Abstract
Myocardial infarction (heart attack) is the number one killer of heart patients. Existing treatments for heart attack do not address the underlying problem of cardiomyocyte (CM) loss and cannot regenerate the myocardium. Introducing exogenous cardiac cells is required for heart regeneration due to the lack of resident progenitor cells and very limited proliferative potential of adult CMs. Poor retention of transplanted cells is the critical bottleneck of heart regeneration. Here, we report the invention of a poly(l-lactic acid)-b-poly(ethylene glycol)-b-poly(N-Isopropylacrylamide) copolymer and its self-assembly into nanofibrous gelling microspheres (NF-GMS). The NF-GMS undergo thermally responsive transition to form not only a 3D hydrogel after injection in vivo, but also exhibit architectural and structural characteristics mimicking the native extracellular matrix (ECM) of nanofibrous proteins and gelling proteoglycans or polysaccharides. By integrating the ECM-mimicking features, injectable form, and the capability of maintaining 3D geometry after injection, the transplantation of hESC-derived CMs carried by NF-GMS led to a striking 10-fold graft size increase over direct CM injection in an infarcted rat model, which is the highest reported engraftment to date. Furthermore, NF-GMS carried CM transplantation dramatically reduced infarct size, enhanced integration of transplanted CMs, stimulated vascularization in the infarct zone, and led to a substantial recovery of cardiac function. The NF-GMS may also serve as advanced injectable and integrative biomaterials for cell/biomolecule delivery in a variety of biomedical applications.
Keywords: Block copolymer, nanofiber, hydrogel, cell transplantation, heart regeneration
Graphical Abstract
A tri-block copolymer is synthesized that self-assembles into porous nanofibrous microspheres. These microspheres in an aqueous suspension form hydrogel upon temperature increase to body temperature. These nanofibrous gelling microspheres are used to deliver cardiomyocytes into an infarcted rat heart and result in the highest cardiomyocyte engraftment to date, dramatically reduce infarct size, and lead to a substantial cardiac functional recovery.

INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death in the world [1]. In particular, myocardial infarction (MI), commonly known as heart attack, results in permanent heart muscle damage or death, and is the number one killer of heart patients. About 695,000 Americans suffer a new heart attack and about 325,000 Americans suffer a recurrent heart attack every year. A typical human heart attack causes the loss of approximately 1 billion cardiomyocytes (CMs). Patients who survive the heart attack often develop heart failure and sudden cardiac arrest due to severely weakened pumping function of the heart. Unfortunately, existing treatments for heart attack are primarily pharmacological or device-based, do not address the underlying problem of CM loss, cannot regenerate the myocardium and rescue the injured ventricle [2]. Indeed, the prevalence of chronic cardiomyopathy is steadily increasing [3]. Although re-entry of cell cycle is found in cardiomyocytes (CMs) after myocardial injury in adult mammalian heart, it does not lead to effective regeneration [4]. The outcome is age-dependent [5]. Recent studies show a lack of resident stem or progenitor cells in adult hearts that can regenerate the myocardium [6]. The introduction of exogenous CMs or their progenitor cells derived from pluripotent stem cells is a highly desired strategy for heart regeneration because of their potential to regenerate the myocardium and restore the pumping function [7]. Commonly used methods for cell transplantation include intravenous, intracoronary, and intramyocardial injections. Among them, intramyocardial injection directly delivers cells into the infarcted region, is more efficient and is more widely used [8]. But even with intramyocardial injection, cell retention remains minimal (only about 2% of injected cells) and does not lead to efficient engraftment [9]. While various approaches including hydrogel delivery [10], suturing [11], engineered tissue patches and survival-improving cocktails [12] have been explored, the efficiency of cell transplantation, functional integration and subsequent maturation of transplanted CMs remain disappointing. One major challenge to heart regeneration is the harsh environment in the infarcted heart, which prevents either repopulation of endogenous CMs or/and retention and integration of transplanted CMs or their progenitor cells.
We hypothesize that an injectable cell carrier that mimics both the nanofibrous (NF) architecture of extracellular matrix (ECM) fibrillar proteins and the gelling property of extracellular proteoglycans or polysaccharides, will enable minimally invasive delivery of cells, enhance retention/integration, and provide regenerative microenvironment for cardiac regeneration and functional recovery. To address the critical challenge facing the field of heart regeneration, we successfully synthesized biodegradable poly(l-lactic acid)-b-poly(ethylene glycol)-b-poly(N-isopropyl acrylamide) (PLLA-PEG-PNIPAm) tri-block copolymers for the first time in this work and demonstrated that they could self-assemble into NF microspheres and in the presence of water could further form a hydrogel upon change from room temperature to body temperature. We termed this cell carrier nanofibrous gelling microspheres (NF-GMS) and examined the CM retention, engraftment, cardiac regeneration and functional recovery.
RESULTS
The design and synthesis of PLLA-PEG-PNIPAm tri-block copolymers
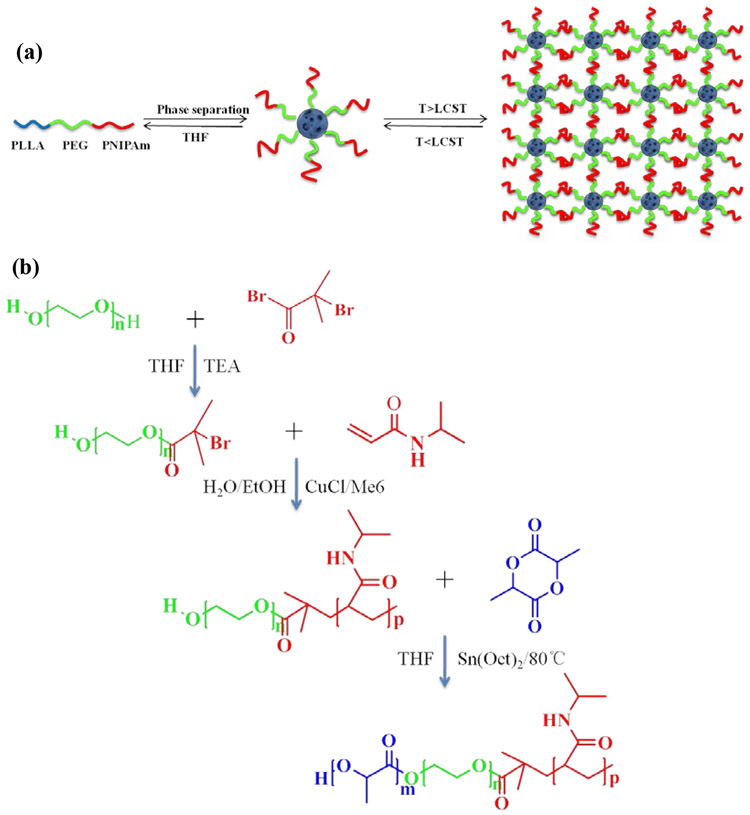
The wounds in an infarcted heart are irregular in shape. Injectable carriers can be advantageous for minimally invasive cell delivery [13]. We designed the PLLA-PEG-PNIPAm tri-block copolymer based on the hypothesis that the PLLA block could be tailored to form NF microspheres, the PEG block could provide hydrophilicity to bind water and the PNIPAm block could become physical crosslinks to integrate these microspheres into a 3D hydrogel once delivered in vivo at the body temperature (Fig. 1a).
Figure 1. Triblock PLLA-PEG-PNIPAm copolymers and nanofibrous gelling microspheres (NF-GMS):
(a) A schematic of PLLA-PEG-PNIPAm triblock copolymer, its self-assembly into NF-GMS, and thermally induced physical crosslinking into hydrogel. (b) A schematic of PLLA-PEG-PNIPAm triblock copolymer synthesis, which involves synthesis of double-headed PEG initiator, ATRP of NIPAm monomer and ROP of l-lactide.
The synthesis of amphiphilic ABC triblock copolymers is highly challenging due to the sharp contrast in polarity between these blocks [14]. Reversible Addition-Fragmentation chain Transfer (RAFT) polymerization [15] and Atom Transfer Radical Polymerization (ATRP) [16] have been used to synthesize di-block and tri-block copolymers. However, for RAFT, an challenge is that a particular RAFT agent is only suitable for a limited set of monomers, but often not suitable for the subsequent two monomers [15a] in the case of triblock copolymer synthesis. For ATRP, termination and other side reactions occur in each step and are more prominent in multi-step ATRP reactions, resulting in less controlled structure of the aimed tri-block copolymers [17]. With the above considerations, we took a new approach, i.e., ATRP and ROP (ring-opening polymerization) two-step polymerization to synthesize the aimed PLLA-PEG-PNIPAm triblock copolymers, utilizing an asymmetric bifunctional macromolecular initiator, Br-PEG-OH. ATRP of NIPAm and the ROP of l-lactide are combined because the two types of polymerization are fully orthogonal (do not interfere with one another) [16, 18]. The presence of both the bromine end group and the hydroxyl end group of Br-PEG-OH ensures fast initiation of both ATRP and ROP. The synthesis of PLLA-PEG-PNIPAm copolymer was carried out in three steps (Fig. 1b). First, Br-PEG-OH was synthesized using the reaction of bromoisobutyryl bromide (BIBB) with equimolar amount of anhydrous HO-PEG-OH in the presence of triethylamine (Et3N) according to the literature [19]. Second, hydroxyl-terminated diblock copolymer HO-PEG-PNIPAm was synthesized by ATRP of NIPAm monomer initiated by the bromine end group of Br-PEG-OH. Third, PLLA-PEG-PNIPAm triblock copolymer was synthesized by ROP of l-lactide initiated using the hydroxyl end group of HO-PEG-PNIPAm. With the above new approach, we successfully developed a reliable synthesis route for PLLA-PEG-PNIPAm triblock copolymers (Supplementary Section 1: Figs. S1&S2, Table S1, Table S2 and Table S3).
Thermo-responsive properties
By carefully examining the thermal responsive hydrodynamic diameter (Dh) changes of the diblock PEG-PNIPAm and triblock PLLA-PEG-PNIPAm copolymers, we confirmed that the PNIPAm block in the triblock PLLA-PEG-PNIPAm copolymer underwent a rapid hydrophilic-hydrophobic transition to serve the aimed physical crosslinking function for hydrogel formation upon change from room temperature to body temperature (Supplementary Section 2: Fig. S3).
PLLA-PEG-PNIPAm NF-GMS fabrication
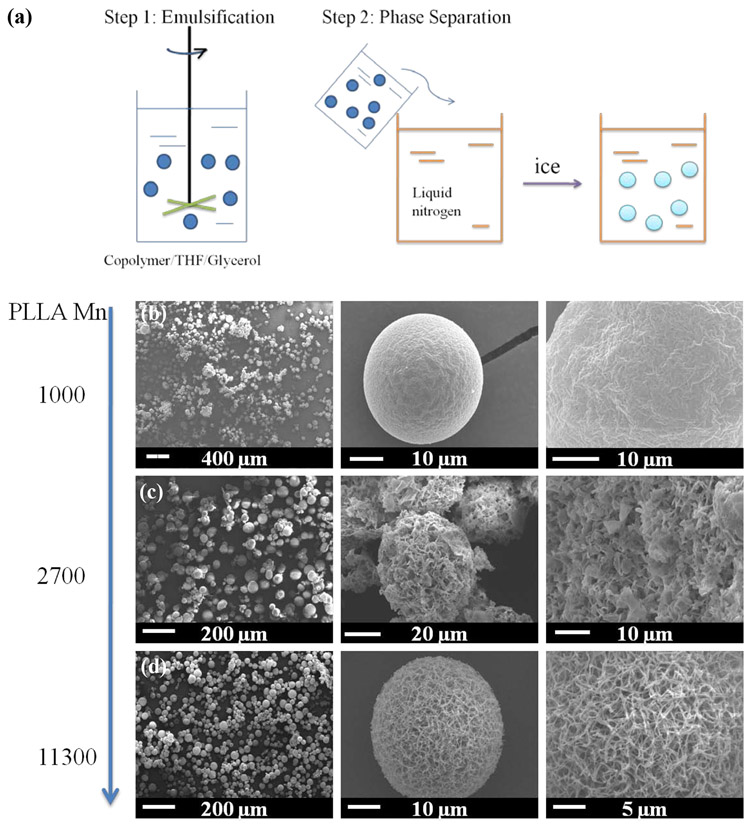
PLLA-PEG-PNIPAm NF-GMS were fabricated through two-step self-assembling procedures (Fig. 2a). First, the polymer was dissolved in tetrahydrofuran (THF) and emulsified into micro-scale liquid spheres in glycerol under rigorous stirring. The mixture was then quenched in liquid nitrogen to induce nano-scale phase separation for nanofiber formation. Next, the NF microspheres were obtained after solvent extraction with distilled water and freeze-drying. To achieve the NF feature, PLLA-PEG-PNIPAm triblock copolymers with varying compositions were synthesized for microsphere fabrication. When a PLLA-PEG-PNIPAma copolymer was synthesized with a PEG block of a number average molecular weight (Mn) of about 1550 (the unit for all molecular weights is g/mol), a PNIPAm block of about 3800, and a PLLA block of about 1000, the fabricated microspheres had a smooth surface instead of an NF structure (Fig. 2b). The failure to achieve the NF feature was attributed to short chain length of the PLLA. By controlling the PEG-PNIPAm/l-lactide ratio in the ROP of l-lactide, PLLA-PEG-PNIPAmb with the identical PEG (Mn = ~1550) and PNIPAm (Mn = ~3800) lengths but a longer PLLA length (Mn = ~2700), microspheres with a platelet-like morphology were formed (Fig. 2c). When the Mn of PLLA block was further increased to ~11300 (PLLA-PEG-PNIPAmc), microspheres with a typical NF structure were achieved (Fig. 2d). The average diameter of the nanofibers in the microspheres was ~150 nm, similar to those of ECM nanofibers. When the microsphere diameter reached ~30μm, one or multiple holes formed in the nanofibrous microspheres (Supplementary Fig. S4).
Figure 2. Molecular weight effect on nanofiber formation of PLLA-PEG-PNIPAm microspheres.
(a) A schematic of PLLA-PEG-PNIPAm microsphere fabrication; SEM micrographs of representative microspheres (30-60 μm) fabricated from (b) PLLA-PEG-PNIPAma (Table1, Mn = ~6376), (c) PLLA-PEG-PNIPAmb (Mn = ~8042), (d) PLLA-PEG-PNIPAmc (Mn = ~16665).
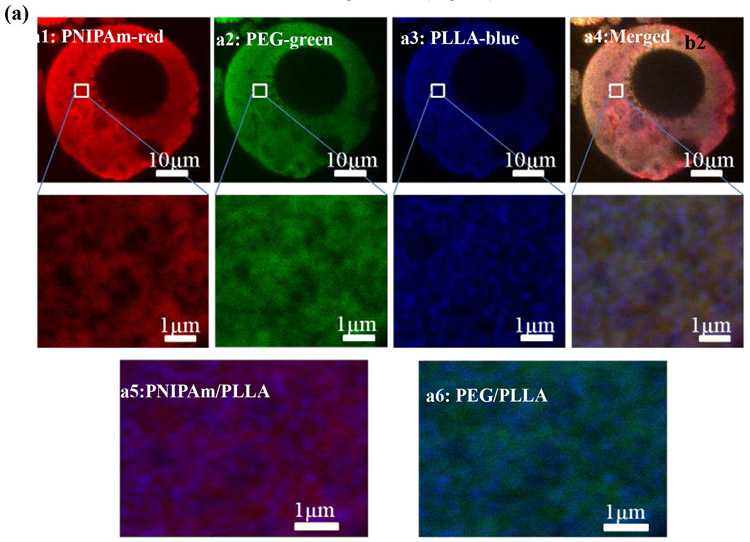
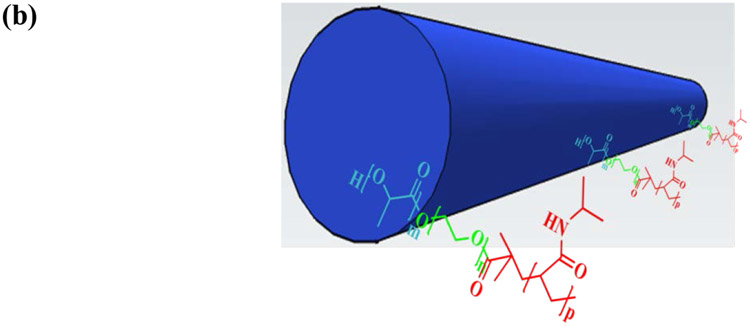
To visualize PLLA, PNIPAm, and PEG blocks in PLLA-PEG-PNIPAm NF-GMS, each of them was “chemically stained” with fluorescent monomer individually: Nile blue acrylamide (blue) was copolymerized into PLLA block, fluorescein o-acrylate (green) was copolymerized into PEG block, and acryloxyethyl thiocarbamoyl rhodamine B (red) was copolymerized into PNIPAm block (see detailed methods in Supplementary Information). As shown in Fig. 3a, all of the three colors distributed throughout the microspheres. However, when observed at a higher magnification using a high-resolution confocal fluorescence microscope (Leica SP8), PLLA blocks (blue) were observed to form a typical nanofibrous structure with more defined lines and dots (cross-sections of nanofibers, high mag images in Fig. 3a), while both PEG (green) and PNIPAm (red) blocks formed diffusive cloud-like structures surrounding the nanofibers. This observation was more obvious when the PLLA block fluorescent micrograph and PNIPAm or PEG block fluorescent micrographs were merged, where the PLLA fibers (blue) were surrounded by PNIPAm (red) or PEG (green) clouds, respectively (Fig. 3 a5&a6). These results suggested that self-assembled PLLA nanofibers with a fiber core-corona structure were formed in the NF-GMS, where PEG and PNIPAm blocks became the surrounding corona (Fig. 3b).
Figure 3. Confocal fluorescence micrographs of NF-GMS fabricated from PLLA-PEG-PNIPAm.
(a) 2D cross-sectional confocal fluorescence micrographs of NF-GMS fabricated from PLLA-PEG-PNIPAmc (60-90 μm based on SEM/confocal microscopy observation): (a1) The PNIPAm blocks were stained by Acryloxyethyl Thiocarbamoyl Rhodamine B (red), (a2) The PEG blocks were stained by Fluorescein o-acrylate (green), (a3) The PLLA blocks were stained by Nile Blue Acrylamide (blue), (a4) A merged fluorescence micrograph of three blocks. (a5) A merged fluorescence micrograph of PNIPAm and PLLA blocks, (a6) A merged fluorescence micrograph of PEG and PLLA blocks. (b) A schematic of a nanofiber in the PLLA-PEG-PNIPAm NF-GMS with a core-corona structure.
The degradation property of a microcarrier is an important feature for consideration in tissue regeneration [20]. The PLLA-PEG-PNIPAmc NF-GMS disintegrated entirely into small pieces with about 50% weight loss after 8 weeks and about 70% weight loss after 15 weeks of incubation in PBS, whereas PLLA NF microspheres (with equivalent molecular weight) remained intact with only about 10% weight loss after 15 weeks (Supplementary Fig. S5&S6), likely due to the substantial difference in hydrophilicity.
Gelation property of PLLA-PEG-PNIPAm NF-GMS
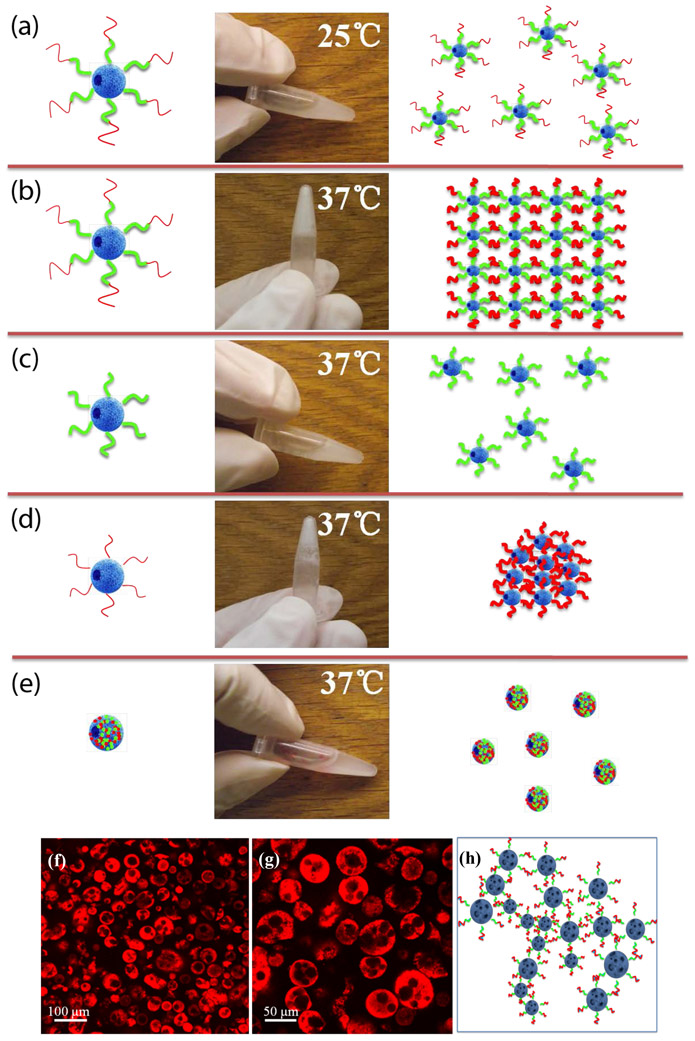
The novel PLLA-PEG-PNIPAm triblock copolymers with PLLA and PNIPAm as terminal blocks and PEG as the middle block were designed to assemble into nanofibrous microspheres that subsequently form a hydrogel (NF-GMS) upon temperature change from room temperature to body temperature. We selected a NF-GMS diameter range of 60-90 μm (based on SEM observation) for subsequent cell transplantation studies. The size distribution was further measured using a Beckman Coulter Multisizer 4 (Supplementary Fig. S7). At room temperature (25°C), the PLLA-PEG-PNIPAmc microspheres were a free-flowing liquid (Fig. 4a), whereas at the body temperature (37 °C), they formed a 3D hydrogel (Fig. 4b). However, the control two-block copolymers either remained a liquid suspension without PNIPAm (Fig. 4c) or precipitated out without PEG (Fig. 4d). The random copolymer of these three components could not form hydrogel either (Fig. 4e), showing the requirement of the tri-block structure for the hydrogel formation.
Figure 4. Thermoresponsive gelation of PLLA-PEG-PNIPAm NF-GMS:
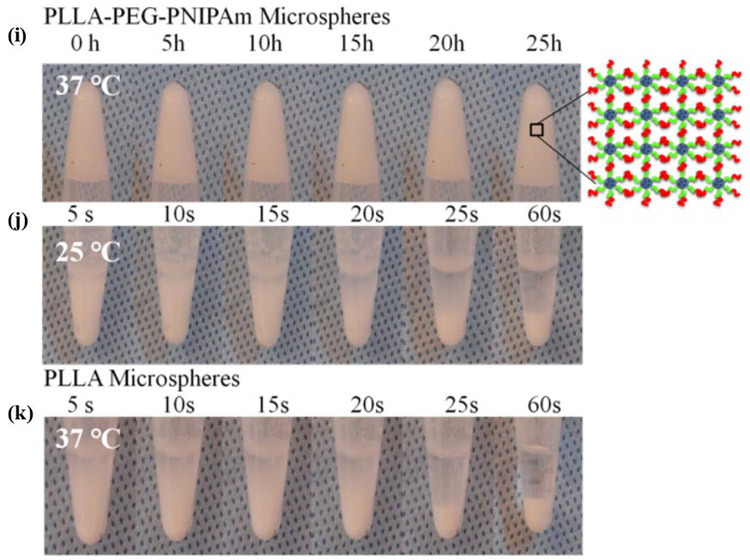
a) aqueous dispersions with 5 w/v % of PLLA-PEG-PNIPAmc (68/9/23 wt%) NF-GMS at 25°C, b) PLLA-PEG-PNIPAmc (68/9/23 wt%) NF-GMS at 37°C, c) PLLA-PEG (89/11 wt%) NF-GMS at 37°C, d) PLLA-PNIPAm (73/27 wt%) NF-HMS at 37°C, and e) PLLA-PEG-PNIPAm (52/14/34 wt%) random copolymer microspheres at 37°C. Confocal fluorescence micrographs of hydrogels: f-g) 5 w/v % PLLA-PEG-PNIPAmc NF-GMS (60-90 μm) at 37°C, the microspheres were stained by Rhodamine-BSA, h) Schematic representation of the crosslinking of PLLA-PEG-PNIPAmc (68/9/23 wt%) NF-GMS inside the hydrogel at 37°C. Stability of aqueous dispersions with 5 w/v % of i) PLLA-PEG-PNIPAmc (68/9/23 wt%) NF-GMS at 37°C, j) PLLA-PEG-PNIPAmc (68/9/23 wt%) NF-GMS at 25°C, and k) PLLA NF-HMS at 37°C.
The microstructure of PLLA-PEG-PNIPAm NF-GMS (60-90 μm, 5 w/v% concentration, stained with Rhodamine-BSA) was observed on a heated glass slide using confocal laser scanning microscopy. NF-GMS remained microspheres inside the formed 3D hydrogel (Fig. 4f-h). The NF-GMS were able to stably maintain the 3D shape at 37°C (Fig. 4i). The PLLA nanofibrous hollow microspheres (NF-HMS) precipitated without hydrogel formation at 37°C (Fig. 4j, k), showing the requirement of water-binding PEG and physical crosslink-forming PNIPAmc.
Rheological studies were carried out to measure the sol-gel transition temperature and viscoelastic properties of NF-GMS (Supplementary Section S5 and Fig. S8). The results confirmed that the aqueous suspension of PLLA-PEG-PNIPAm NF-GMS was a liquid at room temperature and became a free-standing hydrogel at body temperature. This hydrogel was shear-thinning, injectable, and its storage modulus could be modulated over more than two orders of magnitude at the body temperature by varying the composition and the polymer concentration.
Effect of PLLA-PEG-PNIPAm composition on NF structure and gelation of NF-GMS
To systematically investigate the effect of PLLA-PEG-PNIPAm composition on the nanofibrous feature and gelation property of NF-GMS, a library of PLLA-PEG-PNIPAm triblock copolymers with varying PEG, PNIPAm and PLLA block lengths (Supplementary Section 1: Table S1, Table S2), and linear PLLA homopolymers with three different molecular weights (Table S3) were synthesized and fabricated into microspheres. The nanofibrous structure and gelation property of these microspheres were carefully evaluated (Supplementary Information Section S6, Fig. S9 and Table S1). It was found that the nanofibrous structure and the gelation property of NF-GMS strongly depended on the composition of PLLA-PEG-PNIPAm copolymer. Two threshold requirements should be met simultaneously for the nanofiber formation (PLLA block molecular weight and its percentage) and two additional threshold requirements should be met simultaneously for the hydrogel formation (PEG and PNIPAm percentages). Only when the Mn of PLLA block was higher than about 5521, and the weight percentages of PLLA, PEG, and PNIPAm in the copolymer were about or higher than 68, 5, and 11 wt%, respectively, NF-GMS became nanofibrous and formed a free-standing hydrogel at body temperature.
NF-GMS significantly enhanced CM-transplantation mediated heart regeneration
To determine the important role of NF-GMS in supporting CM maintenance and function based on our hypothesis driven design, CMs were cultured with NF-GMS for 7 days in vitro. The open and hollow structure of NF-GMS facilitated CM incorporation, even distribution, and attachment to the NF-GMS. The gel formation of NF-GMS prevented CM leakage and allowed CM interactions. CMs in the gel retained the normal CM structure with the expression of cardiac troponin T (Supplementary Fig. S10). Moreover, we also co-cultured CMs with collagen-I (the major ECM in heart and particularly after MI injury) or NF-GMS with 50 μM H2O2 treatment to mimic in vivo MI injury. NF-GMS significantly increased cell survival after H2O2 treatment compared to collagen-I by Western blot with Bcl-2 (anti-apoptosis marker) and Bax (pro-apoptosis marker) antibodies (Supplementary Fig. S11). High-magnification images showed the cell-cell connections, suggesting cardiac tissue maturation and integration (Supplementary Fig. S12). Importantly, CMs in the gel maintained the cardiac beating property (VedioS1).
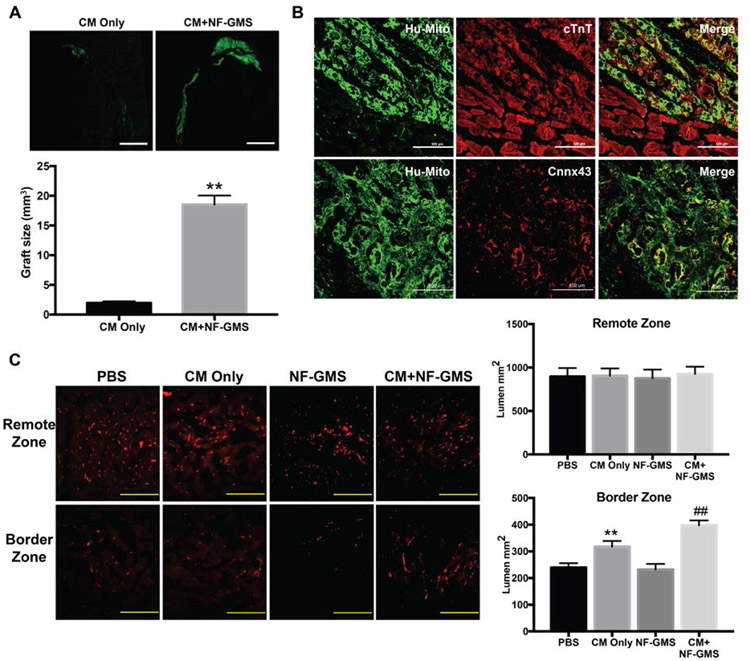
To evaluate the long-term cell retention and in vivo engraftment of NF-GMS carried CMs, we transplanted hESC-derived CMs with NF-GMS. 1×107 hESC-CMs mixed with NF-GMS at the number ratio of 30:1 were suspended in 100 μl PBS and injected into a rat heart after MI injury. 100 μl PBS, NF-GMS alone, and 1×107 hESC-CMs suspended in 100 μl PBS without NF-GMS were injected as controls. The engraftment size of the transplanted hESC-CMs was assessed at day 28 after cell transplantation by immunofluorescence staining with human specific mitochondrial antibody (Hu-mito). In the CMs only group, CMs were found mainly in the infarct border zone and occasionally in the infarct zone (Fig. 5A). In the CM+ NF-GMS group, much larger and confluent CM grafts were identified in both the border zone and the infarct zone (Fig. 5 A&B). The graft volume was calculated using a published method [21] by combining the stained slides every 0.5 mm away from the apex to the base of the heart. The graft area in each slide was measured using ImageJ software. There was a 10-fold higher graft size in the CM+NF-GMS group than that in the CM only group in infarcted rat heart (Fig. 5A). The engraftment of CMs carried by NF-GMS was also detected using immunofluorescence staining against cTnT and Anti-Hu-mito antibodies (Fig. 5B, upper panel). Furthermore, abundant gap junctions formed among CMs in the transplanted areas as indicated by Connexin 43 staining (Fig. 5B, lower panel as well as higher magnification images in Supplementary Fig. S12), indicating that NF-GMS carried CM transplantation also promoted cell-cell integrations.
Figure 5. NF-GMS carried hESC-CM engraftment and angiogenesis.
CM engraftment was illustrated by human specific antigen (Hu-mito) staining and quantitative analysis (A), and immunofluorescence staining against Hu-mito and cTnT (B). Rat I/R hearts were analyzed 4 weeks after transplantation. N=9, **, P<0.01, compared with CM group. Much larger and confluent CM grafts were identified predominantly in both the border zone and the infarct zone in the CM+NF-GMS group, with a 10-fold increase of engraftment over the CM only group (A&B). Furthermore, abundant gap junctions were formed as indicated by Connexin 43 staining (B, lower panel). (C, D) Vessel-like lumens were quantified based on CD31 staining. Scale bars: 200 μm, n = 9, **, p < 0.01, compared with PBS group; ##, p < 0.01, compared with CM group.
The long-term survival and integration of transplanted CMs would require adequate vascular network support in the engrafted areas. Therefore, we evaluated the vascular density in the infarction border zone and the remote zone (non-infarct zone) in the infarcted rat heart by staining with endothelial cell marker CD31. The number of vessel-like lumens was calculated to assess the vascular density 28 days after cell transplantation (Fig. 5 C&D). Vascular density in the remote zone was greater than in the border zone in all groups. No statistical significance of vascular density was observed in the remote zone among PBS, CM alone, NF-GMS alone, and CM + NF-GMS groups. A significantly higher vascular density was observed in the border zone of the CM+ NF-GMS group (398 ± 18) than those of the CM alone (318 ± 21), NF-GMS (231 ± 22) alone and PBS (239 ± 16) groups (P < 0.01). Thus, NF-GMS carried CM transplantation promoted revascularization in the border zone of the infarcted heart. The CD31/αSMA double-staining showed stable vasculature 4 weeks after transplantation (Supplementary Fig. S13).
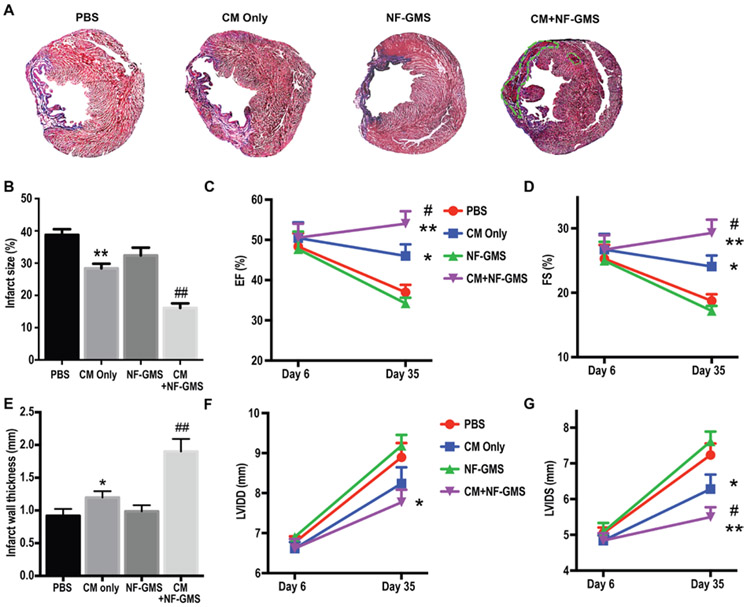
To further evaluate heart regeneration by NF-GMS carried CM transplantation, Masson’s Trichrome staining was performed to identify blue scar tissue and red live tissue 28 days after transplantation (Fig. 6). Live tissue was hardly observed in the infarcted areas of the PBS and NF-GMS only groups. Some clusters of live cells were found in the CM only group but were mainly near the border zone. In contrast, large clusters of live cells were identified in both border zone and infarct zone in the CM + NF-GMS group, leading to a substantially thicker ventricle wall (see areas surrounded by the green line in Fig. 6A). Strikingly, one month after NF-GMS carried CM injection, the infarct size in the CM+ NF-GMS group was only 16% of LV, leading to a 58% reduction compared to PBS group, 50% reduction compared to NF-GMS only group, and a 43% reduction compared to CM only group (P < 0.01). Furthermore, echocardiography was performed at day 6 to get the infarction baseline before cell transplantation. Myocardial infarction was confirmed in all groups with LVFS to be less than 35%. Twenty-eight days after cell transplantation, a significant increase in cardiac function was found in the CM+ NF-GMS group with left ventricle ejection fractions (EF) = 54% and fractional shortening (FS) = 29% indicating a striking functional recovery with 39% increase in EF and 46% increase in FS compared to PBS group (P < 0.01). The cardiac function of CM+NF-GMS group was also significantly greater than that of CM only group with a 17% higher EF and a 22% higher FS (P < 0.05) (Fig. 6). The high-resolution image of Masson’s Trichrome staining (Supplementary Fig. S14) showed reduction in fibrosis (blue) in CM+NF-GMS group. CD68 staining showed modest immune cell infiltration (CD68+) in MI heart 4 weeks after cell transplantation, likely resulted from mainly I/R injury because there were no significant differences between PBS, NF-GMS only, CM only, and CM+NF-GMS groups (Supplementary Fig. S15). Collectively, our studies showed that NF-GMS carried CM transplantation achieved the highest CM engraftment to date, substantially reduced infarct size, enhanced revascularization, and resulted in significant functional recovery compared to CM transplantation alone.
Figure 6. NF-GMS carried hESC-CM injection dramatically decreased infarct size and increased heart function as indicated by Masson Trichrome staining.
(A), quantitative analysis (B), EF (C) and FS (D), wall thickness (E), LVIDD (F), and LVIDS (G) evaluations. (E) Rat MI hearts were analyzed 4 weeks after transplantation. N=9, *, p < 0.05, **, p < 0.01, compared with PBS group; ##, p < 0.01, #, p < 0.05, compared with CM group. NF-GMS alone had little effect whereas CM+NF-MGS transplantation led to a 60% reduction in infarct size compared to PBS control.
DISCUSSION
In order to more comprehensively mimic ECM and obtain superb injectable cell carriers for heart regeneration after a heart attack, we successfully developed a new biodegradable tri-block PLLA-PEG-PNIPAm copolymer that self-assembles into novel nanofibrous microspheres NF-GMS, which in an aqueous suspension are an injectable free-flowing liquid at room temperature and undergo thermally-responsive gelation after injection in vivo to reconstruct complex 3D wounds. The development of the NF-GMS represents several breakthroughs: 1) a route to synthesize PLLA-based tri-block copolymers with drastically different chemical and physical properties among the three blocks, resulting in a new biodegradable and biocompatible thermoresponsive polymer; 2) a new microcarrier that mimics the ECM in both nanofibrous feature and its hydrogel properties; 3) drastic increases in CM retention and engraftment, which have been the critical bottleneck of cardiac regeneration; 4) a substantial reduction of infarct size and a substantial recovery of cardiac function of a infarcted heart.
Pure PLLA has been previously fabricated into macroscale 3D nanofibrous scaffolds using a novel phase-separation technique in our lab [22] for various tissue regeneration applications [23]. PLLA is the major component of the new tri-block copolymer and contributes to the microsphere body and nanofibrous structure. While various NF microspheres have been developed using star-shaped PLLA by our group [24], those microspheres are discrete particles and unable to form the desired integrative hydrogels. While ATRP and ROP have been used in combination to synthesize other types of triblock copolymers to form nanosized drug delivery particles[25], none of them has been reported to form nanofibrous microparticles such as the NF-GMS in this work. By copolymerize PLLA with PEG and PNIPAm using ATRP and ROP, we successfully synthesized PLLA-PEG-PNIPAm triblock copolymer for the first time, imparting hydrophilicity and temperature-triggered physical crosslinks to enable macroscale gel formation. However, chemical modification of PLLA is known to interfere with its fiber formation [26]. More challengingly, due to the limited contact area between spheres, there is a sharp increase in difficulty to build strong interactions between spheres to construct a stable 3D network for hydrogel formation when the diameter of spheres is large, such as on the micrometer scale in this study. By examining the competing requirements for nanofiber and gel formation, structural requirements of NF-GMS formation are revealed. Macroscopic hydrogel of the NF-GMS is developed and its modulus can be tuned over several orders of magnitude. In addition, the PLLA-PEG-PNIPAm NF-GMS degrade in a desired time duration for heart regeneration. Furthermore, NF-GMS carried CM delivery leads to remarkably efficient CM engraftment and significantly improves heart function after infarction in an acute rat MI model. Nanofibrous structure is known to advantageously support various tissue regeneration [20b], gel properties can affect cell behavior [27] and could have protective effect on injured heart [28].
The superior engraftment efficiency of NF-GMS carried cell transplantation appears due to the two most important ECM-mimicking characteristics of this advanced CM carrier: the nanofiber feature and the gelling property. Consistent with this notion, we previously showed porous and nanofibrous scaffolds to enhance cardiac differentiation over control non-nanofibrous scaffolds [29]. CM delivery using NF microspheres without gelling property resulted in significantly lower enhancement in CM engraftment (3-4-fold enhancement over CM only, unpublished data) compared to the approximately 10-fold CM engraftment increase using the NF-GMS hydrogel. Various hydrogels were reported to improve CM retention or protection [28, 30], but their improvements were not at the same level as NF-GMS in this work. For example, we previously showed that a fibrin gel without the nanofibrous microspheres had significantly lower enhancement in CM engraftment (about 2-3 fold over CM suspension alone) [31] compared to the 10-fold CM engraftment enhancement of the NF-GMS hydrogel. NF-GMS achieved the highest CM engraftment enhancement among all carriers reported this far.
There was no statistically significant difference in cardiac function between NF-GMS alone and PBS alone control groups without cells, suggesting that the NF-GMS did not mechanically contribute to the cardiac function of the infarcted heart. However, when CMs were transplanted, NF-GMS enhanced the CM engraftment and improved cardiac function vs. all control groups. The temperature induced NF-GMS hydrogel (at 37°C) has low modulus, which may not enhance cardiac function mechanically by itself. However, the low modulus may facilitate cardiac differentiation [32]. The CM+NF-GMS group enhanced cardiac tissue regeneration (reduced infarct size) over CM alone or NF-GMS alone groups. Importantly, CM+NF-GMS group showed the best functional properties among all groups after 35 days of implantation.
Collectively, the novel NF-GMS self-assembled from the new triblock copolymer, integrating the ECM-mimicking NF architecture with a temperature-responsive in situ gel-forming property, are a highly promising microcarrier for heart regeneration and likely for many other types of tissue regeneration.
MATERIALS AND METHODS
PEG macroinitiator (HO-PEG-Br) synthesis (reaction 1):
A typical procedure to synthesize HO-PEG-Br is as follows: Dry tetrahydrofuran (THF) (25 mL), dry polyethylene glycol (PEG) (6.68 mmol) and dry triethylamine (TEA) (20 mmol, 1.5 mL) were placed in a 250 mL round-bottom flask, kept under a nitrogen atmosphere. Within 1 h, bromoisobutyryl bromide (BIBB) (6.68 mmol, 0.83 ml) was slowly added via a dropping funnel. After the addition was complete, the mixture was stirred at room temperature overnight. The precipitated salts were filtered off, and the filtrate was evaporated in vacuum. Then 1 M hydrochloric acid (HCl) (30 mL) was added and the mixture was extracted with dichloromethane (3 × 20 mL). The combined organic layers were washed three times with water (50 mL) to remove salt. The organic layer was dried over anhydrous Na2SO4 overnight. After removal of the solvent, the polymer was precipitated into cold ethyl ether and collected by filtration. The resultant white powder was dried in vacuum for 24 hours to give HO-PEG-Br.
PEG-PNIPAm (reaction 2: ATRP):
A typical procedure to synthesize PEG-PNIPAM (Mn=5371) is as follows: PEG macroinitiator (Mn=1551) (0.6 mmol, 1g), NIPAm (26.5 mmol, 3 g), and CuCl (0.170 mmol, 0.016.8 g) were placed in a 250 mL round-bottom flask under nitrogen protection and sealed with rubber septum stoppers. Milli-Q water (20 mL) and Me6TREN (0.174 mmol, 0.04 g) were placed in a Schlenk tube and purged with N2 gas for 40 min. The solution was transferred to the round-bottom flask using a syringe under nitrogen protection. The reaction mixture was then stirred under nitrogen atmosphere for 24 h. The reaction was then stopped by opening the vessel to air. The reaction mixture was precipitated into ethyl ether, filtered, and dried. The resulting solid was then dissolved in H2O and dialyzed (MW cut-off 3.5 kDa) against de-ionized water for 3 days to remove unreacted PEG-macroinitiator. The mixture was then lyophilized for three days to give PEG-PNIPAm copolymer.
PLLA-PEG-PNIPAm (reaction 3: ROP):
A typical procedure to synthesize PLLA-PEG-PNIPAmc is as follows: Dry THF (10mL), L-lactide (139 mmol, 2g), PEG-PNIPAm (Mn=5371) (0.0559 mmol, 0.3g) and Sn(Oct)2 (0.4 mmol, 0.162 g) were mixed in a 50 mL round-bottom flask with stirring and nitrogen purging. The mixture was heated to 80°C under nitrogen protection for complete melting. The polymerization was carried out at 80°C under nitrogen protection for 24 h. The crude product was dissolved in 20 mL chloroform, precipitated in 100 mL cold methanol, and then vacuum dried.
Fabrication of microspheres.
PLLA-PEG-PNIPAm copolymer was dissolved in THF at 60 °C with a concentration of 2.0% (wt/v). Under rigorous mechanical stirring (speed 7, MAXIMA, Fisher Scientific), glycerol (60°C) with three times the volume of the PLLA-PEG-PNIPAm copolymer solution was gradually added into the PLLA-PEG-PNIPAm copolymer solution. Stirring was continued for 5 min afterwards. The mixture was then quickly poured into liquid nitrogen. After 10 min, a water ice mixture (1,000 ml) was added for solvent exchange for 24 h. The spheres were sieved and washed with an excessive amount of distilled water six times to remove glycerol residue. The spheres were then lyophilized for three days.
Differentiation and characterization of human embryonic stem cell derived cardiomyocytes (hESC-CMs)
hESCs (H7 cell line) were differentiated into cardiomyocytes (CMs) using chemically defined culture similar to that described in literature [33]. Full confluent single layer hESCs were cultured in CDM3 medium to induce CM differentiation. Lactate instead of glucose medium was applied to purify CMs from day 12 to day 18 [33b, 34]. At day 20, the derived CMs were digested by trypsin for flow cytometry assay or subsequent transplantation. Typically, 90% or more resulting cells were cTnT+ CMs using the above described differentiation process.
SEM and immunofluorescence assay of co-cultured CMs and NF-GMS
Five million CMs mixed with NF-GMS at the ratio of 30:1 were co-cultured in 35 mm petri dishes (Falcon) with CDM3 medium for 7 days. For scanning electron microscope (SEM) assay, the samples were collected and fixed in 2.5% glutaraldehyde and 2% paraformaldehyde overnight at 4 °C. After post-fixation in 1% osmium tetroxide at room temperature for 1 h, samples were dehydrated in increasing concentrations of ethanol and dried with hexamethyldisilazane. NF-GMS only or the CM+NF-GMS samples were sputter-coated with gold and observed under an SEM (Phillips XL30 FEG).
For immunofluorescence staining, samples were fixed with 4% paraformaldehyde at room temperature for 20 min, frozen in Tissue-Plus O.C.T Compound (Fisher Scientific), and cryosectioned into 7 μm sections. Slide sections were permeabilized with 0.3% Triton X-100 for 15 min at room temperature, blocked with 5% horse serum in DPBS-T for 1 h at room temperature and incubated with primary antibodies against cTnT (ab45932, Abcam) at 4°C overnight in 2% horse serum. Sections were then washed 3 times with PBS for 15 min each time, incubated with Alexa Fluor 488-conjugated secondary antibodies (Thermo Fisher Scientific) in 2% horse serum in DPBS-T for 1 h at room temperature, washed with PBS for 3 times and 15 min each time, then stained with DAPI, and images were obtained by fluorescence microscope (Olympus, Japan).
Generation of myocardial infarction (MI) rats and cell transplantation
All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Michigan. Eight-week-old (190-210 g) Female Sprague Dawley rats were purchased from ENVIGO. Myocardial infarction was induced by ischemia reperfusion (I/R) surgery [8b, 35]. In brief, the animal heart was exposed by an open thoracotomy and the left anterior descending artery was ligated with 6-0 sutures for 60 min and reperfused by loosening the suture. Animals were randomly divided into 4 groups: PBS control, CM only, NF-GMS only, and CM+NF-GMS group. Cell transplantation was carried out following the literature [8b, 35b] with minor modifications. Briefly, seven days after I/R (which is within the reported best time window of 4 day to 2 weeks after MI for cardiomyocyte transplantation [7a, 8c, 36]), animals underwent a second thoracotomy and 100 μl PBS or cell suspension containing 1×107 hESC-CMs were injected at 5 sites into the border zone of the infarction. In the CM+NF-GMS group, CMs and NF-GMS at the ratio of 30:1 were mixed before injection. Immunosuppressor cyclosporine A was subcutaneously administered 10 mg/kg/day from two days before cell transplantation until animals were sacrificed.
Histology and Immunohistochemistry
Hearts were fixed in 4% paraformaldehyde, frozen in Tissue-Plus O.C.T Compound (Fisher Scientific), and cryosectioned into 7 μm sections for immunohistochemistry and histological analyses. For immunofluorescence staining, the procedure was the same as for those in vitro CM+NF-GMS samples described above. Staining with primary antibodies against human mitochondrion (MAB1273, EMD Millipore) was performed to identify the transplanted CMs in rat heart. Staining with cTnT (ab45932, Abcam) and Cnnx43 (sc-9059, Santa Cruz Biotechnology) antibodies was performed to characterize CM structure and cell-cell connection. After staining, slides were mounted using ProLong® Diamond Antifade Mountant (P36970, Thermo Fisher Scientific) and imaged using a Nikon A1 Confocal Laser Microscope. Staining with anti-CD31 (sc-1506, Santa Cruz Biotechnology) antibody was used to investigate vascular density. In addition, Masson’s Trichrome staining was performed to calculate the infarct size in rat hearts.
Assessment of cardiac function
Echocardiography was performed at day 6 and day 35 to evaluate cardiac function. Left ventricular internal diameter at end-diastole (LVIDD) and left ventricular internal diameter at end-systole (LVIDS) were measured using a Vevo® 2100 system [37]. Left ventricle ejection fractions (EF) and fractional shortening (FS) were calculated using the equations: EF (%) = (LVIDD3−LVIDS3)/ LVIDD3×100%; and FS (%) = (LVIDD−LVIDS)/ LVIDD×100%. All echocardiography measurement and analysis were performed by a single-blinded investigator in Frankel Cardiovascular Center of University of Michigan.
Statistical Analysis
The data are presented as the mean ± SEM. All results were assessed using Graphpad Prism software. Statistical analyses were performed using a Student’s t-test or a one-way ANOVA test. A p value < 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGEMENTS
Funding:
The authors would like to acknowledge the financial support from the NIH (HL114038, HL136231, HL109054, and HL139735).
Footnotes
SUPPLEMENTARY INFORMATION
1) Supplementary Information including supplementary figures, tables and methods accompanies this manuscript as a PDF file (NF-GMS-SI-v29-clean.pdf).
2) A supplementary video showing in vitro beating CMs in the NF-GMS gel is also provided (Video-S1).
COMPETING INTERESTS
University of Michigan filed an US Patent application on the new polymers, carriers, methods of synthesis, fabrication, and their applications (Inventors: PXM, CZ, ZW).
REFERENCES
- [1].Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, C. American Heart Association Statistics, S. Stroke Statistics, Circulation 2017, 135, e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lin Z, Pu WT, Sci Transl Med 2014, 6, 239rv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Writing Group M, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, C. American Heart Association Statistics, S. Stroke Statistics, Circulation 2016, 133, 447. [DOI] [PubMed] [Google Scholar]
- [4].a) Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P, The New England journal of medicine 2001, 344, 1750; [DOI] [PubMed] [Google Scholar]; b) Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT, Nature 2013, 493, 433; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Tzahor E, Poss KD, Science 2017, 356, 1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kaushik G, Spenlehauer A, Sessions AO, Trujillo AS, Fuhrmann A, Fu Z, Venkatraman V, Pohl D, Tuler J, Wang M, Lakatta EG, Ocorr K, Bodmer R, Bernstein SI, Van Eyk JE, Cammarato A, Engler AJ, Sci Transl Med 2015, 7, 292ra99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].a) van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marban E, Molkentin JD, Nature 2014, 509, 337; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sultana N, Zhang L, Yan J, Chen J, Cai W, Razzaque S, Jeong D, Sheng W, Bu L, Xu M, Huang GY, Hajjar RJ, Zhou B, Moon A, Cai CL, Nat Commun 2015, 6, 8701; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Zhou B, Wu SM, Circ Res 2018, 123, 9; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Kretzschmar K, Post Y, Bannier-Helaouet M, Mattiotti A, Drost J, Basak O, Li VSW, van den Born M, Gunst QD, Versteeg D, Kooijman L, van der Elst S, van Es JH, van Rooij E, van den Hoff MJB, Clevers H, Proceedings of the National Academy of Sciences of the United States of America 2018, DOI: 10.1073/pnas.1805829115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].a) Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, Van Biber B, Dardas T, Mignone JL, Izawa A, Hanna R, Viswanathan M, Gold JD, Kotlikoff MI, Sarvazyan N, Kay MW, Murry CE, Laflamme MA, Nature 2012, 489, 322; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Weinberger F, Breckwoldt K, Pecha S, Kelly A, Geertz B, Starbatty J, Yorgan T, Cheng KH, Lessmann K, Stolen T, Scherrer-Crosbie M, Smith G, Reichenspurner H, Hansen A, Eschenhagen T, Sci Transl Med 2016, 8, 363ra148. [DOI] [PubMed] [Google Scholar]
- [8].a) Elhami E, Dietz B, Xiang B, Deng J, Wang F, Chi C, Goertzen AL, Mzengeza S, Freed D, Arora RC, Tian G, EJNMMI Research 2013, 3, 72; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE, Nature Biotechnology 2007, 25, 1015; [DOI] [PubMed] [Google Scholar]; c) Fernandes S Naumova AV, Zhu WZ, Laflamme MA, Gold J, Murry CE, Journal of Molecular and Cellular Cardiology 2010, 49, 941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].a) Aicher A, Brenner W, Zuhayra M, Badorff C, Massoudi S, Assmus B, Eckey T, Henze E, Zeiher AM, Dimmeler S, Circulation 2003, 107, 2134; [DOI] [PubMed] [Google Scholar]; b) Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, Yeung AC, Johnstone BH, Yock PG, March KL, Circulation 2005, 112, I150. [DOI] [PubMed] [Google Scholar]
- [10].a) Dong R, Zhao X, Guo B, Ma PX, ACS Appl Mater Interfaces 2016, 8, 17138; [DOI] [PubMed] [Google Scholar]; b) Pena B, Laughter M Jett S, Rowland TJ, Taylor MRG, Mestroni L, Park D, Macromol Biosci 2018, 18, e1800079; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Weinberger F, Mannhardt I, Eschenhagen T, Circ Res 2017, 120, 1487. [DOI] [PubMed] [Google Scholar]
- [11].Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE, Nature 2014, 510, 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].a) Ye L, Chang YH, Xiong Q, Zhang P, Zhang L, Somasundaram P, Lepley M, Swingen C, Su L, Wendel JS, Guo J, Jang A, Rosenbush D, Greder L, Dutton JR, Zhang J, Kamp TJ, Kaufman DS, Ge Y, Zhang J, Cell stem cell 2014, 15, 750; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Tang J, Wang J, Huang K, Ye Y, Su T, Qiao L, Hensley MT, Caranasos TG, Zhang J, Gu Z, Cheng K, Sci Adv 2018, 4, eaat9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Purcell BP, Lobb D, Charati MB, Dorsey SM, Wade RJ, Zellars KN, Doviak H, Pettaway S, Logdon CB, Shuman JA, Freels PD, Gorman Iii JH, Gorman RC, Spinale FG, Burdick JA, Nat Mater 2014, 13, 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wolf FF, Friedemann N, Frey H, Macromolecules 2009, 42, 5622. [Google Scholar]
- [15].a) Semsarilar M, Perrier S, Nat Chem 2010, 2, 811; [DOI] [PubMed] [Google Scholar]; b) Gregory A, Stenzel MH, Progress in Polymer Science 2012, 37, 38. [Google Scholar]
- [16].Matyjaszewski K, Tsarevsky NV, Nat Chem 2009, 1, 276. [DOI] [PubMed] [Google Scholar]
- [17].Xiu-fang W, Lan J.-l., Ying L, Pihui P, Zhi-Qi C, Shou-ping X, Li-juan Z, Yu Q, Polymer International 2014, 63, 1238. [Google Scholar]
- [18].a) Kamber NE, Jeong W, Waymouth RM, Pratt RC, Lohmeijer BGG, Hedrick JL, Chemical Reviews 2007, 107, 5813; [DOI] [PubMed] [Google Scholar]; b) Dechy-Cabaret O, Martin-Vaca B, Bourissou D, Chemical Reviews 2004, 104, 6147. [DOI] [PubMed] [Google Scholar]
- [19].Zhao C, Li L, Zheng J, Langmuir 2010, 26, 17375. [DOI] [PubMed] [Google Scholar]
- [20].a) Langer R, Vacanti J, Science 1993, 260, 920; [DOI] [PubMed] [Google Scholar]; b) Ma PX, Advanced Drug Delivery Reviews 2008, 60, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tambara K, Premaratne GU, Sakaguchi G, Kanemitsu N, Lin X, Nakajima H, Sakakibara Y, Kimura Y, Yamamoto M, Tabata Y, Ikeda T, Komeda M, Circulation 2005, 112, I129. [DOI] [PubMed] [Google Scholar]
- [22].a) Ma PX, Zhang R, J Biomed Mater Res 1999, 46, 60; [DOI] [PubMed] [Google Scholar]; b) Ma H, Hu J, Ma PX, Advanced Functional Materials 2010, 20, 2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wei G, Ma PX, Adv Funct Mater 2008, 18, 3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].a) Liu X, Jin X, Ma PX, Nat Mater 2011, 10, 398; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhang Z, Gupte MJ, Jin X, Ma PX, Advanced Functional Materials 2015, 25, 350; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Zhang Z, Marson RL, Ge Z, Glotzer SC, Ma PX Adv Mater 2015, 27, 3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen J, Liu M, Gong H, Huang Y, Chen C, J Phys Chem B 2011, 115, 14947. [DOI] [PubMed] [Google Scholar]
- [26].Liu X, Ma PX, Biomaterials 2010, 31, 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wen JH, Vincent LG, Fuhrmann A, Choi YS, Hribar KC, Taylor-Weiner H, Chen S, Engler AJ, Nat Mater 2014, 13, 979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ifkovits JL, Tous E, Minakawa M, Morita M, Robb JD, Koomalsingh KJ, Gorman JH 3rd, Gorman RC, Burdick JA, Proceedings of the National Academy of Sciences of the United States of America 2010, 107, 11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Liu Q, Tian S, Zhao C, Chen X, Lei I, Wang Z, Ma PX, Acta Biomaterialia 2015, 26, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].a) Kofidis T, de Bruin JL, Hoyt G, Lebl DR, Tanaka M, Yamane T, Chang CP, Robbins RC, J Thorac Cardiovasc Surg 2004, 128, 571; [DOI] [PubMed] [Google Scholar]; b) Christman KL, Fok HH, Sievers RE, Fang Q, Lee J, Tissue Eng 2004, 10, 403; [DOI] [PubMed] [Google Scholar]; c) Jackman CP, Carlson AL, Bursac N, Biomaterials 2016, 111, 66; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Li Z, Guo X, Matsushita S, Guan J, Biomaterials 2011, 32, 3220; [DOI] [PubMed] [Google Scholar]; e) Tokunaga M, Liu ML, Nagai T, Iwanaga K, Matsuura K, Takahashi T, Kanda M, Kondo N, Wang P, Naito AT, Komuro I, Journal of Molecular and Cellular Cardiology 2010, 49, 972; [DOI] [PubMed] [Google Scholar]; f) Yoshizumi T, Zhu Y, Jiang H, D'Amore A, Sakaguchi H, Tchao J, Tobita K, Wagner WR, Biomaterials 2016, 83, 182; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Wang H, Liu Z, Li D, Guo X, Kasper FK, Duan C, Zhou J, Mikos AG, Wang C, J Cell Mol Med 2012, 16, 1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li Y, Tian S, Lei I, Liu L, Ma PX, Wang Z, Am J Transl Res 2017, 9, 1530. [PMC free article] [PubMed] [Google Scholar]
- [32].Engler AJ, Sen S, Sweeney HL, Discher DE, Cell 2006, 126, 677. [DOI] [PubMed] [Google Scholar]
- [33].a) Lian X Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, Hsiao C, Kamp TJ, Palecek SP, Nature Protocols 2013, 8, 162; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, Plews JR, Abilez OJ, Cui B, Gold JD, Wu JC, Nature Methods 2014, 11, 855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tohyama S, Hattori F, Sano M, Hishiki T, Nagahata Y, Matsuura T, Hashimoto H, Suzuki T, Yamashita H, Satoh Y, Egashira T, Seki T, Muraoka N, Yamakawa H, Ohgino Y, Tanaka T, Yoichi M, Yuasa S, Murata M, Suematsu M, Fukuda K, Cell Stem Cell 2013, 12, 127. [DOI] [PubMed] [Google Scholar]
- [35].a) Tian S, Lei I, Gao W, Liu L, Guo Y, Creech J, Herron TJ, Xian S, Ma PX, Eugene Chen Y, Li Y, Alam HB, Wang Z, EBioMedicine 2018, DOI: 10.1016/j.ebiom.2018.12.003; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Fernandes S, Chong JJ, Paige SL, Iwata M, Torok-Storb B, Keller G, Reinecke H, Murry CE, Stem Cell Reports 2015, 5, 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, Forder JR, Anderson RD, Hatzopoulos AK, Penn MS, Perin EC, Chambers J, Baran KW, Raveendran G, Lambert C, Lerman A, Simon DI, Vaughan DE, Lai D, Gee AP, Taylor DA, Cogle CR, Thomas JD, Olson RE, Bowman S, Francescon J, Geither C, Handberg E, Kappenman C, Westbrook L, Piller LB, Simpson LM, Baraniuk S, Loghin C, Aguilar D, Richman S, Zierold C, Spoon DB, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD, Cardiovascular N Cell Therapy Research, JAMA 2012, 308, 2380.23129008 [Google Scholar]
- [37].Bhan A, Sirker A, Zhang J, Protti A, Catibog N, Driver W, Botnar R, Monaghan MJ, Shah AM, American journal of physiology. Heart and Circulatory Physiology 2014, 306, H1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.