Abstract
Background
Cytokine storm is a marker of coronavirus disease 2019 (COVID-19) illness severity and increased mortality. Immunomodulatory treatments have been repurposed to improve mortality outcomes.
Research Question
Do immunomodulatory therapies improve survival in patients with COVID-19 cytokine storm (CCS)?
Study Design and Methods
We conducted a retrospective analysis of electronic health records across the Northwell Health system. COVID-19 patients hospitalized between March 1, 2020, and April 24, 2020, were included. CCS was defined by inflammatory markers: ferritin, > 700 ng/mL; C-reactive protein (CRP), > 30 mg/dL; or lactate dehydrogenase (LDH), > 300 U/L. Patients were subdivided into six groups: no immunomodulatory treatment (standard of care) and five groups that received either corticosteroids, anti-IL-6 antibody (tocilizumab), or anti-IL-1 therapy (anakinra) alone or in combination with corticosteroids. The primary outcome was hospital mortality.
Results
Five thousand seven hundred seventy-six patients met the inclusion criteria. The most common comorbidities were hypertension (44%-59%), diabetes (32%-46%), and cardiovascular disease (5%-14%). Patients most frequently met criteria with high LDH (76.2%) alone or in combination, followed by ferritin (63.2%) and CRP (8.4%). More than 80% of patients showed an elevated D-dimer. Patients treated with corticosteroids and tocilizumab combination showed lower mortality compared with patients receiving standard-of-care (SoC) treatment (hazard ratio [HR], 0.44; 95% CI, 0.35-0.55; P < .0001) and with patients treated with corticosteroids alone (HR, 0.66; 95% CI, 0.53-0.83; P = .004) or in combination with anakinra (HR, 0.64; 95% CI, 0.50-0.81; P = .003). Corticosteroids when administered alone (HR, 0.66; 95% CI, 0.57-0.76; P < .0001) or in combination with tocilizumab (HR, 0.43; 95% CI, 0.35-0.55; P < .0001) or anakinra (HR, 0.68; 95% CI, 0.57-0.81; P < .0001) improved hospital survival compared with SoC treatment.
Interpretation
The combination of corticosteroids with tocilizumab showed superior survival outcome when compared with SoC treatment as well as treatment with corticosteroids alone or in combination with anakinra. Furthermore, corticosteroid use either alone or in combination with tocilizumab or anakinra was associated with reduced hospital mortality for patients with CCS compared with patients receiving SoC treatment.
Key Words: anakinra, coronavirus, corticosteroids, infection, SARS-CoV-2, tocilizumab
Abbreviations: A, anakinra only; CCS, coronavirus disease 2019 cytokine storm; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; HR, hazard ratio; IMV, invasive mechanical ventilation; LDH, lactate dehydrogenase; S, corticosteroids only; SA, corticosteroids and anakinra; SoC, standard-of-care; ST, corticosteroids and tocilizumab; T, tocilizumab only
In March 2020, New York City and its metropolitan area became the epicenter for coronavirus disease 2019 (COVID-19) in the United States, with more than 250,000 cases and more than 17,000 deaths by early May 2020.2 Throughout this outbreak, physicians and scientists have struggled to understand the pathogenesis and clinical course of this infection. Early retrospective data from China and Italy showed increased mortality in those with elevated inflammatory markers, such as ferritin, C-reactive protein (CRP), lactate dehydrogenase (LDH), IL-6, and D-dimer.3 Uncontrolled and unabated cytokine release and a hyperinflammatory response, termed the COVID-19 cytokine storm (CCS), was described as a major determinant of poor survival.4
Limited data existed to guide clinical decision-making in the absence of Food and Drug Administration-approved COVID-19-specific therapies. Faced with rapidly increasing rates of infection and hospitalizations, physicians repurposed immunomodulatory treatments in an attempt to curtail morbidity and mortality. Although initial reports discouraged the use of corticosteroids, later publications suggested survival benefits.3 , 5 , 6 Small retrospective studies reported improved outcomes in CCS by using anti-IL-6 (ie, tocilizumab [Roche]) and anti-IL-1 therapies (ie, anakinra [Sobi])7, 8, 9 that are used commonly for inflammatory conditions such as cytokine release syndrome and macrophage-activation syndrome. Further evidence supporting the use of anti-IL-1 was based on previous reports of improved survival in a subgroup of patients with sepsis and hyperferritinemia.10
Within Northwell Health, the largest private nonprofit health system in New York State, a multidisciplinary committee consisting of pulmonology, infectious disease, immunology, and rheumatology specialists was formed to create COVID-19 treatment protocols. This included the identification of CCS, which we defined as ferritin > 700 ng/mL11 or CRP > 30 mg/dL3 , 12 or LDH > 300 U/L.3 Treatment protocols with corticosteroids, tocilizumab, and anakinra as potential immunomodulatory therapies were based on the available literature at the time.3 , 11 , 12 Because of the rapidly evolving data and surge of patients in a short period, wide variation in the use of these drugs occurred across the health system. In this retrospective study, we leveraged this natural experiment to compare mortality in patients meeting criteria for CCS who received different combinations of these immunomodulatory drugs.
Methods
Study Population
We retrospectively analyzed electronic health record data of patients admitted to the 12 hospitals and EDs within the Northwell Health system between March 1, 2020, and April 24, 2020. The institutional review board for the Feinstein Institutes of Medical Research at Northwell Health approved this study as minimal-risk research and waived the requirement for informed consent. Inclusion criteria were: COVID-19 positivity as determined by polymerase chain reaction testing of nasopharyngeal swabs; age older than 18 years; and meeting CCS criteria of ferritin > 700 ng/mL11 or CRP > 30 mg/dL3 , 12 or LDH > 300 U/L3 (e-Fig 1). T0 was identified as the time at which a patient first met this definition. Patients who received any of the prespecified immunomodulatory drugs before T0 were excluded from this study.
Group Definition
Six groups were identified based on whether they received any of the predefined immunomodulatory drugs. One group consisted of those who received none of the medications, labeled as the standard-of-care (SoC) group. Five treatment groups received varying combinations of the three immunomodulatory drugs: corticosteroids only (S), corticosteroids and tocilizumab (ST), corticosteroids and anakinra (SA), tocilizumab only (T), and anakinra only (A). In the timeframe of this analysis, hydroxychloroquine, azithromycin, colchicine, and vitamin C, either alone or in combination, were administered to COVID-19 patients as part of institutional protocols (e-Table 1).
Statistical Methods
The primary objective was to compare in-hospital mortality among COVID-19 patients with CCS who received combinations of immunomodulatory treatments vs SoC treatment. Potentially confounding variables (covariates) were included in the multivariate model based on clinical experience and the COVID-19 literature at the time. These included demographic data such as age, sex, race or ethnicity, smoking history, insurance status, and whether patients were treated in a tertiary vs community medical center. Comorbidities examined included chronic lung disease (ie, asthma, COPD), cardiovascular disease, hypertension, diabetes, renal disease, hemodialysis, liver disease, cancer, autoimmune disease, Charlson comorbidity index, and BMI. Laboratory data included CRP, ferritin, D-dimer, LDH, hemoglobin, platelet count, serum sodium, serum transaminases, and neutrophil-to-lymphocyte ratio. We also included disease severity surrogates, such as use of invasive mechanical ventilation (IMV; at any time before T0) and vasopressor use (within 24 h of T0).
Statistical Analyses
Treatment groups were compared using demographic variables, comorbidities, and baseline laboratory values using the χ 2, Fisher exact, or Kruskal-Wallis tests, as appropriate. Categorical variables were summarized using percentages. Continuous variables were summarized using medians with 25th to 75th percentiles. Laboratories considered clinically important were included in the analysis. Baseline laboratory values in this study were defined as the value closest to T0 within the 96 h before T0. Exceptions were for CRP, ferritin, and D-dimer, which were defined as within 96 h before T0 and up to 12 h after T0 because of laboratory ordering practices. Patient survival was calculated from T0 to the time of in-hospital death. Data from patients discharged from the hospital or remaining in the hospital on April 24, 2020, were considered censored.
Patient survival was compared between treatment groups using the Cox regression model, adjusting for all covariates outlined above. The proportional hazards assumption was assessed and deemed acceptable. PROC MI (SAS version 9.4 software [SAS Institute]), with all variables from Table 1 , was used for the multiple imputation. Missing laboratory values were handled using multiple imputation, using 50 imputed datasets. We used the fully conditional method with a discriminant function for the imputation of the laboratory categories (eg, low, normal, or high, as specified in Table 2 ). Holm’s stepdown procedure for multiple comparisons was used to account for the 15 pairwise tests resulting from the six groups. The final model included all clinically important covariates regardless of their statistical significance (the full model). SAS version 9.4 software was used for the statistical analysis. Results were considered statistically significant if P < .05.
Table 1.
Patient Demographics
| Variable | Missing Data | Standard of Care (N = 3,076) | Steroids Only (n = 1,383) | Steroids Plus Tocilizumab (n = 454) | Steroids Plus Anakinra (n = 733) | Tocilizumab Only (n = 73) | Anakinra Only (n = 57) | P Value |
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Age, y | 64.6 (53.5-76.4) | 66.5 (55.8-76.9) | 64.5 (54.9-73.1) | 65.7 (56.5-74.7) | 62.4 (55.1-68.7) | 66.7 (57.6-74.6) | .01 | |
| Sex | ||||||||
| Female | 1,185 (38.5) | 489 (35.4) | 123 (27.1) | 238 (32.5) | 21 (28.8) | 19 (33.3) | < .0001 | |
| Male | 1,891 (61.5) | 894 (64.6) | 331 (72.9) | 495 (67.5) | 52 (71.2) | 38 (66.7) | ||
| Race | ||||||||
| White | 1,021 (33.2) | 474 (34.3) | 163 (35.9) | 208 (28.4) | 239(39.7) | 12 (21.1) | .0013 | |
| Black | 656 (21.3) | 293 (21.2) | 68 (15) | 139 (19) | 8 (11) | 17 (29.8) | ||
| Asian | 372 (12.1) | 161 (11.6) | 64 (14.1) | 98 (13.4) | 8 (11) | 1 (1.8) | ||
| Other/multiracial | 867 (28.2) | 372 (26.9) | 132 (29.1) | 243 (33.2) | 24 (32.9) | 23 (40.4) | ||
| Unknown | 160 (5.2) | 83 (6) | 27 (5.9) | 45 (6.1) | 4 (5.5) | 4 (7) | ||
| Ethnicity | ||||||||
| Hispanic or Latino | 671 (21.8) | 319 (23.1) | 119 (26.2) | 171 (23.3) | 13 (17.8) | 12 (21.1) | .41 | |
| Non-Hispanic or Latino | 2,141 (69.6) | 944 (68.3) | 295 (65) | 513 (70) | 55 (75.3) | 42 (73.7) | ||
| Other/unknown | 264 (8.6) | 120 (8.7) | 40 (8.8) | 49 (6.7) | 5 (6.8) | 3 (5.3) | ||
| Insurance | ||||||||
| Commercial | 916 (29.8) | 410 (29.6) | 159 (35) | 230 (31.4) | 35 (47.9) | 15 (26.3) | < .0001 | |
| Medicare | 1,354 (44) | 656 (47.4) | 178 (39.2) | 319 (43.5) | 26 (35.6) | 27 (47.4) | ||
| Medicaid | 634 (20.6) | 271 (19.6) | 103 (22.7) | 162 (22.1) | 12 (16.4) | 14 (24.6) | ||
| Self-pay | 49 (1.6) | 30 (2.2) | 7 (1.5) | 15 (2) | 0 (0) | 0 (0) | ||
| Other | 123 (4) | 16 (1.2) | 7 (1.5) | 7 (1) | 0 (0) | 1 (1.8) | ||
| Smoking status | ||||||||
| Active | 67 (2.2) | 22 (1.6) | 10 (2.2) | 18 (2.5) | 1 (1.4) | 2 (3.5) | .15 | |
| Former | 426 (13.8) | 212 (15.3) | 71 (15.6) | 107 (14.6) | 13 (17.8) | 9 (15.8) | ||
| Never | 2,203 (71.6) | 971 (70.2) | 305 (67.2) | 523 (71.4) | 54 (74) | 41 (71.9) | ||
| Smoker/status unknown | 122 (4) | 38 (2.7) | 17 (3.7) | 14 (1.9) | 3 (4.1) | 2 (3.5) | ||
| Unknown | 258 (8.4) | 140 (10.1) | 51 (11.2) | 71 (9.7) | 2 (2.7) | 3 (5.3) | ||
| Hospital status | ||||||||
| Community | 1,108 (36) | 414 (29.9) | 142 (31.3) | 169 (23.1) | 17 (23.3) | 15 (26.3) | < .0001 | |
| Tertiary | 1,968 (64) | 969 (70.1) | 312 (68.7) | 564 (76.9) | 56 (76.7) | 42 (73.7) | ||
| Comorbidities | ||||||||
| BMI, kg/m2 | 808 (14) | |||||||
| 18.5-24.9 | 666 (25.9) | 270 (22.1) | 75 (18.2) | 152 (23.2) | 23 (37.1) | 18 (36.7) | .004 | |
| < 18.5 | 286 (11.1) | 139 (11.4) | 41 (10) | 71 (10.8) | 1 (1.6) | 5 (10.2) | ||
| 25-29.9 | 821 (31.9) | 405 (33.2) | 149 (36.3) | 215 (32.8) | 14 (22.6) | 12 (24.5) | ||
| ≥ 30 | 799 (31.1) | 405 (33.2) | 146 (35.5) | 217 (33.1) | 24 (38.7) | 14 (28.6) | ||
| Charlson comorbidity index | 1 (0.02) | |||||||
| 0 | 333 (10.8) | 102 (7.4) | 30 (6.6) | 48 (6.5) | 4 (5.5) | 3 (5.3) | < .0001 | |
| 1-2 | 683 (22.2) | 184 (20.5) | 127 (28) | 182 (24.8) | 14 (19.2) | 15 (26.3) | ||
| 3-4 | 710 (23.1) | 353 (25.5) | 133 (29.3) | 221 (30.2) | 26 (35.6) | 14 (24.6) | ||
| ≥ 5 | 1,349 (43.9) | 644 (46.6) | 164 (36.1) | 282 (38.5) | 29 (39.7) | 25 (43.9) | ||
| Asthma | 134 (4.4) | 105 (7.6) | 25 (5.5) | 48 (6.5) | 9 (12.3) | 2 (3.5) | .01 | |
| COPD | 88 (2.9) | 67 (4.8) | 14 (3.1) | 31 (4.2) | 1 (1.4) | 3 (5.3) | .02 | |
| HTN | 1,454 (47.3) | 682 (49.3) | 224 (49.3) | 379 (51.7) | 43 (58.9) | 25 (43.9) | .11 | |
| DM | 980 (31.9) | 460 (33.3) | 154 (33.9) | 241 (32.9) | 27 (37) | 26 (45.6) | .27 | |
| Cardiovascular disease | 393 (12.8) | 181 (13.1) | 59 (13) | 88 (12) | 10 (13.7) | 3 (5.3) | .63 | |
| CKD_ESRD | 11 (0.2) | 356 (11.6) | 145 (10.5) | 29 (6.4) | 55 (7.5) | 4 (5.5) | 5 (8.8) | .001 |
| Hemodialysis | 43 (1.4) | 4 (0.3) | 1 (0.2) | 7 (1) | 0 (0) | 0 (0) | .01 | |
| Cancer | 178 (5.8) | 86 (6.2) | 33 (7.3) | 49 (6.7) | 8 (11) | 5 (8.8) | .35 | |
| Chronic liver disease | 19 (0.6) | 5 (0.4) | 4 (0.9) | 4 (0.5) | 0 (0) | 0 (0) | .75 | |
| Autoimmune disease | 38 (1.2) | 31 (2.2) | 8 (1.8) | 15 (2) | 0 (0) | 1 (1.8) | .14 | |
| Interstitial lung disease | 52 (1.7) | 64 (4.6) | 43 (9.5) | 27 (3.7) | 7 (9.6) | 1 (1.8) | < .0001 | |
| Severity of illness surrogates | ||||||||
| Mechanical ventilation | 143 (4.6) | 82 (5.9) | 35 (7.7) | 30 (4.1) | 7 (9.6) | 1 (1.8) | .01 | |
| Vasopressor use | 89 (2.9) | 50 (3.6) | 18 (4) | 18 (2.5) | 3 (4.1) | 1 (1.8) | .49 | |
| Laboratory data | ||||||||
| CRP, mg/dL | 738 (12.8) | |||||||
| 0-0.5 | 21 (0.8) | 1 (0.1) | 1 (0.2) | 3 (0.5) | 0 (0) | 0 (0) | < .0001 | |
| > 0.5-2.5 | 164 (6.3) | 32 (2.6) | 3 (0.7) | 4 (0.6) | 0 (0) | 0 (0) | ||
| > 2.5 | 2,405 (92.9) | 1,211 (97.3) | 427 (99.1) | 645 (98.9) | 68 (100) | 53 (100) | ||
| D-dimer, ng/mL DDU | 2,026 (35.1) | |||||||
| < 230 | 350 (19.1) | 112 (12.5) | 56 (16) | 80 (14.1) | 18 (30) | 4 (8.3) | .0002 | |
| 230-1150 | 1,129 (61.8) | 582 (65.1) | 222 (63.2) | 360 (63.3) | 31 (51.7) | 33 (68.8) | ||
| > 1150 | 349 (19.1) | 200 (22.4) | 73 (20.8) | 129 (22.7) | 11 (18.3) | 11 (22.9) | ||
| Serum ferritin, ng/mL | 615 (10.7) | |||||||
| < 30 | 1 (0) | 2 (0.1) | 1 (0.2) | 1 (0.1) | 0 (0) | 0 (0) | < .0001 | |
| 30-400 | 423 (15.7) | 182 (14.5) | 28 (6.5) | 59 (8.8) | 7 (10.9) | 4 (7.7) | ||
| > 400-2000 | 1,783 (66.3) | 805 (64.2) | 307 (71.6) | 457 (68) | 49 (76.6) | 34 (65.4) | ||
| > 2000 | 484 (18) | 264 (21.2) | 93 (21.7) | 155 (23.1) | 8 (12.5) | 14 (26.9) | ||
| LDH, U/L | 991 (17.1) | |||||||
| < 242 | 106 (4.2) | 20 (1.7) | 1 (0.3) | 5 (0.8) | 3 (5.5) | 3 (5.6) | < .0001 | |
| ≥ 242 | 2,413 (95.8) | 1,170 (98.3) | 341 (99.7) | 620 (99.2) | 52 (94.5) | 51 (94.4) | ||
| Hemoglobin, g/dL | 121 (2.1) | |||||||
| < 11.5 | 767 (25.4) | 304 (22.5) | 55 (12.4) | 117 (16.3) | 16 (21.9) | 11 (20.8) | < .0001 | |
| 11.5-15.5 | 2,025 (67.1) | 938 (69.4) | 343 (77.6) | 546 (76.3) | 52 (71.2) | 34 (64.2) | ||
| > 15.5 | 227 (7.5) | 110 (8.1) | 44 (10) | 53 (7.4) | 5 (6.8) | 8 (15.1) | ||
| Eosinophils, K/μL | 310 (5.4) | |||||||
| 0-0.5 | 2,891 (99.6) | 1,307 (99.8) | 435 (100) | 697 (99.9) | 69 (100) | 52 (100) | .56 | |
| > 0.5 | 12 (0.4) | 2 (0.2) | 0 (0) | 1 (0.1) | 0 (0) | 0 (0) | ||
| Neutrophil-to-lymphocyte ratio | 321 (5.6) | |||||||
| < 0.75 | 23 (0.8) | 5 (0.4) | 2 (0.5) | 2 (0.3) | 0 (0) | 0 (0) | < .0001 | |
| 0.75-4 | 823 (28.4) | 227 (17.4) | 70 (16.1) | 93 (13.5) | 15 (21.4) | 12 (23.5) | ||
| > 4-20 | 1,879 (64.7) | 939 (72) | 309 (70.9) | 518 (75) | 48 (68.6) | 34 (66.7) | ||
| > 20 | 177 (6.1) | 134 (10.3) | 55 (12.6) | 78 (11.3) | 7 (10) | 5 (9.8) | ||
| Platelets, K/μL | 129 (2.2) | |||||||
| < 150 | 615 (20.4) | 271 (20.1) | 91 (20.6) | 128 (17.9) | 15 (20.5) | 9 (17) | .42 | |
| 150-500 | 2,348 (77.9) | 1,062 (78.8) | 347 (78.5) | 572 (80) | 58 (79.5) | 42 (79.2) | ||
| > 500 | 53 (1.8) | 15 (1.1) | 4 (0.9) | 15 (2.1) | 0 (0) | 2 (3.8) | ||
| Serum sodium, mM | 53 (0.9) | |||||||
| < 135 | 913 (29.9) | 440 (32.1) | 191 (42.6) | 314 (43.3) | 25 (34.7) | 22 (39.3) | < .0001 | |
| 135-145 | 1,921 (62.9) | 835 (60.9) | 248 (55.4) | 380 (52.3) | 43 (59.7) | 32 (57.1) | ||
| > 145 | 218 (7.1) | 95 (6.9) | 9 (2) | 32 (4.4) | 4 (5.6) | 2 (3.6) | ||
| Alanine aminotransferase, IU/L | 153 (2.7) | |||||||
| < 40 | 1,667 (55.8) | 741 (54.9) | 219 (49.5) | 371 (51.5) | 44 (63.8) | 31 (55.4) | .06 | |
| 40-200 | 1,235 (41.4) | 578 (42.8) | 208 (47.1) | 338 (46.9) | 24 (34.8) | 23 (41.1) | ||
| > 200 | 84 (2.8) | 30 (2.2) | 15 (3.4) | 12 (1.7) | 1 (1.4) | 2 (3.6) | ||
| Aspartate aminotransferase, IU/L | 151 (2.6) | |||||||
| < 40 | 1,090 (36.5) | 360 (26.7) | 97 (21.9) | 173 (24) | 30 (43.5) | 13 (23.2) | < .0001 | |
| 40-200 | 1,767 (59.1) | 946 (70.1) | 327 (74) | 526 (73) | 30 (43.5) | 39 (69.6) | ||
| > 200 | 131 (4.4) | 43 (3.2) | 18 (4.1) | 22 (3.1) | 4 (5.8) | 4 (7.1) | ||
| eGFR, mL/min/1.73 m2 | 54 (0.9) | |||||||
| < 15 | 313 (10.3) | 118 (8.6) | 17 (3.8) | 49 (6.7) | 3 (4.2) | 3 (5.4) | < .0001 | |
| 15-60 | 839 (27.5) | 444 (32.4) | 123 (27.5) | 228 (31.4) | 26 (36.1) | 24 (42.9) | ||
| > 60 | 1,788 (58.6) | 761 (55.5) | 301 (67.2) | 438 (60.3) | 41 (56.9) | 29 (51.8) | ||
| > 120 | 110 (3.6) | 47 (3.4) | 7 (1.6) | 11 (1.5) | 2 (2.8) | 0 (0) |
Data are presented as No. (%) or median (25th-75th percentiles) unless otherwise indicated. P values > .05, shown in bold, were considered statistically significant. CKD_ESRD = chronic kidney disease_end-stage renal disease, indication patient on dialysis; CRP = C-reactive protein; DDU = D-dimer unit; DM = diabetes mellitus; eGFR = estimate glomerular filtration rate; HTN = hypertension; LDH = lactate dehydrogenase. Chi-square, Fisher exact, or Kruskal-Wallis tests were used to compare statistical significance, between groups, as appropriate. Demographics and comorbidity data were obtained at baseline on admission. Vasopressor and invasive mechanical ventilation use was within 24 h before T0. Laboratory values included the closest value to T0 from within 96 h before T0. CRP, ferritin, LDH, and D-dimer were defined within 96 h before T0 and up to 12 h after T0 because of laboratory ordering practices.
Table 2.
Hazard Ratios With 95% CIs for Cox Regression Model
| Variable | Hazard Ratio (95% Confidence Limits) | P Value |
|---|---|---|
| Treatment groupsa | . . . | . . . |
| Standard of care | Reference | . . . |
| Steroids only | 0.66 (0.57-0.76) | < .0001 |
| Steroids plus tocilizumab | 0.44 (0.35-0.55) | < .0001 |
| Steroids plus anakinra | 0.68 (0.57-0.81) | < .0001 |
| Tocilizumab only | 0.79 (0.47-1.32) | 0.36 |
| Anakinra only | 0.79 (0.44-1.42) | 0.43 |
| Demographics | . . . | . . . |
| Age | 1.03 (1.02-1.04) | < .0001 |
| Sex | . . . | . . . |
| Female | Reference | . . . |
| Male | 1.13 (0.99-1.29) | .07 |
| Race | . . . | . . . |
| White | Reference | . . . |
| Asian | 0.94 (0.78-1.14) | .53 |
| Black | 0.80 (0.68-0.95) | .01 |
| Other/multiracial | 0.84 (0.70-1.02) | .08 |
| Unknown | 0.91 (0.64-1.30) | .61 |
| Ethnicity | . . . | . . . |
| Not Hispanic or Latino | Reference | . . . |
| Hispanic or Latino | 1.02 (0.83-1.24) | .88 |
| Other/unknown | 0.84 (0.61-1.17) | .30 |
| Insurance | . . . | . . . |
| Commercial | Reference | . . . |
| Medicaid | 1.25 (1.01-1.56) | .04 |
| Medicare | 1.13 (0.94-1.35) | .20 |
| Other | 0.91 (0.49-1.70) | .76 |
| Self-pay | 2.28 (1.45-3.56) | .0003 |
| Smoking status | . . . | . . . |
| Never | Reference | . . . |
| Active | 1.43 (0.94-2.21) | .11 |
| Former | 0.93 (0.78-1.11) | .42 |
| Smoker (unknown active/former) | 1.42 (1.09-1.83) | .01 |
| Unknown | 3.02 (2.58-3.56) | < .0001 |
| Disease severity indexes | . . . | . . . |
| Mechanical ventilation | . . . | . . . |
| No | Reference | . . . |
| Yes | 1.49 (1.18-1.87) | .0007 |
| On vasopressors | . . . | . . . |
| No | Reference | . . . |
| Yes | 0.97 (0.74-1.27) | .83 |
| Laboratory parameters | . . . | . . . |
| Eosinophils, K/uL | . . . | . . . |
| 0-0.5 | Reference | . . . |
| > 0.5 | 1.16 (0.29-4.61) | .84 |
| Platelets, K/uL | . . . | . . . |
| 150-500 | Reference | . . . |
| < 150 | 1.20 (1.05-1.37) | .01 |
| > 500 | 1.10 (0.66-1.84) | .71 |
| Hemoglobin, g/dL | . . . | . . . |
| 11.5-15.5 | Reference | . . . |
| < 11.5 | 1.01 (0.88-1.17) | .90 |
| > 15.5 | 1.05 (0.84-1.31) | .64 |
| eGFR, mL/min/1.73 m2 | . . . | . . . |
| 60-120 | Reference | . . . |
| < 15 | 2.30 (1.83-2.89) | < .0001 |
| 15-60 | 1.74 (1.50-2.01) | < .0001 |
| > 120 | 1.09 (0.59-2.00) | .79 |
| AST, U/L | . . . | . . . |
| 0-40 | Reference | . . . |
| > 40 | 1.35 (1.15-1.58) | .0002 |
| > 200 | 1.58 (1.13-2.21) | .01 |
| ALT, U/L | . . . | . . . |
| 0-40 | Reference | . . . |
| > 40 | 0.84 (0.72-0.97) | .02 |
| > 200 | 1.07 (0.71-1.62) | .76 |
| Sodium, mM | . . . | . . . |
| 135-145 | Reference | . . . |
| < 135 | 1.20 (0.96-1.26) | .19 |
| > 145 | 1.24 (1.03-1.50) | .03 |
| Ferritin, ng/mL | . . . | . . . |
| 30-400 | Reference | . . . |
| < 30 | 2.26 (0.27-18.92) | .45 |
| > 400 | 1.04 (0.84-1.30) | .73 |
| > 2000 | 1.21 (0.94-1.56) | .14 |
| CRP, mg/dL | . . . | . . . |
| 0-0.5 | Reference | . . . |
| > 0.5 | 3.12 (0.34-28.33) | .31 |
| > 2.5 | 4.11 (0.47-35.70) | .20 |
| D-dimer, ng/mL DDU | . . . | . . . |
| 0-230 | Reference | . . . |
| > 230 | 1.34 (1.03-1.75) | .03 |
| > 1150 | 1.67 (1.24-2.26) | .0008 |
| LDH, U/L | . . . | . . . |
| <242 | Reference | . . . |
| ≥ 242 | 1.59 (0.96-2.63) | .07 |
| NLR | . . . | . . . |
| 0.75-4 | Reference | . . . |
| < 0.75 | 2.10 (1.17-3.77) | .01 |
| > 4 | 1.22 (1.03-1.46) | .03 |
| > 20 | 1.17 (0.92-1.49) | .20 |
| Hospital status | . . . | . . . |
| Community | Reference | . . . |
| Tertiary | 0.64 (0.56-0.73) | < .0001 |
| Comorbidities | . . . | . . . |
| Charlson comorbidity index | . . . | . . . |
| 0 | Reference | . . . |
| 1-2 | 1.00 (0.62-1.63) | 1.00 |
| 3-4 | 1.11 (0.68-1.82) | .69 |
| ≥ 5 | 1.42 (0.84-2.40) | .19 |
| Asthma | . . . | . . . |
| No | Reference | . . . |
| Yes | 1.35 (1.01-1.79) | .04 |
| COPD | . . . | . . . |
| No | Reference | . . . |
| Yes | 1.23 (0.95-1.59) | .12 |
| Chronic liver disorder | . . . | . . . |
| No | Reference | . . . |
| Yes | 0.95 (0.46-1.95) | .89 |
| DM | . . . | . . . |
| No | Reference | . . . |
| Yes | 1.02 (0.89-1.17) | .79 |
| HTN | . . . | . . . |
| No | Reference | . . . |
| Yes | 0.83 (0.73-0.94) | .0045 |
| ILD | . . . | . . . |
| No | Reference | . . . |
| Yes | 2.17 (1.76-2.69) | < .0001 |
| Autoimmune disorder | . . . | . . . |
| No | Reference | . . . |
| Yes | 1.21 (0.74-1.98) | .44 |
| Cardiovascular disease | . . . | . . . |
| No | Reference | . . . |
| Yes | 1.13 (0.96-1.33) | .13 |
| CKD | . . . | . . . |
| No | Reference | . . . |
| Yes | 0.88 (0.72-1.07) | .21 |
| Cancer | . . . | . . . |
| No | Reference | . . . |
| Yes | 1.20 (0.96-1.50) | .10 |
| Hemodialysis | . . . | . . . |
| No | Reference | . . . |
| Yes | 0.99 (0.58-1.69) | .96 |
| BMI, kg/m2 | . . . | . . . |
| 18.5-24.9 | Reference | . . . |
| < 18.5 | 1.15 (0.93-1.44) | .20 |
| 25-29.9 | 0.98 (0.83-1.42) | .77 |
| ≥ 30 | 1.07 (0.90-1.27) | .46 |
Results of the multivariate model of in-hospital mortality for coronavirus disease 2019 patients meeting inclusion criteria with coronavirus disease 2019 cytokine storm. Hazard ratios for treatment groups represent adjustment for covariates in the model, comparing with standard of care treatment as reference. Treatment group hazard ratios are not adjusted for multiple comparisons between treatment groups. Refer to Figure 3 (and e-Table 2) for treatment differences using Tukey’s adjustment for multiple comparisons between treatment groups. P values < .05, shown in bold, were considered statistically significant. ALT = alanine aminotransferase; AST = aspartate aminotransferase; CKD = chronic kidney disease; CRP = C-reactive protein; DDU = D-dimer unit; DM = diabetes mellitus; eGFR = estimate glomerular filtration rate; HTN = hypertension; ILD = interstitial lung disease; LDH = lactate dehydrogenase; NLR = neutrophil-to-lymphocyte ratio. aCox regression analysis was performed to identify covariables that were associated with increased mortality in our population.
Results
Patient Characteristics
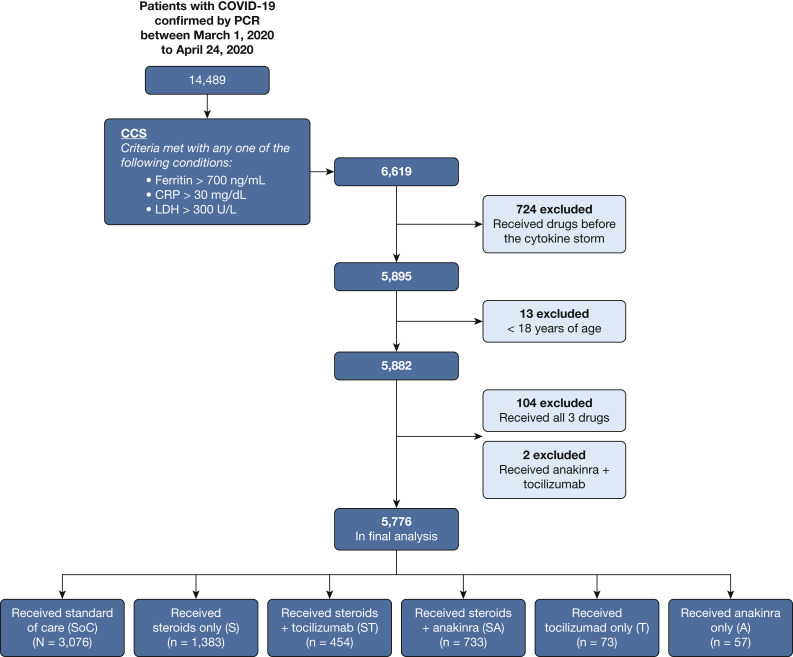
Of the 14,489 patients with COVID-19 seen in EDs or admitted to hospitals within the Northwell Health system during the study period, 6,619 (45.7%) patients met at least one criterion for the definition of CCS. Of these, 5,776 patients were included in the final analysis (Fig 1 ).
Figure 1.
Consort diagram showing selection of patients, inclusion criteria, and exclusion criteria applied to form the final cohort of 3,098 patients. Exclusion criteria included receiving any of the immunomodulatory drugs before the diagnosis of cytokine storm, age younger than 18 years, having received all three study drugs, having received the combination of anakinra and tocilizumab, or missing clinically relevant covariates. Three thousand ninety-eight patients remained in the final analysis. CCS = coronavirus disease 2019 cytokine storm; COVID-19 = coronavirus disease 2019; CRP = C-reactive protein; LDH = lactate dehydrogenase; PCR = polymerase chain reaction.
Demographic characteristics and distribution of covariates across groups are reported in Table 1. Men outnumbered women by a ratio of 2:1. A significant difference in the racial distribution across treatment groups was noted, with more Black people in the A group and White people in the T group. A higher proportion of patients identifying as other or multiracial race were noted in the A group. Most of the cohort (> 65%) had never smoked. The most common comorbidities across groups were: hypertension (44%-59%), diabetes (32%-46%), cardiovascular disease (5%-14%), chronic kidney disease (5%-12%), cancer (5%-11%), and asthma (3%-12%). Less than 2% of patients were receiving hemodialysis before T0. Approximately 40% of the patients in the cohort demonstrated a low predicted 10-year survival rate based on Charlson comorbidity index score (≥ 5). More patients had a moderate to high Charlson comorbidity index score (≥ 3) in the T group as compared with other treatment groups. More patients in the S, ST, and T treatment groups were receiving IMV and vasopressors at T0.
More than 80% of the patients who met criteria for CCS showed elevated D-dimer levels, of which approximately 20% showed levels more than five times the upper limit of normal. The most common criterion met for CCS definition was high LDH, which was found in 76.2% of patients, either alone or in combination with other criteria, followed by high ferritin (63.2%) and CRP (8.4%). The definition of CCS was met by only one criterion in 56.0% of patients, by two criteria in 40.2% of patients, and by three criteria in 3.8% of patients. The distribution of CRP, ferritin, and LDH levels is provided in e-Figure 1. A statistically significant difference was found between treatment arms with respect to CRP, ferritin, and LDH levels (P < .0001), with the SoC group showing lower median CRP, ferritin, and LDH levels compared with the S, ST, and SA groups.
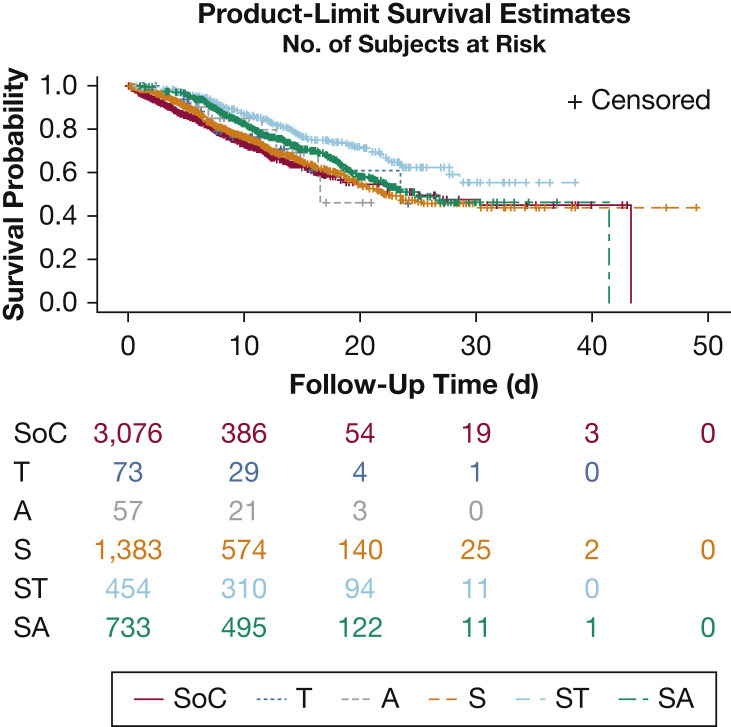
Kaplan-Meier (unadjusted) survival estimates for treatment groups are presented in Figure 2 . A Cox proportional hazards regression model was used to compare treatment groups, adjusting for clinically important variables. In this model, demographic covariates that had a statistically significantly associated with increased mortality were older age, unknown smoking status, Medicaid, and self-pay insurance (Table 2). Higher mortality was associated with the presence of asthma, interstitial lung disease, and the need for IMV at T0. Higher mortality also was associated with elevated D-dimer level, thrombocytopenia, low glomerular filtration rate, transaminitis, hypernatremia, and abnormal neutrophil-to-lymphocyte ratio. Lower mortality was noted in patients with hypertension and Black race.
Figure 2.
Model-based Kaplan-Meier plots showing treatment groups (adjusted for covariates). This figure represents the unadjusted Kaplan-Meier plots for treatment groups with number of patients at risk (ie, patients who remained admitted at the hospital at that time point). The treatment groups are as follows: A = anakinra only; S = steroid only; SA = steroids plus anakinra; SoC = standard of care; ST = steroids plus tocilizumab; T = tocilizumab only.
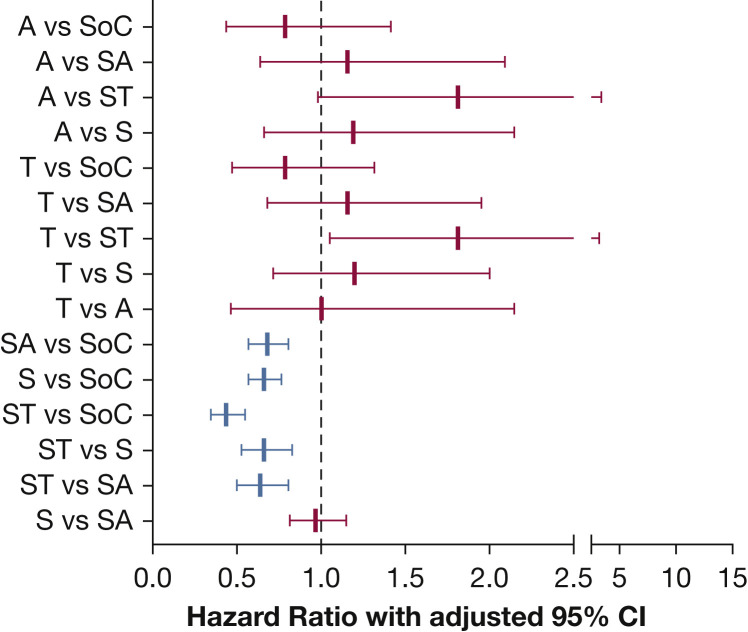
Pairwise comparisons between treatment groups are presented in Figure 3 and e-Table 2. Patients in the ST, SA, and S groups showed significantly improved survival compared with the SoC group (ST vs SoC: hazard ratio [HR], 0.44; 95% CI, 0.35-0.55; P < .0001; SA vs SoC: HR, 0.68; 95% CI, 0.57-0.81; P < .0001; S vs SoC: HR, 0.66; 95% CI, 0.57-0.76; P < .0001). When comparing the treatment groups with each other, the ST group showed significantly improved survival compared with SA or S groups (ST vs SA: HR, 0.64; 95% CI, 0.50-0.81; P = .003; ST vs S: HR, 0.66; 95% CI, 0.53-0.83; P = .004). No significant differences were seen between the other treatment groups.
Figure 3.
Graph showing hazard ratios for treatment differences using Tukey’s adjustment for multiple comparisons. The figure represents pairwise comparisons for all treatment groups with Tukey’s adjustment for multiple comparison. Groups in red are statistically significant. The groups are as follows: A = anakinra only; S = steroid only; SA = steroids plus anakinra; SoC = standard of care; ST = steroids plus tocilizumab; T = tocilizumab only.
At T0, in patients receiving only one of the three treatments, corticosteroids were started earlier (median, 27.6 h; 25th-75th percentiles, 7.6-77.9 h) than either tocilizumab (median, 54.4 h; 25th-75th percentiles, 25.0-99.2 h) or anakinra (median, 66.3 h; 25th-75th percentiles, 23.9-97.6 h). In both groups that received combination therapy with corticosteroids, on average corticosteroids were started before the second drug and at a similar interval from T0 (e-Fig 2; e-Table 3). The time from T0 to tocilizumab dosing was comparable when used alone (median, 54.4 h; 25th-75th percentiles, 25.0-99.2 h) or in combination with corticosteroids (median, 58.5 h; 25th-75th percentiles, 23.6-129.7 h). Anakinra alone was begun earlier (median, 66.3 h; 25th-75th percentiles, 23.9-97.6 h) than anakinra in the SA group (median, 77.5 h; 25th-75th percentiles, 36.6-130.7 h). Patients received oral or IV dexamethasone, IV methylprednisolone, or oral prednisone for corticosteroid therapy (e-Table 4). The average number of days of steroids use was approximately 4.5 days, except for methylprednisolone in the ST and SA groups, in which the average duration was 6.5 days. The average steroid dose used was 12 to 15 mg for dexamethasone, 85 to 89 mg for methylprednisolone, and 29 to 33 mg for prednisone.
Rates of culture-positive bloodstream infections in the treatment groups are reported in e-Table 5. Approximately 5% of patients in the S group demonstrated bacteremia compared with 10% in the SA and ST groups. Similarly, 2% to 3% of patients in the steroid groups S, ST, and SA were noted to have fungemia. In comparison, the rate of bacteremia in the SoC group was 1.6% and the rate of fungemia was 0.4%. No bacteremia or fungemia were reported in the T or A groups.
Discussion
This large retrospective, observational study leveraged natural heterogeneity in practice patterns for CCS patients. We described hospital survival outcomes in patients receiving different combinations of immunomodulatory therapy with careful consideration of potential confounders available in the electronic health records. Our findings suggested that corticosteroids used alone or in combination with tocilizumab or anakinra were associated with lower mortality as compared with SoC treatment. This association remained after controlling for covariates that influence mortality in COVID-19.
Age was associated with increased mortality regardless of treatment group, consistent with other COVID-19 survival analyses. Contradictory to previous reports, Black race was associated with better overall survival compared with White race. Inherent differences may have existed in clinically important covariates in this population that may have contributed to better survival and that could not be analyzed further. Medicaid and self-pay insurance were associated with increased mortality. We speculate that this may be because of factors such as hospital admission later in disease course or socioeconomic disadvantages. For surrogates of illness severity, the need for IMV before T0 was associated with increased mortality, whereas the need for vasopressors was not.
Prior diagnosis of interstitial lung disease was associated with increased mortality, consistent with existing literature.13 Surprisingly, those with comorbid hypertension showed lower mortality, which is contradictory to other reports.1 , 14 One study suggested that use of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers via renin-angiotensin pathway modulation may confer a protective effect in the setting of CCS.15 Our analysis did not include consideration of home medications. Alternatively, adjustments for covariates in our model may have uncovered an association between hypertension and COVID-19 outcomes that could be investigated further. Interestingly, increased mortality was associated with asthma, but not with COPD. Early in the pandemic, chronic lung disease, including asthma, was reported as one of the comorbidities associated with hospital admissions.16 Later studies failed to demonstrate increased mortality in patients with asthma and COPD,17 although pre-existent asthma was reported to be associated with prolonged intubation time. Atopic asthma and treatment with inhaled corticosteroids were reported to correlate with lower sputum cell expression of Angiotensin concerting enzyme 2 (ACE2),18, 19, 20 implying decreased susceptibility and morbidity in these patients.
High D-dimer level was associated significantly with in-hospital mortality. This is consistent with known evidence that elevated D-dimer level is associated with worse outcomes21 and predicts a higher chance of requiring ICU admission and increased 28-day mortality.5 , 22 , 23 Thrombocytopenia is associated with severity of SARS-CoV-2 infection.24 We also found thrombocytopenia to be associated with higher mortality. Both thrombocytopenia and elevated D-dimer level reflect the known coagulopathy in COVID-19.25
IL-6 is an important mediator of inflammation that plays an essential role in host response to viral infection.26 Higher IL-6 levels were observed in patients with severe COVID-19 compared with those with mild disease.3 , 27 Therefore, tocilizumab was proposed early in the pandemic as a potential treatment for those with CCS.28 , 29 Small retrospective, observational studies of tocilizumab use in COVID-19 have been published with continued controversy.7 , 30 Biran et al31 and Guaraldi et al32 published larger reports with 210 and 544 patients, respectively, who received either intravenous or subcutaneous tocilizumab. Per Biran et al, tocilizumab seemed to decrease hospital-related mortality (HR, 0.64; 95% CI, 0.47-0.87; P = .0040). Guaraldi et al32 reported a reduced requirement of IMV or death (adjusted HR, 0.61; 95% CI, 0.40-0.92; P = .020). More recently, Mikulska et al33 examined the combined effect of steroids and tocilizumab and noted an overall survival benefit as compared with SoC treatment (HR, 0.41; 95% CI, 0.19-0.89; P = .025). Supporting this result, patients in the ‘ST’ cohort were more likely to survive compared to ‘SoC’. Notably, ST treatment seemed to show an augmented survival effect compared with S treatment alone. Tocilizumab alone did not improve survival.
Although corticosteroids are used in the treatment of hyperinflammatory syndromes and ARDS,34 their use in viral infections is controversial. Although initially not recommended by the World Health Organization35 for use in COVID-19 pneumonia, corticosteroids have become a widely accepted treatment option after the Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial demonstrated improved survival compared with SoC treatment both in patients receiving IMV (29.3% vs 41.4%; rate ratio, 0.64; 95% CI, 0.51-0.81) and in patients without IMV (23.3% vs 26.2%; rate ratio, 0.82; 95% CI, 0.72-0.94).36 More recently, the World Health Organization Rapid Evidence Appraisal for COVID-19 Therapies published a meta-analysis supporting the independent use of corticosteroids in patients with COVID-19. However, the RECOVERY trial contributed 59.1% of patients to this analysis, which favors dexamethasone over hydrocortisone (OR, 0.69; 95% CI, 0.43-1.12; P = .13) and methylprednisolone (OR, 0.91; 95% CI, 0.29-2.87; P = .87).37 Overall, our study findings support the use of corticosteroids in COVID-19 and may add to the data presented by the RECOVERY trial and the World Health Organization Rapid Evidence Appraisal for COVID-19 Therapies data.
Anti-IL-1 therapy has been an attractive choice in the treatment of COVID-19 because of its short half-life, safety, and tolerability profile. IL-1β has been implicated in lung inflammation, fibrosis,38 and indirectly, with activation of the inflammatory cascade.39, 40, 41, 42 A study examining cytokine kinetics during COVID-19 showed an IL-1 peak before the apex of respiratory distress and the surge of other inflammatory cytokines.43 Anakinra also has been shown to improve survival in a subset of sepsis patients with hyperferritinemia and hepatobiliary dysfunction10 when compared with placebo.44
Small studies report improvement in clinical outcomes with use of anakinra in COVID-19.8 , 45 , 46 Cavalli et al8 evaluated 36 hospitalized non-ICU patients with CCS and observed improvements in respiratory function, inflammatory markers, and intubation avoidance in 72% of patients receiving high-dose intravenous anakinra as compared with low-dose IV anakinra or SoC treatment. Huet et al45 described a prospective study with a historical comparison group in which anakinra was dosed subcutaneously at 100 mg twice daily for 72 h followed by 100 mg daily for 7 days. IMV or death was reduced when compared with SoC treatment (HR, 0.22; 95% CI, 0.11-0.41; P < .0001). Most recently, Cauchois et al46 reported that 12 patients who received intravenous anakinra 300 mg for 5 days, tapered to 200 mg daily for 2 days, and finally 100 mg for 1 day showed similar beneficial results.
In our study, although patients treated with anakinra in combination with corticosteroids showed improved survival compared with patients receiving SoC treatment, patients receiving anakinra alone did not. The dose of anakinra suggested in our health system protocol (100 mg subcutaneous four times daily for 3 days, followed by a taper) was modest in comparison with that used in some of the above studies. The lack of benefit with anakinra may have been the result of lower doses, delayed time to treatment, and subcutaneous administration, leading to decreased drug availability, especially in the critically ill.
Biological effects of anakinra and tocilizumab are slower when compared with steroids. Also with anakinra, we observed a delay in drug initiation when combined with corticosteroids. This leads us to question whether the timing to drug administration and the time to onset of action influenced the outcome among our treatment groups. Statistical analysis of the variation of drug administration across treatment groups was not feasible in this study. Further analysis of our data is needed to evaluate the effects of immunomodulatory treatments on disease progression, including rates of thrombosis.
Given the small sample sizes in the groups receiving tocilizumab or anakinra only, we should be cautious in interpreting the relative lack of survival advantage in these groups. To test the robustness of the model, we performed sensitivity analyses by removing groups with small sample sizes (either the A or T groups). The results remained consistent with those of the full model.
Increased rates of bacteremia and fungemia were found in the steroid groups compared with the SoC group (e-Table 5). However, despite this increase in the infection rate, improved survival remained in these cohorts.
Although we were rigorous in our approach to the study design and data analysis, intrinsic limitations exist that preclude definitive conclusions in retrospective studies. Although the effect of variability in systematic practices across the individual hospitals in the health system could not be evaluated, we did look at differences between tertiary vs community hospitals. Despite similar use of immunomodulatory therapies in tertiary and community centers, tertiary facilities showed a higher survival. Potential explanations for this could include a greater number of ICU beds, subspecialist availability, or differences in patient demographics between hospitals.
To our knowledge, our study is the largest retrospective analysis to date reporting on outcomes comparing the use of immunomodulatory therapies such as corticosteroids, tocilizumab, and anakinra in the treatment of COVID-19 CCS. Our findings suggest that patients receiving steroids and tocilizumab experienced the lowest mortality of all treatment groups. Corticosteroid use, either alone or in combination with tocilizumab or anakinra, was associated with lower hospital mortality compared with SoC treatment. A randomized clinical trial with head-to-head comparison of tocilizumab plus corticosteroids vs corticosteroids alone is warranted. Further investigation into the effect of dosing and timing of these drugs also needs to be elucidated.
Acknowledgments
Author contributions: S. N., D. G. S., A. S. C., A. G. W., G. M., B. K., P. M., O. B., A. L., M. L. L., and N. H. contributed to the concept, design, drafting, and editing of the manuscript. S. L. C, J. C., D. A. H., N. I. M., S. K. S., C. S., M. T., and M. Q. edited the manuscript.
Financial/nonfinancial disclosures: None declared.
∗Northwell COVID-19 Research Consortium Collaborators: Stuart L. Cohen, MD (Biostatistics Unit, Northwell Health, Manhasset, NY; the Institute of Health Innovations and Outcomes Research and Barbara Zucker School of Medicine at Hofstra/Northwell, Northwell Health, Hempstead, New York); Jennifer Cookingham, MHA (Institute of Health Innovations and Outcomes Research, Northwell Health, Manhasset, NY); David A. Hirschwerk, MD (Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Northwell Health, Hempstead, New York); Naomi I. Maria, PhD (the Institute of Molecular Medicine, Northwell Health, Manhasset, NY); Sanjaya K Satapathy, MD (Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, and the Feinstein Institute for Medical Research, the Division of Hepatology, Northwell Health, Hempstead, New York); Cristina Sison, PhD; Matthew Taylor, MD (the Institute of Cancer Research and the Division of Critical Care Medicine, Cohen Children’s Medical Center of New York, Northwell Health, New Hyde Park, NY); and Michael Qiu, MD, PhD (Department of Medicine, the Department of Information Services, Northwell Health, Manhasset, NY).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank the Northwell COVID-19 Research Consortium for facilitating the study. The initial characteristics of 5,700 patients from Northwell Health are presented elsewhere (Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5,700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059). Our manuscript discusses, in-depth, the mortality outcomes of patients treated with corticosteroids, tocilizumab and/or anakinra that was not presented in that article.
Additional information: The e-Figures and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: A. S. C. was supported by The Primary Immune Deficiency Treatment Consortium [Grant U54 AI 082973], funded jointly by the National Center for Advancing Translational Sciences and the National Institute of Allergy and Infectious Diseases. O. B. was supported by the United States Department of Defense [Grant W81XWH-15-1-0614] and the New York State Spinal Cord Injury Research Board [Grant DOH01-ISSCI6-2016-00018]. N. H. was supported by a grant from the Patient Centered Outcomes Research Institute [Grant AD-1511-33066].
Contributor Information
Northwell COVID-19 Research Consortium:
Stuart L. Cohen, Jennifer Cookingham, David A. Hirschwerk, Naomi I. Maria, Sanjaya K. Satapathy, Cristina Sison, Matthew Taylor, and Michael Qiu
Supplementary Data
References
- 1.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang X., Mei Q., Yang T., et al. Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-1. J Infect. 2020;81(1):147–178. doi: 10.1016/j.jinf.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toniati P., Piva S., Cattalini M., et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19(7):102568. doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavalli G., De Luca G., Campochiaro C., et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2(6):e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aouba A., Baldolli A., Geffray L., et al. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann Rheum Dis. 2020;79(10):1381–1382. doi: 10.1136/annrheumdis-2020-217706. [DOI] [PubMed] [Google Scholar]
- 10.Shakoory B., Carcillo J.A., Chatham W.W., et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med. 2016;44(2):275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machowicz R., Janka G., Wiktor-Jedrzejczak W. Similar but not the same: differential diagnosis of HLH and sepsis. Crit Rev Oncol Hematol. 2017;114:1–12. doi: 10.1016/j.critrevonc.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Chen G., Wu D., Guo W., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209-218. [DOI] [PMC free article] [PubMed]
- 14.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng J., Xiao G., Zhang J., et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9(1):757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garg S., Kim L., Whitaker M., et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 states, March 1–30, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ssentongo P., Ssentongo A.E., Heilbrunn E.S., Ba D.M., Chinchilli V.M. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: a systematic review and meta-analysis. PLOS One. 2020;15(8) doi: 10.1371/journal.pone.0238215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camiolo M.J., Gauthier M., Kaminski N., Ray A., Wenzel S.E. Expression of SARS-CoV-2 receptor ACE2 and coincident host response signature varies by asthma inflammatory phenotype. J Allergy Clin Immunol. 2020;146(2):315–324.e7. doi: 10.1016/j.jaci.2020.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters M.C., Sajuthi S., Deford P., et al. COVID-19 related genes in sputum cells in asthma: relationship to demographic features and corticosteroids. Am J Respir Crit Care Med. 2020;202(1):83–90. doi: 10.1164/rccm.202003-0821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson D.J., Busse W.W., Bacharier L.B., et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol. 2020;146(1):203–206.e3. doi: 10.1016/j.jaci.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lippi G., Favaloro E.J. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. 2020;120(5):876–878. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu P., Zhou Q., Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020;99(6):1205–1208. doi: 10.1007/s00277-020-04019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chau AS, Weber AG, Maria NI, et al. The longitudinal immune response to coronavirus disease 2019: Chasing the cytokine storm [published online ahead of print September 15, 2020]. Arthritis Rheumatol. 10.1002/art.41526. [DOI] [PubMed]
- 27.Coomes EA, Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30(6):1-9. [DOI] [PMC free article] [PubMed]
- 28.Fu B., Xu X., Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020;18:164. doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang S., Li L., Shen A., Chen Y., Qi Z. Rational use of tocilizumab in the treatment of novel coronavirus pneumonia. Clin Drug Invest. 2020;40(6):511–518. doi: 10.1007/s40261-020-00917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colaneri M., Bogliolo L., Valsecchi P., et al. Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE) Microorganisms. 2020;8(5):695–707. doi: 10.3390/microorganisms8050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biran N., Ip A., Ahn J., et al. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;2(10):e603–e612. doi: 10.1016/S2665-9913(20)30277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guaraldi G., Meschiari M., Cozzi-Lepri A., et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2(8):e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mikulska M., Nicolini L.A., Signori A., et al. Tocilizumab and steroid treatment in patients with COVID-19 pneumonia. Plos one. 2020;15(8):e0237831. doi: 10.1371/journal.pone.0237831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meduri GU, Bridges L., Shih M.C., Marik P.E., Siemieniuk R.A.C., Kocak M. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: analysis of individual patients’ data from four randomized trials and trial-level meta-analysis of the updated literature. Intensive Care Med. 2016;42(5):829–840. doi: 10.1007/s00134-015-4095-4. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization . World Health Organization; 27 May 2020. Clinical Management of COVID-19: Interim Guidance. https://apps.who.int/iris/handle/10665/332196. Accessed October 27, 2020. [Google Scholar]
- 36.Group R.C., Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020 Jul 17 NEJMoa2021436. [Google Scholar]
- 37.Sterne J.A.C., Murthy S., Diaz J.V., et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kolb M., Margetts P.J., Anthony D.C., Pitossi F., Gauldie J. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest. 2001;107(12):1529–1536. doi: 10.1172/JCI12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warnatsch A., Ioannou M., Wang Q., Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349(6245):316–320. doi: 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meher A.K., Spinosa M., Davis J.P., et al. Novel role of IL (interleukin)-1beta in neutrophil extracellular trap formation and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2018;38(4):843–853. doi: 10.1161/ATVBAHA.117.309897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lachowicz-Scroggins M.E., Dunican E.M., Charbit A.R., et al. Extracellular DNA, neutrophil extracellular traps, and inflammasome activation in severe asthma. Am J Respir Crit Care Med. 2019;199(9):1076–1085. doi: 10.1164/rccm.201810-1869OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo L., Rondina M.T. The era of thromboinflammation: platelets are dynamic sensors and effector cells during infectious diseases. Front Immunol. 2019;10:2204–2218. doi: 10.3389/fimmu.2019.02204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ong E.Z., Chan Y.F.Z., Leong W.Y., et al. A dynamic immune response shapes COVID-19 progression. Cell Host Microbe. 2020;27(6):879–882.e2. doi: 10.1016/j.chom.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fisher C.J., Jr., Dhainaut J.F., Opal S.M., et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA. 1994;271(23):1836–1843. [PubMed] [Google Scholar]
- 45.Huet T., Beaussier H., Voisin O., et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cauchois R., Koubi M., Delarbre D., et al. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc Natl Acad Sci U S A. 2020;117(32):18951–18953. doi: 10.1073/pnas.2009017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.