ABSTRACT
Dysregulation of gene expression, often interpreted by gene transcription as an endpoint response, is tightly associated with human cancer. Long noncoding RNAs (lncRNAs), derived from the noncoding elements in the genome and appeared no less than 200nt in length, have emerged as a novel class of pivotal regulatory component. Recently, great attention has been paid to the cancer-related lncRNAs and growing evidence have shown that lncRNAs act as key transcriptional regulators in cancer cells through diverse mechanisms. Here, we focus on the nucleus-expressed lncRNAs and summarize their molecular mechanisms in transcriptional control during tumorigenesis and cancer metastasis. Six major mechanisms will be discussed in this review: association with transcriptional factor, modulating DNA methylation or histone modification enzyme, influencing on chromatin remodelling complex, facilitating chromosomal looping, interaction with RNA polymerase and direct association with promoter.
KEYWORDS: Long noncoding RNA, Transcription, Cancer
Introduction
The completion of the 'Human Genome Project’ and the development of the ‘ENCODE Program’ have revealed a large portion of noncoding elements in the human genome. RNA products derived from these regions are not further translated into typical proteins and collectively referred to as noncoding RNAs (ncRNAs), which account for approximately 72% of the entire human genome [1]. Long noncoding RNA (lncRNA), a heterogenous class of ncRNAs that are larger than 200nt, is a young member in the ncRNA family. The current definition of lncRNA was generated and world-wide accepted in the first decade of the twenty-first century, although the first lncRNA can be traced back to 1970s. The discovery of lncRNA quickly attracted the research attention, particularly in the transcriptional regulation, as that the majority of reported lncRNAs are nucleus-expressed, a feature that is distinct from mRNAs, which are predominantly expressed in the cytoplasm. Dysregulation of gene program is tightly linked with cancer biology. Cancer-associated gene dysregulation affects both protein-encoding genes and ncRNA molecules [2]. To date, emerging evidence have shown that cancer misregulated lncRNAs often act as critical regulatory molecules in cancer development, and are potential therapeutic targets in the diagnosis and treatment of cancer [3–5]. The regulatory mechanisms of these lncRNAs are diverse and complicated. They are deeply involved in almost all the steps during the gene expression, transcriptionally and post-transcriptionally. Generally at the transcriptional level, these lncRNAs can regulate the neighbouring genes in cis by trapping transcription factors, influencing epigenetic modulation enzymes, forming chromatin looping, or direct association with promoter [6,7]. They can also regulate distal genes in trans through similar mechanisms [8–11]. In this review, we will focus on these cancer-related lncRNAs that are expressed in the nucleus and discuss their working mechanisms as transcriptional modulators. We synthesized their acts into six main mechanisms: association with transcriptional factor, modulating DNA methylation or histone modification enzyme, influencing on chromatin remodelling complex, facilitating chromosomal looping, interaction with RNA polymerase, and direct association with promoter.
Association with transcriptional factor (TF)
Association with TFs to regulate gene transcription is a widely observed mechanism for the nucleus-expressed lncRNAs. lncRNAs interplay with the TFs via various mechanisms (Table 1). By direct or indirect interaction with the TFs [12–30], lncRNAs may control the neighbouring intrachromosomal genes in cis or affect the genes on different chromosomes in trans.
Table 1.
Association with transcriptional factor (TF).
| lncRNAs | Cancer type | Functions and phenotype | References |
|---|---|---|---|
| lncSOX4 | Liver cancer | Recruits transcriptional factor STAT3 to the promoter of SOX4 gene for SOX4 activation, promotes hepatocellular carcinoma development | [22] |
| HOXC-AS3 | Gastric cancer | Induces transcriptional factor YBX1 to the target genes’ promoters that contain CCAAT-box, activatess cancer cell proliferation and migration. | [23] |
| HOTAIR | Breast cancer | Serves as a scaffold to form a complex with HBXIP, c-myc and LSD1, activates transcription of c-myc target genes and drives carcinogenesis. | [27] |
| PCAT19-long | Prostate cancer | Recruits HNRNPAB and other transcription factors to upregulate cell-cycle genes, promotes cell proliferation, migration and invasion. | [30] |
| CCAT1 | Squamous cell carcinomas (SCCs) | Acts as a scaffold, forms a complex with TP63 and SOX2, activates the expression of EGFR, MEK/ERK1/2 and PI3K/AKT cell signal. It promotes SCC cell proliferation both in vitro and in vivo | [33] |
| SLNCR | Melanoma | Recruits AR to EGR1-bound genomic loci and switches EGR1-mediated transcriptional activation of p21Waf1/Cip1 to repressive status, promotes the development of melanoma | [34] |
| PCAT1 | Prostate cancer | Recruits AR and LSD1 to the enhancers of androgen late-response genes, promotes prostate cancer development. | [35] |
| MALAT1 | Breast cancer | Serves as a decoy to interact TEAD preventing TEAD from associating with its co-activator YAP and target gene promoters, suppresses cell metastasis. | [37] |
| GAS5 | Breast cancer | Acts as a decoy of GRE, prevents GR binding to GRE, controls cell apoptosis and inhibits breast cancer development. | [38,39] |
| MALAT1 | Liver cancer | Acts as a RNA decoy and releases DBC1 from SIRT1 to activate the enzymatic activity of SIRT1. It promotes deacetylation of p53 and cell proliferation and inhibits cell apoptosis. | [40] |
RNA guide
Some lncRNAs achieve their roles in an ‘RNA guide’ manner (Fig. 1A). In this regulatory mechanism, the lncRNAs usually interact with a single TF and facilitate the loading of the TF to promoter or enhancer of the target genes to participate in transcriptional activation [22,23] (Fig. 1A). For instance, liver tumour-initiating cells (TICs) play an important role in initiation and recurrence of liver cancer. lncSOX4 is highly expressed in TICs to promote hepatocellular carcinoma (HCC) development. Mechanistically, lncSOX4 recruits transcriptional factor STAT3 to the promoter of SOX4 gene for SOX4 activation [22]. HOXC-AS3 is a gastric cancer cell highly expressed lncRNA and plays an oncogenic role in gastric cancer cells [23]. It acts as an RNA guide to induce transcriptional factor YBX1 to the target genes’ promoters that contain CCAAT-box. As a consequence, HOXC-AS3 activates a set of genes that promote cancer cell proliferation and migration, including CDK2, HOXB13, IGFBP4, ATF5, MAPK4, MMP7, MMP24, BIRC2, WNT10B and HDAC5.
Figure 1.
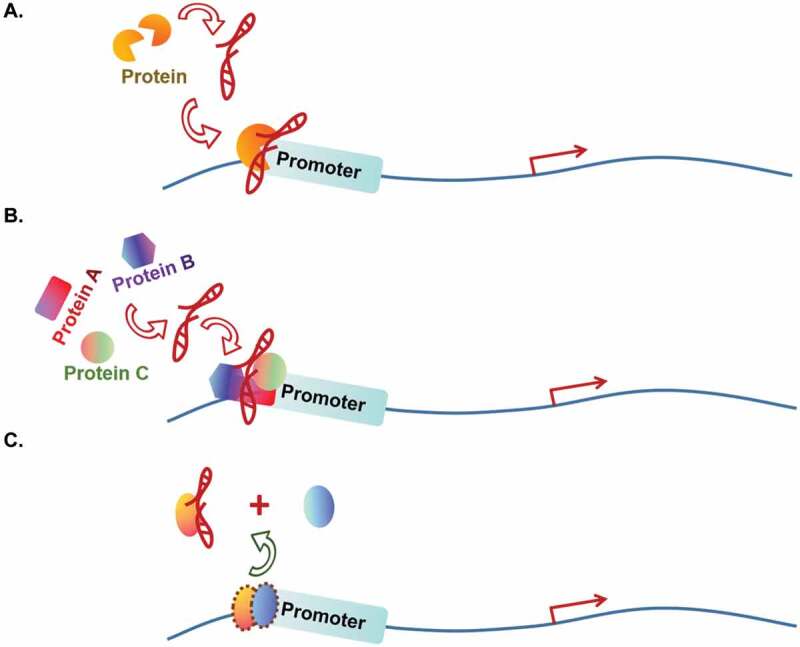
Association with transcriptional factors (TFs).
lncRNAs regulate gene transcription via influencing on TFs. (a) RNA guide: An lncRNA interacts with a single TF directly or indirectly and recruits the TF to the target gene promoters. (b) Molecular scaffold: An lncRNA is able to interact with multiple transcriptional regulators and promotes their recruitment to the target gene’s promoter. (c) RNA decoy: An lncRNA sequesters its associated TF from the target gene’s promoter or the latter’s complex.
Molecular scaffold
Other lncRNAs function as ‘molecular scaffolds’. These lncRNAs are capable to interact with multiple TFs, or TF and other transcriptional regulators simultaneously. The resulting protein:lncRNA:protein complex provides a platform for the involved transcriptional modulators to coordinate on the target genes’ transcription [10,12,24–35] (Fig. 1B). For example, lncRNA HOTAIR usually acts as a scaffold molecule to regulate genes’ expression. During cancer development, HOTAIR recruits PRC2 and LSD1 at new chromatin target sites and inhibits the transcription of multiple anti-metastasis genes [10,31,32]. In breast cancer, HOTAIR forms a complex with HBXIP, c-myc and LSD1. This complex activates transcription of the c-myc target genes to drive carcinogenesis [27]. The lncRNA PCAT19 has two different isoforms, a short isoform of lncRNA PCAT19 (PCAT19-short) and a long isoform of lncRNA PCAT19 (PCAT19-long). In prostate cancer (PCa), lncRNA PCAT19-long interacts with HNRNPAB to activate a subset of cell-cycle genes that are associated with PCa progression, thus, promoting PCa tumour growth and metastasis [30]. As an up-regulated lncRNA in squamous cell carcinomas (SCCs), lncRNA CCAT1 promotes SCC cell proliferation both in vitro and in vivo. Mechanistically, CCAT1 acts as a scaffold by recruiting two TFs, TP63 and SOX2, to form TP63-SOX2-CCAT1 complex. This complex occupies at the supper-enhancer region of EGFR, activates the expression of EGFR and promotes SCC tumorigenesis through activating MEK/ERK1/2 and PI3K/AKT cell signal [33]. Similarly, lncRNA SLNCR builds a scaffold for androgen receptor (AR) and EGR1, two TFs responsible for the transcriptional regulation of tumour suppressor p21Waf1/Cip1 [34]. Under physiological condition, EGR1 works as an activator to promote the expression of p21Waf1/Cip1. In addition, the authors also found that ligand-free AR is enriched on the SLNCR-regulated melanoma genes by ChIP-sequencing assay and that AR genomic occupancy significantly overlaps with EGR1 at consensus EGR1 binding sites. During tumorigenesis in melanoma cells, SLNCR recruits AR to EGR1-bound genomic loci and switches EGR1-mediated transcriptional activation of p21Waf1/Cip1 to repressive status. Thus, lncRNA SLNCR plays an important role in promoting the development of melanoma. The oncogenic lncRNA PCAT1 acts as a scaffold molecule to link AR and histone modifier LSD1 (lysine-specific demethylatase1) in prostate cancer cell and is required for AR and LSD1’s recruitment to the enhancers of androgen late-response genes [35]. RNA decoy: Still other lncRNAs are found to serve as ‘RNA decoys’. These lncRNAs act as intermediates to sequester TFs from their functional sites [36–40] (Fig. 1C). For example, glucocorticoid receptor (GR) works with glucocorticoid response element (GRE) to regulate apoptosis and the cell cycle. It has been reported that the lncRNA GAS5 represses GR activity by acting as a decoy of GRE. GAS5 prevents GR binding to GRE and suppresses the expression of targeted genes in breast cancer [38,39]. For another example, lncRNA MALAT1 is a broadly expressed lncRNA involved in many aspects of cellular processes through multiple mechanisms. Kim J.et al. reported that MALAT1 can inactivate the prometastatic transcription factor TEAD through the RNA decoy mechanism [37]. At the molecular level, interaction of MALAT1 to TEAD prevents TEAD from associating with its co-activator YAP and target gene promoters. In this work, the expression level of MALAT1 is inversely correlated with breast cancer progression and metastatic ability and was demonstrated as a metastasis-suppressing lncRNA in breast cancer. It is worth mentioning that MALAT1 can also function as an RNA decoy by releasing the MALAT1-associated protein DBC1 from the latter’s association with SIRT1 to reactivate the enzymatic activity of SIRT1 [40], a mechanism that overlaps with ‘modulating histone modification enzyme’. Several other lncRNAs have been reported to use this mechanism for transcritpional regulation: releasing the interacting proteins from their complexes, thus altering the activation of the protein complexes during transcriptional regulation [41–43]. In addition, besides TFs, decoy lncRNAs can also regulate gene expression by sequestering RNA-binding proteins that are not typical TFs, as well as microRNAs, catalytic proteins and subunits of larger modifying complexes in transcription level and post-transcription level [44].
Modulating DNA methylation or histone modification enzyme
Epigenetic modification is recognized as a stable genetic phenotype caused by chromosome changes without changing the DNA sequence [45]. DNA methylation and histone modification, the two most studied epigenetic modifications, are reversible processes and dynamically regulated [46]. Numerous lncRNAs have been reported to work with the DNA methylation or histone modification enzymes to mediate gene transcriptional events in tumorigenesis [12,47–56] (Fig. 2, Table 2). Similar to the ‘association with transcriptional factor’ mechanism, the lncRNAs in this category can also function as RNA guide, scaffold or decoy.
Figure 2.
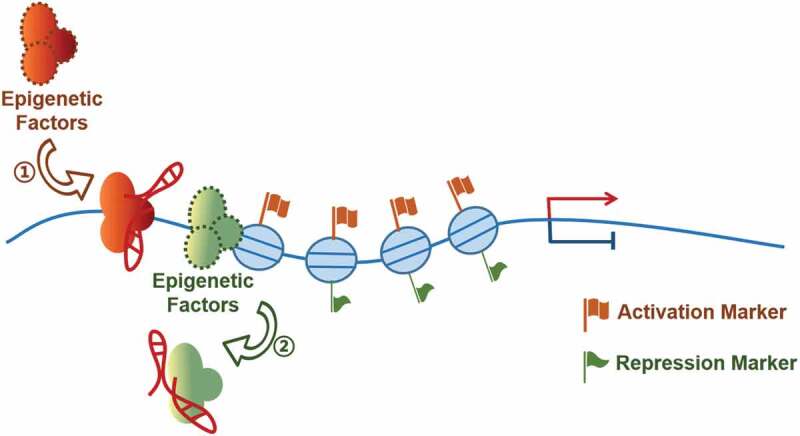
Modulating DNA methylation and histone modification enzymes.
lncRNAs mediate gene activation or repression through recruiting (1) or sequestering (2) epigenetic factors to influence the process of DNA methylation and/or histone modifications.
Table 2.
Modulating DNA methylation or histone modification enzyme.
| lncRNAs | Cancer type | Functions and phenotype | References |
|---|---|---|---|
| lnc-β-Catm | Liver cancer | lnc-β-Catm serves as a co-activator of EZH2 to mediate the methylation of β-catenin and activates Wnt–β-catenin signalling in liver cancer stem cells (CSCs). | [47] |
| BCAR4 | Breast cancer | Interacts with SNIP1 and releases the SNIP1’s inhibition of p300-dependent histone acetylation, it promotes H3K18 acetylation of the GLI2 target genes promoter. Besides, BCAR4 recruits PNUTS to interact with H3K18ac and disrupts inhibition of RNA Pol II, activating target genes of GLI2 expression, promoting tumour invasion and metastasis. | [53] |
| SANT1 | Renal cell carcinoma | Removes SFPQ/E2F1/HDAC1 suppressor complex from the promoter region and increases the acetylation of H3K27 to promote SLC47A2 expression | [55] |
| TARID | Non-small cell lung cancer, head and neck squamous cell carcinoma, ovarian cancer | Induces an R-loop formation at the TCF21 promoter, recruits GADD45A and TET1. It leads to demethylation of TCF21 promoter, activates the tumour suppressor TCF21 and inhibit tumour development. | [68,69] |
| EBIC | Cervical cancer | Recruits EZH2 to E-cadherin promoter, promotes cell metastasis and invasion | [72] |
| AGAP2-AS1 | Non-small cell lung cancer | recruits EZH2 and LSD1, represses tumour suppressors KLF2 and LATS2 transcription via H3K27me3, promotes cell proliferation and inhibits cell apoptosis. | [80] |
| APC | Liver cancer | Recruits EZH2 to APC promoter, represses the expression of APC and activates Wnt/β-catenin signalling to promote the self-renewal of TIC cells. | [81] |
| TUG1 | Non-small cell lung cancer, liver cancer, glioma | Recruits EZH2 to target genes’ promoters, inhibits genes’ expression and cancer development. It also acts as a scaffold to recruit EZH2 and YY1 to repress differentiation genes in glioma stem cells (GSCs) | [82–84] |
| LINC00152 | Gastric cancer | Binds to EZH2 and represses p15 and p21 transcription to accelerate the cell cycle and promote cell proliferation. | [85] |
| lncRNA-p21 | Prostate cancer | Switches the activity of EZH2 from histone-methyltransferase to non-histone methyltransferase, induces EZH2 methylating STAT3 to promote neuroendocrine differentiation (NED). | [86] |
| LNMAT1 | Bladder cancer | Recruits hnRNPL to CCL2 promoter to enhance the expression of CCL2 by increasing H3K4me3. It induces CCL2 recruiting macrophages into the tumour and promotes lymphatic metastasis. | [87] |
| EZR-AS1 | Human oesophageal squamous cell carcinoma | Recruits histone methyltransferase SMYD3 to a binding site that is presented in a GC-rich region downstream of the EZR promoter. It causes the local enrichment of H3K4me3, promotes cell proliferation and invasion. | [88] |
| LINC00473 | Breast cancer | Recruits phosphorylated CREB or FUS to CCND1 promoter, maintaining the expression levels of CCND1. It can promote cell proliferation. | [90] |
| BCAR4 | Breast cancer | Recruits GLI2 to promoters of HK2 and PFBFK3, promotes H3K27 acetylation, cell migration and invasion. | [91] |
| SATB2-AS1 | Colorectal carcinoma | Recruits p300, acetylates H3K27 and H3K9 at the SATB2 promoter for SATB2 activation. It suppresses cell proliferation, migration and invasion. | [56] |
Through DNA methylation
More than 24 million autosomal CpG sites exist on the human genome, of which, 60–90% are methylated [57–59]. DNA methylation is highly dynamic dependent on the cell type, physiological or pathophysiological condition, and developmental stage [60–64]. In mammalian somatic cells, the DNA methylation is presumably maintained by DNMT1. Recently, DNMT1 has been re-grouped as an RNA-binding protein [65–67]. Several DNMT1-associated lncRNAs have been reported [66,68,69]. Among which, ecCEBPA has been shown to regulate the activity of DNMT1 in a site-specific manner in leukaemia cells [66], suggesting a possibility that the site-specificity of DNA methylation may be tightly regulated by the DNMTs-associated RNAs. In addition, some lncRNAs have been reported to promote the demethylation event in cancer. For example, lncRNA TARID (TCF21 antisense RNA inducing promoter demethylation) has been initially found to facilitate the demethylation of the tumour suppressor TCF21 promoter through GADD45A and thymine-DNA glycosylase [68]. Recently, the activation of TARID has been further shown to be able to induce an R-loop formation at the TCF21 promoter. The R-loop triggers the recruitment of GADD45A and TET1 to the TCF21 promoter and results in the activation of TCF21 [69].
Through histone modification
Abundant research articles have linked lncRNAs with histone modification. (1) Histone methylation: Interestingly, although the regulatory mechanisms are diverse at molecular level, about 11–20% of the reported lncRNAs achieved their roles via associating with polycomb repressive complexes, including PCR1 and PRC2 [10,70]. PRC2, consisting of enhancer of zeste homolog 2 (EZH2), suppressor of zeste 12 (SUZ12), and embryonic ectoderm development (EED) [68], methylates the 27th lysine of histone H3 to inhibit the transcription of the target genes. (i) Promoting H3K27me3: Many PRC1/PRC2-associated lncRNAs can serve as RNA guide or scaffold molecule to recruit PCR2 complex by interacting with EZH2 directly and promote H3K27me3 [28,71–79]. For instance, the tumour up-regulated lncRNA AGAP2-AS1 functions as a transcriptional repressor of the tumour suppressors KLF2 and LATS2 by recruiting EZH2 and LSD1 to the promoter regions of these two tumour suppressors, and repressing their transcription via H3K27me3 in non-small-cell lung cancer (NSCLC) cells [80]; LncAPC, located near APC locus, recruits EZH2 to APC promoter, represses the expression of APC and activates Wnt/β-catenin signalling to promote the self-renewal of TIC cells [81]; LncRNA-EBIC promotes the metastasis and invasion of cervical cancer cells by recruiting EZH2 to E-cadherin promoter [72]; lncRNA TUG1, a tumour suppressor regulated by p53 in NSCLC, recruits EZH2 to inhibit the expression of HOXB7 [82]; TUG1 regulates the downstream gene KLF2 through EZH2 recruitment to the promoter of KLF2 in liver cancer [83] or acts as a scaffold to recruit EZH2 and YY1 to repress differentiation genes in glioma stem cells (GSCs) [84]; LINC00152 overexpression facilitates cell proliferation through accelerating the cell cycle by binding to EZH2 and repressing p15 and p21 transcription in gastric cancer cells [85]. Intriguingly, a couple of reports have showed that lncRNA can also mediate the non-histone methylation activity of the PRC2 complex. Enzalutamide (Enz) is an anti-tumour drug that may extend the castration-resistant prostate cancer (CRPC) patients’ survival up to an extra 4.8 months. However, Enz might also result in some adverse effects via inducing the neuroendocrine differentiation (NED). Luo J et al. found that lncRNA-p21 is an Enz-induced lncRNA and is able to interact with EZH2 [86]. Upregulation of lncRNA-p21 switches the activity of EZH2 from histone-methyltransferase to non-histone methyltransferase. As a result, EZH2 methylated STAT3 to promote NED. These results further support the earlier identification by Zhu P et al. that lnc-β-Catm serves as a co-activator of EZH2 to mediate the methylation of non-histone substrate. The lnc-β-Catm-mediated methylation of β-catenin can suppress the ubiquitination of β-catenin, and promote the latter’s stability to result in an activation of Wnt–β-catenin signalling in liver cancer stem cells (CSCs) [47]. (ii) Promoting histone modifications other than H3K27me3: Compared with those PRC complexes-associated lncRNAs, relatively less reports have showed that lncRNAs can mediate other histone methylations [50,52,87–89]. For example, lncRNA LNMAT1 recruits hnRNPL to CCL2 promoter to enhance the expression of CCL2 by increasing H3K4me3. The resulted up-regulation of CCL2 recruits macrophages into the tumour and promotes lymphatic metastasis [87]. In human oesophageal squamous cell carcinoma (ESCC) cells, lncRNA EZR-AS1 promotes cell proliferation and invasion. Mechanistically, EZR-AS1 recruits histone methyltransferase SMYD3 to a binding site that is presented in a GC-rich region downstream of the EZR promoter, which causes the local enrichment of H3K4me3. In addition, EZR-AS1 can also interact with RNA polymerase II to promote EZR transcription [88], a mechanism that will be discussed later.(2) Histone acetylation: Histone acetylation is widely accepted as an important epigenetic marker for gene activation. A large amount of lncRNAs have been reported to be involved in mediating histone acetylation as well [40,53,55,56,89–91]. For example, ncRNA transcripts derived from the 5ʹ-regualtory regions of the cyclinD1/CCND1 (ncRNACCND1) were found to have the capacity to interact with the RNA-binding protein TLS upon DNA damage signal. The lncRNA:TLS association induces an allosteric modulation of TLS and induces the repressive activity of TLS on the histone acetyltransferases to inhibit the expression of cyclinD1 in Hela cells [89] . Recently, Shi X et al. further discovered that the regulatory role of ncRNACCND1 is controlled by an upstream lncRNA molecule LINC00473 in breast cancer cells [90]. In breast cancer, lncRNA BCAR4 is responsible for GLI2-controlled gene activation. After being induced by CCL21, BCAR4 interacts with SNIP1 and releases the SNIP1’s inhibition of p300-dependent histone acetylation. By CIT/GLI2/SNIP1/p300 signal axis, BCAR4 promotes H3K18 acetylation of the GLI2 target gene promoter. Moreover, BCAR4 recruits PNUTS to interact with H3K18ac and disrupts inhibition of RNA Pol II, activating target genes of GLI2 expression, promoting tumour invasion and metastasis [53]. lncRNA BCAR4 also responses to Hippo-Yap signal during tumour metabolism. BCAR4 is activated by Hippo-Yap signal, and up-regulated BCAR4 recruits GLI2 to the promoter of HK2 and PFBFK3, promoting H3K27 acetylation. Thereby BCAR4 is involved in the regulation of tumour metabolic microenvironment and promotes the migration and invasion of breast cancer [91]. LncRNA SATB2-AS1 is a suppressor to cell proliferation, migration and invasion in colorectal carcinoma. Mechanistically, SATB2-AS1 acts as a scaffold to recruit p300, acetylates H3K27 and H3K9 at the SATB2 promoter for SATB2 activation [56]. In renal cell carcinoma, lncRNA SANT1 in cis regulates SLC47A2 expression by removing SFPQ/E2F1/HDAC1 suppressor complex from the promoter region and increasing the acetylation of H3K27 to promoting SLC47A2 expression [55]. As having been briefly mentioned under the heading of ‘RNA decoy’, MALAT1 interacts with DCB1 directly and competes with SIRT1 for DCB1 binding, which results in a release of SIRT1 and enhances its deacetylation activity. Consequently, the deacetylation of p53 reduces the transcription activity of target genes, promotes cell proliferation and inhibits cell apoptosis [40].
Influencing on chromatin remodelling complex
Chromatin remodelling is an important mechanism regulating the transcription of eukaryotic genes, which switches the chromatin status between condensed and loose conditions. Chromatin remodelling complexes rely on hydrolysis of ATP to provide energy. Based on the type of subunits for hydrolysing ATP, chromatin remodelling complexes can be divided into three categories: SWI/SNF complex, ISW complex and other complexes. During tumorigenesis, some lncRNAs have been shown to be involved in the process of SWI/SNF-mediated chromatin remodelling events [92–97] (Table 3). For example, lncRNA LncTCF7 is highly expressed in CSC cells. It activates TCF7 by recruiting the SWI/SNF complex to the promoter of TCF7, which triggers the Wnt signalling pathway to increase CSC self-renewal and cause cancer recurrence [96] (Fig. 3A). Conversely, some SWI/SNF-associated lncRNAs function through molecule decoy mechanism (Fig. 3B). As an example of this type of lncRNAs, lncRNA SChLAP1 antagonizes the genome-wide localization and regulatory functions of the SWI/SNF chromatin-modifying complex. Particularly, SChLAP1 represses the expression of the PTEN gene and promotes the process of tumorigenesis in prostate cancer cells through a direct association with SNF5, a subunit of SWI/SNF complex [97]. Other factors such as INO80 can also be induced by lncRNAs [98–104]. For instance, lncHand2-AS1 recruits INO80 chromatin remodelling complex to the promoter region of BMP signalling receptor BMPR1A, promotes the expression of BMPR1A, activates BMP signalling pathway, and maintains self-renewal of liver cancer stem cells [104].
Table 3.
Influencing on chromatin remodelling complex.
| lncRNAs | Cancer type | Functions and phenotype | References |
|---|---|---|---|
| LncTCF7 | Liver cancer | Recruits SWI/SNF complex to the promoter of TCF7, triggers Wnt signalling pathway to increase CSC self-renewal and cause cancer recurrence. | [96] |
| SChLAP1 | Pprostate cancer | Interacts with SWI/SNF complex, represses the expression of PTEN gene and promotes the process of tumorigenesis in prostate cancer cells. | [97] |
| lncHand2-AS1 | Liver cancer | Recruits INO80 chromatin remodelling complex to the promoter region of BMP signalling receptor BMPR1A, promotes the expression of BMPR1A, activates BMP signalling pathway, and maintains self-renewal of liver cancer stem cells. | [104] |
Figure 3.
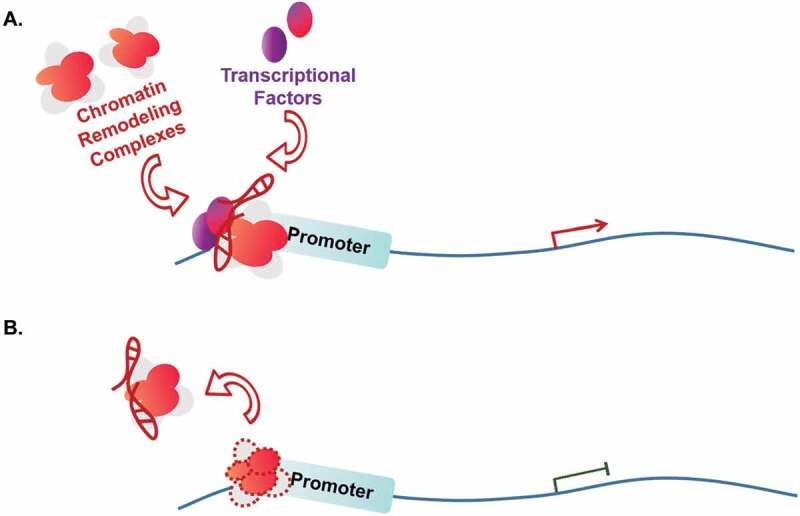
Influencing on chromatin remodelling complex.
(a) lncRNAs can recruit chromatin remodelling complexes such as SWI/SNF, ISW, INO80 to the target gene’s promoter, resulting in an alteration of chromatin structure that enables tightly condensed DNA to be accessed by transcription factors and gene activation. (b.) Conversely, other lncRNAs can sequester chromatin remodelling complexes from the targeting cis elements to trigger gene repression.
Facilitating chromosomal looping
The spatial organization of the human genome plays an important role in gene transcription [105–107]. In eukaryotes, gene expression depends on the interaction between transcription factors and DNA, which can be regulated by changing the three-dimensional (3D) conformations of chromatin [108]. The chromosomal looping may trigger reversible interaction between enhancer and promoter of distal genes [109] (Fig. 4). Recently, some lncRNAs or the act of transcription from the lncRNA loci have been suggested to actively direct the formation of specific nuclear conformations, including XIST in X chromosome inactivation, NEAT1 in forming paraspeckle [110–113] and FIRRE [114,115]. Studies from lncRNA CCAT1-L, IRAIN and Khps1 further indicate that cancer-associated lncRNAs may utilize a similar mechanism to affect the transcriptional output of the downstream genes and tumorigenesis [116–118] (Table 4). LncRNA CCAT1-L is transcribed from an enhancer region located at 515 kb upstream of MYC. In human colorectal cancer cells, CCAT1-L promotes the long-range chromatin looping between the MYC enhancers and promoter to regulate MYC expression. Through interaction with CTCF, CCAT1-L modulates the structural information of the surrounding chromatin near the resulted looping domains [119]. LncRNA IRAIN interacts with chromatin DNA, forming an intra-chromosomal enhancer/promoter loop to down-regulate the expression of IGF1R in acute myeloid leukaemia (AML) cells [116]. LncRNA Khps1 acts as an oncogenic molecule by up-regulating its deriving gene SPHK1’s expression [118]. At the molecular level, Khps1 forms an RNA-DNA triplex via a homopurine stretch upstream of the transcription start site of SPHK1, recruits p300/CBP and E2H1 to the SPHK1 promoter, and mediate the chromatin structure change of the target regions. Overall, these lncRNAs act as a transcriptional regulator by localizing to target loci, attracting regulatory proteins or chromatin modifiers, and shaping 3D nuclear organization (Fig. 4).
Figure 4.
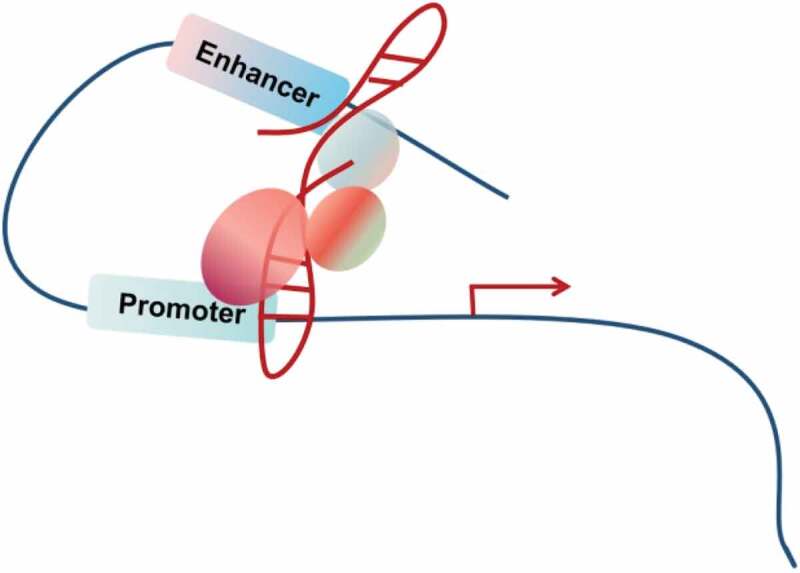
Facilitating chromosomal looping.
lncRNAs helps to form enhancer:promoter looping to activate the transcription of the target genes.
Table 4.
Facilitating chromosomal looping.
| lncRNAs | Cancer type | Functions and phenotype | References |
|---|---|---|---|
| IRAIN | acute myeloid leukaemia | Interacts with chromatin DNA, forming an intra-chromosomal enhancer/promoter loop to down-regulate the expression of IGF1R. | [116] |
| Khps1 | Osteosarcoma | Forms a RNA-DNA triplexes via a homopurine stretch upstream of the transcription start site of SPHK1, recruits p300/CBP and E2H1 to the SPHK1 promoter, and mediate the chromatin structure change of the target regions. It acts as an oncogenic molecule by up-regulating its deriving gene SPHK1’s expression. | [118] |
| CCAT1-L | Colorectal cancer | Forms chromatin looping between the MYC enhancer and promoter, recruits CTCF to regulate MYC expression. | [119] |
Interaction with RNA polymerase
RNA polymerases catalyse the transcription of DNA to synthesize precursors of mRNAs and ncRNAs [120]. As the two most studied polymerases in eukaryotic cells, Pol I is responsible for the synthesis of ribosomal RNA, while RNA Pol II is responsible for most of the pre-mRNA and lncRNAs synthesis [121]. Taken polymerase II as an example, it is a 550-kDa complex, composed of 12 subunits. A broad range of transcription factors are required for the association of polymerase and promoter. They help the construction of RNA polymerase complex on the gene’s promoter during initiation and in the process of transcription. In addition, chromatin structure, regulated by chromatin structure-oriented factors (e.g. histone modifiers), is also linked with RNA polymerase II-recorded transcription. Therefore, the above discussed lncRNAs through regulating transcriptional modulators and chromosomal looping, although not been proven, are likely to have an influence on polymerases. Here, we will summarize those lncRNAs that have been reported to participate in gene transcription by influencing polymerase (Table 5), and synthesize the recent findings into two main categories (Fig. 5): direct influence and indirect influence.
Table 5.
Interaction with RNA polymerase.
| lncRNAs | Cancer type | Functions and phenotype | References |
|---|---|---|---|
| SLERT | Teratoma | Interacts with single DDX21 molecule to adjust the size of the DDX21 ring and relieve the inhibition of pol I, activates pol I -mediated transcription initiation directly and promote tumorgenesis. | [122] |
| lncRNA AY | Hepatocellular carcinoma | Interacts with histone 1FX (H1FX) to the ITGAV promoter, enhances modification of H3K4Me3 and acH3K9/14 but reduces H3K27Me3 modification and H1FX occupation on ITGAV promoter. This causes a change of chromatin structure and pol II recruitment to target gene promoter for gene activation. | [125] |
| NRCP | Ovarian cancer | Recruits both TF (STAT1) and RNA polymerase II as an intermediate to target gene’s promoter. The complex then increases the expression of downstream genes including glucose-6-phosphate isomerase to modulate cancer metabolism and promote ovarian cancer development. | [128] |
Figure 5.
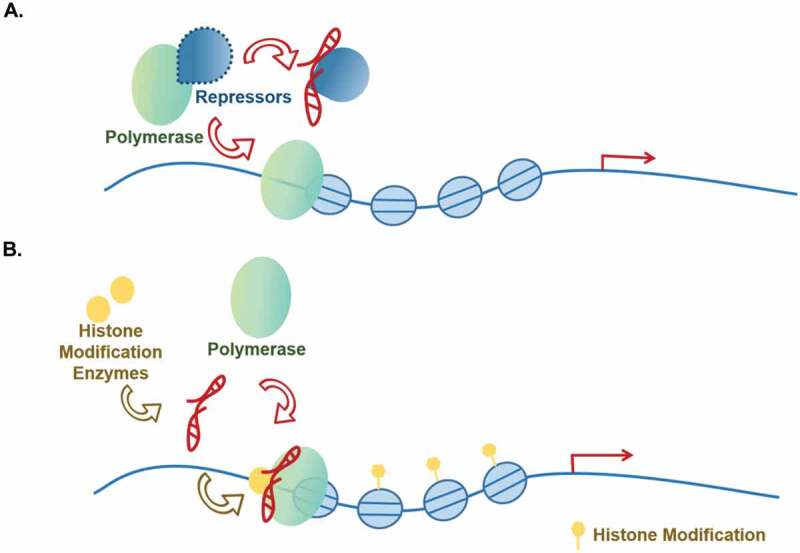
Associated with polymerases.
lncRNAs participate in gene transcription by influencing the function of polymerases through direct (a) or indirect (b) manner.
Direct regulation
In the case of direct regulation, lncRNAs can alter the structure of the protein complexes and deactivate the inhibition of the complexes in RNA polymerase-mediated transcription initiation, exampled by lncRNA SLERT (Fig. 5A). Human cells contain about 400 copies of ribosomal DNA (rDNA) sequences, but only half can be converted into rRNA. Recently, a new mechanism has been reported to control rRNA transcriptional differences by lncRNA SLERT-DDX21 looping structure and Pol ӏ transcriptional activation. Under normal physiological conditions, the RNA helicase DDX21, which is present in the nucleolus, forms a circular structure around RNA polymerase I. This circular structure traps RNA polymerase I, and its ‘encirclement’ size directly affects RNA pol I transcriptional activity. In cancer cells, the conformation of DDX21 is changed by up-regulated lncRNA SLERT. SLERT can interact with single DDX21 molecule to adjust the size of the DDX21 ring and relieve the inhibition of pol I. Thereby, SLERT can activate pol I-mediated transcription initiation directly and promote tumorgenesis [122]. Indirect regulation: lncRNAs that infulence polymerases indirectly, on the other hand, recruit histone modification enzymes and alter chromatin structure to affect the RNA polymerases’ binding to target genes’ promoters [88,123–125] (Fig. 5B). lncRNA AY [125] and EZR-AS1 [88] belong to this category. In hepatocellular carcinoma, lncRNA AY promotes transcription of ITGAV and the expression of αVβ3 to induce tumour metastasis by specifically interacting with the promoter of ITGAV and stimulating its activity. AY interacts with histone 1FX (H1FX) through the latter’s central domain (371–522) to the ITGAV promoter, resulting in enhanced modification of H3K4Me3 and acH3K9/14 but reducing H3K27Me3 and H1FX occupation on ITGAV promoter. This causes a change of chromatin structure and pol II recruitment to target gene promoter for gene activation [125]. EZR-AS1 functions through multiple mechanisms and have been discussed above under the heading ‘Promoting histone modifications other than H3K27me3’ [88]. In addition, some lncRNAs can also regulate gene transcription by acting as scaffolds to recruit TF or histone modification enzyme and RNA polymerase together to downstream gene’s promoter, activating gene transcription [73,126–128]. For instance, in ovarian cancer, lncRNA NRCP recruits both TF (STAT1) and RNA polymerase II as an intermediate to target gene’s promoter. The complex then increases the expression of downstream genes including glucose-6-phosphate isomerase to modulate cancer metabolism and promote ovarian cancer development [128].
Direct association with promoter
Some lncRNAs can directly bind the target gene’s promoter to influence transcription [129–136] (Fig. 6, Table 6). Currently, lncRNAs fallen in this category take up a very small proportion among the reported lncRNAs and the underlying mechanisms of how RNA:DNA association affects transcription is largely unclear. Recently, a study from Engreitz JM and colleagues [136] suggests that the lncRNA:promoter association may affect the loading or activity of the neighbouring transcriptional factors. In this work, the investigated lncRNA is initially named as MIR205HG, for being derived from the host gene of miR-205. Interestingly, in human prostate basal cells, MIR205HG is capable of regulating cell differentiation through an autonomous role from miR-205 and therefore re-annotated as LEADeR (for Long Epithelial Alu-interacting Differentiation-related RNA) based on its molecular function. Mechanistically, LEADeR directly binds to the Alu elements, which are located at the proximity of the Interferon-Regulatory Factor (IRF) binding site on the LEADeR target genes’ promoters. This results in a transcriptional repression of the target gene possibly via buffering the activity of IRF1 on the promoter. Several other lncRNAs have also been suggested to have the capacity of direct association with promoter [129–135]. For example, ARHGAP5-AS1 is a natural antisense transcript (NAT) derived from ARHGAP5 and associated with autophagy [129]. Autophagy is a catabolic process that captures and degrades damaged proteins and organelles in lysosomes. Under normal physiological conditions, the activity of autophagy is at a low basal level to sustain cellular homoeostasis [137]. Changes of autophagy activity can lead to a variety of disorders including metabolic disease, neurodegenerative disease, and infectious disease [138]. Recently, it has been found that numerous cancer cell lines have a high level of autophagy activity, which is necessary to meet elevated metabolic demand and allow cell survival in vitro and tumorigenesis in vivo [137]. ARHGAP5-AS1 is up-regulated when autophagy is inhibited in chemoresistant gastric cancer cells. In vitro and in vivo experiments demonstrate that SQSTN directly binds and recruits ARHGAP5-AS1 for autophagy degradation. ARHGAP5-AS1 interacts with ARHGAP5 promoter directly to promote the transcriptional expression of ARHGAP5. In addition, ARHGAP5-AS1 can stabilize ARHGAP5 mRNA by enhancing the interaction of ARHGAP5 mRNA with Hur and recruiting METTL3 to mediate M6A methylation of ARHGAP5 mRNA [129].
Figure 6.
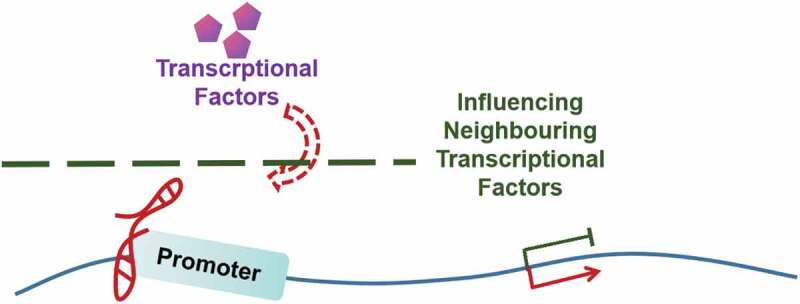
Direct association with promoter.
An lncRNA binds to the target gene promoter directly and influences gene transcription likely through influencing the neighbouring transcriptional factor’s loading or activity.
Table 6.
Direct association with promoter.
| lncRNAs | Cancer type | Functions and phenotype | References |
|---|---|---|---|
| ARHGAP5-AS1 | Gastric cancer | ARHGAP5-AS1 interacts with ARHGAP5 promoter directly to promote the transcriptional expression of ARHGAP5. It promotes autophagy activity and allows cell survival in vitro and tumorigenesis in vivo. | [129] |
| LEADeR (MIR205HG) |
Prostate cancer | Directly binds to the Alu elements, leads to a transcriptional repression of the target gene possibly via buffering the activity of IRF1 on the promoter. | [136] |
Conclusion
Propelled by the HGP and ENCODE Programs, the discovery of lncRNAs as an entire new class of ncRNAs adds a rich layer to the complexity of the human genome. It has become clear that many lncRNAs are critical regulators in almost all the tested physiological and pathophysiological events. There are several databases that contain comprehensive information on disease-related lncRNAs, such as DIANA-LncBase [139], LncRNADisease [140,141], LNCipedia [142], and LncRNAWiki [143]. Recently, researchers have developed the Lnc2Cancer database (http://www.bio-bigdata.net/lnc2cancer) [144,145], which is a manually curated database that provides experimentally associations between lncRNAs and cancers. Up to October 2019, the Lnc2Cancer database has included 1057 associations between 531 lncRNAs and 86 human cancers. With the development of chromatin isolation by RNA purification (CHIRP), capture hybridization analysis of RNA targets (CHART), as well as the combined utilization with mass-spectrometry and next-generation sequencing, researchers have more technical tools to investigate the mechanism of lncRNA molecules. In this review, we discussed the nucleus-expressed lncRNAs and their roles in cancer-related transcriptional regulation events. Although around 55% of the tested lncRNAs are found to be located in the nucleus [146], still an abundant portion of lncRNAs are found to be expressed in the cytoplasm or asscociated with cell membranes. It has become clearer now that besides transcriptional controlling, lncRNAs participate in genomic stability, post-transcriptional RNA processing events, protein modifications, and metabolisms through interacting with other types of RNA molecules and proteins in cancer development [147–161]. lncRNAs are becoming hot targets for developing diagnostic markers and therapeutic targets for human cancers.
Acknowledgments
We apologize to researchers whose work was not able to be included in this review due to space limitations. Research in the X. Wang laboratory is funded by the Ministry of Science and Technology of China (2016YFA0100502), National Natural Science Foundation of China (31970598, 31671306), the Fundamental Research Funds for the Central Universities (YD2070002010) and the Major/Innovative Program of Development Foundation of Hefei Center for Physical Science and Technology (2018CXFX006).
Funding Statement
This work was supported by the National Natural Science Foundation of China [31970598]; The Ministry of Science and Technology of China [2016YFA0100502]; The Fundamental Research Funds for the Central Universities [YD2070002010]; The Major/Innovative Program of Development Foundation of Hefei Center for Physical Science and Technology [2018CXFX006].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Consortium, E. P. The ENCODE (ENCyclopedia Of DNA Elements) Project. Science. 2004;306:636–640. [DOI] [PubMed] [Google Scholar]
- [2].Maurano MT, Humbert R, Rynes E, et al. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337:1190–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Iyer MK, Niknafs YS, Malik R, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sanchez Y, Huarte M.. Long non-coding RNAs: challenges for diagnosis and therapies. Nucleic Acid Ther. 2013;23:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. [DOI] [PubMed] [Google Scholar]
- [6].Sigova AA, Abraham BJ, Ji X, et al. Transcription factor trapping by RNA in gene regulatory elements. Science. 2015;350:978–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang KC, Yang YW, Liu B, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hung T, Wang Y, Lin MF, et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet. 2011;43:621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yang L, Lin C, Jin C, et al. lncRNA-dependent mechanisms of androgen-receptor-regulated gene activation programs. Nature. 2013;500:598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tsai MC, Manor O, Wan Y, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sun -T-T, He J, Liang Q, et al. LncRNA GClnc1 promotes gastric carcinogenesis and May act as a modular scaffold of WDR5 and KAT2A complexes to specify the histone modification pattern. Cancer Discov. 2016;6:784–801. [DOI] [PubMed] [Google Scholar]
- [13].Zhang H, Zhang N, Liu Y, et al. Epigenetic regulation of NAMPT by NAMPT-AS drives metastatic progression in triple-negative breast cancer. Cancer Res. 2019;79:3347–3359. [DOI] [PubMed] [Google Scholar]
- [14].Sakai S, Ohhata T, Kitagawa K, et al. Long noncoding RNA ELIT-1 Acts as a Smad3 cofactor to facilitate TGFβ/Smad signaling and promote Epithelial–mesenchymal TRansition. Cancer Res. 2019;79:2821–2838. [DOI] [PubMed] [Google Scholar]
- [15].Ma R, Zhai X, Zhu X, et al. LINC01585 functions as a regulator of gene expression by the CAMP/CREB signaling pathway in breast cancer. Gene. 2019;684:139–148. [DOI] [PubMed] [Google Scholar]
- [16].Pavlaki I, Alammari F, Sun B, et al. The long non-coding RNA Paupar promotes KAP1-dependent chromatin changes and regulates olfactory bulb neurogenesis. Embo J. 2018;37. DOI: 10.15252/embj.201798219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lingadahalli S, Jadhao S, Sung YY, et al. Novel lncRNA LINC00844 regulates prostate cancer cell migration and invasion through AR signaling. Mol Cancer Res. 2018;16:1865–1878. [DOI] [PubMed] [Google Scholar]
- [18].Li Y, Li J, Luo M, et al. Novel long noncoding RNA NMR promotes tumor progression via NSUN2 and BPTF in esophageal squamous cell carcinoma. Cancer Lett. 2018;430:57–66. [DOI] [PubMed] [Google Scholar]
- [19].Xu Z, Yang F, Wei D, et al. Long noncoding RNA-SRLR elicits intrinsic sorafenib resistance via evoking IL-6/STAT3 axis in renal cell carcinoma. Oncogene. 2017;36:1965–1977. [DOI] [PubMed] [Google Scholar]
- [20].Luo G, Liu D, Huang C, et al. LncRNA GAS5 inhibits cellular proliferation by targeting P27(Kip1). Mol Cancer Res. 2017;15:789–799. [DOI] [PubMed] [Google Scholar]
- [21].Fernando TR, Contreras JR, Zampini M, et al. The lncRNA CASC15 regulates SOX4 expression in RUNX1-rearranged acute leukemia. Mol Cancer. 2017;16:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen Z.Z, Huang L, Wu Y-H, et al. LncSox4 promotes the self-renewal of liver tumour-initiating cells through Stat3-mediated Sox4 expression. Nat Commun. 2016;7:12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang E, He X, Zhang C, et al. A novel long noncoding RNA HOXC-AS3 mediates tumorigenesis of gastric cancer by binding to YBX1. Genome Biol. 2018;19:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sun Y, Wei G, Luo H, et al. The long noncoding RNA SNHG1 promotes tumor growth through regulating transcription of both local and distal genes. Oncogene. 2017;36:6774–6783. [DOI] [PubMed] [Google Scholar]
- [25].Liu Z, Chen Z, Fan R, et al. Over-expressed long noncoding RNA HOXA11-AS promotes cell cycle progression and metastasis in gastric cancer. Mol Cancer. 2017;16:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Puvvula PK, Desetty RD, Pineau P, et al. Long noncoding RNA PANDA and scaffold-attachment-factor SAFA control senescence entry and exit. Nat Commun. 2014;5:5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li Y, Wang Z, Shi H, et al. HBXIP and LSD1 scaffolded by lncRNA hotair mediate transcriptional activation by c-Myc. Cancer Res. 2016;76:293–304. [DOI] [PubMed] [Google Scholar]
- [28].Battistelli C, Cicchini C, Santangelo L, et al. The Snail repressor recruits EZH2 to specific genomic sites through the enrollment of the lncRNA HOTAIR in epithelial-to-mesenchymal transition. Oncogene. 2017;36:942–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Martinez-Moreno M, O’Shea TM, Zepecki JP, et al. Regulation of peripheral myelination through transcriptional buffering of Egr2 by an antisense long non-coding RNA. Cell Rep. 2017;20:1950–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hua JT, Ahmed M, Guo H, et al. Risk SNP-mediated promoter-enhancer switching drives prostate cancer through lncRNA PCAT19. Cell. 2018;174:564–575 e518. [DOI] [PubMed] [Google Scholar]
- [31].Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kogo R, Shimamura T, Mimori K, et al. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6326. [DOI] [PubMed] [Google Scholar]
- [33].Jiang Y, Jiang YY, Xie JJ, et al. Co-activation of super-enhancer-driven CCAT1 by TP63 and SOX2 promotes squamous cancer progression. Nat Commun. 2018;9:3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Schmidt K, Carroll JS, Yee E, et al. The lncRNA SLNCR recruits the androgen receptor to EGR1-bound genes in melanoma and inhibits expression of tumor suppressor p21. Cell Rep. 2019;27:2493-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Guo H, Ahmed M, Zhang F, et al. Modulation of long noncoding RNAs by risk SNPs underlying genetic predispositions to prostate cancer. Nat Genet. 2016;48:1142–1150. [DOI] [PubMed] [Google Scholar]
- [36].Liu N, Liu Q, Yang X, et al. Hepatitis B virus-upregulated LNC-HUR1 promotes cell proliferation and tumorigenesis by blocking p53 activity. Hepatology. 2018;68:2130–2144. [DOI] [PubMed] [Google Scholar]
- [37].Kim J, Piao H-L, Kim B-J, et al. Long noncoding RNA MALAT1 suppresses breast cancer metastasis. Nat Genet. 2018;50:1705–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Garabedian MJ, Logan SK. Glucocorticoid receptor DNA binding decoy is a gas. Sci Signal. 2010;3:pe5. [DOI] [PubMed] [Google Scholar]
- [39].Mourtadamaarabouni M, Pickard MR, Hedge VL, et al. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene. 2009;28:195–208. [DOI] [PubMed] [Google Scholar]
- [40].Chen R, Liu Y, Zhuang H, et al. Quantitative proteomics reveals that long non-coding RNA MALAT1 interacts with DBC1 to regulate p53 acetylation. Nucleic Acids Res. 2017;45:9947–9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kung JT, Colognori D, Lee JT. Long noncoding RNAs: past, present, and future. Genetics. 2013;193:651–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chen LJTIBS. Linking long noncoding RNA localization and function. Trends Biochem Sci. 2016;41:761–772. [DOI] [PubMed] [Google Scholar]
- [44].Sun W, Yang Y, Xu C, et al. Regulatory mechanisms of long noncoding RNAs on gene expression in cancers. Cancer Genet. 2017;216–217:105–110. [DOI] [PubMed] [Google Scholar]
- [45].Berger SL, Kouzarides T, Shiekhattar R, et al. An operational definition of epigenetics. Genes Dev. 2009;23:781–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhang H, Tian XJ, Mukhopadhyay A, et al. Statistical mechanics model for the dynamics of collective epigenetic histone modification. Phys Rev Lett. 2014;112:068101. [DOI] [PubMed] [Google Scholar]
- [47].Zhu P, Wang Y, Huang G, et al. lnc-beta-Catm elicits EZH2-dependent beta-catenin stabilization and sustains liver CSC self-renewal. Nat Struct Mol Biol. 2016;23:631–639. [DOI] [PubMed] [Google Scholar]
- [48].Gao Y, Wang T, Li Y, et al. Lnc-chop promotes immunosuppressive function of myeloid-derived suppressor cells in tumor and inflammatory environments. J Immunol. 2018;200:2603–2614. [DOI] [PubMed] [Google Scholar]
- [49].Tang J, Xie Y, Xu X, et al. Bidirectional transcription of Linc00441 and RB1 via H3K27 modification-dependent way promotes hepatocellular carcinoma. Cell Death Dis. 2017;8:e2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yang MH, Zhao L, Wang L, et al. Nuclear lncRNA HOXD-AS1 suppresses colorectal carcinoma growth and metastasis via inhibiting HOXD3-induced integrin 3 transcriptional activating and MAPK/AKT signalling. Mol Cancer. 2019;18. DOI: 10.1186/s12943-019-0955-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Su W, Xu M, Chen X, et al. Long noncoding RNA ZEB1-AS1 epigenetically regulates the expressions of ZEB1 and downstream molecules in prostate cancer. Mol Cancer. 2017;16:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Terashima M, Ishimura A, Wanna-Udom S, et al. MEG8 long noncoding RNA contributes to epigenetic progression of the epithelial-mesenchymal transition of lung and pancreatic cancer cells. J Biol Chem. 2018;293:18016–18030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Xing Z, Lin A, Li C, et al. lncRNA directs cooperative epigenetic regulation downstream of chemokine signals. Cell. 2014;159:1110–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Dong H, Wang W, Mo S, et al. SP1-induced lncRNA AGAP2-AS1 expression promotes chemoresistance of breast cancer by epigenetic regulation of MyD88. J Exp Clin Cancer Res. 2018;37:202. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [55].Gao Z, Chen M, Tian X, et al. A novel human lncRNA SANT1 cis-regulates the expression of SLC47A2 by altering SFPQ/E2F1/HDAC1 binding to the promoter region in renal cell carcinoma. RNA Biol. 2019;16:940–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wang YQ, Jiang DM, Hu SS, et al. SATB2-AS1 Suppresses colorectal carcinoma aggressiveness by inhibiting SATB2-dependent snail transcription and epithelial-mesenchymal transition. Cancer Res. 2019;79:3542–3556. [DOI] [PubMed] [Google Scholar]
- [57].Komaki S, Shiwa Y, Furukawa R, et al. iMETHYL: an integrative database of human DNA methylation, gene expression, and genomic variation. Hum Genome Var. 2018;5:18008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Xiao C, Gao L, Hou Y, et al. Chromatin-remodelling factor Brg1 regulates myocardial proliferation and regeneration in zebrafish. Nat Commun. 2016;7:13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ziller MJ, Gu H, Müller F, et al. Charting a dynamic DNA methylation landscape of the human genome. Nature. 2013;500:477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Duan L, Liu Y, Wang J, et al. The dynamic changes of DNA methylation in primordial germ cell differentiation. Gene. 2016;591:305–312. [DOI] [PubMed] [Google Scholar]
- [61].Liu XS, Wu H, Ji X, et al. Editing DNA methylation in the mammalian genome. Cell. 2016;167:233–247 e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Miyazaki K, Mapendano CK, Fuchigami T, et al. Developmentally dynamic changes of DNA methylation in the mouse Snurf/Snrpn gene. Gene. 2009;432:97–101. [DOI] [PubMed] [Google Scholar]
- [63].Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38:23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wu H, Zhang Y. Reversing DNA methylation: mechanisms, genomics, and biological functions. Cell. 2014;156:45–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Chalei V, Sansom SN, Kong L, et al. The long non-coding RNA Dali is an epigenetic regulator of neural differentiation. Elife. 2014;3:e04530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Di Ruscio A, Ebralidze AK, Benoukraf T, et al. DNMT1-interacting RNAs block gene-specific DNA methylation. Nature. 2013;503:371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wang L, Zhao Y, Bao X, et al. LncRNA Dum interacts with Dnmts to regulate Dppa2 expression during myogenic differentiation and muscle regeneration. Cell Res. 2015;25:335–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Arab K, Park Y, Lindroth A, et al. Long noncoding RNA TARID directs demethylation and activation of the tumor suppressor TCF21 via GADD45A. Mol Cell. 2014;55:604–614. [DOI] [PubMed] [Google Scholar]
- [69].Arab K, Karaulanov E, Musheev M, et al. GADD45A binds R-loops and recruits TET1 to CpG island promoters. Nat Genet. 2019;51:217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Khalil AM, Guttman M, Huarte M, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106:11667–11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Yang F, Zhang L, Huo X-S, et al. Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology. 2011;54:1679–1689. [DOI] [PubMed] [Google Scholar]
- [72].Sun NX, Ye C, Zhao Q, et al. Long noncoding RNA-EBIC promotes tumor cell invasion by binding to EZH2 and repressing E-cadherin in cervical cancer. PLoS One. 2014;9:e100340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Melo CA, Léveillé N, Rooijers K, et al. A p53-bound enhancer region controls a long intergenic noncoding RNA required for p53 stress response. Oncogene. 2016;35:4399–4406. [DOI] [PubMed] [Google Scholar]
- [74].Yang XJ, Huang CQ, Peng CW, et al. Long noncoding RNA HULC promotes colorectal carcinoma progression through epigenetically repressing NKD2 expression. Gene. 2016;592:172–178. [DOI] [PubMed] [Google Scholar]
- [75].Portoso M, Ragazzini R, Brenčič Ž, et al. PRC2 is dispensable for HOTAIR-mediated transcriptional repression. Embo J. 2017;36:981–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].He W, Cai Q, Sun F, et al. linc-UBC1 physically associates with polycomb repressive complex 2 (PRC2) and acts as a negative prognostic factor for lymph node metastasis and survival in bladder cancer. Biochim Biophys Acta. 2013;1832:1528–1537. [DOI] [PubMed] [Google Scholar]
- [77].Marin-Bejar O, Mas AM, González J, et al. The human lncRNA LINC-PINT inhibits tumor cell invasion through a highly conserved sequence element. Genome Biol. 2017;18:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Li JK, Chen C, Liu J-Y, et al. Long noncoding RNA MRCCAT1 promotes metastasis of clear cell renal cell carcinoma via inhibiting NPR3 and activating p38-MAPK signaling. Mol Cancer. 2017;16:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Jin L, He Y, Tang S, et al. LncRNA GHET1 predicts poor prognosis in hepatocellular carcinoma and promotes cell proliferation by silencing KLF2. J Cell Physiol. 2018;233:4726–4734. [DOI] [PubMed] [Google Scholar]
- [80].Li W, Sun M, Zang C, et al. Upregulated long non-coding RNA AGAP2-AS1 represses LATS2 and KLF2 expression through interacting with EZH2 and LSD1 in non-small-cell lung cancer cells. Cell Death Dis. 2016;7:e2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Fu X, Lin J, Qin F, et al. LncAPC drives Wnt/β-catenin activation and liver TIC self-renewal through EZH2 mediated APC transcriptional inhibition. Mol Carcinog. 2018;57:408–418. [DOI] [PubMed] [Google Scholar]
- [82].Zhang EB, Yin -D-D, Sun M, et al. P53-regulated long non-coding RNA TUG1 affects cell proliferation in human non-small cell lung cancer, partly through epigenetically regulating HOXB7 expression. Cell Death Dis. 2014;5:e1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Huang MD, Chen W-M, Qi F-Z, et al. Long non-coding RNA TUG1 is up-regulated in hepatocellular carcinoma and promotes cell growth and apoptosis by epigenetically silencing of KLF2. Mol Cancer. 2015;14:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Katsushima K, Natsume A, Ohka F, et al. Targeting the Notch-regulated non-coding RNA TUG1 for glioma treatment. Nat Commun. 2016;7:13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Chen QN, Chen X, Chen Z-Y, et al. Long intergenic non-coding RNA 00152 promotes lung adenocarcinoma proliferation via interacting with EZH2 and repressing IL24 expression. Mol Cancer. 2017;16:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Luo J, Wang K, Yeh S, et al. LncRNA-p21 alters the antiandrogen enzalutamide-induced prostate cancer neuroendocrine differentiation via modulating the EZH2/STAT3 signaling. Nat Commun. 2019;10:2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Chen C, He W, Huang J, et al. LNMAT1 promotes lymphatic metastasis of bladder cancer via CCL2 dependent macrophage recruitment. Nat Commun. 2018;9:3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zhang XD, Huang G-W, Xie Y-H, et al. The interaction of lncRNA EZR-AS1 with SMYD3 maintains overexpression of EZR in ESCC cells. Nucleic Acids Res. 2018;46:1793–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Wang X, Arai S, Song X, et al. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature. 2008;454:126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Shi XM, Wang XT. LINC00473 mediates cyclin D1 expression through a balance between activation and repression signals in breast cancer cells. Febs Lett. 2019;593:751–759. [DOI] [PubMed] [Google Scholar]
- [91].Zheng X, Han H, Liu G-P, et al. LncRNA wires up Hippo and Hedgehog signaling to reprogramme glucose metabolism. Embo J. 2017;36:3325–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Tang Y, Wang J, Lian Y, et al. Linking long non-coding RNAs and SWI/SNF complexes to chromatin remodeling in cancer. Mol Cancer. 2017;16:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Lee RS, Roberts CW. Linking the SWI/SNF complex to prostate cancer. Nat Genet. 2013;45:1268–1269. [DOI] [PubMed] [Google Scholar]
- [94].Chen Z, Gao Y, Yao L, et al. LncFZD6 initiates Wnt/beta-catenin and liver TIC self-renewal through BRG1-mediated FZD6 transcriptional activation. Oncogene. 2018;37:3098–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Wang WY, Wang YF, Ma P, et al. Taurineupregulated gene 1: A vital long noncoding RNA associated with cancer in humans (Review). Mol Med Rep. 2017;16:6467–6471. [DOI] [PubMed] [Google Scholar]
- [96].Wang Y, He L, Du Y, et al. The long noncoding RNA lncTCF7 promotes self-renewal of human liver cancer stem cells through activation of Wnt signaling. Cell Stem Cell. 2015;16:413–425. [DOI] [PubMed] [Google Scholar]
- [97].Prensner JR, Iyer MK, Sahu A, et al. The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nat Genet. 2013;45:1392–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Liu T, Han Z, Li H, et al. LncRNA DLEU1 contributes to colorectal cancer progression via activation of KPNA3. Mol Cancer. 2018;17:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Shao M, Yang Q, Zhu W, et al. LncHOXA10 drives liver TICs self-renewal and tumorigenesis via HOXA10 transcription activation. Mol Cancer. 2018;17:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Wang X, GONG Y, JIN BO, et al. Long non-coding RNA urothelial carcinoma associated 1 induces cell replication by inhibiting BRG1 in 5637 cells. Oncol Rep. 2014;32:1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Fang C, He W, Xu T, et al. Upregulation of lncRNA DGCR5 correlates with better prognosis and inhibits bladder cancer progression via transcriptionally facilitating P21 expression. J Cell Physiol. 2019;234:6254–6262. [DOI] [PubMed] [Google Scholar]
- [102].Huang G, Jiang H, Lin Y, et al. LncGPR107 drives the self-renewal of liver tumor initiating cells and liver tumorigenesis through GPR107-dependent manner. J Exp Clin Cancer Res. 2018;37:121. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [103].Zhu P, Wu J, Wang Y, et al. LncGata6 maintains stemness of intestinal stem cells and promotes intestinal tumorigenesis. Nat Cell Biol. 2018;20:1134–1144. [DOI] [PubMed] [Google Scholar]
- [104].Wang Y, Zhu P, Luo J, et al. LncRNA HAND2-AS1 promotes liver cancer stem cell self-renewal via BMP signaling. Embo J. 2019;e101110. DOI: 10.15252/embj.2018101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Cremer T, Cremer C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet. 2001;2:292–301. [DOI] [PubMed] [Google Scholar]
- [106].Sexton T, Schober H, Fraser P, et al. Gene regulation through nuclear organization. Nat Struct Mol Biol. 2007;14:1049–1055. [DOI] [PubMed] [Google Scholar]
- [107].Bickmore WA. The spatial organization of the human genome. Annu Rev Genomics Hum Genet. 2013;14:67–84. [DOI] [PubMed] [Google Scholar]
- [108].Cantone I, Fisher AG. Epigenetic programming and reprogramming during development. Nat Struct Mol Biol. 2013;20:282–289. [DOI] [PubMed] [Google Scholar]
- [109].Debruyne DN, Dries R, Sengupta S, et al. BORIS promotes chromatin regulatory interactions in treatment-resistant cancer cells. Nature. 2019;572:676–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].West JA, Davis C, Sunwoo H, et al. The long noncoding RNAs NEAT1 and MALAT1 bind active chromatin sites. Mol Cell. 2014;55:791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Clemson CM, Hutchinson JN, Sara SA, et al. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol Cell. 2009;33:717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Imamura K, Imamachi N, Akizuki G, et al. Long noncoding RNA NEAT1-dependent SFPQ relocation from promoter region to paraspeckle mediates IL8 expression upon immune stimuli. Mol Cell. 2014;53:393–406. [DOI] [PubMed] [Google Scholar]
- [113].Mao YS, Sunwoo H, Zhang B, et al. Direct visualization of the co-transcriptional assembly of a nuclear body by noncoding RNAs. Nat Cell Biol. 2011;13:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Hacisuleyman E, Goff LA, Trapnell C, et al. Topological organization of multichromosomal regions by the long intergenic noncoding RNA Firre. Nat Struct Mol Biol. 2014;21:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Hacisuleyman E, Shukla CJ, Weiner CL, et al. Function and evolution of local repeats in the Firre locus. Nat Commun. 2016;7:11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Sun J, Li W, Sun Y, et al. A novel antisense long noncoding RNA within the IGF1R gene locus is imprinted in hematopoietic malignancies. Nucleic Acids Res. 2014;42:9588–9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wang XQD, Dostie J. Reciprocal regulation of chromatin state and architecture by HOTAIRM1 contributes to temporal collinear HOXA gene activation. Nucleic Acids Res. 2017;45:1091–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Postepska-Igielska A, Giwojna A, Gasri-Plotnitsky L, et al. LncRNA Khps1 regulates expression of the proto-oncogene SPHK1 via triplex-mediated changes in chromatin structure. Mol Cell. 2015;60:626–636. [DOI] [PubMed] [Google Scholar]
- [119].Younger ST, Rinn JL. ‘Lnc’-ing enhancers to MYC regulation. Cell Res. 2014;24:643–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Roeder RG, Rutter WJ. Specific nucleolar and nucleoplasmic RNA polymerases. Proc Natl Acad Sci U S A. 1970;65:675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Cramer P. Multisubunit RNA polymerases. Curr Opin Struct Biol. 2002;12:89–97. [DOI] [PubMed] [Google Scholar]
- [122].Xing YH, Yao R-W, Zhang Y, et al. SLERT regulates DDX21 rings associated with Pol I Transcription. Cell. 2017;169:664–678 e616. [DOI] [PubMed] [Google Scholar]
- [123].Pan W, Zhang N, Liu W, et al. The long noncoding RNA GAS8-AS1 suppresses hepatocarcinogenesis by epigenetically activating the tumor suppressor GAS8. J Biol Chem. 2018;293:17154–17165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Krawczyk M, Emerson BM. p50-associated COX-2 extragenic RNA (PACER) activates COX-2 gene expression by occluding repressive NF-kappaB complexes. Elife. 2014;3:e01776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kang CL, Qi B, Cai QQ, et al. LncRNA AY promotes hepatocellular carcinoma metastasis by stimulating ITGAV transcription. Theranostics. 2019;9:4421–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Zhu XX, Liu Y, Yu J, et al. LncRNA HOXA-AS2 represses endothelium inflammation by regulating the activity of NF-kappa B signaling. Atherosclerosis. 2019;281:38–46. [DOI] [PubMed] [Google Scholar]
- [127].Huang G, Jiang H, He Y, et al. LncMAPK6 drives MAPK6 expression and liver TIC self-renewal. J Exp Clin Cancer Res. 2018;37:105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [128].Rupaimoole R, Lee J, Haemmerle M, et al. Long noncoding RNA ceruloplasmin promotes cancer growth by altering glycolysis. Cell Rep. 2015;13:2395–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Zhu L, Zhu Y, Han S, et al. Impaired autophagic degradation of lncRNA ARHGAP5-AS1 promotes chemoresistance in gastric cancer. Cell Death Dis. 2019;10:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Su K, Zhao Q, Bian A, et al. A novel positive feedback regulation between long noncoding RNA UICC and IL-6/STAT3 signaling promotes cervical cancer progression. Am J Cancer Res. 2018;8:1176–1189. [PMC free article] [PubMed] [Google Scholar]
- [131].Sun QM, Hu B, Fu P-Y, et al. Long non-coding RNA 00607 as a tumor suppressor by modulating NF-kappaB p65/p53 signaling axis in hepatocellular carcinoma. Carcinogenesis. 2018;39:1438–1446. [DOI] [PubMed] [Google Scholar]
- [132].Wang J, Zhou J, Jiang C, et al. LNRRIL6, a novel long non-coding RNA, protects colorectal cancer cells by activating the IL-6-STAT3 pathway. Mol Oncol. 2019;13:2344–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Atmadibrata B, Liu PY, Sokolowski N, et al. The novel long noncoding RNA linc00467 promotes cell survival but is down-regulated by N-Myc. Plos One. 2014;9:e88112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Su H, Liu L, Zhang Y, et al. Long noncoding RNA NPCCAT1 promotes nasopharyngeal carcinoma progression via upregulating YY1. Biochimie. 2019;157:184–194. [DOI] [PubMed] [Google Scholar]
- [135].Ding LJ, Li Y, Wang S-D, et al. Long noncoding RNA lncCAMTA1 promotes proliferation and cancer stem cell-like properties of liver cancer by inhibiting CAMTA1. Int J Mol Sci. 2016;17:1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Profumo V, Forte B, Percio S, et al. LEADeR role of miR-205 host gene as long noncoding RNA in prostate basal cell differentiation. Nat Commun. 2019;10:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Poillet-Perez L, White E. Role of tumor and host autophagy in cancer metabolism. Genes Dev. 2019;33:610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Mizushima N, Levine B, Cuervo AM, et al. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Paraskevopoulou MD, Georgakilas G, Kostoulas N, et al. DIANA-LncBase: experimentally verified and computationally predicted microRNA targets on long non-coding RNAs. Nucleic Acids Res. 2013;41:D239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Chen G, Wang Z, Wang D, et al. LncRNADisease: a database for long-non-coding RNA-associated diseases. Nucleic Acids Res. 2013;41:D983–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Bao Z, Yang Z, Huang Z, et al. LncRNADisease 2.0: an updated database of long non-coding RNA-associated diseases. Nucleic Acids Res. 2019;47:D1034–D1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Volders PJ, Verheggen K, Menschaert G, et al. An update on LNCipedia: a database for annotated human lncRNA sequences. Nucleic Acids Res. 2015;43:4363–4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Ma L, Li A, Zou D, et al. LncRNAWiki: harnessing community knowledge in collaborative curation of human long non-coding RNAs. Nucleic Acids Res. 2015;43:D187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Ning S, Zhang J, Wang P, et al. Lnc2Cancer: a manually curated database of experimentally supported lncRNAs associated with various human cancers. Nucleic Acids Res. 2016;44:D980–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Gao Y, Wang P, Wang Y, et al. Lnc2Cancer v2.0: updated database of experimentally supported long non-coding RNAs in human cancers. Nucleic Acids Res. 2019;47:D1028–D1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Cabili MN, Dunagin MC, McClanahan PD, et al. Localization and abundance analysis of human lncRNAs at single-cell and single-molecule resolution. Genome Biol. 2015;16. DOI: 10.1186/s13059-015-0586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Lee S, Kopp F, Chang T-C, et al. Noncoding RNA NORAD regulates genomic stability by sequestering PUMILIO proteins. Cell. 2016;164:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Shahabi S, Kumaran V, Castillo J, et al. LINC00261 is an epigenetically regulated tumor suppressor essential for activation of the DNA damage response. Cancer Res. 2019;79:3050–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Li D, Chen Y, Mei H, et al. Ets-1 promoter-associated noncoding RNA regulates the NONO/ERG/Ets-1 axis to drive gastric cancer progression. Oncogene. 2018;37:4871–4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Klingenberg M, Groß M, Goyal A, et al. The long noncoding RNA cancer susceptibility 9 and RNA binding protein heterogeneous nuclear ribonucleoprotein l form a complex and coregulate genes linked to AKT signaling. Hepatology. 2018;68:1817–1832. [DOI] [PubMed] [Google Scholar]
- [151].Zhang WY, Liu YJ, He Y, et al. Suppression of long noncoding RNA NCK1-AS1 increases chemosensitivity to cisplatin in cervical cancer. J Cell Physiol. 2019;234:4302–4313. [DOI] [PubMed] [Google Scholar]
- [152].Yu T, Zhao Y, Hu Z, et al. MetaLnc9 facilitates lung cancer metastasis via a PGK1-activated AKT/mTOR pathway. Cancer Res. 2017;77:5782–5794. [DOI] [PubMed] [Google Scholar]
- [153].Lu Z, Li Y, Che Y, et al. The TGFbeta-induced lncRNA TBILA promotes non-small cell lung cancer progression in vitro and in vivo via cis-regulating HGAL and activating S100A7/JAB1 signaling. Cancer Lett. 2018;432:156–168. [DOI] [PubMed] [Google Scholar]
- [154].Huang JF, Guo Y-J, Zhao C-X, et al. Hepatitis B virus X protein (HBx)-related long noncoding RNA (lncRNA) down-regulated expression by HBx (Dreh) inhibits hepatocellular carcinoma metastasis by targeting the intermediate filament protein vimentin. Hepatology. 2013;57:1882–1892. [DOI] [PubMed] [Google Scholar]
- [155].Liu B, Sun L, Liu Q, et al. A cytoplasmic NF-kappaB interacting long noncoding RNA blocks IkappaB phosphorylation and suppresses breast cancer metastasis. Cancer Cell. 2015;27:370–381. [DOI] [PubMed] [Google Scholar]
- [156].Yuan JH, Yang F, Wang F, et al. A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666–681. [DOI] [PubMed] [Google Scholar]
- [157].Yuan JH, Liu X-N, Wang -T-T, et al. The MBNL3 splicing factor promotes hepatocellular carcinoma by increasing PXN expression through the alternative splicing of lncRNA-PXN-AS1. Nat Cell Biol. 2017;19:820–832. [DOI] [PubMed] [Google Scholar]
- [158].Evdokimova V, Ruzanov P, Anglesio MS, et al. Akt-mediated YB-1 phosphorylation activates translation of silent mRNA species. Mol Cell Biol. 2006;26:277–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Carpenter S, Aiello D, Atianand MK, et al. A long noncoding RNA mediates both activation and repression of immune response genes. Science. 2013;341:789–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Rapicavoli NA, Qu K, Zhang J, et al. A mammalian pseudogene lncRNA at the interface of inflammation and anti-inflammatory therapeutics. Elife. 2013;2:e00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Bi X, Xu Y, Li T, et al. RNA targets ribogenesis factor WDR43 to chromatin for transcription and pluripotency control. Mol Cell. 2019;75:102–116 e109. [DOI] [PubMed] [Google Scholar]


