Abstract
The anti-inflammatory effects of vagus nerve stimulation are well known. It has recently been shown that low-level, transcutaneous stimulation of vagus nerve at the tragus (LLTS) reduces cardiac inflammation in a rat model of heart failure with preserved ejection fraction (HFpEF). The mechanisms by which LLTS affect the central neural circuits within the brain regions that are important for the regulation of cardiac vagal tone are not clear. Female Dahl salt-sensitive rats were initially fed with either low salt (LS) or high salt (HS) diet for a period of 6 weeks, followed by sham or active stimulation (LLTS) for 30 min daily for 4 weeks. To study the central effects of LLTS, four brainstem (SP5, NAb, NTS, and RVLM) and two forebrain sites (PVN and SFO) were examined. HS diet significantly increased the gene expression of proinflammatory cytokines in the SP5 and SFO. LLTS reversed HS diet-induced changes at both these sites. Furthermore, LLTS augmented the levels of antioxidant Nrf2 in the SP5 and SFO. Taken together, these findings suggest that LLTS has central anti-inflammatory and antioxidant properties that could mediate the neuromodulation of cardiac vagal tone in the rat model of HFpEF.
Subject terms: Cardiovascular biology, Neurophysiology, Molecular neuroscience
Introduction
Heart failure with preserved ejection fraction (HFpEF) is one of the leading causes of mortality in the elderly1. The prevalence of HFpEF increases from 1% at age 40 to about 10% at age 802. It is the most common cause of hospitalization in patients ≥ 65 years of age and with the growing elderly population, these trends are expected to worsen2,3. The prognosis for patients with HFpEF is poor (5-year mortality rate approximately 50%)4, and so far, no treatment has been demonstrated to achieve positive outcomes on morbidity or mortality in these patients5. Inflammation and fibrosis are the most prominent pathophysiological mechanisms that mediate impairments of cardiac function in heart failure. Identification of treatment options that target these cardiac mechanisms may prove fruitful.
Vagus nerve stimulation (VNS) is a non-pharmacological, device-based therapy that is currently approved by FDA for the treatment of epilepsy and depression6. Recent studies have indicated that low-level VNS suppresses atrial fibrillation and inflammatory cytokines in humans7–9. In addition, several preclinical studies have yielded promising results for the use of non-invasive VNS in improving cardiac outcomes of HFpEF10,11. Using Dahl salt sensitive rats on high salt diet as a rodent model for HFpEF, it was recently shown that chronic, intermittent, low-level, tragus stimulation (LLTS) significantly ameliorated diastolic function and attenuated left ventricular (LV) inflammation and fibrosis10. Although it is well-established that the auricular branch of the vagus nerve (ABVN) is activated during LLTS, the neural pathways and central mechanisms underlying the effects of LLTS on inflammation and heart function have not been fully explored12. In fact, previous studies have shown that increased oxidative stress and inflammation in the brains of Dahl sensitive rats contribute to autonomic imbalance. This has been characterized by increased sympathetic nerve activity13, which is known to play a critical role in the progression of heart failure. However, there is a significant gap in knowledge in understanding the neural circuitry that is modulated by LLTS, which in turn may lead to identification of potential therapeutic targets.
The sensory afferents from ABVN innervate two brainstem regions, the nucleus tractus solitarius (NTS) and spinal trigeminal nucleus (SP5)14–17 and hence present the possibility for the involvement of both these neural centers in LLTS mediated beneficial effects on the heart. The first possibility may involve activation of the NTS by ABVN afferents to inhibit the rostral ventrolateral medulla (RVLM) via stimulation of the caudal ventrolateral medulla (CVLM), resulting in reduced sympathetic output to the heart. This is a well-known arterial baroreflex pathway that provides arterial pressure stabilization through feedback control of cardiac and vascular tone18,19. The second possibility might involve activation of either NTS or SP5 by ABVN afferents resulting in stimulation of vagal efferents (cardiac vagal preganglionic parasympathetic neurons) from the nucleus ambiguus (NAb) to inhibit heart rate15,20. In addition to the brainstem, studies involving direct vagus nerve stimulation have shown c-FOS immunoreactivity in forebrain regions such as the paraventricular nucleus (PVN) and subfornical organ (SFO), to which direct neuronal projections from NTS has been well established21,22. Whether LLTS acts through any of these neural circuits to exert beneficial effects on the heart is not known.
The overall objective of this study was to understand the effects of LLTS on four brainstem (SP5, NAb, NTS and RVLM) and two forebrain regions (PVN and SFO) that are important for the regulation of cardiac vagal tone17 by using a well-established rat model of HFpEF (Dahl salt sensitive rats)23. The hypothesis behind this study is that LLTS would attenuate the pro-oxidant and pro-inflammatory response exerted by HS diet in the brainstem regions that are part of the ABVN-heart neural circuitry.
Methods
Experimental animals and treatment
Fourteen female DS rats were purchased from Charles River Laboratories (Wilmington, MA, USA) and were maintained on 0.3% NaCl (low salt, LS) diet until 7 weeks of age. After 7 weeks, the animals were randomly divided into three groups. All the animals in the first group (n = 4) continued to be fed with LS diet until the end of the experimental period. The remaining animals (n = 10) were fed with 8% NaCl (high salt, HS) diet for 6 weeks. After 6 weeks of HS, animals received either sham (n = 6) or active (n = 4) LLTS treatment for an additional 4 weeks. Heart rate, blood pressure (BP) and body weight were determined at 6 weeks after LS/HS diet (baseline) and 4 weeks after LLTS treatment (endpoint). BP measurements were obtained non-invasively using the tail-cuff method (AD Instrument, PowerLab Data Acquisition System, New South Wales, Australia). Echocardiography (Acuson SC2000, Siemens) was performed at the same time points to assess diastolic function, under isoflurane 2% anesthesia as described previously10. At the end of 4 weeks of LLTS sham or active treatment, the animals were euthanized with 5% isoflurane by inhalation and the brainstems were harvested for analysis. The final LLTS was performed about 24 h before the euthanasia. Water was provided ad libitum throughout the duration of the treatment. All the animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Science Center (protocol number 16‐106) and were carried out under the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
LLTS
LLTS (20 Hz frequency, 0.2 ms pulse duration, 2 mA amplitude) was given through a TENS device (InTENSity Twin Stim, Current Solutions LLC, Austin, TX, USA) daily for 30 min over a 4-week period under 2% isoflurane anesthesia as described previously10. Briefly, in HS sham group, the electrodes were placed on the auricular margin which does not have any vagal innervation whereas in HS active group they were placed over the auricular concha region to stimulate the auricular branch of vagal nerve.
Heart rate variability
At baseline, a 3-min ECG was recorded before and during active stimulation in all the rats. Heart rate variability (HRV) in the time domain (standard deviation of NN interval, SDNN and root mean square of consecutive NN intervals, RMSSD) and in the frequency domain (low frequency, LF, 0.2–0.8 Hz and high frequency, HF, 0.8–2.5 Hz), was calculated, as previously described24, using the Kubios software.
Microdissection of key brainstem nuclei and forebrain regions
Serial coronal sections (300 μm thick) of the brainstem were obtained on super frost plus slides using a cryostat. The spinal trigeminal nucleus (SP5), nucleus ambiguus (NAb), nucleus tractus solitarius (NTS), rostral ventrolateral medulla (RVLM), paraventricular nucleus (PVN) and subfornical organ (SFO) were microdissected by Palkovits’s technique. A rat brain stereotaxic atlas was used as a reference (Paxinos and Watson, 1987).
Real time PCR
Micropunches from brain regions were collected from either side of the 300 μm thick section based on the stereotaxic co-ordinates. Punches were collected from the SP5, NAb, NTS, RVLM, PVN, and SFO regions and used for RNA extraction and real-time PCR analysis. The tissue micropunches were lysed in Trizol reagent and RNA was extracted using Direct-zol RNA Microprep kit (ZymoResearch, Irvine, CA, USA). cDNA was synthesized using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Real time PCR reactions were carried out using iTaq Universal SYBR Green mix (Biorad) with 10 ng cDNA per reaction. The rat primers for IL6, IL1-Beta, TNF-Alpha, MCP1, CXCL1, Nrf2, NQO1, Fra1 and GAPDH (the housekeeping gene) were designed using primer blast software and synthesized by Integrated DNA technologies (primer sequences are listed in Table 1). Data was analyzed by 2−ΔΔCT method.
Table 1.
Primer sequences and their gene accession numbers for real time PCR analysis are listed.
| Rat gene | Forward primer | Reverse primer | Gene accession number |
|---|---|---|---|
| IL6 | CTGGTCTTCTGGAGTTCCGT | TGCTCTGAATGACTCTGGCT | NM_012589.2 |
| IL-1Beta | GACTCGTGGGATGATGACGAC | GAGCTTTCAGCTCACATGGGT | NM_031512.2 |
| TNF-Alpha | ACCATGAGCACGGAAAGCAT | CTTGTTGGGACCGATCACCC | NM_012675.3 |
| MCP1 | CACTCACCTGCTGCTACTCATT | TACAGCTTCTTTGGGACACCTG | NM_031530.1 |
| CXCL1 | GCGCCCGTCGCCAAT | GGCATCACCTTCAAACTCTGGA | NM_030845.1 |
| Nrf2 | AGAGCAACTCCAGAAGGAACAG | TTGGGAGGAATTCTCCGGTCT | NM_031789.2 |
| NQO1 | ATTGTATTGGCCCACGCAGA | GATTCGACCACCTCCCATCC | NM_017000.3 |
| Fra-1 | TGGATGGTGCAGCCTCATTT | GCTCGTATGACTCCTGGTCG | NM_012953.1 |
| GAPDH | GGGTGCCAACCCCAAACGTA | CCATAGGTCCCTTGGCTGCT | NM_023964.1 |
Statistical analysis
All statistical analysis was performed using GraphPad Prism Version 8.3. All data were analyzed using one-way ANOVA followed by post hoc Tukey’s multiple comparison test. Log transformation of the heart rate variability parameters was performed to achieve normality and a paired t-test was used for comparison. All gene expression values were expressed as fold change relative to low salt. A p-value of < 0.05 is considered statistically significant.
Ethical approval
This article does not contain any study with human participants. The experimental protocols using rats were approved by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Science Center (protocol number 16‐106) and were carried out under the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Results
Effects of LLTS on HFpEF phenotype
After 6 weeks of treatment with HS diet, rats developed hypertension, left ventricular hypertrophy and diastolic dysfunction [as indicated by elevated early mitral inflow velocity to early diastolic mitral annulus velocity (E/e’)], compared to LS rats (158.3 ± 12.6 mmHg vs. 123.8 ± 9.8 mmHg, p = 0.0004; 2.4 ± 0.2 mm vs. 1.8 ± 0.1 mm, p = 0.02; 24.0 ± 2.1 vs. 18.3 ± 1.6, p = 0.01, respectively). Consistent with our previous study, 4 weeks of LLTS attenuated the increase in BP (146.0 ± 10.2 mmHg vs. 183.5 ± 9.8 mmHg, respectively, p = 0.04), left ventricular thickness (2.3 ± 0.1 mm vs. 2.7 ± 0.1 mm, p = 0.03, respectively) and diastolic dysfunction (18.8 ± 3.0 vs. 26.5 ± 4.9, p = 0.01, respectively) induced by HS diet, without any change in left ventricular ejection fraction (p > 0.05).
Effects of LLTS on cytokine expression in the brainstem and forebrain regions
To determine the central effects of LLTS on inflammation, the gene expression of cytokines IL-6, IL1-Beta and TNF-Alpha was measured in four brainstem (SP5, NAb, NTS, and RVLM) and two forebrain (PVN and SFO) regions after HS treatment. High salt resulted in significant increases in the level of IL-6 (Fig. 1) in the SP5 and NAb compared to low salt (LS) controls. LLTS attenuated the increases in IL-6 expression in HS rats in the SP5 but not in the NAb region. There were no significant changes in IL1-Beta or TNF-Alpha levels in SP5 and NAb. There was a tenfold increase in the expression of IL-1Beta in the SFO of HS group compared to low salt. LLTS was able to significantly reduce the IL-1Beta in the SFO. No changes were observed in the expression of both of these inflammatory markers in the NTS, RVLM, and PVN among different groups.
Figure 1.
Gene expression of cytokines in the brainstem and forebrain regions after LLTS or sham stimulation. The mRNA levels of cytokines IL-6, IL1-Beta and TNF-Alpha in the brain regions SP5, NAb, NTS, RVLM, PVN, and SFO after low salt (LS), high salt with sham stimulation (HS sham) and high salt with LLTS (HS active) treatment are shown. Values are mean ± SE (n = 4–6/group). *P < 0.05 is considered statistically significant.
Effects of LLTS on chemokine expression in the brainstem and forebrain regions
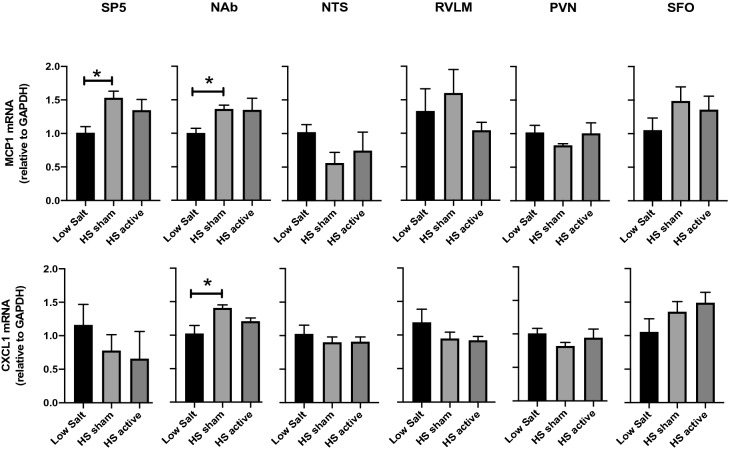
To elucidate the central effects of LLTS on chemokines, we measured the gene expression of MCP1 and CXCL1 after HS treatment. HS rats showed significant increase in the mRNA levels of MCP1 in both SP5 and NAb compared with LS controls (Fig. 2). However, LLTS failed to attenuate MCP1 expression in these brainstem regions. Similarly, CXCL1 was upregulated in the NAb of HS sham rats and LLTS was not able to reduce its expression. Neither HS diet nor LLTS had any effects on chemokine expression in the other regions that were tested.
Figure 2.
Gene expression of chemokines in the brainstem and forebrain regions after LLTS or sham stimulation. The mRNA levels of chemokines MCP1 and CXCL1 in the brainstem regions SP5, NAb, NTS and RVLM and forebrain regions PVN and SFO after LS, HS sham and HS active treatment are shown. Values are mean ± SE (n = 4–6/group). *P < 0.05 is considered statistically significant.
Effects of LLTS on antioxidant expression in the brainstem and forebrain regions
It is widely accepted that vagus nerve stimulation has systemic antioxidant properties25,26. To test whether LLTS has any central antioxidant effects, mRNA expression of the antioxidant gene, NQO1 and its transcriptional regulator Nrf2 were measured in four brainstem and two forebrain regions. HS diet did not produce any changes in the expression of Nrf2 or NQO1 in the SP5 compared to LS controls. However, LLTS produced a significant upregulation of Nrf2 and NQO1 in the SP5 compared to both LS and HS sham (Fig. 3). Similar increases in Nrf2 expression were also seen in the SFO region of the LLTS group suggesting LLTS may be involved in the activation of the antioxidant pathways. Interestingly, LLTS mediated upregulation of antioxidant gene expression was specific only to SP5 and SFO and not observed in the other regions that were tested (NAb, NTS, RVLM, and PVN).
Figure 3.
Gene expression of antioxidants in the brainstem and forebrain regions after LLTS or sham stimulation. The mRNA levels of antioxidants Nrf2 and NQO1 in the brain regions SP5, NAb, NTS, RVLM, PVN and SFO after LS, HS sham and HS active treatment are shown. Values are mean ± SE (n = 4–6/group). *P < 0.05 is considered statistically significant.
Effect of LLTS on markers of neuronal activity in the brainstem and forebrain regions
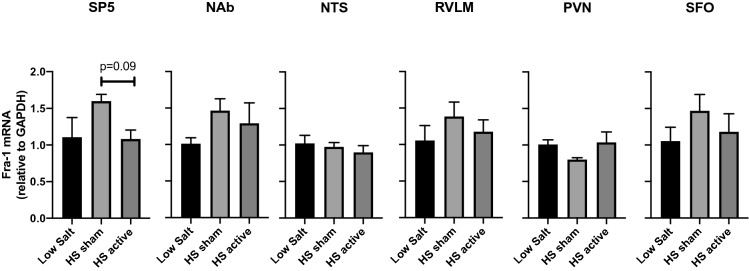
The gene expression of molecular marker of long-term neuronal activity namely Fra-1 was measured in the regions of interest. A numerical trend was observed towards upregulation of Fra-1 gene in the HS sham group followed by an inclination towards downregulation in both the SP5 and SFO regions within the LLTS treated group. However, the data did not reach statistical significance (Fig. 4).
Figure 4.
Gene expression of neuronal activity markers in the brain regions after LLTS or sham stimulation. The mRNA levels of markers Fra-1 in the brain regions SP5, NAb, NTS RVLM, PVN, and SFO after LS, HS sham and HS active treatment are shown. Values are mean ± SE (n = 4–6/group). *P < 0.05 is considered statistically significant.
Acute effects of LLTS on autonomic tone
Heart rate variability was used to assess the acute effects of LLTS on autonomic tone, in order to ensure that LLTS resulted in stimulation of parasympathetic elements. Both SDNN and RMSSD were measured before (baseline) and during active LLTS (stimulation) indicating the long and short-term variations in heart rate respectively. Compared to baseline, SDNN was significantly upregulated during LLTS stimulation. RMSSD showed an increase during active stimulation, however it did not reach statistical significance (Table 2). Both LF and HF powers were increased during LLTS, consistent with acute cardiac autonomic modulation (Fig. 5). Acute changes in heart rate variability may not reflect long-term effects of LLTS. However, it has recently been shown that chronic LLTS results in changes in HRV in humans9.
Table 2.
Heart rate variability parameters before and during stimulation are shown.
| Variable | Baseline | Stimulation | P-value |
|---|---|---|---|
| SDNN (log) (ms) | 1.31 ± 0.13 | 1.72 ± 0.13 | 0.04 |
| RMSSD (log) (ms) | 1.38 ± 0.11 | 1.68 ± 0.11 | 0.09 |
| Low frequency (log) (ms2) | 1.92 ± 0.13 | 2.24 ± 0.14 | 0.04 |
| High frequency (log) (ms2) | 0.83 ± 0.11 | 1.23 ± 0.11 | 0.01 |
Figure 5.
Acute effects of LLTS on heart rate variability parameters. The amplitude of time domain parameters (SDNN and RMSSD) and the power of frequency domain parameters (LH and HF) are shown. Values are mean ± SE. *P < 0.05 is considered statistically significant.
Discussion
The effects of LLTS on the central neural circuits involved in the autonomic control of heart are not well established. In order to investigate the neural circuitry mediating the effects of LLTS on the heart, four brainstem and two forebrain regions were analyzed for alterations in the gene expression of pro-inflammatory and antioxidant markers after 4 weeks of LLTS in a rat model of HFpEF. Results showed that HS diet exerted pro-inflammatory effects marked by increased levels of IL6, MCP1, and CXCL1 specifically in the SP5 and NAb of the brainstem and IL-1Beta in the SFO of the forebrain. LLTS attenuated inflammation by decreasing IL6 levels in the SP5 and IL-1Beta in the SFO. In addition, LLTS stimulated antioxidant defense mechanisms by activating the Nrf2 pathway in the SP5 and SFO of HS diet animals. These results are consistent with a recent study which showed that the cardiovascular autonomic effect of tragus stimulation is mediated at least partly through spinal cervical sensory afferent pathways, including SP516. Moreover, only sparse labeling of transganglionic tracer injected into the tragus of rats was found in NTS16. No strong evidence was found to support the direct involvement of NTS in LLTS mediated autonomic regulation of the heart. The parallel observations in the gene expression of antioxidant, anti-inflammatory and neuronal activity markers seen in the SFO similar to SP5 is novel. The data suggests that ascending inputs from brainstem site (possibly NTS) may function in modulating the response of neurons within the circumventricular organ (SFO) which in turn could be responsible for the observed alterations in blood pressure and heart rate variability after LLTS22,27. These results provide preliminary evidence that LLTS modulates central neural circuits, specifically at the level of SP5 and SFO, and challenge the notion that LLTS activates only the auricular branch of the vagus nerve17. On the other hand, the LLTS effects on heart rate variability, which are consistent with acute cardiac autonomic modulation, support the notion that stimulation engages central vagal pathways. Further studies are warranted to delineate the exact anatomical basis of transcutaneous VNS, in order to better guide future clinical trials.
Numerous studies showed that LLTS had anti-inflammatory effects in a variety of cardiovascular diseases7,28. These effects were attributed to activation of the cholinergic anti-inflammatory pathway, which uses the vagus nerve as the efferent limb29. Recently it has been shown that LLTS alleviates cardiac inflammation and fibrosis in a rat-model of HFpEF, but the neural mechanisms behind the protective effects of LLTS remain unclear10. In the present study, HS diet significantly increased the proinflammatory cytokine IL-6 mRNA in the Dahl salt sensitive rats in both SP5 and Nab, but not in other regions tested. The result showed that HS diet produces inflammation in key brainstem regions (SP5 and NAb) important for cardiac vagal control. After LLTS in HS rats, IL-6 mRNA was significantly downregulated in the SP5 suggesting that LLTS reduces inflammation at the brainstem level. One possible mechanism by which LLTS reduces cardiac inflammation in this HFpEF model could be through modulation of autonomic activity in key brainstem regions involved in cardiac vagal tone. Notably, in the present study, HS diet induced upregulation of IL-6 was observed only at SP5 but not in NTS. LLTS was able to significantly attenuate the increase in IL-6 mRNA. Interestingly, a significant upregulation in IL-6 mRNA was also noted at NAb after HS diet, however LLTS was not able to reverse this increase. NAb is downstream from SP5 and NTS. Afferent signals from LLTS through ABVN first reaches SP5 and NTS, signals from there could be transmitted downstream by direct neuronal connection between these regions and NAb14,20. It has been well documented that NAb neurons directly innervate heart and are involved in the modulation of cardiac vagal tone20.
In addition to inflammatory cytokines, the effects of LLTS on central chemokines after HS treatment in rats was not known. In a recent human study, non-invasive transcutaneous VNS decreased both cytokines and chemokines from whole blood culture30. Furthermore, VNS has been shown to decrease chemokines in rat model of cardiac ischemia and after ischemia–reperfusion injury31,32. In the present study, HS treatment significantly upregulated the mRNA expression of chemokines MCP1 in SP5 and NAb and CXCL1 in the NAb region. In terms of chemokines, there was no statistically significant difference detected between the HS sham and HS active groups. Beneficial effects of LLTS in SP5 and NAb were only restricted to cytokine expression and not chemokines, which is intriguing. Significant changes were not observed in the chemokines tested at the level of NTS, RVLM, PVN and SFO. Taken together, these results demonstrated that LLTS attenuate HS-induced inflammation in a key brainstem and forebrain region, however further studies are needed to confirm these findings.
Central oxidative stress is characterized by accumulation of excess free radicals from reactive oxygen or reactive nitrogen species and has been implicated in several pathological conditions including HS-induced heart failure33. Nrf2 is a master transcription factor that regulates the expression of antioxidant and anti-inflammatory genes34,35. The present study explored whether LLTS activated the central antioxidant pathway in order to reduce the HS-induced oxidative stress. Even though oxidative stress was not measured in the present study, it has been well established by numerous other studies using similar animal model33,36. The present study found that LLTS significantly upregulated the mRNA of Nrf2 in the SP5 and SFO suggesting that LLTS increases antioxidant capacity at the central level. Interestingly, these increases in Nrf2 and NQO1 after LLTS were not observed in other brain regions examined.
Clinical implications
The importance of this data is highlighted by the fact that therapies that improve outcomes in patients with HFpEF are lacking at present5. The study results support the notion that autonomic modulation may be used as a novel non-pharmacological therapeutic modality for HFpEF. Consistent with this notion, an ongoing clinical study (ANTHEM-HFpEF) aims at evaluating the feasibility, tolerability and safety of right cervical VNS in patients with symptomatic HFpEF37. In addition, understanding the effects of LLTS on key brainstem regions that are important for the regulation of cardiac vagal tone may lead to identification of novel targets for therapy in this patient population.
Limitations
One limitation of the current study is that changes induced by LLTS in the brainstem and forebrain regions were not studied at the protein level. The relatively small amount of tissues dissected from each brain region in the present study is the reason for this limitation. In order to uncover which brain regions are actively involved during LLTS, the gene expression of long-term neuronal activity marker Fra-1 was measured. Even though numerical trends were seen, statistically significant differences were not observed between the groups. This is most likely due to decreased power of the study to detect changes in Fra-1 expression and therefore unable to positively confirm the exact brain regions involved while using this approach. Another caveat of the present study is that even though four brainstem and two forebrain regions were tested, other regions that are immunoreactive to vagal nerve stimulation are not examined21. The stimulation parameters used in the study were derived from previous experimental10 and human studies38. The effect of different stimulation parameters were not systemically assessed on outcomes, as such an assessment was beyond the scope of this study. In light of the disappointing results of VNS in patients with heart failure, despite the favorable preclinical data and the clear rationale for correcting the sympathovagal imbalance in this disease39, further research is necessary to optimize the stimulation parameters of autonomic modulation therapies, including VNS and LLTS. Finally, the current study is only able to show an association between the LLTS and its beneficial effects on the antioxidant and anti-inflammatory properties in the brainstem and forebrain. More mechanistic studies are warranted in the future to confirm the functional relevance of our current findings.
Conclusion
In summary, the central pathways by which LLTS could attenuate HFpEF phenotype in Dahl salt sensitive rats was evaluated in this study. The protective mechanism of LLTS in this model might be associated to the reduction of inflammatory and enhancement of antioxidant capacity in the brainstem (SP5) and forebrain (SFO) regions thus modulating the cardiac vagal tone.
Author contributions
Acquisition of data: L.E., A.M., L.B., A.H., M.K.S., R.M., S.S., L.M., K.E., M.N.; Analysis, interpretation and drafting of the work or revising it: M.S., P.B., S.S., Conception or design of the work: M.S., and S.S.
Funding
This work was supported by National Heart, Blood and Lung Institute grant R15HL148844 to MS and National Institute on Aging grant R21AG057879 to SS.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Madhan Subramanian, Email: madhan.subramanian@okstate.edu.
Stavros Stavrakis, Email: stavros-stavrakis@ouhsc.edu.
References
- 1.Loffredo FS, Nikolova AP, Pancoast JR, Lee RT. Heart failure with preserved ejection fraction: molecular pathways of the aging myocardium. Circ. Res. 2014;115:97–107. doi: 10.1161/CIRCRESAHA.115.302929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2011;13:18–28. doi: 10.1093/eurjhf/hfq121hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owan TE, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N. Engl. J. Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 4.Tribouilloy C, et al. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population-based study. Eur. Heart J. 2008;29:339–347. doi: 10.1093/eurheartj/ehm554. [DOI] [PubMed] [Google Scholar]
- 5.Butler J, et al. Developing therapies for heart failure with preserved ejection fraction: current state and future directions. JACC Heart Fail. 2014;2:97–112. doi: 10.1016/j.jchf.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson RL, Wilson CG. A review of vagus nerve stimulation as a therapeutic intervention. J. Inflamm. Res. 2018;11:203–213. doi: 10.2147/JIR.S163248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stavrakis S, et al. Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J. Am. Coll. Cardiol. 2015;65:867–875. doi: 10.1016/j.jacc.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stavrakis S, et al. Low-level vagus nerve stimulation suppresses post-operative atrial fibrillation and inflammation: a randomized study. JACC Clin. Electrophysiol. 2017;3:929–938. doi: 10.1016/j.jacep.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Stavrakis S, et al. TREAT AF (Transcutaneous Electrical Vagus nerve stimulation to suppress atrial fibrillation): a randomized clinical trial. JACC Clin. Electrophysiol. 2020;6:282–291. doi: 10.1016/j.jacep.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou L, et al. Low-level transcutaneous vagus nerve stimulation attenuates cardiac remodelling in a rat model of heart failure with preserved ejection fraction. Exp. Physiol. 2019;104:28–38. doi: 10.1113/EP087351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaumont E, et al. Vagus nerve stimulation mitigates intrinsic cardiac neuronal remodeling and cardiac hypertrophy induced by chronic pressure overload in guinea pig. Am. J. Physiol. Heart Circ. Physiol. 2016;310:H1349–1359. doi: 10.1152/ajpheart.00939.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deuchars SA, et al. Mechanisms underpinning sympathetic nervous activity and its modulation using transcutaneous vagus nerve stimulation. Exp. Physiol. 2018;103:326–331. doi: 10.1113/EP086433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujita M, Ando K, Nagae A, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in salt-sensitive hypertension. Hypertension. 2007;50:360–367. doi: 10.1161/HYPERTENSIONAHA.107.091009. [DOI] [PubMed] [Google Scholar]
- 14.He W, et al. The auriculo-vagal afferent pathway and its role in seizure suppression in rats. BMC Neurosci. 2013;14:85. doi: 10.1186/1471-2202-14-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker, E. & Lui, F. StatPearls (2019).
- 16.Mahadi KM, Lall VK, Deuchars SA, Deuchars J. Cardiovascular autonomic effects of transcutaneous auricular nerve stimulation via the tragus in the rat involve spinal cervical sensory afferent pathways. Brain Stimul. 2019;12:1151–1158. doi: 10.1016/j.brs.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Mercante B, et al. Anatomo-physiologic basis for auricular stimulation. Med. Acupunct. 2018;30:141–150. doi: 10.1089/acu.2017.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreihofer AM, Guyenet PG. The baroreflex and beyond: control of sympathetic vasomotor tone by GABAergic neurons in the ventrolateral medulla. Clin. Exp. Pharmacol. Physiol. 2002;29:514–521. doi: 10.1046/j.1440-1681.2002.03665.x. [DOI] [PubMed] [Google Scholar]
- 19.Balasubramanian P, Hall D, Subramanian M. Sympathetic nervous system as a target for aging and obesity-related cardiovascular diseases. Geroscience. 2019;41:13–24. doi: 10.1007/s11357-018-0048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farmer DG, Dutschmann M, Paton JF, Pickering AE, McAllen RM. Brainstem sources of cardiac vagal tone and respiratory sinus arrhythmia. J. Physiol. 2016;594:7249–7265. doi: 10.1113/JP273164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunningham JT, Mifflin SW, Gould GG, Frazer A. Induction of c-Fos and DeltaFosB immunoreactivity in rat brain by Vagal nerve stimulation. Neuropsychopharmacology. 2008;33:1884–1895. doi: 10.1038/sj.npp.1301570. [DOI] [PubMed] [Google Scholar]
- 22.Zimmerman CA, Leib DE, Knight ZA. Neural circuits underlying thirst and fluid homeostasis. Nat. Rev. Neurosci. 2017;18:459–469. doi: 10.1038/nrn.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horgan S, Watson C, Glezeva N, Baugh J. Murine models of diastolic dysfunction and heart failure with preserved ejection fraction. J. Cardiovasc. Fail. 2014;20:984–995. doi: 10.1016/j.cardfail.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Cho JH, et al. Ventricular arrhythmias underlie sudden death in rats with heart failure and preserved ejection fraction. Circ. Arrhythm Electrophysiol. 2018;11:e006452. doi: 10.1161/CIRCEP.118.006452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samniang B, et al. Vagus nerve stimulation improves cardiac function by preventing mitochondrial dysfunction in obese-insulin resistant rats. Sci. Rep. 2016;6:19749. doi: 10.1038/srep19749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsutsumi T, et al. Modulation of the myocardial redox state by vagal nerve stimulation after experimental myocardial infarction. Cardiovasc. Res. 2008;77:713–721. doi: 10.1093/cvr/cvm092. [DOI] [PubMed] [Google Scholar]
- 27.Young CN, Davisson RL. Angiotensin-II, the brain, and hypertension: an update. Hypertension. 2015;66:920–926. doi: 10.1161/HYPERTENSIONAHA.115.03624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, et al. Chronic intermittent low-level transcutaneous electrical stimulation of auricular branch of vagus nerve improves left ventricular remodeling in conscious dogs with healed myocardial infarction. Circ. Heart Fail. 2014;7:1014–1021. doi: 10.1161/CIRCHEARTFAILURE.114.001564. [DOI] [PubMed] [Google Scholar]
- 29.Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J. Intern. Med. 2011;269:45–53. doi: 10.1111/j.1365-2796.2010.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lerman I, et al. Noninvasive vagus nerve stimulation alters neural response and physiological autonomic tone to noxious thermal challenge. PLoS ONE. 2019;14:e0201212. doi: 10.1371/journal.pone.0201212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hua F, Ardell JL, Williams CA. Left vagal stimulation induces dynorphin release and suppresses substance P release from the rat thoracic spinal cord during cardiac ischemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R1468–1477. doi: 10.1152/ajpregu.00251.2004. [DOI] [PubMed] [Google Scholar]
- 32.Dusi V, Ghidoni A, Ravera A, De Ferrari GM, Calvillo L. Chemokines and heart disease: a network connecting cardiovascular biology to immune and autonomic nervous systems. Mediat. Inflamm. 2016;2016:5902947. doi: 10.1155/2016/5902947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang P, et al. Hydrogen sulfide inhibits high-salt diet-induced myocardial oxidative stress and myocardial hypertrophy in Dahl rats. Front. Pharmacol. 2017;8:128. doi: 10.3389/fphar.2017.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao H, Eguchi S, Alam A, Ma D. The role of nuclear factor-erythroid 2 related factor 2 (Nrf-2) in the protection against lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017;312:L155–L162. doi: 10.1152/ajplung.00449.2016. [DOI] [PubMed] [Google Scholar]
- 35.Balasubramanian P, et al. Obesity-induced sympathoexcitation is associated with Nrf2 dysfunction in the rostral ventrolateral medulla. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019 doi: 10.1152/ajpregu.00206.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li HB, et al. Exercise training attenuates proinflammatory cytokines, oxidative stress and modulates neurotransmitters in the rostral ventrolateral medulla of salt-induced hypertensive rats. Cell Physiol. Biochem. 2018;48:1369–1381. doi: 10.1159/000492095. [DOI] [PubMed] [Google Scholar]
- 37.DiCarlo LA, et al. Autonomic regulation therapy to enhance myocardial function in heart failure patients: the ANTHEM-HFpEF study. ESC Heart Fail. 2018;5:95–100. doi: 10.1002/ehf2.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tran N, et al. Autonomic neuromodulation acutely ameliorates left ventricular strain in humans. J. Cardiovasc. Transl. Res. 2019;12:221–230. doi: 10.1007/s12265-018-9853-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byku M, Mann DL. Neuromodulation of the failing heart: lost in translation? JACC Basic Transl. Sci. 2016;1:95–106. doi: 10.1016/j.jacbts.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]