Abstract
Gross intraoperative assessment can be used to ensure negative margins at the time of surgery. Previous studies of this technique were conducted before the introduction of consensus guidelines defining a “positive” margin. We performed a retrospective study examining the accuracy of this technique since these guidelines were published. We identified all specimens that were grossly examined at the time of breast conserving surgery from January 2014 to July 2020. Gross and final microscopic diagnoses were compared and the performance of intraoperative examination was assessed in terms of false positive and false negative rates. Logistic regression models were used to examine the effect of clinicopathologic covariates on discordance. 327 cases were reviewed. Gross exam prompted re-excision in 166 cases (61%). The rate of false negative discordance was 8.6%. In multivariate analysis, multifocality on final pathology was associated with discordance. We consider the false negative rate acceptable for routine clinical use; however, there is an ongoing need for more accurate methods for the intraoperative assessment of margins.
Subject terms: Breast cancer, Surgical oncology
Introduction
Breast conserving therapy (BCT), defined as breast conserving surgery (BCS) followed by radiation therapy, is one of the standard of care options for early stage breast cancer1–5. In order to minimize the chance of local recurrence, cancer must be completely excised—i.e., the surgical resection margins must be negative for cancer. Therefore, ensuring negative margins at the time of surgery is a major goal of the breast surgeon. Positive surgical resection margins following BCS are a strong risk factor for local recurrence6,7. A positive surgical margin requires additional surgery, with potential for increased morbidity, a less desirable cosmetic outcome, more patient anxiety, and increased cost to the healthcare system8. Persistently positive surgical margins may ultimately require a mastectomy.
The gold standard for assessing breast surgical margins is microscopic pathologic evaluation of the excised tissue following formalin fixation, paraffin embedding, and hematoxylin and eosin (H&E) staining9. Prior to sectioning, the margins of the specimen are typically inked in different colors so that they can be identified microscopically. This is a labor- and time-intensive process that cannot be completed intraoperatively. Therefore, any positive margins identified require a second surgical procedure. A method for rapidly and accurately identifying positive surgical margins intraoperatively would allow the surgeon to immediately excise additional tissue to achieve negative margins, thereby sparing the patient a future second surgery.
Several such methods have been proposed and investigated. The most widely used appear to be gross examination, frozen section, and imprint cytology (“touch prep”). Gross examination entails visual examination and/or palpation of a freshly excised surgical specimen without microscopic evaluation10–12. Frozen section analysis involves rapidly freezing tissue without formalin fixation, followed by thin sectioning and H&E staining13–17. Imprint cytology is performed by touching a glass slide to freshly excised tissue; cancer cells adhere to the slide, which can be immediately stained and interpreted18–21. Gross examination can be performed either by a pathologist or directly by the surgeon in the operating room; the other two techniques are typically performed by a pathologist.
Each of these techniques has been claimed to reduce positive margin rates, but concerns persist regarding accuracy, methodologic issues, and the need for pathologists with specific expertise (e.g. cytopathology)22. The College of American Pathologists (CAP) recently surveyed 866 laboratories regarding their evaluation of breast specimen margin status23. 265 (33%) of respondents reported performing intraoperative assessment of breast specimens; of those, 171 (66%) reported performing gross examination only, while 73 (28%) performed frozen section only, 3 (1%) performed touch prep only, and 12% used some combination of techniques.
Although gross examination seems to be the most commonly used intraoperative method, relatively few studies have analyzed the accuracy of this technique, and there are differing results10,11. In addition, most studies were performed prior to 2014, when there was no consensus definition for what constituted a “positive” margin24. Thus, these studies were forced to use arbitrary definitions which limited the generalizability of their findings (e.g., in the Balch study11, tumor within 2 mm of a margin was defined as “positive”, while in the Fleming study10, a distance of less than 10 mm was considered “positive”). In 2014, the Society of Surgical Oncology (SSO) and the American Society for Radiation Oncology (ASTRO) issued joint consensus guidelines for breast conserving surgery which established tumor touching ink as the definitive definition of a positive margin for invasive ductal carcinoma25. To our knowledge, there has been no study revisiting the accuracy of gross intraoperative assessment since the SSO-ASTRO guidelines were published.
At our institution, gross intraoperative examination by a pathologist is routinely requested for all breast conserving surgery specimens with a pre-operative diagnosis of invasive carcinoma. In this study, the primary aim was to assess the accuracy of this technique in light of the 2014 SSO-ASTRO margin guidelines. We therefore assessed the performance of the gross intraoperative method in terms of false positive and false negative rates, and analyzed clinico-pathologic variables associated with discrepancy between gross and final microscopic margin status.
Results
During the study period, gross intraoperative margin assessment was requested for 405 patients undergoing BCS with a pre-operative diagnosis of invasive carcinoma. Only 5 patients were identified for whom no gross assessment was requested—all these had small tumors which were well visualized on pre-operative imaging. After excluding neoadjuvant treated patients, and patients for whom a quantitative gross margin distance was not provided by the interpreting pathologist, our final cohort comprised 327 patients.
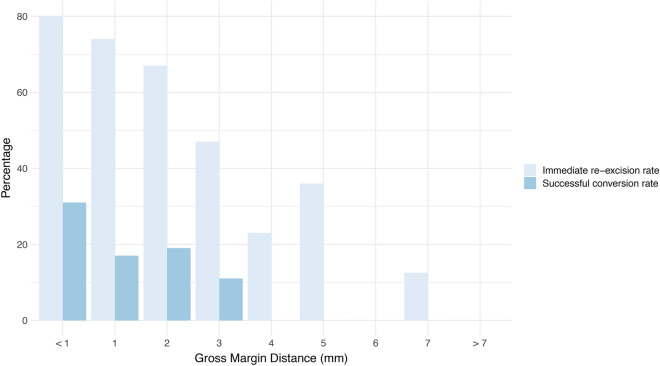
The intraoperative assessment prompted immediate re-excision in 166 cases (51% of the total cohort). Of these 166 cases, 27 (16%) represented patients whose margin status was successfully converted from positive to negative as a result of intraoperative margin assessment. These 27 patients comprised 8.3% of the total cohort, and correspond to the true positive number in Table 1. For 138 (83%) of patients who underwent re-excision, the final pathology indicated negative margins without considering any additionally excised tissue. These included 38 patients who were deemed to have positive margins grossly (the false positive number in Table 1). Figure 1 displays the immediate re-excision rate and the successful conversion rate as a function of the reported gross margin distance.
Table 1.
Confusion matrix for gross intraoperative diagnosis versus final microscopic diagnosis.
| Final microscopic diagnosis | Gross intraoperative diagnosis | |
|---|---|---|
| Negative (262) | Positive (65) | |
| Nsegative (272) | TN = 234 (71.6%) | FP = 38 (11.6%) |
| Positive (55) | FN = 28 (8.6%) | TP = 27 (8.3%) |
Figure 1.
Re-excision rate and successful conversion rate by gross intraoperative margin distance. The rate of immediate re-excision (light blue) is displayed as a function of gross intraoperative margin distance. Also shown (dark blue) is the successful conversion rate as a function of gross intraoperative margin distance. For gross margin distances less than 4 mm, some patients were successfully converted to negative margin status due to gross intraoperative assessment. For gross margin distances of 4 mm or more, no re-excisions resulted in a successful conversion.
The false negative rate was 8.6%, and the false positive rate was 11.6%. The overall test performance metrics were as follows: accuracy 80%, sensitivity 49%, specificity 86%, positive predictive value 42%, negative predictive value 89% (Table 1). In a multivariate logistic regression model, multifocality on final pathology was associated with false negative discordance (odds ratio 3.5, p = 0.02, 95% CI 1.3–9.9) (Table 2).
Table 2.
Logistic regression model results. P-values < 0.5 are highlighted in bold.
| Variable | Odds ratio | p-value | 95% CI |
|---|---|---|---|
| False negative discordance model | |||
| Tumor size | 1.1 | 0.42 | 0.84–1.5 |
| Tumor histologic type | |||
| Ductal (reference) | |||
| Lobular | 1.6 | 0.52 | 0.4–7.1 |
| Tumor grade | |||
| 1 (reference) | |||
| 2 | 1.0 | 0.99 | 0.3–3.4 |
| 3 | 1.1 | 0.90 | 0.3–4.7 |
| Multifocal | |||
| No (reference) | |||
| Yes | 3.5 | 0.02 | 1.3–9.9 |
| Lymphovascular invasion | |||
| No (reference) | |||
| Yes | 0.6 | 0.53 | 0.2–2.6 |
| Lymph node stage | |||
| 0 (reference) | |||
| 1mi | 0.9 | 0.95 | 0.1–8.6 |
| 1a | 2.1 | 0.15 | 0.8–5.6 |
| 2a | 2.4 | 0.40 | 0.3–17.3 |
| Estrogen receptor status | |||
| Positive (reference) | |||
| Negative | 0.4 | 0.48 | 0.04–4.4 |
| Progesterone receptor status | |||
| Positive (reference) | |||
| Negative | 0.9 | 0.86 | 0.2–3.2 |
| HER2 status | |||
| Negative (reference) | |||
| Equivocal | 0.9 | 0.93 | 0.1–7.5 |
| Positive | 0.4 | 0.47 | 0.05–3.9 |
| Patient age > 50 | |||
| No (reference) | |||
| Yes | 0.46 | 0.1 | 0.2–1.1 |
Discussion
To our knowledge, this is the only study examining gross intraoperative evaluation since the 2014 publication of the SSO-ASTRO margin guidelines. In contrast to several earlier studies, our data indicate that the technique is associated with a relatively low false negative rate of 8.6%. In other words, if a margin is deemed grossly negative, it is likely to also be microscopically negative. We therefore believe the technique is clinically useful, and can spare patients future additional surgery to achieve clear margins.
In order to optimize cosmesis, breast surgeons are usually interested in removing the minimal amount of tissue required to achieve clear margins. It would therefore be useful to establish a gross margin distance at which re-excision can be confidently avoided. At our institution, there is no agreed upon standard, and the decision to re-excise is left to the judgement of the individual surgeon. Other institutions do appear to implement a universal standard—e.g. in the study by Bolger et al.26, re-excision was performed for gross margin distances < 5 mm. As seen in Fig. 1, in our cohort the re-excision rate remained > 50% up to a gross margin distance of 4 mm. At a gross margin distance of 4 mm, the re-excision rate fell to approximately 25%. Importantly, re-excising for gross margin distances ≥ 4 mm did not result in any successful conversions. These data suggest that if the gross margin distance is at least 4 mm, re-excision can be avoided in order to optimize cosmesis without risking positive margins.
Are there any clinicopathologic variables that affect the accuracy of gross intraoperative assessment? In particular, are there any variables collected pre-operatively which could guide the surgeon when requesting gross assessment? In our multivariate logistic regression model (Table 2) only multifocality on final pathology was associated with a false negative intraoperative assessment. Multifocality is often (but not always) appreciated on pre-operative imaging and or/clinical exam; our results suggest caution when requesting a gross margin assessment in the setting of a suspected multifocal tumor. Somewhat surprisingly, no other tumor characteristics had a significant effect on the performance of gross assessment (e.g. lobular histology, tumor markers). We did not incorporate pre-operative imaging features into our model; intuitively, such features may affect the results of gross assessment, and would be interesting to explore in a future study.
In our practice, pathologists perform the gross exam and communicate the results to the surgeon. This appears to be a relatively common practice per the CAP survey cited above23. However, the surgeon could perform the procedure themselves, or could examine the specimen in conjunction with the pathologist, as was done in the Balch study11. One would expect that incorporating the surgeon’s judgement would improve the accuracy of the method, but as far as we are aware, there is no study addressing this question.
Previous studies examining the accuracy of gross intraoperative margin assessment have shown varying results. For example, a 2005 study by Balch et al.11 analyzed concordance between gross intraoperative margin status and final microscopic margin status for 254 consecutive patients undergoing breast conserving surgery for carcinoma (both in situ and invasive). 25% of patients ultimately underwent a second surgical procedure for margin clearance despite the use of gross intraoperative assessment. The authors concluded that the technique was not sufficiently accurate, and that other methods were needed. In the 2004 study by Fleming et al.10, 220 patients undergoing BCS underwent gross intraoperative margin assessment. The technique reduced the rate of future re-operation from 21.4% to 9.1%, and was associated with a low false negative rate of 3%. The authors concluded that the technique was effective. A 2011 study by Uecker et al.12 examined intraoperative assessment from a cost saving perspective, and found that the technique decreased total surgical costs as well as the rate of re-operation. A relatively small number of patients (17) underwent intraoperative assessment, and a “positive margin” definition was not provided.
These studies were all conducted prior to 2014, before the SSO-ASTRO consensus guidelines25 on margins were published. Therefore, different definitions of “positive margin” were used, and it is difficult to generalize the findings. The publication of the consensus guidelines appears to have markedly decreased the rate of re-excision for positive margins27–30, and has thereby somewhat decreased the necessity of an intraoperative margin assessment technique. However, a positive margin rate of 10–15% still represents a significant portion of patients who will require a second surgery. Given the high cost in terms of patient distress, potential morbidity, cosmesis, and financial burden, we still believe a rapid and accurate intraoperative tool for margin analysis is needed.
Besides the well-established pathology-assisted intraoperative methods discussed above, various radiographic and surgical tools and techniques have been shown to reduce positive margins22. These include pre-operative localization of the lesion via wires or newer localization devices, as well as intraoperative ultrasound. Cavity shave margin excision, in which the surgeon “shaves” an additional thin rim of tissue from the excision cavity, has also been shown to reduce positive margin rates. In a randomized clinical trial by Chagpar et al., this technique reduced positive margins by nearly 50% without an apparent adverse impact on cosmesis31–33.
Several novel techniques have been proposed for margin assessment, including Raman spectroscopy34,35, optical coherence tomography36,37, and confocal and multiphoton microscopy38,39. The MarginProbe (Dune Medical Devices, Alpharetta, GA) is a commercially available spectroscopy-based device that has been shown to reduce positive margin rates40–42. The Lumicell (Lumicell, Inc., Newton, MA) is a fluorescent protease probe-based system that is currently undergoing evaluation for cancer detection43,44. Other fluorescence-based techniques have also been described—one based on γ-glutamyl hydroxymethyl rhodamine green appears promising45.
Since our data was collected after the adoption of the SSO-ASTRO margin consensus guidelines, we believe our results are more generalizable than previous studies. However, we have not controlled for institution-specific surgical or pathology-related practices (e.g., the use of intraoperative specimen radiograph), and our findings should be interpreted and applied with reference to the workflow outlined above. In addition, while our cohort of 327 patients is one of the largest amongst the existing studies, a significantly larger cohort (perhaps from multiple institutions) would be ideal for bolstering our conclusions.
To conclude, in the era of consensus margin guidelines for BCS which define a “positive” margin as no tumor touching ink, gross intraoperative assessment appears to be a useful tool. In our cohort of 327 patients, 27 (8.3%) were spared a second surgery because of intraoperative assessment. The technique was associated with a false negative rate of 8.6%, which we believe is acceptable. Our data suggest that for gross margin distances of at least 4 mm, re-excision can safely be avoided in order to optimize cosmesis. To our knowledge, this is the only study of the gross intraoperative technique performed since 2014, and thus contributes substantially to the relatively scant existing literature.
While the SSO-ASTRO guidelines have significantly decreased the re-excision rate in BCS, the problem of positive margins persists. We anticipate and encourage the establishment of more evidence-based best practice guidelines for BCS, including pathologic assessment, in order to alleviate the persistent problem of positive margins and create more uniform care. Ultimately some of the novel techniques outlined above may play a role.
Methods
We retrospectively identified all patients at our institution who underwent breast conserving surgery for a preoperative diagnosis of invasive carcinoma (with or without accompanying in situ carcinoma), and for whom a gross intraoperative examination was requested at the time of surgery. Excisions performed only for ductal carcinoma in situ (DCIS) were excluded, since margin guidelines differ for DCIS46 and intraoperative assessment is not routinely requested at our institution for these specimens. The study period was January 2014 through July 2020; the starting date was chosen to allow sufficient time for the SSO-ASTRO guidelines to be implemented by all our breast surgeons.
Specimens from 405 patients underwent gross intraoperative evaluation during the study period. For 23 specimens, a margin distance was not provided by the interpreting pathologist; rather, wording such as “tumor close to anterior margin” was used instead of a precise measurement. These specimens were excluded from the analysis. There were 55 patients who had received neoadjuvant chemotherapy (NAC) prior to gross examination. Surgeons at our institution less commonly request gross examination for NAC-treated cases since the treated tumor mass can be difficult to grossly identify in cases with a complete or near-complete pathologic response. Additionally, NAC-treated patients are likely to differ from non-NAC patients in terms of clinico-pathologic characteristics. These 55 patients were therefore excluded from the main analysis in an effort to achieve a more homogenous cohort. The clinico-pathologic features of these 55 patients are separately presented in Table S1. The final cohort comprised 327 patients.
Target margin distances for BCT at our institution follow the 2014 SSO-ASTRO guidelines46,47—e.g. 2 mm for ductal carcinoma in situ, and no tumor touching ink for invasive carcinoma. Use of intraoperative ultrasonography is surgeon-dependent and is generally used selectively. Sentinel lymph node biopsy is performed in the clinically negative axilla, and frozen sections of sentinel lymph nodes are not performed in the setting of breast conserving surgery. Many patients with clinically positive axillary nodes receive neoadjuvant systemic therapy.
Per our standard workflow, specimen radiographs with two views were obtained and interpreted by the breast surgeon and a radiologist. In our practice, these radiographs serve primarily to confirm excision of any biopsy localization devices and/or localizing wires. They are correlated with the gross intraoperative examination findings, but the latter method is generally used to assess margin status since it is assumed to be more sensitive. Occasionally, surgeons will excise additional tissue based only on the specimen radiograph findings (e.g. if the tumor mass is well visualized and appears to abut a margin). However, radiographic margin assessment is not routinely performed.
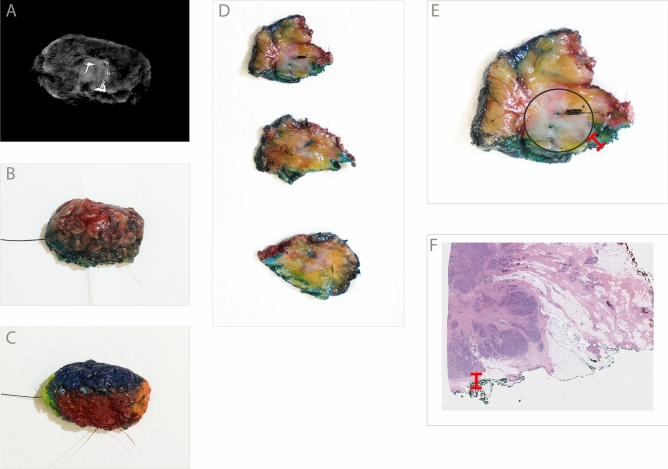
Gross examination was carried out as follows (Fig. 2): after receipt in the pathology gross lab, each specimen was first radiographed to localize any markers placed at the time of biopsy, then inked in six colors to designate margins, and finally serially sectioned perpendicular to the longest axis by a Pathologists’ Assistant (PA). The PA identified any grossly visible and/or palpable lesions, at which point the supervising pathologist was called to examine the specimen as well. The pathologist was ultimately responsible for determining the closest gross margin, which was communicated to the surgeon via telephone and was also documented via a written diagnosis. The written diagnosis was incorporated into the final pathology report. Although the exact wording of the intraoperative diagnosis was left to the discretion of the individual pathologist, in practice nearly all the diagnoses included an estimated measurement in millimeters, as well as an indication of which specific margin appeared closest (e.g. “closest gross margin: anterior, 3 mm”). For 23 specimens in our cohort, a distance to the closest margin was not recorded by the pathologist (e.g. “tumor close to anterior margin”), and these specimens were excluded from the analysis. The decision to re-excise, and the amount of tissue to remove during the re-excision, was left to the discretion of the surgeon.
Figure 2.
Gross intraoperative assessment workflow with example specimen. (A) Radiograph of a freshly excised breast lumpectomy specimen. (B) Excised specimen with orienting sutures and a localization wire. Dye from the sentinel node localization procedure is present. (C) The specimen has been inked in six colors to designate the surgical margins (inferior, superior, anterior, posterior, medial, lateral). (D) Representative serial sections. A centrally located tumor is visible as a vaguely defined area of whitish discoloration. (E) A close-up with the region of tumor annotated (black circle), as well as the grossly identified closest margin (red ruler). In this case the green-inked inferior margin was closest, and grossly measured 1 mm to the tumor. A SAVI SCOUT localization device is present (Cianna Medical, Inc.). (F) Microscopic pathology showing invasive ductal carcinoma. The microscopic distance to the inferior margin was 1 mm (red ruler). Gross intraoperative assessment prompted immediate re-excision of this margin, and the re-excised margin was negative for carcinoma.
The diagnosis rendered during gross assessment was compared with the final microscopic pathologic diagnosis for concordance, which was considered the gold standard. A gross intraoperative diagnosis was considered “positive” if the margin distance was recorded as 0 mm, or if equivalent language was used (this was more common, e.g. “tumor abuts anterior margin”). Otherwise, the gross diagnosis was considered negative. For all final pathology reports, preliminary margins (for the initially excised specimen) and final margins (including any additionally excised tissue) were reported. A patient was deemed to have been successfully converted from positive to negative margin status if the preliminary margin was positive, but the final margins were negative after additional tissue was excised due to gross examination findings.
Test performance metrics were computed to summarize the overall performance of the gross intraoperative assessment technique, and the following clinicopathologic variables were retrieved for each specimen: tumor histologic type, grade, stage, presence of lymphovascular invasion, tumor focality, receipt of neoadjuvant therapy, and biomarker status (ER, PR, and HER2). Tumor focality was based on the final pathologic assessment as defined in the American Joint Committee on Cancer (AJCC) staging manual, 8th edition48, and not a gross assessment of focality. Table 3 displays the clinicopathologic variables for our patient cohort. Multivariate logistic regression models were used to examine the impact of clinicopathologic covariates on discordance. All statistics were performed using the R software package49.
Table 3.
Distribution of clinicopathologic variables (total patients = 327).
| Variable | Number of patients (%) |
|---|---|
| Tumor size | |
| T1mi | 5 (1.5%) |
| T1a | 18 (5.5%) |
| T1b | 51 (15.6%) |
| T1c | 139 (42.5%) |
| T2 | 103 (31.5%) |
| T3 | 11 (3.4%) |
| Tumor histologic type | |
| Invasive ductal carcinoma | 295 (90.2%) |
| Invasive lobular carcinoma | 24 (7.3%) |
| Other | 4 (1.2%) |
| Multifocal | |
| Yes | 38 (11.6%) |
| No | 289 (88.4%) |
| Lymphovascular invasion | |
| Yes | 44 (13.5%) |
| No | 283 (86.5%) |
| Lymph node stage | |
| N0 | 213 (65.1%) |
| N1mi | 15 (4.6%) |
| N1a | 65 (19.9%) |
| N2a | 9 (2.8%) |
| N3a | 5 (1.5%) |
| Unknown | 20 (6.1%) |
| Estrogen receptor status | |
| Positive | 266 (81.3%) |
| Negative | 30 (9.2%) |
| Unknown | 31 (9.5%) |
| Progesterone receptor status | |
| Positive | 225 (68.8%) |
| Negative | 71 (21.7%) |
| Unknown | 31 (9.5%) |
| HER2 status | |
| Negative | 227 (69.4%) |
| Positive | 18 (5.5%) |
| Equivocal | 13 (4%) |
| Unknown | 28 (8.6%) |
| Patient age > 50 years | |
| Yes | 246 (75.2%) |
| No | 79 (24.2%) |
| Unknown | 2 (0.6%) |
The study was approved by the City of Hope institutional review board, and a waiver of informed consent was obtained (IRB #19128). All methods were carried out in accordance with relevant guidelines and regulations.
Supplementary information
Author contributions
D.S., V.J. and K.S.-C. designed the study. A.N. and D.S. collected the data and carried out the data analysis. All authors reviewed the manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-74373-6.
References
- 1.Veronesi U, et al. Breast conservation is the treatment of choice in small breast cancer: Long-term results of a randomized trial. Eur. J. Cancer Clin. Oncol. 1990;26:668–670. doi: 10.1016/0277-5379(90)90113-8. [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U, et al. Breast conservation is a safe method in patients with small cancer of the breast. Long-term results of three randomised trials on 1,973 patients. Eur. J. Cancer. 1995;31:1574–1579. doi: 10.1016/0959-8049(95)00271-J. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson JA, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N. Engl. J. Med. 1995;332:907–911. doi: 10.1056/NEJM199504063321402. [DOI] [PubMed] [Google Scholar]
- 4.Poggi MM, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: The National Cancer Institute randomized trial. Cancer. 2003;98:697–702. doi: 10.1002/cncr.11580. [DOI] [PubMed] [Google Scholar]
- 5.Houssami N, et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur. J. Cancer. 2010;46:3219–3232. doi: 10.1016/j.ejca.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 6.Schnitt SJ, et al. The relationship between microscopic margins of resection and the risk of local recurrence in patients with breast cancer treated with breast-conserving surgery and radiation therapy. Cancer. 1994;74:1746–1751. doi: 10.1002/1097-0142(19940915)74:6<1746::AID-CNCR2820740617>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 7.Park CC, et al. Outcome at 8 years after breast-conserving surgery and radiation therapy for invasive breast cancer: Influence of margin status and systemic therapy on local recurrence. J. Clin. Oncol. 2000;18:1668–1675. doi: 10.1200/JCO.2000.18.8.1668. [DOI] [PubMed] [Google Scholar]
- 8.Abe SE, et al. Margin re-excision and local recurrence in invasive breast cancer: A cost analysis using a decision tree model. J. Surg. Oncol. 2015;112:443–448. doi: 10.1002/jso.23990. [DOI] [PubMed] [Google Scholar]
- 9.Lester SC, et al. Protocol for the examination of specimens from patients with invasive carcinoma of the breast. Arch. Pathol. Lab. Med. 2009;133:1515–1538. doi: 10.5858/133.10.1515. [DOI] [PubMed] [Google Scholar]
- 10.Fleming FJ, et al. Intraoperative margin assessment and re-excision rate in breast conserving surgery. Eur. J. Surg. Oncol. (EJSO) 2004;30:233–237. doi: 10.1016/j.ejso.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Balch GC, Mithani SK, Simpson JF, Kelley MC. Accuracy of intraoperative gross examination of surgical margin status in women undergoing partial mastectomy for breast malignancy. Am. Surg. 2005;71:22–27. doi: 10.1177/000313480507100104. [DOI] [PubMed] [Google Scholar]
- 12.Uecker JM, Bui EH, Foulkrod KH, Sabra JP. Intraoperative assessment of breast cancer specimens decreases cost and number of reoperations. Am. Surg. 2011;77:342–344. doi: 10.1177/000313481107700325. [DOI] [PubMed] [Google Scholar]
- 13.Olson TP, Harter J, Muñoz A, Mahvi DM, Breslin T. Frozen section analysis for intraoperative margin assessment during breast-conserving surgery results in low rates of re-excision and local recurrence. Ann. Surg. Oncol. 2007;14:2953–2960. doi: 10.1245/s10434-007-9437-1. [DOI] [PubMed] [Google Scholar]
- 14.Esbona K, Li Z, Wilke LG. Intraoperative imprint cytology and frozen section pathology for margin assessment in breast conservation surgery: A systematic review. Ann. Surg. Oncol. 2012;19:3236–3245. doi: 10.1245/s10434-012-2492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osako T, et al. Efficacy of intraoperative entire-circumferential frozen section analysis of lumpectomy margins during breast-conserving surgery for breast cancer. Int. J. Clin. Oncol. 2015;20:1093–1101. doi: 10.1007/s10147-015-0827-2. [DOI] [PubMed] [Google Scholar]
- 16.Jorns JM, et al. Intraoperative frozen section analysis of margins in breast conserving surgery significantly decreases reoperative rates: One-year experience at an ambulatory surgical center. Am. J. Clin. Pathol. 2012;138:657–669. doi: 10.1309/AJCP4IEMXCJ1GDTS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber WP, et al. Accuracy of frozen section analysis versus specimen radiography during breast-conserving surgery for nonpalpable lesions. World J. Surg. 2008;32:2599–2606. doi: 10.1007/s00268-008-9757-8. [DOI] [PubMed] [Google Scholar]
- 18.Cox CE. Touch preparation cytology of breast lumpectomy margins with histologic correlation. Arch. Surg. 1991;126:490. doi: 10.1001/archsurg.1991.01410280094014. [DOI] [PubMed] [Google Scholar]
- 19.Muttalib M, et al. Intra-operative assessment of excision margins using breast imprint and scrape cytology. The Breast. 2005;14:42–50. doi: 10.1016/j.breast.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Bakhshandeh M, Tutuncuoglu SO, Fischer G, Masood S. Use of imprint cytology for assessment of surgical margins in lumpectomy specimens of breast cancer patients. Diagn. Cytopathol. 2007;35:656–659. doi: 10.1002/dc.20704. [DOI] [PubMed] [Google Scholar]
- 21.Valdes EK, Boolbol SK, Cohen J-M, Feldman SM. Intra-operative touch preparation cytology; does it have a role in re-excision lumpectomy? Ann. Surg. Oncol. 2007;14:1045–1050. doi: 10.1245/s10434-006-9263-x. [DOI] [PubMed] [Google Scholar]
- 22.Landercasper J, et al. Toolbox to reduce lumpectomy reoperations and improve cosmetic outcome in breast cancer patients: The American Society of breast surgeons consensus conference. Ann. Surg. Oncol. 2015;22:3174–3183. doi: 10.1245/s10434-015-4759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guidi AJ, et al. Breast specimen processing and reporting with an emphasis on margin evaluation: A College of American Pathologists survey of 866 laboratories. Arch. Pathol. Lab. Med. 2018;142:496–506. doi: 10.5858/arpa.2016-0626-CP. [DOI] [PubMed] [Google Scholar]
- 24.McCahill LE, et al. Variability in reexcision following breast conservation surgery. JAMA. 2012;307:467. doi: 10.1001/jama.2012.43. [DOI] [PubMed] [Google Scholar]
- 25.Buchholz TA, et al. Margins for breast-conserving surgery with whole-breast irradiation in stage I and II invasive breast cancer: American Society of Clinical Oncology Endorsement of the Society of Surgical Oncology/American Society for Radiation Oncology Consensus Guideline. J. Clin. Oncol. 2014;32:1502–1506. doi: 10.1200/JCO.2014.55.1572. [DOI] [PubMed] [Google Scholar]
- 26.Bolger JC, Solon JG, Khan SA, Hill ADK, Power CP. A comparison of intra-operative margin management techniques in breast-conserving surgery: A standardised approach reduces the likelihood of residual disease without increasing operative time. Breast Cancer. 2015;22:262–268. doi: 10.1007/s12282-013-0473-3. [DOI] [PubMed] [Google Scholar]
- 27.Landercasper J, Whitacre E, Degnim AC, Al-Hamadani M. Reasons for re-excision after lumpectomy for breast cancer: Insight from the American Society of Breast Surgeons Mastery(SM) database. Ann. Surg. Oncol. 2014;21:3185–3191. doi: 10.1245/s10434-014-3905-1. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberger LH, et al. Early adoption of the SSO-ASTRO consensus guidelines on margins for breast-conserving surgery with whole-breast irradiation in stage I and II invasive breast cancer: Initial experience from memorial sloan kettering cancer center. Ann. Surg. Oncol. 2016;23:3239–3246. doi: 10.1245/s10434-016-5397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schulman AM, et al. Reexcision surgery for breast cancer: An analysis of the American Society of Breast Surgeons (ASBrS) MasterySM database following the SSO-ASTRO “no ink on tumor” guidelines. Ann. Surg. Oncol. 2017;24:52–58. doi: 10.1245/s10434-016-5516-5. [DOI] [PubMed] [Google Scholar]
- 30.Monaghan A, Chapinal N, Hughes L, Baliski C. Impact of SSO-ASTRO margin guidelines on reoperation rates following breast-conserving surgery. Am. Surg. 2019 doi: 10.1016/j.amjsurg.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Kobbermann A, et al. Impact of routine cavity shave margins on breast cancer re-excision rates. Ann. Surg. Oncol. 2011;18:1349–1355. doi: 10.1245/s10434-010-1420-6. [DOI] [PubMed] [Google Scholar]
- 32.Chagpar AB, et al. A randomized, controlled trial of cavity shave margins in breast cancer. N. Engl. J. Med. 2015;373:503–510. doi: 10.1056/NEJMoa1504473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones V, et al. Excising additional margins at initial breast-conserving surgery (BCS) reduces the need for re-excision in a predominantly African American population: A report of a randomized prospective study in a public hospital. Ann. Surg. Oncol. 2016;23:456–464. doi: 10.1245/s10434-015-4789-4. [DOI] [PubMed] [Google Scholar]
- 34.Haka AS, et al. Diagnosing breast cancer by using Raman spectroscopy. Proc. Natl. Acad. Sci. 2005;102:12371–12376. doi: 10.1073/pnas.0501390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haka AS, et al. In vivo margin assessment during partial mastectomy breast surgery using Raman spectroscopy. Can. Res. 2006;66:3317–3322. doi: 10.1158/0008-5472.CAN-05-2815. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen FT, et al. Intraoperative evaluation of breast tumor margins with optical coherence tomography. Can. Res. 2009;69:8790–8796. doi: 10.1158/0008-5472.CAN-08-4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ha R, et al. Optical coherence tomography. Acad. Radiol. 2018;25:279–287. doi: 10.1016/j.acra.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 38.Tao YK, et al. Assessment of breast pathologies using nonlinear microscopy. Proc. Natl. Acad. Sci. 2014;111:15304–15309. doi: 10.1073/pnas.1416955111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshitake T, et al. Direct comparison between confocal and multiphoton microscopy for rapid histopathological evaluation of unfixed human breast tissue. J. Biomed. Opt. 2016;21:126021. doi: 10.1117/1.JBO.21.12.126021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allweis TM, et al. A prospective, randomized, controlled, multicenter study of a real-time, intraoperative probe for positive margin detection in breast-conserving surgery. Am. J. Surg. 2008;196:483–489. doi: 10.1016/j.amjsurg.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 41.Schnabel F, et al. A randomized prospective study of lumpectomy margin assessment with use of MarginProbe in patients with nonpalpable breast malignancies. Ann. Surg. Oncol. 2014;21:1589–1595. doi: 10.1245/s10434-014-3602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sebastian M, Akbari S, Anglin B, Lin EH, Police AM. The impact of use of an intraoperative margin assessment device on re-excision rates. Springerplus. 2015;4:198. doi: 10.1186/s40064-015-0801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitley MJ, et al. A mouse-human phase 1 co-clinical trial of a protease-activated fluorescent probe for imaging cancer. Sci. Transl. Med. 2016;8:320. doi: 10.1126/scitranslmed.aad0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Intraoperative Detection of Residual Cancer in Breast Cancer—Full Text View—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03321929?term=Lumicell.
- 45.Ueo H, et al. Rapid intraoperative visualization of breast lesions with γ-glutamyl hydroxymethyl rhodamine green. Sci. Rep. 2015;5:12080. doi: 10.1038/srep12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrow M, et al. Society of Surgical Oncology-American Society for Radiation Oncology-American Society of Clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. J. Clin. Oncol. 2016;34:4040–4046. doi: 10.1200/JCO.2016.68.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moran MS, et al. Society of Surgical Oncology-American Society for Radiation Oncology Consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014;88:553–564. doi: 10.1016/j.ijrobp.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giuliano AE, Edge SB, Hortobagyi GN. Eighth edition of the AJCC cancer staging manual: Breast cancer. Ann. Surg. Oncol. 2018;25:1783–1785. doi: 10.1245/s10434-018-6486-6. [DOI] [PubMed] [Google Scholar]
- 49.R Development Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, 2009).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.