Abstract
The aim of this study was to assess the prognostic value of baseline clinical and high resolution CT (HRCT) findings in patients with severe COVID-19. In this retrospective, two-center study, we included two groups of inpatients with severe COVID-19 who had been discharged or died in Jin Yin-tan hospital and Wuhan union hospital between January 5, 2020, and February 22, 2020. Cases were confirmed by real-time polymerase chain reaction. Demographic, clinical, and laboratory data, and HRCT imaging were collected and compared between discharged and deceased patients. Univariable and multivariable logistic regression models were used to assess predictors of mortality risk in these patients. 101 patients were included in this study, of whom 66 were discharged and 35 died in the hospital. The mean age was 56.6 ± 15.1 years and 67 (66.3%) were men. Of the 101 patients, hypertension (38, 37.6%), cardiovascular disease (21,20.8%), diabetes (18,17.8%), and chronic pulmonary disease (16,15.8%) were the most common coexisting conditions. The multivariable regression analysis showed older age (OR: 1.142, 95% CI 1.059–1.231, p < 0.001), acute respiratory distress syndrome (ARDS) (OR: 10.142, 95% CI 1.611–63.853, p = 0.014), reduced lymphocyte count (OR: 0.004, 95% CI 0.001–0.306, p = 0.013), and elevated HRCT score (OR: 1.276, 95% CI 1.002–1.625, p = 0.049) to be independent predictors of mortality risk on admission in severe COVID-19 patients. These findings may have important clinical implications for decision-making based on risk stratification of severe COVID-19 patients.
Subject terms: Microbiology, Risk factors
Introduction
In December 2019, a cluster of cases of pneumonia of unknown etiology, now known as coronavirus disease 2019 (COVID-19), and the coronavirus was called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), were reported in Wuhan, Hubei province, China1–3. Epidemiological studies have reported that most initial patients worked at or lived around a local seafood market in Wuhan and had human to human transmission3,4. As of 13 September, 2020, a total of 28,584,158 COVID-19 cases have been confirmed globally, including 916,955 deaths across 200 countries, suggesting an enormous threat to the global public health5.
Similar to human severe acute respiratory syndrome (SARS) and middle east respiratory syndrome (MERS), SARS-CoV-2 mainly causes lower respiratory tract infections6,7. Previous studies have demonstrated the epidemiological, clinical characteristics and clinical outcome in patients with COVID-19, which range from mild to critically ill cases2,3,8–10. Yang et al.10 reported critically ill patients with COVID-19 having a considerable mortality, which puts tremendous pressure on hospital critical care resources. Some studies also revealed11,12 risk factors associated with death of adult inpatients with COVID-19, including older age, high SOFA score, coagulation dysfunction, etc. However, specific information about mortality risk factors in critically ill patients remains unclear. In addition, the MuLBSTA score13 indicated that the multi-lobular infiltrate assessed by chest radiography or CT imaging is the most important mortality risk factors in viral pneumonia patients. And Chen et al.3 reported the characteristics of deceased COVID-19 patients were in line with the score. Currently, High resolution computed tomography (HRCT) has been considered an important imaging modality in assisting the diagnosis and management of patients with COVID-1914. A large sample study showed that HRCT has a high sensitivity for diagnosis of COVID-19 in epidemic area15. However, the prognostic value of radiological findings in severe patients with COVID-19 was not reported, and previous studies10,12 mainly focused on chest radiography rather than the more practical HRCT imaging.
In this study, we aimed to evaluate the prognostic value of baseline clinical and HRCT findings in severe patients with COVID-19.
Results
Demographics and baseline characteristics in severe COVID-19 patients
Between January 5, 2020, to Feb 22, 2020, 101 patients (87 from Jin Yin-tan hospital, 14 from Wuhan Union hospital) with severe COVID-19 who underwent chest CT scans on admission were included in this study. According to the hospital data, of 87 patients from Jin Yin-tan hospital, 16 have been described by Wu et al11 and Zhou et al12.
Of all patients with severe COVID-19, 66 patients (65.3%) have recovered from severe pneumonia and were discharged from hospital, 35 cases (34.7%) died despite supportive treatment. As Table 1 showed, the mean age was 56.6 ± 15.1 years (ranged from 23 to 82 years old). 67 (66.3%) were male, 15 (14.9%) had direct exposure to the Huanan seafood market, 8 cases (7.9%) were familial clusters. The most common symptom at onset were fever (96, 95.0%) and cough (79, 78.2%). Of the 101 patients, the most common coexisting conditions was hypertension (38, 37.6%), followed by cardiovascular disease, diabetes and chronic pulmonary disease. In addition, 14 (13.9%) patients were complicated with bacterial infection.
Table 1.
Demographics and baseline characteristics of patients with severe COVID-19.
| All patients | Survivors | Non-survivors | P value | |
|---|---|---|---|---|
| (n = 101) | (n = 66) | (n = 35) | ||
| Age, years | 56.6 ± 15.1 | 51.1 ± 14.2 | 66.8 ± 10.9 | < 0.001* |
| Sex | 0.430 | |||
| Female | 34 (33.7) | 24 (36.4) | 10 (28.6) | |
| Male | 67 (66.3) | 42 (63.6) | 25 (71.4) | |
| Huanan seafood market exposure | 15 (14.9) | 12 (25.0) | 3 (11.5) | 0.217 |
| Maximum temperature (°C) | 38.7 ± 0.8 | 38.6 ± 0.7 | 38.7 ± 1.0 | 0.799 |
| Heart rate (bmp) | 91.7 ± 13.0 | 91.2 ± 12.4 | 92.5 ± 14.0 | 0.639 |
| Respiratory rate | 23.2 ± 4.9 | 22.4 ± 4.0 | 24.6 ± 5.7 | 0.033* |
| SBP (mmHg) | 131.0 ± 20.0 | 131.7 ± 18.4 | 129.9 ± 23.3 | 0.677 |
| DBP (mmHg) | 80.1 ± 12.1 | 81.0 ± 11.0 | 78.5 ± 13.3 | 0.326 |
| Signs and symptoms | ||||
| Fever | 96 (95.0) | 62 (93.9) | 34 (97.1) | 0.656 |
| Fatigue | 49 (48.5) | 29 (43.9) | 20 (57.1) | 0.206 |
| Cough | 79 (78.2) | 53 (80.3) | 26 (74.3) | 0.486 |
| Expectoration | 48 (47.5) | 31 (47.0) | 17 (48.6) | 0.878 |
| Dyspnea | 17 (16.8) | 8 (12.1) | 9 (25.7) | 0.082 |
| Myalgia | 13 (12.9) | 8 (12.1) | 5 (14.3) | 0.757 |
| Abdominal pain | 2 (2.0) | 0 (0) | 2 (5.7) | 0.118 |
| Diarrhea | 9 (8.9) | 6 (9.1) | 3 (8.6) | 1.000 |
| Dizziness | 11 (10.9) | 9 (13.6) | 2 (5.7) | 0.321 |
| Nausea | 9 (8.9) | 4 (6.2) | 5 (14.3) | 0.271 |
| Vomiting | 7 (6.9) | 4 (6.2) | 3 (8.6) | 0.693 |
| Comorbidities | ||||
| Cardiovascular disease | 21 (20.8) | 10 (15.2) | 11 (31.4) | 0.055 |
| Diabetes | 18 (17.8) | 6 (9.1) | 12 (34.3) | 0.002* |
| Hypertension | 38 (37.6) | 20 (30.3) | 18 (51.4) | 0.037* |
| Chronic pulmonary disease | 16 (15.8) | 9 (25.7) | 7 (10.6) | 0.048* |
| Chronic liver disease | 5 (5.0) | 2 (3.0) | 3 (8.6) | 0.338 |
| Malignancy | 5 (5.0) | 3 (4.5) | 2 (5.7) | 0.797 |
| Bacterial infection | 14 (13.9) | 8 (22.9) | 6 (9.1) | 0.057 |
| Acute respiratory distress syndrome | 44 (43.6) | 16 (24.2) | 28 (80) | < 0.001* |
| Time interval from onset of symptom (days) | ||||
| Hospital admission | 11.2 ± 5.5 | 11.2 ± 5.5 | 11.3 ± 5.5 | 0.907 |
| Acute respiratory distress syndrome | 15.0 ± 6.6 | 11.9 ± 5.8 | 16.7 ± 6.4 | 0.019* |
| ICU admission | 15.4 ± 5.4 | 11.9 ± 5.5 | 17.7 ± 5.1 | < 0.001* |
| hospital day (days) | 15.6 ± 8.1 | 17.1 ± 8.2 | 12.7 ± 7 | 0.008* |
| Duration of disease (days) | 26.6 ± 8.5 | 28.3 ± 8.8 | 23.4 ± 7.1 | 0.006* |
| Treatment | ||||
| Oxygen therapy | 101 (100) | 66 (100) | 35 (100) | – |
| Antiviral agents | 88 (87.1) | 64 (97.0) | 24 (68.6) | < 0.001* |
| Antibacterial agents | 93 (92.1) | 60 (90.9) | 33 (94.3) | 0.550 |
| Glucocorticoids | 49 (50) | 28 (42.4) | 21 (65.6) | 0.031* |
| Immunoglobulin | 45 (86.5) | 10 (58.8) | 35 (100) | < 0.001* |
| Tracheal intubation | 22 (21.8) | 1 (1.5) | 21 (60.0) | < 0.001* |
| ECMO | 4 (4.1) | 0 (0) | 4 (12.5) | 0.004* |
Data are mean (SD) or n (%). p values comparing survivors and non-survivors are from χ2, Fisher’s exact test and independent-samples T test.
SBP systolic blood pressure, DBP diastolic blood pressure, ICU intensive care unit, ECMO extracorporeal membrane oxygenation. *p < 0.05.
The mean duration from onset of symptoms to hospital admission, ARDS and ICU admission were 11.2 ± 5.5 days, 15.0 ± 6.6 days, and 15.4 ± 5.4 days, respectively. The mean hospital day and duration of disease were 15.6 ± 8.1 days and 26.6 ± 8.5 days, respectively. The mean duration from onset of symptoms to ICU admission was significantly longer in deceased patients than discharged patients (p < 0.05). The hospital day and duration of disease were significantly longer in discharged patients than deceased groups (p < 0.05) (Table 1).
Compared with discharged patients, deceased patients were significantly older and were more likely to have ARDS and comorbidities, including cardiovascular disease, diabetes, hypertension, and chronic pulmonary disease (p < 0.05) (Table 1).
All patients were treated oxygen therapy (100%). 93 (92.1%) patients received antibacterial treatment, 88 (87.1%) patients received antiviral treatment, 49 (50%) received glucocorticosteroids, 45 (86.5%) received immunoglobulin, 22 (21.8%) received tracheal intubation, and 4 (4.1%) patients received extracorporeal membrane oxygenation (ECMO). Compared with discharged patients, deceased patients were more likely to receive glucocorticosteroids, immunoglobulin, tracheal intubation and ECMO (Table 1).
The mean survival time in the death group from disease onset to death were 22.6 ± 7.2 days. Among the 35 severe deceased cases, 21 patients (60%) died of respiratory failure, 4 patients (11.4%) with myocardial damage died of circulatory failure, 8 patients (22.9%) died of respiratory and circulatory failure, and 2 (5.7%) with severe sepsis died of multiple organ failure (Fig. 1).
Figure 1.
Comparison of age (a), lymphocyte count (b), total HRCT score (c), and ARDS proportion (d) between the died and discharged patients with severe COVID-19. (e) Survival of patients with severe COVID-19, dashed lines represent 95% CI. (f) Summary of the cause of death of 35 died patients with severe COVID-19.
Baseline laboratory findings in severe COVID-19 patients
The laboratory findings showed the levels of hyper-sensitive C-reactive protein, Serum amyloid A protein, and erythrocyte sedimentation rate were markedly increased in almost all patients (Table 2). The lymphocyte count, hemoglobin, albumin level on admission and oxygen saturation on room air were significantly lower in deceased patients than discharged patients (p < 0.05). Lactate dehydrogenase (LDH), creatinine, D-dimer and hypersensitive troponin I level on admission were higher in deceased patients than discharged patients (p < 0.05) (Table 2).
Table 2.
Baseline laboratory findings of patients with severe COVID-19.
| All patients | Survivors | Non-survivors | Normal range | P value | |
|---|---|---|---|---|---|
| (n = 101) | (n = 66) | (n = 35) | |||
| Leukocyte count (109/L) | 7.6 ± 3.9 | 7.6 ± 3.9 | 7.5 ± 4.0 | 4.0 ~ 10.0 | 0.971 |
| Neutrophil Count (109/L) | 7.2 ± 3.7 | 7.3 ± 3.8 | 7.0 ± 3.6 | 1.8 ~ 6.3 | 0.852 |
| Lymphocyte count (109/L) | 0.70 ± 0.37 | 0.85 ± 0.39 | 0.49 ± 0.18 | 1.1 ~ 3.2 | < 0.001* |
| Platelet count (109/L) | 164.5 ± 52.1 | 169.4 ± 53.2 | 156.3 ± 50.2 | 125.0 ~ 350.0 | 0.333 |
| Hemoglobin (g/L) | 120.4 ± 18.5 | 123.7 ± 16.6 | 114.8 ± 20.5 | 130.0 ~ 175.0 | 0.046* |
| Hyper-sensitive C-reactive protein (mg/L) | 67.3 (24.0, 133.9) | 54.3(16.0, 129.6) | 90.4 (45.1, 141.0) | < 25.0 | 0.057 |
| Serum amyloid A protein (mg/L) | 174.3 ± 86.6 | 165.0 ± 94.5 | 193.9 ± 64.0 | < 10.0 | 0.170 |
| Erythrocyte sedimentation rate (mm/h) | 53.0 ± 25.1 | 52.3 ± 23.4 | 54.3 ± 28.1 | 0 ~ 15.0 | 0.738 |
| Interleukin-6 (pg/ml) | 11.6 ± 9.4 | 10.9 ± 9.9 | 12.9 ± 8.5 | 0.1 ~ 2.9 | 0.407 |
| ALT (U/L) | 54.3 ± 33.3 | 57.4 ± 33.3 | 49.2 ± 33.4 | 5.0 ~ 40.0 | 0.310 |
| AST (U/L) | 58.8 ± 36.9 | 56.6 ± 33.6 | 62.5 ± 42.0 | 8.0 ~ 40.0 | 0.501 |
| Albumin (g/L) | 31.0 ± 4.4 | 32.2 ± 4.0 | 28.3 ± 4.2 | 35.0 ~ 55.0 | < 0.001* |
| Lactate dehydrogenase (U/L) | 476.3 ± 243.4 | 402.9 ± 163.8 | 583.8 ± 299.9 | 109.0 ~ 254.0 | 0.001* |
| Glucose (mmol/L) | 8.5 ± 5.0 | 8.3 ± 5.7 | 8.7 ± 3.8 | 3.9 ~ 6.1 | 0.718 |
| Creatinine (umol/L) | 80.0 (66.5, 99.8) | 75.0 (65.2, 83.6) | 93.0 (74.2, 125.3) | 44.0 ~ 133.0 | 0.017* |
| Prothrombin time (s) | 16.8 (16.2, 21.1) | 16.8 (16.2, 23.1) | 17.7 (16.1, 20.7) | 11.0 ~ 16.0 | 0.691 |
| Activated partial thromboplastin time(s) | 26.1 ± 8.9 | 24.5 ± 8.3 | 28.3 ± 9.4 | 28.0 ~ 43.5 | 0.189 |
| Thrombin time (s) | 19.0 ± 7.2 | 18.2 ± 5.7 | 20.0 ± 9.7 | 14.0 ~ 21.0 | 0.463 |
| D-dimer (mg/L) | 3.09 (0.8, 7.1) | 1.5 (0.6, 3.1) | 7.0 (3.3, 28.0) | < 0.5 | < 0.001* |
| Hypersensitive troponin I(pg/mL) | 15.3 (3.4, 37.7) | 3.6 (0.3, 18.9) | 31.4 (11.0, 94.2) | < 0.6 | < 0.001* |
| Myoglobin (ug/L) | 118.3 (54.3, 189.2) | 65.5 (38.7, 183.3) | 141.3 (59.3, 194.8) | 50.0 ~ 85.0 | 0.141 |
| Oxygen saturation on room air (%) | 87.7 ± 9.7 | 90.7 ± 8.5 | 84.1 ± 9.9 | 95.0 ~ 99.0 | 0.005* |
| PH | 7.5 ± 0.1 | 7.5 ± 0.05 | 7.4 ± 0.1 | 7.35 ~ 7.45 | 0.168 |
| PO2 (mmHg) | 70.6 ± 18.2 | 71.6 ± 20.9 | 69.3 ± 14.2 | 80 ~ 100 | 0.665 |
| PCO2 (mmHg) | 35.7 ± 11.8 | 36.4 ± 12.4 | 34.7 ± 11.2 | 35 ~ 45 | 0.638 |
Data are median (IQR), mean (SD) or n (%). p values comparing Non-survivors and survivors are from χ2, Fisher’s exact test, independent-samples T test or Mann–Whitney U test.
ALT alanine transaminase, AST aspartate aminotransferase.
*p < 0.05.
Baseline HRCT findings in severe COVID-19 patients
The median time interval from admission to baseline CT scan in all patients were 4 days (IQR 1-9), with no difference between discharged and deceased patients (Table 3). The typical chest CT findings of severe COVID-19 on admission were diffuse bilateral GGO and consolidation in peripheral areas (Figs. 2, 3, 4, 5, 6, 7). GGO (90, 89.1%) is main diffusion lesion characteristics in all patients, and consolidation proportion (8 [22.9] vs 3 [4.5], p = 0.005) was comparatively higher in deceased patients than discharged patients (Table 3). The mean total CT scores in all patients was 17.4 ± 5.1. Deceased patients had higher CT scores than discharged patients (20.9 ± 3.0 vs 15.6 ± 5.0, p < 0.001) (Fig. 1).
Table 3.
Baseline HRCT findings of patients with severe COVID-19.
| All patients | Survivors | Non-survivors | P value | |
|---|---|---|---|---|
| (n = 101) | (n = 66) | (n = 35) | ||
| Time from admission to CT scan, days | 4 (1–9) | 4 (1–9) | 5 (3–9) | 0.436 |
| HRCT score | – | |||
| Left upper lobe | 3.4 ± 1.3 | 3.0 ± 1.4 | 4.1 ± 1.0 | < 0.001* |
| Left lower lobe | 3.7 ± 1.2 | 3.4 ± 1.3 | 4.2 ± 1.0 | 0.001* |
| Right upper lobe | 3.5 ± 1.5 | 3.1 ± 1.4 | 4.4 ± 1.2 | < 0.001* |
| Right middle lobe | 3.0 ± 1.3 | 2.6 ± 1.2 | 3.8 ± 1.0 | < 0.001* |
| Right lower lobe | 3.8 ± 1.1 | 3.5 ± 1.2 | 4.4 ± 0.9 | < 0.001* |
| Total lesions score | 17.4 ± 5.1 | 15.6 ± 5.0 | 20.9 ± 3.0 | < 0.001* |
| Lung involvement | – | |||
| Unilateral | 0 | 0 | 0 | – |
| Bilateral | 101 (100) | 66 (100) | 35 (100) | – |
| Predominant distribution | – | |||
| Diffusely septal/subpleural area | 101 (100) | 66 (100) | 35 (100) | – |
| Diffusely central hilar area | 0 | 0 | 0 | – |
| Main lesion component | 0.005* | |||
| GGO | 90 (89.1) | 63 (95.5) | 27 (77.1) | – |
| Consolidation | 11 (10.9) | 3 (4.5) | 8 (22.9) | – |
| Other typical signs | – | |||
| Interlobular septal thickening | 72 (71.3) | 40 (60.6) | 32 (91.4) | 0.001* |
| Crazy-paving sign | 48 (47.5) | 25 (37.9) | 23 (65.7) | 0.008* |
| Air bronchogram | 75 (74.3) | 40 (60.6) | 35 (100) | < 0.001* |
| Coexisting lesion | – | |||
| Pleural effusion | 28 (28.0) | 11 (16.9) | 17 (48.6) | 0.001* |
| Emphysema | 15 (14.9) | 7 (10.6) | 8 (22.9) | 0.099 |
| Hydropericardium | 10 (9.9) | 5 (7.6) | 5 (14.3) | 0.283 |
| Pneumomediastinum | 3 (3.0) | 2 (3.0) | 1 (2.9) | 1.000 |
Data are mean (SD) or n (%). p values comparing discharged patients and died patients are from χ2, Fisher’s exact test, or independent-samples T test.
GGO ground-glass opacities.
*p < 0.05.
Figure 2.
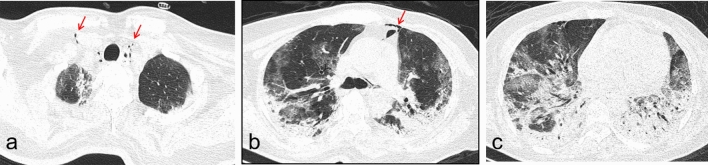
Thin-section CT scans in a man infected with SARS-CoV-2. Scan obtained on 7th day from onset of symptoms shows diffuse ground glass opacities that affected bilateral lung parenchyma. The patient died 10 days after this scan due to respiratory failure.
Figure 3.
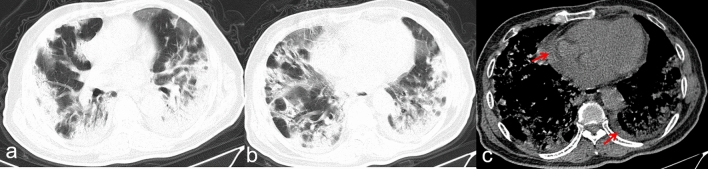
Transverse CT scans in a man infected with SARS-CoV-2 and with type 2 diabetes for 20 years. Scan obtained on 19th day from onset of symptoms shows diffuse, heterogeneous consolidation that affected bilateral lung parenchyma (a,b). The mediastinal window (c) shows a small pleural effusion at the right (arrow) and a small pericardial effusion (arrow). The patient died 4 days after this scan due to respiratory failure.
Figure 4.
Transverse CT scans in a woman infected with SARS-CoV-2 and suffering type 2 diabetes for 10 years. Scan obtained on 16th day from onset of symptoms shows bilateral, diffuse consolidation, which predominantly involved the lower lobes. There was pneumomediastinum were also seen (a,b arrows). The patient died 7 days after this scan because of both circulatory and respiratory failure.
Figure 5.
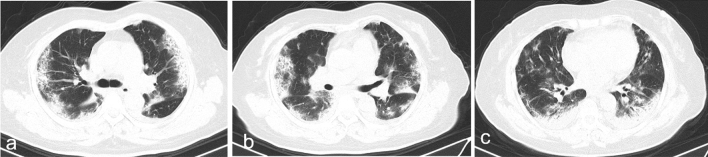
Transverse CT scans in a man with infected with SARS-CoV-2. Scan (a–c) obtained on 9th day from onset of symptoms shows bilateral, consolidation combined with ground glass opacities. Scan (d–f) obtained on 20th day shows previous consolidation being dissipated into ground-glass opacities. The patient was discharged from hospital 6 days after the final scan.
Figure 6.
Transverse CT scans in a man infected with SARS-CoV-2. Scan obtained on 10th day from onset of symptoms shows diffuse, heterogeneous consolidation that affected bilateral, subpleural lung parenchyma. The patient was discharged from hospital 7 days the scan.
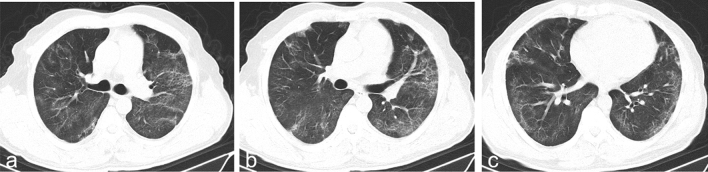
Figure 7.
Transverse CT scans in a man with infected with SARS-CoV-2. Scan obtained on 20th day from onset of symptoms shows bilateral, light ground glass opacities with irregular linear opacities. The patient was discharged from hospital 11 days after the scan.
The other common CT findings were interlobular septal thickening (72/101, 71.3%), crazy paving (48/101, 47.5%) and air bronchograms (75/101, 74.3%). The relatively less common CT findings were pleural effusion (28/101, 28%), emphysema (15/101, 14.9%), hydropericardium (10/101, 9.9%) and pneumomediastinum (3/101, 3.0%) (Table 3).
Interlobular septal thickening, crazy-paving, air bronchogram, and pleural effusion were significantly more common in deceased patients than discharged patients (p < 0.05) (Table 3).
Univariate and multivariable analysis of predictors of mortality risk
By univariate regression analysis in severe COVID-19 patients, the following baseline characteristics were predictors of mortality risk: older age, faster respiratory rate, ARDS, history of diabetes, history of hypertension; the following laboratory findings were predictors of mortality risk: reduced lymphocyte count, reduced albumin, elevated lactate dehydrogenase, elevated D-dimer, reduced SpO2 at room air; the following baseline HRCT findings were predictors of mortality risk: elevated total HRCT score, higher consolidation proportion, pleural effusion (Table 4).
Table 4.
Univariate analysis of predictors of mortality risk in patients with severe COVID-19.
| Odds ratio | 95% CI | P value | |
|---|---|---|---|
| Age | 1.102 | 1.055–1.151 | < 0.001* |
| Sex | 1.429 | 0.588–3.473 | 0.431 |
| Huanan seafood market exposure | 0.391 | 0.100–1.538 | 0.179 |
| Maximum temperature at admission | 1.071 | 0.638–1.796 | 0.796 |
| Heart rate at admission | 1.008 | 0.976–1.040 | 0.636 |
| Respiratory rate | 1.095 | 1.001–1.198 | 0.048* |
| SBP | 0.996 | 0.975–1.016 | 0.674 |
| DBP | 0.983 | 0.950–1.017 | 0.324 |
| Fever | 2.194 | 0.236–20.417 | 0.490 |
| Fatigue | 1.701 | 0.744–3.891 | 0.208 |
| Cough | 0.709 | 0.268–1.871 | 0.487 |
| Expectoration | 1.066 | 0.469–2.422 | 0.878 |
| Dyspnea | 2.510 | 0.871–7.235 | 0.089 |
| Myalgia | 1.208 | 0.364–4.016 | 0.757 |
| Diarrhea | 0.938 | 0.220–4.000 | 0.931 |
| Dizziness | 0.384 | 0.078–1.884 | 0.238 |
| Nausea | 2.542 | 0.636–10.159 | 0.187 |
| Vomiting | 1.430 | 0.301–6.782 | 0.653 |
| Acute respiratory distress syndrome | 12.500 | 4.592–34.028 | < 0.001* |
| Cardiovascular disease | 2.567 | 0.962–6.844 | 0.060 |
| Diabetes | 5.217 | 1.752–15.542 | 0.003* |
| Hypertension | 2.435 | 1.046–5.672 | 0.039* |
| Chronic pulmonary disease | 2.918 | 0.981–8.679 | 0.054 |
| Chronic liver disease | 3.000 | 0.477–18.867 | 0.242 |
| Malignancy | 1.273 | 0.203–7.999 | 0.797 |
| Bacterial infection | 2.963 | 0.936–9.375 | 0.065 |
| Leukocyte count | 0.998 | 0.888–1.121 | 0.971 |
| Lymphocyte count | 0.003 | 0.000–0.064 | < 0.001* |
| Neutrophil count | 0.982 | 0.815–1.183 | 0.847 |
| Platelet count | 0.995 | 0.985–1.005 | 0.329 |
| Hemoglobin | 0.973 | 0.946–1.000 | 0.052 |
| Hyper-sensitive C-reactive protein | 1.006 | 0.999–1.013 | 0.113 |
| Serum amyloid A protein | 1.004 | 0.998–1.010 | 0.171 |
| Erythrocyte sedimentation rate | 1.003 | 0.985–1.022 | 0.734 |
| Interleukin-6 | 1.023 | 0.969–1.079 | 0.415 |
| ALT | 0.992 | 0.977–1.007 | 0.307 |
| AST | 1.004 | 0.992–1.017 | 0.498 |
| Albumin | 0.790 | 0.694–0.899 | < 0.001* |
| Lactate dehydrogenase | 1.004 | 1.001–1.006 | 0.004* |
| Glucose | 1.018 | 0.926–1.118 | 0.716 |
| Creatinine | 1.004 | 0.997–1.010 | 0.257 |
| Activated partial thromboplastin time | 1.051 | 0.974–1.134 | 0.200 |
| Thrombin time | 1.039 | 0.938–1.150 | 0.464 |
| D-dimer | 1.039 | 1.004–1.076 | 0.031* |
| Hypersensitive troponin I | 1.001 | 0.998–1.003 | 0.620 |
| Myoglobin | 1.001 | 0.997–1.005 | 0.749 |
| Oxygen saturation on room air | 0.916 | 0.855–0.981 | 0.012* |
| PH | 0.001 | 0.001–7.861 | 0.135 |
| PO2 | 0.993 | 0.963–1.024 | 0.658 |
| PCO2 | 0.987 | 0.933–1.043 | 0.633 |
| Total lesions score | 1.389 | 1.196–1.613 | < 0.001* |
| Main lesion component (GGO/consolidation) | 6.222 | 1.532–25.268 | 0.011* |
| Pleural effusion | 4.636 | 1.834–11.718 | 0.001* |
| Emphysema | 2.497 | 0.821–7.592 | 0.107 |
| Hydropericardium | 0.941 | 0.082–10.757 | 0.961 |
| Pneumomediastinum | 2.033 | 0.546–7.569 | 0.290 |
SBP systolic blood pressure, DBP diastolic blood pressure, ALT alanine transaminase, AST aspartate aminotransferase.
*p < 0.05.
The multivariable regression analysis showed older age (OR: 1.142, 95% CI 1.059–1.231, p < 0.001), ARDS (OR: 10.142, 95% CI 1.611–63.853, p = 0.014), reduced lymphocyte count (OR: 0.004, 95% CI 0.001–0.306, p = 0.013), and elevated HRCT score (OR: 1.276, 95% CI 1.002–1.625, p = 0.049) independent predictors of mortality risk on admission in severe COVID-19 patients (Table 5).
Table 5.
Multivariable study of predictors of mortality risk in patients with severe COVID-19.
| Odds ratio | 95% CI | P values | |
|---|---|---|---|
| Model 1: baseline characteristics | |||
| Age | 1.154 | 1.068–1.248 | < 0.001* |
| Respiratory rate | 1.127 | 0.990–1.284 | 0.171 |
| Dyspnea | 1.257 | 0.218–7.259 | 0.798 |
| Acute respiratory distress syndrome | 14.951 | 3.539–63.171 | < 0.001* |
| Cardiovascular disease | 0.439 | 0.081–2.387 | 0.341 |
| Diabetes | 1.239 | 0.231–6.638 | 0.802 |
| Hypertension | 0.733 | 0.151–3.562 | 0.700 |
| Chronic pulmonary disease | 1.795 | 0.323–9.969 | 0.504 |
| Bacterial infection | 3.257 | 0.558–19.031 | 0.190 |
| Model 2: laboratory findings | |||
| Lymphocyte count | 0.001 | 0.001–0.194 | 0.019* |
| Hemoglobin | 0.914 | 0.800–1.044 | 0.183 |
| Albumin | 0.872 | 0.586–1.299 | 0.501 |
| Lactate dehydrogenase | 1.004 | 0.996–1.013 | 0.305 |
| D-dimer | 0.981 | 0.925–1.041 | 0.530 |
| Oxygen saturation on room air | 0.914 | 0.800–1.044 | 0.183 |
| Model 3: HRCT findings | |||
| Total lesions score | 1.332 | 1.138–1.559 | < 0.001* |
| Main lesion component (GGO/consolidation) | 3.408 | 0.710–16.360 | 0.126 |
| Pleural effusion | 2.070 | 0.693–6.184 | 0.193 |
| Emphysema | 2.581 | 0.628–10.610 | 0.189 |
| Model 4: baseline characteristics + Laboratory findings + HRCT findings | |||
| Age | 1.142 | 1.059–1.231 | 0.001* |
| Acute respiratory distress syndrome | 10.142 | 1.611–63.853 | 0.014* |
| Lymphocyte count | 0.004 | 0.001–0.306 | 0.013* |
| Total lesions score | 1.276 | 1.002–1.625 | 0.049* |
*p < 0.05.
Discussion
In this study, we have investigated the prognostic value of baseline clinical and HRCT findings in severe patients with COVID-19. In two previously published prognostic studies11,12, included groups were not all severe patients and HRCT findings associated with poor clinical outcomes have not been specified. The main finding of our study was that older age, developed ARDS, lymphocytopenia, and high CT score were the independent predictors of mortality risk in severe patients with COVID-19.
In our study, the mean age of severe patients with COVID-19 was 56.6 ± 15.1 years old, and more than 60% of patients were men. As described in previous studies2,3,9,10, elderly male patients are more susceptible to SARS-CoV-2 infection, which were supported by our severe patient cohort. But there was no sexual distinction between deceased and discharged patients in our study, which indicated gender is not a risk factor of death. In concert with a recent study10, we observed that deceased group was significantly older than the discharged group in severe COVID-19. And in the multivariable regression analysis, we have demonstrated the older age was an independent predictor of mortality risk in severe COVID-19 patients. Therefore, the clinical imperatively management for severe elderly patients requires further attention. Fever is the most common clinical symptom in severe patients with COVID-19, but 5% patients had no fever at the onset of disease, which is also in line with previous studies8,16. If patients are asymptomatic SARS-CoV-2 carriers, early diagnosis is relatively difficult for areas with less medical specialties1. Absence of typical initial symptoms for few patients may delay the optimal quarantine and treatment time and lead to poor outcome. However, in our present study, no significant differences were found between the initial symptoms of deceased patients and discharged patients.
ARDS characterized by an acute, diffuse, inflammatory lung damage is one of the most common causes of respiratory failure in critically ill patients with viral pneumonia17. In our study, compared with discharged patients with severe COVID-19, the deceased group has significantly higher incidence of ARDS. ARDS was an independent predictor of mortality risk in severe COVID-19 patients, which is similar to critically ill patients with SARS18. The incidence of ARDS in the present study is consistent with previous critically ill patients with COVID-1910, but which is slightly higher than that previously seen in patients with SARS18,19 and MERS20. Such a discrepancy may be due to failure to timely diagnosis and treatment in the situation of sudden increase in infected patients. Of note, the incidence of ARDS of survivors in the present study is significantly higher than that recently reported in Zhou’s study12. The different inclusion criteria, our discharged group were all severe patients, may result in the problem. In future, the earlier recognition of ARDS and ongoing efforts to study potential mechanisms of pulmonary damage in severe COVID-19 are the keys to reduce mortality rate. In addition, hypertension, cardiovascular disease and diabetes are the most common comorbidities in severe patients, which is in keep with previous studies9,10. Similarly, deceased patients had more comorbidities, such as hypertension, diabetes, and chronic pulmonary disease, than those discharged. Although comorbidities were not risk factors for death event in multivariable regression analysis, clinicians still require to focus on the clinical progression in severe patients.
In terms of laboratory indexes, more than 80% of severe patients in the present study have lymphocytopenia on admission, and the lymphocyte count was significantly lower in deceased patients than discharged patients. This finding is similar to previous COVID-19, SARS and MERS patients10,21,22. Unlike Chen’s study showed less than 40% of non-severe patients had only mild lymphocytopenia, the present study indicated the extent of lymphocytopenia may reflect the severity of SARS-CoV-2 infection. Furthermore, our study first demonstrated lymphocytopenia is an independent predictor of mortality risk in severe patients with COVID-19. Prior studies reported that severe SARS or MERS patients were prone to have lymphocytopenia. It may due to virus particle directly invading and damaging the lymphocyte cytoplasmic component or lymphocytes apoptosis16,22. Unfortunately, the pathogenesis of lymphocytopenia in severe COVID-19 is still unclear by now, later basic immunology may be the key to solve the problem. Additionally, compared with discharged patients, deceased patients had lower hemoglobin and albumin level, higher LDH, Creatinine, D-dimer and hypersensitive troponin I level. These abnormal results suggest that the severity of SARS-CoV-2 infection may be related to coagulation activation, myocardial damage and kidney damage. Unlike a previous study9, although more than 50% severe patients have elevated ALT and AST, no significant differences of ALT and AST level were found between deceased and discharged patients with severe COVID-19. Liver damage in patients with COVID-19 is common, especially in critically ill patients. The possible reasons are as follows: (1) liver cells are possibly directly infected with virus, (2) drug hepatotoxicity in the treatment, (3) immune-mediated inflammation, such as cytokine storm23. Although, liver damage is not a prognostic factor in the present study, further research still should pay close attention to the causes and evolution of liver injury in COVID-19.
Of note, the present study showed that elevated baseline HRCT score is an independent prognostic factor of mortality risk in severe COVID-19 patients. CT score24 was a semi quantitative parameter, which could quantify the severity of pulmonary abnormalities (GGO and consolidation dominated in COVID-19). Previous studies22,25 have demonstrated that the score was correlated with the degree of pulmonary lesions in pathologic specimens. CT score has been applied in the quantitative assessment of the evolution of pulmonary lesions in previous SARS26 and COVID-19 patients27. To the best of our knowledge, this is the first study to assess the prognostic value of baseline CT score, which has a significant value for clinical decisions based on radiological risk stratification in severe COVID-19 patients. We have reasons to believe that CT scan after hospital admission may be an important imaging modality in assisting the diagnosis and assessment of outcome in patients with severe COVID-19. However, given the infectious risk of transporting patients with COVID-19 for other hospitalized patients and health care workers, it's necessary to select the appropriate timing for immediate CT imaging. First, in the outbreak stage of COVID-19, RT-PCR for SARS-CoV-2 viral nucleic acid was reported to have a sensitivity of less than 60%15,28. Based on a higher sensitivity (80–90%) for COVID-1915,29, HRCT can be considered as a primary tool for the current COVID-19 detection in epidemic areas. Second, With the improvement of the accuracy and rapidity of the RT-PCR testing, immediate CT imaging is mainly used for suspected positive patients30,31. In addition, there were no pregnant women among the study, however, according to previous studies, HRCT examination for pregnant patients may be performed in the appropriate clinical settings32. Given the concern of radiation exposure to the fetus, for asymptomatic pregnant patients, self-quarantine until the results of RT‐PCR (not CT) are available may be considered. For pregnant patients who are prone to severe COVID-19, CT scan after hospital admission may be an important tool in assisting reasonable risk stratification and timely clinical management.
Like SARS and MERS, the CT findings of severe COVID-19 patients were nonspecific, which included diffusely bilateral GGO and consolidation of subsegmental areas. However, the extent of consolidation in severe COVID-19 patients was less severe than SARS patients33. Interestingly, although GGO was mainly diffuse in all patients, the consolidation proportion was comparatively higher in died patients than discharged patients. In a recent autopsy report of a deceased COVID-19 patient, Liu et al.34 demonstrated that edema, inflammatory infiltrate, and exudation were common in affected lung areas. Those may explain the predominant pattern of GGO on CT images35. At present, the relation between the extent of consolidation and outcome is unclear, which needs further large sample autopsy and HRCT studies. In this study, 28% patients with severe COVID-19 had pleural effusion, and which was significantly more common in deceased patients than discharged patients. Due to the present study mainly included severe patients, the incidence of pleural effusion may be higher than previous studies36–38. Das et al. study39 showed that the presence of pleural effusion was a poor prognostic factor in patients with MERS. Consequently, severe patients having abnormal pleural effusion deserve the attention of clinicians. All in all, baseline CT may be an important imaging modality in assisting the diagnosis and assessment of outcome in patients with severe COVID-19.
This study has several limitations. First, the sample size was small. Second, this is a retrospective study design, a few of clinical and laboratory results were missing in some patients, which may lead to unavoidable biases in analysis. Third, some survivors still hospitalized were not included the present study, the mortality rate of severe patients was overestimated in this study.
In conclusion, older age, ARDS, lymphocytopenia and elevated CT score were strong predictors of mortality risk in severe COVID-19 patients. Patients who are prone to severe COVID-19, CT scan after hospital admission may be an important imaging modality for assisting assessment of outcome in patients with severe COVID-19. Our findings may have important implications for clinical decisions based on risk stratification in severe pneumonia patients. As our study was retrospectively designed with possible selection bias, further prospective studies are needed to confirm our findings.
Materials and methods
Study design and participants
A retrospective two-center study was performed in Wuhan Jin Yin-tan hospital and Wuhan Union hospital (Wuhan, Hubei province, China). From January 5, 2020, to Feb 22, 2020, we enrolled two groups of patients (deceased and discharged patients) with severe COVID-19, who underwent HRCT scan at admission. All patients of confirmed infection with SARS-CoV-2 are in accordance with World Health Organization (WHO) interim guidance40. Throat swab samples were collected for confirmation of SARS-CoV-2 by the real-time polymerase chain reaction assay as previously described2,3. The diagnostic criteria for adult severe pneumonia was according to WHO interim guidance41, which includes fever or suspected respiratory tract infection, plus one of followings: respiratory rate > 30 breaths/min, SpO2 < 90% on room air or severe respiratory distress. The discharge criteria for patients was according to the sixth edition of “pneumonia diagnosis and treatment plan for new coronavirus infection” in China42, which includes normal temperature for more than 3 days, both the respiratory symptoms and chest imaging showing significant improvement, and negative two consecutive respiratory pathogen nucleic acid tests with the interval at least 1 day. This study had ethics approval of the Ethics Commission of Wuhan Jin Yin-tan hospital and Wuhan Union hospital. All participants remained anonymous, and written informed consent was waived by ethics commission for rapid emerging infectious diseases. This study was conducted in compliance with the Declaration of Helsinki.
Clinical data collection
We reviewed the electronic medical records, nursing records, laboratory findings, and radiology findings in all patients. All data were independently reviewed and checked by four physicians (J.L., Y.L., X.H., Y.C.). We collected age, sex, exposure history, underlying disease, maximum body temperature, vital signs, onset of symptoms, and the laboratory finding at admission (complete blood count, liver and kidney function, coagulation profile, C-reactive protein, erythrocyte sedimentation rate, blood gas analysis etc.) and treatment measures [oxygen therapy, antiviral agents, antibacterial agents, corticosteroids, immunoglobulin, tracheal intubation and extracorporeal membrane oxygenation (ECMO)]. In addition, the durations from onset of disease to hospital admission, chest HRCT scan, ARDS and ICU admission were recorded. ARDS was diagnosed according to the Berlin definition43.
HRCT image acquisition
All patients underwent HRCT examination in the supine position on one of the three CT scanners: SOMATOM Definition AS+, SOMATOM Perspective, or SOMATOM Spirit (Siemens Healthineers, Germany). Scans were done from the thoracic inlet to the diaphragm, and no contrast medium was used. CT scan parameters were as described in our previous study38. All images were reconstructed using standard reconstruction algorithms with a slice thickness of 2 mm or 5 mm. The multiplanar reconstruction postprocessing was implemented on workstation and picture archiving and communication systems.
HRCT image interpretation
All HRCT images were reviewed in random order by three radiologists (H.S., J.G., Y.F.) who were senior cardiothoracic radiologists. The readers independently assessed the CT features using both axial and MPR images, who were unaware of any other clinical and laboratory findings or outcome of the patients. After independent evaluation, discussion and consensus resolved the disagreements.
For each severe pneumonia patients, the predominant HRCT patterns defined by the Fleischner Society glossary44 were as following: ground-glass opacities (GGO), consolidation, interlobular septal thickening, crazy-paving pattern, air bronchogram, pneumonectasis, pleural effusion and pneumomediastinum. The distribution of lesion was categorized as diffuse central area and peripheral area.
To quantify the extent of pulmonary abnormalities, a HRCT score system24 was assigned on the basis of the area involved. And a previous study22 has demonstrated that this system was correlated well with the degree of pulmonary lesions in pathologic specimens. Specifically, each of the five lung lobes was visually scored from 0 to 5 as: 0, no involvement; (1) < 5% of lobe (minimal but not normal), (2) 5–25% of lobe, (3) 26–49% of lobe; (4) 50–75% of lobe; (5) > 75% of lobe. Finally, the total CT severity score was calculated by summing the individual lobar scores (range of possible scores from 0 to 25).
Statistical analysis
The Kolmogorov–Smirnov test was used to checked the normality of all continuous data. Normally and non-normally distributed data and categorical variables are expressed as the mean (SD) and the medians (IQR) and number (%), respectively. We assessed differences between two groups of normally distributed variables using two-sample t test or Mann–Whitney U test depending on normally and non-normally distributed data for continuous variables and Fisher’s exact test for categorical variables. The association between baseline clinical or HRCT Findings and incidence of death events was assessed with univariate logistic regression model (P < 0.1 in Tables 1, 2, 3). From a clinical point of view, to ensure proper independent variable number and avoid variable overfitting of the final multivariable model, we implemented the following step: (1) first, a multivariable model (Model 1: baseline characteristics) was tested, including those baseline variables that showed an association (p < 0.1 in Table 4) with incidence of death events. (2) A second multivariable model (Model 2: laboratory findings) was tested, including those baseline laboratory findings that showed an association (p < 0.1 in Table 4) with incidence of death events. (3) A third multivariable model (Model 3: HRCT findings) was tested, including those baseline HRCT findings that showed an association (p < 0.1 in Table 4) with incidence of death events. (4) A final multivariable model (Model 4: baseline characteristics plus laboratory findings plus HRCT findings) included variables from Model 1–3 that were independently related to the incidence of death events. The minimum sample size of the study should be 10 times greater than the included independent variable number. Co-linearity of variables tested in multivariate regression model (Table 4) was evaluated using the tolerance statistic and the variance inflation factor (excessive if < 0.1 and if > 10, respectively). Statistical significance was considered a p value < 0.05 (2-tailed). Analyses were conducted using SPSS software (SPSS 21.0 for Windows, IBM, Chicago, IL, USA).
Ethics declarations
This study was approved by both ethics committees of Union hospital of Tongji Medical College of Huazhong University of Science and Technology and Wuhan Jin-yintan hospital. The written informed consent was waived by ethics commission for rapid emerging infectious diseases.
Acknowledgements
We would like to thank all colleagues for helping us during the current study. This study was supported by Nation Science Foundation of China (82071921), Zhejiang University special scientific research fund for COVID-19 prevention and control and the Fundamental Research Funds for the Central Universities (2020kfyXGYJ019).
Author contributions
Conception and design: Y.F., H.W., H.S., Y.C., X.H.; Collection and assembly of data: J.G., X.H., J.L., Y.L., Y.C., X.Z., O.A.; Data analysis and interpretation: J.G., Y.F., Y.C., C.Z.; Manuscript writing: Y.C., X.H.; Final approval of manuscript: All authors; Accountable for all aspects of the work: All authors.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yukun Cao, Xiaoyu Han and Jin Gu.
Contributor Information
Yanqing Fan, Email: 1024932023@qq.com.
Hanping Wu, Email: hanpingwumd@gmail.com.
Heshui Shi, Email: heshuishi@hust.edu.cn.
References
- 1.Zhu N, et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. (2020) Novel Coronavirus (2019-nCoV): situation report. World Health Organization.https://covid19.who.int/
- 6.Yin Y, Wunderink R. MERS, SARS and other coronaviruses as causes of pneumonia: MERS, SARS and coronaviruses. Respirology. 2017 doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 8.Xu XW, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: Retrospective case series. BMJ. 2020 doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu C, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020 doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo L, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front. Microbiol. 2019;10:2752. doi: 10.3389/fmicb.2019.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee EYP, Ng MY, Khong PL. COVID-19 pneumonia: what has CT taught us? Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ai T, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan WJ, et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthay MA, et al. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lew TW, et al. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:374–380. doi: 10.1001/jama.290.3.374. [DOI] [PubMed] [Google Scholar]
- 19.Gomersall CD, et al. Short-term outcome of critically ill patients with severe acute respiratory syndrome. Intens. Care Med. 2004;30:381–387. doi: 10.1007/s00134-003-2143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arabi YM, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann. Intern. Med. 2014;160:389–397. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 21.Chu H, et al. Middle east respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J. Infect. Dis. 2015;6:2. doi: 10.1093/infdis/jiv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kazerooni EA, et al. Thin-section CT obtained at 10-mm increments versus limited three-level thin-section CT for idiopathic pulmonary fibrosis: Correlation with pathologic scoring. AJR. 1997;169(4):977–983. doi: 10.2214/ajr.169.4.9308447. [DOI] [PubMed] [Google Scholar]
- 23.Zhang C, Shi L, Wang F-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol. Hepatol. 2020 doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang Y-C, et al. Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: Evaluation with thin-section CT. Radiology. 2005;236:1067–1075. doi: 10.1148/radiol.2363040958. [DOI] [PubMed] [Google Scholar]
- 25.Flaherty KR, et al. Fibroblastic foci in usual interstitial pneumonia: Idiopathic versus collagen vascular disease. Am. J. Respir. Crit. Care Med. 2003;167:1410–1415. doi: 10.1164/rccm.200204-373oc. [DOI] [PubMed] [Google Scholar]
- 26.Qian Y, et al. Multicenter cohort study demonstrates more consolidation in upper lungs on initial CT increases the risk of adverse clinical outcome in COVID-19 patients. Theranostics. 2020;10:5641–5648. doi: 10.7150/thno.46465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan F, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020 doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long C, et al. Diagnosis of the Coronavirus disease (COVID-19): rRT-PCR or CT? Eur. J. Radiol. 2020;126:108961. doi: 10.1016/j.ejrad.2020.108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai HX, et al. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 2020 doi: 10.1148/radiol.2020200823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mossa-Basha M, et al. Radiology department preparedness for COVID-19: Radiology scientific expert panel. Radiology. 2020 doi: 10.1148/radiol.2020200988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin X, et al. Novel coronavirus pneumonia outbreak in 2019: Computed tomographic findings in two cases. Korean J. Radiol. 2020;21(3):365–368. doi: 10.3348/kjr.2020.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahu KK, et al. A twin challenge to handle: COVID-19 with pregnancy. J. Med. Virol. 2020 doi: 10.1002/jmv.25784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong KT, et al. Severe acute respiratory syndrome: Radiographic appearances and pattern of progression in 138 patients. Radiology. 2003;228:401–406. doi: 10.1148/radiol.2282030593. [DOI] [PubMed] [Google Scholar]
- 34.Q L, et al. Autopsy report in a dead patient with COVID-19. J. Forens. Med. 2020 doi: 10.12116/j.issn.1004-5619.2020.01.00. [DOI] [Google Scholar]
- 35.Li M, et al. Coronavirus disease (COVID-19): Spectrum of CT findings and temporal progression of the disease. Acad Radiol. 2020 doi: 10.1016/j.acra.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei J, et al. Novel coronavirus (COVID-19) pneumonia: Serial computed tomography findings. Korean J. Radiol. 2019;21(4):501–504. doi: 10.3348/kjr.2020.0112(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernheim A, et al. Chest CT findings in coronavirus disease-19 (COVID-19): Relationship to duration of infection. Radiology. 2020 doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi H, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet. Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Das KM, et al. CT correlation with outcomes in 15 patients with acute middle east respiratory syndrome coronavirus. AJR Am. J. Roentgenol. 2015;204:736–742. doi: 10.2214/AJR.14.13671. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. Published January 28, 2020. https://apps.who.int/iris/handle/10665/330893.
- 41.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf Published on January 12, 2020.
- 42.General Office of National Health Committee. Notice on the issuance of a program for the diagnosis and treatment of novel coronavirus (2019-nCoV) infected pneumonia (trial sixth edition) 2020.2.18. https://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml?from=timeline (accessed Feb 24, 2020)
- 43.Force ADT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 44.Hansell DM, et al. Fleischner Society: Glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.