Abstract
Purpose
Accurate delineation of intraprostatic gross tumor volume (GTV) is mandatory for successful fusion biopsy guidance and focal therapy planning of prostate cancer (PCa). Multiparametric magnetic resonance imaging (mpMRI) is the current gold standard for GTV delineation; however, prostate-specific membrane antigen positron emission tomography (PSMA-PET) is emerging as a promising alternative. This study compares GTV delineation between mpMRI and 68Ga-PSMA-PET in a large number of patients using validated contouring approaches.
Methods
One hundred one patients with biopsy-proven primary PCa who underwent mpMRI and 68Ga-PSMA-PET within 3 months before primary treatment were retrospectively enrolled. Clinical parameters (age, PSA, Gleason score in biopsy) were documented. GTV based on MRI and PET images were delineated; volumes measured and laterality determined. Additionally, biopsy data from 77 patients was analyzed. Univariate and multivariate binary logistic regression analyses were performed using concordance in laterality as the endpoint.
Results
In total mpMRI and 68Ga-PSMA-PET detected 151 and 159 lesions, respectively. Median GTV-MRI (2.8 ml, 95% CI 2.31–3.38 ml) was significantly (p < 0.0001) smaller than median GTV-PET (4.9 ml, 95% CI 3.9–6.6 ml). 68Ga-PSMA-PET detected significantly more bilateral lesions than mpMRI (71 vs 57, p = 0.03). Analysis of patients with bilateral lesions in biopsy showed a significant higher concordance of laterality in 68Ga-PSMA-PET (p = 0.03). In univariate analysis, PSA level and volume of GTV-MRI had an impact on concordance in laterality (p = 0.02 and p = 0.01), whereas in multivariate analysis, only GTV-MRI volume remained significant (p = 0.04).
Conclusion
MpMRI and 68Ga-PSMA-PET detect a similar amount of PCa lesions. However, GTV-PET had approximately twice the volume (median 4.9 ml vs 2.8 ml) and detected significantly more bilateral lesions than mpMRI. Thus, 68Ga-PSMA-PET gives highly important complementary information. Since we could not find any strong evidence for parameters to guide when 68Ga-PSMA-PET is dispensable, it should be performed additionally to MRI in patients with intermediate and high-risk PCa according to D’Amico classification to improve GTV delineation.
Keywords: Prostate cancer, MRI, PSMA PET/CT
Introduction
Prostate cancer (PCa) is the most common tumor entity for men in North America [1] and Europe [2], and PCa incidence rates are rising steadily in Asian countries as well [3]. The accurate delineation of intraprostatic tumor burden is mandatory for successful fusion biopsy guidance [4] and for focal therapy planning such as focal dose escalation in radiotherapy (RT), high-intensity focused ultrasound (HIFU) focal laser ablation (FAL), cryotherapy, or irreversible electroporation (IRE) [5].
Multiparametric magnetic resonance imaging (mpMRI) is the current gold standard for PCa detection [4] but has been shown to miss pivotal tumor lesions and underestimates their volume [6, 7]. Instead prostate-specific membrane antigen positron emission tomography (PSMA-PET) is emerging as a promising technique to improve tumor lesion detection [8–15], focal therapy guidance [16], and non-invasive PCa characterization [17].
In histopathologic comparison studies combining T2-weighted spin echo (T2) and diffusion-weighted (DWI) and dynamic contrast-enhanced (DCE) MRI, with median sensitivity of 61.5% and median specificity of 85.5%, was found. For PSMA-PET, these studies identified similar specificity scores and slightly higher sensitivity scores with a median sensitivity of 76% [15, 18–25]. Additionally, tumor volumes delineated in PSMA-PET and mpMRI differed significantly, with smaller volumes in mpMRI [24, 25].
However, these studies only included small patient numbers (range, 7–53 patients) and used not validated contouring approaches for delineation of intraprostatic tumor according to PSMA PET. The aim of this study is an intraindividual comparison of tumor volume delineation between mpMRI and 68Ga-PSMA-PET in a large number of patients (n = 101) using validated contouring approaches for both imaging modalities [16, 26]. We analyzed differences in the number of detected PCa lesions and their gross tumor volume (GTV). Additionally, laterality in imaging and biopsies was assessed on a lobe level. Finally, we investigated whether clinical parameters correlate with the concordance of laterality between mpMRI and 68Ga-PSMA-PET.
Patients and methods
Study design and patient population
A total of 101 patients with biopsy-proven primary PCa who underwent mpMRI and 68Ga-PSMA-PET/CT (68Ga-PSMA-PET) within 3 months were retrospectively studied. Seven patients had received a transurethral resection of the prostate (TUR-P) more than 2 years prior to imaging and had visible tumor burden in imaging. Exclusion criteria were any therapeutic interventions prior imaging (except for TUR-P > 2 years ago), including neoadjuvant androgen deprivation therapy. Clinical parameters (age, prostate-specific antigen (PSA) serum levels prior to conduction of first imaging method (PSA), Gleason score in biopsy) were acquired. The study was approved by the institutional review board under registration number 203/19.
MR imaging
Prostate MRI was performed in 50 patients on a clinical 3 T system (MAGNETOM Trio Trim, MAGNETOM Skyra, MAGNETOM Vida, Siemens; Discovery MR750, GE Healthcare) and in 51 patients on a 1.5 T MRI (MAGNETOM Area, MAGNETOM Avanto fit, Siemens). For image acquisition, a surface phased array (body matrix) was combined with integrated spine array coil. Endorectal coils were not used to increase both patient compliance and signal homogeneity over the prostate. As part of the imaging protocol, T2-weighted, diffusion-weighted (DWI), and dynamic contrast-enhanced (DCE) image data was acquired. From the DWI images, maps of the apparent diffusion coefficient (ADC) were calculated. A detailed description of the imaging protocols can be found in [25]. Due to the time period of data (2013–2019) acquisition, MR protocols were heterogeneous in terms of slice thickness (3–3,5 mm), gap between slices (0–≤ 1 mm), and b values (low, 0 s/mm2 and 50 s/mm2; high, 800 s/mm2, 1000 s/mm2, and 1500s/mm2).
PET/CT imaging
The PSMA-HBED-CC radiolabeling with 68GaCl3 was performed according to good laboratory practice using a fully automated synthesis module (Ecker & Ziegler, Germany) and sterile single-use cassettes. The decay-corrected yield was > 95%, and radiochemical purity of the final product was ≥ 97%. The mean injected activity of 68Ga-HBED-CC-PSMA was 205.4 MBq (95% CI 200.1–210.7 MBq). Patients underwent a whole-body PET scan starting 1 h after injection. Scans were performed with either a 64-slice Gemini TF PET/CT scanner, a 16-slice Gemini TF big bore, or Vereos PET/CT scanner (all Philips Healthcare, USA). Cross-calibration of the three scanners was performed to ensure comparability of the quantitative measurements. At the time of the PET scan, either a contrast-enhanced diagnostic CT scan (120 kVp, 100–400 mAs, dose modulation) or a low-dose CT scan (120 kVp, 25 mAs) was performed for attenuation correction (depending on previous CT scans and contraindications). The uptake of 68Ga-PSMA was quantified in terms of standardized uptake values (SUV) normalized body weight.
Image analysis
MpMRI (T2-w and ADC) and PET (attenuation-corrected)/CT DICOM datasets were imported into a radiation treatment planning system (iPLAN RT image 4.1.2 BrainLAB, Germany; or Eclipse™ Treatment Planning System, Varian, USA). Images were co-registered according to in-house protocols [27].
GTV based on MRI T2-w and ADC images (GTV-MRI) was delineated in consensus by two experienced readers. Standardized imaging criteria (PI-RADSv2) were applied, and only lesions with a PI-RADS score of ≥ 3 were considered as relevant [26]. Access to the full MRI datasets including DCE and DWI images side by side was granted to the delineating physician.
GTV based on PET images (GTV-PET) was delineated by two experienced readers in consensus. Any monofocal or multifocal uptake greater than the adjacent background uptake in more than one slice within the CT-defined prostate gland was defined as the presence of PCa. GTVs were delineated manually in every single slice using PET image scaling of SUVmin−max: 0–5 [16]. For an example, see Fig. 1.
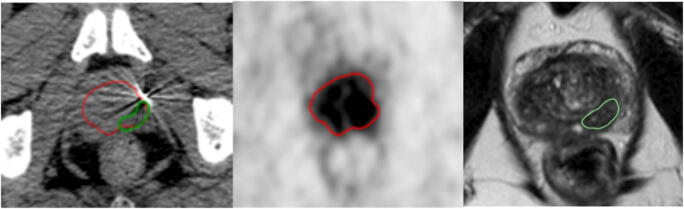
Fig. 1.
Example of differences in tumor volumes and laterality. (Left) axial CT slice with fused gross tumor volumes (GTV) delineated in PET (GTV-PET, red) and mpMRI (GTV MRI, green). (Middle) GTV delineated in PSMA-PET (scaling SUVmin−max 0–5). (Right) GTV delineated in mpMRI, image shows axial T2-weighted MR image. GTV-PET is larger than GTV delineated in MRI. Additionally, the GTV-PET extents to the right and left lobe, whereas the GTV-MRI is restricted to left lobe only
Subsequently delineated volumes were measured, and laterality (left lobe, right lobe, both lobes) as well as the number of lesions in both modalities was determined. Additionally, laterality of PCa-positive biopsies was extracted from 77 patients. The data was available in a form of histopathological or tumor boards reports.
Statistical analysis
Statistical analysis was performed using SPSS v25 (IBM, Armonk, NY) for the regression analysis and GraphPad PRISM v7.01 (GraphPad software, San Diego, CA) for all other analyses. D’Agostino-Pearson normality test was performed, and the data was not normally distributed. Comparison of GTV volumes was performed with Wilcoxon matched-pairs signed-rank test. Fisher’s exact test was performed to evaluate concordance in laterality. Univariate and multivariate analysis was performed with binary logistic regression analysis. Concordance in laterality was defined as the endpoint for regression analyses at a statistical significance threshold of < 0.05.
Figures were created with SPSS v25 (IBM, Armonk, NY), GraphPad PRISM v7.01 (GraphPad software, San Diego, CA), and Microsoft Office 2010 (Microsoft, Redmond, WA, USA).
Results
Between November 2013 and April 2019, 101 patients with primary PCa underwent mpMRI of the pelvis and 68Ga-PSMA PET/CT and fulfilled the required inclusion and exclusion criteria. Please see Table 1 for patient characteristics.
Table 1.
Patient characteristics
| patients, n = 101 | Median (95% CI) or (%) |
|---|---|
| Age (years) | 70 (68–72) |
| PSA (ng/ml) | 10.9 (9.39–13.03) |
| Patients, n = 101 | n (%) |
| Gleason score in biopsy, n | |
| 6 | 6 (6) |
| 7a | 37 (37) |
| 7b | 28 (28) |
| 8 | 19 (19) |
| 9 | 11 (11) |
| D’Amico risk group, n | |
| Low risk | 1 (1) |
| Intermediate risk | 35(35) |
| High risk | 65 (65) |
Analysis of GTV, laterality, and biopsies
In MRI, 63 lesions were detected in the left lobe only, 48 lesions in the right lobe only, and 40 lesions in both lobes (in total 151 lesions). In 68Ga-PSMA-PET, 51 lesions were detected in the left lobe only, 48 lesions in the right lobe only, and 60 lesions in both lobes (in total 159 lesions).
Analysis of tumor volumes revealed that GTV-MRI (median, 2.8 ml; range, 0.1–67.7 ml) was statistically significant smaller than GTV-PET (median 4.9 ml, range 0.5–140.0 ml, p < 0.0001). GTVs showed a difference in volume of ≥ 25% in 83.2% of patients and a difference ≥ 50% in 73.3% of patients. For details, see Table 2 and Fig. 2.
Table 2.
Volume differences
| Volume difference > 25% (in % of patients) | Volume difference > 50% (in % of patients) | |
|---|---|---|
| GTV-PET>CTV-MRI | 62.4% | 54.5% |
| GTV-MRI>GTV-PET | 20.8% | 19.3% |
| Total | 83.2% | 73.8% |
Fig. 2.
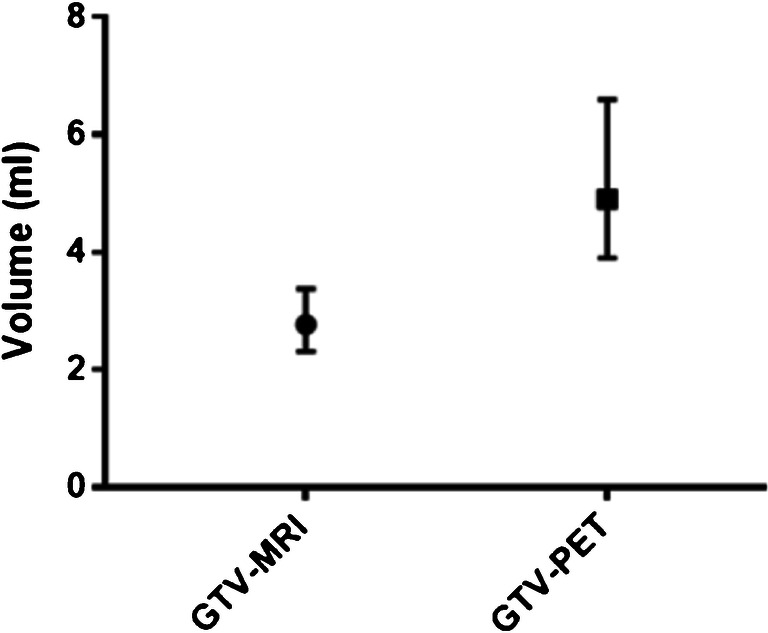
GTV volumes. (Left) median GTV-MRI was 2.8 ml (95% CI 2.31–3.38 ml), and median GTV-PET was 4.9 ml (95% CI 3.9–6.6 ml), respectively. Tumor volume was significantly smaller in mpMRI than in PSMA-PET/CT, p < 0.0001
Distribution and laterality of lesions in one lobe only (unilateral) or both lobes (bilateral) were analyzed. MpMRI showed 44 patients (43.6%) with lesions in one lobe only and 57 patients (56.4%) with lesions in both lobes. 68Ga-PSMA-PET showed 30 patients (29.7%) with lesions in one lobe only and 71 patients (70.3%) with lesions in both lobes, respectively (Fig. 3). PSMA-PET detected significantly more bilateral lesions than MRI (p = 0.03). In 37.6% of patients, PET and MRI showed different distribution of lesions. PSMA-PET detected lesions in both lobes, whereas MRI did not in 26 patients (26%), and conversely, MRI detected lesions in both lobes, whereas PET did not in 12 patients (12%). Subsequently laterality was concordant in 62.4%.
Fig. 3.
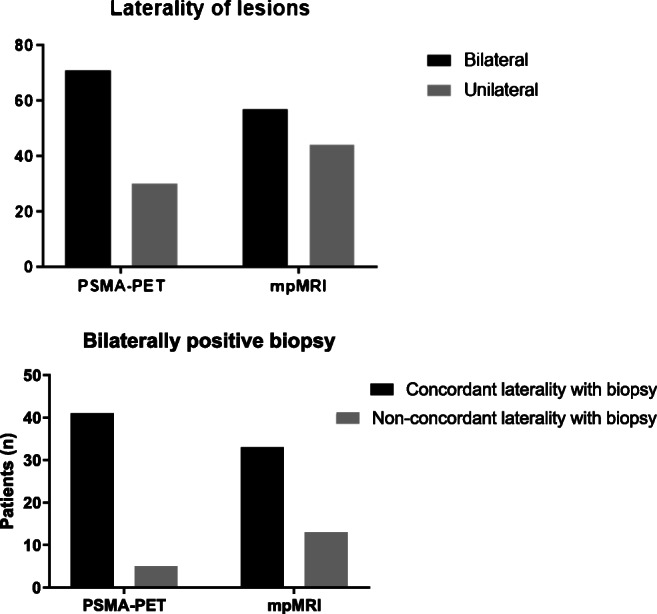
(Upper graph) laterality of lesions: PSMA-PET/CT showed 71 patiens with lesions in both lobes (bilateral) and 30 patients with lesions in one lobe only (unilateral), whereas mpMRI showed 57 patients with lesions in both lobes (bilateral) and 44 patients with lesions in one lobe only (unilateral) lesions. Laterality was significantly different between PSMA-PET/CT and mpMRI, p = 0.03. (Lower graph) analysis of biopsy data with bilateral lesions showed 41 concordant and 5 non-concordant cases in PSMA-PET/CT and 33 concordant and 13 non-concordant cases in mpMRI. There is a significant difference between concordance of laterality between PSMA-PET/CT and mpMRI with a higher concordance in PSMA-PET/CT, p = 0.03
Biopsy data from histopathological or tumor board reports were available in 77 patients and were analyzed in terms of laterality. Biopsy data showed lesions in one lobe only in 31 patients (40.3%) and lesions on both lobes in 46 patients (59.7%).There is no significant difference between concordance of laterality when analyzing all biopsy data. Subgroup analysis of cases with bilateral lesions in biopsy showed a significant higher concordance of laterality in 68Ga-PSMA-PET p = 0.03. For details, see Fig. 3.
Regression analysis
Regression analyses are presented in Table 3. In univariate analysis, PSA level and volume of GTV-MRI had an impact on concordance between 68Ga-PSMA-PET and mpMRI in terms of laterality (p = 0.02 and p = 0.01). Gleason score, MRI technique (1.5 vs 3 T), and age did not show to be significant. In multivariate analysis, including PSA and GTV-MRI volume, only the volume significantly predicted concordance of laterality (p = 0.04).
Table 3.
Regression analysis
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Parameters | OR | 95% CI | p value | OR | 95% CI | p value |
| PSA (metric variable) | 0.93 | 0.88–0.99 | 0.15 | 0.96 | 0.90–1.01 | 0.14 |
| GTV-MRI (categorical variable) | 0.79 | 0.66–0.94 | 0.01 | 0.82 | 0.69–0.99 | 0.04 |
| GS (categorical variable) | 0.80 | 0.52–1.23 | 0.31 | |||
| Age (continuous variable) | 1.0 | 0.95–1.05 | 0.92 | |||
| 0.74 | 0.33–1.65 | 0.46 | ||||
Discussion
Accurate identification of intraprostatic tumor burden is the key to successful biopsy guidance as well as planning of focal therapies. While mpMRI is the current gold standard, PSMA-PET has been shown to improve detection of intraprostatic tumor lesions. We compared delineation of GTVs in 68Ga-PSMA-PET/CT and mpMRI in 101 patients and analyzed clinical and technical parameters in terms of concordance of GTV volumes. Our study included 101 patients and showed that 68Ga-PSMA-PET gives complementary information to mpMRI due to identification of larger tumor volumes and more bilateral lesions, which show a higher concordance with biopsies.
mpMRI is the current gold standard for PCa detection [4] and detects significant lesions, since local recurrences occur at the site of primary tumor lesions detected by mpMRI [28, 29]. Nevertheless, Priester et al. demonstrated that mpMRI consistently underestimates the extent of tumor foci [6], and Johnsen et al. showed that mpMRI misses clinically significant tumor lesions in approximately 35% of the patients [7].
A study using whole-mount histopathology as the reference (16) proved that image segmentation based on PET does not overestimate the tumor burden. This study showed that the identified total number of lesions does not differ significantly between 68Ga-PSMA-PET and mpMRI. However, GTVs based on MRI were significantly smaller than GTV-PET, with approximately twice the volume in 68Ga-PSMA-PET. In more than half of the patients, GTV-PET was ≥ 50% larger than GTV-MRI, and in approximately one fifth of patients GTV-MRI was ≥ 50% larger than GTV-PET. These data confirm that GTVs in mpMRI are smaller than 68Ga-PET/CT [24, 25]. These results suggest that the use of margins to the GTV-MRI might deliver a better coverage of the tumor lesions detected by 68Ga-PSMA-PET. This approach could be assessed in future studies, which should ideally use whole-mount histology as the reference to draw convincing conclusion.
Additionally, our study showed differences in laterality in imaging modalities with significantly more patients with bilateral lesions in 68Ga-PSMA-PET/CT (71 vs 57 patients). Differences in laterality were confirmed by analysis of biopsy data. Comparison with positive bilateral biopsies showed that there is a significantly higher concordance of 68Ga-PSMA-PET with biopsy results. A bias in favor of mpMRI is noteworthy, since most biopsies were MRI-guided.
Previous histopathologic comparison studies have shown an increased sensitivity of intraprostatic tumor lesion delineation in small patient numbers. Additionally, the union of GTV-PET and GTV-MRI further improved sensitivity [18, 22, 24]. Consequently, we could prove that there is a significant difference in tumor volume, and laterality in a large number of patients and PET might detect clinically significant tumor lesions in the contralateral prostate lobe, which are not detected by mpMRI. Vice versa mpMRI is not dispensable, due to the substantial number of patients (12%), where mpMRI identified larger volumes and bilateral lesions exclusively. It is important to consider that in this study, contouring in both 68Ga-PSMA-PET and MRI was based on guidelines [16, 26]. To our knowledge, this study is the first to compare MRI and 68Ga-PET tumor delineation in a large cohort using validated contouring techniques for both imagings. The scaling used for PET results neither in over- nor in underestimation of tumor volumes validated in whole-mount histology reference (16). Our findings show that PSMA-PET gives highly important complimentary information.
Possible reasons for the difference in PCa detection are that mpMRI misses lesions in the transition zone, where conditions such as benign prostatic hypertrophy aggravate identification of tumor lesions [30]. Additionally, artifacts caused by prior biopsies affect mpMRI more than PSMA-PET, since T2-weighted MRI cannot differentiate between PCa lesions and hemorrhage [31]. Plausible biological or physical explanations for differences in volume delineation are not available to our knowledge.
Since the conduction of both imaging techniques in parallel is leading to increased financial burden for the healthcare system and PET-CT causes a radiation exposure, we analyzed the correlation of clinical parameters with the concordance between mpMRI and PET-CT. Thus, we aimed to identify parameters to guide in which cases PET-CT would be dispensable. Univariate analysis showed a significant association for PSA level as well as for volume of GTV-MRI with concordance of laterality between both imaging techniques. In multivariate analysis, only GTV-MRI was significant. Hence, merely in patients with large tumor volumes in mpMRI, 68Ga-PSMA-PET/CT might give little complimentary information. However, these patients bear a high risk for lymph node or distant metastases [32], and 68Ga-PSMA-PET shows high sensitivities and specificities for lymph node detection, especially in metastases smaller than 10 mm and thus is beneficial for lymph node staging [33, 34]. Consequently, our data encourage the usage of 68Ga-PSMA-PET and mpMRI for biopsy guidance and planning of focal therapies and show that 68Ga-PSMA-PET should at least be performed in patients with intermediate and high-risk PCa since it gives highly complementary information.
The study’s limitations are the retrospective design and the lack of information about laterality in biopsy samples in 24% of patients. Surely, whole-mount histopathologic data would be the reference gold standard for comparison of intraprostatic tumor burden detection. Conduction of heterogeneous MRI protocols with 1.5 T (without endorectal coil) and 3 T systems is another limitation of our study. However, we performed analyses with data from 50 patients, who received a 3 T mpMRI and obtained comparable results (data not shown). Additionally, we highlight that for the dominant sequences of mpMRI [35], a reasonable and recommended slice thickness of 3 mm was acquired in the vast majority of patients. Furthermore, the inclusion of 1.5 T and 3 T systems reflects real-world prostate diagnostic practice. Potential sources of differences in in visual 68Ga-PSMA-PET interpretation were the usage of three different PET-CT scanners and the use of contrast-enhanced as well as low-dose CT-scans. However, cross-calibration between PET-scanners was performed to optimize comparability. 68Ga-PSMA accumulation in the bladder intensifies background signal and thus aggravates tumor lesion delineation in areas adjacent to the bladder. New PSMA-tracers like 18F-PSMA-1007 show lesser renal elimination and might further improve intraprostatic tumor lesions detection [36, 37].
Conclusion
Our study is the first study to perform an intraindividual comparison between 68Ga-PSMA-PET/CT and mpMRI in a large cohort of > 100 patients by using validated contouring approaches. Taking into account previous findings from histopathologic comparison studies, our study empowers the use of 68Ga-PSMA-PET-CT and the combination with mpMRI for delineation of GTVs for planning of focal therapies and biopsy guidance in patients with primary PCa. 68Ga-PSMA-PET/CT gives highly important complementary information, which improves coverage of intraprostatic tumor lesions due to identification of larger tumor volumes and bilateral lesions missed by mpMRI. Additionally, 68Ga-PSMA-PET detects metastasis with high accuracy and thus is also beneficial for staging. So far there is no strong evidence for parameters to guide in which cases 68Ga-PSMA-PET/CT is dispensable.
Funding information
Open Access funding provided by Projekt DEAL. This work was funded by the Klaus Tschira Foundation.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
This article is part of the Topical Collection on Oncology – Genitourinary
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel, R.L., Miller, K.D. and Jemal, A. (2019), Cancer statistics, 2019. CA A Cancer J Clin, 69: 7–34. [DOI] [PubMed]
- 2.Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer (Oxford, England : 1990) 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Ha Chung B, Horie S, Chiong E. The incidence, mortality, and risk factors of prostate cancer in Asian men. Prostate Int. 2019;7(1):1–8. doi: 10.1016/j.prnil.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378(19):1767–1777. doi: 10.1056/NEJMoa1801993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahdoot M, Lebastchi AH, Turkbey B, Wood B, Pinto PA. Contemporary treatments in prostate cancer focal therapy. Curr Opin Oncol. 2019;31(3):200–206. doi: 10.1097/CCO.0000000000000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Priester A, Natarajan S, Khoshnoodi P, Margolis DJ, Raman SS, Reiter RE, et al. Magnetic resonance imaging underestimation of prostate cancer geometry: use of patient specific molds to correlate images with whole mount pathology. J Urol. 2017;197(2):320–326. doi: 10.1016/j.juro.2016.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson DC, Raman SS, Mirak SA, Kwan L, Bajgiran AM, Hsu W, et al. Detection of individual prostate cancer foci via multiparametric magnetic resonance imaging. Eur Urol. 2019;75(5):712–720. doi: 10.1016/j.eururo.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Eder M, Neels O, Muller M, Bauder-Wust U, Remde Y, Schafer M, et al. Novel preclinical and radiopharmaceutical aspects of [68Ga]Ga-PSMA-HBED-CC: a new PET tracer for imaging of prostate cancer. Pharmaceuticals (Basel) 2014;7(7):779–796. doi: 10.3390/ph7070779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mhawech-Fauceglia P, Zhang S, Terracciano L, Sauter G, Chadhuri A, Herrmann FR, et al. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using mutiple tumour tissue microarray technique. Histopathology. 2007;50(4):472–483. doi: 10.1111/j.1365-2559.2007.02635.x. [DOI] [PubMed] [Google Scholar]
- 10.Eder M, Schafer M, Bauder-Wust U, Hull WE, Wangler C, Mier W, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 2012;23(4):688–697. doi: 10.1021/bc200279b. [DOI] [PubMed] [Google Scholar]
- 11.Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid (6)(8)Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56(5):668–674. doi: 10.2967/jnumed.115.154153. [DOI] [PubMed] [Google Scholar]
- 12.Ceci F, Uprimny C, Nilica B, Geraldo L, Kendler D, Kroiss A, et al. (68)Ga-PSMA PET/CT for restaging recurrent prostate cancer: which factors are associated with PET/CT detection rate? Eur J Nucl Med Mol Imaging. 2015;42(8):1284–1294. doi: 10.1007/s00259-015-3078-6. [DOI] [PubMed] [Google Scholar]
- 13.Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019;5(6):856–863. doi: 10.1001/jamaoncol.2019.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt-Hegemann NS, Stief C, Kim TH, Eze C, Kirste S, Strouthos I, et al. Outcome after PSMA PET/CT based salvage radiotherapy in patients with biochemical recurrence after radical prostatectomy: a bi-institutional retrospective analysis. J Nucl Med. 2019;60:227–233 [DOI] [PMC free article] [PubMed]
- 15.Civelek AC. (68)Ga-PSMA-11 PET: better at detecting prostate cancer than multiparametric MRI? Radiology. 2018;289(3):738–739. doi: 10.1148/radiol.2018181981. [DOI] [PubMed] [Google Scholar]
- 16.Zamboglou C, Fassbender TF, Steffan L, Schiller F, Fechter T, Carles M, et al. Validation of different PSMA-PET/CT-based contouring techniques for intraprostatic tumor definition using histopathology as standard of reference. Radiother Oncol. 2019 Dec;141:208–213 [DOI] [PubMed]
- 17.Zamboglou C, Carles M, Fechter T, Kiefer S, Reichel K, Fassbender TF, et al. Radiomic features from PSMA PET for non-invasive intraprostatic tumor discrimination and characterization in patients with intermediate- and high-risk prostate cancer - a comparison study with histology reference. Theranostics. 2019;9(9):2595–2605. doi: 10.7150/thno.32376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eiber M, Weirich G, Holzapfel K, Souvatzoglou M, Haller B, Rauscher I, et al. Simultaneous (68)Ga-PSMA HBED-CC PET/MRI improves the localization of primary prostate cancer. Eur Urol. 2016;70(5):829–836. doi: 10.1016/j.eururo.2015.12.053. [DOI] [PubMed] [Google Scholar]
- 19.Rhee H, Thomas P, Shepherd B, Gustafson S, Vela I, Russell PJ, et al. Prostate specific membrane antigen positron emission tomography may improve the diagnostic accuracy of multiparametric magnetic resonance imaging in localized prostate cancer. J Urol. 2016;196(4):1261–1267. doi: 10.1016/j.juro.2016.02.3000. [DOI] [PubMed] [Google Scholar]
- 20.Berger I, Annabattula C, Lewis J, Shetty DV, Kam J, Maclean F, et al. (68)Ga-PSMA PET/CT vs. mpMRI for locoregional prostate cancer staging: correlation with final histopathology. Prostate Cancer Prostatic Dis. 2018;21(2):204–211. doi: 10.1038/s41391-018-0048-7. [DOI] [PubMed] [Google Scholar]
- 21.Kesch C, Vinsensia M, Radtke JP, Schlemmer HP, Heller M, Ellert E, et al. Intraindividual comparison of (18)F-PSMA-1007 PET/CT, multiparametric MRI, and radical prostatectomy specimens in patients with primary prostate cancer: a retrospective, proof-of-concept study. J Nucl Med. 2017;58(11):1805–1810. doi: 10.2967/jnumed.116.189233. [DOI] [PubMed] [Google Scholar]
- 22.Chen M, Zhang Q, Zhang C, Zhao X, Marra G, Gao J, et al. Combination of (68)Ga-PSMA PET/CT and multiparametric MRI improves the detection of clinically significant prostate cancer: a lesion-by-lesion analysis. J Nucl Med. 2019;60(7):944–949. doi: 10.2967/jnumed.118.221010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hicks RM, Simko JP, Westphalen AC, Nguyen HG, Greene KL, Zhang L, et al. Diagnostic accuracy of (68)Ga-PSMA-11 PET/MRI compared with multiparametric MRI in the detection of prostate cancer. Radiology. 2018;289(3):730–737. doi: 10.1148/radiol.2018180788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bettermann AS, Zamboglou C, Kiefer S, Jilg CA, Spohn S, Kranz-Rudolph J, et al. [(68)Ga-]PSMA-11 PET/CT and multiparametric MRI for gross tumor volume delineation in a slice by slice analysis with whole mount histopathology as a reference standard - Implications for focal radiotherapy planning in primary prostate cancer. Radiother Oncol. 2019 Dec;141:214–219. [DOI] [PubMed]
- 25.Zamboglou C, Wieser G, Hennies S, Rempel I, Kirste S, Soschynski M, et al. MRI versus (6)(8)Ga-PSMA PET/CT for gross tumour volume delineation in radiation treatment planning of primary prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43(5):889–897. doi: 10.1007/s00259-015-3257-5. [DOI] [PubMed] [Google Scholar]
- 26.Turkbey B, Choyke PL. PIRADS 2.0: what is new? Diagn Interv Radiol. 2015;21(5):382–384. doi: 10.5152/dir.2015.15099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zamboglou C, Drendel V, Jilg CA, Rischke HC, Beck TI, Schultze-Seemann W, et al. Comparison of (68)Ga-HBED-CC PSMA-PET/CT and multiparametric MRI for gross tumour volume detection in patients with primary prostate cancer based on slice by slice comparison with histopathology. Theranostics. 2017;7(1):228–237. doi: 10.7150/thno.16638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pucar D, Hricak H, Shukla-Dave A, Kuroiwa K, Drobnjak M, Eastham J, et al. Clinically significant prostate cancer local recurrence after radiation therapy occurs at the site of primary tumor: magnetic resonance imaging and step-section pathology evidence. Int J Radiat Oncol Biol Phys. 2007;69(1):62–69. doi: 10.1016/j.ijrobp.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 29.Zamboglou C, Klein CM, Thomann B, Fassbender TF, Rischke HC, Kirste S, et al. The dose distribution in dominant intraprostatic tumour lesions defined by multiparametric MRI and PSMA PET/CT correlates with the outcome in patients treated with primary radiation therapy for prostate cancer. Radiat Oncol (London, England) 2018;13(1):65. doi: 10.1186/s13014-018-1014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bates A, Miles K. Prostate-specific membrane antigen PET/MRI validation of MR textural analysis for detection of transition zone prostate cancer. Eur Radiol. 2017;27(12):5290–5298. doi: 10.1007/s00330-017-4877-x. [DOI] [PubMed] [Google Scholar]
- 31.Eiber M, Nekolla SG, Maurer T, Weirich G, Wester H-J, Schwaiger M. 68Ga-PSMA PET/MR with multimodality image analysis for primary prostate cancer. Abdom Imaging. 2015;40(6):1769–1771. doi: 10.1007/s00261-014-0301-z. [DOI] [PubMed] [Google Scholar]
- 32.Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71(4):618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Cook GJR, Kulkarni M, Warbey VS. PSMA PET/CT imaging for primary staging of intermediate and high-risk prostate cancer. BJU Int. 2019;124(3):357–358. doi: 10.1111/bju.14856. [DOI] [PubMed] [Google Scholar]
- 34.Meißner S, Janssen J-C, Prasad V, Diederichs G, Hamm B, Brenner W, et al. Accuracy of standard clinical 3T prostate MRI for pelvic lymph node staging: comparison to 68Ga-PSMA PET-CT. Sci Rep. 2019;9(1):10727. doi: 10.1038/s41598-019-46386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol. 2019;76(3):340–351. doi: 10.1016/j.eururo.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 36.Kuten J, Fahoum I, Savin Z, Shamni O, Gitstein G, Hershkovitz D, et al. Head- to head Comparison of (68)Ga-PSMA-11 with (18)F-PSMA-1007 PET/CT in Staging Prostate Cancer Using Histopathology and Immunohistochemical Analysis as Reference-Standard. J Nucl Med. 2020 Apr;61(4):527–532. [DOI] [PubMed]
- 37.Rahbar K, Weckesser M, Ahmadzadehfar H, Schafers M, Stegger L, Bogemann M. Advantage of (18)F-PSMA-1007 over (68)Ga-PSMA-11 PET imaging for differentiation of local recurrence vs. urinary tracer excretion. Eur J Nucl Med Mol Imaging. 2018;45(6):1076–1077. doi: 10.1007/s00259-018-3952-0. [DOI] [PubMed] [Google Scholar]