly proves the critical role that Br-LPS has in virulence (133). The core oligosaccharide of this attenuated mutant lacks the positively charged glucosamine branch linked to the second 2-keto-3-deoxyoctulosonic acid (KDO) while keeping the O chain bound to the first KDO (Fig. 3A); hence, the negatively charged molecules become exposed. Consequently, bactericidal molecules of PMNs and other cells can attack and kill the WadC mutant more efficiently (Fig. 4A). The WadC mutant also induces higher production of proinflammatory cytokines in infected phagocytic cells, a phenomenon that is reproduced by its Br-LPS. Like the Ochrobactrum LPS, the WadC LPS binds to the MD-2 coreceptor of TLR4 with higher affinity, triggering the activation of NF-κB. As expected, the WadC mutants are also killed more efficiently by PMNs than virulent wild-type (WT) Brucella organisms (Fig. 4A).
These examples suggest that the free-living Brucella alphaproteobacterial ancestor evolved and adapted to infect animal cells by fine-tuning its virulence mechanisms. Some of these adaptations involved structural and functional modifications of the surface molecules and PAMPs and other virulent systems such as VirB, BvrS/BvrR, and VjbR. Thanks to these molecular adaptations, Brucella organisms were capable of circumventing the activation of PMNs, became stealthy pathogens, and avoid the early recognition of the innate immune system. This strategy opened a “window” for the dispersion and expansion of Brucella organisms before activation.
PMN RECRUITMENT IN THE COURSE OF BRUCELLOSIS
Mucosal epithelial cells, Mϕ, DCs, and PMNs are the first cells to encounter Brucella organisms in the mucous membranes (16, 157). Despite this, brucellosis seldom progresses with the characteristic neutrophilia of many other bacterial infections (Fig. 5) (19, 31, 100, 101, 158–161). At the onset of systemic infection (the first 48 h) of experimental murine brucellosis, there is no recruitment of PMNs at the infection site, and the number of PMNs in blood or target organs, such as the spleen and liver, remains low. Five days after infection, at the beginning of the acute brucellosis phase (43), there are still negligible numbers of infected PMNs in the target organs but significant recruitment of infected mononuclear phagocytes (42). After 2 weeks of bacterial replication in cells of the reticuloendothelial system, granulomas composed of lymphocytes and infected Mϕs and DCs become evident, while PMNs are at low numbers and are not infected (42, 162).
FIG 5.
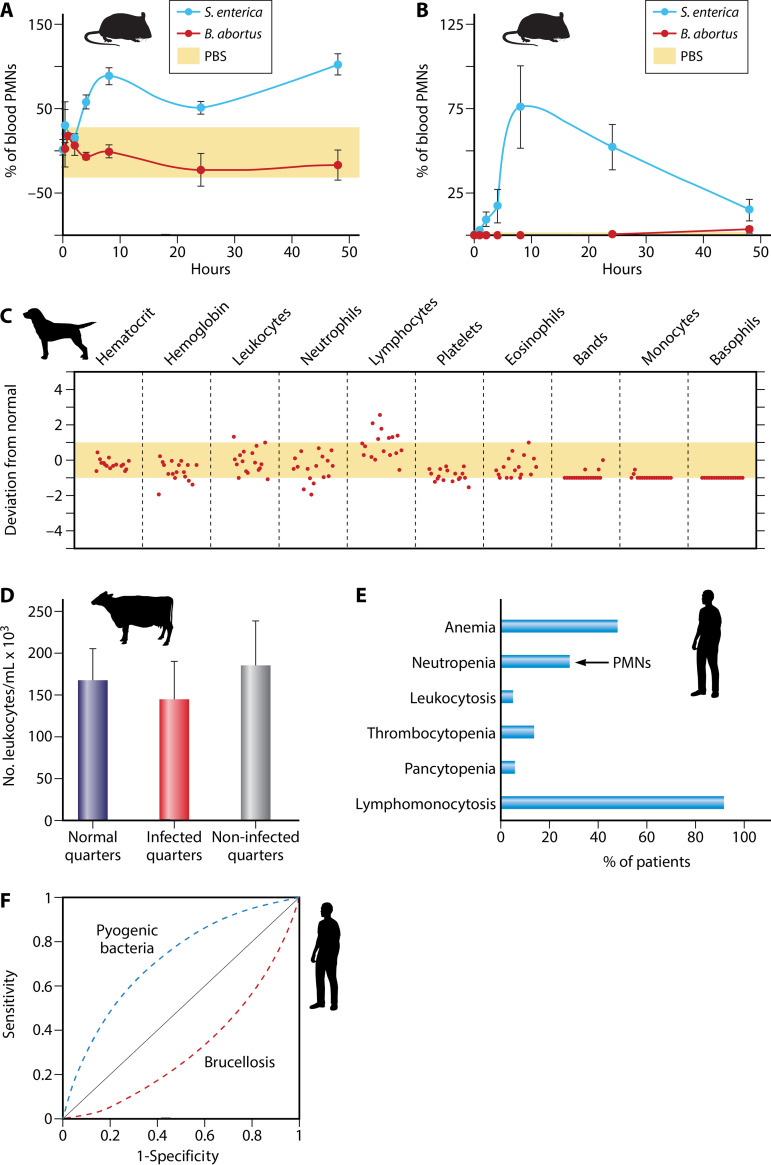
Leukocyte counts in brucellosis. (A) Blood PMN counts in S. enterica Typhimurium- and B. abortus-infected mice. The yellow area demarcates average blood PMN normal values. PBS, phosphate-buffered saline. (Adapted from reference 100, published under the terms of the Creative Commons Attribution license [http://creativecommons.org/licenses/by/2.0].) (B) PMN counts in the peritoneal fluids of five mice infected with S. enterica Typhimurium or B. abortus. The yellow area (close to the x axis) depicts the normal maximum upper and lower limits. (Adapted from reference 100, published under the terms of the Creative Commons Attribution license [http://creativecommons.org/licenses/by/2.0].) (C) Hematological profiles of 17 B. canis-infected dogs. The average maximum upper and lower limits correspond to the yellow area for each cell type. Each circle represents one dog, numbered from 1 to 17, with number 1 being the farthest to the left in each panel. (Adapted from reference 101.) (D) Leukocyte counts in infected quarters (with isolation of Brucella spp.) and noninfected quarters of udders of 18 cows with brucellosis and comparison with quarters from udders from noninfected cows. (Based on data from reference 158.) (E) Blood cell profile of 530 human patients with brucellosis. Neutropenia, due to the absolute low PMN number (<2,500/mm3), is observed in about one-third of the patients, while relative lymphomonocytosis is observed in close to 90% of the patients. (Based on data from reference 159.) (F) Comparison of receiver operating characteristic (ROC) curves estimated by regression analysis of lymphocyte/PMN ratios in human patients with brucellosis and infected with pyogenic bacteria. Note the frequent neutropenia as a predictive indicator for brucellosis diagnoses in contrast to pyogenic bacterial infections. (Based on data from references 160 and 161.)
The recruitment of PMNs depends on the route of infection. Natural Brucella infection through the skin is rare, since these organisms cannot cross this epithelial barrier (8, 10). Despite this, experimental subcutaneous inoculation of Brucella organisms into the skin of humans induces the recruitment of PMNs and histiocytes to the site of infection, within a few hours (33). Likewise, subcutaneous Brucella injection in mice promotes transient recruitment of PMNs at the site of infection. However, this effect is 10 times lower than the recruitment of PMNs induced by subcutaneous injection of Salmonella (100). Infection with high doses of bacteria (107 CFU) by the intradermal route in the footpad of mice recruits some PMNs and fibroblasts in the skin (35). However, intradermal administration of Brucella crude extracts in mice after 21 days of bacterial infection promotes only the migration of Mϕs at the injection site after 24 and 48 h, with no early or late migration of PMNs (163). A similar inhibitory effect occurs in PMN mobility in the presence of Brucella extracts in human patients (164). Moreover, intradermal infections with Brucella organisms or extracts containing Br-LPS and other components do not promote a Shwartzman reaction or the characteristic immediate local inflammatory response and recruitment of PMNs observed after the injection of Gram-negative bacteria or enterobacterial endotoxin (6, 8, 10, 165).
As stated before, the natural route of Brucella infection is through mucosal membranes. In humans and the preferred host, the mucosal infection seldom promotes dense PMN recruitment within the next 6 to 8 days (157). Brucella infection of mice by the intravenous, intraperitoneal, intranasal, intragastric, or oral route barely promotes the recruitment of PMNs in the blood (Fig. 4A), peritoneum (Fig. 5B), lungs (35), regional lymph nodes (35), spleen (35, 42, 162), or gastric mucosa (166). Intratracheal inoculation with a high number of Brucella organisms (108 CFU) induces microgranulomas and necrosis in the lungs and granulomas in the liver in the presence of PMNs. However, this does not occur with a lower number of infecting bacteria (<106 CFU) (167).
We ignore why the subcutaneous inoculation of Brucella organisms promotes transient recruitment of PMNs at the site of infection while systemic infections by other routes commonly do not. Although mast cells do not phagocytize Brucella organisms, these bacteria are capable of inducing degranulation of these leukocytes by an unknown mechanism (168). Resident mast cells, beneath the skin, recruit PMNs to release cytokines and chemokines and coordinate the movement of these phagocytic leukocytes to the site of infection (169). Therefore, it is feasible that the subcutaneous inoculation of large numbers of Brucella organisms promotes the degranulation of mast cells and the concomitant recruitment of PMNs in the skin. It seems that live Brucella organisms are required to recruit PMNs in the skin, since the injection of bacterial extracts or Br-LPS at this site does not induce the same effect.
Epididymitis, orchitis, placentitis, osteoarthritis, BM pancytopenia, meningoencephalomyelitis, and other severe tissue pathologies are mainly observed in long-lasting brucellosis cases (8, 10). While the recruitment of mononuclear phagocytes at the infection site is a constant feature, the arrival of PMNs is related to the disease's duration and is reliant on the local cytokine and chemokine response and tissue injury (35, 37, 170–174). Mϕs, DCs, and fibroblasts produce chemotactic signals, while intensely infected tissues generate damage-associated molecular patterns known as DAMPs. These two substances are potent mediators for recruiting PMNs and other inflammatory cells (174).
Abortion is the consequence of placental destruction and cytokine release in the last trimester of pregnancy. At this stage, there is an intense Brucella parasitism of trophoblasts, characterized by necrosis and the presence of a high number of extracellular bacteria, accompanied by a conspicuous inflammatory exudate and cellular debris (Fig. 5) (170, 172, 175–177). After an abortion, the infected placenta shows vasculitis and necrotizing placentitis, with infiltration of Mϕs and PMNs with a large number of intracellular Brucella organisms (Fig. 6A and B). The destruction of the trophoblast layer promotes the release of DAMPs and cytokines and the subsequent recruitment of PMNs (175, 178, 179). The release of proinflammatory mediators by B. abortus-infected bovine trophoblasts in vivo and in vitro is dampened at an earlier but not later stage of the infection (171).
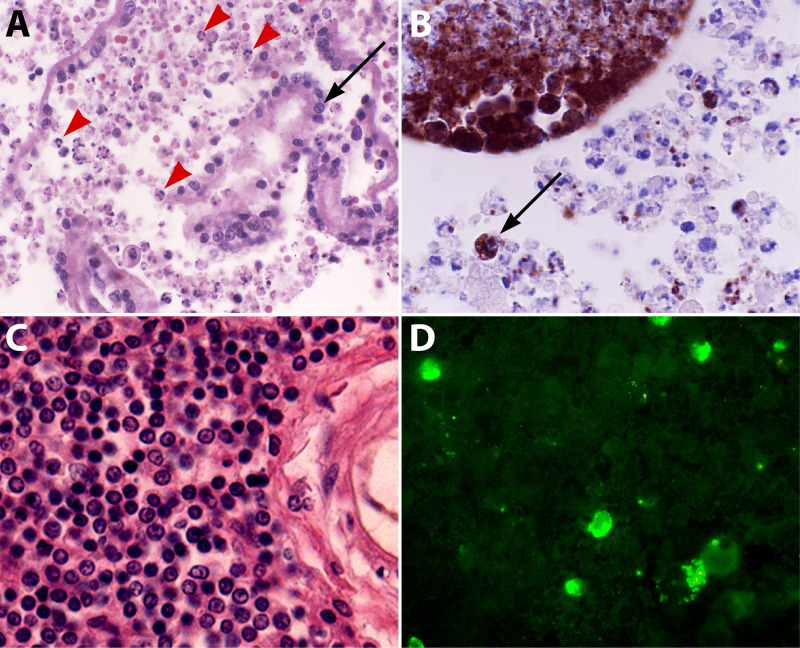
FIG 6.
Histopathological findings in the placenta and brain of a Brucella ceti-infected striped dolphin. (A) Placental villi with mononuclear and PMN inflammatory infiltrate (red arrowheads) and some detached placental epithelial cells (arrow). Hematoxylin and eosin (HE) stain. Magnification, ×40. (B) Immunoperoxidase labeling of intracellular Brucella antigens inside inflammatory PMNs and mononuclear cells (dark brown) invading the placental villi (example indicated by an arrow). Immunohistochemistry. Magnification, ×40. (C) Mononuclear infiltrate in infected brain meninges with the absence of PMNs. HE stain. Magnification, ×40. (D) Immunofluorescence of infected mononuclear cells (in green) in the cerebrospinal fluid of infected meninges. HE stain. Magnification, ×40. The expected outcomes of stranded striped dolphins with brucellosis are abortion, meningoencephalomyelitis, and death caused by the invading bacteria (172). (All photos courtesy of Rocio Gonzalez-Barrientos and Gabriela Hernández-Mora, reproduced with permission.)
In contrast to the infected placenta, the lungs and other tissues of aborted fetuses from bovines with brucellosis display a predominant inflammatory infiltrate of Mϕs with just a few PMNs (176). Likewise, B. canis-infected dogs do not show neutrophilia or significant hematological alterations (Fig. 5C), and the placentae of bitches after abortion display a low inflammatory reaction, with very few or no PMNs (180). Brucella-infected mice also develop moderate multifocal necrotic placentitis (181). The infiltrate is not particularly purulent; still, the infected mouse placenta shows Brucella within trophoblasts and signs of inflammation, with infiltration of few PMNs and an abundance of other immune cells (181). Purulent placentae associated with endometritis and abortion are rare outcomes in murine brucellosis (182), and many pups come to term and are born alive (28, 182, 183).
At the initial stages of testicular Brucella infection in men, dogs, bulls, and rams, the inflammatory exudates of the epididymis and cortical testicular tissue seldom show a PMN predominance (31, 184). Later, severe epididymitis and orchitis, with infiltration of PMNs and mononuclear cells with intracellular bacteria, become evident (185). As in other tissues, the recruitment of PMNs may be due to the combination of cytokines and DAMPs released by the injured testicular tissue.
B. abortus and B. melitensis also have a strong tropism for the sinuses of the mammary gland in ungulates (175, 186). Despite this, there are no classical signs of mastitis, and macroscopically the udders do not demonstrate significant pathological signs. Although PMNs and other cells in the udder of Brucella-infected animals may harbor intracellular bacteria, PMNs are not particularly abundant in this tissue (170, 186). The infected quarters of cows do not shed more leukocytes than normal or noninfected quarters (Fig. 5D). Resident udder PMNs display significantly lesser bactericidal activity and lower ROS formation against Brucella than those induced by other pathogens such as Escherichia coli, Salmonella enterica, Streptococcus agalactiae, Staphylococcus aureus, Listeria monocytogenes, and Mycobacterium bovis (187, 188). This picture contrasts with human breast brucellosis. In rare cases of long-lasting disease, the bacterium may invade patients' breasts, with the generation of granulomas containing giant cells and PMNs (189).
Neurobrucellosis in humans and animals such as dolphins seldom progresses with neutrophilia and inflammatory exudates in the cerebrospinal fluid, and the meninges have mononuclear cells of lymphocytic and macrophagic origin, some of them containing Brucella and bacterial debris but seldom PMNs (Fig. 6C and D) (172, 190). This observation is relevant, since parenchymal nerve and glial cells do not become infected with Brucella organisms and inflammation of the brain is limited to the blood vessels and the meninges. Brucella invasion of the brain is a severe disease that may cause death (172).
In contrast to what happens in bacterially induced arthritis (191), Brucella infections in humans are not a frequent cause of bacterial septic or reactive arthritides, and most brucellosis patients with arthritis do not show significant hematological changes. Brucella organisms are seldom isolated from the mononuclear cell-enriched synovial liquids that generally are devoid of PMNs (192, 193). In long-lasting brucellosis arthritis and bursitis, PMNs may infiltrate the synovial fluid. Inflammation of the joints such as arthritis, bursitis, and hygromas may also be present in old bovines with a long history of brucellosis, mainly from low-income countries (194, 195). The most frequent histopathological lesions in bursitis with the isolation of Brucella from the synovial fluid correspond with multifocal necrosis, hemorrhage, and signs of a granulomatous process with a lymphohistiocytic inflammatory infiltrate containing Mϕs and some PMNs (194). As expected, joint infections also involve the local immune response and release of DAMPs due to tissue destruction (174).
B and T lymphocytes are dispensable in brucellar arthritis, while CXCR2 (receptor for IL-8/CXCL8 chemokine) is necessary for focal PMN recruitment and inflammation in the joints of mice (196). Although murine PMNs may participate in arthritis, they do not seem important in Brucella elimination, and CXCR2 in cells is not necessary for the clearance of these microorganisms. Brucella-infected human fibroblast-like synoviocytes produce proinflammatory cytokines and chemokine CXCL8, and supernatants from these infected cells recruit PMNs and Mo (197). Likewise, human osteoblasts upregulate their cytokine and CXCL8 production after coculture with supernatant from Brucella-infected Mo. In turn, Brucella-infected osteoblasts also induce proinflammatory cytokines and CXCL8 by Mo (36).
It seems that the ability of Brucella organisms to develop long-lasting infections is linked to their persistence in the BM (198, 199). In humans, the BM shows histopathological alterations such as granulomas and an augmented number of inflammatory cells. Later, neutropenia, thrombocytopenia, anemia, and, in severe cases, pancytopenia, myelodysplasia, and hemophagocytosis are common BM alterations (198, 200, 201). Brucella organisms can persist in the BM of infected mice for protracted periods and induce significant changes in this tissue. Granulomas and augmented numbers of BM myeloid granulocyte-monocyte progenitors, PMNs, and CD4+ lymphocytes are present during the acute phase (199). The most abundant infected BM cells at the early phases of murine infection (once antibodies against Brucella have developed) are PMNs, followed by granulocyte-monocyte progenitors and Mo. At later times, the number of infected PMNs in the BM decreases considerably, and the numbers become similar to those of infected Mo. The neutropenia observed in about one-third of patients with long-lasting infections (Fig. 5E and F) may be due to the sustained death of Brucella-infected PMNs and granulocyte-monocyte progenitor cells in the BM (39). Monocytes and granulocyte-monocyte progenitors are likely to serve as host cells for Brucella replication, remaining in this hematopoietic tissue for protracted periods. It is unlikely that PMNs function as bacterial reservoirs in the BM, since the number of these infected cells significantly decreases after the initial infection periods, along with the fact that Brucella organisms do not replicate in these leukocytes. Instead, according to the Trojan horse model, PMNs may function as vehicles for dispersion (39).
Repeated injections of antibodies against PMNs eliminate these leukocytes from blood, lymphatics, and other organs of mice but only partially remove them from the BM in Brucella-infected mice (93). Nevertheless, those PMNs that remain in the BM barely become infected (93). This result suggests that anti-PMN antibodies select functional and fully phagocytic BM PMNs, leaving a population of resting, not fully phagocytic PMNs in the BM. This observation is relevant since BM Mϕs phagocytize bacterial antigen-laden PMNs and subsequently present antigen to CD8+ T lymphocytes to generate memory T cells (202).
The clinical and experimental observations demonstrate that the recruitment of PMNs in brucellosis depends on the route of infection, the course of the disease, infected organs, and tissue destruction. At the onset of infection, and during the long incubation period, the recruitment of PMNs at the infection site and target organs is low. At these sites, mononuclear phagocytes and lymphocytes are the primary inflammatory cells. Throughout this period, Brucella organisms avoid the recruitment and full activation of PMNs, behaving as silent pathogens. Once the disease becomes evident and clinical signs appear, the infection progresses without neutrophilia hematological alterations or coagulopathies (19, 31, 100, 101). Like the brain or udder, some organs seldom present septic PMN infiltration, despite the large numbers of bacteria present in these tissues. Later, PMNs may arrive at the damaged, infected organs, such as the testes, placenta, joints, or BM. However, PMNs do not contribute to the resolution of the Brucella infection, and these leukocytes are dispensable for bacterial clearance and the cellular immune response (87, 93).
RESISTANCE OF BRUCELLA TO THE BACTERICIDAL ACTION OF PMNs
After infection, Brucella organisms are opsonized and quickly phagocytized by naive human, bovine, caprine, rat, and canine PMNs (6, 14, 15, 100, 203–207). Human and bovine PMNs internalize opsonized Brucella cells with complement or immunoglobulins or with integrins, C-type lectins, and fibronectin-binding receptors (6, 41, 117, 208, 209). In contrast, murine PMNs require specific antibodies to phagocytize smooth (but not rough) Brucella organisms, since N-formyl-perosamine oligosaccharides of the LPSs and related native haptens constitute a shelter for complement and fibronectin opsonization in these animals (205).
Brucella organisms can live in large numbers inside PMNs and resist the bactericidal action of these leukocytes (6, 13, 16, 100, 203, 209, 210). Still, Brucella resistance to PMNs is high but not absolute (Fig. 4A), and it depends upon various factors. Brucella is slightly more susceptible to IFN-γ-activated PMNs and PMN-granule extracts supplemented with H2O2 and halide (13, 117, 211). The bactericidal response of activated PMNs correlates with larger amounts of superoxide anion and H2O2 secretion. The production in Brucella organisms is regulated, and catalase mutant bacterial strains have high sensitivity to H2O2 (212). Extensive contacts with O2−, myeloperoxidase-H2O2-halide, and other components of the ROS system are lethal for Brucella organisms (14). Therefore, it seems that activated PMNs may circumvent, in part, the furtive behavior of Brucella once adaptive immunity has initiated and killed a proportion of the bacteria.
The bactericidal activities of PMNs also depend on the animal species and Brucella strain. For instance, bovine PMNs are more bactericidal than those of humans and caprine, and all these cells are more bactericidal than those of guinea pigs and mice (13–15, 204, 205). Likewise, bovine PMNs are more bactericidal after ingesting antibody-opsonized Brucella in the presence or absence of complement (208). In contrast, murine and guinea pig PMNs show low bactericidal activity against Brucella, regardless of antibody opsonization (13, 205). B. melitensis, B. abortus, B. canis, and B. suis display similar resistance against PMNs (117); however, the rough counterparts and some other attenuated mutants show increased sensitivity to these leukocytes.
The Brucella VirB attenuated mutants are resistant to PMNs for at least 2 h (Fig. 3C). In contrast, some mutants (e.g., HtrA) susceptible to PMNs keep their virulence in mice (210). This difference demonstrates decoupling between the capacity of Brucella to cause systemic infections and its resistance to PMNs and suggests that PMNs are dispensable for immune defense in brucellosis. It also indicates that the ability to control intracellular trafficking is independent of bacterial resistance to PMNs. The VirB mutants inside PMNs persist longer in the skin (35). This resistance suggests that Brucella organisms do not require the type IV secretion machinery for survival inside PMNs but rather depend on the other two virulent strategies. The relatively neutral conditions of PMN phagosomes in comparison to those of Mϕs may preclude the turning on of the Brucella type IV secretion system in PMNs (213). Indeed, the expression of the VirB system requires the acidic conditions of the Mϕ phagosomes (123). Therefore, the vacuolar milieu of PMNs constitutes a shelter for the bacterium rather than a replication niche.
The unique structure of the Brucella cell envelope protects these bacteria from the bactericidal action of PMNs; defects in this layer favor the destruction of these pathogens (133, 153, 203, 205). Rough Brucella mutants lack the outer membrane protective O-polysaccharide layer and display pleiotropic membrane alterations, exposing otherwise hidden molecular determinants (118, 152, 214, 215). As a consequence, rough mutants are more susceptible to bactericidal substances and PMNs (13, 15). As explained before, WadC exposes negative charges on its outer membrane, a property that makes the WadC mutant susceptible to the cationic bactericidal molecules of PMNs (133). Likewise, the smooth Brucella BvrR and BvrS mutants that display Br-LPS alterations and cell envelope defects show high susceptibility to bactericidal substances and PMNs (114, 124, 153). After the phagocytosis of these cell envelope-defective Brucella mutants, PMNs degranulate and generate a more robust ROS response than when infected with the fully virulent counterparts (13). Some investigators proposed that a nucleotide-like material derived from Brucella extracts was responsible for blocking PMN degranulation, with preferential inhibition of primary granule release (216). However, this proposal does not explain why the attenuated mutant brucellae that also produce this material, or even killed organisms, do not inhibit degranulation (13) or ROS activation (Fig. 4B).
As mentioned before, the opportunistic Ochrobactrum is more sensitive than Brucella organisms to bactericidal molecules and PMNs (138, 217), and ochrobacteriosis progresses with moderate to high neutrophilia in humans and mice (138, 218–220). In contrast to Brucella, Ochrobactrum replicates in neutropenic mice and promotes the secretion of cytokines but still less than Salmonella (138). These differences are due in part by the broader diversity of PAMPs in Ochrobactrum, which binds PRRs, promoting the activation of NF-κB (138, 221).
PMN DEACTIVATION BY BRUCELLA ORGANISMS
Vigorous proinflammatory activities characterize the biological properties of PMNs during bacterial infections. Among the most conspicuous are the robust production of cytokines and chemokines, oxidative burst with potent ROS generation, degranulation, the release of ectosomes, and NET formation (222). Even heavily infected Brucella PMNs, with up to 25 to 50 bacteria/cell, barely become activated or display significant phenotypic alterations (6, 16, 139, 205). Brucella-infected PMNs do not undergo NETosis, degranulation, necrosis, oncosis, or classical apoptosis morphology, and large quantities of Br-LPS do not promote noticeable phenotypic changes associated with activation (100, 139, 223). Antibiotics used to treat brucellosis, such as doxycycline, streptomycin, and rifampin, which influence the bactericidal functions of PMNs (224), do not promote the activation or degranulation of these Brucella-infected cells (225). These phenomena correlate with low ROS formation (Fig. 4B), negligible activation of the monophosphate pathway, and low myeloperoxidase activity of Brucella-infected PMNs (100, 188, 203, 204, 225, 226). These processes may be partially overcome, just by very high numbers of Brucella organisms (>100/PMN) (227). Moreover, PMNs from human patients with an early evolution of brucellosis do not show the robust activation observed in other bacterial diseases (228–230). In long-lasting Brucella infections, the PMNs of some patients show augmented migration against zymosan but diminished chemokinesis against specific antigens. Likewise, the phagocytosis mediated by PMNs diminishes, and the oxidative burst of unstimulated leukocytes is not augmented before treatment (164).
PMNs infected with Brucella organisms or treated with Br-LPS practically do not release proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α), IL-1β, and IL-6 (139) or regulatory IL-10 (231). This phenomenon is consistent with the low cytokine production of Brucella-infected mice at early time points of the infection (100, 137, 232). The small amounts of cytokines, particularly IL-1β, and the absence of caspase-1 involvement preclude the generation of the inflammasome pathway in Brucella-infected PMNs (139). One exception is the release of low but consistent amounts of IL-8 (CXCL8) (139). This chemokine, constitutively produced by PMNs, is readily available after Brucella infection (233). The CXCL8 may function as a “find me” signal for Brucella-infected PMNs by Mϕs (234).
The mechanisms behind the overall low activation of PMNs relate to the furtive nature of Brucella organisms. On the one hand, the putative Brucella PAMPs are not agonistic for PMN PRRs, hampering the activation of these leukocytes. On the other hand, Br-LPS promotes the premature cell death of infected PMNs (139). These two effects work together, triggering a short-term, nonphlogistic process that allows Brucella dispersion and infection of other cells.
BRUCELLA-INFECTED NEUTROPHILS AS VEHICLES FOR MACROPHAGE INFECTION
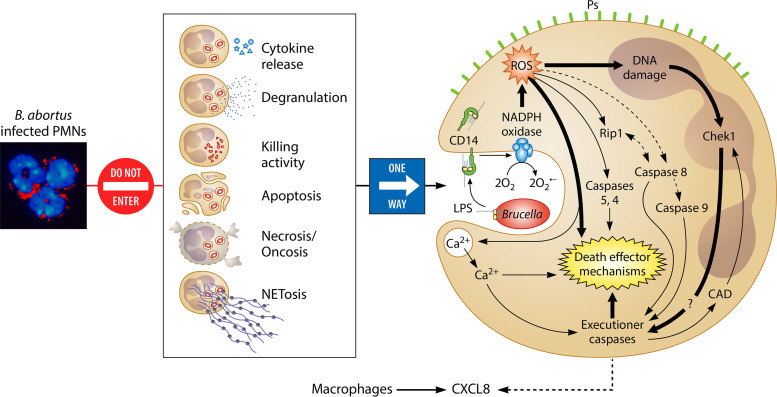
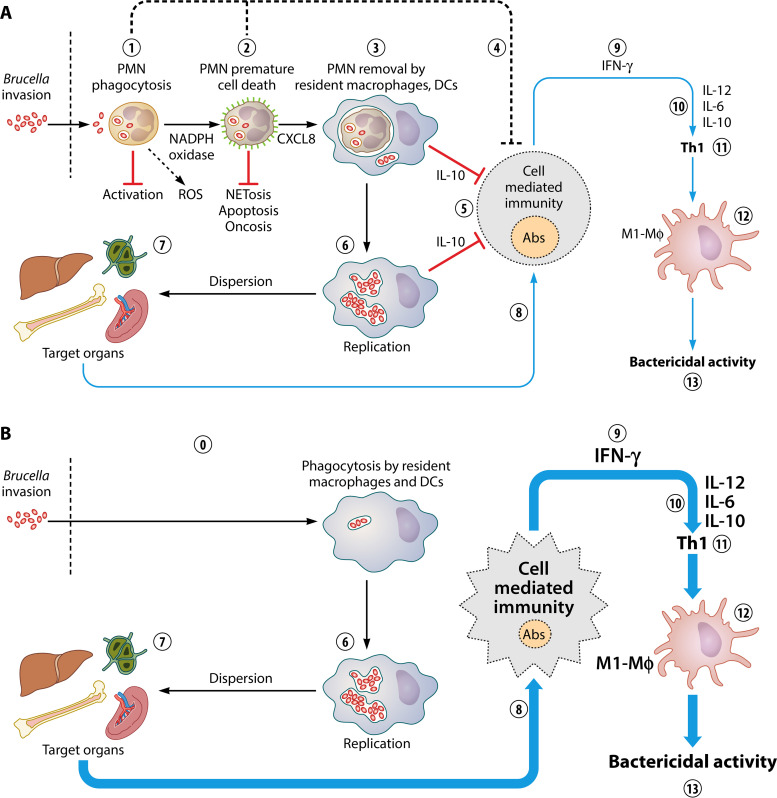
Intracellular Brucella organisms are nontoxic for host cells (Fig. 2A); instead, they inhibit apoptosis and prolong the life of Mo and Mϕs, induce the maturation of DCs, and do not hamper mitosis of the infected cells (100, 104–106, 235). Despite this, Brucella provokes the premature cell death of human and mouse PMNs in a dose-dependent manner, an event that is reproduced by Br-LPS and its lipid A (Fig. 6). The premature cell death of Brucella-infected PMNs initiates after phagocytosis of the bacterium and the subsequent intracellular release of Br-LPS. Once inside PMNs, Br-LPS fails to interact with TLR4; instead, it moves to CD14 lipoprotein domains in the plasma membrane or intracellular vesicles, as observed in Mϕs (122). Then, the nonendotoxic lipid A moiety of Br-LPS recruits NADPH oxidase, which promotes the controlled generation of small amounts of ROS, triggering the cell death mechanisms (Fig. 7). This phenomenon occurs without the conspicuous phenotypic and functional changes characteristic of PMN apoptosis morphology, NETosis, or oncosis (39, 139, 223, 226). Alternatively, the Brucella-infected PMNs proceed to nonphlogistic premature cell death that resembles, in some features, apoptosis, but without condensation of the nucleus or cell fragmentation (139). First, the Brucella-infected PMNs release CXCL8 chemokine as a “find me” signal, and second, they translocate phosphatidylserine to the membrane external surface as an “eat me” signal (Fig. 7) (39). As demonstrated in other systems, dying PMNs still perform oxidative metabolism, release CXCL8, and express phosphatidylserine on the external surface in a time-dependent manner (61, 236). After this process, Mϕs find infected PMNs and specifically detect phosphatidylserine on these cells (Fig. 8A). Then, Mϕs internalize the Brucella-infected PMNs in a nonphlogistic manner by a homeostatic-like procedure for PMN recycling (see Movie S1 in the supplemental material) (39, 51). Finally, at early times of infection, Mϕs release low quantities of proinflammatory cytokines but larger amounts of regulatory IL-10 (39).
FIG 7.
Brucella organisms do not activate PMNs but induce the premature cell death of these leukocytes. PMNs (nuclei in blue) heavily infected with B. abortus (in red) do not display significant phenotypic changes characteristic of degranulation, apoptosis, necrosis/oncosis, or NETosis and do not display significant bactericidal activities or release proinflammatory cytokines. However, through the release of nonendotoxic Br-LPS inside infected PMNs, B. abortus organisms promote the premature cell death of these leukocytes. After fusion with cell membranes, Br-LPS interacts with CD14 lipoprotein, progressively recruiting the action of NADPH oxidase and promoting the slow generation of controlled amounts of ROS mediators, which induce oxidative fragmentation of nuclear DNA and the recruitment of Chek1 protein, which is mainly responsible for coordinating the DNA damage after the activation of caspase-activated DNases (CAD). At the same time, some of the ROS effectors may induce recruitment of the RIP1 kinase/FADD cell death routes and caspase 8 and promote the release of Ca2+ to the cytosol. These mediators also recruit cell death executioner caspases and, together with ROS mediators, trigger additional death effector mechanisms (e.g., activation of calpains and cathepsins). Finally, the initiator caspase 9 of the intrinsic cell death pathway is activated downstream by caspase 8, contributing to the premature PMN cell death mechanism. In this process, the infected PMNs release chemokine CXCL8 to attract mononuclear phagocytic cells and expose “eat me” signals (e.g., phosphatidylserine [Ps]) on the surface to promote their phagocytosis. Since Br-LPS does not interact with TLR4 on PMNs, then these cells do not become activated through this pathway. (Adapted in part from supplemental material for reference 139, published under the terms of the Creative Commons Attribution license [http://creativecommons.org/licenses/by/2.0]. The photo [far left] is reproduced from reference 205.)
FIG 8.
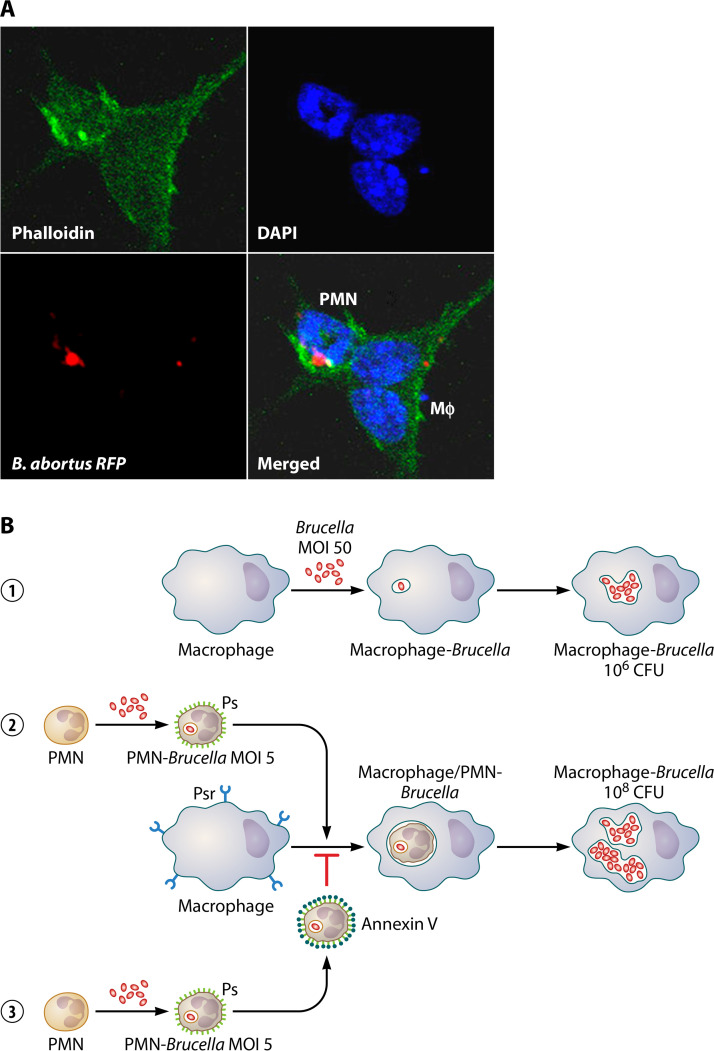
PMNs are efficient vehicles for dispersing Brucella organisms. (A) The Mϕ (cell with two nuclei and the actin cytoskeleton in green) phagocytizes a Brucella-infected PMN (cell with a donut-shaped nucleus, actin cytoskeleton in green, and with intracellular bacteria in red) with high efficiency. RFP, red fluorescent protein. (Adapted from reference 39, published under the terms of the Creative Commons Attribution license [http://creativecommons.org/licenses/by/2.0].) (B) B. abortus propagates at higher rates in Mϕs colonized through infected PMNs (1). Brucella infects Mϕs and replicates intracellularly (2). Mϕs recognize Brucella-infected PMNs, exposing phosphatidylserine (Ps), through their Ps receptors (Psr). Once the infected PMNs are phagocytized, bacteria move to the Mϕ phagosomes and replicate intracellularly at rates 100- to 1,000-fold higher than in Mϕs infected with bacteria alone (3). The blockage of Ps with annexin V on the PMN surface hampers the recognition of Mϕ-Psr and the subsequent bacterial colonization.
The invasion of Mϕs by Brucella organisms via infected PMNs is 100 to 1,000 times more efficient than when these mononuclear phagocytic cells become infected with bacteria alone (Fig. 8B). After several hours, once bacteria are inside their replicative niche in Mϕs, a simultaneous release of TNF-α and regulatory IL-10 cytokines is produced. Bacteria inside endoplasmic reticulum-derived compartments are protected from destruction, even if Mϕs become activated afterward (100, 106). As expected, blockage of phosphatidylserine on the surface of the Brucella-infected PMNs hinders their association with Mϕs and hampers phagocytosis (Fig. 9B). It seems, therefore, that the infected PMNs may serve as efficient Trojan horse vehicles for bacterial dispersal, as suggested by Wesley W. Spink 70 years ago on the basis of clinical observations (8).
FIG 9.
The Trojan horse hypothesis and modulation of the Th1 response. (A) After the invasion of Brucella organisms, resident PMNs phagocytize the bacteria without inducing significant activation or degranulation (1) nor signs of necrosis, oncosis, or NETosis. Infected PMNs, however, release chemokine CXCL8 and expose phosphatidylserine on the cell surface as an “eat me” signal (in green) (2). Then, in the primarily affected organs, such as the liver, spleen, and BM, Mϕs and DCs phagocytize the Brucella-infected PMNs in a nonphlogistic manner (3). Infected PMNs may have a direct regulatory influence over cells of the adaptive immune response by an unknown mechanism that may involve lipoxins and other mediators (3). Those Mϕs colonized by Brucella-infected PMNs control adaptive immunity by releasing the regulatory cytokine IL-10 (5). This event allows bacterial replication inside mononuclear phagocytic cells such as Mϕs, Mo, and DCs (6), opening a window for bacterial dispersion in the target organs (7). Then, the adaptive immune response begins, with activation of the cell-mediated immunity and release of anti-Brucella antibodies (8), significant quantities of IFN-γ (9), and the subsequent release of IL-12, IL-6, and the regulatory IL-10 (10), promoting a regulated Th1 response (11). The Th1 immune response induces polarization toward M1-type Mϕs (12), which exert an effective bactericidal action, controlling the infection (13). (B) In the absence of PMNs, there is no such regulatory function over the immune system, allowing the direct interaction of Mϕs with the bacterium (0). The fast activation of Mϕs and other mononuclear cells narrows the window for the immune system to respond, favoring an exacerbation of Th1 cell-mediated immunity with very large amounts of IFN-γ, IL-12, and IL-6 cytokines, smaller amounts of antibodies, and increased polarization of bactericidal M1-type Mϕs. The large amounts of IL-10 control the cytokine storm and promote the fast resolution of inflammation. As a consequence of this enhanced activation, the number of bacteria in the target organs decreases, favoring fast resolution of the infection.
BRUCELLA-INFECTED NEUTROPHILS DAMPEN ADAPTIVE IMMUNITY
As in other diseases caused by intracellular bacteria, the activation of cell-mediated immunity followed by the release of agonistic cytokines and recruitment of mononuclear phagocytic cells is essential for eliminating Brucella organisms (22, 23). However, before this process, PMNs and other elements of innate immunity that are essential for controlling the first stages of infection recognize invading bacteria (48). Besides the central role exerted by PMNs in the innate immune response, these leukocytes also modulate adaptive immunity (48, 80–83). In most bacterial infections, the absence of PMNs favors bacterial replication and increases lethality (99, 100, 237–239). In murine brucellosis, however, the absence of PMNs (either in neutropenic mutant Genista mice or in mice depleted of PMNs with anti-Ly6G antibodies) does not favor Brucella replication but instead promotes the elimination of these pathogens from the target organs and fast resolution of the disease (87, 93). This phenomenon correlates with the transient and greater liability for infection observed in some Brucella-susceptible mouse strains. In these animals, the recruitment of PMNs in the spleen results from a higher bacterial burden, without efficient bacterial elimination from this organ (240).
After Brucella infection of neutropenic mice, there is a mild transient increase in bacterial numbers in the spleen at the onset of infection, a phenomenon that is rapidly reversed after 2 to 3 days (87). At this time, the absence of PMNs provokes the exacerbation of cell immunity, with augmented spleen swelling and higher infiltration of Mϕs and DCs. The numbers of CD8+ CD4+ B cells, monocytes, and Th1 cytokines significantly increase in both the Brucella-infected, neutropenic Genista mice and the PMN-depleted mice. Removal of PMNs in infected mice, once the adaptive immune response has initiated, promotes an even faster resolution of the spleen inflammation. These phenomena are associated with the potent activation of the cellular immune response, with increasing levels of IFN-γ, IL-12, and IL-6 and efficient recruitment of Mo, Mϕs, and DCs, as well as enhanced Mϕ differentiation toward M1-type cells (87, 93). At later times of the Brucella infection, once the number of bacteria has diminished in the neutropenic mice, the amounts of IFN-γ decrease, while IL-12, IL-10, and IL-6 increase and IL-4, IL-1β, and TNF-α remain at background levels. In murine brucellosis, augmented IL-10 is related to the CD25+ CD4+ T cell function that balances proinflammatory and anti-inflammatory cytokines (231). Likewise, the increase of IL-10 and IL-6 cytokines at later times of infection in neutropenic mice is linked to the decrease of IFN-γ. Low levels of IL-10 and large amounts of IFN-γ correlate with reduced Brucella survival, while the opposite connects to the resolution of the infection at later times (231, 241). The rise of IL-6 at later stages of infection limits recruitment of innate immune cells, and it is a critical regulatory event in inflammation (242).
Neutropenic infected mice dampen the overall antibody titers and all the immunoglobulin isotype responses against Brucella antigens, a phenomenon that is commensurate with the recorded robust cell-mediated immunity. Therefore, the enhanced removal of Brucella in these mice is not due to increased levels of antibodies but to a more efficient cellular response. The low levels of antibodies correlate with the absence of IL-4 and the rise of the Th1 response (241). Furthermore, the presence of anti-Brucella antibodies in neutropenic infected mice did not affect the levels of IFN-γ, although they dampen the IL-6, IL-12, and IL-10 response. Taken together, this indicates that PMNs play a significant role in regulating adaptive immunity during the infection process (87, 93).
Under the effect of IFN-γ and IL-12 cytokines, CD4+ lymphocytes become activated, promoting Th1 polarization and the differentiation of Mϕs to M1-type cells, with increased brucellicidal function (243–245). Since in murine brucellosis, all these factors increase in the absence of PMNs, then the obvious conclusion is that these leukocytes exert modulatory functions on the adaptive immune response (Fig. 8). However, the hyperactivation of adaptive immunity, with IFN-γ levels that exceed 8,000 pg/ml, is not without a price, since the neutropenic Brucella-infected mice develop cachexia in the early phases of the disease (93). Therefore, the physiological removal of infected PMNs in brucellosis may keep the immune response's equilibrium, avoiding the cytokine storm, which may be lethal (see Movie S2 in the supplemental material).
Given the role that PMNs have in controlling many bacterial infections, the inhibitory effect that these leukocytes have on the immune response against Brucella organisms is counterintuitive (87, 99, 237–239). In legionellosis, the absence of PMNs, rather than promoting a Th1 response, led to Th2 skewing and more disease (99). This shift is a remarkable phenomenon, since IFN-γ also plays a central role in the immune response in legionellosis (246) and the intracellular life cycle of Legionella pneumophila resembles that of Brucella organisms (247). Likewise, the absence of PMN apoptosis is linked to the slow activation of naive CD4+ T cells in M. tuberculosis infections (88), and the role of PMNs as antigen-presenting cells in other bacterial infections (92) does not fit with the inhibitory functions observed in brucellosis. Mechanisms like regulation through PMN cytokines or the release of ectosomes or exosomes (248) seem unlikely due to the absence of degranulation and the negligible amounts of regulatory and proinflammatory cytokines released by Brucella-infected PMNs (100, 139, 231).
Despite this, there are inhibitory actions throughout the immune system mediated by PMNs that offer alternative mechanisms. Activated or dying PMNs hamper the proliferation of IFN-γ-producing T cells by the release of arginase, which, over time, depletes extracellular l-arginine in a NO-dependent manner (82). Similarly, subsets of mature and activated PMNs inhibit T cell proliferation through the release of ROS in the immunological synapse between the PMNs and T cells (94). In cancer patients, activated PMNs suppress cytokines and inhibit T cell functions through ROS generation (90). PMNs instructed by Mycobacterium-infected DCs produce IL-10 and shut down Th17 cells through their IL-10 receptor (249). Primed PMNs may compete for the availability of antigen with DCs and Mϕs and then hamper antigen presentation in lymph nodes (85). PMN-mediated T cell regulation in adjuvant immunization may be dependent on prostanoids. Indeed, adjuvant-primed PMNs control the spread of T cell responses to distal lymph nodes. This effect depends on prostanoids, since, in neutropenic mice, there is mobilization of, and an increase in, CD4+ T cell response-dependent IL-2 or IFN-γ production (86). Although these mechanisms somewhat fit with inhibitory regulatory functions, they require fully activated PMNs, which is not the case in brucellosis.
One alternative that deserves attention is the release by PMNs of anti-inflammatory lipid mediators, such as lipoxins, particularly LXA4 (250). 5-Lipoxygenase mediates the synthesis and production of lipoxins through a mechanism that involves the interaction of PMNs with the endothelium and platelets in the vascular lumen or resident tissue mucosal cells (250). It has been demonstrated that Brucella-infected, 5-lipoxygenase knockout mice deficient in the synthesis of lipid mediators, such as lipoxin LXA4, release larger amounts of IFN-γ and IL-12 cytokines than infected wild-type mice, promoting the fastest resolution of inflammation and more efficient removal of bacteria in the target organs (251). This phenomenon, which is reminiscent of the Th1 modulatory function exerted by PMNs in murine brucellosis, may be due to the inhibitory action that lipoxins exert on Mϕs, Mos, and DCs and on the differentiation of T effector cell responses as well as T helper activities (250, 252). Therefore, in the absence of PMNs and the concomitant lower levels of lipoxins, the exacerbation of the Th1 response may be the anticipated outcome in neutropenic mice.
THE TROJAN HORSE HYPOTHESIS AND MODULATION OF THE Th1 RESPONSE
A comprehensive model of the role of PMNs in the Brucella infection process is illustrated in Fig. 9A. After invasion through mucous membranes, resident Mϕs/DCs and PMNs phagocytize Brucella organisms. While the former are suitable host cells for Brucella replication (253, 254), the latter leukocytes do not kill the bacterium and avoid the proinflammatory functions at the onset of the infection. The removal of infected PMNs by phagocytic cells occurs mainly in the affected organs, such as the liver, spleen, and BM. Then, Brucella organisms are delivered inside mononuclear phagocytic cells through the infected PMNs. Once inside, the bacteria escape to their replicating niche without significant activation of the host Mϕs, Mo, or DCs (Fig. 9A). The internalization of Brucella-infected dying PMNs promotes the release of IL-10 and hampers the activation of the phagocytic mononuclear cells early in the infection process (255–257). The regulatory IL-10 cytokine dampens Mϕ effector functions and limits the production of IFN-γ (241). This nonphlogistic process at the onset of infection opens a window for bacterial dispersion before the adaptive immune system becomes fully activated. Later in the infection, the Th1 adaptive immunity fully develops with a predominant production of IFN-γ and other cytokines that promote Mϕ polarization toward M1-type cells, which are the most brucellicidal cells (243–245). This model does not rule out the direct influence of Brucella-infected PMNs on immune cells as a complementary mechanism (e.g., through lipoxins or other mediators).
In the absence of PMNs, mononuclear phagocytic cells interact directly with free Brucella organisms that display lower invasion efficiency (Fig. 8B). The direct recognition and phagocytosis of the bacterial cells promote a faster and more powerful Th1 immune response, with the release of larger amounts of IFN-γ and other cytokines and stronger polarization toward M1-type Mϕs. The exacerbated immune response readily eliminates brucellae from the target organs. After that, larger IL-10 and IL-6 amounts are released by the neutropenic host, modulating the enhanced immune response and resolving the infection in a shorter period.
A short time after infection, PMN-containing brucellae aggregate around Kupffer Mϕs in the liver sinusoids, which then become fully infected with bacteria (12). Likely, this phenomenon also occurs in other organs of the mononuclear phagocytic system (42). The replication of Brucella organisms in the primarily affected organs induces granulomas composed mainly of Mϕs and DCs (epithelioid cells), many of them heavily infected, with just a few PMNs present (43). Most PMNs in these granulomatous inflammations do not harbor bacteria (42), suggesting that phagocytic cells of the mononuclear phagocytic system remove the infected PMNs.
FINAL REMARKS
Experimental designs related to infectious diseases must fit the realm and reality of the episodes to unveil the hidden mechanisms behind the disease; otherwise, the results become a curiosity and are irrelevant in the context and scope of the events (258). In this direction, the use of neutropenic experimental murine models has contributed significantly to defining the role of PMNs at the onset of Brucella infection or in the early phases of the disease, explaining some of the outcomes observed in human and animal brucellosis. While PMNs are dispensable for combating Brucella, these infected PMNs dampen the adaptive immune response against brucellosis. Nevertheless, PMNs are needed to modulate the cytokine storm that risks the lives of infected animals, as demonstrated by weight loss in neutropenic infected mice (93). Although PMNs are not critical for bacterial clearance, they still modulate the immune response to avoid an exacerbated reaction.
Our comprehensive model is compatible with a large number of clinical observations, which include a lack of overt clinical signs at the onset of infection and a long incubation period, which in humans and domestic ruminants may last from weeks to months (31, 259). The absence of neutrophilia in the initial phases and the concomitant neutropenia in a significant number of long-lasting brucellosis cases suggest the effective removal of Brucella-infected PMNs by mononuclear phagocytic cells of the reticuloendothelial system. It is noteworthy that Br-LPS, the molecule that triggers the premature cell death of PMNs, circulates for several months in mice without being destroyed (122), a condition that may be associated with the neutropenia observed in some brucellosis patients.
This scenario is not without precedent. For instance, other microbial pathogens also use PMNs as Trojan horse vehicles for dispersion (79, 260), and the presence of CD14 on PMNs and DCs has been related to LPS-induced apoptosis (261, 262). Some of the strategies followed by Brucella organisms are present in other pathogenic alphaproteobacteria related to Brucella, such as Anaplasma and Bartonella. These bacteria also display furtive strategies used to avoid activation of the innate immune system and the microbicidal action of PMNs (65, 66, 69, 263). Finally, some investigations have shown the negative role of PMNs in controlling chronic bacterial infections (97).
Once installed in humans, brucellosis is a long-lasting bacterial infection that is seldom eliminated by the sole action of the immune system and requires an aggressive dual antibiotic treatment for several weeks (8, 10, 149). If not treated, the bacteria may remain in tissues for extended periods and cause relapses, some of them many years apart (264, 265). Immunized animals with the live attenuated-Brucella vaccines can mount an efficient cellular adaptive immune response against brucellosis (266), and activated phagocytic cells can eliminate the pathogenic organisms (254, 267).
The stealthy strategy followed by Brucella organisms opens a window into the innate immune response and gives the bacteria time to establish and hide within host tissues before adaptive immunity becomes fully activated (46). This event is a sine qua non requirement for the development of long-lasting brucellosis. Finally, our model describing Brucella-infected PMNs as Trojan horse vehicles for bacterial dispersal and as modulators of the Th1 adaptive immunity in the infection fits with the “Occam's razor” principle of parsimony, in which the most straightforward explanation that agrees with the experimental data is also the most probable one.
ACKNOWLEDGMENTS
We thank Esteban Chaves-Olarte, Caterina Guzmán-Verri, Carlos Chacón Díaz, Cristina Gutierrez-Jiménez, and Ricardo Mora-Cartín for their support and participation in developing this topic of research over the years. We also thank Jean Pierre Gorvel from Centre d'Immunologie de Marseille-Luminy for his collaboration in research activities and for the photograph of infected BM Mϕ displayed in Fig. 2A. We are deeply grateful to the research teams of the Centro de Investigación en Enfermedades Tropicales (CIET) and Programa de Investigación en Enfermedades Tropicales (PIET) for supporting our work. We thank Pamela Altamirano-Silva and Esteban Chaves for the photographs of infected HeLa and RAW Mϕ, Gabriela Hernández-Mora for the histopathology photographs of Brucella-infected dolphins, and Ricardo Mora-Cartín for Movie S2.
We do not have any conflicts of interest to disclose, including any financial support or any other matter related to the manuscript's content.
Biographies

Edgardo Moreno received his Ph.D. at the University of Wisconsin–Madison. He had postdoctoral training at the Max Planck Institute for Immunobiology, Freiburg, Germany, was an Orange-Fellow at the Center for Immunology, Marseille-Luminy, France, and a DAAD-Fellow at the Max Planck Institute for Infectious Diseases, Berlin, Germany. His main contributions are in the field of brucellosis, including the pathobiology, the structure and function of Br-LPS, the proinflammatory response, the diagnoses, and the evolution of the genus Brucella. He acted as Director of the Tropical Disease Research Program of the National University, as Dean of Student Affairs of the Graduate Program between the Central American universities and the Karolinska Institute, Sweden, Coordinator of the Network for Research and Training in Tropical Diseases in Central America, and a scientific manager of the Iberoamerican Program for Science and Technology Development. He holds the chair of Humboldt Honorary Professor.

Elías Barquero-Calvo received his Ph.D. at the University of Costa Rica. He had an internship at the Center for Immunology, Marseille-Luminy, France. His foremost interest is in the pathogenesis of bacterial infections and host-parasite interactions of zoonotic diseases of veterinary and public health relevance. His research focuses on the mechanisms behind the early response of innate immunity and the role of neutrophils in brucellosis. His investigations are partly devoted to analysis of the antibiotic resistance profile of some bacterial pathogens. Presently, he is the coordinator of the Bacteriology Laboratory at the Veterinary Hospital of the School of Veterinary Medicine. He has received several awards, including an Honorable Mention from the University of Costa Rica and the National Science Prize Clodomiro Picado Twight, Costa Rica.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Bassett-Smith PW. 1902. Malta fever. Br Med J 2:861–867. [Google Scholar]
- 2.Bassett-Smith PW. 1903. Duration of Mediterranean fever. Br Med J 2:1589. doi: 10.1136/bmj.2.2242.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans AC. 1934. Chronic brucellosis. JAMA 103:665–667. doi: 10.1001/jama.1934.02750350029008. [DOI] [Google Scholar]
- 4.Bassett-Smith PW. 1907. The treatment of Mediterranean fever by means of vaccines, with illustrative cases. J Hyg (Lond) 7:115–144. doi: 10.1017/s0022172400033179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metschnikoff H. 1906. Prof. Metschnikoff’s Harben Lectures. Nature 74:107–107. [Google Scholar]
- 6.Huddleson IF, Johnson HW, Hamann EE. 1933. A study of the opsono-cytophagic power of the blood and allergic skin reaction in Brucella infection and immunity in man. Am J Public Health Nations Health 23:917–929. doi: 10.2105/ajph.23.9.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calder RM, Steen C, Baker L. 1939. Blood studies in brucellosis. JAMA 112:1893–1898. doi: 10.1001/jama.1939.02800190007002. [DOI] [Google Scholar]
- 8.Spink WW. 1956. The nature of brucellosis. University of Minnesota Press, Minneapolis, MN. [Google Scholar]
- 9.Munger M. 1941. Study of the effect of the toxic fraction from Brucella cells on the leucocytic picture in normal guinea pigs. Tech Bull Mich State Coll Agric Exp Stn 177:45–55. [Google Scholar]
- 10.Ruiz-Castañeda M. 1986. Brucelosis, 3rd ed La Prensa Médica Mexicana, S.A, Mexico City, Mexico. [Google Scholar]
- 11.Wise B, Kerby GP. 1943. Phenomenon of local skin-reactivity (Shwartzman) to organisms of the Brucella group. J Immunol 46:225–233. [Google Scholar]
- 12.Braude AI. 1951. Studies in the pathology and pathogenesis of experimental brucellosis. II. The formation of the hepatic granuloma and its evolution. J Infect Dis 89:87–94. doi: 10.1093/infdis/89.1.87. [DOI] [PubMed] [Google Scholar]
- 13.Kreutzer DL, Dreyfus LA, Robertson DC. 1979. Interaction of polymorphonuclear leukocytes with smooth and rough strains of Brucella abortus. Infect Immun 23:737–742. doi: 10.1128/IAI.23.3.737-742.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riley LK, Robertson DC. 1984. Ingestion and intracellular survival of Brucella abortus in human and bovine polymorphonuclear leukocytes. Infect Immun 46:224–230. doi: 10.1128/IAI.46.1.224-230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riley LK, Robertson DC. 1984. Brucellacidal activity of human and bovine polymorphonuclear leukocyte granule extracts against smooth and rough strains of Brucella abortus. Infect Immun 46:231–236. doi: 10.1128/IAI.46.1.231-236.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ackermann MR, Cheville NF, Deyoe BL. 1988. Bovine ileal dome lymphoepithelial cells: endocytosis and transport of Brucella abortus strain 19. Vet Pathol 25:28–35. doi: 10.1177/030098588802500104. [DOI] [PubMed] [Google Scholar]
- 17.Enright FM. 1990. The pathogenesis and pathobiology of Brucella infection in domestic animals, p 301–320. In Nielsen K, Duncan J (ed), Animal brucellosis. CRC Press Inc, Boca Raton, FL. [Google Scholar]
- 18.Ahmed K, Al-Matrouk KA, Martinez G, Oishi K, Rotimi VO, Nagatake T. 1999. Increased serum levels of interferon-γ and interleukin-12 during human brucellosis. Am J Trop Med Hyg 61:425–427. doi: 10.4269/ajtmh.1999.61.425. [DOI] [PubMed] [Google Scholar]
- 19.Ariza J. 1999. Brucellosis: an update. The perspective from the Mediterranean basin. Rev Med Microbiol 10:125–135. [Google Scholar]
- 20.Celli J. 2019. The intracellular life cycle of Brucella spp. Microbiol Spectr 7:101–111. doi: 10.1128/microbiolspec.BAI-0006-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreno E, Gorvel J-P. 2004. Invasion, intracellular trafficking and replication of Brucella organisms in professional and non-professional phagocytes, p 287–312. In López-Goñi I, Moriyón I (ed), Brucella: molecular and cellular biology. Horizon Scientific Press, Wymondham, United Kingdom. [Google Scholar]
- 22.Baldwin CL, Goenka R. 2006. Host immune responses to the intracellular bacteria Brucella: does the bacteria instruct the host to facilitate chronic infection? Crit Rev Immunol 26:407–442. doi: 10.1615/critrevimmunol.v26.i5.30. [DOI] [PubMed] [Google Scholar]
- 23.Martirosyan A, Gorvel J-PP. 2013. Brucella evasion of adaptive immunity. Future Microbiol 8:147–154. doi: 10.2217/fmb.12.140. [DOI] [PubMed] [Google Scholar]
- 24.Moreno E, Moriyón I. 2006. The genus Brucella, p 315–456. In Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (ed), The prokaryotes, 3rd ed Springer-Verlag, New York, NY. [Google Scholar]
- 25.Franc KA, Krecek RC, Häsler BN, Arenas-Gamboa AM. 2018. Brucellosis remains a neglected disease in the developing world: a call for interdisciplinary action. BMC Public Health 18:125. doi: 10.1186/s12889-017-5016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitten TV, Brayshaw G, Patnayak D, Alvarez J, Larson CM, Root Kustritz M, Holzbauer SM, Torrison J, Scheftel JM. 2019. Seroprevalence of Brucella canis antibodies in dogs entering a Minnesota humane society, Minnesota, 2016–2017. Prev Vet Med 168:90–94. doi: 10.1016/j.prevetmed.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norman FF, Monge-Maillo B, Chamorro-Tojeiro S, Pérez-Molina JA, López-Vélez R. 2016. Imported brucellosis: a case series and literature review. Travel Med Infect Dis 14:182–199. doi: 10.1016/j.tmaid.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Bosseray N, Plommet M, De Rycke J. 1982. Evolution de l’infection de la souris par Brucella abortus, Brucella melitensis et Brucella suis vers l’état chronique et la guérison. Ann Rech Vet 13:153–161. [PubMed] [Google Scholar]
- 29.Braude AI. 1951. Studies in the pathology and pathogenesis of experimental brucellosis. I. A comparison of the pathogenicity of Brucella abortus, Brucella melitensis, and Brucella suis for guinea pigs. J Infect Dis 89:76–86. doi: 10.1093/infdis/89.1.76. [DOI] [PubMed] [Google Scholar]
- 30.Gorvel JP, Moreno E, Moriyón I. 2009. Is Brucella an enteric pathogen? Nat Rev Microbiol 7:250–250. doi: 10.1038/nrmicro2012-c1. [DOI] [PubMed] [Google Scholar]
- 31.Megid J, Mathias L, Robles C. 2010. Clinical manifestations of brucellosis in domestic animals and humans. The Open Vet Sci J 4:119–126. doi: 10.2174/1874318801004010119. [DOI] [Google Scholar]
- 32.Moreno E, Moriyon I. 2002. Brucella melitensis: a nasty bug with hidden credentials for virulence. Proc Natl Acad Sci U S A 99:1–3. doi: 10.1073/pnas.022622699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arias J. 1951. Contribución al conocimiento de la patología de la brucelosis. Observaciones anátomo-patológicas en casos mortales humanos y estudio patogénico experimental. An Fac Med Lima 34:429–517. [PubMed] [Google Scholar]
- 34.Moreno E, Stackebrandt E, Dorsch M, Wolters J, Busch M, Mayer H. 1990. Brucella abortus 16S rRNA and lipid A reveal a phylogenetic relationship with members of the alpha-2 subdivision of the class Proteobacteria. J Bacteriol 172:3569–3576. doi: 10.1128/jb.172.7.3569-3576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demars A, Lison A, Machelart A, Van Vyve M, Potemberg G, Vanderwinden JM, De Bolle X, Letesson JJ, Muraille E. 2019. Route of infection strongly impacts the host-pathogen relationship. Front Immunol 10:1589. doi: 10.3389/fimmu.2019.01589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delpino MV, Fossati CA, Baldi PC. 2009. Proinflammatory response of human osteoblastic cell lines and osteoblast-monocyte interaction upon infection with Brucella spp. Infect Immun 77:984–995. doi: 10.1128/IAI.01259-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delpino MV, Barrionuevo P, Scian R, Fossati CA, Baldi PC. 2010. Brucella-infected hepatocytes mediate potentially tissue-damaging immune responses. J Hepatol 53:145–154. doi: 10.1016/j.jhep.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 38.Goenka R, Guirnalda PD, Black SJ, Baldwin CL. 2012. B lymphocytes provide an infection niche for intracellular bacterium Brucella abortus. J Infect Dis 206:91–98. doi: 10.1093/infdis/jis310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gutiérrez-Jiménez C, Mora-Cartín R, Altamirano-Silva P, Chacón-Díaz C, Chaves-Olarte E, Moreno E, Barquero-Calvo E. 2019. Neutrophils as Trojan horse vehicles for Brucella abortus macrophage infection. Front Immunol 10:1012. doi: 10.3389/fimmu.2019.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vitry M-A, Hanot Mambres D, De Trez C, Akira S, Ryffel B, Letesson J-J, Muraille E. 2014. Humoral immunity and CD4+ Th1 cells are both necessary for a fully protective immune response upon secondary infection with Brucella melitensis. J Immunol 192:3740–3752. doi: 10.4049/jimmunol.1302561. [DOI] [PubMed] [Google Scholar]
- 41.Campbell GA, Adams LG, Sowa BA. 1994. Mechanisms of binding of Brucella abortus to mononuclear phagocytes from cows naturally resistant or susceptible to brucellosis. Vet Immunol Immunopathol 41:295–306. doi: 10.1016/0165-2427(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 42.Copin R, Vitry M-A, Hanot Mambres D, Machelart A, De Trez C, Vanderwinden J-M, Magez S, Akira S, Ryffel B, Carlier Y, Letesson J-J, Muraille E. 2012. In situ microscopy analysis reveals local innate immune response developed around Brucella infected cells in resistant and susceptible mice. PLoS Pathog 8:e1002575. doi: 10.1371/journal.ppat.1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grilló M-J, Blasco J-M, Gorvel J-P, Moriyon I, Moreno E. 2012. What have we learned from brucellosis in the mouse model? Vet Res 43:29. doi: 10.1186/1297-9716-43-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy EA, Sathiyaseelan J, Parent MA, Zou B, Baldwin CL. 2001. Interferon-γ is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology 103:511–518. doi: 10.1046/j.1365-2567.2001.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goenka R, Parent MA, Elzer PH, Baldwin CL. 2011. B cell-deficient mice display markedly enhanced resistance to the intracellular bacterium Brucella abortus. J Infect Dis 203:1136–1146. doi: 10.1093/infdis/jiq171. [DOI] [PubMed] [Google Scholar]
- 46.Martirosyan A, Moreno E, Gorvel J-P. 2011. An evolutionary strategy for a stealthy intracellular Brucella pathogen. Immunol Rev 240:211–234. doi: 10.1111/j.1600-065X.2010.00982.x. [DOI] [PubMed] [Google Scholar]
- 47.Moreno E. 2014. Retrospective and prospective perspectives on zoonotic brucellosis. Front Microbiol 5:213. doi: 10.3389/fmicb.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mantovani A, Cassatella MA, Costantini C, Jaillon S. 2011. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 49.Tecchio C, Micheletti A, Cassatella MA. 2014. Neutrophil-derived cytokines: facts beyond expression. Front Immunol 5:508. doi: 10.3389/fimmu.2014.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petri B, Sanz MJ. 2018. Neutrophil chemotaxis. Cell Tissue Res 371:425–436. doi: 10.1007/s00441-017-2776-8. [DOI] [PubMed] [Google Scholar]
- 51.Hidalgo A, Chilvers ER, Summers C, Koenderman L. 2019. The neutrophil life cycle. Trends Immunol 40:584–597. doi: 10.1016/j.it.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 52.Liu L, Sun B. 2019. Neutrophil pyroptosis: new perspectives on sepsis. Cell Mol Life Sci 76:2031–2042. doi: 10.1007/s00018-019-03060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kobayashi SD, Voyich JM, Burlak C, DeLeo FR. 2005. Neutrophils in the innate immune response. Arch Immunol Ther Exp (Warsz) 53:505–517. [PubMed] [Google Scholar]
- 54.Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. 2000. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest 80:617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 55.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. 2010. Neutrophil kinetics in health and disease. Trends Immunol 31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kennedy AD, de Leo FR. 2009. Neutrophil apoptosis and the resolution of infection. Immunol Res 43:25–61. doi: 10.1007/s12026-008-8049-6. [DOI] [PubMed] [Google Scholar]
- 57.Pillay J, den Braber I, Vrisekoop N, Kwast LM, de Boer RJ, Borghans JAM, Tesselaar K, Koenderman L. 2010. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 116:625–627. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- 58.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 59.Simon H-U. 2003. Neutrophil apoptosis pathways and their modifications in inflammation. Immunol Rev 193:101–110. doi: 10.1034/j.1600-065x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 60.Ellis TN, Beaman BL. 2004. Interferon-γ activation of polymorphonuclear neutrophil function. Immunology 112:2–12. doi: 10.1111/j.1365-2567.2004.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Savill J, Fadok V. 2000. Corpse clearance defines the meaning of cell death. Nature 407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 62.Byrne A, Reen DJ. 2002. Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. J Immunol 168:1968–1977. doi: 10.4049/jimmunol.168.4.1968. [DOI] [PubMed] [Google Scholar]
- 63.Anwar S, Whyte MKBB. 2007. Neutrophil apoptosis in infectious disease. Exp Lung Res 33:519–528. doi: 10.1080/01902140701756620. [DOI] [PubMed] [Google Scholar]
- 64.DeLeo FR. 2004. Modulation of phagocyte apoptosis by bacterial pathogens. Apoptosis 9:399–413. doi: 10.1023/B:APPT.0000031448.64969.fa. [DOI] [PubMed] [Google Scholar]
- 65.von Loewenich FD, Scorpio DG, Reischl U, Dumler JS, Bogdan C. 2004. Frontline: control of Anaplasma phagocytophilum, an obligate intracellular pathogen, in the absence of inducible nitric oxide synthase, phagocyte NADPH oxidase, tumor necrosis factor, Toll-like receptor (TLR)2 and TLR4, or the TLR adaptor molecule MyD88. Eur J Immunol 34:1789–1797. doi: 10.1002/eji.200425029. [DOI] [PubMed] [Google Scholar]
- 66.Rikihisa Y. 2010. Anaplasma phagocytophilum and Ehrlichia chaffeensis: subversive manipulators of host cells. Nat Rev Microbiol 8:328–339. doi: 10.1038/nrmicro2318. [DOI] [PubMed] [Google Scholar]
- 67.Fumarola D, Pece S, Fumarulo R, Petruzzelli R, Greco B, Giuliani G, Maffione AB, Jirillo E. 1994. Downregulation of human polymorphonuclear cell activities exerted by microorganisms belonging to the α2 subgroup of proteobacteria (Afipia felis and Rochalimaea henselae). Immunopharmacol Immunotoxicol 16:449–461. doi: 10.3109/08923979409007104. [DOI] [PubMed] [Google Scholar]
- 68.Stützer B, Hartmann K. 2012. Chronic bartonellosis in cats: what are the potential implications? J Feline Med Surg 14:612–621. doi: 10.1177/1098612X12458208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anderson BE, Neuman MA. 1997. Bartonella spp. as emerging human pathogens. Clin Microbiol Rev 10:203–219. doi: 10.1128/CMR.10.2.203-219.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fontana C, Conde-Álvarez R, Ståhle J, Holst O, Iriarte M, Zhao Y, Arce-Gorvel V, Hanniffy S, Gorvel J-P, Moriyón I, Widmalm G. 2016. Structural studies of lipopolysaccharide-defective mutants from Brucella melitensis identify a core oligosaccharide critical in virulence. J Biol Chem 291:7727–7741. doi: 10.1074/jbc.M115.701540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Casabuono AC, Czibener C, Del Giudice MG, Valguarnera E, Ugalde JE, Couto AS. 2017. New features in the lipid a structure of Brucella suis and Brucella abortus lipopolysaccharide. J Am Soc Mass Spectrom 28:2716–2723. doi: 10.1007/s13361-017-1805-x. [DOI] [PubMed] [Google Scholar]
- 72.Gil-Ramírez Y, Conde-Álvarez R, Palacios-Chaves L, Zúñiga-Ripa A, Grilló M-JJ, Arce-Gorvel V, Hanniffy S, Moriyón I, Iriarte M. 2014. The identification of wadB, a new glycosyltransferase gene, confirms the branched structure and the role in virulence of the lipopolysaccharide core of Brucella abortus. Microb Pathog 73:53–59. doi: 10.1016/j.micpath.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 73.Zähringer U, Lindner B, Knirel YA, van den Akker WMR, Hiestand R, Heine H, Dehio C. 2004. Structure and biological activity of the short-chain lipopolysaccharide from Bartonella henselae ATCC 49882T. J Biol Chem 279:21046–21054. doi: 10.1074/jbc.M313370200. [DOI] [PubMed] [Google Scholar]
- 74.Kastowsky M, Gutberlet T, Bradaczek H. 1992. Molecular modelling of the three-dimensional structure and conformational flexibility of bacterial lipopolysaccharide. J Bacteriol 174:4798–4806. doi: 10.1128/jb.174.14.4798-4806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.François M, Le Cabec V, Dupont MA, Sansonetti PJ, Maridonneau-Parini I. 2000. Induction of necrosis in human neutrophils by Shigella flexneri requires type III secretion, IpaB and IpaC invasins, and actin polymerization. Infect Immun 68:1289–1296. doi: 10.1128/iai.68.3.1289-1296.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dacheux D, Toussaint B, Richard M, Brochier G, Croize J, Attree I. 2000. Pseudomonas aeruginosa cystic fibrosis isolates induce rapid, type III secretion-dependent, but ExoU-independent, oncosis of macrophages and polymorphonuclear neutrophils. Infect Immun 68:2916–2924. doi: 10.1128/iai.68.5.2916-2924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Usher LR, Lawson RA, Geary I, Taylor CJ, Bingle CD, Taylor GW, Whyte MKB. 2002. Induction of neutrophil apoptosis by the Pseudomonas aeruginosa exotoxin pyocyanin: a potential mechanism of persistent infection. J Immunol 168:1861–1868. doi: 10.4049/jimmunol.168.4.1861. [DOI] [PubMed] [Google Scholar]
- 78.Register KB, Morgan PA, Wyrick PB. 1986. Interaction between Chlamydia spp. and human polymorphonuclear leukocytes in vitro. Infect Immun 52:664–670. doi: 10.1128/IAI.52.3.664-670.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rupp J, Pfleiderer L, Jugert C, Moeller S, Klinger M, Dalhoff K, Solbach W, Stenger S, Laskay T, van Zandbergen G. 2009. Chlamydia pneumoniae hides inside apoptotic neutrophils to silently infect and propagate in macrophages. PLoS One 4:e6020. doi: 10.1371/journal.pone.0006020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rajeeve K, Das S, Prusty BK, Rudel T. 2018. Chlamydia trachomatis paralyses neutrophils to evade the host innate immune response. Nat Microbiol 3:824–835. doi: 10.1038/s41564-018-0182-y. [DOI] [PubMed] [Google Scholar]
- 81.Johnson MB, Criss AK. 2011. Resistance of Neisseria gonorrhoeae to neutrophils. Front Microbiol 2:77. doi: 10.3389/fmicb.2011.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Darcy CJ, Minigo G, Piera KA, Davis JS, McNeil YR, Chen Y, Volkheimer AD, Weinberg JB, Anstey NM, Woodberry T. 2014. Neutrophils with myeloid derived suppressor function deplete arginine and constrain T cell function in septic shock patients. Crit Care 18:R163. doi: 10.1186/cc14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li Y, Wang W, Yang F, Xu Y, Feng C, Zhao Y. 2019. The regulatory roles of neutrophils in adaptive immunity. Cell Commun Signal 17:147. doi: 10.1186/s12964-019-0471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leliefeld PHC, Koenderman L, Pillay J. 2015. How neutrophils shape adaptive immune responses. Front Immunol 14:471. doi: 10.3389/fimmu.2015.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang C-W, Strong BSI, Miller MJ, Unanue ER. 2010. Neutrophils influence the level of antigen presentation during the immune response to protein antigens in adjuvants. J Immunol 185:2927–2934. doi: 10.4049/jimmunol.1001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang CW, Unanue ER. 2013. Neutrophils control the magnitude and spread of the immune response in a thromboxane A2-mediated process. J Exp Med 210:375–387. doi: 10.1084/jem.20122183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barquero-Calvo E, Martirosyan A, Ordoñez-Rueda D, Arce-Gorvel V, Alfaro-Alarcón A, Lepidi H, Malissen B, Malissen M, Gorvel J-P, Moreno E. 2013. Neutrophils exert a suppressive effect on Th1 responses to intracellular pathogen Brucella abortus. PLoS Pathog 9:e1003167. doi: 10.1371/journal.ppat.1003167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blomgran R, Desvignes L, Briken V, Ernst JD. 2012. Mycobacterium tuberculosis inhibits neutrophil apoptosis, leading to delayed activation of naive CD4 T cells. Cell Host Microbe 11:81–90. doi: 10.1016/j.chom.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Diana J, Simoni Y, Furio L, Beaudoin L, Agerberth B, Barrat F, Lehuen A. 2013. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med 19:65–73. doi: 10.1038/nm.3042. [DOI] [PubMed] [Google Scholar]
- 90.Hock BD, Taylor KG, Cross NB, Kettle AJ, Hampton MB, Mckenzie JL. 2012. Effect of activated human polymorphonuclear leucocytes on T lymphocyte proliferation and viability. Immunology 137:249–258. doi: 10.1111/imm.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kalyan S, Kabelitz D. 2014. When neutrophils meet T cells: beginnings of a tumultuous relationship with underappreciated potential. Eur J Immunol 44:627–633. doi: 10.1002/eji.201344195. [DOI] [PubMed] [Google Scholar]
- 92.Lin A, Loré K. 2017. Granulocytes: new members of the antigen-presenting cell family. Front Immunol 8:1781. doi: 10.3389/fimmu.2017.01781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mora-Cartín R, Gutiérrez-Jiménez C, Alfaro-Alarcón A, Chaves-Olarte E, Chacón-Díaz C, Barquero-Calvo E, Moreno E. 2019. Neutrophils dampen adaptive immunity in brucellosis. Infect Immun 87:e118-19. doi: 10.1128/IAI.00118-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pillay J, Kamp VM, Van Hoffen E, Visser T, Tak T, Lammers JW, Ulfman LH, Leenen LP, Pickkers P, Koenderman L. 2012. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest 122:327–336. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jaeger BN, Donadieu J, Cognet C, Bernat C, Ordoñez-Rueda D, Barlogis V, Mahlaoui N, Fenis A, Narni-Mancinelli E, Beaupain B, Bellanne-Chantelot C, Bajenoff M, Malissen B, Malissen M, Vivier E, Ugolini S. 2012. Neutrophil depletion impairs natural killer cell maturation, function, and homeostasis. J Exp Med 209:565–580. doi: 10.1084/jem.20111908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sporri R, Joller N, Hilbi H, Oxenius A. 2008. A novel role for neutrophils as critical activators of NK cells. J Immunol 181:7121–7130. doi: 10.4049/jimmunol.181.10.7121. [DOI] [PubMed] [Google Scholar]
- 97.Zhang X, Majlessi L, Deriaud E, Leclerc C, Lo-Man R. 2009. Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity 31:761–771. doi: 10.1016/j.immuni.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 98.Soehnlein O, Steffens S, Hidalgo A, Weber C. 2017. Neutrophils as protagonists and targets in chronic inflammation. Nat Rev Immunol 17:248–261. doi: 10.1038/nri.2017.10. [DOI] [PubMed] [Google Scholar]
- 99.Tateda K, Moore T. a, Deng JC, Newstead MW, Zeng X, Matsukawa A, Swanson MS, Yamaguchi K, Standiford TJ. 2001. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J Immunol 166:3355–3361. doi: 10.4049/jimmunol.166.5.3355. [DOI] [PubMed] [Google Scholar]
- 100.Barquero-Calvo E, Chaves-Olarte E, Weiss DS, Guzmán-Verri C, Chacón-Díaz C, Rucavado A, Moriyón I, Moreno E. 2007. Brucella abortus uses a stealthy strategy to avoid activation of the innate immune system during the onset of infection. PLoS One 2:e631. doi: 10.1371/journal.pone.0000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chacón-Díaz C, Altamirano-Silva P, González-Espinoza G, Medina M-C, Alfaro-Alarcón A, Bouza-Mora L, Jiménez-Rojas C, Wong M, Barquero-Calvo E, Rojas N, Guzmán-Verri C, Moreno E, Chaves-Olarte E. 2015. Brucella canis is an intracellular pathogen that induces a lower proinflammatory response than smooth zoonotic counterparts. Infect Immun 83:4861–4870. doi: 10.1128/IAI.00995-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Freer E, Moreno E, Moriyón I, Pizarro-Cerdá J, Weintraub A, Gorvel JP. 1996. Brucella-Salmonella lipopolysaccharide chimeras are less permeable to hydrophobic probes and more sensitive to cationic peptides and EDTA than are their native Brucella sp. counterparts. J Bacteriol 178:5867–5876. doi: 10.1128/jb.178.20.5867-5876.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martínez de Tejada G, Pizarro-Cerdá J, Moreno E, Moriyón I. 1995. The outer membranes of Brucella spp. are resistant to bactericidal cationic peptides. Infect Immun 63:3054–3061. doi: 10.1128/IAI.63.8.3054-3061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Galdiero E, Romano Carratelli C, Vitiello M, Nuzzo I, Del Vecchio E, Bentivoglio C, Perillo G, Galdiero F. 2000. HSP and apoptosis in leukocytes from infected or vaccinated animals by Brucella abortus. New Microbiol 23:271. [PubMed] [Google Scholar]
- 105.Gross A, Terraza A, Ouahrani-Bettache S, Liautard JP, Dornand J. 2000. In vitro Brucella suis infection prevents the programmed cell death of human monocytic cells. Infect Immun 68:342–351. doi: 10.1128/iai.68.1.342-351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Salcedo SP, Marchesini MI, Lelouard H, Fugier E, Jolly G, Balor S, Muller A, Lapaque N, Demaria O, Alexopoulou L, Comerci DJ, Ugalde RA, Pierre P, Gorvel J-P. 2008. Brucella control of dendritic cell maturation is dependent on the TIR-containing protein Btp1. PLoS Pathog 4:e21. doi: 10.1371/journal.ppat.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Starr T, Child R, Wehrly TD, Hansen B, Hwang S, López-Otin C, Virgin HW, Celli J. 2012. Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe 11:33–45. doi: 10.1016/j.chom.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cha SB, Rayamajhi N, Lee WJ, Shin MK, Jung MH, Shin SW, Kim JW, Yoo HS. 2012. Generation and envelope protein analysis of internalization defective Brucella abortus mutants in professional phagocytes, RAW 264.7. FEMS Immunol Med Microbiol 64:244–254. doi: 10.1111/j.1574-695X.2011.00896.x. [DOI] [PubMed] [Google Scholar]
- 109.Hernández-Castro R, Verdugo-Rodríguez A, Luis Puente J, Suárez-Güemes F, Puente JL, Suárez-Güemes F, Luis Puente J, Suárez-Güemes F. 2008. The BMEI0216 gene of Brucella melitensis is required for internalization in HeLa cells. Microb Pathog 44:28–33. doi: 10.1016/j.micpath.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 110.Iannino F, Ugalde JE, Iñón de Iannino N. 2012. Brucella abortus efp gene is required for an efficient internalization in HeLa cells. Microb Pathog 52:31–40. doi: 10.1016/j.micpath.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 111.Marchesini MI, Connolly J, Delpino MV, Baldi PC, Mujer CV, DelVecchio VG, Comerci DJ. 2011. Brucella abortus choloylglycine hydrolase affects cell envelope composition and host cell internalization. PLoS One 6:e28480. doi: 10.1371/journal.pone.0028480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martín-Martín AI, Caro-Hernández P, Orduña A, Vizcaíno N, Fernández-Lago L. 2008. Importance of the Omp25/Omp31 family in the internalization and intracellular replication of virulent B. ovis in murine macrophages and HeLa cells. Microbes Infect 10:706–710. doi: 10.1016/j.micinf.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 113.Posadas DM, Ruiz-Ranwez V, Bonomi HR, Martín FA, Zorreguieta A. 2012. BmaC, a novel autotransporter of Brucella suis, is involved in bacterial adhesion to host cells. Cell Microbiol 14:965–982. doi: 10.1111/j.1462-5822.2012.01771.x. [DOI] [PubMed] [Google Scholar]
- 114.Sola-Landa A, Pizarro-Cerdá J, Grilló MJ, Moreno E, Moriyón I, Blasco JM, Gorvel JP, López-Goñi I. 1998. A two-component regulatory system playing a critical role in plant pathogens and endosymbionts is present in Brucella abortus and controls cell invasion and virulence. Mol Microbiol 29:125–138. doi: 10.1046/j.1365-2958.1998.00913.x. [DOI] [PubMed] [Google Scholar]
- 115.Ruiz-Ranwez V, Posadas DM, Van der Henst C, Estein SM, Arocena GM, Abdian PL, Martín FA, Sieira R, De Bolle X, Zorreguieta A. 2013. BtaE, an adhesin that belongs to the trimeric autotransporter family, is required for full virulence and defines a specific adhesive pole of Brucella suis. Infect Immun 81:996–1007. doi: 10.1128/IAI.01241-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim S, Watarai M, Suzuki H, Makino SI, Kodama T, Shirahata T. 2004. Lipid raft microdomains mediate class A scavenger receptor-dependent infection of Brucella abortus. Microb Pathog 37:11–19. doi: 10.1016/j.micpath.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 117.Keleher LL, Skyberg JA. 2016. Activation of bovine neutrophils by Brucella spp. Vet Immunol Immunopathol 177:1–6. doi: 10.1016/j.vetimm.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 118.Chaves-Olarte E, Guzmán-Verri C, Paramithiotis E, Moreno E. 2011. What have we learned from Brucella proteomics? p 103–132. In López-Goñi I, O’Callaghan D (ed), Brucella: molecular and cellular biology. Horizon Scientific Press, Wymondham, United Kingdom. [Google Scholar]
- 119.Byndloss MX, Tsolis RM. 2016. Brucella spp. virulence factors and immunity. Annu Rev Anim Biosci 4:111–127. doi: 10.1146/annurev-animal-021815-111326. [DOI] [PubMed] [Google Scholar]
- 120.von Bargen K, Gorvel J-PP, Salcedo SP. 2012. Internal affairs: investigating the Brucella intracellular lifestyle. FEMS Microbiol Rev 36:533–562. doi: 10.1111/j.1574-6976.2012.00334.x. [DOI] [PubMed] [Google Scholar]
- 121.Porte F, Naroeni A, Ouahrani-Bettache S, Liautard J-P. 2003. Role of the Brucella suis lipopolysaccharide O antigen in phagosomal genesis and in inhibition of phagosome-lysosome fusion in murine macrophages. Infect Immun 71:1481–1490. doi: 10.1128/iai.71.3.1481-1490.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Forestier C, Moreno E, Pizarro-Cerda J, Gorvel J-P. 1999. Lysosomal accumulation and recycling of lipopolysaccharide to the cell surface of murine macrophages, an in vitro and in vivo study. J Immunol 162:6784–6791. [PubMed] [Google Scholar]
- 123.Altamirano-Silva P, Meza-Torres J, Castillo-Zeledón A, Ruiz-Villalobos N, Zuñiga-Pereira AM, Chacón-Díaz C, Moreno E, Guzmán-Verri C, Chaves-Olarte E. 2018. Brucella abortus senses the intracellular environment through the BvrR/BvrS two-component system, which allows B. abortus to adapt to its replicative niche. Infect Immun 86:e00713-17. doi: 10.1128/IAI.00713-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lamontagne J, Butler H, Chaves-Olarte E, Hunter J, Schirm M, Paquet C, Tian M, Kearney P, Hamaidi L, Chelsky D, Moriyón I, Moreno E, Paramithiotis E. 2007. Extensive cell envelope modulation is associated with virulence in Brucella abortus. J Proteome Res 6:1519–1529. doi: 10.1021/pr060636a. [DOI] [PubMed] [Google Scholar]
- 125.Martínez-Núñez C, Altamirano-Silva P, Alvarado-Guillén F, Moreno E, Guzmán-Verri C, Chaves-Olarte E. 2010. The two-component system BvrR/BvrS regulates the expression of the type IV secretion system VirB in Brucella abortus. J Bacteriol 192:5603–5608. doi: 10.1128/JB.00567-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Viadas C, Rodríguez MC, Sangari FJ, Gorvel J-P, García-Lobo JM, López-Goñi I. 2010. Transcriptome analysis of the Brucella abortus BvrR/BvrS two-component regulatory system. PLoS One 5:e10216. doi: 10.1371/journal.pone.0010216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Christie PJ, Vogel JP. 2000. Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol 8:354–360. doi: 10.1016/s0966-842x(00)01792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Celli J, de Chastellier C, Franchini D-M, Pizarro-Cerda J, Moreno E, Gorvel J-P. 2003. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J Exp Med 198:545–556. doi: 10.1084/jem.20030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Delrue R-M, Deschamps C, Léonard S, Nijskens C, Danese I, Schaus J-M, Bonnot S, Ferooz J, Tibor A, De Bolle X, Letesson J-J. 2005. A quorum-sensing regulator controls expression of both the type IV secretion system and the flagellar apparatus of Brucella melitensis. Cell Microbiol 7:1151–1161. doi: 10.1111/j.1462-5822.2005.00543.x. [DOI] [PubMed] [Google Scholar]
- 130.Ke Y, Wang Y, Li W, Chen Z. 2015. Type IV secretion system of Brucella spp. and its effectors. Front Cell Infect Microbiol 5:72. doi: 10.3389/fcimb.2015.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fretin D, Fauconnier A, Köhler S, Halling S, Léonard S, Nijskens C, Ferooz J, Lestrate P, Delrue R-M, Danese I, Vandenhaute J, Tibor A, DeBolle X, Letesson J-J. 2005. The sheathed flagellum of Brucella melitensis is involved in persistence in a murine model of infection. Cell Microbiol 7:687–698. doi: 10.1111/j.1462-5822.2005.00502.x. [DOI] [PubMed] [Google Scholar]
- 132.Arellano-Reynoso B, Lapaque N, Salcedo S, Briones G, Ciocchini AE, Ugalde R, Moreno E, Moriyón I, Gorvel J-P. 2005. Cyclic beta-1,2-glucan is a Brucella virulence factor required for intracellular survival. Nat Immunol 6:618–625. doi: 10.1038/ni1202. [DOI] [PubMed] [Google Scholar]
- 133.Conde-Álvarez R, Arce-Gorvel V, Iriarte M, Manček-Keber M, Barquero-Calvo E, Palacios-Chaves L, Chacón-Díaz C, Chaves-Olarte E, Martirosyan A, von Bargen K, Grilló M-J, Jerala R, Brandenburg K, Llobet E, Bengoechea JA, Moreno E, Moriyón I, Gorvel J-P. 2012. The lipopolysaccharide core of Brucella abortus acts as a shield against innate immunity recognition. PLoS Pathog 8:e1002675. doi: 10.1371/journal.ppat.1002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Oliveira FS, Carvalho NB, Zamboni DS, Oliveira SC. 2012. Nucleotide-binding oligomerization domain-1 and -2 play no role in controlling Brucella abortus infection in mice. Clin Dev Immunol 2012:861426. doi: 10.1155/2012/861426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Palacios-Chaves L, Conde-Álvarez R, Gil-Ramírez Y, Zúñiga-Ripa A, Barquero-Calvo E, Chacón-Díaz C, Chaves-Olarte E, Arce-Gorvel V, Gorvel J-P, Moreno E, de Miguel M-J, Grilló M-J, Moriyón I, Iriarte M. 2011. Brucella abortus ornithine lipids are dispensable outer membrane components devoid of a marked pathogen-associated molecular pattern. PLoS One 6:e16030. doi: 10.1371/journal.pone.0016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Campos MA, Rosinha GMS, Almeida IC, Salgueiro XS, Jarvis BW, Splitter GA, Qureshi N, Bruna-Romero O, Gazzinelli RT, Oliveira SC, Rene P. 2004. Role of toll-like receptor 4 in induction of cell-mediated immunity and resistance to Brucella abortus infection in mice. Infect Immun 72:176–186. doi: 10.1128/iai.72.1.176-186.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Weiss DS, Takeda K, Akira S, Zychlinsky A, Moreno E. 2005. MyD88, but not toll-like receptors 4 and 2, is required for efficient clearance of Brucella abortus. Infect Immun 73:5137–5143. doi: 10.1128/IAI.73.8.5137-5143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Barquero-Calvo E, Conde-Alvarez R, Chacón-Díaz C, Quesada-Lobo L, Martirosyan A, Guzmán-Verri C, Iriarte M, Mancek-Keber M, Jerala R, Gorvel JP, Moriyón I, Moreno E, Chaves-Olarte E. 2009. The differential interaction of Brucella and Ochrobactrum with innate immunity reveals traits related to the evolution of stealthy pathogens. PLoS One 4:e5893. doi: 10.1371/journal.pone.0005893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Barquero-Calvo E, Mora-Cartín R, Arce-Gorvel V, de Diego JL, Chacón-Díaz C, Chaves-Olarte E, Guzmán-Verri C, Buret AG, Gorvel J-P, Moreno E. 2015. Brucella abortus induces the premature death of human neutrophils through the action of its lipopolysaccharide. PLoS Pathog 11:e1004853. doi: 10.1371/journal.ppat.1004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Copin R, De Baetselier P, Carlier Y, Letesson J-J, Muraille E. 2007. MyD88-dependent activation of B220-CD11b+LY-6C+ dendritic cells during Brucella melitensis infection. J Immunol 178:5182–5191. doi: 10.4049/jimmunol.178.8.5182. [DOI] [PubMed] [Google Scholar]
- 141.Gomes MT, Campos PC, Pereira G de S, Bartholomeu DC, Splitter G, Oliveira SC. 2016. TLR9 is required for MAPK/NF-κB activation but does not cooperate with TLR2 or TLR6 to induce host resistance to Brucella abortus. J Leukoc Biol 99:771–780. doi: 10.1189/jlb.4A0815-346R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Arias MA, Santiago L, Costas-Ramon S, Jaime-Sánchez P, Freudenberg M, Jiménez De Bagüés MP, Pardo J. 2016. Toll-Like receptors 2 and 4 cooperate in the control of the emerging pathogen Brucella microti. Front Cell Infect Microbiol 6:205. doi: 10.3389/fcimb.2016.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lapaque N, Forquet F, de Chastellier C, Mishal Z, Jolly G, Moreno E, Moriyon I, Heuser JE, He H-T, Gorvel J-P. 2006. Characterization of Brucella abortus lipopolysaccharide macrodomains as mega rafts. Cell Microbiol 8:197–206. doi: 10.1111/j.1462-5822.2005.00609.x. [DOI] [PubMed] [Google Scholar]
- 144.Terwagne M, Ferooz J, Rolán HG, Sun Y-H, Atluri V, Xavier MN, Franchi L, Núñez G, Legrand T, Flavell RA, De Bolle X, Letesson J-J, Tsolis RM. 2013. Innate immune recognition of flagellin limits systemic persistence of Brucella. Cell Microbiol 15:942–960. doi: 10.1111/cmi.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Okemoto K, Hanada K, Nishijima M, Kawasaki K. 2008. The preparation of a lipidic endotoxin affects its biological activities. Biol Pharm Bull 31:1952–1954. doi: 10.1248/bpb.31.1952. [DOI] [PubMed] [Google Scholar]
- 146.Degos C, Gagnaire A, Banchereau R, Moriyón I, Gorvel J-P. 2015. Brucella CβG induces a dual pro- and anti-inflammatory response leading to a transient neutrophil recruitment. Virulence 6:19–28. doi: 10.4161/21505594.2014.979692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Martirosyan A, Pérez-Gutierrez C, Banchereau R, Dutartre H, Lecine P, Dullaers M, Mello M, Pinto Salcedo S, Muller A, Leserman L, Levy Y, Zurawski G, Zurawski S, Moreno E, Moriyón I, Klechevsky E, Banchereau J, Oh S, Gorvel J-P. 2012. Brucella β 1,2 cyclic glucan is an activator of human and mouse dendritic cells. PLoS Pathog 8:e1002983. doi: 10.1371/journal.ppat.1002983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Martínez de Tejada G, Moriyón I. 1993. The outer membranes of Brucella spp. are not barriers to hydrophobic permeants. J Bacteriol 175:5273–5275. doi: 10.1128/jb.175.16.5273-5275.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ariza X. 1988. Brucelosis perspectiva actual de la enfermedad. Perfil de las inmunoglobulinas especificas en el curso de su evolucion. PhD thesis, Universitat Autònoma de Barcelona, Barcelona, Spain. [Google Scholar]
- 150.Bundle DR, Cherwonogrodzky JW, Gidney MAJ, Meikle PJ, Perry MB, Peters T. 1989. Definition of Brucella A and M epitopes by monoclonal typing reagents and synthetic oligosaccharides. Infect Immun 57:2829–2836. doi: 10.1128/IAI.57.9.2829-2836.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kubler-Kielb J, Vinogradov E. 2013. The study of the core part and non-repeating elements of the O-antigen of Brucella lipopolysaccharide. Carbohydr Res 366:33–37. doi: 10.1016/j.carres.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schurig GG, Pringle AT, Breese SS. 1981. Localization of Brucella antigens that elicit a humoral immune response in Brucella abortus-infected cells. Infect Immun 34:1000–1007. doi: 10.1128/IAI.34.3.1000-1007.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Manterola L, Guzmán-Verri C, Chaves-Olarte E, Barquero-Calvo E, de Miguel M-J, Moriyón I, Grilló M-J, López-Goñi I, Moreno E. 2007. BvrR/BvrS-controlled outer membrane proteins Omp3a and Omp3b are not essential for Brucella abortus virulence. Infect Immun 75:4867–4874. doi: 10.1128/IAI.00439-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Páramo L, Lomonte B, Pizarro-Cerdá J, Bengoechea JA, Gorvel JP, Moreno E. 1998. Bactericidal activity of Lys49 and Asp49 myotoxic phospholipases A2 from Bothrops asper snake venom–synthetic Lys49 myotoxin II-(115-129)-peptide identifies its bactericidal region. Eur J Biochem 253:452–461. doi: 10.1046/j.1432-1327.1998.2530452.x. [DOI] [PubMed] [Google Scholar]
- 155.Poncin K, Roba A, Jimmidi R, Potemberg G, Fioravanti A, Francis N, Willemart K, Zeippen N, Machelart A, Biondi EG, Muraille E, Vincent SP, De Bolle X. 2019. Occurrence and repair of alkylating stress in the intracellular pathogen Brucella abortus. Nat Commun 10:847. doi: 10.1038/s41467-019-12516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Graham RLJ, Pollock CE, O'Loughlin SN, Ternan NG, Weatherly DB, Jackson PJ, Tarleton RL, McMullan G. 2006. Multidimensional proteomic analysis of the soluble subproteome of the emerging nosocomial pathogen Ochrobactrum anthropi. J Proteome Res 5:3145–3153. doi: 10.1021/pr060293g. [DOI] [PubMed] [Google Scholar]
- 157.Enright FM, Araya LN, Elzer PH, Rowe GE, Winter AJ. 1990. Comparative histopathology in BALB/c mice infected with virulent and attenuated strains of Brucella abortus. Vet Immunol Immunopathol 26:171–182. doi: 10.1016/0165-2427(90)90065-z. [DOI] [PubMed] [Google Scholar]
- 158.Prouty CC. 1934. Studies on the leucocyte content of milk drawn from Brucella abortus infected udders. J Bacteriol 27:293–301. doi: 10.1128/JB.27.3.293-301.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Colmenero JD, Reguera JM, Martos F, Sánchez-De-Mora D, Delgado M, Causse M, Martín-Farfán A, Juárez C. 1996. Complications associated with Brucella melitensis infection: a study of 530 cases. Medicine (Baltimore, MD) 75:195–211. doi: 10.1097/00005792-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 160.Kofoed K, Andersen O, Kronborg G, Tvede M, Petersen J, Eugen-Olsen J, Larsen K. 2007. Use of plasma C-reactive protein, procalcitonin, neutrophils, macrophage migration inhibitory factor, soluble urokinase-type plasminogen activator receptor, and soluble triggering receptor expressed on myeloid cells-1 in combination to diagnose infections. Crit Care 11:R38. doi: 10.1186/cc5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Olt S, Ergenç H, Açikgöz SB. 2015. Predictive contribution of neutrophil/lymphocyte ratio in diagnosis of brucellosis. Biomed Res Int 2015:210502. doi: 10.1155/2015/210502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Machelart A, Khadrawi A, Demars A, Willemart K, De Trez C, Letesson J-J, Muraille E. 2017. Chronic Brucella infection induces selective and persistent IFN-γ-dependent alterations of marginal zone macrophages in the spleen. Infect Immun 85:e00115-17. doi: 10.1128/IAI.00115-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bureau JP, Caravano R, Garelli L, Lemaire J. 1989. Cellular responses to “brucelline INRA” during the experimental brucellosis of the mouse. Immunobiology 179:8–16. doi: 10.1016/S0171-2985(89)80003-8. [DOI] [PubMed] [Google Scholar]
- 164.Ocon P, Reguera JM, Morata P, Juarez C, Alonso a, Colmenero JD. 1994. Phagocytic cell function in active brucellosis. Infect Immun 62:910–914. doi: 10.1128/IAI.62.3.910-914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fullerton JN, Segre E, De Maeyer RPH, Maini AAN, Gilroy DW. 2016. Intravenous endotoxin challenge in healthy humans: an experimental platform to investigate and modulate systemic inflammation. J Vis Exp 111:53913. doi: 10.3791/53913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Paixão TA, Roux CM, den Hartigh AB, Sankaran-Walters S, Dandekar S, Santos RL, Tsolis RM. 2009. Establishment of systemic Brucella melitensis infection through the digestive tract requires urease, the type IV secretion system, and lipopolysaccharide O antigen. IAI 77:4197–4208. doi: 10.1128/IAI.00417-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hensel ME, Garcia-Gonzalez DG, Chaki SP, Samuel J, Arenas-Gamboa AM. 2019. Characterization of an intratracheal aerosol challenge model of Brucella melitensis in guinea pigs. PLoS One 14:e0212457. doi: 10.1371/journal.pone.0212457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cao Z, Yan W, Wang B, Rong R, Ding J, Zhang L, Hao M, Mao K, Wang J. 2013. Pattern recognition and activation effect of mast cells in vitro infected by Brucella suis S2. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 29:1137–1140. (In Chinese.) [PubMed] [Google Scholar]
- 169.Schramm R, Thorlacius H. 2004. Neutrophil recruitment in mast cell-dependent inflammation: inhibitory mechanisms of glucocorticoids. Inflamm Res 53:644–652. doi: 10.1007/s00011-004-1307-8. [DOI] [PubMed] [Google Scholar]
- 170.Carvalho Neta AV, Mol JPS, Xavier MN, Paixão TA, Lage AP, Santos RL. 2010. Pathogenesis of bovine brucellosis. Vet J 184:146–155. doi: 10.1016/j.tvjl.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 171.Carvalho Neta AV, Stynen APR, Paixão TA, Miranda KL, Silva FL, Roux CM, Tsolis RM, Everts RE, Lewin HA, Adams LG, Carvalho AF, Lage AP, Santos RL. 2008. Modulation of the bovine trophoblastic innate immune response by Brucella abortus. Infect Immun 76:1897–1907. doi: 10.1128/IAI.01554-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.González-Barrientos R, Morales J-A, Hernández-Mora G, Barquero-Calvo E, Guzmán-Verri C, Chaves-Olarte E, Moreno E. 2010. Pathology of striped dolphins (Stenella coeruleoalba) infected with Brucella ceti. J Comp Pathol 142:347–352. doi: 10.1016/j.jcpa.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 173.Silva APC, Macêdo AA, Costa LF, Rocha CE, Garcia LNN, Farias JRD, Gomes PPR, Teixeira GC, Fonseca KWJ, Maia ARF, Neves GG, Romão EL, Silva TMA, Mol JPS, Oliveira RM, Araújo MSS, Nascimento EF, Martins-Filho OA, Brandão HM, Paixão TA, Santos RL. 2015. Encapsulated Brucella ovis lacking a putative ATP-binding cassette transporter (ΔabcBA) protects against wild type Brucella ovis in rams. PLoS One 10:e0136865. doi: 10.1371/journal.pone.0136865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sokolove J, Lepus CM. 2013. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis 5:77–94. doi: 10.1177/1759720X12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Anderson TD, Meador VP, Cheville NF. 1986. Pathogenesis of placentitis in the goat inoculated with Brucella abortus. I. Gross and histologic lesions. Vet Pathol 23:219–226. doi: 10.1177/030098588602300301. [DOI] [PubMed] [Google Scholar]
- 176.Pérez J, Quezada M, López J, Casquet O, Sierra MA, Martín de las Mulas J. 1998. Immunohistochemical detection of Brucella abortus antigens in tissues from aborted bovine fetuses using a commercially available polyclonal antibody. J Vet Diagn Invest 10:17–21. doi: 10.1177/104063879801000104. [DOI] [PubMed] [Google Scholar]
- 177.Xavier MN, Paixão TA, Poester FP, Lage AP, Santos RL. 2009. Pathological, immunohistochemical and bacteriological study of tissues and milk of cows and fetuses experimentally infected with Brucella abortus. J Comp Pathol 140:149–157. doi: 10.1016/j.jcpa.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 178.Khan RN, Hay DP. 2015. A clear and present danger: inflammasomes DAMPing down disorders of pregnancy. Hum Reprod Update 21:388–405. doi: 10.1093/humupd/dmu059. [DOI] [PubMed] [Google Scholar]
- 179.Fernández AG, Ferrero MC, Hielpos MS, Fossati CA, Baldi PC, Fernandez AG, Ferrero MC, Hielpos MS, Fossati CA, Baldi PC. 2016. Proinflammatory response of human trophoblastic cells to Brucella abortus infection and upon interactions with infected phagocytes. Biol Reprod 94:48. doi: 10.1095/biolreprod.115.131706. [DOI] [PubMed] [Google Scholar]
- 180.de Souza TD, De Carvalho TF, Mol JPDS, Lopes JVM, Silva MF, da Paixão TA, Santos RL. 2018. Tissue distribution and cell tropism of Brucella canis in naturally infected canine foetuses and neonates. Sci Rep 8:7203. doi: 10.1038/s41598-018-25651-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kim S, Lee DS, Watanabe K, Furuoka H, Suzuki H, Watarai M, Dong SL, Watanabe K, Furuoka H, Suzuki H, Watarai M. 2005. Interferon-γ promotes abortion due to Brucella infection in pregnant mice. BMC Microbiol 5:22. doi: 10.1186/1471-2180-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tobias L, Cordes DO, Schurig GG. 1993. Placental pathology of the pregnant mouse inoculated with Brucella abortus strain 2308. Vet Pathol 30:119–129. doi: 10.1177/030098589303000204. [DOI] [PubMed] [Google Scholar]
- 183.Bosseray N. 1980. Colonization of mouse placentas by Brucella abortus inoculated during pregnancy. Br J Exp Pathol 61:361–368. [PMC free article] [PubMed] [Google Scholar]
- 184.Colmenero JD, Muñoz-Roca NL, Bermudez P, Plata A, Villalobos A, Reguera JM. 2007. Clinical findings, diagnostic approach, and outcome of Brucella melitensis epididymo-orchitis. Diagn Microbiol Infect Dis 57:367–372. doi: 10.1016/j.diagmicrobio.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 185.Abu-Seida AM, Ahmed KA, Torad FA, Marouf SA. 2015. Ultrasonographic and histopathological findings in rams with epididymo-orchitis caused by Brucella melitensis. Pak Vet J 35:456–460. [Google Scholar]
- 186.Meador VP, Deyoe BL, Cheville NF. 1989. Pathogenesis of Brucella abortus infection of the mammary gland and supramammary lymph node of the goat. Vet Pathol 26:357–368. doi: 10.1177/030098588902600501. [DOI] [PubMed] [Google Scholar]
- 187.Silva ID, Jain NC. 1988. Phagocytic and nitroblue tetrazolium reductive properties of bovine neutrophils for mammary pathogens. J Dairy Sci 71:1625–1631. doi: 10.3168/jds.S0022-0302(88)79726-X. [DOI] [PubMed] [Google Scholar]
- 188.Stevens MG, Olsen SC. 1993. Comparative analysis of using MTT and XTT in colorimetric assays for quantitating bovine neutrophil bactericidal activity. J Immunol Methods 157:225–231. doi: 10.1016/0022-1759(93)90091-k. [DOI] [PubMed] [Google Scholar]
- 189.Nemenqani D, Yaqoob N, Khoja H. 2009. Breast brucellosis in Taif, Saudi Arabia: cluster of six cases with emphasis on FNA evaluation. J Infect Dev Ctries 3:255–259. doi: 10.3855/jidc.121. [DOI] [PubMed] [Google Scholar]
- 190.Haji-Abdolbagi M, Rasooli-Nejad M, Jafari S, Hasibi M, Soudbakhsh A. 2008. Clinical and laboratory findings in neurobrucellosis: review of 31 cases. Arch Iran Med 11:21–25. [PubMed] [Google Scholar]
- 191.Mathews CJ, Kingsley G, Field M, Jones A, Weston VC, Phillips M, Walker D, Coakley G. 2008. Management of septic arthritis: a systematic review. Postgrad Med J 84:265–270. doi: 10.1136/ard.2006.058909. [DOI] [PubMed] [Google Scholar]
- 192.Khateeb MI, Araj GF, Majeed SA, Lulu AR. 1990. Brucella arthritis: a study of 96 cases in Kuwait. Ann Rheum Dis 49:994–998. doi: 10.1136/ard.49.12.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mavridis AK, Drosos AA, Tsolas O, Moutsopoulos HM. 1984. Lactate levels in Brucella arthritis. Rheumatol Int 4:169–171. doi: 10.1007/BF00541209. [DOI] [PubMed] [Google Scholar]
- 194.de Macedo AA, Galvão NR, Sá JC, de Carvalho da Silva AP, da Silva Mol JP, dos Santos LS, Santos RL, de Carvalho Neta AV. 2019. Brucella-associated cervical bursitis in cattle. Trop Anim Health Prod 51:697–702. doi: 10.1007/s11250-018-1745-x. [DOI] [PubMed] [Google Scholar]
- 195.Musa MT, Jahans KL, Fadalla ME. 1990. Clinical manifestations of brucellosis in cattle of the Southern Darfur Province, Western Sudan. J Comp Pathol 103:95–99. doi: 10.1016/S0021-9975(08)80139-9. [DOI] [PubMed] [Google Scholar]
- 196.Lacey CA, Keleher LL, Mitchell WJ, Brown CR, Skyberg JA. 2016. CXCR2 mediates Brucella-induced arthritis in IFN-γ-deficient mice. J Infect Dis 214:151–160. doi: 10.1093/infdis/jiw087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Scian R, Barrionuevo P, Giambartolomei GH, De Simone EA, Vanzulli SI, Fossati CA, Baldi PC, Delpino MV. 2011. Potential role of fibroblast-like synoviocytes in joint damage induced by Brucella abortus infection through production and induction of matrix metalloproteinases. Infect Immun 79:3619–3632. doi: 10.1128/IAI.05408-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Demir C, Karahocagil MK, Esen R, Atmaca M, Gönüllü H, Akdeniz H. 2012. Bone marrow biopsy findings in brucellosis patients with hematologic abnormalities. Chin Med J (Engl) 125:1871–1876. [PubMed] [Google Scholar]
- 199.Gutiérrez-Jiménez C, Hysenaj L, Alfaro-Alarcón A, Mora-Cartín R, Arce-Gorvel V, Moreno E, Gorvel JP, Barquero-Calvo E. 2018. Persistence of Brucella abortus in the bone marrow of infected mice. J Immunol Res 2018:5370414–5370418. doi: 10.1155/2018/5370414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.El-Koumi MA, Afify M, Al-Zahrani SH. 2013. A prospective study of brucellosis in children: relative frequency of pancytopenia. Mediterr J Hematol Infect Dis 5:e2013011. doi: 10.4084/MJHID.2013.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Makis A, Perogiannaki A, Chaliasos N. 2017. Severe thrombocytopenic purpura in a child with brucellosis: case presentation and review of the literature. Case Rep Infect Dis 2017:1–5. doi: 10.1155/2017/3416857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Duffy D, Perrin H, Abadie V, Benhabiles N, Boissonnas A, Liard C, Descours B, Reboulleau D, Bonduelle O, Verrier B, Van Rooijen N, Combadière C, Combadière B. 2012. Neutrophils transport antigen from the dermis to the bone marrow, initiating a source of memory CD8+ T cells. Immunity 37:917–929. doi: 10.1016/j.immuni.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 203.Canning PC, Roth JA, Tabatabai LB, Deyoe BL. 1985. Isolation of components of Brucella abortus responsible for inhibition of function in bovine neutrophils. J Infect Dis 152:913–921. doi: 10.1093/infdis/152.5.913. [DOI] [PubMed] [Google Scholar]
- 204.Gallego MC, Lapeña MA. 1990. The interaction of Brucella melitensis 16-M and caprine polymorphonuclear leukocytes. Comp Immunol Microbiol Infect Dis 13:59–65. doi: 10.1016/0147-9571(90)90517-W. [DOI] [PubMed] [Google Scholar]
- 205.Mora-Cartín R, Chacón-Díaz C, Gutiérrez-Jiménez C, Gurdián-Murillo S, Lomonte B, Chaves-Olarte E, Barquero-Calvo E, Moreno E. 2016. N-formyl-perosamine surface homopolysaccharides hinder the recognition of Brucella abortus by mouse neutrophils. Infect Immun 84:1712–1721. doi: 10.1128/IAI.00137-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Victor J, Pollack AD, Raymond R, Valliant JR. 1952. Studies on phagocytosis; determination of blood opsonin for Brucella. J Bacteriol 64:121–130. doi: 10.1128/JB.64.1.121-130.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Young EJ, Borchert M, Kretzer FL, Musher DM. 1985. Phagocytosis and killing of Brucella by human polymorphonuclear leukocytes. J Infect Dis 151:682–690. doi: 10.1093/infdis/151.4.682. [DOI] [PubMed] [Google Scholar]
- 208.Bounous DI, Enright FM, Gossett KA, Berry CM, Kearney MT. 1992. Comparison of oxidant production by bovine neutrophils and monocyte-derived macrophages stimulated with Brucella abortus strain 2308. Inflammation 16:215–225. doi: 10.1007/BF00918811. [DOI] [PubMed] [Google Scholar]
- 209.Gallego MC, Cuello F, Garrido A. 1989. In vitro determination of phagocytosis and intracellular killing of Brucella melitensis by goat’s polymorphonuclear phagocytes. Comp Immunol Microbiol Infect Dis 12:9–15. doi: 10.1016/0147-9571(89)90004-0. [DOI] [PubMed] [Google Scholar]
- 210.Elzer PH, Phillips RW, Robertson GT, Roop RM. 1996. The HtrA stress response protease contributes to resistance of Brucella abortus to killing by murine phagocytes. Infect Immun 64:4838–4841. doi: 10.1128/IAI.64.11.4838-4841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Canning PC, Roth JA. 1989. Effects of in vitro and in vivo administration of recombinant bovine interferon-gamma on bovine neutrophil responses to Brucella abortus. Vet Immunol Immunopathol 20:119–133. doi: 10.1016/0165-2427(89)90093-7. [DOI] [PubMed] [Google Scholar]
- 212.Kim JA, Sha Z, Mayfield JE. 2000. Regulation of Brucella abortus catalase. Infect Immun 68:3861–3866. doi: 10.1128/iai.68.7.3861-3866.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nordenfelt P, Tapper H. 2011. Phagosome dynamics during phagocytosis by neutrophils. J Leukoc Biol 90:271–284. doi: 10.1189/jlb.0810457. [DOI] [PubMed] [Google Scholar]
- 214.Bowden RA, Cloeckaert A, Zygmunt MS, Bernard S, Dubray G. 1995. Surface exposure of outer membrane protein and lipopolysaccharide epitopes in Brucella species studied by enzyme-linked immunosorbent assay and flow cytometry. Infect Immun 63:3945–3952. doi: 10.1128/IAI.63.10.3945-3952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cloeckaert A, de Wergifosse P, Dubray G, Limet JN. 1990. Identification of seven surface-exposed Brucella outer membrane proteins by use of monoclonal antibodies: immunogold labeling for electron microscopy and enzyme-linked immunosorbent assay. Infect Immun 58:3980–3987. doi: 10.1128/IAI.58.12.3980-3987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bertram TA, Canning PC, Roth JA. 1986. Preferential inhibition of primary granule release from bovine neutrophils by a Brucella abortus extract. Infect Immun 52:285–292. doi: 10.1128/IAI.52.1.285-292.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Velasco J, Romero C, López-Goñi I, Leiva J, Díaz R, Moriyón I. 1998. Evaluation of the relatedness of Brucella spp. and Ochrobactrum anthropi and description of Ochrobactrum intermedium sp. nov., a new species with a closer relationship to Brucella spp. Int J Syst Bacteriol 48:759–768. doi: 10.1099/00207713-48-3-759. [DOI] [PubMed] [Google Scholar]
- 218.Arora U, Kaur S, Devi P. 2008. Ochrobactrum anthropi septicaemia. Indian J Med Microbiol 26:81–83. doi: 10.4103/0255-0857.38868. [DOI] [PubMed] [Google Scholar]
- 219.Ozdemir D, Soypacaci Z, Sahin I, Bicik Z, Sencan I. 2006. Ochrobactrum anthropi endocarditis and septic shock in a patient with no prosthetic valve or rheumatic heart disease: case report and review of the literature. Jpn J Infect Dis 59:264–265. [PubMed] [Google Scholar]
- 220.Wheen L, Taylor S, Godfrey K. 2002. Vertebral osteomyelitis due to Ochrobactrum anthropi. Intern Med J 32:426–428. doi: 10.1046/j.1445-5994.2002.00248.x. [DOI] [PubMed] [Google Scholar]
- 221.Chain PSG, Lang DM, Comerci DJ, Malfatti SA, Vergez LM, Shin M, Ugalde RA, Garcia E, Tolmasky ME. 2011. Genome of Ochrobactrum anthropi ATCC 49188 T, a versatile opportunistic pathogen and symbiont of several eukaryotic hosts. J Bacteriol 193:4274–4275. doi: 10.1128/JB.05335-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Nauseef WM, Borregaard N. 2014. Neutrophils at work. Nat Immunol 15:602–611. doi: 10.1038/ni.2921. [DOI] [PubMed] [Google Scholar]
- 223.Rasool O, Freer E, Moreno E, Jarstrand C. 1992. Effect of Brucella abortus lipopolysaccharide on oxidative metabolism and lysozyme release by human neutrophils. Infect Immun 60:1699–1702. doi: 10.1128/IAI.60.4.1699-1702.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gemmell CG. 1993. Antibiotics and neutrophil function—potential immnnomodulating activities. J Antimicrob Chemother 31:23–33. doi: 10.1093/jac/31.suppl_B.23. [DOI] [PubMed] [Google Scholar]
- 225.Orduña A, Orduña C, Zarzosa P, Bratos MA, Ortiz de Lejarazu R, Rodríguez-Torres A. 1989. Influence of doxycycline, streptomycin, cotrimoxazole and rifamycin on the functions of normal human neutrophils against Brucella melitensis. J Chemother 1:429–431. [PubMed] [Google Scholar]
- 226.Orduña A, Orduña C, Eiros JM, Bratos MA, Gutiérrez P, Alonso P, Rodríguez Torres A. 1991. Inhibition of the degranulation and myeloperoxidase activity of human polymorphonuclear neutrophils by Brucella melitensis. Microbiologia 7:113–119. [PubMed] [Google Scholar]
- 227.Iyankan L, Singh DK. 2002. The effect of Brucella abortus on hydrogen peroxide and nitric oxide production by bovine polymorphonuclear cells. Vet Res Commun 26:93–102. doi: 10.1023/A:1014039500378. [DOI] [PubMed] [Google Scholar]
- 228.Dabdoob WA, Abdulla ZA. 2000. A panel of eight tests in the serodiagnosis and immunological evaluation of acute brucellosis. East Mediterr Health J 6:304–312. [PubMed] [Google Scholar]
- 229.Gordon AM, Rowan RM, Brown T, Carson HG. 1973. Routine application of the nitroblue tetrazolium test in the clinical laboratory. J Clin Pathol 26:52–56. doi: 10.1136/jcp.26.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hellum KB. 1977. Nitroblue tetrazolium test in bacterial and viral infections. Scand J Infect Dis 9:269–276. doi: 10.3109/inf.1977.9.issue-4.03. [DOI] [PubMed] [Google Scholar]
- 231.Xavier MN, Winter MG, Spees AM, Nguyen K, Atluri VL, Silva TMA, Bäumler AJ, Müller W, Santos RL, Tsolis RM. 2013. CD4(+) T Cell-derived IL-10 promotes Brucella abortus persistence via modulation of macrophage function. PLoS Pathog 9:e1003454. doi: 10.1371/journal.ppat.1003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Tumurkhuu G, Koide N, Takahashi K, Hassan F, Islam S, Ito H, Mori I, Yoshida T, Yokochi T. 2006. Characterization of biological activities of Brucella melitensis lipopolysaccharide. Microbiol Immunol 50:421–427. doi: 10.1111/j.1348-0421.2006.tb03810.x. [DOI] [PubMed] [Google Scholar]
- 233.Altstaedt J, Kirchner H, Rink L. 1996. Cytokine production of neutrophils is limited to interleukin-8. Immunology 89:563–568. doi: 10.1046/j.1365-2567.1996.d01-784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Cullen SP, Henry CM, Kearney CJ, Logue SE, Feoktistova M, Tynan GA, Lavelle EC, Leverkus M, Martin SJ. 2013. Fas/CD95-induced chemokines can serve as “find-me” signals for apoptotic cells. Mol Cell 49:1034–1048. doi: 10.1016/j.molcel.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 235.Eskra L, Mathison A, Splitter G. 2003. Microarray analysis of mRNA levels from RAW264.7 macrophages infected with Brucella abortus. Infect Immun 71:1125–1133. doi: 10.1128/iai.71.3.1125-1133.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Koch CC, Esteban DJ, Chin AC, Olson ME, Read RR, Ceri H, Morck DW, Buret AG. 2000. Apoptosis, oxidative metabolism and interleukin-8 production in human neutrophils exposed to azithromycin: effects of Streptococcus pneumoniae. J Antimicrob Chemother 46:19–26. doi: 10.1093/jac/46.1.19. [DOI] [PubMed] [Google Scholar]
- 237.Conlan JW. 1997. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella Typhimurium, and Yersinia enterocolitica. Infect Immun 65:630–635. doi: 10.1128/IAI.65.2.630-635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ordoñez-Rueda D, Jönsson F, Mancardi DA, Zhao W, Malzac A, Liang Y, Bertosio E, Grenot P, Blanquet V, Sabrautzki S, de Angelis MH, Méresse S, Duprez E, Bruhns P, Malissen B, Malissen M. 2012. A hypomorphic mutation in the Gfi1 transcriptional repressor results in a novel form of neutropenia. Eur J Immunol 42:2395–2408. doi: 10.1002/eji.201242589. [DOI] [PubMed] [Google Scholar]
- 239.Robertson CM, Perrone EE, McConnell KW, Dunne WM, Boody B, Brahmbhatt T, Diacovo MJ, Van Rooijen N, Hogue LA, Cannon CL, Buchman TG, Hotchkiss RS, Coopersmith CM. 2008. Neutrophil depletion causes a fatal defect in murine pulmonary Staphylococcus aureus clearance. J Surg Res 150:278–285. doi: 10.1016/j.jss.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Guilloteau LA, Dornand J, Gross A, Olivier M, Cortade F, Le Vern Y, Kerboeuf D. 2003. Nramp1 is not a major determinant in the control of Brucella melitensis infection in mice. Infect Immun 71:621–628. doi: 10.1128/iai.71.2.621-628.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Fernandes DM, Baldwin CL. 1995. Interleukin-10 downregulates protective immunity to Brucella abortus. Infect Immun 63:1130–1133. doi: 10.1128/IAI.63.3.1130-1133.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, Ernst M, Jones SA, Topley N, Jenkins BJ. 2008. IL-6 regulates neutrophil trafficking during acute Inflammation via STAT3. J Immunol 181:2189–2195. doi: 10.4049/jimmunol.181.3.2189. [DOI] [PubMed] [Google Scholar]
- 243.Hamza T, Barnett JB, Li B. 2010. Interleukin 12 a key immunoregulatory cytokine in infection applications. Int J Mol Sci 11:789–806. doi: 10.3390/ijms11030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Muraille E, Leo O, Moser M. 2014. Th1/Th2 paradigm extended: macrophage polarization as an unappreciated pathogen-driven escape mechanism? Front Immunol 26:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhan Y, Cheers C. 1995. Endogenous interleukin-12 is involved in resistance to Brucella abortus infection. Infect Immun 63:1387–1390. doi: 10.1128/IAI.63.4.1387-1390.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Shinozawa Y, Matsumoto T, Uchida K, Tsujimoto S, Iwakura Y, Yamaguchi K. 2002. Role of interferon-gamma in inflammatory responses in murine respiratory infection with Legionella pneumophila. J Med Microbiol. 51:225–230. doi: 10.1099/0022-1317-51-3-225. [DOI] [PubMed] [Google Scholar]
- 247.Roy CR, Salcedo SP, Gorvel JPE. 2006. Pathogen-endoplasmic-reticulum interactions: in through the out door. Nat Rev Immunol 6:136–147. doi: 10.1038/nri1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Sadallah S, Eken C, Schifferli JA. 2011. Ectosomes as modulators of inflammation and immunity. Clin Exp Immunol 163:26–32. doi: 10.1111/j.1365-2249.2010.04271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Doz E, Lombard R, Carreras F, Buzoni-Gatel D, Winter N. 2013. Mycobacteria-infected dendritic cells attract neutrophils that produce IL-10 and specifically shut down Th17 CD4 T cells through their IL-10 receptor. J Immunol 191:3818–3826. doi: 10.4049/jimmunol.1300527. [DOI] [PubMed] [Google Scholar]
- 250.Kieran NE, Maderna P, Godson C. 2004. Lipoxins: potential anti-inflammatory, proresolution, and antifibrotic mediators in renal disease. Kidney Int 65:1145–1154. doi: 10.1111/j.1523-1755.2004.00487.x. [DOI] [PubMed] [Google Scholar]
- 251.Fahel JS, de Souza MB, Ribeiro Gomes MT, Corsetti PP, Carvalho NB, Marinho FAV, de Almeida LA, Caliari MV, Machado FS, Oliveira SC. 2015. 5-Lipoxygenase negatively regulates Th1 response during Brucella abortus infection in mice. Infect Immun 83:1210–1216. doi: 10.1128/IAI.02592-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wei J, Mattapallil MJ, Horai R, Jittayasothorn Y, Modi AP, Sen HN, Gronert K, Caspi RR. 2020. A novel role for lipoxin A4 in driving a lymph node-eye axis that controls autoimmunity to the neuroretina. Elife 9:e51102. doi: 10.7554/eLife.51102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Billard E, Cazevieille C, Dornand J, Gross A. 2005. High susceptibility of human dendritic cells to invasion by the intracellular pathogens Brucella suis, B. abortus, and B. melitensis. Infect Immun 73:8418–8424. doi: 10.1128/IAI.73.12.8418-8424.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Jiang X, Baldwin CL. 1993. Effects of cytokines on intracellular growth of Brucella abortus. Infect Immun 61:124–134. doi: 10.1128/IAI.61.1.124-134.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Marwick JA, Mills R, Kay O, Michail K, Stephen J, Rossi AG, Dransfield I, Hirani N. 2018. Neutrophils induce macrophage anti-inflammatory reprogramming by suppressing NF-κB activation article. Cell Death Dis 9:665. doi: 10.1038/s41419-018-0710-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. 1998. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest 101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. 1997. Immunosuppressive effects of apoptotic cells. Nature 390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 258.Gräbner C. 2018. How to relate models to reality? An epistemological framework for the validation and verification of computational models. J Artif Soc Soc Simul 21:8. doi: 10.18564/jasss.3772. [DOI] [Google Scholar]
- 259.Georghiou PR, Young EJ. 1991. Prolonged incubation in brucellosis. Lancet 337:1543. doi: 10.1016/0140-6736(91)93231-w. [DOI] [PubMed] [Google Scholar]
- 260.Laskay T, van Zandbergen G, Solbach W. 2003. Neutrophil granulocytes—Trojan horses for Leishmania major and other intracellular microbes? Trends Microbiol 11:210–214. doi: 10.1016/s0966-842x(03)00075-1. [DOI] [PubMed] [Google Scholar]
- 261.Chen KW, Lawlor KE, von Pein JB, Boucher D, Gerlic M, Croker BA, Bezbradica JS, Vince JE, Schroder K. 2018. Cutting edge: blockade of inhibitor of apoptosis proteins sensitizes neutrophils to TNF- but not lipopolysaccharide-mediated cell death and IL-1β secretion. J Immunol 200:3341–3346. doi: 10.4049/jimmunol.1701620. [DOI] [PubMed] [Google Scholar]
- 262.Zanoni I, Ostuni R, Capuano G, Collini M, Caccia M, Ronchi AE, Rocchetti M, Mingozzi F, Foti M, Chirico G, Costa B, Zaza A, Ricciardi-Castagnoli P, Granucci F. 2009. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature 460:264–268. doi: 10.1038/nature08118. [DOI] [PubMed] [Google Scholar]
- 263.Popa C, Abdollahi-Roodsaz S, Joosten LAB, Takahashi N, Sprong T, Matera G, Liberto MC, Foca A, van Deuren M, Kullberg BJ, van den Berg WB, van der Meer JWM, Netea MG. 2007. Bartonella quintana lipopolysaccharide is a natural antagonist of Toll-like receptor 4. Infect Immun 75:4831–4837. doi: 10.1128/IAI.00237-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Franco MP, Mulder M, Smits HL. 2007. Persistence and relapse in brucellosis and need for improved treatment. Trans R Soc Trop Med Hyg 101:854–855. doi: 10.1016/j.trstmh.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 265.Pedro-Pons A, Valenti PF. 1944. La brucelosis humana, p 19–29. Salvat S.A, Barcelona, Spain. [Google Scholar]
- 266.Nicoletti P, Milward FW. 1983. Protection by oral administration of Brucella abortus strain 19 against an oral challenge exposure with a pathogenic strain of Brucella. Am J Vet Res 44:1641–1643. [PubMed] [Google Scholar]
- 267.Eze MO, Yuan L, Crawford RM, Paranavitana CM, Hadfield TL, Bhattacharjee AK, Warren RL, Hoover DL. 2000. Effects of opsonization and gamma interferon on growth of Brucella melitensis 16M in mouse peritoneal macrophages in vitro. Infect Immun 68:257–263. doi: 10.1128/iai.68.1.257-263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]