Abstract
Recently, paradoxical combinations of colistin with anti-Gram-positive bacterial agents were introduced as a treatment alternative for multidrug-resistant Acinetobacter baumannii (MDRAB) infection. We assessed the therapeutic efficacy of the colistin–linezolid combination regimen in vitro and in a murine model of Acinetobacter baumannii pneumonia. A multidrug-resistant clinical strain (MDRAB31) and an extensively drug-resistant clinical strain (XDRAB78) were used in this study. The survival rates of mice and bacterial counts in lung tissue were used to assess the effects of colistin–linezolid combination. The survival rates of colistin–linezolid combination groups significantly increased compared with colistin groups for MDRAB31 (72% versus 32%, P = 0.03) and for XDRAB78 (92% versus 68%, P = 0.031). The colistin–linezolid combination groups significantly reduced the bacterial counts in lung tissue compared with colistin groups for MDRAB31 and for XDRAB78 (P < 0.05). The colistin–linezolid combination had a bactericidal and synergistic effect compared with colistin alone in time-kill assay and in murine model of pneumonia. Our data demonstrated the synergistic effect of colistin–linezolid combination regimen as a treatment alternative for the severe pulmonary infection caused by MDRAB and XDRAB.
Subject terms: Infectious diseases, Antimicrobials, Bacteria, Clinical microbiology
Introduction
Acinetobacter baumannii (A. baumannii) has become an important cause of hospital-associated infections affecting critically ill patients all over the world over the last decades1, and is mainly responsible for hospital-associated and ventilator-associated pneumonia2. The number of drugs that retain activity against A. baumannii has dramatically reduced for the global widespread of multidrug-resistant A. baumannii (MDRAB) and extensively drug-resistant A. baumannii (XDRAB) in hospital environment3. Since it is difficult to develop new classes of antibiotics, colistin is considered the last alternative for the treatment of MDR strains, and has been used increasingly4. However, for the sake of its heteroresistance, lipopolysaccharide modification, low plasma concentrations and toxicity, colistin monotherapy should be avoided5. Consequently, colistin-based combination therapies of existing drugs, including paradoxical combinations of colistin with anti-Gram-positive bacterial agents, have been made exploration and research to combat MDR A. baumannii infection6–11.
Since linezolid natural resistance to Gram-negative bacteria is due to the inability of drug to achieve effective intracytoplasmic concentrations, colistin can be used to increase the accumulation of linezolid by disrupting the permeability barrier of outer membrane and by inhibiting bacterial efflux pumps activities12. In a recent study, positive results of in vitro colistin–linezolid combination have been demonstrated in Pseudomonas aeruginosa, Escherichia coli and A. baumannii12–15.
Concurrently, little in vivo data exists concerning the effectiveness of colistin with linezolid for the treatment of A. baumannii infection. Since linezolid has been approved mainly for the hospital-acquired pneumonia treatment, our objective was to investigate the efficacy of the combination of colistin with linezolid in a murine model of A. baumannii pneumonia.
Results
Susceptibility tests and checkerboard assays
The MICs results of the colistin, linezolid and colistin–linezolid combination in A. baumannii are shown in Table 1. Both strains of A. baumannii resulted susceptible to colistin and showed high values to linezolid. The colistin–linezolid combination resulted in synergy for MDRAB31, additivity/indifference for XDRAB78.
Table 1.
The results of MICs and checkerboard assays for A. baumannii strains and control strain.
| Strain | MIC (mg/ml) | FICI | ||
|---|---|---|---|---|
| COL | LNZ | COL/LNZ | LNZ + COL | |
| MDRAB 31 | 0.5 | > 256 | 0.125/8 | 0.281 |
| XDRAB 78 | 0.5 | > 256 | 0.25/8 | 0.531 |
| PA ATCC 27853 | 0.5 | > 256 | 0.25/64 | 0.75 |
COL colistin, LNZ linezolid, FICI fractional inhibitory concentration index.
Time-kill assays
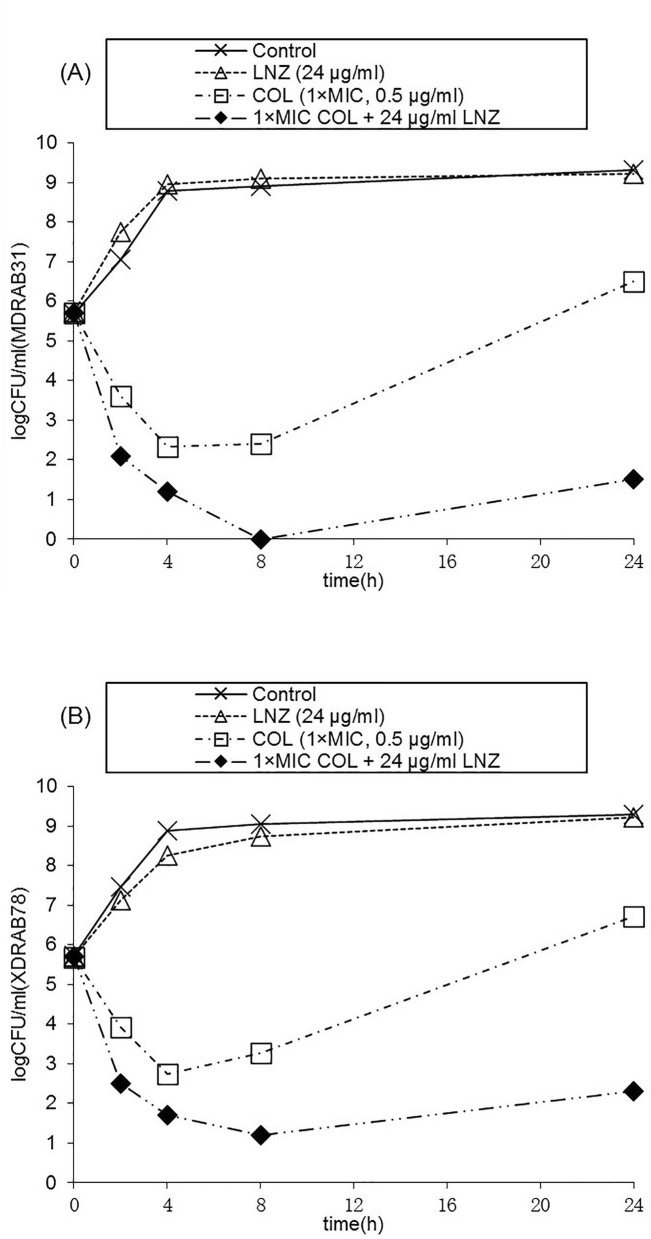
Figure 1 shows the time-kill curves of both A. baumannii strains. Although bactericidal action against MDRAB31 and XDRAB78 was detected using colistin alone at 0.5 μg/ml (1 × MIC), rapid regrowth was recorded after 8 h. The colistin–linezolid combination showed bactericidal and synergistic effects against MDRAB31 and XDRAB78, with minimal regrowth.
Figure 1.
Time-kill curves. Effects of linezolid, colistin and colistin–linezolid combination on the burden for strains MDRAB31 (A) and XDRAB78 (B). LNZ linezolid, COL colistin.
Survival rates
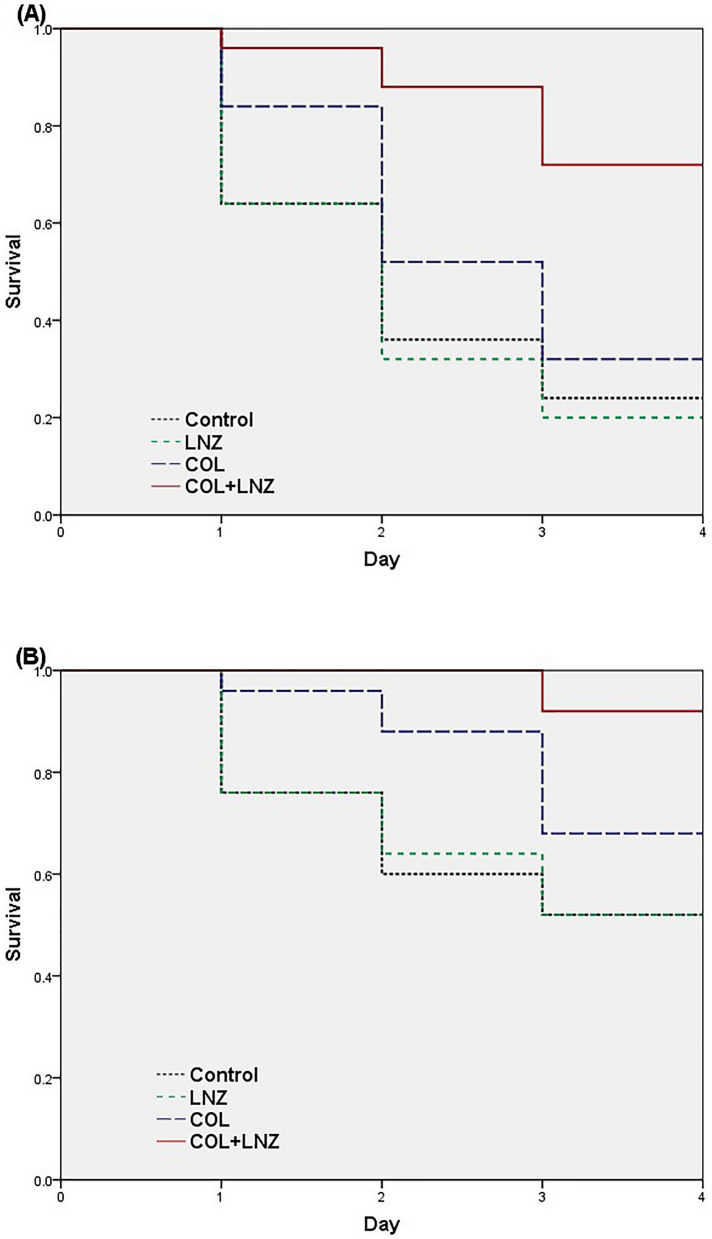
The survival curves of both strains are shown in the Fig. 2. For MRDAB31 strain, the survival rates within 4 days were 24% in control group, 20% in linezolid group, 32% in colistin group and 72% in colistin–linezolid combination group. The survival rate of colistin–linezolid combination group significantly increased compared with colistin group (72% versus 32%, P = 0.03). Significant differences in survival rates were observed between colistin–linezolid combination group and the other groups (P < 0.05).
Figure 2.
Survival curves. Mice survival with control, linezolid, colistin and colistin–linezolid combination in the murine model of pneumonia for strains MDRAB31 (A) and XDRAB78 (B). COL colistin, LNZ linezolid.
Comparing with MRDAB31 strain, a lower lethality rate of 48% was observed in control group for XDRAB78 strain. Survival rates of 52%, 52%, 68% and 92% were observed in the control group, linezolid group, colistin group, colistin–linezolid combination group, respectively. There were significant differences in survival rates between colistin–linezolid combination group and colistin group (92% versus 68%, P = 0.031), and between the colistin–linezolid combination group and the other two groups (P < 0.05).
Effects on lung bacterial counts
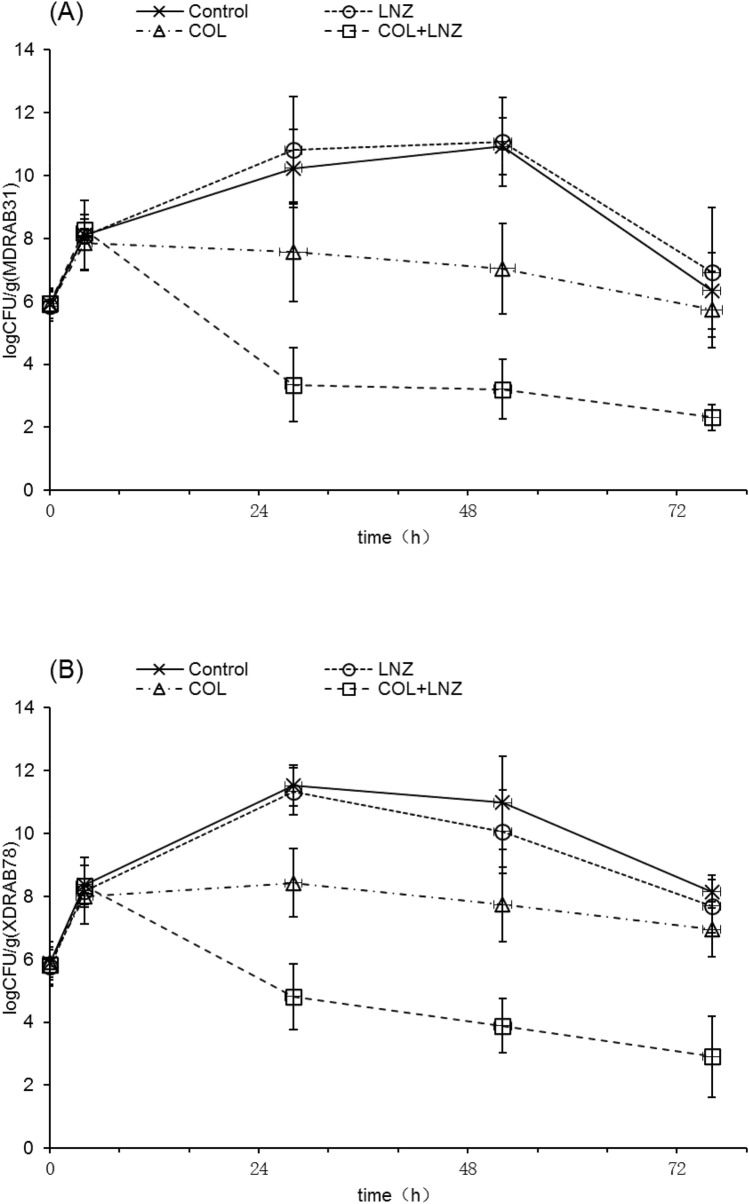
Figure 3 shows the evolution of bacterial loads of both strains in each group in lungs after intra-tracheal inoculation. The mean bacterial counts (log10 CFU/g of lung) after inoculation 0 h and 4 h were respectively 5.89 ± 0.41 log10 CFU/g and 8.08 ± 0.65 log10 CFU/g for MDRAB31 strain, 5.84 ± 0.49 log10 CFU/g and 8.22 ± 0.57 log10 CFU/g for XDRAB78 strain. Colistin monotherapy did not show bacterial effects against both strains. The colistin–linezolid combination groups significantly reduced the bacterial counts in lung tissue compared with colistin groups for MDRAB31 and for XDRAB78 (P < 0.05). The colistin–linezolid combination demonstrated bactericidal effects and synergistic effects compared with colistin monotherapy on both strains. The bacterial counts continued to drop, and fell to 2.32 ± 0.41 log10 CFU/g for MDRAB31 and 2.91 ± 1.29 log10 CFU/g for XDRAB78 at the 76 h.
Figure 3.
Bacterial counts in lungs (log10 CFU/g) with control, linezolid, colistin and colistin–linezolid combination in the murine model of pneumonia for strains MDRAB31 (A) and XDRAB78 (B). LNZ linezolid, COL colistin.
Discussion
Our study demonstrated the synergistic activity of colistin–linezolid combination in a murine model of MDR and XDR A. baumannii pneumonia for the first time.
Colistin, which was abandoned due to its neurotoxicity and nephrotoxicity, has been reused to fight MDR strains infection16. Like in other previous studies17, colistin exhibited good activity against A. baumannii at first and rapid regrowth was recorded subsequently in time-kill curves. In addition, in Dudhani et al. study18, similar phenomenon of colistin monotherapy appeared in murine model of A. baumannii infection. In 2006, Li et al.19 defined colistin heteroresistance of A. baumannii as the emergence of resistant subpopulations from an otherwise susceptible population. Mutant prevention concentration (MPC) studies and pharmacokinetics (PKs)/pharmacodynamics (PDs) studies revealed that monotherapy of colistin was unable to prevent the development of resistance, and may be substantially caused therapeutic failure20,21. Those views may explain the phenomenon of bacterial regrowth in vitro observed here when using colistin-monotherapy.
Linezolid is widely used against most Gram-positive bacteria by binding to rRNA on the 30S and 50S ribosomal subunits to prevent the synthesis of protein22. The intrapulmonary concentrations of linezolid in epithelial lining fluid were 64.3 ± 33.1 μg/ml at 4 h and 24.3 ± 13.3 μg/ml at 12 h in healthy volunteers for the recommended dosage regimen (600 mg every 12 h)23. And an important part of the application of linezolid was the treatment of pulmonary infection24. In this study, the MICs of linezolid in combination were significantly lower than the intrapulmonary concentrations in epithelial lining fluid for the recommended dosage regimen. So it provided the possibility of the linezolid-colistin combination to fight A. baumannii pneumonia.
Recently, linezolid was introduced as a colistin-combination option for A. baumannii infection basing on the mechanism that colistin exerted a subinhibitory permeabilizing effect allowing the second drug to enter cells14. Armengol et al.12 from biophysical point of view, and Ritcher et al.25 from physicochemical properties point of view, demonstrated that colistin had a synergistic effect and possibility with linezolid against A. baumannii strains. Although all the A. baumannii strains were high resistance to linezolid, synergy between linezolid and colistin was observed in checkerboard assay and time-kill assay15. In addition, another study in checkerboard assay found when the sub-inhibitory concentrations of colistin were incorporated to colistin–linezolid combination, the MICs of linezolid in combination against all A. baumannii strains decreased dramatically, ranging from 4 to 16 μg/ml13. And in our time-kill assay and in vivo study, the colistin–linezolid combination also showed bactericidal and synergistic effects. More importantly, there were significant differences in survival rates between colistin–linezolid combination group and colistin group in a murine model of A. baumannii pneumonia. Further clinical trials are necessary to confirm this result.
In this study, the result of colistin–linezolid combination for XDRAB78 strain was additive in checkerboard assay, while the result of combination was synergy in time-kill assay, when we used 1 × MIC colistin in combination. Sub-inhibitory concentrations of colistin could increase linezolid uptake in A. baumannii, and the accumulation of linezolid was colistin-concentration dependent12. Moreover, in the emergence of high concentrations of colistin (7 μg/ml), antimicrobials accumulation was obviously increased in E. coli25. This may explain above phenomenon and highly lights, even in combination therapies, the importance of optimizing the therapeutic regimen of colistin basing on PK/PD.
Although we did not detect the PKs of the bacterial agents used in this study, antibiotic doses were based on other published studies of same species murine model of pneumonia.
Conclusion
Colistin–linezolid combination therapy had a bactericidal and synergistic effect in vivo in a murine model of MDR and XDR A. baumannii pneumonia.
Materials and methods
Strains
Two clinical isolates of A. baumanni, which were isolated from two unrelated pulmonary infection patients with bacteremia, were studied. The first (MDRAB31) was tested to be a MDR A. baumanni strain (resistant to mezlocillin, piperacillin-tazobactam, cefepime, ceftazidime, ciprofloxacin, levofloxacin) and the second (XDRAB78) was tested to be a XDR A. baumanni strain (resistant to mezlocillin, piperacillin-tazobactam, cefepime, ceftazidime, ciprofloxacin, levofloxacin, gentamicin, amikacin, imipenem, meropenem) by the VITEK 2 testing system (bioMérieux, Craponne, France). The quality control stain used as internal standard for each batch of tests was Pseudomonas aeruginosa ATCC 27853. All strains were stored separately at − 70 °C in form of powder in airtight vials before being subcultured on containing 5% sheep blood Columbia plates (bioMérieux, Shanghai, China).
XuZhou Central Hospital Ethics Committee approved all experimental protocols, and the methods were carried out in strict accordance with the approved protocols. Informed consent was obtained from all subjects.
Antibiotic susceptibility tests
The minimum inhibitory concentrations (MICs) of linezolid (Pfizer Inc., NY, USA) and colistin (Sigma-Aldrich, St. Louis, MO, USA) was determined in triplicate using microdilution method, according to the protocol of the Clinical and Laboratory Standards Institute (CLSI)26. Because there are no CLSI breakpoint criteria for linezolid against A. baumannii, the linezolid breakpoints for A. baumannii could not be assessed.
Checkerboard assays
The synergy testing of linezolid in combination with colistin was assessed by standard checkerboard assay using 96-well microtiter plates27. The final concentration range of each antimicrobial varied and based on the MIC of each strain. The concentration of the final bacterial suspension was adjusted to 5 × 105 colony-forming units (CFU) per ml in a 100 ml final volume. The assay was performed in triplicate for each isolate. Interaction between colistin combinations with linezolid was calculated using formula as Odds et al. previously described28. The interpretation of fractional inhibitory concentration index (FICI) result was as follows: synergy, FICI ≤ 0.5; additivity/indifference, 0.5 < FICI ≤ 4; and antagonism FICI > 4.
Time-kill assays
Bactericidal activity of both agents and their combinations against each isolate was assessed by standard time-kill assay. The bacteria were diluted to a concentration about 5 × 105 CFU/ml in fresh Ca–Mueller–Hinton broth (MHBCA, Oxoid, Ltd., Hampshire, England). 0.5 μg/ml (1 × MIC) colistin was added and linezolid was added at 24 μg/ml to simulate the lowest intrapulmonary concentrations of linezolid in epithelial lining fluid under standard linezolid dosing regimen (intravenous administration 600 mg/12 h)23. Viable bacterial counts were performed at 0 h, 2 h, 4 h, 8 h and 24 h. The definition of bactericidal activity of agents was a ≥ 3 log10 reduction in CFU/ml compared with the bacterial concentration of starting inoculum. The definition of synergy was a ≥ 2 log10 reduction in CFU/ml for the bacterial concentration of combination compared with the most active agent at 24 h29. The experiments were repeated in triplicate on separate days.
Murine model of A. baumannii pneumonia
Healthy female, specific pathogen-free, immunocompetent, 6-week-old female C57BL/6 J mice (Animal Core Facility, Nanjing Medical University, Nanjing, Jiangsu, China) weighing 17–19 g were used for the A. baumannii pneumonia model. The mice were housed 5 per cage and had access to chow and drink ad libitum throughout the study. Cyclophosphamide (150 mg/kg of body weight in 0.15 ml) was intraperitoneally injected into the animals to render transiently neutropenic on days 4 and 1 before inoculation. All mice were anesthetized by a mixture of isoflurane and oxygen. Then 50 μl bacterial suspension containing 109 CFU/ml was inoculated by a needle through the nose. Antibiotics were initiated 4 h after inoculation and were administered intraperitoneally.
Experimental Animal Welfare and Ethics Committee of the Nanjing Medical University approved all animal experiments, and the methods were carried out in strict accordance with the approved protocols.
Study groups
In the first section of the experiment, the mice were randomized into four groups of 25 mice each group for each A. baumannii strain. The first group received colistin (125,000 UI/kg, every 6 h; 500,000 UI/kg/day)30. The second group received linezolid (50 mg/kg, every 12 h)31. The third group received the combination of colistin (125,000 UI/kg, every 6 h) and linezolid (50 mg/kg, every 12 h). The control group received saline (every 12 h). The outcome was observed by survival rate.
In the second section of the experiment, the mice were also randomized into four groups (20 mice in each group): saline, colistin, linezolid, colistin–linezolid combination, for each A. baumannii strain. Three mice per group at 0 h, 4 h, 28 h, 52 h, 76 h were euthanized before next dosing and lungs were removed for quantitative bacteriological studies. Lungs were weighted and then homogenized in 1 ml of saline. 0.01 ml serial tenfold dilutions of homogenates were plated on containing 5% sheep blood Columbia plates at 37 °C for 24 h. The definition of bactericidal activity of agents was a ≥ 3 log10 reduction compared with the bacterial concentration before first dosing. The definition of synergy was a ≥ 2 log10 reduction in CFU/g for the combination compared with the most active single agent32.
Statistical analysis
The data were presented as the means ± SD (standard deviations). Log-rank test and Kruskal–Wallis test were used to compare survival and bacterial counts in lung tissue respectively between groups of each strain. In all experiments, statistical significance was accepted when the P value was < 0.05.
Acknowledgements
We gratefully acknowledge XuZhou Central Hospital for financial support.
Author contributions
X.L.M., W.T.G., Y.Z.G. and Y.M.W. designed the experiments. X.L.M. conducted Experiment with the support of W.T.G. and Y.Z.G. X.L.M., J.S. and Z.H. analyzed the data of experiments and prepared the manuscript. W.T.G. and Y.M.W. supervised the research and edited the manuscript. All authors discussed and approved the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Perez F, et al. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2007;51:3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrer M, Torres A. Epidemiology of ICU-acquired pneumonia. Curr. Opin. Crit. Care. 2018;24:325–331. doi: 10.1097/MCC.0000000000000536. [DOI] [PubMed] [Google Scholar]
- 3.Lee CR, et al. Biology of Acinetobacter baumannii: Pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front. Cell Infect. Microbiol. 2017;7:55. doi: 10.3389/fcimb.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu W, Wang Y, Cao W, Cao S, Zhang J. In vitro evaluation of antimicrobial combinations against imipenem-resistant Acinetobacter baumannii of different MICs. J. Infect. Public Health. 2018;11:856–860. doi: 10.1016/j.jiph.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Cai Y, Chai D, Wang R, Liang B, Bai N. Colistin resistance of Acinetobacter baumannii: Clinical reports, mechanisms and antimicrobial strategies. J. Antimicrob. Chemother. 2012;67:1607–1615. doi: 10.1093/jac/dks084. [DOI] [PubMed] [Google Scholar]
- 6.Cirioni O, et al. Colistin enhances therapeutic efficacy of daptomycin or teicoplanin in a murine model of multiresistant Acinetobacter baumannii sepsis. Diagn. Microbiol. Infect Dis. 2016;86:392–398. doi: 10.1016/j.diagmicrobio.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Gordon NC, Png K, Wareham DW. Potent synergy and sustained bactericidal activity of a vancomycin-colistin combination versus multidrug-resistant strains of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2010;54:5316–5322. doi: 10.1128/AAC.00922-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hornsey M, Wareham DW. In vivo efficacy of glycopeptide-colistin combination therapies in a Galleria mellonella model of Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 2011;55:3534–3537. doi: 10.1128/AAC.00230-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenhard JR, Nation RL, Tsuji BT. Synergistic combinations of polymyxins. Int. J. Antimicrob. Agents. 2016;48:607–613. doi: 10.1016/j.ijantimicag.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrosillo N, Ioannidou E, Falagas ME. Colistin monotherapy vs combination therapy: Evidence from microbiological, animal and clinical studies. Clin. Microbiol. Infect. 2008;14:816–827. doi: 10.1111/j.1469-0691.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- 11.Sanderink D, et al. Colistin-glycopeptide combinations against multidrug-resistant Acinetobacter baumannii in a mouse model of pneumonia. Future Microbiol. 2019;14:581–586. doi: 10.2217/fmb-2019-0022. [DOI] [PubMed] [Google Scholar]
- 12.Armengol E, et al. Efficacy of combinations of colistin with other antimicrobials involves membrane fluidity and efflux machinery. Infect. Drug Resist. 2019;12:2031–2038. doi: 10.2147/IDR.S207844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armengol E, Asuncion T, Vinas M, Sierra JM. When combined with colistin, an otherwise ineffective rifampicin-linezolid combination becomes active in Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii. Microorganisms. 2020 doi: 10.3390/microorganisms8010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brennan-Krohn T, Pironti A, Kirby JE. Synergistic activity of colistin-containing combinations against colistin-resistant enterobacteriaceae. Antimicrob. Agents Chemother. 2018 doi: 10.1128/AAC.00873-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu B, et al. Colistin and anti-Gram-positive bacterial agents against Acinetobacter baumannii. Rev. Soc. Bras. Med. Trop. 2014;47:451–456. doi: 10.1590/0037-8682-0081-2014. [DOI] [PubMed] [Google Scholar]
- 16.Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect Dis. 2005;40:1333–1341. doi: 10.1086/429323. [DOI] [PubMed] [Google Scholar]
- 17.Owen RJ, Li J, Nation RL, Spelman D. In vitro pharmacodynamics of colistin against Acinetobacter baumannii clinical isolates. J. Antimicrob. Chemother. 2007;59:473–477. doi: 10.1093/jac/dkl512. [DOI] [PubMed] [Google Scholar]
- 18.Dudhani RV, Turnidge JD, Nation RL, Li J. fAUC/MIC is the most predictive pharmacokinetic/pharmacodynamic index of colistin against Acinetobacter baumannii in murine thigh and lung infection models. J. Antimicrob. Chemother. 2010;65:1984–1990. doi: 10.1093/jac/dkq226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, et al. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2006;50:2946–2950. doi: 10.1128/AAC.00103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai Y, et al. In vitro antimicrobial activity and mutant prevention concentration of colistin against Acinetobacter baumannii. Antimicrob. Agents Chemother. 2010;54:3998–3999. doi: 10.1128/AAC.00264-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan CH, Li J, Nation RL. Activity of colistin against heteroresistant Acinetobacter baumannii and emergence of resistance in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob. Agents Chemother. 2007;51:3413–3415. doi: 10.1128/AAC.01571-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batts DH. Linezolid—A new option for treating gram-positive infections. Oncology (Williston Park) 2000;14:23–29. [PubMed] [Google Scholar]
- 23.Conte JE, Jr, Golden JA, Kipps J, Zurlinden E. Intrapulmonary pharmacokinetics of linezolid. Antimicrob. Agents Chemother. 2002;46:1475–1480. doi: 10.1128/aac.46.5.1475-1480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashemian SMR, Farhadi T, Ganjparvar M. Linezolid: A review of its properties, function, and use in critical care. Drug Des. Devel. Ther. 2018;12:1759–1767. doi: 10.2147/DDDT.S164515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richter MF, et al. Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature. 2017;545:299–304. doi: 10.1038/nature22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing 27th informational supplement. Approved standard M100-S27. Clinical and Laboratory Standards Institute, Wayne (2017).
- 27.Rand KH, Houck HJ, Brown P, Bennett D. Reproducibility of the microdilution checkerboard method for antibiotic synergy. Antimicrob. Agents Chemother. 1993;37:613–615. doi: 10.1128/aac.37.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 29.Pillai, S. K., Moellering, R. C. & Eliopoulos, G. M. Chapter 9: Antimicrobial combinations. In: Antibiotics in Laboratory Medicine ed Lorian, 365–440. Philadelphia, PA: Lippincott Williams & Wilkins (2005).
- 30.Montero A, et al. Efficacy of colistin versus beta-lactams, aminoglycosides, and rifampin as monotherapy in a mouse model of pneumonia caused by multiresistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2002;46:1946–1952. doi: 10.1128/aac.46.6.1946-1952.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, et al. Linezolid exerts greater bacterial clearance but no modification of host lung gene expression profiling: A mouse MRSA pneumonia model. PLoS ONE. 2013;8:e67994. doi: 10.1371/journal.pone.0067994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan B, Guan J, Wang X, Cong Y. Activity of colistin in combination with meropenem, tigecycline, fosfomycin, fusidic acid, rifampin or sulbactam against extensively drug-resistant Acinetobacter baumannii in a murine thigh-infection model. PLoS ONE. 2016;11:e0157757. doi: 10.1371/journal.pone.0157757. [DOI] [PMC free article] [PubMed] [Google Scholar]