Polymer-MOF hybrid enables simultaneous and uninterrupted sorption and release of atmospheric water.
Abstract
The atmosphere contains an abundance of fresh water, but this resource has yet to be harvested efficiently. To date, passive atmospheric water sorbents have required a desorption step that relies on steady solar irradiation. Since the availability and intensity of solar radiation vary, these limit on-demand desorption and hence the amount of harvestable water. Here, we report a polymer–metal-organic framework that provides simultaneous and uninterrupted sorption and release of atmospheric water. The adaptable nature of the hydro-active polymer, and its hybridization with a metal-organic framework, enables enhanced sorption kinetics, water uptake, and spontaneous water oozing. We demonstrate continuous water delivery for 1440 hours, producing 6 g of fresh water per gram of sorbent at 90% relative humidity (RH) per day without active condensation. This leads to a total liquid delivery efficiency of 95% and an autonomous liquid delivery efficiency of 71%, the record among reported atmospheric water harvesters.
INTRODUCTION
Atmospheric water is a sustainable source for freshwater supply that replenishes continuously via the global hydrological cycle (1). With a capacity of about 1018 liters in the form of moisture and suspended water droplets (e.g., fog and clouds), the atmosphere offers a ubiquitous yet generally overlooked source for potable water (2–4). Typical air-to-water capture technologies, such as fog (5, 6) and dew water collection (7, 8), enable decentralized water collection and onsite production. However, so far, energy intensity, production intermittency, and climatic constraints (e.g., temperature, wind, and humidity) have imposed extra costs on the generation of clean water (9–11). Substantial fluctuations in wind speed and direction stand in the way of efficient fog water collection. For dew water collection, there is a trade-off between energy input and water yield; for example, passive dew water collectors rely on radiative cooling, making their applications and water yields restricted by climatic conditions, while using active condensers can produce higher yields under a wider range of climatic conditions but at a high energy penalty [650 to 850 watt-hour (electricity) per kilogram of water] (9, 12).
With the advancement of materials design, in the past few years, desiccant-based atmospheric water extraction has received renewed attention. This method uses liquid or solid desiccants [e.g., metal-organic frameworks (MOFs) and hygroscopic solutions] to capture water from humid air in the atmosphere during nighttime and desorb/evaporate water in a closed container during daytime, resulting in an increased vapor pressure within the container and subsequent water condensation (13–18). Using photothermic materials and sunlight with this technology boosts the passive collection of atmospheric water. Nevertheless, the overall water collection rate is still bounded by the sluggish kinetics of sorption-desorption, the availability of sunlight only during the day, the need for iterative sorption-desorption steps, and the need to open and close the water collection chamber during sorption-desorption cycles.
In this work, we report an atmospheric water collection process based on polymer-MOF (PC-MOF) mixed-matrix materials. These collect water spontaneously and efficiently at minimum external energy expenditure without ancillary evaporators/condensers (table S1). The tunable hydrophilic chain of the PC-MOF matrix enables us to design for enhanced water uptake and reversible hydrophilic-hydrophobic transitions. These, in turn, enable autonomous water release and stand-alone airborne water supply without moving parts (Fig. 1). Continuous sorption-desorption is maintained through the direct release of weakly bound water clusters to support the uninterrupted regeneration of hydro-active sites and to further the sorption process—a requirement for a self-sustained water uptake-and-delivery system. We report self-sustained water production that delivers water at 6.39 g g−1 at 90% RH per day, of which 95% (6.04 g g−1) leads to direct water collection, and only 5%, 0.35 g g−1, is solar desorbed. The synchronous water uptake by the PC-MOF matrix provides a continuous water supply that is independent of the number of solar-assisted adsorption-desorption cycles.
Fig. 1. Design of the autonomous airborne water supplier.
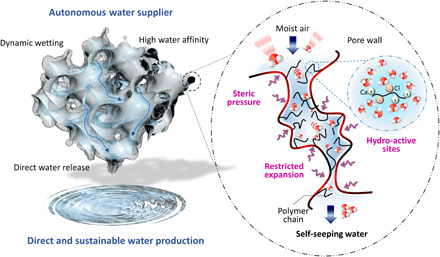
Schematic illustration shows the hydro-active sites on polymer chains that capture atmospheric moisture. Under steric pressure and restricted expansion, the polymer-MOF hybrid pore enables self-seepage for direct water harvesting from ambient.
RESULTS
Design and characterization of the PC-MOF airborne water supplier
The PC-MOF design uses a nonswelling cross-linked polymer to enable restricted volumetric transition and steric pressure. The activation of this polymer via ionic grafting improves the moisture harvesting affinity, while the hybridization of this polymer with a MOF accelerates the moisture harvesting rate. These design principles enable water coalescence and transport within the MOF-polymer pores, resulting in autonomous water collection.
MIL-101(Cr) is a MOF having remarkable water stability (i.e., over months under air and weeks in boiling water), high equilibrium water uptake [i.e., >1.5 g g−1 above 2.4 kPa (p/p0 = 0.57) at 30°C], and fast sorption kinetics, making it a promising candidate for atmospheric water harvesting (19–21). It has a zeotype structure with mesoporous cages (diameters of 29 and 34 Å) and microporous windows (diameters up to 16 Å) accessible to water molecules (Fig. 2A) (21, 22). MIL-101(Cr) nanoparticles (figs. S1 and S2) were incorporated as fillers into the PNIPAM [poly(N-isopropylacrylamide)] matrix to combine the superior water sorption of MOF with the dynamic conformational water repelling feature of PNIPAM. A porous P-MOF mixed-matrix structure is realized through in situ free-radical polymerization and cross-linking (Fig. 2B and figs. S3 to S5). MIL-101(Cr) nanoparticles show good adhesion to the polymer matrix without obvious interfacial voids, a finding that we assign to rich chemical functionality of the polymer matrix (isopropyl and amide groups) and the filler nanoparticles (organic ligand and coordinatively unsaturated sites) (20, 23).
Fig. 2. Fabrication and characterization of the PC-MOF.
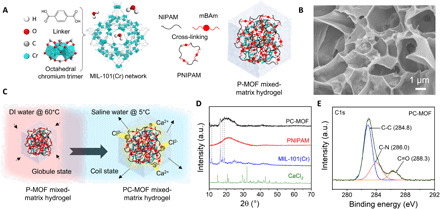
(A) Units and crystal structure of MIL-101(Cr), schematic cross-linking process of the NIPAM monomer by the mBAm cross-linker, and schematic preparation of the P-MOF mixed-matrix hydrogel. (B) SEM image of the freeze-dried P-MOF mixed-matrix hydrogel. (C) Salinization process of the P-MOF mixed-matrix hydrogel to the PC-MOF mixed-matrix hydrogel. (D) XRD patterns of the MIL-101(Cr), PNIPAM, CaCl2, and PC-MOF. (E) High-resolution C1s XPS spectrum of the PC-MOF. a.u., arbitrary units.
To further improve the water sorption properties of the P-MOF, hydro-active sorption sites with high water affinity were ionically grafted by leveraging the dynamic conformational change of PNIPAM chains at its lower critical solution temperature (LCST) (~32°C) and interaction of the polymer with CaCl2 (Fig. 2C) (24, 25). P-MOF hydrogel-water interaction was minimized by collapsing the polymer chains into the globular state at 60°C, inducing a hydrophobic characteristic accompanied by intramolecular hydrogen bond formation (C═O···H─N) (fig. S6) (26). The globule-to-coil state transformation was then initiated by immersing the collapsed P-MOF gel in cold saline solution (5°C) to unlock the functional polymer chains to enable intermolecular interaction. This simultaneously activates the salinization of P-MOF to PC-MOF.
The x-ray diffraction (XRD) pattern of the PC-MOF (Fig. 2D) exhibits the peak characteristic of MIL-101(Cr) and a broad peak corresponding to amorphous PNIPAM (fig. S7) (22). During the salinization process, the characteristic amide and carbonyl groups of thermo-responsive PNIPAM are preserved. X-ray photoelectron spectroscopy (XPS) of the PC-MOF for C1s (Fig. 2E) and N1s (fig. S8) core levels further prove the chemical structures (14, 27).
Airborne water uptake and direct water collection
Atmospheric water uptake and collection in the PC-MOF is realized through two simultaneous processes: (i) direct water release and (ii) water retention (Fig. 3A). When the PC-MOF is exposed to a humid atmosphere, water vapor adsorbed on the pore surface saturates, condenses, and exudes as the gravitational force and surface energy overcome the coalescence of water. The wet gel releases water while confining a small amount of the strongly coordinated water molecules within the pores.
Fig. 3. Atmospheric water uptake and direct release performance of the PC-MOF.
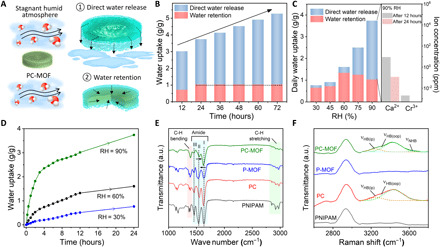
(A) Schematic illustration of the water harvesting processes; ① direct water release and ② water retention. (B) Continuous water uptake performance of the PC-MOF for 72 hours at 90% RH. (C) Left: Water uptake mechanism, and performance of the PC-MOF under various RH environments after 24 hours. Right: Measured concentrations of Ca2+ and Cr3+ ions in the water collected after 12 and 24 hours of continuous sorption and direct release process at 90% RH. (D) Water uptake rate of the PC-MOF at RHs of 30, 60, and 90%. (E) FTIR spectra of the PNIPAM, PC, P-MOF, and PC-MOF. (F) Raman spectra of the PNIPAM, PC, P-MOF, and PC-MOF after 1-hour adsorption at 90% RH. All moisture sorption experiments were conducted at 25°C.
MIL-101(Cr) nanoparticles loading and CaCl2 concentration in PC-MOF mixed-matrix aerogels were optimized to prepare a self-standing three-dimensional network with high water uptake (figs. S9 to S11). The optimized PC-MOF harvests a total water amount of 3.01 g g−1 after 12 hours of sorption at 90% RH, which consists of 2.31 g g−1 directly released water and 0.70 g g−1 confined water (Fig. 3B). After 72 hours of water harvesting at 90% RH, water retention is 1.02 g g−1, and the passively collected water amount reaches 4.25 g g−1. This is efficient compared with pristine MOFs and hygroscopic materials that require extra energy to evaporate, condense, and collect the captured moisture (16, 17, 28–31). Direct water release is the predominant process at environmental humidity values higher than 60% RH, while, at RH ≤ 60%, water retention increases with increasing humidity up to 1.3 g g−1 at 60% RH (Fig. 3C).
In addition to the promising water uptake and direct release attributes of the sorbent, another essential factor is the quality of the collected water; it must not be contaminated by the ionic and polymeric impurities released from the PC-MOF. Liquid water collected from the PC-MOF was analyzed by inductively coupled plasma mass spectrometry (Fig. 3C) and Fourier transform infrared (FTIR) spectroscopy (fig. S12). The polymer impurity was not detected, and Ca2+ and Cr3+ ion concentrations are below the level of drinking water standard defined by the World Health Organization (32).
Kinetic analysis was conducted to elucidate the water uptake dynamics of the PC-MOF at different RHs (Fig. 3D). At 30 and 60% RH, water uptake gradually increases through sorption process under the effect of moisture adsorption and confinement. At 90% RH, fast uptake is realized within the first 3 hours with a sorption rate of 0.72 g g−1 hour−1. The PC-MOF is capable of maintaining the uptake with a slower yet linear profile after 3 hours that is formed by the coinvolvement of direct water release and the water retention processes (fig. S13).
To investigate the changes in the microenvironment with salinization, FTIR (Fig. 3E and fig. S14) and XPS (fig. S15) measurements were conducted. FTIR spectra display characteristic C─H bending, C─H stretching, and amide bands over the frequency range of 1000 to 3000 cm−1 (25). The amide I band (1635 cm−1) shifts to a lower wave number upon interaction of the coiled polymer chain (PNIPAM and P-MOF) with the CaCl2 below LCST (5°C), which could be attributed to the binding of Ca+2 to the amide carbonyl oxygen (fig. S14) (33). On the other hand, anions (Cl−) tend to bind to the amide II moiety, which is reflected by the shift in amide II band peak position observed in Fig. 3E (33–35). These findings are further evidenced by XPS (fig. S15) (36–39).
To better elucidate the salinization-based functionalization, aerogel prepared by direct mixing of CaCl2 with P-MOF (denoted as PCD-MOF) was studied (fig. S16). In contrast to that of the PC-MOF, the XRD pattern of the PCD-MOF shows sharp crystalline peaks that match the patterns of CaCl2.6H2O. The PCD-MOF harvests 1.19 g g−1 atmospheric water in 12 hours at 90% RH through a water retention process, and liquid water is not released in the course of sorption. These results indicate that the excellent airborne water uptake and direct release properties of the PC-MOF originate from the hydro-active sorption sites of the chemically cross-linked polymer (fig. S17) (40–43).
The water structure on the sorption sites is confirmed by Raman spectroscopy (Fig. 3F). The PC and PC-MOF exhibit an intense O─H broad band around 3400 cm−1 after 1 hour of sorption under 90% RH (44–46). The O─H band of the PC is deconvolved into two components centered at 3255 and 3435 cm−1, which are associated with the in-phase [νHB(ip)] and out-of-phase [νHB(oop)] O─H stretching vibration modes of bound water in tetrahedral hydrogen (44).
Compared with that of the PC, the PC-MOF spectrum shows curve fittings with νHB(ip) (3245 cm−1) and νHB(oop) (3428 cm−1) at lower vibrational energies, and a new O─H mode appears at 3642 cm−1 that corresponds to free or weakly hydrogen-bonded water molecules (νNHB) in the form of partially and entirely broken water structure (44, 45). This reveals that the PC-MOF is capable of capturing water in fully and weakly hydrogen-bonded states in a short sorption period, corroborating its high water affinity and capability of generating water network (47).
Thermal activation of PC-MOF for complete water desorption
In addition to the passively collected water obtained through the direct release process, the detained water in the gel can also be optionally desorbed. Confined water molecules in the polymeric chain detach from the PC-MOF, nucleate in liquid form, and grow, migrate, and coalesce into larger clusters, forming a liquid layer on the surface at temperature >25°C (Fig. 4A and movie S1). We used atomic force microscopy (AFM) to examine the change in water thickness as a function of temperature (Fig. 4B and fig. S18) (48, 49). The PC-MOF displays a sharp increase in water thickness at 32°C, indicating that detained water is rapidly expelled at this particular temperature, which corresponds to the LCST of PNIPAM (24).
Fig. 4. Optional thermal activation of the PC-MOF for complete water desorption.
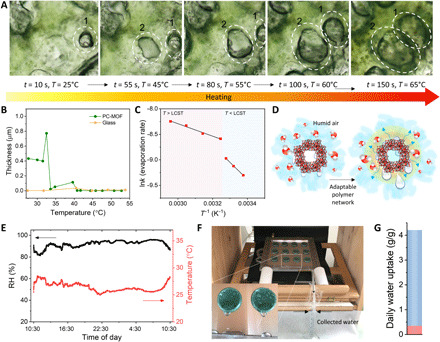
(A) Optical microscope images demonstrating the temperature-dependent dynamic phase separation behavior of the PC-MOF containing detained water. (B) The thickness of water layer on the PC-MOF surface at various temperatures as determined by the AFM force curve measurements. (C) Arrhenius plot representing the evaporation rate of detained water in PC-MOF as a function of temperature. (D) Schematic illustration of the water evaporation through photothermal effect. (E) The ambient temperature and humidity as functions of time during water harvesting. (F) Photograph of the proof-of-concept prototype for atmospheric water harvesting. (G) Water uptake performance of the PC-MOF; blue and pink portions represent direct water release and water retention, respectively. Photo credit: Connor Kangnuo Peh, National University of Singapore.
The stimuli response at LCST and water removal energy requirement were further verified using an Arrhenius plot (Fig. 4C and fig. S19). The water evaporation profile of the PC-MOF exhibits two regions divided at the LCST over the temperature range 23° to 65°C, resulting in different activation energy values below (27.7 kJ mol−1) and above (10.6 kJ mol−1) the LCST. Lower activation energy above LCST is attributed to the formation of a hydrophobic surface that facilitates the expulsion process of the weakly bound water molecules at the solid/liquid interface compared to the more strongly bound water molecules in the hydrophilic structure (Fig. 4D). The Arrhenius plot of the PC exhibits a similar dual trend with a similar activation energy above LCST (18.2 kJ mol−1), confirming that water removal is aided by the hydrophilic to hydrophobic phase transition (fig. S19). However, the activation energy requirement of the PC below the LCST (56.0 kJ mol−1) is higher than that of PC-MOF, suggesting that the MOF actively contributes to the desorption process, specifically below the LCST, where discharging of liquid water via the polymeric phase transition does not occur. Figure S20 shows that MIL-101(Cr) nanoparticles exhibit not only a fast desorption rate compared to PC but also a fast sorption rate, ultimately accelerating the sorption-desorption kinetics of PC-MOF when incorporated into the polymer (19).
An outdoor test was carried out by deploying a prototype that consists of an array of PC-MOF aerogels. The prototype was placed outdoors for 24 hours to achieve simultaneous uptake and release (Fig. 4, E and F, and movie S2). Thermal activation was subsequently applied to further collect detained water (fig. S21 and movie S3). The prototype is capable of harvesting 4.20 g g−1 water (3.07 liter m−2 day−1) and can attain a substantial water delivery along with thermal activation in 15 min, which is 92% of the total uptake (Fig. 4G and fig. S22).
Photothermal engineering and structural design
MIL-101(Cr) was loaded with Au nanoparticles and incorporated into the polymer (PCA-MOF) to realize the photothermal effect (fig. S23). PCA-MOF with a cone array geometry was also fabricated to realize directional migration of water droplets for accelerated removal and refreshing the sorbent’s surface by gravity effect (Fig. 5, A and B, and figs. S24 and S25). This, in turn, enables the rapid movement and coalescence of the seeped droplets and eases the release of accumulated ones at the cone tip (50, 51).
Fig. 5. Photothermal engineering and structural design.
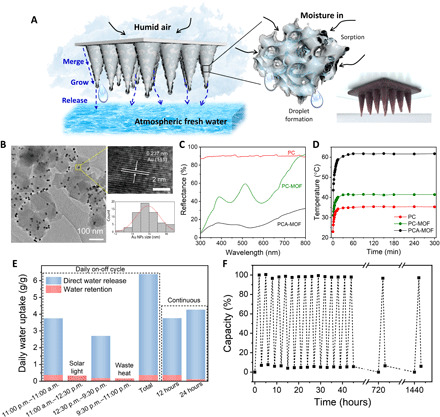
(A) Schematic illustration and digital image of the PCA-MOF cone array. (B) TEM image of the Au@MIL-101(Cr) nanoparticles. The enlarged image shows the HRTEM of Au nanoparticles, and the histogram shows the corresponding size distribution. (C) Reflectance spectra of the PC, PC-MOF, and PCA-MOF. (D) Temperature changes of the PC, PC-MOF, and PCA-MOF over time under 1 sun solar irradiation. (E) Daily on-off cycle: Daily water uptake performance of the PCA-MOF cone array using solar-assisted regeneration. Continuous: Total amount of water uptake after 12 hours (11:00 p.m. to 11:00 a.m.) and 24 hours (11:00 p.m. to 11:00 p.m., next day) without solar-assisted regeneration. (F) Water sorption (2 hours)–release (1 hour) cycles for the PCA-MOF. Photo credit: Gamze Yilmaz, National University of Singapore.
As seen in Fig. 5C, the PCA-MOF suppresses light reflectance compared to the PC and PC-MOF, and its temperature can rise up to 53°C from room temperature in 5 min under solar irradiation (Fig. 5D and fig. S26), indicating the excellent light-to-heat conversion capability. An all-day water harvesting process is carried out (Fig. 5E). From 11:00 p.m. to 11:00 a.m. (90% RH and 25°C), a water uptake of 3.74 g g−1 is attained with a remarkable direct water release that constitutes 90% of the total uptake (fig. S27). The total water uptake and directly released water amount obtained from the PCA-MOF cone array are 24 and 46% higher than that obtained from the regular circular design (Fig. 3B), respectively. From 12:30 p.m. to 9:30 p.m. (90% RH and 25°C), the desorbed PCA-MOF (fig. S28) yields 2.52 g g−1 by direct release at 90% RH. Altogether, in a daily cycle, the PCA-MOF cone array exhibits a water sorption capacity of 6.39 g g−1, of which a total of 6.04 g g−1 is collected as liquid water, while 0.35 g g−1 detained water is desorbed. Apart from the daily solar-assisted process, continuous water collection can also be carried out in areas where solar radiation is scarce (Fig. 5E, continuous). The liquid delivery performance of the PCA-MOF is evaluated by the total liquid delivery efficiency (TLDE) and autonomous liquid delivery efficiency (ALDE)
| (1) |
| (2) |
The PCA-MOF achieves a TLDE of 95% and an ALDE of 71% (note S1). The sorbent reusability was also tested by performing consecutive atmospheric water capturing-releasing cycles for 45 hours (Fig. 5F). Continuous cycling performance does not present any notable capacity loss as the gel performs well even after a prolonged time (1440 hours), indicating its potential in long-term operational stability (fig. S29).
DISCUSSION
In summary, we have demonstrated a self-sustained atmospheric water harvesting process enabled by polymer-MOF mixed-matrix membrane. The cross-linked polymer chain is grafted with hydro-active sites and integrated with a water-stable MOF that serves as a catalyst to accelerate the sorption/desorption kinetics and augment the water uptake. The gel exhibits a remarkable water uptake capability and direct water release property induced through the firm porous water pathways and reduced activation energy. These unique properties enable a continuous water collection at a rate of 4.16 g g−1 day−1, and 6.04 g g−1 liquid water is collected in a day at 90% RH by regenerating the sorbent. The as-presented stand-alone airborne water supplier gel offers a promising solution for achieving robust, sustainable, and decentralized water production in wideband climatic conditions at minimal energy cost.
MATERIALS AND METHODS
Preparation of PNIPAM aerogel
In a typical synthesis, N-isopropylacrylamide (NIPAM) monomer (0.75 g) and N,N′-methylenebisacrylamide (mBAm) cross-linker (38 mg) were dissolved in deinozed (DI) water (7.5 ml) and purged with N2 gas for 15 min by keeping the solution in an ice bath. Then, ammonium persulfate (23 mg, acts as the initiator) and N,N,N’,N’-tetramethylethylenediamine (TEMED) (45 μl, acts as the catalyst) were added into the purged solution to start the polymerization. The polymer solution was poured into a small petri dish with a size of 35 mm by 10 mm. The polymerization was allowed to proceed for 12 hours at 4°C. After the polymerization was complete, the PNIPAM hydrogel was washed with copious amount of DI water and freeze-dried to obtain the PNIPAM aerogel.
Synthesis of MIL-101(Cr)
The MIL-101(Cr) was synthesized through an alkaline-mediated route (22). Briefly, 5 ml of alkaline aqueous solution containing 400 mg of Cr(NO3)3.9H2O, 166 mg of H2BDC, and 0.1 ml of tetramethyl ammonium hydroxide [ρ: 1.016 g/ml; 25 weight % (wt %) in H2O] was added into a 25-ml autoclave reactor and kept at 180°C for 24 hours. After the reaction, the solution was cooled to room temperature and centrifuged to collect the green precipitate. For further activation and removal of linker impurities, the as-collected green precipitate was first dispersed in N,N′-dimethylformamide and kept for 2 hours at 120°C under stirring. Then, it was successively dispersed in ethanol at 60°C for 2 hours and water at 90°C for 2 hours under stirring. The collected material was dried under vacuum at room temperature for further use.
Preparation of P-MOF aerogel
MIL-101(Cr) (120 mg) was dispersed in 7.5 ml of DI before adding 750 mg of NIPAM and 38 mg of mBAm to prepare 16 wt % NIPAM/MIL-101(Cr) solution. The solution was then purged with N2 gas for 15 min in an ice bath. Ammonium persulfate (23 mg) and TEMED (45 μl) were added into the purged solution to start the polymerization. The polymer solution was poured into a small petri dish with a size of 35 mm by 10 mm. The polymerization was carried out for 12 hours at 4°C. Then, the polymerized PNIPAM/MIL-101(Cr) (P-MOF) hydrogel was washed with copious amount of DI water and freeze-dried to obtain the P-MOF aerogel.
Preparation of PC and PC-MOF aerogels
In a typical salinization treatment, PNIPAM or P-MOF hydrogel was first immersed into cold DI water (5°C) for 15 min to obtain polymeric hydrogel in coil conformation surrounded with water molecules. The hydrogel was then transferred into hot DI water (60°C) and kept for another 15 min to transform the polymer coins into globule state and squeeze out the bound and surrounding water molecules. This process was repeated three times. Subsequently, the hydrogel in globular conformation was quickly immersed into aqueous solution of CaCl2 (160 mg/ml) at 5°C and kept for 12 hours for interaction of the ions with the polymeric chain. To remove the unreacted CaCl2, the hydrogel was successively washed three times with copious amount of hot (60°C) (kept in for 15 min) and cold DI (5°C) (kept in for 15 min) water. Last, PC and PC-MOF hydrogels were freeze-dried to obtain the PC or PC-MOF aerogels, respectively.
Synthesis of Au nanoparticles
Au nanoparticles were prepared by a previously reported sodium citrate reduction method (51). In a typical procedure, aqueous solution of 150 ml of HAuCl4 (2.5 × 10−4 M) was heated to boiling under continuous stirring in an oil bath. After 5 min of boiling, aqueous solution of 4.5 ml of trisodium citrate (0.034 M) was added, and the resulting solution was kept in boiling state with continuous stirring. After 20 min, the reaction was stopped and cooled to room temperature. The as-cooled Au solution was then added dropwise into an aqueous solution of 20 ml of polyvinylpyrrolidone (PVP) (0.5 g, Mw = 55,000) with stirring, and stirring was continued for another 24 hours. Au nanoparticles were then centrifuged and washed three times with water at 14,000 rpm for 30 min. Last, the Au nanoparticles were dispersed in water.
Preparation of Au@MIL-101(Cr) nanoparticles
MIL-101(Cr) (120 mg) was dispersed in 18 ml of DI water and stirred at 45°C for 1 hour. Then, aqueous solution of 2 ml of Au nanoparticles (0.005 M) was added dropwise, and the solution was stirred at 45°C for another 8 hours. After the reaction, the solution was cooled to room temperature and centrifuged to collect the Au@MIL-101(Cr) nanoparticles. The collected material was dried under vacuum at room temperature for further use.
Preparation of PCA-MOF aerogel
The preparation of the PCA-MOF aerogel is similar to the PC-MOF aerogel preparation except for the use of Au@MIL-101(Cr) instead of MIL-101(Cr).
Preparation of PCD-MOF aerogel
CaCl2 (1.2 g) was dissolved in 7.5 ml of DI water before adding 120 mg of MIL-101(Cr), 750 mg of NIPAM, and 38 mg of mBAm. The solution was then purged with N2 gas for 15 min in an ice bath. Ammonium (23 mg) persulfate and 45 μl of TEMED were added into the purged solution to start the polymerization. The polymer solution was poured into a small petri dish with a size of 35 mm by 10 mm. The polymerization was carried out for 12 hours at 4°C. Then, the polymerized PCD-MOF hydrogel was washed with copious amount of DI water and freeze-dried to obtain the PCD-MOF aerogel.
Characterization
Field-emission scanning electron microscopy was performed on a JEOL FEG JSM-7001F, equipped with an Oxford/INCA EDS, to study the morphology of the materials. Optical microscope images were obtained with an Olympus BX53M (Olympus, Tokyo, Japan) microscope equipped with a homemade heating stage. Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images were recorded by a JEOL JEM-2010F transmission electron microscope to investigate the nanostructural morphology. The crystal structure was obtained by x-ray powder diffraction (XRD) by collecting the patterns using a diffractometer [General Area Detector Diffraction System (GADDS) XRD system, Bruker AXS] equipped with a CuKα radiation source (λ = 1.54 Å). XPS measurements were performed on a PHI Quantera x-ray photoelectron spectrometer with a monochromated Al Kα radiation. The XPS binding energies were calibrated using the C1s level of 284.5 eV. Nitrogen adsorption-desorption isotherms were obtained at 77.3 K using the (Quantachrome) NOVA-1200 System and pore size distribution was calculated by the Barrett-Joyner-Halenda method on the basis of desorption isotherm. FTIR spectroscopy was conducted on a Shimadzu IR Prestige-21 spectrophotometer. Raman spectrum was obtained using the Renishaw system coupled with a 532-nm excitation laser. Infrared (IR) images were captured using a FLIR E50 IR camera. The optical properties of the samples were analyzed using an ultraviolet (UV)–visible spectrophotometer (Shimadzu, UV-3600). Inductively coupled plasma optical emission spectrometry was used to determine the concentration ions, which was measured through iCAP 6000 Series (Thermo Fisher Scientific).
Water uptake and removal experiments
The moisture in the aerogels was evaporated at 100°C for 24 hours in an oven before the atmospheric water harvesting experiments. The mass of the dry aerogel is carefully recorded and put in a petri dish with known mass. The water uptake and direct release experiments were carried out by placing the petri dish with gel in an acrylic airtight humidity chamber, in which the relative humidity was controlled using HumiSys low flow humidity generator (InstruQuest Inc., USA) at 25°C. The weight of the gel in petri dish was measured by an analytical balance at certain time intervals. The released water amount was carefully determined by removing the gel from the petri dish and weighing the mass of the water remaining in the petri dish.
Outdoor experiment
The outdoor atmospheric water harvesting experiments were carried out using a proof-of-concept prototype at the National University of Singapore on 11 to 12 February 2020 from 10:30 a.m. (11 February) to 10:30 a.m. (12 February). The ambient temperature and humidity were recorded automatically using a TSI Q-TRAK IAQ monitor (Model 7575). Twelve pieces of PC-MOF aerogel disks were attached to the metal plate, and the device was placed outdoors under shade. The temperature/humidity sensor is placed ~5 to 10 cm away from the proof-of-concept prototype. The released water was collected to a graduated cylinder. For thermal activation of the PC-MOF and removal of the detained water, hot water (55°C) was circulated for 15 min (figs. S21 and S22) and released water was collected in another graduated cylinder.
Simulated daily water harvesting of PCA-MOF
The PCA-MOF cones array was fixed over a container (fig. S25) and placed in an acrylic airtight humidity chamber, in which the relative humidity was set to 90%. For photothermal removal of detained water and regeneration of PCA-MOF gels, a simulated sunlight with a radiation intensity of 1 kW m−2 (1 sun, 300-W xenon arc lamp) was used. The mass and temperature changes were monitored by an analytical balance and IR camera, respectively. The waste heat was simulated by placing the PCA-MOF aerogel in an oven at 50°C, 30% RH. In the cycling test, 2 hours of capture (90% RH, 25°C) was followed by 1 hour of release (30% RH, 50°C) process for the first 45 hours. After 720 and 1440 hours, the same sample was cycled again to check for any performance and/or cyclability loss of the sample after inactive storage.
Supplementary Material
Acknowledgments
Funding: We gratefully acknowledge the financial support from the Ministry of Education Singapore (MOE) (MOE2017-T2-1-140 and MOE2017-T2-2-102). E.H.S. and J.A. acknowledge the University of Toronto International Doctoral Cluster on Urban Water, Waste and Energy Solutions (DSF19-22); and NSERC Discovery Grant (RGPIN-2017-06477). Author contributions: G.Y., G.W.H., and E.H.S. conceived the idea and cowrote the manuscript. G.Y. prepared the materials and carried out the experiments. W.L. performed the AFM experiments. J.A., F.L.M., and M.G. contributed to materials characterization. C.K.N.P. designed the prototype and assisted in outdoor experiments. All authors reviewed and edited the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/42/eabc8605/DC1
REFERENCES AND NOTES
- 1.Chahine M. T., The hydrological cycle and its influence on climate. Nature 359, 373–380 (1992). [Google Scholar]
- 2.Elimelech M., Phillip W. A., The future of seawater desalination: Energy, technology, and the environment. Science 333, 712–717 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Werber J. R., Osuji C. O., Elimelech M., Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 1, 16018 (2016). [Google Scholar]
- 4.P. H. Gleick, Water in Crisis: A Guide to the World's Fresh Water Resources (Oxford Univ. Press, 1993). [Google Scholar]
- 5.Domen J. K., Stringfellow W. T., Camarillo M. K., Gulati S., Fog water as an alternative and sustainable water resource. Clean Technol. Environ. Policy 16, 235–249 (2014). [Google Scholar]
- 6.Fessehaye M., Abdul-Wahab S. A., Savage M. J., Kohler T., Gherezghiher T., Hurni H., Fog-water collection for community use. Renew. Sustain. Energy Rev. 29, 52–62 (2014). [Google Scholar]
- 7.Lee A., Moon M.-W., Lim H., Kim W.-D., Kim H.-Y., Water harvest via dewing. Langmuir 28, 10183–10191 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Gido B., Friedler E., Broday D. M., Assessment of atmospheric moisture harvesting by direct cooling. Atmos. Res. 182, 156–162 (2016). [Google Scholar]
- 9.Tu Y., Wang R., Zhang Y., Wang J., Progress and expectation of atmospheric water harvesting. Joule 2, 1452–1475 (2018). [Google Scholar]
- 10.LaPotin A., Kim H., Rao S. R., Wang E. N., Adsorption-based atmospheric water harvesting: Impact of material and component properties on system-level performance. Acc. Chem. Res. 52, 1588–1597 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Kaseke K. F., Wang L., Fog and dew as potable water resources: Maximizing harvesting potential and water quality concerns. GeoHealth 2, 327–332 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khalil B., Adamowski J., Shabbir A., Jang C., Rojas M., Reilly K., Ozga-Zielinski B., A review: Dew water collection from radiative passive collectors to recent developments of active collectors. Sustain. Water Resour. Manag. 2, 71–86 (2016). [Google Scholar]
- 13.Nandakumar D. K., Zhang Y., Ravi S. K., Guo N., Zhang C., Tan S. C., Solar energy triggered clean water harvesting from humid air existing above sea surface enabled by a hydrogel with ultrahigh hygroscopicity. Adv. Mater. 31, 1806730 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Zhao F., Zhou X., Liu Y., Shi Y., Dai Y., Yu G., Super moisture-absorbent gels for all-weather atmospheric water harvesting. Adv. Mater. 31, 1806446 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Fathieh F., Kalmutzki M. J., Kapustin E. A., Waller P. J., Yang J., Yaghi O. M., Practical water production from desert air. Sci. Adv. 4, eaat3198 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X., Li X., Liu G., Li J., Hu X., Xu N., Zhao W., Zhu B., Zhu J., Interfacial solar heating assisted liquid sorbent atmospheric water generator. Angew. Chem. Int. Ed. 58, 12054–12058 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Qi H., Wei T., Zhao W., Zhu B., Liu G., Wang P., Lin Z., Wang X., Li X., Zhang X., Zhu J., An interfacial solar-driven atmospheric water generator based on a liquid sorbent with simultaneous adsorption-desorption. Adv. Mater. 31, 1903378 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Karmakar A., Mileo P. G. M., Bok I., Peh S. B., Zhang J., Yuan H., Maurin G., Zhao D., Thermo-responsive MOF/polymer composites for temperature-mediated water capture and release. Angew. Chem. Int. Ed. 59, 11003–11009 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Seo Y.-K., Yoon J. W., Lee J. S., Hwang Y. K., Jun C.-H., Chang J.-S., Wuttke S., Bazin P., Vimont A., Daturi M., Bourrelly S., Llewellyn P. L., Horcajada P., Serre C., Férey G., Energy-efficient dehumidification over hierachically porous metal–organic frameworks as advanced water adsorbents. Adv. Mater. 24, 806–810 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Hong D.-Y., Hwang Y. K., Serre C., Ferey G., Chang J.-S., Porous chromium terephthalate MIL-101 with coordinatively unsaturated sites: Surface functionalization, encapsulation, sorption and catalysis. Adv. Funct. Mater. 19, 1537–1552 (2009). [Google Scholar]
- 21.Férey G., Mellot-Draznieks C., Serre C., Millange F., Dutour J., Surblé S., Margiolaki I., A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 309, 2040–2042 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Yang J., Zhao Q., Li J., Dong J., Synthesis of metal-organic framework MIL-101 in TMAOH-Cr(NO3)3-H2BDC-H2O and its hydrogen-storage behavior. Micropor. Mesopor. Mater. 130, 174–179 (2010). [Google Scholar]
- 23.Lin R., Hernandez B. V., Ge L., Zhu Z., Metal organic framework based mixed matrix membranes: An overview on filler/polymer interfaces. J. Mater. Chem. A 6, 293–312 (2018). [Google Scholar]
- 24.Katsumoto Y., Tanaka T., Sato H., Ozaki Y., Conformational change of poly (N-isopropylacrylamide) during the coil-globule transition investigated by attenuated total reflection/infrared spectroscopy and density functional theory calculation. J. Phys. Chem. A 106, 3429–3435 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Maeda Y., Higuchi T., Ikeda I., Change in hydration state during the coil–globule transition of aqueous solutions of poly (N-isopropylacrylamide) as evidenced by FTIR spectroscopy. Langmuir 16, 7503–7509 (2000). [Google Scholar]
- 26.Sun B., Lin Y., Wu P., Siesler H. W., A FTIR and 2D-IR spectroscopic study on the microdynamics phase separation mechanism of the poly(N-isopropylacrylamide) aqueous solution. Macromolecules 41, 1512–1520 (2008). [Google Scholar]
- 27.Kang E. T., Neoh K. G., Tan K. L., The intrinsic redox states in polypyrrole and polyaniline: A comparative study by XPS. Surf. Interface Anal. 19, 33–37 (1992). [Google Scholar]
- 28.Furukawa H., Gándara F., Zhang Y.-B., Jiang J., Queen W. L., Hudson M. R., Yaghi O. M., Water adsorption in porous metal-organic frameworks and related materials. J. Am. Chem. Soc. 136, 4369–4381 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Li R., Shi Y., Shi L., Alsaedi M., Wang P., Harvesting water from air: Using anhydrous salt with sunlight. Environ. Sci. Technol. 52, 5398–5406 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Kim H., Rao S. R., Kapustin E. A., Zhao L., Yang S., Yaghi O. M., Wang E. N., Adsorption-based atmospheric water harvesting device for arid climates. Nat. Commun. 9, 1191–1198 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li R., Shi Y., Alsaedi M., Wu M., Shi L., Wang P., Hybrid hydrogel with high water vapor harvesting capacity for deployable solar-driven atmospheric water generator. Environ. Sci. Technol. 52, 11367–11377 (2018). [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization, Guidelines for Drinking-water Quality (World Health Organization, ed. 4, 2011).
- 33.Okur H. I., Kherb J., Cremer P. S., Cations bind only weakly to amides in aqueous solutions. J. Am. Chem. Soc. 135, 5062–5067 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y., Furyk S., Bergbreiter D. E., Cremer P. S., Specific ion effects on the water solubility of macromolecules: PNIPAM and the Hofmeister series. J. Am. Chem. Soc. 127, 14505–14510 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y., Furyk S., Sagle L. B., Cho Y., Bergbreiter D. E., Cremer P. S., Effects of Hofmeister anions on the LCST of PNIPAM as a function of molecular weight. J. Phys. Chem. C 111, 8916–8924 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J., Wang N., Yu L.-J., Karton A., Li W., Zhang W., Guo F., Hou L., Cheng Q., Jiang L., Weitz D. A., Zhao Y., Bioinspired graphene membrane with temperature tunable channels for water gating and molecular separation. Nat. Commun. 8, 2011–2019 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang F., Srinivasan M. P., Multilayered gold-nanoparticle/polyimide composite thin film through layer-by-layer assembly. Langmuir 23, 10102–10108 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Kang E. T., Neoh K. G., Khor S. H., Tan K. L., Tan B. T. G., Structural determination of polyaniline by X-ray photoelectron spectroscopy. J. Chem. Soc. Chem. Commun., 695–697 (1989). [Google Scholar]
- 39.Hasik M., Bernasik A., Drelinkiewicz A., Kowalski K., Wenda E., Camra J., XPS studies of nitrogen-containing conjugated polymers–palladium systems. Surf. Sci. 507–510, 916–921 (2002). [Google Scholar]
- 40.Myers A. L., Thermodynamics of adsorption in porous materials. AIChE J. 48, 145–160 (2002). [Google Scholar]
- 41.Cracknell R. F., Nicholson D., Adsorption of gas mixtures on solid surfaces, theory and computer simulation. Adsorption 1, 7–16 (1995). [Google Scholar]
- 42.Van Ness H. C., Adsorption of gases on solids. Review of role of thermodynamics. Ind. Eng. Chem. Fundamen. 8, 464–473 (1969). [Google Scholar]
- 43.Morrow N. R., Physics and thermodynamics of capillary action in porous media. Ind. Eng. Chem. 62, 32–56 (1970). [Google Scholar]
- 44.Monosmith W. B., Walrafen G. E., Temperature dependence of the Raman OH-stretching overtone from liquid water. J. Chem. Phys. 81, 669–674 (1984). [Google Scholar]
- 45.Sekine Y., Ikeda-Fukazawa T., Structural changes of water in a hydrogel during dehydration. J. Chem. Phys. 130, 034501 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Zhou X., Zhao F., Guo Y., Rosenberger B., Yu G., Architecting highly hydratable polymer networks to tune the water state for solar water purification. Sci. Adv. 5, eaaw5484 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun Q., The Raman OH stretching bands of liquid water. Vib. Spectrosc. 51, 213–217 (2009). [Google Scholar]
- 48.Dey F. K., Cleaver J. A. S., Zhdan P. A., Atomic force microscopy study of adsorbed moisture on lactose particles. Adv. Powder Technol. 11, 401–413 (2000). [Google Scholar]
- 49.Mate C. M., Lorenz M. R., Novotny V. J., Determination of lubricant film thickness on a particulate disk surface by atomic force microscopy. IEEE Trans. Magn. 26, 1225–1228 (1990). [Google Scholar]
- 50.Xu T., Lin Y., Zhang M., Shi W., Zheng Y., High-efficiency fog collector: Water unidirectional transport on heterogeneous rough conical wires. ACS Nano 10, 10681–10688 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Lu G., Li S., Guo Z., Farha O. K., Hauser B. G., Qi X., Wang Y., Wang X., Han S., Liu X., Du Chene J. S., Zhang H., Zhang Q., Chen X., Ma J., Loo S. C. J., Wei W. D., Yang Y., Hupp J. T., Huo F., Imparting functionality to a metal–organic framework material by controlled nanoparticle encapsulation. Nat. Chem. 4, 310–316 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/42/eabc8605/DC1


