Abstract
This study aimed to evaluate the association of interleukin-6 (IL-6) level with the poor outcomes in coronavirus disease 2019 (COVID-19) patients by utilizing a meta-analysis based on adjusted effect estimates. We searched the keywords from PubMed, Web of Science, and EMBASE on August 14, 2020. The pooled effects and 95% confidence interval (95% CI) were estimated by Stata 11.2. Subgroup analysis and meta-regression were performed to explore the source of heterogeneity. Sensitivity analysis was implemented to assess the stability of the results. Begg’s test and Egger’s test were conducted to assess the publication bias. Sixteen articles with 8752 COVID-19 patients were finally included in the meta-analysis. The results based on random-effects model indicated that elevated value of IL-6 was significantly associated with adverse outcomes in patients with COVID-19 (pooled effect = 1.21, 95% CI 1.13–1.31, I2 = 90.7%). Subgroup analysis stratified by disease outcomes showed consistent results (severe: pooled effect = 1.18, 95% CI 1.05–1.31; ICU (intensive care unit) admission: pooled effect = 1.90, 95% CI 1.04–3.47; death: pooled effect = 3.57, 95% CI 2.10–6.07). Meta-regression indicated that study design was a source of heterogeneity. Publication bias was existent in our analysis (Begg’s test: P = 0.007; Egger’s test: P < 0.001). In conclusion, the elevated IL-6 level is an independent risk factor associated with adverse outcomes in patients with COVID-19.
Electronic supplementary material
The online version of this article (10.1007/s00251-020-01179-1) contains supplementary material, which is available to authorized users.
Keywords: COVID-19, IL-6, Adverse outcomes, Meta-analysis, Adjusted effect estimates
With the developing of the epidemic caused by coronavirus disease 2019 (COVID-19), biomarkers which might predict the adverse outcomes of COVID-19 patients gradually attract researchers’ attention. Interleukin-6 (IL-6) is one of the main pro-inflammatory factors in the formation of cytokine storm, which increases permeability to a great extent and damages organ function (Liu et al. 2020d). As a result, IL-6, as a possible indicator of the poor prognosis in patients with COVID-19, has been noticed, and many relevant articles have been published. Recently, a meta-analysis conducted by Zeng et al. aroused our interests, which reported the significant association between IL-6 levels and severe COVID-19 (weighted mean difference (WMD): − 21.32 ng/L, 95% confidence interval (CI) (− 28.34, − 14.31); P < 0.001, I2 = 99.1%) (Zeng et al. 2020). However, this meta-analysis was based on unadjusted effect estimates. As we all know, there are several factors affecting the disease progression, such as gender, age, and comorbidities (Del Valle et al. 2020). Moreover, in the paper reported by Wang et al., the univariate logistic analysis suggested that the baseline levels of IL-6 were significantly associated with the disease progression of COVID-19 patients, while the multivariate logistic analysis indicated that only high levels of IL-6 were a risk factor for disease progression of COVID-19 patients (Wang et al. 2020). Therefore, it is necessary to evaluate the association of IL-6 level with the adverse outcomes of COVID-19 patients by utilizing a meta-analysis on the basis of adjusted effect estimates.
A scientific literature search of the electronic databases including PubMed, Web of Science, and EMBASE was carried out on August 14, 2020, to enroll all eligible publications which reported the association between elevated IL-6 levels and adverse outcomes in patients with COVID-19. The following terms were used as our search strategy: (“coronavirus disease 2019” OR “COVID-19” OR “SARS-CoV-2” OR “2019-nCoV” OR “novel coronavirus”) AND (“IL-6” OR “interleukin-6”) AND (“mortality” OR “death” OR “fatality” OR “demise” OR “severe” OR “severity” OR “critical” OR “poor outcome” OR “poor prognosis” OR “adverse outcome” OR “progression”). We incorporated the articles that reported the correlation of IL-6 level with the poor outcomes of COVID-19 patients based on adjusted effect estimates. The meta-analysis was performed by the software Stata 11.2 to obtain the pooled effect and 95% CI. We used the I2 test to evaluate the heterogeneity among the included articles. The fixed-effects model was chosen if I2 < 50%, while the random-effects model was used if I2 ≥ 50%. Meta-regression and subgroup analysis were conducted to identify the source of heterogeneity. Sensitivity analysis was implemented by taking out one study each time to assess the stability of the results. Additionally, we used the Begg’s test and Egger’s test to assess the publication bias and conducted a trim and fill analysis to adjust the effect size.
Figure S1 presents the process of study selection. The initial search produced 1044 articles with 603 excluded because of duplication. We excluded 198 articles after assessing the titles and abstracts, because some of the articles are reviews; some are correspondences, commentaries, or letters; and others are case reports, study protocols for clinical trial, or no-human studies. After assessing the full text, 176 were excluded because they did not report the association between IL-6 level and poor outcomes in COVID-19 patients, and 51 were excluded because original data were not reported or adjusted effect was not used. Finally, 16 articles consisting of 8752 COVID-19 patients were included in the meta-analysis (Ayanian et al. 2020; Bellmann-Weiler et al. 2020; Cummings et al. 2020; Del Valle et al. 2020; Li et al. 2020; Liu et al. 2020a, b, c, d, e; Phipps et al. 2020; Sardu et al. 2020; Song et al. 2020; Tian et al. 2020; Wang et al. 2020; Yan et al. 2020). The characteristics of the 16 eligible studies are shown in Table 1.
Table 1.
Characteristics of the included studies
| Author | Country | Cases (n) | Age (years) | Male, n (%) | Study design | Cutoff value | Outcomes | Adjusted effect estimate (95% CI) | Confounders |
|---|---|---|---|---|---|---|---|---|---|
| Cummings MJ (PMID: 32442528) | USA | 257 | 62 (51, 72) | 171 (67) | P | Per decile increase | Death | HR 1.11 (1.02, 1.20) | Age, gender, symptom duration before hospital presentation, hypertension, chronic cardiac disease, COPD or interstitial lung disease, diabetes, D-dimer |
| Liu X (PMID: 32475880) | China | 88 | 60.45 ± 11.51 | 51 (58) | P | 7 pg/ml | Severe | OR 1.31 (1.032, 1.687) | Lymphocyte count, LDH level, erythrocyte count, albumin level, A/G ratio, blood glucose level |
| Phipps MM (PMID: 32473607) | USA | 3381 | 65 (52, 76) | 1297 (57) | R | NR | Death | OR 1.45 (1.1, 1.93) | Age, gender, BMI, peak ferritin, peak D-dimer, peak CRP, peak PCT, peak creatinine kinase, peak high sensitivity troponin |
| Del Valle DM (PMID: 32511562) | USA | 1268 | 63 (53, 72) | 787 (60.1) | P | 70 pg/ml | Death | HR 2.06 (1.33, 3.18) | Age, gender, race/ethnicity, BMI, smoking status, TNF-a, IL-8, IL-1b, CRP, D-dimer, ferritin, diabetes, hypertension, CKD, asthma, CHF, COPD, sleep apnea, atrial fibrillation, cancer, severity scores |
| Tian J (PMID: 32479790) | China | 232 | 64 (58, 69) | 119 (51) | R | NR | Severe | OR 1.03 (1, 1.05) | Age, ECOG performance status, tumor stage, antitumor treatments, TNF-α, IL-6, IL-2R, procalcitonin, CRP, lymphocytes, leukocyte count, neutrophils, monocytes, LDH, albumin, A/G ratio, NT-proBNP, myoglobin, hs-cTnI, platelet count, activated partial thromboplastin time, prothrombin time, D-dimer |
| Wang F (PMID: 32620125) | China | 323 | 46 (33, 59) | 154 (47.7) | P | 7 pg/ml | Progressive | OR 1.03 (1, 1.05) | NLR, T lymphocyte, CRP, IL-6, ESR |
| Liu J (PMID: 32622796) | China | 107 | 68 (61, 76) | 52 (49) | R | 10 pg/ml | Poor outcomes | OR 7.228 (2.222, 23.514) | Age, calcium, CRP, PCT, IL-6, D-dimer |
| ICU admission | HR 1.617 (1.094, 2.389) | ||||||||
| Sardu C (PMID: 32633594) | Italy | 62 | 58 ± 18 | 41 (66.1) | P | NR | ICU admission | HR 1.617 (1.094, 2.389) | Demographic variables that were significantly different amongst the groups and values of left ventricle ejection fraction |
| Mechanical ventilation | HR 1.149 (1.082, 1.219) | ||||||||
| Cardiac injury | HR 1.367 (1.054, 1.772) | ||||||||
| Death | HR 4.742 (1.788, 8.524) | ||||||||
| Ayanian S (PMID: 32677844) | USA | 299 | NR | 161 (54) | R | 50 pg/ml | ICU admission | OR 5.9 (2.7, 13.1) | D-dimer, ferritin, CRP, LDH |
| Intubation | OR 4.6 (1.7, 12.4) | ||||||||
| Death | OR 5.4 (2.4, 11.8) | ||||||||
| Li T (PMID: 32688107) | China | 312 | 69.2 ± 7.3 | 187 (59.9) | R | NR | Severe | OR 4.32 (2.07, 7.13) | Age, SOFA score, APACHE II score, platelet count, D-dimer, creatinine, lung consolidation |
| Liu D (PMID: 32696591) | China | 115 | 62.0 (51.0, 70.0) | 115 (100) | R | 14 pg/ml | Fatal outcome | OR 5.21 (2.65, 10.27) | Hypertension, age, WBC count, lymphocyte count, D-dimer, procalcitonin, CRP |
| 120 | 0 (0) | OR 12.89 (4.71, 35.3) | IL-2R, IL-8, WBC count, lymphocyte count, high-sensitivity cardiac troponin I | ||||||
| Liu SP (PMID: 32712122) | China | 255 | 64 (24, 92) | 136 (53.3) | P | NR | ICU admission | OR 1.01 (1.002, 1.018) | Diabetes, high FPG at admission, high IL-6, D-dimer |
| Song Y (PMID: 32733921) | China | 64 | 64.8 ± 12.2 | 42 (65.6) | R | 703.9 pg/ml | Myocardial injury | OR 13.63 (3.33, 55.71) | hs-CRP, IL-2R, IL-8, TNF-α |
| Bellmann-Weiler (PMID: 32751400) | USA | 259 | NR | 156 (60.6) | R | NR | ICU admission | OR 1.961 (1.088, 3.535) | Gender, temperature, SpO2, DM, COPD, ferritin, transferrin, leukocytes |
| Yan Q (PMID: 32766817) | China | 882 | 71 (68, 77) | 440 (49.9) | R | 6 pg/l | Death | HR 25.53 (3.5, 186.04) | Age, gender, cardiovascular diseases, chronic respiratory disease, CKD, cerebrovascular disease, diabetes, malignancy, lymphocytes, D-dimer, LDH, cardiac injury, liver injury, AKI |
| Liu Z (PMID: 32765283) | China | 728 | 58 (49, 68) | 342 (46.98) | R | 7 pg/ml | Death | HR 10.39 (1.09, 99.23) | Age, cardiovascular disease, lymphocyte count, D-dimer, LDH |
| Severe | OR 3.56 (2.06, 6.19) | Age, cardiovascular disease, lymphocyte count, D-dimer, LDH |
The values of age are mean ± standard deviation (SD) or median (interquartile range, IQR). The values of male are n (%). P prospective study, R retrospective study, CI confidence interval, ICU intensive care unit, HR hazard ratio, OR odds ratio, LDH lactate dehydrogenase, A/G ratio albumin-globulin ratio, BMI body mass index, CRP C-reactive protein, PCT procalcitonin, TNF tumor necrosis factor, IL interleukin, CKD chronic kidney disease, CHF congestive heart failure, COPD chronic obstructive pulmonary disease, ECOG Eastern Cooperative Oncology Group, IL-2R IL-2 receptor, NT-proBNP N-terminal pro-B-type natriuretic peptide, hs-cTnI high-sensitivity cardiac troponin I, NLR neutrophil to lymphocyte ratio, ESR erythrocyte sedimentation rate, SOFA sequential organ failure assessment, APACHE acute physiologic and chronic health evaluation, WBC white blood cell, FPG fasting plasma glucose, hs-CRP high-sensitivity C-reactive protein, DM diabetes mellitus, AKI acute kidney injury, SpO2 peripheral capillary oxygen saturation, NR not reported.
Our results indicated that elevated values of IL-6 were significantly associated with adverse outcomes in patients with COVID-19 (pooled effect = 1.21, 95% CI 1.13–1.31, I2 = 90.7%, random-effects model, Fig. 1a). Subgroup analysis stratified by disease outcomes showed consistent results (severe: pooled effect = 1.18, 95% CI 1.05–1.31, Fig. 1b; ICU (intensive care unit) admission: pooled effect = 1.90, 95% CI 1.04–3.47, Fig. 1c; death: pooled effect = 3.57, 95% CI 2.10–6.07, Fig. 1d). Since most of the studies were from China, we performed subgroup analysis stratified by countries, which also demonstrated consistent results (China: pooled effect = 1.12, 95% CI 1.04–1.20; USA: pooled effect = 1.78, 95% CI 1.15–2.77, Fig. S2a). The results of subgroup analysis only based on prospective studies showed that elevated IL-6 values were also significantly associated with adverse outcomes in COVID-19 patients (pooled effect = 1.07, 95% CI 1.01–1.14) (Fig. S2b). Meta-regressions revealed that different study designs (retrospective study or prospective study) contributed to the heterogeneity among studies (P = 0.026), while others had no contribution, such as effect estimate model (odds ratio (OR) and hazard ratio (HR)) (P = 0.677), disease outcomes (P = 0.916), country (P = 0.458), as well as adjusted factors and so on. In addition, sensitivity analysis indicated that there was few influence of individual study on pooled effects when we eliminated each of the included studies. However, publication bias was existent in our analysis (Begg’s test: P = 0.013; Egger’s test: P < 0.001, respectively). The trim and fill analysis revealed that after adjusting the asymmetry, the results were still stability (pooled effect = 1.116, 95% CI 1.022–1.220).
Fig. 1.
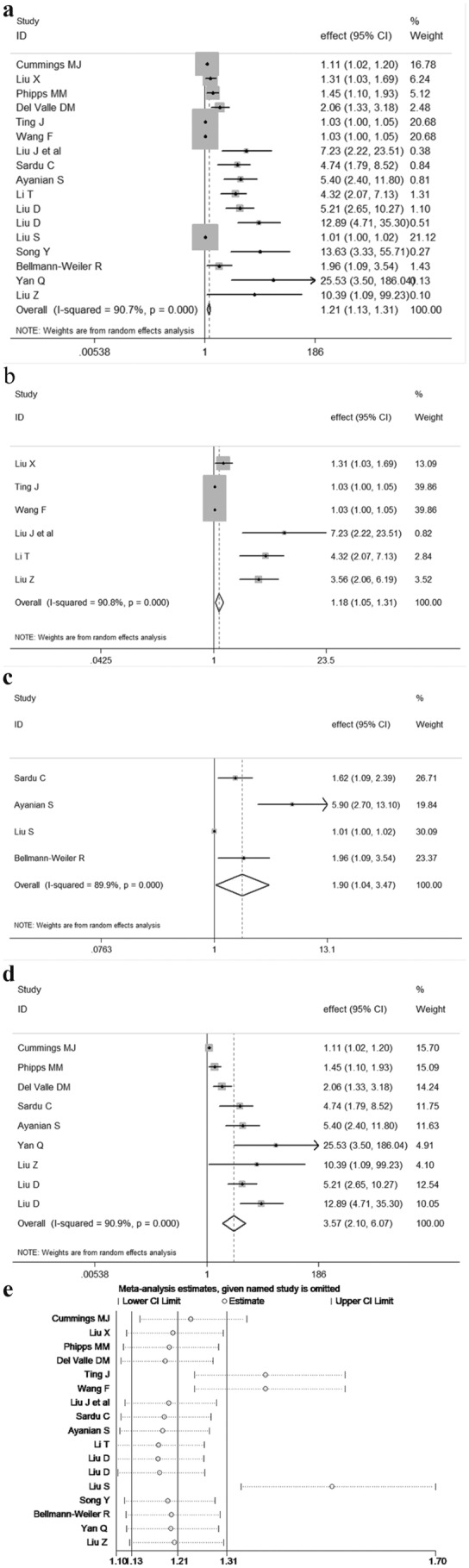
Forest plot with pooled effects and 95% confidence interval (CI) indicating the association between interleukin-6 (IL-6) level and adverse outcomes in coronavirus disease 2019 patients (a). Subgroup analysis based on different disease outcomes (severe (b), ICU (intensive care unit) admission (c), death (d)). Sensitivity analysis (e).
Based on our analysis taking the confounders into account, elevated IL-6 value was significantly associated with severe COVID-19 and can be regard as an independent risk factor for adverse outcomes in COVID-19 patients. IL-6, as a cytokine, has been previously verified elevating in inflammatory state for multiple conditions. The pathophysiological hallmark of COVID-19 is the severe inflammation and cytokine storm, which explains the elevation of IL-6 levels (Cai et al. 2020; Mo et al. 2020). Thus, IL-6 can be used as a significant indicator of adverse prognosis reminding the clinicians to pay more attention to the patients with COVID-19 who might have a poor outcome in the early stage.
However, there are still some limitations in our study. One of the main defects is that the adjusted factors were different among the selected studies. Additionally, the publication bias existed in our study. The generation of publication bias is probably owing to that studies with positive results are more likely to be published than negative ones and the number of relevant studies is still not enough. Thus, further well-designed studies with more available articles are required to verify our current findings in the future.
In conclusion, elevated IL-6 was an independent risk factor associated with the adverse outcomes in patients with COVID-19. Thus, COVID-19 patients with high levels of IL-6 were worth noticing and needed more clinical attention. Furthermore, the biomarkers indicting poor prognosis in patients with COVID-19 should be further researched in order to help clinicians reasonably arrange the medical resource.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding Information
This work was supported by a grant from the National Natural Science Foundation of China (grant number 81973105). The funder has no role in data collection, data analysis, preparation of manuscript and decision to manuscript submission.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ayanian S, Reyes J, Lynn L, Teufel K. The association between biomarkers and clinical outcomes in novel coronavirus pneumonia in a US cohort. Biomark Med. 2020 doi: 10.2217/bmm-2020-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellmann-Weiler R, Lanser L, Barket R, Rangger L, Schapfl A, Schaber M, Fritsche G, Wöll E, Weiss G. Prevalence and predictive value of anemia and dysregulated iron homeostasis in patients with COVID-19 infection. J Clin Med. 2020;9:E2429. doi: 10.3390/jcm9082429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, Su Y, Ma Z, Zhang Y, Li Z, He Q, Liu L, Fu Y, Chen J. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742–1752. doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, Aaron JG, Claassen J, Rabbani LE, Hastie J, Hochman BR, Salazar-Schicchi J, Yip NH, Brodie D, O'Donnell MR. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle DM, Kim-Schulze S, Hsin-Hui H, Beckmann ND, Nirenberg S, Wang B, Lavin Y, Swartz T, Madduri D, Stock A, Marron T, Xie H, Patel MK, van Oekelen O, Rahman A, Kovatch P, Aberg J, Schadt E, Jagannath S, Mazumdar M, Charney A, Firpo-Betancourt A, Mendu DR, Jhang J, Reich D, Sigel K, Cordon-Cardo C, Feldmann M, Parekh S, Merad M, Gnjatic S (2020) An inflammatory cytokine signature helps predict COVID-19 severity and death. medRxiv 10.1101/2020.05.28.20115758
- Li T, Lu L, Zhang W, Tao Y, Wang L, Bao J, Liu B, Duan J. Clinical characteristics of 312 hospitalized older patients with COVID-19 in Wuhan. China Arch Gerontol Geriatr. 2020;91:104185. doi: 10.1016/j.archger.2020.104185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Li R, Yu R, Wang Y, Feng X, Yuan Y, Wang S, Zeng S, Gao Y, Xu S, Li H, Jiao X, Chi J, Yu Y, Song C, Jin N, Cui P, Liu J, Zheng X, Gong W, Liu X, Cai G, Song J, Kwan SY, Desai A, Li C, Gao Q. Alteration of serum markers in COVID-19 and implications on mortality. Clin Transl Med. 2020;10:e119. doi: 10.1002/ctm2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Han P, Wu J, Gong J, Tian D. Prevalence and predictive value of hypocalcemia in severe COVID-19 patients. J Infect Public Health. 2020;13:1224–1228. doi: 10.1016/j.jiph.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SP, Zhang Q, Wang W, Zhang M, Liu C, Xiao X, Liu Z, Hu WM, Jin P. Hyperglycemia is a strong predictor of poor prognosis in COVID-19. Diabetes Res Clin Pract. 2020;167:108338. doi: 10.1016/j.diabres.2020.108338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Shi S, Xiao J, Wang H, Chen L, Li J, Han K. Prediction of the severity of corona virus disease 2019 and its adverse clinical outcomes. Jpn J Infect Dis. 2020 doi: 10.7883/yoken.JJID.2020.194. [DOI] [PubMed] [Google Scholar]
- Liu Z, Li J, Chen D, Gao R, Zeng W, Chen S, Huang Y, Huang J, Long W, Li M, Guo L, Wang X, Wu X. Dynamic interleukin-6 level changes as a prognostic indicator in patients with COVID-19. Front Pharmacol. 2020;11:1093. doi: 10.3389/fphar.2020.01093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, Wang H, Xiong Y, Cheng Z, Gao S, Liang K, Luo M, Chen T, Song S, Ma Z, Chen X, Zheng R, Cao Q, Wang F, Zhang Y (2020) Clinical characteristics of refractory COVID-19 pneumonia in Wuhan China . Clin Infect Dis 10.1093/cid/ciaa270 [DOI] [PMC free article] [PubMed]
- Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute liver injury in COVID-19: prevalence and association with clinical outcomes in a large US cohort. Hepatology. 2020 doi: 10.1002/hep.31404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardu C, Maggi P, Messina V, Iuliano P, Sardu A, Iovinella V, Paolisso G, Marfella R. Could anti-hypertensive drug therapy affect the clinical prognosis of hypertensive patients with COVID-19 infection? Data from centers of southern Italy. J Am Heart Assoc. 2020;9:e016948. doi: 10.1161/JAHA.120.016948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Gao P, Ran T, Qian H, Guo F, Chang L, Wu W, Zhang S. High inflammatory burden: a potential cause of myocardial injury in critically ill patients with COVID-19. Front Cardiovasc Med. 2020;7:128. doi: 10.3389/fcvm.2020.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Yuan X, Xiao J, Zhong Q, Yang C, Liu B, Cai Y, Lu Z, Wang J, Wang Y, Liu S, Cheng B, Wang J, Zhang M, Wang L, Niu S, Yao Z, Deng X, Zhou F, Wei W, Li Q, Chen X, Chen W, Yang Q, Wu S, Fan J, Shu B, Hu Z, Wang S, Yang XP, Liu W, Miao X, Wang Z. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:893–903. doi: 10.1016/S1470-2045(20)30309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Qu M, Zhou X, Zhao K, Lai C, Tang Q, Xian W, Chen R, Li X, Li Z, He Q, Liu L. The timeline and risk factors of clinical progression of COVID-19 in Shenzhen. China J Transl Med. 2020;18:270. doi: 10.1186/s12967-020-02423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Zuo P, Cheng L, Li Y, Song K, Chen Y, Dai Y, Yang Y, Zhou L, Yu W, Li Y, Xie M, Zhang C, Gao H. Acute kidney injury is associated with in-hospital mortality in older patients with COVID-19. The Journals of Gerontology: Series A; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F, Huang Y, Guo Y, Yin M, Chen X, Xiao L, Deng G. Association of inflammatory markers with the severity of COVID-19: A meta-analysis. Int J Infect Dis. 2020;96:467–474. doi: 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


