Abstract
Collapsing focal segmental glomerulosclerosis (cFSGS) is a progressive kidney disease characterized by glomerular collapse with epithelial hyperplasia. Here we used a transgenic mouse model of cFSGS with immunotoxin-induced podocyte-specific injury to determine the role for Notch signaling in its pathogenesis. The mice exhibited progressive loss of podocytes and severe proteinuria concomitant with histological features of cFSGS. Hyperplastic epithelium was negative for genetic podocyte tags, but positive for the parietal epithelial cell marker claudin-1, and expressed Notch1, Jagged1, and Hes1 mRNA and protein. Enhanced Notch mRNA expression induced by transforming growth factor-β1 in cultured parietal epithelial cells was associated with mesenchymal markers (α-smooth muscle actin, vimentin, and Snail1). Notch inhibition in vitro suppressed these phenotypic transcripts and Notch-dependent cell migration. Moreover, Notch inhibition in vivo significantly decreased parietal epithelial cell lesions but worsened proteinuria and histopathology in our cFSGS model. Thus, aberrant Notch1-mediated parietal epithelial cell migration with phenotypic changes appears to underlie the pathogenesis of cFSGS. Parietal epithelial cell hyperplasia may also represent an adaptive response to compensate for a disrupted filtration barrier with progressive podocyte loss.
Keywords: focal segmental glomerulosclerosis, Notch signaling, parietal epithelial cell, podocyte loss
Focal segmental glomerulosclerosis (FSGS), a progressive kidney disease, is the major cause of end-stage renal failure.1 Progressive decrease in podocytes is considered a factor underlying FSGS.2,3 The sequence of events in podocyte loss–induced FSGS is known for the remnant kidney.4 In this model, slow podocyte loss results in presclerotic synechia formation, as observed in postadaptive secondary FSGS.4
Collapsing FSGS (cFSGS), a distinct form of FSGS, is characterized by more pronounced proteinuria and more rapid renal dysfunction as compared with other variants in the Columbia FSGS classification.1,5–7 Although several causes, including viruses and drugs, have been suggested,1 the molecular mechanism underlying cFSGS remains unclear. cFSGS has a distinguishing morphological feature known as tuft collapse that is accompanied by marked epithelial cell hyperplasia, which is supposedly related to its progression.5,7,8 Thus, the cellular mechanism underlying hyperplastic epithelium may be important for understanding the pathogenesis of cFSGS.
Using human and murine models of cFSGS, our group as well as Smeets et al.9–15 showed that hyperplastic cells expressed parietal epithelial cell (PEC) markers but not podocyte markers, although others have interpreted lack of podocyte markers as an indicator of podocyte dedifferentiation.8 Probably, heterogeneous origins are responsible for these lesions.16 Genetically tagged podocytes in murine cFSGS models showed that hyperplastic lesions were essentially negative for podocyte-tagged cells but positive for PEC markers.9,14,15 More importantly, in a transgenic mouse expressing human CD25 on podocyte (NEP25 mouse), specific antibody to human CD25 (an immunotoxin called LMB2) treatment induced extensive podocyte loss and histology similar to human cFSGS.14,17 These findings indicate that acute decrease in podocytes followed by PEC hyperplasia may be a pathogenic route leading to cFSGS.
In this study, we focused on the Notch signaling pathway as a possible mechanism underlying these events. Notch signaling is a highly conserved determinant of cell-fate decisions during organogenesis via the asymmetrical differentiation of developing cells.18,19 It is also involved in cell migration, proliferation, phenotypic changes, and apoptosis in many diseases.20–23 During kidney development, Notch signaling expression is detected transiently in candidate podocytes and PECs, and is important for glomerulogenesis.24,25 Moreover, Notch signaling is involved in several kidney diseases and was detected in podocytes in which it induces apoptosis.23 On the basis of these observations, we hypothesized that the Notch signaling pathway might induce significant epithelial changes that could provide new insights into cFSGS pathogenesis.
For this aim, we used NEP25 mouse as an experimental cFSGS model and a recently established immortalized PEC line to determine the role of Notch signaling in PEC responses to podocyte loss.14,17,26 The therapeutic potential of Notch inhibition was also tested in our mouse cFSGS model. Our results suggest that acute podocyte loss followed by glomerular collapse and PEC migration via Notch1-dependent phenotypic changes is a novel pathogenic mechanism underlying cFSGS. Furthermore, inhibition of Notch signaling in vivo potentially worsens cFSGS, suggesting an underlying wound healing mechanism for cFSGS pathology.
RESULTS
Podocyte-specific injury causes progressive proteinuria, podocyte loss, and PEC hyperplasia
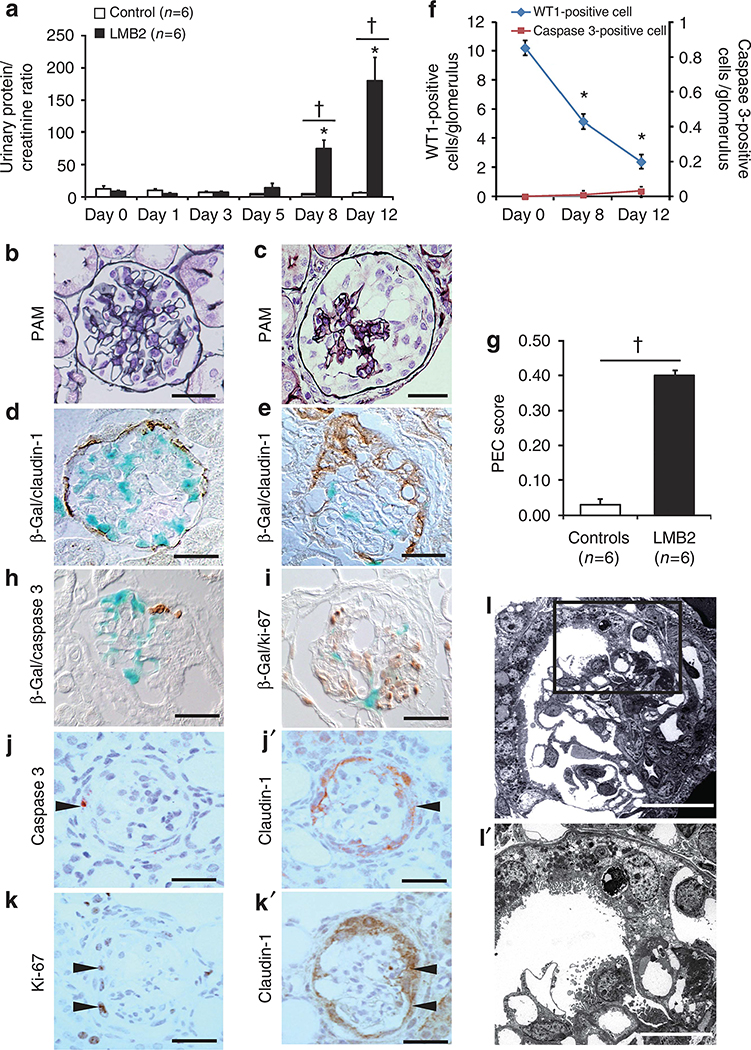
NEP25 mice administered the immunotoxin (LMB2) developed progressive proteinuria beginning on day 8 (Figure 1a). Histological analysis showed normal kidney morphology in vehicle-treated NEP25 mice (controls; Figure 1b). Conversely, in LMB2-treated mice (LMB2 mice), 9.44±0.62% glomeruli exhibited segmental or global collapse and 27.67±2.12% glomeruli exhibited extracapillary hypercellularity that formed adhesions with the glomerular tuft. These glomeruli closely resembled those in human cFSGS (Figure 1c). LMB2 mice, but not controls (Figure 1d), had marked decrease in podocytes on β-galactosidase (β-gal) staining (Figure 1e). In the sections examined, >95% of the cells in the extracapillary lesions expressed claudin-1 (a PEC marker) and were negative for β-gal, a podocyte label. In LMB2 mice, the numbers of WT1-positive cells (podocytes) progressively decreased and cleaved caspase 3–positive cells were rarely observed throughout the observation period (Figure 1f). The PEC score was significantly higher for LMB2 mice than for controls on day 12 (Figure 1g). Cleaved caspase 3– and Ki-67–positive cells were considered PECs but not podocytes according to results of double labeling for β-gal and claudin-1 (Figure 1h–k). Electron micrographs revealed severe podocyte injury and initial PEC migration toward the bared glomerular capillary during the early events in this model (Figure 1ll′). These results showed that PEC hyperplasia occurred concurrently with acute podocyte depletion and that proliferating/apoptotic cells were PECs but not podocytes.
Figure 1 |. Podocyte-selective injury induces progressive proteinuria and histological features of collapsing focal segmental glomerulosclerosis associated with hyperplastic parietal epithelial cells.
(a) Total proteinuria was determined from the urinary protein/urinary creatinine ratio. NEP25 transgenic mice that received immunotoxin (LMB2 mice, n = 6) showed progressive proteinuria beginning at days 8–12 compared with vehicle-treated NEP25 mice (controls, n = 6). Periodic acid-silver methenamine (PAM) staining showed normal morphology in (b) controls, whereas (c) global tuft collapse was observed along with epithelial hyperplasia on day 12 in LMB2 mice. (d) Double labeling for β-galactosidase (β-gal) and claudin-1 showed podocyte-limited β-gal staining (blue) in controls. (e) In LMB2 mice, marked decrease of podocytes on β-gal staining and expression of claudin-1 (brown) in the hyperplastic lesions were observed. Bars = 15 μm. (f) The number of WT1-positive cells per glomerulus was significantly and progressively reduced in LMB2 mice on days 8 and 12 compared with day 0 (day 0: 10.24±0.22; day 8: 5.17±0.1; day 12: 2.4±0.05 cells per glomerulus). Cleaved caspase 3–positive cells were rarely observed throughout the observation period (day 0: 0±0; day 8: 0.009±0.006; day 12: 0.03±0.006 cells per glomerulus). (g) The PEC score on day 12 for LMB2 mice was significantly higher than that for controls (0.40±0.04 vs. 0.03±0.01 per glomerulus; P<0.001). *P<0.001 for day 0 versus day 8 or 12; †P<0.001 for controls versus LMB2 mice. Double labeling for (h) β-gal (blue) and cleaved caspase 3 and (i) Ki-67 (brown) did not reveal colocalization. Mirror images of claudin-1 with (j, j′) cleaved caspase 3 and (k, k′) Ki-67 showed that these markers were colocalized (arrowheads) (bars = 15 μm). For electron microscopy, ultrathin sections were stained with uranyl acetate and lead citrate. (l) A low-magnification view of an early glomerular lesion in an LMB2 mice showed initial PEC migration toward the glomerular tuft (original magnification ×200; bar = 28 μm). (l′) Higher magnification view of the inset in l showing adhesion of a podocyte-lost glomerular capillary with a migrated PEC, and remnant podocytes on the glomerular tuft revealed severe foot process effacement, pseudocyst, and villous transformation representing severe podocyte damage (original magnification ×1000; bar = 12 μm).
Aberrant Notch signaling is activated in hyperplastic PEC lesions in murine cFSGS
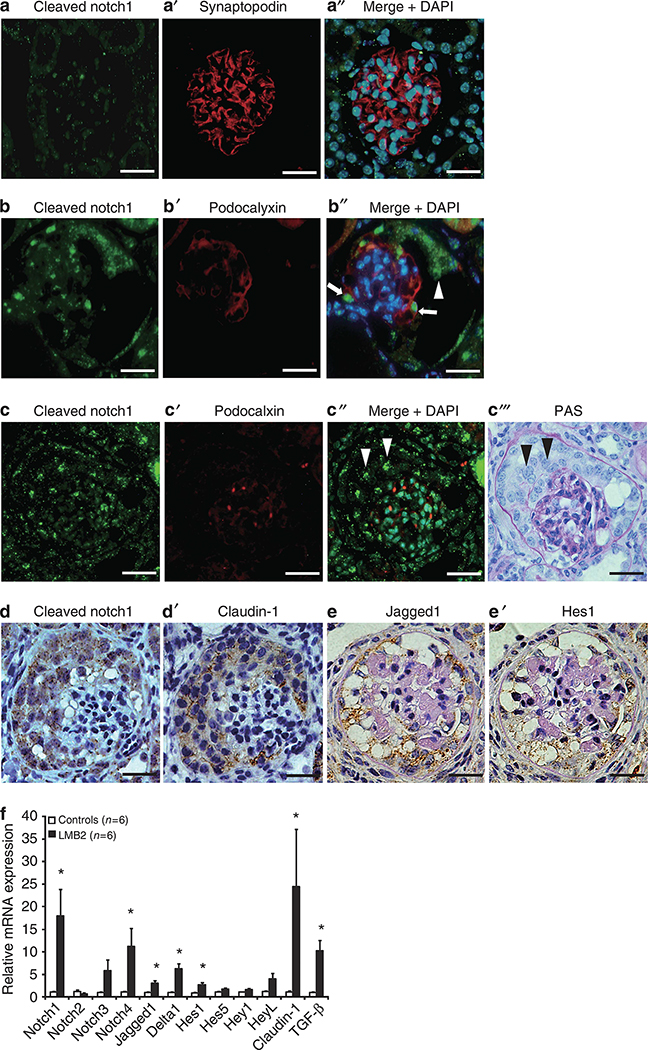
Immunofluorescence staining for cleaved Notch1, which reflects Notch signaling activation, was barely detectable in control glomeruli (Figure 2aa′′). Conversely, cleaved Notch1 staining intensity was markedly increased in podocytes and PECs during the early disease phase (Figure 2bb′′) and was rarely colocalized with podocalyxin (a podocyte marker) during the advanced phase in LMB2 mice (Figure 2cc′′′). Immunohistochemical staining of serial sections revealed that cleaved Notch1 exclusively colocalized with claudin-1 (Figure 2dd′). Similarly, Jagged1 (a Notch ligand) and Hes1 (a Notch target gene) were specifically increased in PEC lesions in LMB2 mice (Figure 2ee′). These results demonstrated that active Notch signaling proteins were present in hyperplastic lesions comprising PECs in diseased LMB2 mice.
Figure 2 |. Aberrant Notch expression is observed in hyperplastic parietal epithelial cells (PECs) in LMB2 mice.
(a) Only minimal cleaved Notch1 immunostaining (green) and abundant expression of synaptopodin (a’, red) were observed in the glomeruli of controls, even in a merged image (a′′). (b) In contrast, cleaved Notch1 expression was apparent both in podocytes and migrating PECs in an early glomerular lesion of LMB2 mice, and coexpression with decreasing podocyte marker podocalyxin (b′, red) was observed in a merged image (b′′, white arrows: podocytes, white arrowhead: PECs). (c) Cleaved Notch1 expression was more obvious in an advanced glomerular lesion and the coexpression of cleaved Notch1 and the podocyte marker podocalyxin (nearly disappeared) (c′, red) was rarely observed in a merged image. Cleaved Notch1 was observed predominantly in extracapillary hyperplastic cells, presumably PECs (c′′, white arrowheads), and in the same glomerulus with periodic acid–Schiff (PAS) staining (c′′′, black arrowheads). On serial sections, cleaved Notch1 (d, brown dots) expression was colocalized with claudin-1 (d′, brown) expression, similar to Jagged1 (e, brown) and Hes1 (e′, brown) expression. (f) The relative mRNA expression of Notch pathway genes from isolated glomeruli on day 12 was scored for each group (n = 6). Notch1, Notch4, Jagged1, Delta1, and Hes1 mRNAs were significantly increased in LMB2 mice (17.99±5.85, 11.21±4.00, 3.07±0.56, 6.25±1.11, and 2.73-fold respective increases vs. controls; P = 0.016, 0.029, 0.005, 0.001, and 0.003, respectively). Both Notch3 and HeyL mRNAs appeared to be increased, but the differences were not statistically significant. Claudin-1 mRNA levels were markedly increased in LMB2 mice (24.43±12.68-fold increases vs. controls). All results are expressed in arbitrary units and standard errors (*P<0.05, controls vs. LMB2 mice). DAPI, 4′,6-diamino-2-phenylindole. Bars = 15 μm.
To determine any changes in mRNA levels of components of the Notch signaling pathway, quantitative reverse transcriptase–PCR (qRT–PCR) was performed for isolated glomeruli. Statistically significant increases in mRNA expression of Notch-related genes (Notch1, Notch4, Jagged1, Delta1, and Hes1) were observed in LMB2 mice on day 12 compared with controls (Figure 2f). Among Notch receptors, Notch1 was most significantly increased. Among the Notch ligands, Jagged1 and Delta1 exhibited significant increases. Hes1 was the only significantly enhanced downstream gene. Consistent with its protein expression in immunohistochemistry, claudin-1 mRNA levels were markedly increased in LMB2 mice. These results demonstrated that the mRNA and protein levels were increased for several Notch receptors and ligands in hyperplastic lesions comprising PECs in this mouse model.
Notch signaling is also activated in PECs of human cFSGS
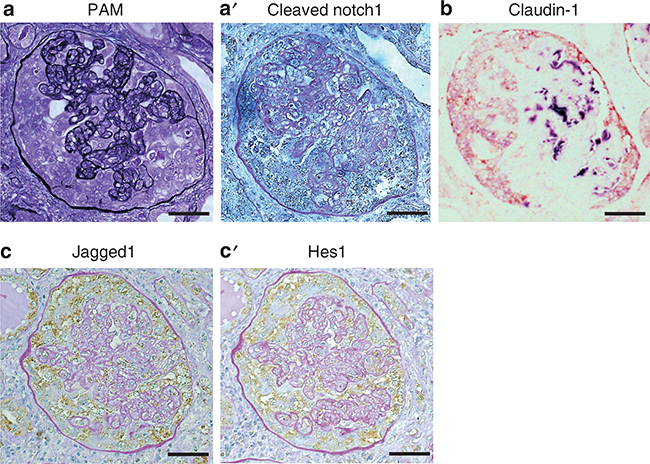
Human cFSGS biopsy samples with typical glomerular features were also examined (Figure 3a). Immunostaining revealed that cleaved Notch1 was increased in extracapillary cells and colocalized with claudin-1 but not with the podocyte marker Nestin (Figure 3aa′ and b). Jagged1 and Hes1 also colocalized in this lesion (Figure 3cc′). These results showed that Notch signaling expression in PECs were similar in our mouse model and human cFSGS.
Figure 3 |. Aberrant Notch expression is observed in hyperplastic parietal epithelial cells (PECs) in human collapsing focal segmental glomerulosclerosis.
(a) Periodic acid-silver methenamine (PAM) staining showed severely collapsed glomerular capillaries with extracapillary hyperplasia. On serial sections, cleaved Notch1 staining (a’, brown dots) was observed in hyperplastic epithelial cells but was almost completely absent in glomerular tufts. (b) Extracapillary hyperplastic epithelial cells expressed claudin-1 (red) but were negative for Nestin (violet). On serial sections, Jagged1 (c) and Hes1 (c’) colocalized in hyperplastic PECs (brown), as shown with periodic acid–Schiff (PAS) counterstaining. Bars = 40 μm.
PECs undergo Notch-dependent mesenchymal phenotypic changes mediated by transforming growth factor-β1 (TGF-β1) in vitro
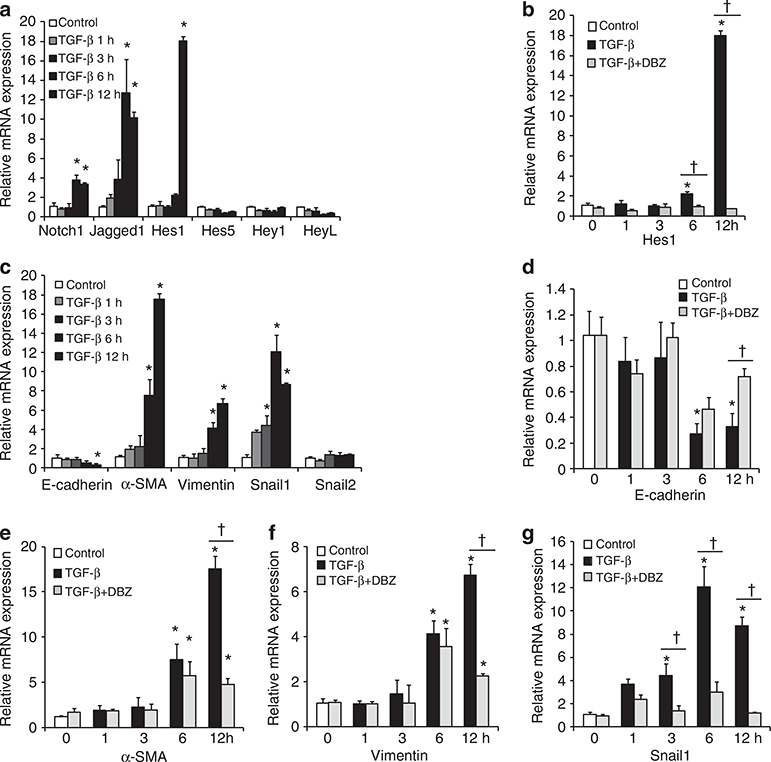
To investigate a functional role for Notch signaling in PECs at the cellular level, we used a recently established immortalized PEC cell line.26 We also examined a possible role for TGF-β1 because it could induce Notch signaling in numerous cell types,27–29 and is increased in FSGS.30 Treating cultured PECs with TGF-β1 resulted in increased Jagged1, Notch1, and Hes1 mRNA levels after 6–12 h, as per qRT–PCR (Figure 4a). Hes1 mRNA exhibited a significantly higher expression (17.99±5.85-fold increase), whereas other downstream genes were not significantly affected. This specificity was confirmed using pharmacological inhibition of Notch signaling by preincubation with dibenzazepine (DBZ), a γ-secretase inhibitor that significantly reduced Hes1 mRNA expression (Figure 4b).
Figure 4 |. Transforming growth factor (TGF)-β1 induces Notch signaling mRNAs in cultured parietal epithelial cells (PECs) and Notch inhibition effectively suppresses mesenchymal phenotypic change–related gene expressions.
(a) Relative Notch signaling mRNA expression at 1, 3, 6, and 12 h with or without (control) TGF-β1 treatment. TGF-β1 treatment resulted in significantly increased Notch1, Jagged1, and Hes1 mRNA expression (P<0.001, = 0.004, and <0.001, respectively). (b) Relative Hes1 mRNA levels in control, TGF-β1-treated, and TGF-β1 + dibenzazepine (DBZ)-treated cells showed that TGF-β1-induced Hes1 mRNA expression was significantly suppressed by DBZ at 6 and 12 h. (c) Relative mRNA expression of E-cadherin, smooth muscle actin (α-SMA), vimentin, and Snail1 were significantly changed 3, 6, and 12 h after TGF-β1 treatment. (d–g) Time course changes in phenotypic marker mRNAs in control, TGF-β1-treated, and TGF-β1 + DBZ-treated cells. TGF-β1-induced mRNA expression of mesenchymal markers were significantly suppressed by DBZ at 6 and 12 h. All results are expressed in arbitrary units and standard errors (n = 3 for each group; *P<0.05 vs. control; †P<0.05 for TGF-β1-treated group vs. TGF-β1 + DBZ-treated group).
In association with Notch induction by TGF-β1 in cultured PECs, the mRNA levels of the epithelial cell marker E-cadherin decreased, whereas those of the mesenchymal markers α-smooth muscle actin, vimentin, and Snail1 increased (Figure 4c). Preincubation with DBZ clearly preserved E-cadherin and restricted the expression of mesenchymal markers (Figure 4d–g), indicating that Notch-dependent phenotypic changes were mediated by TGF-β1 in PECs.
Inhibition of Notch signaling suppresses PEC migration in vitro
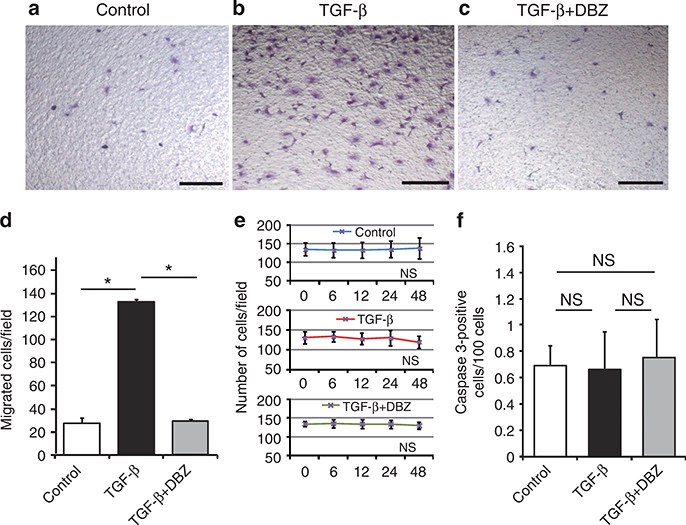
Notch signaling is involved in cell migration, proliferation, phenotypic changes, and apoptosis.20–23 Cultured PEC migration was assessed using a Boyden chamber motility assay for three cell groups: control, TGF-β1-treated, and TGF-β1 + DBZ-treated cells. The number of migrating PECs was significantly increased after TGF-β1 treatment compared with control treatment (Figure 5a, b, and d). Notably, DBZ pretreatment significantly reduced cell migration (Figure 5c and d). Conversely, a cell proliferation assay showed no significant differences among these three groups within 48 h (Figure 5e). The proportion of apoptotic cells, as determined by cleaved caspase 3 expression, was also not significantly different among these three groups (Figure 5f). These results indicated that TGF-β1-induced PEC migration was dependent on the Notch signaling pathway and that TGF-β1-mediated Notch signaling had no effect on cell proliferation or apoptosis.
Figure 5 |. Inhibition of Notch signaling suppresses transforming growth factor (TGF)-β1-induced cell migration, but does not affect cultured parietal epithelial cell (PEC) proliferation or apoptosis.
(a–c) Representative images from a Boyden chamber motility assay (original magnification ×40): (a) control (no treatment), (b) TGF-β1, (c) TGF-β1 + dibenzazepine (DBZ). (d) Quantitative analysis showed that the accelerated cell migration due to TGF-β was effectively inhibited by DBZ (27.44±5.71, 132.44±2.44, and 29.22±2.03 cells per field, respectively; *P<0.001). DBZ treatment did not significantly affect (e) cell proliferation or (f) apoptosis of the cultured PEC line. Results are averages with standard errors (n = 3 for each treatment group). NS, not significant. Bars = 200 μm.
Inhibition of Notch signaling worsens the disease in vivo
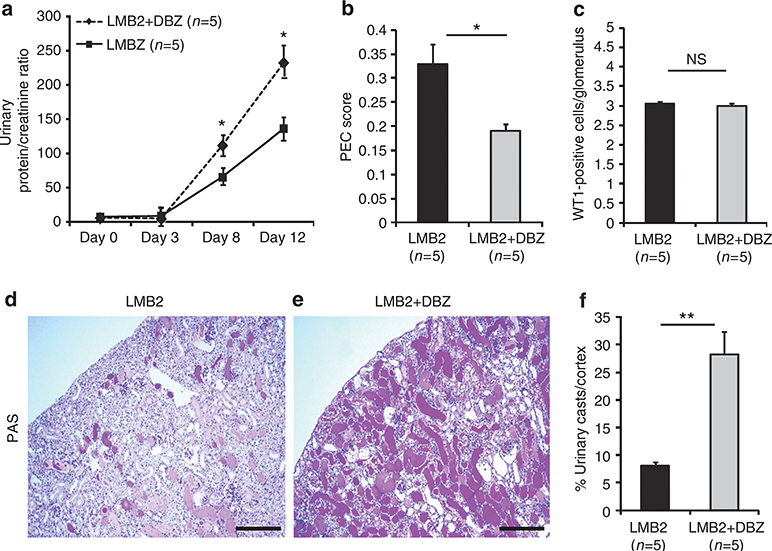
To determine the consequences of hyperplastic PECs on disease outcomes and a role for Notch in our cFSGS model, we administered DBZ (5 μg/g body weight (BW)) to LMB2 mice. DBZ-treated LMB2 mice had significantly increased proteinuria (Figure 6a) that was associated with reduced PEC score compared with untreated LMB2 mice (Figure 6b). The numbers of WT1-positive cells were not significantly different (Figure 6c). However, urinary casts reflecting histological damage were far more frequent in DBZ-treated LMB2 mice than in untreated mice (Figure 6d and e). The proportions of urinary casts in the cortex, which reflected tubulointerstitial damage, were significantly higher in DBZ-treated LMB2 mice than in untreated mice (Figure 6f). Electron micrographs showed PEC migration and that these cells covered a glomerular tuft with or without underlying podocytes in LMB2 mice (Figure 7a and b). Conversely, DBZ-treated LMB2 mice had bare PEC lesions and severe podocyte damage, including villous transformation, diffuse foot process effacement, and vacuolation (Figure 7c and d).
Figure 6 |. Notch inhibition suppresses parietal epithelial cell (PEC) lesions but worsens proteinuria and histopathology.
(a) Time course for the urinary protein/urinary creatinine ratio. Proteinuria was not different in both groups until day 3, but became significantly higher on days 8 and 12 in dibenzazepine (DBZ)-treated LMB2 mice. (b) PEC scores were significantly reduced in DBZ-treated LMB2 mice; the frequency and severity of PEC lesion were lower in the DBZ-treated group (0.33±0.03 vs. 0.19±0.02; P = 0.028, LMB2 vs. LMB2+DBZ mice). (c) The numbers of WT1-positive cells per glomerulus were not significantly different between these groups (2.87±0.19 vs. 2.69±0.19 cells per glomerulus; P = 0.54). A low-magnification view of the renal cortex of a nontreated LMB2 mouse showed a few urinary casts by (d) periodic acid–Schiff (PAS) staining, whereas far more abundant urinary casts were observed in (e) DBZ-treated LMB2 mice. (f) Planimetry of urinary casts in the renal cortex revealed significantly more protein casts in the DBZ-treated LMB2 mice (8.01±0.81 vs. 28.24±4.17%; P<0.001, LMB2 vs. LMB2+DBZ mice). In each group, n = 5; *P<0.05 and **P<0.001 for LMB2 vs. LMB2+DBZ mice. NS, not significant. Bars = 150 μm.
Figure 7 |. Transmission electron micrographs of glomeruli in LMB2 and dibenzazepine (DBZ)-treated LBM2 mice.
(a) A low-magnification view of a glomerulus in an LMB2 mouse showed a collapsed glomerular tuft with segmental hyperplastic parietal epithelial cells (PECs; original magnification ×200; bar = 38 μm). PECs migrated onto the glomerular tuft from the presumed entry site (arrowhead), and clusters or single PECs had attached to the collapsed tuft surface. (b) Higher magnification view of the inset in a showing the collapsed tuft covered by migrated PECs (original magnification ×1000; bar = 12 μm). (c) A low-magnification view of a glomerulus in a DBZ-treated LMB2 mouse showed a segmentally collapsed glomerular tuft and no PEC lesion (original magnification ×200; bar = 38 μm). (d) Higher magnification view of the inset in c (original magnification ×600; bar = 12 μm). Podocytes on the collapsed glomerular tuft exhibited severe foot process effacement, a pseudocyst, and villous transformation.
DISCUSSION
cFSGS in our murine model was initiated by podocyte-specific injury. Genetically tagging podocytes with β-gal and WT1 immunostaining indicated notable podocyte depletion. Hyperplastic epithelium essentially expressed claudin-1 and was negative for β-gal, indicating that PECs are major component of cFSGS. It is noteworthy that glomerular and tubulointerstitial histology was similar to that observed in human cFSGS. Because epithelial hyperplasia is the major characteristic of presclerotic lesions in cFSGS, understanding the molecular mechanism that induces PEC hyperplasia could provide important insights into cFSGS pathogenesis. Our in vivo and in vitro findings strongly suggest that the Notch signaling pathway plays a novel role in cFSGS pathogenesis.
First, hyperplastic PECs in LMB2-treated NEP25 mice distinctly expressed cleaved Notch1 (an activated Notch signaling molecule), Jagged1, and Hes1, whereas cleaved Notch1 expression was minimal in mice without LMB2 treatment. This aberrant Notch signaling expression was confirmed by increased mRNA levels in isolated glomeruli. Claudin-1-positive hyperplastic PECs in human cFSGS also expressed these molecules. These results suggest that Notch signaling molecules are aberrantly expressed in activated PECs in cFSGS in mice and humans. Among the pleiotropic functions regulated by Notch signaling,20–23 proliferation, migration, and trans-differentiation were likely responsible for the PEC lesions in our model. Although PEC lesion formation depends on cell proliferation and migration, our previous work with a cFSGS model of anti-glomerular basement membrane serum–treated p21-deficient mice found only a single PEC attached to naked glomerular basement membrane without hyperplasia.9 This suggests that proliferation and migration of PECs do not always occur together or that they may be mediated by different mechanisms.
To investigate the cellular mechanisms of Notch signaling in PEC lesions, we used the recently established immortalized PEC line with TGF-β1 as a Notch inducer.26 The Boyden chamber migration assay demonstrated TGF-β1-mediated PEC migration, which was significantly reduced by Notch inhibition. Conversely, TGF-β1-induced Notch activation did not lead to cell proliferation. TGF-β1 also induces cell cycle inhibitors, such as p21, in vitro.31 Thus, lack of cell proliferation with TGF-β1-induced Notch activation does not exclude the possibility that Notch signals have no effect on PEC proliferation in vivo. We propose that no single factor mediates PEC lesion formation in cFSGS. Further studies are required to establish this hypothesis.
Cell migration is occasionally associated with trans-differentiation into different cell types (for example, the epithelial–mesenchymal transition) and is considered a cell motility phenotype.32–34 The immortalized PEC line exhibited significant increases in mesenchymal phenotype mRNAs, including those for α-smooth muscle actin, vimentin, and Snail1, during TGF-β1-induced Notch activation, which were significantly reduced by DBZ, a Notch inhibitor. These results suggest that PEC lesions in cFSGS are promoted by aberrant Notch-dependent cell migration and mesenchymal phenotypic changes.
Notch signaling has various functions in many cell types during kidney development and in diseases.19,22,23,35 Niranjan et al.23 showed that Notch1 expression in podocytes was associated with their apoptosis via the TGF-β–p53 cascade in rat puromycin aminonucleoside nephrosis and that inhibiting Notch signaling prevented podocyte apoptosis and ameliorated proteinuria. We also found occasional Notch1 expression in remnant podocytes during the early disease stage, although the majority of Notch1-expressing cells were PECs in our cFSGS model. Furthermore, we also found minimal apoptosis in PECs, but never found in podocytes with genetic tagging. Our previous observation revealed cell necrosis, but not apoptosis as a cause of podocyte depletion in this model. In addition, puromycin aminonucleoside nephrosis which Niranjan et al. used is a model of minimal change disease that is not characterized by podocyte depletion. Thus, we propose that different Notch functions are involved in the pathogenesis of these different diseases.
PEC hyperplasia is a hallmark of progressive glomerular disease and is typically found in epithelial hyperplasia in FSGS and crescent formation in glomerulonephritis.10,12,13,36 However, it is unknown whether limiting PEC hyperplasia in glomerular disease has any beneficial effects on slowing disease progression. Thus, we were particularly interested in the possible therapeutic benefits of Notch inhibition during cFSGS development. Notch inhibition with DBZ significantly reduced PEC lesions in LMB2 mice; however, proteinuria and renal histopathology in these mice were significantly worsened. WT1-positive cells were decreased to similar levels regardless of DBZ treatment; therefore, the significant decrease in PEC lesions in DBZ-treated LMB2 mice appears to have been caused by the direct effect of DBZ on PECs rather than DBZ-mediated podocyte protection. The worsening proteinuria and renal histopathology by Notch inhibition highlights the potential protective consequences of PEC hyperplasia within the setting of progressive podocyte loss.
Notch signaling and phenotypic changes (for example, the epithelial–mesenchymal transition) play important roles in tissue repair.32,34,37 Notch signaling also affected wound healing and Notch inhibition delayed wound closure by suppressing cell migration in a skin scratch-scarring model.37 In our in vitro study using cultured PECs, Notch inhibition blocked migration and mesenchymal phenotypic changes in these cells. This suggests that Notch1-mediated mesenchymal phenotypic changes and cell migration compensate for the injured filtration barrier in vivo and supports an earlier hypothesis that PEC lesions in FSGS may participate in glomerular scarring.38,39
Wound healing is a basic biological phenomenon commonly observed in many species, and glomerular pathology reflects glomerular responses against various insults. Because we found identical expression of Notch molecules in PECs in human cFSGS, this process may also explain cFSGS pathology in humans. According to the results of our in vivo and in vitro experiments, we suggest that Notch signaling mediates PEC migration and phenotypic changes to compensate for podocyte-lost filtration barrier. We used TGF-β1 as a Notch inducer in our in vitro studies. Basically, TGF-β1 has many different functions other than Notch pathway activation. Indeed, we observed immunohistological localization of TGF-β1 in PEC lesions in vivo (data not shown) and it is known that glomerular cells synthesize TGF-β1 in FSGS.30 However, the actual inducer of Notch signaling in PECs in response to podocyte loss in vivo requires further investigation.
In conclusion, progressive podocyte depletion leads to PEC hyperplasia in a mouse cFSGS model via a Notch-mediated pathway. Aberrantly expressed Notch signaling in PECs may repair a podocyte-lost filtration barrier as in wound healing, which may account for the characteristic pathology of cFSGS.
MATERIALS AND METHODS
Animal experiments
NEP25 mice genetically express human CD25 specifically on podocytes.17 Podocyte-specific injury is induced after administering a human CD25-specific antibody, LMB2. We mated Nphs1-Cre/ROSA26-loxP mice with NEP25 mice to obtain Nphs1-Cre/ROSA26-loxP/NEP25 triple transgenic mice for lineage-specific podocyte tagging.14 Mice were treated humanely and housed in animal facilities with free access to food and water according to the protocols approved by the institutional animal use and care committee of the University of Tsukuba (registered number: 11–305).
Nphs1-Cre/ROSA26-loxP/NEP25 mice (10–24 weeks old) were used for experiments. On day 0, mice underwent general anesthesia with isoflurane and were then injected LMB2 (n = 6; LMB2 mice) through the tail vein (4 ng/g BW diluted in 0.1 ml phosphate-buffered saline containing 0.1% bovine serum albumin). Mice injected vehicle alone were used as controls (n = 6). Urine samples (24-h sampling) were collected in metabolic cages on days 0, 1, 3, 5, 8, and 12 after injection. Total urinary protein and creatinine concentrations were determined using an automated analyzer (SRL, Tokyo, Japan). Mice were humanely killed on day 12. Kidney cortices and isolated glomeruli were harvested and used for further experiments. DBZ is an inhibitor that specifically blocks Notch signaling by inhibiting γ-secretase, which assumes proteolytic cleavage of Notch receptors into extra- and intracellular domains.40 DBZ was purchased from Merck (Darmstadt, Germany) and Syncom (Groningen, The Netherlands). DBZ was suspended in water containing 0.5% hydroxypropyl methylcellulose (Methocel E4M; Dow Chemicals, Midland, MI) and 0.1% Tween 80. This was injected intraperitoneally (5 μg/g BW) once per day on days 6–12 after LMB2 administration. We selected these DBZ administration time points because severe proteinuria and hyperplastic PEC lesions were observed between 8 and 12 days after LMB2 injection according to the time course assessment of histology in the same mice combined with sequential renal biopsy on days 0, 8, and 12 (Supplementary Figure S1a online).
Staining and morphological analysis
β-gal activity was detected by enzymatic X-gal staining as described previously.14 Paraffin sections (2-μm thick) were processed for periodic acid–Schiff staining, periodic acid-silver methenamine staining, and immunostaining with specific primary antibodies listed in Supplementary Table S1 online. Anti-WT1, anti-podocalyxin, and anti-nestin antibodies were used to identify podocytes. Anti-claudin-1 antibody was used to indentify PECs. Anti-cleaved Notch1, anti-Jagged1, and anti-Hes1 antibodies were visualized using a CSA II Biotin-Free Catalyzed Amplification System (Dako, Glostrup, Denmark). For immunofluorescence analysis, primary antibodies were labeled with fluorescein isothiocyanate or rhodamine-conjugated secondary antibodies (MP Biomedicals, Morgan Irvine, CA). For immunohistochemistry, primary antibodies were incubated with EnVision labeled polymer-HRP (Dako), Histofine kit (Nichirei Bioscience, Tokyo, Japan), or biotinylated rabbit anti-rat IgG (1:100 dilution; Chemicon, Temecula, CA) followed by reaction with peroxidase-conjugated streptavidin (Nichirei), depending on the host species used to generate the antibody. Peroxidase activity was visualized using liquid diaminobenzidine substrate (Dako). Nuclear staining used hematoxylin or 4′,6-diamino-2-phenylindole.
To estimate the frequency and extent of PEC lesions (defined as hyperplastic PECs bridging toward a glomerular tuft), we devised an index designated the ‘PEC score,’ which was a semiquantitative score modified from a previously reported glomerular sclerosis index.41 This score was graded from 0 to 4 as follows: 0, no lesion; 1, extracapillary PEC lesions of <25%; 2, 25–50%; 3, 50–75%; and 4, >75% of the urinary space in each glomerulus. The number of podocytes in each glomerulus was determined by counting WT1-positive cells. The average for the whole kidney was calculated by averaging the values for >70 glomeruli in each mouse. The extent of urinary casts in kidney cortices was analyzed using Lumina Vision (Mitani, Fukui, Japan). For electron microscopy, kidney samples were fixed in glutaraldehyde and processed using standard procedures.
Glomerulus isolation
Our glomerulus isolation technique was a modification of a method reported previously.42 Briefly, anesthetized mice were perfused through the heart with Hanks’ balanced salt solution (Life Technologies, Carlsbad, CA) containing 1 mg/ml of ion powder. Kidneys were minced into 1-mm3 pieces and digested in Hanks’ balanced salt solution containing 1 mg/ml collagenase A (Roche, Basel, Switzerland) and 100 U/ml DNase I (Life Technologies) for 30 min at 37 °C. Digested tissue was pressed through a 100-μm cell strainer. Finally, ion powder–containing glomeruli were isolated using a magnetic particle concentrator. All procedures were performed at 4 °C on ice, except during collagenase digestion.
RNA extraction and qRT–PCR
Total RNAs from isolated glomeruli and cultured PECs were extracted using the RNeasy Mini Kit (Qiagen, Hilden, Germany). The amount of RNA was determined using NanoDrop 1000 (Thermo Scientific, Rockford, IL). Total RNA (1 μg) was reverse transcribed using the Thermoscript RT–PCR System (Life Technologies) for first-strand cDNA. Then, 10 ng of cDNA template and 0.25 mmol/l of sequence-specific primers (listed in Supplementary Table S2 online) were used for qRT–PCR with a KAPA SYBR Fast qPCR Kit (Nippon Genetics, Tokyo, Japan) and an ABI 7300 real time PCR system (Life Technologies). All measured values were normalized with glyceraldehyde-3-phosphate dehydrogenase and calculated using the ΔΔCT method.
Cultured mouse PEC line
A previously established conditionally immortalized mouse PEC line was used for cell culture studies.26 Differentiated PECs were maintained under low serum conditions (0.5% fetal bovine serum) for at least 24 h before stimulation with recombinant human TGF-β1 (2.5 ng/ml; R&D Systems, Minneapolis, MN) in the absence or presence of DBZ (1 μmol/l), which was added 3 h before TGF-β1.
Cell migration, proliferation, and apoptosis assays
Cell migration assays used Boyden chambers (Transwell, Corning, Lowell, MA). The lower surfaces of membrane filters were precoated with mouse collagen IV (BD Biosciences, Bedford, MA). After incubation with TGF-β1 for 12 h with/without DBZ or no treatment (control), harvested cells (5000 cells) were seeded on the top of a chamber and allowed to migrate for 12 h at 37 °C. Then, the membrane was fixed in 4% paraformaldehyde and stained with crystal violet. The upper membrane surface was wiped using a cotton swab to remove nonmigrated cells. Cells on the lower membrane surface were counted in at least three fields of view and then averaged.
A proliferation assay was performed by counting cells in the same field of view 0, 6, 12, 24, and 48 h after incubation with TGF-β1 with/without DBZ or control. Cell counts were performed in at least three fields of view and then averaged. Apoptotic cells were identified by cleaved caspase 3 staining. The frequency of apoptotic cells was determined by counting the numbers of cleaved caspase 3–positive nuclei per 100 nuclei at 12 h after incubation with TGF-β1 and with/without DBZ or control. Apoptotic cell counts were made in at least three fields of view and then averaged.
Each experiment was performed in triplicate and repeated at least twice.
Statistical analysis
Results are presented as means±standard errors. Comparisons between two groups were made by two-tailed Student’s t-tests. Comparisons between three or more groups were made by nonrepeated measures analysis of variance. The results were analyzed to generate hazard ratios with 95% confidence intervals and P-values. P<0.05 was considered statistically significant.
Supplementary Material
Figure S1.
Table S1. Primary antibodies for immunostaining.
Table S2. Sequence-specific primers for quantitative RT-PCR.
ACKNOWLEDGMENTS
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, and was also supported by Grants-in-Aid for Scientific Research of Japan Society for the Promotion of Science (KAKEN; research project no. 22590877) and Progressive Renal Disease Research of the Ministry of Health, Labour and Welfare of Japan.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
REFERENCES
- 1.D’Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med 2011; 365: 2398–2411. [DOI] [PubMed] [Google Scholar]
- 2.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 2005; 67: 404–419. [DOI] [PubMed] [Google Scholar]
- 3.Wharram BL, Goyal M, Wiggins JE et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 2005; 16: 2941–2952. [DOI] [PubMed] [Google Scholar]
- 4.Nagata M, Kriz W. Glomerular damage after uninephrectomy in young rats. II. Mechanical stress on podocytes as a pathway to sclerosis. Kidney Int 1992; 42: 148–160. [DOI] [PubMed] [Google Scholar]
- 5.D’Agati VD, Fogo AB, Bruijn JA et al. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis 2004; 43: 368–382. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz MM, Lewis EJ. Focal segmental glomerular sclerosis: the cellular lesion. Kidney Int 1985; 28: 968–974. [DOI] [PubMed] [Google Scholar]
- 7.Stokes MB, Valeri AM, Markowitz GS et al. Cellular focal segmental glomerulosclerosis: clinical and pathologic features. Kidney Int 2006; 70: 1783–1792. [DOI] [PubMed] [Google Scholar]
- 8.Barisoni L, Kriz W, Mundel P et al. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 1999; 10: 51–61. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki T, Matsusaka T, Nakayama M et al. Genetic podocyte lineage reveals progressive podocytopenia with parietal cell hyperplasia in a murine model of cellular/collapsing focal segmental glomerulosclerosis. Am J Pathol 2009; 174: 1675–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagata M, Hattori M, Hamano Y et al. Origin and phenotypic features of hyperplastic epithelial cells in collapsing glomerulopathy. Am J Kidney Dis 1998; 32: 962–969. [DOI] [PubMed] [Google Scholar]
- 11.Smeets B, Te Loeke NA, Dijkman HB et al. The parietal epithelial cell: a key player in the pathogenesis of focal segmental glomerulosclerosis in Thy-1.1 transgenic mice. J Am Soc Nephrol 2004; 15: 928–939. [DOI] [PubMed] [Google Scholar]
- 12.Nagata M, Horita S, Shu Y et al. Phenotypic characteristics and cyclin-dependent kinase inhibitors repression in hyperplastic epithelial pathology in idiopathic focal segmental glomerulosclerosis. Lab Invest 2000; 80: 869–880. [DOI] [PubMed] [Google Scholar]
- 13.Dijkman H, Weening JJ, Smeets B et al. Proliferating cells in HIV and pamidronate-associated collapsing focal segmental glomerulosclerosis are parietal epithelial cells. Kidney Int 2006; 70: 338–344. [DOI] [PubMed] [Google Scholar]
- 14.Asano T, Niimura F, Pastan I et al. Permanent genetic tagging of podocytes: fate of injured podocytes in a mouse model of glomerular sclerosis. J Am Soc Nephrol 2005; 16: 2257–2262. [DOI] [PubMed] [Google Scholar]
- 15.Smeets B, Uhlig S, Fuss A et al. Tracing the origin of glomerular extracapillary lesions from parietal epithelial cells. J Am Soc Nephrol 2009; 20: 2604–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bariéty J, Bruneval P. Activated parietal epithelial cells or dedifferentiated podocytes in FSGS: can we make the difference? Kidney Int 2006; 69: 194. [DOI] [PubMed] [Google Scholar]
- 17.Matsusaka T, Xin J, Niwa S et al. Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol 2005; 16: 1013–1023. [DOI] [PubMed] [Google Scholar]
- 18.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 2006; 7: 678–689. [DOI] [PubMed] [Google Scholar]
- 19.Cheng HT, Kim M, Valerius MT et al. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development 2007; 134: 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonoshita M, Aoki M, Fuwa H et al. Suppression of colon cancer metastasis by Aes through inhibition of Notch signaling. Cancer Cell 2011; 19: 125–137. [DOI] [PubMed] [Google Scholar]
- 21.Bao B, Wang Z, Ali S et al. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett 2011; 307: 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Bielesz B, Sirin Y, Si H et al. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest 2010; 120: 4040–4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niranjan T, Bielesz B, Gruenwald A et al. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med 2008; 14: 290–298. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Al-Awqati Q. Segmental expression of Notch and Hairy genes in nephrogenesis. Am J Physiol Renal Physiol 2005; 288: F939–F952. [DOI] [PubMed] [Google Scholar]
- 25.Piscione TD, Wu MY, Quaggin SE. Expression of Hairy/Enhancer of Split genes, Hes1 and Hes5, during murine nephron morphogenesis. Gene Expr Patterns 2004; 4: 707–711. [DOI] [PubMed] [Google Scholar]
- 26.Ohse T, Pippin JW, Vaughan MR et al. Establishment of conditionally immortalized mouse glomerular parietal epithelial cells in culture. J Am Soc Nephrol 2008; 19: 1879–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuxe J, Vincent T, Garcia de Herreros A. Transcriptional crosstalk between TGF-β and stem cell pathways in tumor cell invasion: role of EMT promoting Smad complexes. Cell Cycle 2010; 9: 2363–2374. [DOI] [PubMed] [Google Scholar]
- 28.Zavadil J, Cermak L, Soto-Nieves N et al. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J 2004; 23: 1155–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 2010; 21: 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JH, Kim BK, Moon KC et al. Activation of the TGF-beta/Smad signaling pathway in focal segmental glomerulosclerosis. Kidney Int 2003; 64: 1715–1721. [DOI] [PubMed] [Google Scholar]
- 31.Wada T, Pippin JW, Terada Y et al. The cyclin-dependent kinase inhibitor p21 is required for TGF-beta1-induced podocyte apoptosis. Kidney Int 2005; 68: 1618–1629. [DOI] [PubMed] [Google Scholar]
- 32.Acloque H, Adams MS, Fishwick K et al. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 2009; 119: 1438–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 2009; 119: 1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 2004; 15: 1–12. [DOI] [PubMed] [Google Scholar]
- 35.Sirin Y, Susztak K. Notch in the kidney: development and disease. J Pathol 2012; 226: 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimizu M, Kondo S, Urushihara M et al. Role of integrin-linked kinase in epithelial-mesenchymal transition in crescent formation of experimental glomerulonephritis. Nephrol Dial Transplant 2006; 21: 2380–2390. [DOI] [PubMed] [Google Scholar]
- 37.Chigurupati S, Arumugam TV, Son TG et al. Involvement of notch signaling in wound healing. PLoS One 2007; 2: e1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smeets B, Kuppe C, Sicking EM et al. Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis. J Am Soc Nephrol 2011; 22: 1262–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagata M Pathogenesis of glomerulosclerosis: role of epithelial interactions. Clin Exp Nephrol 2000; 4: 173–181. [Google Scholar]
- 40.van Es JH, van Gijn ME, Riccio O et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 2005; 435: 959–963. [DOI] [PubMed] [Google Scholar]
- 41.Ma LJ, Marcantoni C, Linton MF et al. Peroxisome proliferator-activated receptor-gamma agonist troglitazone protects against nondiabetic glomerulosclerosis in rats. Kidney Int 2001; 59: 1899–1910. [DOI] [PubMed] [Google Scholar]
- 42.Takemoto M, Asker N, Gerhardt H et al. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 2002; 161: 799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Table S1. Primary antibodies for immunostaining.
Table S2. Sequence-specific primers for quantitative RT-PCR.