Abstract
Purpose
In vivo Alzheimer’s disease (AD) biomarkers for tau pathology are cerebrospinal fluid (CSF) phosphorylated tau (p-tau) and [18F]flortaucipir positron emission tomography (PET). Our aim was to assess associations between CSF p-tau with [18F]flortaucipir PET and the associations of both tau biomarkers with cognition and atrophy.
Methods
We included 78 amyloid positive cognitively impaired patients (clinical diagnoses mild cognitive impairment (MCI, n = 8) and AD dementia (n = 45) and 25 cognitively normal subjects with subjective cognitive decline (SCD) (40% amyloid-positive)). Dynamic 130 min [18F]flortaucipir PET scans were acquired to generate binding potential (BPND) images using receptor parametric mapping and standardized uptake values ratios of 80–100 min (SUVr80-100min) post injection. We obtained regional BPND and SUVr from entorhinal, limbic, and neocortical regions-of-interest (ROIs), closely aligning to the neuropathological tau staging schemes. Cognition was assessed using MMSE and composite scores of four cognitive domains, and atrophy was measured using gray matter volume covering the major brain lobes. First, we used linear regressions to investigate associations between CSF p-tau (independent variable) and tau PET (dependent variable). Second, we used linear regressions to investigate associations between CSF p-tau, tau PET (separate independent variables, model 1), and cognition (dependent variable). We then assessed the independent effects of CSF p-tau and tau PET on cognition by simultaneously adding the other tau biomarker as a predictor (model 2). Finally, we performed the same procedure for model 1 and 2, but replaced cognition with atrophy. Models were adjusted for age, sex, time lag between assessments, education (cognition only), and total intracranial volume (atrophy only).
Results
Higher [18F]flortaucipir BPND was associated with higher CSF p-tau (range of standardized betas (sβ) across ROIs, 0.43–0.46; all p < 0.01). [18F]flortaucipir BPND was more strongly associated with cognition and atrophy than CSF p-tau. When [18F]flortaucipir BPND and CSF p-tau were entered simultaneously, [18F]flortaucipir BPND (range sβ = − 0.20 to – 0.57, all p < 0.05) was strongly associated with multiple cognitive domains and atrophy regions. SUVr showed comparable results to BPND.
Conclusion
Regional [18F]flortaucipir BPND correlated stronger with cognition and neurodegeneration than CSF p-tau, suggesting that tau PET more accurately reflects disease severity in AD.
Electronic supplementary material
The online version of this article (10.1007/s00259-020-04758-2) contains supplementary material, which is available to authorized users.
Keywords: [18F]flortaucipir, PET, CSF, Tau, Cognition, Atrophy
Introduction
Alzheimer’s disease (AD) is characterized neuropathologically by depositions of amyloid plaques and hyperphosphorylated tau. Postmortem studies have revealed that tau pathology is highly associated with the degree of cognitive impairment [1–3] and thus may serve as a biomarker of disease severity. In vivo tau can be captured in cerebrospinal fluid (CSF) and by positron emission tomography (PET). CSF phosphorylated tau (p-tau) is thought to reflect an AD-specific hyperphosphorylated state of tau [4]. [18F]flortaucipir is currently the most widely used PET tau tracer, which binds with high affinity to the paired helical filaments (PHFs) of tau in AD [5–7].
Although both are in vivo biomarkers for tau pathology, it remains unclear whether CSF p-tau and [18F]flortaucipir PET provide interchangeable or complementary information. So far, a number of studies have investigated associations between [18F]flortaucipir PET and CSF p-tau. Some studies found moderate to strong correlations across groups which consisted mainly of cognitively normal individuals [8, 9], while others could not replicate these findings [10]. Across the AD continuum, strongest associations between tau PET and CSF p-tau were mostly observed in later, dementia stages [11]. In addition, similar high levels were observed in CSF p-tau in prodromal AD and AD dementia, while [18F]flortaucipir uptake values continued to increase with progression of AD [12]. Taken together these results indicate that the relationship between CSF p-tau biomarkers and [18F]flortaucipir may depend on disease severity.
Both CSF p-tau [13, 14] and [18F]flortaucipir [15–21] have been related separately to proxies of disease severity, for example, to worse cognitive performance [13, 15–19] and a greater degree of atrophy [14, 18–21]. Despite these similarities, they may still differ on various aspects. [18F]flortaucipir PET is able to capture regional uptake patterns and mirrors established neuropathological staging schemes of tau [15, 22], while correlations of CSF p-tau with neuropathological tau burden have been modest [23, 24]. However, CSF p-tau may be more sensitive in detecting early changes in AD (tau) pathology [11, 25, 26]. Few studies have described the relationship between both CSF p-tau and [18F]flortaucipir binding with proxies of disease severity, and it remains to be established whether [18F]flortaucipir PET and p-tau can be viewed as equivalent markers for AD staging.
Therefore, we aimed to compare CSF and PET markers of tau pathology and to assess their relationship with cognitive impairment and atrophy as proxies of disease severity. We first examined regional associations between CSF p-tau and quantitative [18F]flortaucipir binding in cognitively normal and impaired subjects on the AD pathophysiological continuum [27, 28]. Second, to investigate whether CSF p-tau biomarkers and [18F]flortaucipir PET provide complementary information regarding disease severity, we investigated associations of both CSF p-tau and [18F]flortaucipir PET with multiple cognitive domains and regional atrophy.
Methods
Participants
We included 78 subjects from the Amsterdam Dementia Cohort [29, 30], of whom 25 were cognitively normal with subjective cognitive decline (SCD), and 53 were cognitively impaired (mild cognitive impairment (MCI) due to AD [31] (n = 8) or probable AD dementia [32] (n = 45)).
We grouped the MCI-AD and probable AD dementia subjects into one MCI/AD group.
All subjects underwent a standardized dementia screening, including medical history, extensive neuropsychological assessment, physical and neurological examination, lumbar puncture, blood tests, electroencephalography, and brain magnetic resonance imaging (MRI) [30]. Diagnosis was established by consensus in a multidisciplinary meeting [29, 30].
The label of SCD [33], which were used as controls, was provided based on self-reported cognitive complaints but without any objective impairment in performance on cognitive or neurological tasks or brain damage as evidenced by MRI. SCD subjects with evidence of substantial Aß pathology after visual reading of SUVR50–70 of [18F]florbetapir Aß-PET scans [34] were classified as amyloid positive subjects.
The diagnosis of MCI/AD met core clinical criteria [31, 32] according to the National Institute on Aging and Alzheimer’s Association (NIA-AA) and were biomarker supported, an AD like CSF (i.e., tau/Aβ42 fraction > 0.52 [35]) and/or a positive Aß-PET ([11C]PiB or [18F]florbetaben) scan by visual assessment [36, 37].
Exclusion criteria for all participants were (1) diagnosis of dementia not due to AD, (2) significant cerebrovascular disease on MRI (e.g., major CVA), (3) major traumatic brain injury, (4) major psychiatric or neurological disorders other than AD, (5) recent substance abuse, and (6) a time lag between LP and tau-PET imaging > 2 years. The study protocol was approved by the Medical Ethics Review Committee of the Amsterdam UMC, location VU Medical center. All patients provided written informed consent.
CSF biomarkers
CSF samples were obtained from LP between the L3/L4, L4/L5, or L5/S1 intervertebral space and collected in polypropylene tubes using a 25-gauge needle and syringe [29]. To assess CSF biomarker levels, analyses were performed at the Neurochemistry Laboratory of the Department of Clinical Chemistry of the Amsterdam UMC according to international guidelines [38]. Levels of Aß1–42, t-tau, and p-tau (phosphorylated at threonine 181) in CSF were determined using sandwich ELISAs (Innotest ß-AMYLOID (1–42), Innotest hTAU-Ag, and Innotest PhosphoTAU-181p, Fujirebio (formerly Innogenetics), Belgium), and Aβ1–42 levels were corrected for drift in kit performance over time [39]. Cutoff values for abnormal CSF (Aβ1–42 < 813 pg/mL [39]), t-tau (> 375 pg/mL), and p-tau (> 52 pg/mL) [40] were used to define an AD like positive CSF profile (i.e., tau/Aβ42 fraction > 0.52 [35]) supportive of the diagnosis of MCI/AD. In this sample, p-tau and t-tau were highly correlated (r = 0.98, p < 0.01); therefore, all main analyses were performed with p-tau, and results for t-tau are provided in the Supplementary Material.
Neuropsychological examination
The Mini-Mental State Examination (MMSE) was used as a measure of global cognitive status [41]. In addition, we assessed four cognitive domains [42] including memory (Dutch version of the Rey Auditory Verbal Learning Test immediate recall and delayed recall, Visual Association Test (VAT) version A), attention (Digit span Forward, Trial Making Test [TMT] version A), Stroop word and color naming), executive functioning (Digit span Backward, TMT version B, letter fluency test (D-A-T) and Stroop color-word interference test), and language (VAT-A naming and category fluency version animals). Trail Making Tests and Stroop tests were inverted so that lower scores indicated worse performance. For cognitive domain scores, we created composite scores by averaging Z-scores for each individual test within a domain (with a minimum of two tests per domain). The percentages of missing data for the cognitive domains were 10.3% (attention), 11.5% (memory), 12.8% (language), and 19.2% (executive functioning). As a sensitivity analysis we repeated our analyses including subjects without missing data (n = 59, see Supplementary Table 1).
MRI imaging and processing
All subjects underwent a 3D-T1 weighted sequence on a 3.0 Tesla MRI scanner (Ingenuity TF PET/MR, Philips Medical Systems, Best, The Netherlands, n = 75), Vantage Titan (Toshiba Medical Systems, Otawara, Japan, n = 2), and a Signa HDxT (GE Healthcare, Milwaukee, WI, n = 1). All MR images were performed within a maximum of 12 months from the [18F]flortaucipir PET scan for SCD subjects (median = 0.2, IQR = 1.3) and a maximum of 6 months from the [18F]flortaucipir PET scan for subjects with MCI/AD (median = 0.1, IQR = 0.2).
We calculated gray matter (GM) volumes using voxel-based morphometry (VBM) implemented in Statistical Parametric Mapping (SPM) version 12 software (Wellcome Trust Center for Neuroimaging, University College London, UK) as described previously [43]. Structural T1-weighted MR images were segmented into gray matter, white matter, and cerebrospinal fluid. Quality control was performed on all gray matter native space images. Next, images were normalized to Montreal Neurological Institute (MNI) 152 space, using a study specific template created with the DARTEL toolbox. Whole brain gray matter maps were smoothed with an 8 mm full-width at half-maximum Gaussian kernel. We extracted regional gray matter volumes from the automatic anatomic labeling (AAL) using a priori defined cortical ROIs covering all the major brain lobes [43]: medial/lateral temporal, medial/lateral parietal, and occipital and frontal lobes (see Supplementary Table 2) based on the AAL atlas.
Total intracranial volume (TIV) was calculated by combining the native space segmentations (gray matter, white matter, and CSF) for each subject. We used gray matter density (i.e., gray matter volume corrected for TIV) as a proxy of atrophy in the analyses.
[18F]flortaucipir imaging and processing
Dynamic 130 min [18F]flortaucipir PET scans were acquired on a Philips Ingenuity TF-64 PET/CT scanner. The scanning protocol consisted of two dynamic PET scans of 60 and 50 min, respectively, with a 20-min break in between [44, 45]. The first 60 min dynamic scan started simultaneously with a bolus injection 234 ± 16 MBq [18F]flortaucipir (injected mass 1.17 ± 0.78 μg). The second PET scan was co-registered to the first dynamic PET scan using Vinci software [46]. PET list mode data were rebinned into a total of 29 frames (1 × 15, 3 × 5, 3 × 10, 4 × 60, 2 × 150, 2 × 300, 4 × 600, and 10 × 300 seconds) and reconstructed using 3D RAMLA with a matrix size of 128 × 128 × 90 and a final voxel size of 2x2x2 mm3, including standard corrections for dead time, decay, attenuation, random, and scatter. The 3D-T1 MR images were co-registered to the averaged images (frame 8–29) of the PET scan.
The T1-weighted images were co-registered to their corresponding PET images using Vinci (volume imaging in neurological imaging) software [46]. Volumes of interest (VOI, Hammers template [47]) were subsequently delineated on the MR images and superimposed on the PET scan using PVElab [48]. Binding potential (BPND) was generated using receptor parametric mapping (RPM) with whole cerebellar gray matter as a reference region (extracted from the Hammers template [47]). SUVr images were generated for the time interval 80–100 min post injection. BPND (or SUVr) images were resliced to FreeSurfer coordinates and using a MarsBar package in SPM12, BPND (or SUVr) was extracted in the following a priori defined FreeSurfer regions-of-interest (ROIs) on spatially normalized MR images: entorhinal, limbic, and neocortical regions. These ROIs closely aligning the neuropathological tau staging schemes, adjusted from Scholl et al. [15] (see Supplementary Table 3).
Statistical analysis
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS, IBM version 22) and R (v. 3.2.3, The R foundation for Statistical computing).
Differences in demographic, clinical, and AD biomarker characteristics between disease groups were assessed using analyses of variance for continuous variables and χ2 for dichotomous data.
Linear regression models were performed to assess the relationship between entorhinal, limbic, and neocortical [18F]flortaucipir BPND (as dependent variable) and CSF p-tau (as independent variable), adjusted for age, sex, and time lag between LP and tau PET. We performed the analyses across all subjects and additionally stratified for diagnosis (i.e., SCD subjects and MCI/AD) and amyloid status for SCD subjects only. We did not stratify for amyloid status for the rest of the analyses because of the limited amount of amyloid positive SCD subjects (n = 10).
In order to investigate spatial associations between CSF p-tau (independent variable) and [18F]flortaucipir BPND (or SUVr) (dependent variable), we performed a voxel-wise multiple regression analysis for CSF p-tau and [18F]flortaucipir PET, adjusted for age, sex, and time lag between LP and [18F]flortaucipir PET scan. All resulting t-maps were thresholded with p < 0.001 at the voxel-level. Data are shown with and without correction for family-wise error p < 0.05.
Linear regression models were performed to assess the relationships between CSF p-tau and 3 a priori defined [18F]flortaucipir ROIs on PET with cognition. First, we assessed the predictive effects of CSF p-tau and entorhinal, limbic, and neocortical [18F]flortaucipir separately (model 1), followed by a model including both predictors (model 2: CSF p-tau + neocortical [18F]flortaucipir or entorhinal/limbic/neocortical [18F]flortaucipir + CSF p-tau as predictors). Additionally, both models were adjusted for age, sex, education, and time lag between PET/CSF assessments. Finally, we repeated the aforementioned procedure, but replaced cognition with gray matter atrophy (dependent variable) additionally adjusted for TIV.
In addition, all regressions were repeated by replacing BPND with SUVr80-100min and were repeated by replacing CSF p-tau by t-tau. The results for t-tau are presented in the Supplementary Material. Results were comparable for CSF p-tau and t-tau for all analysis.
For all linear regressions, standardized beta’s (sβ) were used as an outcome variable. By standardizing all variables in the equation, we obtain an easy interpretable outcome measure, which is comparable across regressions. The sβ is the change in the outcome variable for 1 standard deviation in change of the predictor. All sβ’s are tested against the null hypothesis that an sβ of 0 yields no effect [49].
Results
Demographics
Subject characteristics are presented in Table 1. Subjects were 64.8 ± 6.5 years old and as expected MCI/AD participant had a lower MMSE score of 23 ± 4 (p < 0.01). In addition, CSF p-tau and [18F]flortaucipir BPND within all brain regions were higher in MCI/AD patients compared to SCD subjects (all p < 0.01).
Table 1.
Demographic, clinical, and AD biomarker characteristics over the total sample and per disease group
| SCD Aβ + (n=10) | SCD Aβ-(n=15) | MCI/AD (n = 53) | Total Sample (n = 78) | Total SCD (n = 25) | |
|---|---|---|---|---|---|
| Age, years | 67 ± 6 | 64 ± 6 | 65 ± 7 | 65 ± 7 | 65 ± 6 |
| Female, % | 60% | 60% | 53% | 43% | 60% |
| No. Aß positive subjects | 10(100%) | 0(0%) | 53 (100%) | 63 (81%) | 10 (40%) |
| Education, Verhage scale, median(range) | 6 (4-7) | 6 (2-7) | 6 (3-7) | 6 (2-7) | 6 (2-7) |
| Time lag LP/PET, years | 0.7 ± 0.6 | 0.9 ± 0.5 | 0.6 ± 0.5 | 0.7 ± 0.5 | 0.8 ± 0.7 |
| Neuropsychological measures | |||||
| MMSE (n=78) | 28 ± 1 | 28 ± 1 | 23 ± 4b | 25 ± 4 | 28 ± 1 |
| Memoryc (n=78) | -0.5 ± 0.8 | 0.3 ± 0.6 | -3.1 ± 2.1b | -2.1 ± 2.3 | -0.0 ± 0.8 |
| Attentiond (n=73) | -0.2 ± 0.6 | 0.1 ± 0.6 | -1.3 ± 1.2b | -0.9 ± 1.2 | -0.0 ± 0.6 |
| Languagee (n=68) | -0.0 ± 0.4 | 0.0 ± 0.8 | -1.0 ± 1.0b | -0.6 ± 1.0 | -0.0 ± 0.7 |
| Executive functioningf (n=73) | -0.1 ± 0.9 | -0.1 ± 0.7 | -2.4 ± 1.0b | -0.9 ± 1.1 | 0.0 ± 0.8 |
| Tau biomarkers | |||||
| CSF | |||||
| CSF Aß1-42 | 779 ± 197 | 1067 ± 217 | 541 ± 113b | 677 ± 260 | 966 ± 247 |
| CSF t-tau | 615 ± 383 | 257 ± 201 | 760 ± 412bd | 645 ± 422 | 401 ± 333 |
| CSF p-tau | 83 ± 36 | 43 ± 23 | 90 ± 35b | 80 ± 38 | 59 ± 38 |
| [18F]flortaucipir PET | |||||
| Entorhinal cortex BPND | 0.2 ± 0.2 | -0.1 ± 0.1 | 0.3 ± 0.2b | 0.2 ± 0.2 | -0.0 ± 0.2 |
| Limbic region BPND | 0.2 ± 0.1 | 0.0 ± 0.0 | 0.4 ± 0.2b | 0.3 ± 0.2 | 0.1 ± 0.1 |
| Neocortex BPND | 0.1 ± 0.1 | -0.0 ± 0.0 | 0.3 ± 0.3b | 0.2 ± 0.3 | 0.0 ± 0.1 |
| Entorhinal cortex SUVr | 1.0 ± 0.1 | 1.3 ± 0.2 | 1.5 ± 0.2b | 1.4 ± 0.3 | 1.1 ± 0.2 |
| Limbic region SUVr | 1.1 ± 0.1 | 1.3 ± 0.2 | 1.5 ± 0.2b | 0.4 ± 0.3 | 1.2 ± 0.1 |
| Neocortex SUVr | 1.1 ± 0.1 | 1.2 ± 0.2 | 1.4 ± 0.3b | 1.3 ± 0.3 | 1. ± 0.1 |
Continuous data shown as mean ± standard deviation, unless specified otherwise. Differences in demographic, clinical, and AD biomarker characteristics between disease groups were assessed using ANOVA for continuous variables and χ2 for dichotomous data
aSignificantly different from SCD subjects at p < 0.05. bSignificantly different from SCD subjects at p < 0.01
cZ-score memory domain, dZ-score attention domain, eZ-score language domain, fZ-score executive functioning domain
The relationship between CSF p-tau and [18F]flortaucipir BPND
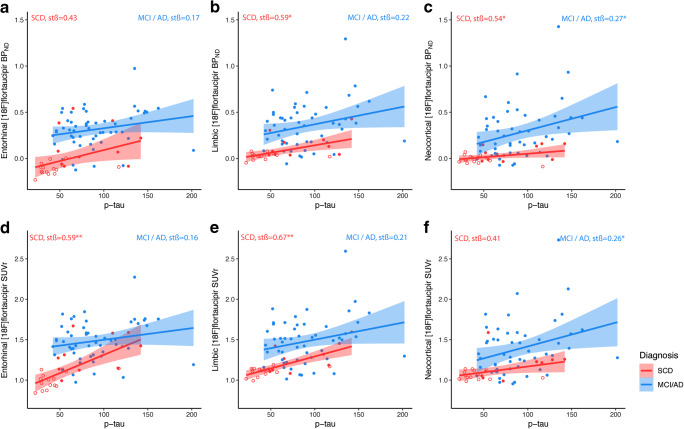
Associations between CSF p-tau and tau PET are presented in Table 2. Using all subjects CSF p-tau was associated with higher [18F]flortaucipir BPND in the entorhinal cortex (sβ = 0.46), limbic (sβ = 0.45), and neocortical region (sβ = 0.43), all p < 0.01. Within-group correlations were stronger for SCD subjects than for MCI/AD patients, with strongest correlations seen in the limbic region (sβ = 0.59, p < 0.01, Table 2, Fig. 1). Within the SCD subjects, the relationship between CSF p-tau and [18F]flortaucipir BPND was driven by the amyloid positive individuals (Fig. 1). Note that the variance of the SCD subjects patients is smaller than for MCI/AD patients, therefore a more gradual slope is observed for association between CSF p-tau and limbic, neocortical tau, although sβs are stronger for SCD. Comparable results were seen for CSF t-tau (Supplementary Table 4, Supplementary Fig. 1).
Table 2.
Standardized ß coefficients for the relationship between CSF p-tau and entorhinal, limbic, and neocortical [18F]flortaucipir BPND or SUVr over the total sample and stratified per disease group
| Total sample (n = 78) |
SCD (n = 25) |
MCI/AD (n = 53) |
|
|---|---|---|---|
| CSF p-tau | CSF p-tau | CSF p-tau | |
| Entorhinal[18F]flortaucipir BPND | 0.46 (p <0.01) | 0.43 (p = 0.07) | 0.17(p = 0.17) |
| Limbic [18F]flortaucipir BPND | 0.45 (p = <0.01) | 0.59a (p = 0.01) | 0.22 (p = 0.08) |
| Neocortical [18F]flortaucipir BPND | 0.43 (p = <0.01) | 0.54 (p = 0.02) | 0.27 (p = 0.03) |
| Entorhinal[18F]flortaucipir SUVr | 0.50 (p = <0.01) | 0.59 (p<0.01) | 0.16 (p = 0.21) |
| Limbic [18F]flortaucipir SUVr | 0.47 (p = <0.01) | 0.67(p = 0.00) | 0.21 (p = 0.09) |
| Neocortical [18F]flortaucipir SUVr | 0.43 (p = <0.01) | 0.41 (p = 0.08) | 0.26 (p = 0.03) |
Standardized ß coefficients (significant in bold) from regression analysis with [18F]flortaucipir BPND or SUVr as the dependent variables and CSF p-tau as predictor.
Effects adjusted for age, sex, and time lag between LP and [18F]flortaucipir PET
Fig. 1.
Scatterplots of the observed relationship between CSF p-tau with entorhinal, limbic, and neocortical [18F]flortaucipir BPND (top row, a–c) and SUVr (bottom row, d–f). Each symbol represents one subject. The fitted lines are stratified over AD (blue) and SCD subjects (red); closed circles are Aβ positive, open circles are Aβ negative. Correlations were adjusted for age, sex, and time lag between LP and [18F]flortaucipir PET scan
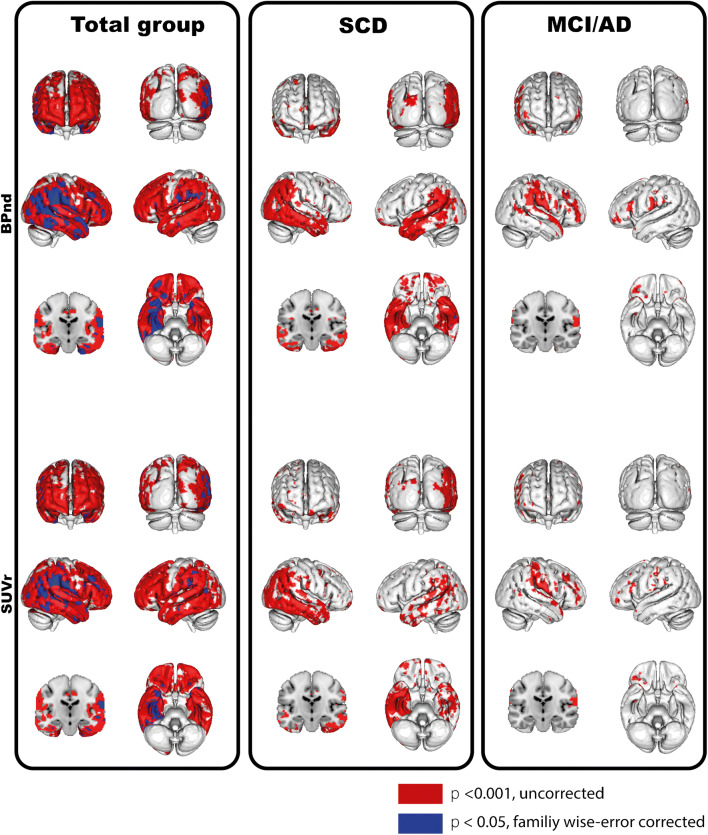
Voxel-wise analyses across all subjects showed significant associations between widespread cortical [18F]flortaucipir binding (right > left binding) and CSF p-tau. In SCD subjects, associations between tau PET and CSF p-tau were mainly observed in temporoparietal regions, whereas in MCI/AD this association was observed in the fronto-temporo-parietal areas (Fig. 2). All results survived family-wise error corrections, except for the results in the MCI/AD group. CSF t-tau showed a comparable, although marginally more widespread pattern than p-tau (Supplementary Fig. 2).
Fig. 2.
Voxel-wise associations between CSF p-tau and [18F]flortaucipir BPND (top row) and SUvr (bottom row). Voxel-wise associations are shown using a threshold puncorrected < 0.001(red) and pFWEcorrected < 0.05 (blue) at the voxel level. Contrasts were adjusted for age, sex, and time lag between LP and [18F]flortaucipir PET scan. The associations were assessed in the total sample, within SCD subjects only and within MCI/AD subjects only
Associations between CSF p-tau and [18F]flortaucipir BPND and cognition
Associations between CSF p-tau and tau PET and cognition are presented in Table 3 and Supplementary Table 1. Across the total group, all tau PET ROIs correlated to all cognitive scores: MMSE and the memory, attention and executive functioning, and language (model 1 with tau PET as a predictor; range sβ = − 0.23 to – 0.64, all p < 0.05). Contrary, CSF p-tau showed only associations with MMSE and memory (model 1; range sβ’s = − 0.26 to – 0.44, all p < 0.01, Table 3).
Table 3.
Standardized ß coefficients for the relationship between cognitive outcome and CSF p-tau or entorhinal, limbic, and neocortical [18F]flortaucipir BPND over the total sample and stratified per disease group
| Total sample (n = 78) |
SCD (n = 25) |
MCI/AD (n = 53) |
||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| CSF p-tau | ||||||
| MMSE | − 0.44b | − 0.21a | 0.07 | 0.20 | − 0.29a | − 0.17 |
| Memory | − 0.26a | − 0.12 | − 0.10 | 0.10 | − 0.05 | − 0.04 |
| Attention | − 0.18 | − 0.01 | − 0.16 | 0.08 | − 0.05 | 0.03 |
| Executive functioning | − 0.14 | 0.06 | 0.28 | 0.48a | − 0.03 | 0.07 |
| Language | − 0.10 | − 0.00 | 0.11 | 0.32 | − 0.00 | 0.01 |
| [18F]flortaucipir BPND | ||||||
| Entorhinal region | ||||||
| MMSE | − 0.41b | − 0.25a | − 0.23 | − 0.21 | − 0.01 | − 0.04 |
| Memory | − 0.54b | − 0.52b | − 0.50b | − 0.50a | − 0.27 | − 0.27 |
| Attention | − 0.14 | − 0.35 | − 0.17 | − 0.14 | 0.43b | 0.45b |
| Executive functioning | − 0.29b | 0.25a | 0.07 | − 0.01 | 0.13 | 0.14 |
| Language | − 0.23a | − 0.22 | − 0.18 | − 0.41 | 0.20 | 0.21 |
| Limbic region | ||||||
| MMSE | − 0.64b | − 0.54b | − 0.22 | − 0.20 | − 0.50b | − 0.45b |
| Memory | − 0.46b | − 0.41b | − 0.24 | − 0.22 | − 0.10 | − 0.09 |
| Attention | − 0.45b | − 0.41b | − 0.29 | − 0.32 | − 0.22 | − 0.21 |
| Executive functioning | − 0.52b | − 0.52b | − 0.06 | − 0.23 | − 0.34a | − 0.34a |
| Language | − 0.33b | − 0.33a | − 0.10 | − 0.24 | − 0.11 | − 0.11 |
| Neocortical region | ||||||
| MMSE | − 0.64b | − 0.53b | − 0.17 | − 0.13 | − 0.50b | − 0.43b |
| Memory | − 0.38b | − 0.29a | − 0.32 | − 0.29 | 0.04 | 0.07 |
| Attention | − 0.55b | − 0.52b | − 0.40a | − 0.42 | − 0.38a | − 0.39a |
| Executive functioning | − 0.56b | − 0.57b | − 0.16 | − 0.31 | − 0.39a | − 0.41a |
| Language | − 0.27a | − 0.25 | − 0.25 | − 0.39 | 0.00 | 0.02 |
Standardized ß coefficients (significant in bold) from multiple regression analysis with cognitive measures as the dependent variables and either CSF p-tau and/or [18F]flortaucipir BPND as predictors using separate analyses
Model 1 = Either CSF p-tau or entorhinal/limbic/neocortical [18F]flortaucipir BPND was used as a predictor. Effects adjusted for age, sex, education, and time lag between cognitive testing and LP or [18F]flortaucipir PET
Model 2 = CSF p-tau + neocortical [18F]flortaucipir BPND or entorhinal/limbic/neocortical [18F]flortaucipir BPND + CSF p-tau were used as predictors. Effects adjusted as model 1
aSignificant standardized ß coefficient at p < 0.05
bSignificant standardized ß coefficient at p < 0.01
When entered simultaneously (model 2), associations between [18F]flortaucipir BPND and cognition (model 2, MMSE, memory, attention, executive functioning, and language; range sβ’s = − 0.25 to – 0.57, all p < 0.05, Table 3) remained essentially comparable, but appeared strongly attenuated for CSF p-tau (model 2, MMSE; sβ = − 0.21, p < 0.05, Table 3).
After stratification, stronger associations were seen in MCI/ AD patients compared to SCD subjects. After correcting for the other biomarker (model 2), regional tau PET, but not CSF p-tau, was related to MMSE (range sβ’s = − 0.43 to – 0.45, all p < 0.01), attention (range sβ’s = − 0.39 to – 0.45, all p < 0.05), and executive functioning (range sβ’s = − 0.34 to – 0.41, all p < 0.05) within the MCI/AD patients. Comparable results for model 1 and model 2 were seen when replacing p-tau with t-tau (Supplementary Table 5).
Associations between CSF p-tau and [18F]flortaucipir BPND and atrophy
Associations between CSF p-tau and tau PET and atrophy are presented in Table 4. Across all subjects, associations were seen between high CSF p-tau with temporal, parietal, and occipital atrophy (model 1; range sβ’s = − 0.20 to – 0.22, all p < 0.05), but disappeared when adjusting for neocortical tau PET. All [18F]flortaucipir ROIs were related to the different atrophy ROIs (model 1; range sβ’s = − 0.27 to – 0.49, all p < 0.05), even after adjusting for CSF p-tau (model 2; range sβ’s = − 0.20 to – 0.51, all p < 0.05). After stratification, these results appeared largely attributable to MCI/AD participants, no significant associations were seen for the SCD subjects. Comparable results for t-tau are presented in Supplementary Table 6.
Table 4.
Standardized ß coefficients for the relationship between regional gray matter atrophy and CSF p-tau or [18F]flortaucipir BPND over the total sample and stratified per disease group
| Total sample (n = 78) |
SCD (n = 25) |
MCI/AD (n = 53) |
||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| CSF p-tau | ||||||
| Medial temporal | − 0.15 | − 0.01 | 0.18 | 0.25 | 0.03 | 0.08 |
| Lateral temporal | − 0.21a | − 0.00 | 0.15 | − 0.01 | − 0.13 | − 0.01 |
| Medial parietal | − 0.17 | 0.04 | 0.17 | 0.29 | − 0.09 | 0.04 |
| Lateral parietal | − 0.20a | 0.02 | 0.25 | 0.33 | − 0.13 | − 0.00 |
| Frontal | − 0.10 | 0.09 | 0.21 | 0.33 | − 0.07 | 0.04 |
| Occipital | − 0.22a | − 0.15 | 0.15 | 0.30 | − 0.18 | − 0.07 |
| [18F]flortaucipir BPND | ||||||
| Entorhinal region | ||||||
| Medial temporal | − 0.42b | − 0.45b | − 0.14 | − 0.22 | − 0.26a | − 0.27a |
| Lateral temporal | − 0.33b | − 0.31b | − 0.09 | − 0.16 | − 0.23 | − 0.20 |
| Medial parietal | − 0.27b | − 0.27b | − 0.08 | − 0.16 | 0.18 | − 0.15 |
| Lateral parietal | − 0.31b | − 0.29b | − 0.10 | − 0.21 | − 0.16 | − 0.12 |
| Frontal | − 0.23a | − 0.25a | − 0.19 | − 0.32 | − 0.11 | − 0.09 |
| Occipital | − 0.27b | − 0.20a | − 0.21 | − 0.32 | − 0.14 | − 0.09 |
| Limbic region | ||||||
| Medial temporal | − 0.38b | − 0.40b | − 0.14 | − 0.20 | − 0.26a | − 0.23 |
| Lateral temporal | − 0.48b | − 0.46b | − 0.07 | − 0.18 | − 0.50b | − 0.49b |
| Medial parietal | − 0.41b | − 0.43b | − 0.11 | − 0.26 | − 0.41b | − 0.40b |
| Lateral parietal | − 0.40b | − 0.41b | − 0.01 | − 0.26 | − 0.37b | − 0.35b |
| Frontal | − 0.31b | − 0.34b | − 0.08 | − 0.26 | − 0.29a | − 0.28a |
| Occipital | − 0.38b | − 0.37b | − 0.16 | − 0.32 | − 0.37b | − 0.34b |
| Neocortical region | ||||||
| Medial temporal | − 0.32b | − 0.33b | − 0.01 | − 0.08 | − 0.18 | − 0.20 |
| Lateral temporal | − 0.47b | − 0.48b | − 0.06 | − 0.13 | − 0.48b | − 0.47b |
| Medial parietal | − 0.45b | − 0.48b | − 0.09 | − 0.18 | − 0.49b | − 0.49b |
| Lateral parietal | − 0.49b | − 0.51 b | − 0.00 | − 0.12 | − 0.50b | − 0.49b |
| Frontal | − 0.39b | − 0.44b | − 0.11 | − 0.24 | − 0.41b | − 0.40b |
| Occipital | − 0.47b | − 0.47b | − 0.18 | − 0.28 | − 0.50b | − 0.42b |
Standardized ß coefficients (significant in bold) from multiple regression analysis with gray matter density as the dependent variable and either CSF p-tau and/or [18F]flortaucipir BPND as predictors using separate analyses
Model 1 = Either CSF p-tau or entorhinal/limbic/neocortical [18F]flortaucipir BPND was used as a predictor. Effects adjusted for age, sex, intracranial volume, and time lag between MRI and LP or [18F]flortaucipir PET
Model 2 = CSF p-tau + neocortical [18F]flortaucipir BPND or entorhinal/limbic/neocortical [18F]flortaucipir BPND + CSF p-tau were used as predictors. Effects adjusted as model 1
aSignificant standardized ß coefficient at p < 0.05
bSignificant standardized ß coefficient at p < 0.01
SUVr vs BPND
Although SUVr overestimated BPND values (Table 1), comparable results were obtained for SUVr and BPND. Overall, higher p-tau was related to higher entorhinal (sβ = 0.50), limbic (sβ = 0.47), and neocortical (sβ = 0.43) [18F]flortaucipir SUVr, all p < 0.01 (Table 2). In line with BPND, within groups, correlations were stronger for SCD subjects, and strongest correlations were seen in the limbic region (sβ = 0.67 p < 0.01, Table 2, Fig. 1). Voxel-wise analysis confirmed comparable associations between [18F]flortaucipir BPND or SUVr and CSF p-tau (Fig. 2), and the associations with cognition and atrophy were essentially the same for SUVr (Supplementary Tables 7, 8) and BPND (Tables 3 and 4).
Discussion
In this study, we examined cross-sectional associations between CSF p-tau and quantitative [18F]flortaucipir PET and their associations with cognition and atrophy. First, we found that higher CSF p-tau was only moderately correlated to higher [18F]flortaucipir binding across the total group. Both tau markers were associated with disease severity, but we found stronger associations for [18F]flortaucipir compared to CSF p-tau in relation to cognition and atrophy, particularly for the MCI/AD group. Taken together, our findings suggest that tau PET may be a better biomarker for tracking Alzheimer’s disease severity.
Our main finding was that tau PET, independently of CSF p-tau, was related to proxies of disease severity in AD. We found associations between various [18F]flortaucipir ROIs, recapitulating the neuropathological tau stages, and attention, memory, language, and executive functioning as well as with brain atrophy. Associations were most pronounced for MCI/AD dementia patients, especially with regard to limbic and neocortical tau deposition and widespread latrophy, which suggest that later neuropathological tau stages measured by [18F]flortaucipir BPND are tightly related to neurodegeneration in clinical AD. In line with the previous studies [15, 22], we showed that tau PET aligns with the neuropathological tau stages by showing a temporoparietal pattern in the SCD subjects and greater involvement of frontal areas in AD subjects. Previous studies also showed stronger associations for frontal, temporal, and parietal tau PET with cognition [11, 50] and temporoparietal [18F]flortaucipir with atrophy [11] compared to CSF p-tau in AD, further supporting the use of especially tau PET to track disease severity. A possible explanation for these differences in associations could be that each tau biomarker reflects different aspects of tau pathology. CSF p-tau rises early in the disease course, possibly even before Aβ PET positivity [51]. Additionally, extracellular soluble forms of pathological tau (as can be measured using CSF) may precede the intracellular aggregated hyperphosphorylated form of tau (as measured by [18F]flortaucipir PET). Therefore, CSF may show greater disparity than tau PET with later markers of disease severity, such as atrophy and cognition [52, 53].
After the initial early rise of CSF p-tau, it may have limited longitudinal changes in prodromal / AD dementia stages [54–56]. However, [18F]flortaucipir binding continues to increase over time [57, 58] suggesting a better ability for [18F]flortaucipir PET to dynamically track neurodegeneration and/or (changes in) cognition [59, 60] on the clinical stage of the disease. This is in line with our study, in which we observed that regional [18F]flortaucipir PET values were higher for MCI/AD patients than for SCD subject with similar p-tau values (Fig. 1). These findings underscore that tau PET may be a more robust marker for disease severity in clinical AD than CSF p-tau, and that tau PET may serve as an important outcome variable for tracking disease severity, for instance, during treatment with disease-modifying or symptomatic drugs. The early plateauing of CSF p-tau in the prodromal phase of AD may also explain the lack of correlation of p-tau and tau PET in MCI/AD patients. Indeed CSF p-tau tends to solely increase in Aβ + cognitively normal individuals and patients with MCI and stabilize or even decrease in AD dementia [54]. Alternatively, results may be partly be explained by differences in age (65 years (present study) vs. 74–77 years) [10, 11]. Early onset AD harbors a greater tau pathology burden and faster progression than late onset AD [61, 62], and studies have shown that age could affect the levels of p-tau [63] and the amount of [18F]flortaucipir uptake [18, 64].
In line with earlier studies [8], we observed a tight link between CSF p-tau and [18F]flortaucipir BPND in SCD. However, other studies did not find a relationship between CSF p-tau and [18F]flortaucipir within cognitively unimpaired controls [9–11], which could be related to the notion that individuals with SCD are at increased risk for AD [65, 66, 67]. However, p-tau may be more sensitive in early stages than [18F]flortaucipir [11, 26, 52], as subtle increases in [18F]flortaucipir binding has been shown in the preclinical stages of AD [15, 19, 60, 68].
Additionally, by using quantitative [18F]flortaucipir BPND, we may have been able to capture modest increases in [18F]flortaucipir uptake in SCD subjects, since we are able to measure small effects with BPND with higher test-rest accuracy than SUVr [69]. Although in this study, we observed comparable results if we replace BPND with SUVr. In line with the previous studies, we found that SUVr overestimated BPND [70]; however, this appeared to only marginally affect the associations of p-tau and tau PET.
Strengths and limitations
The strength of this study is that we used the dynamic tau PET scans resulting in specific tracer to tau binding rather than a semi-quantitative approach (i.e., SUVr) which overestimates true tracer binding as shown in this study.
In addition, we examined these associations between the different tau biomarkers in SCD subjects instead of cognitively unimpaired individuals without cognitive complaints. By not using controls from a populations study, these results may be more generalizable to a memory clinic cohort.
We acknowledge that there was a time lag in our study between CSF tau sampling and performing of the tau PET scan. Ideally, CSF would be collected on the day of the PET scan, and although our results were comparable if we adjusted the time lag between CSF p-tau and tau PET to a maximum of 1 year and we entered the time lag as a covariate in our main analysis, this time lag may have affected our results. Since p-tau levels may rise before tau PET [11, 26, 52], associations may have been stronger when there was a greater time lag.
Conclusion
Regional [18F]flortaucipir PET, more than CSF p-tau, relates to important clinical parameters of disease severity of AD, i.e., cognition and neurodegeneration. As such, tau PET may more accurately reflect disease severity in AD than p-tau.
Electronic supplementary material
(DOCX 18 kb)
(DOCX 14 kb)
(DOCX 16 kb)
(DOCX 14 kb)
(DOCX 18 kb)
(DOCX 18 kb)
(DOCX 18 kb)
(DOCX 18 kb)
Scatterplots of the observed relationship between CSF p-tau with entorhinal, limbic, and neocortical [18F]flortaucipir BPND. Each symbol represents one subject. The fitted lines are stratified over AD (blue) and SCD subjects (red); closed circles are Aβ positive, open circles are Aβ negative. Correlations were adjusted for age, sex, and time lag between LP and [18F]flortaucipir PET scan. (PNG 12830 kb)
Voxel-wise associations between CSF t-tau and [18F]flortaucipir BPND. Voxel-wise associations are shown using a threshold puncorrected < 0.001(red) and pFWEcorrected < 0.05 (blue) at the voxel level. Contrasts were adjusted for age, sex, and time lag between LP and [18F]flortaucipir PET scan. The associations were assessed in the total sample, within SCD subjects only and within MCI/AD subjects only. (PNG 2198 kb)
Acknowledgments
We kindly thank all participants for their contribution. Research of Amsterdam Alzheimer Center is part of the Neurodegeneration program of Amsterdam Neuroscience. The Amsterdam Alzheimer Center is supported by Alzheimer Nederland and Stichting VUmc funds. [18F]Flortaucipir PET scans were made possible by Avid Radiopharmaceuticals Inc.
Funding information
This study was funded by a ZonMW Memorabel grant.
Compliance with ethical standards
Conflict of interest
Wolters, Ossenkoppele, Verfaillie, Coomans, Timmers, Visser, Tuncel, Boellaard, Windhorst, and Teunissen declare that they have no conflict of interest. Van der Flier received grant support from ZonMW, NWO, EU-FP7, Alzheimer Nederland, CardioVascular Onderzoek Nederland, Stichting Dioraphte, Gieskes-Strijbis Fonds, Boehringer Ingelheim, Piramal Neuroimaging, Roche BV, Janssen Stellar, Combinostics. All funding is paid to the institution. WvdF holds the Pasman chair. Van Berckel received research support from ZON-MW, AVID radiopharmaceuticals, CTMM and Janssen Pharmaceuticals. He is a trainer for Piramal and GE. He receives no personal honoraria. Scheltens received grant support (to the institution) from GE Healthcare, Danone Research, Piramal, and MERCK. In the past 2 years he has received consultancy/speaker fees from Lilly, GE Healthcare, Novartis, Forum, Sanofi, Nutricia, Probiodrug, and EIP Pharma. All funding is paid to the institution. No other potential conflicts of interest relevant to this article exist.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
This article is part of the Topical Collection on Neurology
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/9/2020
A Correction to this paper has been published: 10.1007/s00259-020-04849-0
References
- 1.Giannakopoulos P, Herrmann FR, Bussiere T, Bouras C, Kovari E, Perl DP, et al. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology. 2003;60:1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- 2.Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631. doi: 10.1212/WNL.42.3.631. [DOI] [PubMed] [Google Scholar]
- 4.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 5.Chien DT, Bahri S, Szardenings AK, Walsh JC, Mu F, Su MY, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimers Dis. 2013;34:457–468. doi: 10.3233/JAD-122059. [DOI] [PubMed] [Google Scholar]
- 6.Xia CF, Arteaga J, Chen G, Gangadharmath U, Gomez LF, Kasi D, et al. [(18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer's disease. Alzheimers Dement. 2013;9:666–676. doi: 10.1016/j.jalz.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Marquie M, Normandin MD, Vanderburg CR, Costantino I, Bien EA, Rycyna LG, et al. Validating novel tau PET tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 2015. 10.1002/ana.24517. [DOI] [PMC free article] [PubMed]
- 8.Chhatwal JP, Schultz AP, Marshall GA, Boot B, Gomez-Isla T, Dumurgier J, et al. Temporal T807 binding correlates with CSF tau and phospho-tau in normal elderly. Neurology. 2016;87:920–926. doi: 10.1212/Wnl.0000000000003050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon BA, Friedrichsen K, Brier M, Blazey T, Su Y, Christensen J, et al. The relationship between cerebrospinal fluid markers of Alzheimer pathology and positron emission tomography tau imaging. Brain. 2016;139:2249–2260. doi: 10.1093/brain/aww139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, et al. Tau and Abeta imaging, CSF measures, and cognition in Alzheimer's disease. Sci Transl Med. 2016;8:338ra66. doi: 10.1126/scitranslmed.aaf2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattsson N, Scholl M, Strandberg O, Smith R, Palmqvist S, Insel PS, et al. (18)F-AV-1451 and CSF T-tau and P-tau as biomarkers in Alzheimer's disease. EMBO Mol Med. 2017;9:1212–1223. doi: 10.15252/emmm.201707809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattsson N, Smith R, Strandberg O, Palmqvist S, Scholl M, Insel PS, et al. Comparing (18)F-AV-1451 with CSF t-tau and p-tau for diagnosis of Alzheimer disease. Neurology. 2018;90:e388–ee95. doi: 10.1212/WNL.0000000000004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallin AK, Blennow K, Zetterberg H, Londos E, Minthon L, Hansson O. CSF biomarkers predict a more malignant outcome in Alzheimer disease. Neurology. 2010;74:1531–1537. doi: 10.1212/WNL.0b013e3181dd4dd8. [DOI] [PubMed] [Google Scholar]
- 14.Herukka SK, Pennanen C, Soininen H, Pirttila T. CSF A beta 42, tau and phosphorylated tau correlate with medial temporal lobe atrophy (vol 14, pg 51, 2008) J Alzheimers Dis. 2008;15:347. doi: 10.3233/JAD-2008-15216. [DOI] [PubMed] [Google Scholar]
- 15.Scholl M, Lockhart SN, Schonhaut DR, O'Neil JP, Janabi M, Ossenkoppele R, et al. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89:971–982. doi: 10.1016/j.neuron.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aschenbrenner AJ, Gordon BA, Benzinger TLS, Morris JC, Hassenstab JJ. Influence of tau PET, amyloid PET, and hippocampal volume on cognition in Alzheimer disease. Neurology. 2018;91:e859–ee66. doi: 10.1212/WNL.0000000000006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79:110–119. doi: 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ossenkoppele R, Schonhaut DR, Scholl M, Lockhart SN, Ayakta N, Baker SL, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. 2016;139:1551–1567. doi: 10.1093/brain/aww027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ossenkoppele R, Smith R, Ohlsson T, Strandberg O, Mattsson N, Insel PS, et al. Associations between tau, Abeta, and cortical thickness with cognition in Alzheimer disease. Neurology. 2019;92:e601–ee12. doi: 10.1212/WNL.0000000000006875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Benzinger TL, Su Y, Christensen J, Friedrichsen K, Aldea P, et al. Evaluation of tau imaging in staging Alzheimer disease and revealing interactions between beta-amyloid and tauopathy. Jama Neurol. 2016;73:1070–1077. doi: 10.1001/jamaneurol.2016.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iaccarino L, Tammewar G, Ayakta N, Baker SL, Bejanin A, Boxer AL, et al. Local and distant relationships between amyloid, tau and neurodegeneration in Alzheimer's disease. Neuroimage Clin. 2018;17:452–464. doi: 10.1016/j.nicl.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maass A, Landau S, Baker SL, Horng A, Lockhart SN, La Joie R, et al. Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer's disease. Neuroimage. 2017;157:448–463. doi: 10.1016/j.neuroimage.2017.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buerger K, Alafuzoff I, Ewers M, Pirttila T, Zinkowski R, Hampel H. No correlation between CSF tau protein phosphorylated at threonine 181 with neocortical neurofibrillary pathology in Alzheimer's disease. Brain. 2007;130:e82. doi: 10.1093/brain/awm140. [DOI] [PubMed] [Google Scholar]
- 24.Engelborghs S, Sleegers K, Cras P, Brouwers N, Serneels S, De Leenheir E, et al. No association of CSF biomarkers with APOEepsilon4, plaque and tangle burden in definite Alzheimer's disease. Brain. 2007;130:2320–2326. doi: 10.1093/brain/awm136. [DOI] [PubMed] [Google Scholar]
- 25.Sato C, Barthelemy NR, Mawuenyega KG, Patterson BW, Gordon BA, Jockel-Balsarotti J, et al. Tau kinetics in neurons and the human central nervous system. Neuron. 2018;97:1284–1298. doi: 10.1016/j.neuron.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer PF, Binette AP, Gonneaud J, Breitner JCS, Villeneuve S. Characterization of Alzheimer disease biomarker discrepancies using cerebrospinal fluid phosphorylated tau and AV1451 positron emission tomography. JAMA Neurol. 2020. 10.1001/jamaneurol.2019.4749. [DOI] [PMC free article] [PubMed]
- 27.Golla SSV, Timmers T, Ossenkoppele R, Groot C, Verfaillie S, Scheltens P, et al. Quantification of tau load using [(18)F]AV1451 PET. Mol Imaging Biol. 2017;19:963–971. doi: 10.1007/s11307-017-1080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lammertsma AA. Forward to the past: the case for quantitative PET imaging. J Nucl Med. 2017;58:1019–1024. doi: 10.2967/jnumed.116.188029. [DOI] [PubMed] [Google Scholar]
- 29.van der Flier WM, Pijnenburg YA, Prins N, Lemstra AW, Bouwman FH, Teunissen CE, et al. Optimizing patient care and research: the Amsterdam dementia cohort. J Alzheimers Dis. 2014;41:313–327. doi: 10.3233/JAD-132306. [DOI] [PubMed] [Google Scholar]
- 30.van der Flier WM, Scheltens P. Amsterdam dementia cohort: performing research to optimize care. J Alzheimers Dis. 2018;62:1091–1111. doi: 10.3233/JAD-170850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chetelat G, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golla SS, Verfaillie SC, Boellaard R, Adriaanse SM, Zwan MD, Schuit RC, et al. Quantification of [(18)F]florbetapir: a test-retest tracer kinetic modelling study. J Cereb Blood Flow Metab. 2018:271678X18783628. 10.1177/0271678X18783628. [DOI] [PMC free article] [PubMed]
- 35.Duits FH, Teunissen CE, Bouwman FH, Visser PJ, Mattsson N, Zetterberg H, et al. The cerebrospinal fluid "Alzheimer profile": easily said, but what does it mean? Alzheimers Dement. 2014;10:713–723. doi: 10.1016/j.jalz.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 36.Seibyl J, Catafau AM, Barthel H, Ishii K, Rowe CC, Leverenz JB, et al. Impact of training method on the robustness of the visual assessment of 18F-Florbetaben PET scans: results from a Phase-3 study. J Nucl Med. 2016;57:900–906. doi: 10.2967/jnumed.115.161927. [DOI] [PubMed] [Google Scholar]
- 37.Zwan M, van Harten A, Ossenkoppele R, Bouwman F, Teunissen C, Adriaanse S, et al. Concordance between cerebrospinal fluid biomarkers and [11C]PIB PET in a memory clinic cohort. Journal of Alzheimer's disease : JAD. 2014;41:801–807. doi: 10.3233/JAD-132561. [DOI] [PubMed] [Google Scholar]
- 38.Teunissen CE, Petzold A, Bennett JL, Berven FS, Brundin L, Comabella M, et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology. 2009;73:1914–1922. doi: 10.1212/WNL.0b013e3181c47cc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tijms BM, Willemse EAJ, Zwan MD, Mulder SD, Visser PJ, van Berckel BNM, et al. Unbiased approach to counteract upward drift in cerebrospinal fluid Amyloid-beta 1-42 analysis results. Clin Chem. 2018;64:576–585. doi: 10.1373/clinchem.2017.281055. [DOI] [PubMed] [Google Scholar]
- 40.Mulder C, Verwey NA, van der Flier WM, Bouwman FH, Kok A, van Elk EJ, et al. Amyloid-beta(1-42), total tau, and phosphorylated tau as cerebrospinal fluid biomarkers for the diagnosis of Alzheimer disease. Clin Chem. 2010;56:248–253. doi: 10.1373/clinchem.2009.130518. [DOI] [PubMed] [Google Scholar]
- 41.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 42.Groot C, van Loenhoud AC, Barkhof F, van Berckel BN, Koene T, Teunissen CC, et al. Differential effects of cognitive reserve and brain reserve on cognition in Alzheimer disease. Neurology. 2018;90:e149–ee56. doi: 10.1212/WNL.0000000000004802. [DOI] [PubMed] [Google Scholar]
- 43.Timmers T, Ossenkoppele R, Wolters EE, Verfaillie SCJ, Visser D, Golla SSV, et al. Associations between quantitative [(18)F]flortaucipir tau PET and atrophy across the Alzheimer's disease spectrum. Alzheimers Res Ther. 2019;11:60. doi: 10.1186/s13195-019-0510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golla SSV, Timmers T, Ossenkoppele R, Groot C, Verfaillie S, Scheltens P, et al. Quantification of tau load using [18F]AV1451 PET. Mol Imaging Biol. 2017;19:963–971. doi: 10.1007/s11307-017-1080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolters EE, Golla SSV, Timmers T, Ossenkoppele R, van der Weijden CWJ, Scheltens P, et al. A novel partial volume correction method for accurate quantification of [(18)F] flortaucipir in the hippocampus. EJNMMI Res. 2018;8:79. doi: 10.1186/s13550-018-0432-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vollmar S, Michel C, Treffert JT, Newport DF, Casey M, Knoss C, et al. HeinzelCluster: accelerated reconstruction for FORE and OSEM3D. Phys Med Biol. 2002;47:2651–2658. doi: 10.1088/0031-9155/47/15/307. [DOI] [PubMed] [Google Scholar]
- 47.Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19:224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Svarer C, Madsen K, Hasselbalch SG, Pinborg LH, Haugbol S, Frokjaer VG, et al. MR-based automatic delineation of volumes of interest in human brain PET images using probability maps. Neuroimage. 2005;24:969–979. doi: 10.1016/j.neuroimage.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 49.Standardized LR, Coefficients R. Encyclopedia of statistics in behavioral Science. 2005. [Google Scholar]
- 50.La Joie R, Bejanin A, Fagan AM, Ayakta N, Baker SL, Bourakova V, et al. Associations between [(18)F]AV1451 tau PET and CSF measures of tau pathology in a clinical sample. Neurology. 2018;90:e282–ee90. doi: 10.1212/WNL.0000000000004860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palmqvist S, Insel PS, Stomrud E, Janelidze S, Zetterberg H, Brix B, et al. Cerebrospinal fluid and plasma biomarker trajectories with increasing amyloid deposition in Alzheimer's disease. EMBO Mol Med. 2019:e11170. 10.15252/emmm.201911170. [DOI] [PMC free article] [PubMed]
- 52.Sato C, Barthelemy NR, Mawuenyega KG, Patterson BW, Gordon BA, Jockel-Balsarotti J, et al. Tau kinetics in neurons and the human central nervous system. Neuron. 2018;98:861–864. doi: 10.1016/j.neuron.2018.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jack CR, Holtzman DM. Biomarker modeling of Alzheimer's disease. Neuron. 2013;80:1347–1358. doi: 10.1016/j.neuron.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sutphen CL, McCue L, Herries EM, Xiong C, Ladenson JH, Holtzman DM, et al. Longitudinal decreases in multiple cerebrospinal fluid biomarkers of neuronal injury in symptomatic late onset Alzheimer's disease. Alzheimers Dement. 2018;14:869–879. doi: 10.1016/j.jalz.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O. Cerebrospinal fluid levels of β-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry. 2012;69:98–106. doi: 10.1001/archgenpsychiatry.2011.155. [DOI] [PubMed] [Google Scholar]
- 56.Blennow K, Zetterberg H, Minthon L, Lannfelt L, Strid S, Annas P, et al. Longitudinal stability of CSF biomarkers in Alzheimer's disease. Neurosci Lett. 2007;419:18–22. doi: 10.1016/j.neulet.2007.03.064. [DOI] [PubMed] [Google Scholar]
- 57.Jack CR, Jr, Wiste HJ, Schwarz CG, Lowe VJ, Senjem ML, Vemuri P, et al. Longitudinal tau PET in ageing and Alzheimer's disease. Brain. 2018;141:1517–1528. doi: 10.1093/brain/awy059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pontecorvo MJ, Devous MD, Kennedy I, Navitsky M, Lu M, Galante N, et al. A multicentre longitudinal study of flortaucipir (18F) in normal ageing, mild cognitive impairment and Alzheimer's disease dementia. Brain. 2019;142:1723–1735. doi: 10.1093/brain/awz090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harrison TM, La Joie R, Maass A, Baker SL, Swinnerton K, Fenton L, et al. Longitudinal tau accumulation and atrophy in aging and alzheimer disease. Ann Neurol. 2019;85:229–240. doi: 10.1002/ana.25406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanseeuw BJ, Betensky RA, Jacobs HIL, Schultz AP, Sepulcre J, Becker JA, et al. Association of amyloid and tau with cognition in preclinical Alzheimer disease: a longitudinal study. JAMA Neurol. 2019. 10.1001/jamaneurol.2019.1424. [DOI] [PMC free article] [PubMed]
- 61.Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 62.Ho GJ, Hansen LA, Alford MF, Foster K, Salmon DP, Galasko D, et al. Age at onset is associated with disease severity in Lewy body variant and Alzheimer's disease. Neuroreport. 2002;13:1825–1828. doi: 10.1097/00001756-200210070-00028. [DOI] [PubMed] [Google Scholar]
- 63.Mattsson N, Rosen E, Hansson O, Andreasen N, Parnetti L, Jonsson M, et al. Age and diagnostic performance of Alzheimer disease CSF biomarkers. Neurology. 2012;78:468–476. doi: 10.1212/WNL.0b013e3182477eed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scholl M, Ossenkoppele R, Strandberg O, Palmqvist S. Swedish bio Fs, Jogi J, et al. distinct 18F-AV-1451 tau PET retention patterns in early- and late-onset Alzheimer's disease. Brain. 2017;140:2286–2294. doi: 10.1093/brain/awx171. [DOI] [PubMed] [Google Scholar]
- 65.Slot RER, Verfaillie SCJ, Overbeek JM, Timmers T, Wesselman LMP, Teunissen CE, et al. Subjective cognitive impairment cohort (SCIENCe): study design and first results. Alzheimers Res Ther. 2018;10:76. 10.1186/s13195-018-0390-y. [DOI] [PMC free article] [PubMed]
- 66.Slot RER, Sikkes SAM, Berkhof J, Brodaty H, Buckley R, Cavedo E, et al. Subjective cognitive decline and rates of incident Alzheimer's disease and non-Alzheimer's disease dementia. Alzheimers Dement. 2019;15:465–476. doi: 10.1016/j.jalz.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Timmers T, Ossenkoppele R, Verfaillie SCJ, van der Weijden CWJ, Slot RER, Wesselman LMP, et al. Amyloid PET and cognitive decline in cognitively normal individuals: the SCIENCe project. Neurobiol Aging. 2019;79:50–58. doi: 10.1016/j.neurobiolaging.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 68.Sperling RA, Mormino EC, Schultz AP, Betensky RA, Papp KV, Amariglio RE, et al. The impact of amyloid-beta and tau on prospective cognitive decline in older individuals. Ann Neurol. 2019;85:181–193. doi: 10.1002/ana.25395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Timmers T, Ossenkoppele R, Visser D, Tuncel H, Wolters E, Verfaillie SCJ, et al. Test-retest repeatability of [18F]flortaucipir PET in Alzheimer’s disease and cognitively normal individuals. J Cereb Blood Flow Metab. 2019; in press. [DOI] [PMC free article] [PubMed]
- 70.van Berckel BN, Ossenkoppele R, Tolboom N, Yaqub M, Foster-Dingley JC, Windhorst AD, et al. Longitudinal amyloid imaging using 11C-PiB: methodologic considerations. J Nucl Med. 2013;54:1570–1576. doi: 10.2967/jnumed.112.113654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 18 kb)
(DOCX 14 kb)
(DOCX 16 kb)
(DOCX 14 kb)
(DOCX 18 kb)
(DOCX 18 kb)
(DOCX 18 kb)
(DOCX 18 kb)
Scatterplots of the observed relationship between CSF p-tau with entorhinal, limbic, and neocortical [18F]flortaucipir BPND. Each symbol represents one subject. The fitted lines are stratified over AD (blue) and SCD subjects (red); closed circles are Aβ positive, open circles are Aβ negative. Correlations were adjusted for age, sex, and time lag between LP and [18F]flortaucipir PET scan. (PNG 12830 kb)
Voxel-wise associations between CSF t-tau and [18F]flortaucipir BPND. Voxel-wise associations are shown using a threshold puncorrected < 0.001(red) and pFWEcorrected < 0.05 (blue) at the voxel level. Contrasts were adjusted for age, sex, and time lag between LP and [18F]flortaucipir PET scan. The associations were assessed in the total sample, within SCD subjects only and within MCI/AD subjects only. (PNG 2198 kb)