Abstract
We present an extension of the Individual Brain Charting dataset –a high spatial-resolution, multi-task, functional Magnetic Resonance Imaging dataset, intended to support the investigation on the functional principles governing cognition in the human brain. The concomitant data acquisition from the same 12 participants, in the same environment, allows to obtain in the long run finer cognitive topographies, free from inter-subject and inter-site variability. This second release provides more data from psychological domains present in the first release, and also yields data featuring new ones. It includes tasks on e.g. mental time travel, reward, theory-of-mind, pain, numerosity, self-reference effect and speech recognition. In total, 13 tasks with 86 contrasts were added to the dataset and 63 new components were included in the cognitive description of the ensuing contrasts. As the dataset becomes larger, the collection of the corresponding topographies becomes more comprehensive, leading to better brain-atlasing frameworks. This dataset is an open-access facility; raw data and derivatives are publicly available in neuroimaging repositories.
Subject terms: Brain imaging, Cognitive neuroscience, Human behaviour, Functional magnetic resonance imaging
| Measurement(s) | functional brain measurement • regional part of brain • brain measurement • cognition |
| Technology Type(s) | functional magnetic resonance imaging |
| Factor Type(s) | type of task |
| Sample Characteristic - Organism | Homo sapiens |
Machine-accessible metadata file describing the reported data: 10.6084/m9.figshare.12958181
Background & Summary
Understanding the fundamental principles that govern human cognition requires mapping the brain in terms of functional segregation of specialized regions. This is achieved by measuring local differences of brain activation related to behavior. Functional Magnetic Resonance Imaging (fMRI) has been used for this purpose as an attempt to better understand the neural correlates underlying cognition. However, while there is a rich literature concerning performance of isolated tasks, little is still known about the overall functional organization of the brain.
Meta- and mega-analyses constitute active efforts at providing accumulated knowledge on brain systems, wherein data from different studies are pooled to map regions consistently linked to mental functions1–9 Because data are impacted by both intra- and inter-subject plus inter-site variability, these approaches still limit the exact demarcation of functional territories and, consequently, formal generalizations about brain mechanisms. Several large-scale brain-imaging datasets are suitable for atlasing, wherein differences can be mitigated across subjects and protocols together with standardized data-processing routines. Yet, as they have different scopes, not all requirements are met for cognitive mapping. For instance, the Human Connectome Project (HCP)10,11 and CONNECT/Archi12,13 datasets provide large subject samples as they are focused in population analysis across different modalities; task-fMRI data combine here 24 and 28 conditions, respectively, which is scarce for functional atlasing. Another example is the studyforrest dataset14–17, that includes a variety of task data on complex auditory and visual information, but restricted to naturalistic stimuli. Additionally, one shall note that within-subject variability reduces task-fMRI replicability; thus, more data per subject can in fact facilitate reliability of group-level results18.
To obtain as many cognitive signatures as possible and simultaneously achieve a wide brain coverage at a fine scale, extensive functional mapping of individual brains over different psychological domains is necessary. Within this context, the Individual Brain Charting (IBC) project pertains to the development of a 1.5mm-resolution, task-fMRI dataset acquired in a fixed environment, on a permanent cohort of 12 participants. Data collection from a broad range of tasks, at high spatial resolution, yields a sharp characterization of the neurocognitive components common to the different tasks. This extension corresponds to the second release of the IBC dataset, meant to increase the number of psychological domains of the first one19. It both aims at a consistent mapping of elementary spatial components, extracted from all tasks, and a fine characterization of the individual architecture underlying this topographic information.
The first release encompassed a sample of modules ranging from perception to higher-level cognition, e.g. retinotopy, calculation, language and social reasoning10,12,20. The second release refers to tasks predominantly focused on higher-level functions, like mental time travel, reward, theory-of-mind, self-reference effect and speech recognition. Nonetheless, a subset dedicated to lower-level processes is also included, covering pain, action perception and numerosity. These tasks are intended to complement those from the first release, such that a considerable cognitive overlap is attained, while new components are introduced. For instance, components concerning social cognition, already found in ARCHI Standard, ARCHI Social and HCP Social tasks from the previous release, are now present in tasks about theory-of-mind and self-reference effect. Likewise, components on incentive salience, already tackled in the HCP Gambling task, are now included in a task battery addressing positive-incentive value. Yet also, a battery on mental time travel brings in new modules pertaining to time orientation and cardinal-direction judgment. Data from both releases are organized in 25 tasks –most of them reproduced from other studies– and they amount for 205 contrasts described on the basis of 110 cognitive atoms, extracted from the Cognitive Atlas21.
Here, we give an account –focused on the second release– of the experimental procedures and the dataset organization and show that raw task-fMRI data and their derivatives represent functional activity in direct response to behavior. Data collection is ongoing and more releases are planned for the next years. Despite being a long-term project, IBC is not dedicated to longitudinal surveys; acquisitions of the same tasks will not be conducted systematically.
The IBC dataset is an open-access facility devoted to providing high-resolution, functional maps of individual brains as basis to support investigations in human cognition.
Methods
To avoid ambiguity with MRI-related terms used throughout this manuscript, definitions of such terms follow the Brain-Imaging-Data-Structure (BIDS) Specification version 1.2.122.
Complementary information about dataset organization and MRI-acquisition protocols can be found in the IBC documentation available online: https://project.inria.fr/IBC/data/
Participants
The present release of the IBC dataset consists of brain fMRI data from eleven individuals (one female), acquired between April 2017 and July 2019. The two differences from the cohort of the first release are: (1) the replacement of participant 2 (sub-02) by participant 15 (sub-15); and (2) the absence of data from participant 8 (sub-08). Regarding the latter, data will be acquired in the future and included in one of the upcoming releases.
Age, sex and handedness of this group of participants is given on Table 1. Handedness was determined with the Edinburgh Handedness Inventory23.
Table 1.
Demographic data of the participants.
| Subject ID | Year of recruitment | Age | Sex | Handedness score |
|---|---|---|---|---|
| sub-01 | 2015 | 39.5 | M | 0.3 |
| sub-04 | 2015 | 26.9 | M | 0.8 |
| sub-05 | 2015 | 27.4 | M | 0.6 |
| sub-06 | 2015 | 33.1 | M | 0.7 |
| sub-07 | 2015 | 38.8 | M | 1 |
| sub-09 | 2015 | 38.5 | F | 1 |
| sub-11 | 2016 | 35.8 | M | 1 |
| sub-12 | 2016 | 40.8 | M | 1 |
| sub-13 | 2016 | 28.2 | M | 0.6 |
| sub-14 | 2016 | 28.3 | M | 0.7 |
| sub-15 | 2017 | 30.3 | M | 0.9 |
Age stands for the participants’ age upon recruitment. All acquisitions of the present release took place between April 2017 and July 2019.
All experimental procedures were approved by a regional ethical committee for medical protocols in Île-de-France (“Comité de Protection des Personnes” - no. 14-031) and a committee to ensure compliance with data-protection rules (“Commission Nationale de l’Informatique et des Libertés” - DR-2016-033). They were undertaken with the informed written consent of each participant according to the Helsinki declaration and the French public health regulation. For more information, consult19.
Materials
Stimulation
For all tasks (see Section “Experimental Paradigms” for details), the stimuli were delivered through custom-made scripts that ensure a fully automated environment and computer-controlled collection of the behavioral data. Two software tools were used for the development of such protocols: (1) Expyriment (versions 0.7.0 and 0.9.0, Python 2.7); and (2) Psychophysics Toolbox Version 3 for GNU Octave version 4.2.1. The visual and auditory stimuli presented in the Theory-of-Mind and Pain Matrices battery as well as in the Bang task (see respectively Sections “Theory-of-Mind and Pain Matrices task battery” and task “Bang task” for details) were translated into French. The corresponding material is publicly available, as described in Section “Code Availability”.
MRI Equipment
The fMRI data were acquired using an MRI scanner Siemens 3 T Magnetom Prismafit along with a Siemens Head/Neck 64-channel coil. Behavioral responses were obtained with two MR-compatible, optic-fiber response devices that were interchangeably used according to the type of task employed: (1) a five-button ergonomic pad (Current Designs, Package 932 with Pyka HHSC-1 × 5-N4); and (2) a pair of in-house custom-made sticks featuring one-top button. MR-Confon package was used as audio system in the MRI environment.
All sessions were conducted at the NeuroSpin platform of the CEA Research Institute, Saclay, France.
Experimental Procedure
Upon arrival to the research institute, participants were instructed about the execution and timing of the tasks referring to the upcoming session.
All sessions were composed of several runs dedicated to one or a group of tasks as described in Section “Experimental Paradigms”. The structure of the sessions according to the MRI modality employed at every run is detailed in Table 2. Specifications about imaging parameters of the referred modalities as well as procedures undertaken toward recruitment of participant 15 plus handling and training of all participants are described in19. As a side note, data pertaining to tasks of the first release were also acquired for participant 15.
Table 2.
Plan of the MRI-data acquisitions for the sessions pertaining the second release of the IBC dataset.
| Session | Modality | Task * | Duration** (min:sec) | Repetitions |
|---|---|---|---|---|
| MTT1 | 2D Spin-Echo | — | 00:31 | PA(×2) + AP(×2) |
| BOLD fMRI | MTT WE† | 13:08 | PA(×2) + AP | |
| MTT2 | 2D Spin-Echo | — | 00:31 | PA(×2) + AP(×2) |
| BOLD fMRI | MTT SN‡ | 13:08 | PA(×2) + AP | |
| Preference | 2D Spin-Echo | — | 00:31 | PA(×2) + AP(×2) |
| BOLD fMRI | Food | 08:16 | PA + AP | |
| BOLD fMRI | Painting | 08:16 | PA + AP | |
| BOLD fMRI | Face | 08:16 | PA + AP | |
| BOLD fMRI | House | 08:16 | PA + AP | |
| TOM | 2D Spin-Echo | — | 00:31 | PA(×2) + AP(×2) |
| BOLD fMRI | TOM localizer | 06:12 | PA + AP | |
| BOLD fMRI | Emotional Pain localizer | 05:12 | PA + AP | |
| BOLD fMRI | Pain Movie localizer | 05:56 | PA + AP | |
| Enumeration | 2D Spin-Echo | — | 00:31 | PA(×2) + AP(×2) |
| BOLD fMRI | VSTM | 08:40 | PA(×2) + AP(×2) | |
| BOLD fMRI | Enumeration | 16:20 | PA + AP | |
| Self | 2D Spin-Echo | — | 00:31 | PA(×2) + AP(×2) |
| BOLD fMRI | Self 1–2 | 12:00 | PA(×2) | |
| BOLD fMRI | Self 3 | 12:00 | AP | |
| BOLD fMRI | Self 4 | 15:58 | AP | |
| BOLD fMRI | Bang | 08:06 | PA |
A BOLD-fMRI run refers to the acquisition of fMRI data on one single task. At least, there were two BOLD runs, corresponding to PA- and AP- phase-encoding directions for each task during a session. The 2D Spin-Echo PA/AP maps were always acquired before the runs dedicated to the collection of BOLD-fMRI data and repeated afterwards.
*Full descriptions of task siglas are provided in Section “Experimental Paradigms”.
**For BOLD fMRI sequences, the durations presented here account only for the period of the actual acquisition. The full duration of each run also included ∼45 s of calibration scans, always performed at their beginning.
†Mental Time Travel task featuring “West-East island” stimuli.
‡Mental Time Travel task featuring “South-North island” stimuli.
§The run “Self 4” relates to a longer version of the others runs of the same task. Thus, “Self 4” contains four pairs of Encoding + Recognition phases, whereas the remaining runs contain only three pairs.
Experimental Paradigms
Tasks were aggregated in different sessions according to their original studies24–33. Most of the paradigms are composed by trials usually separated by the display of a fixation cross. All trials within each task were randomized in order to avoid the extensively consecutive repetition of trials containing conditions of the same kind. For some tasks, trials were in fact pseudo-randomized by following specific criteria relative to the experimental design of those tasks.
The following sections are thus dedicated to a full description of the set of paradigms employed for each task, including description of the experimental conditions, temporal organization of the trials and their (pseudo-)randomization. Moreover, Table 3 provides an overview of the tasks, which includes a short description and motivation of their inclusion in terms of psychological domains covered. Ideally and as mentioned in Section “Background and Summary”, the main purpose of each release is to provide the dataset with a greater variety of cognitive modules from as many new psychological domains as possible, at the same time that a better coverage with the already existing ones is also attained.
Table 3.
Overview of the tasks featuring the second release of the IBC dataset.
| Tasks | Description | Psychological Domains covered | References |
|---|---|---|---|
| MTT battery | Assess the mental time and space shifts involved in the allocentric mapping of fictional events described in terms of audio narratives. | Existing: auditory cognition, spatial cognition memory New: temporal cognition (e.g. time orientation) spatial cognition (e.g. cardinal orientation). | 24–26 |
| Preference battery | Assess decision-making associated with the positive-incentive value and level of confidence in the evaluation of visual constructs. | Existing: incentive salience, visual cognition perception New: confidence, food-cue responsiveness. | 27 |
| TOM battery | Assess theory-of-mind and pain-matrix networks. | Existing: language, social cognition, theory-of-mind New: pain. | 28–30 |
| VSTM + Enumeration | Assess numerosity with and without encoding of object features. | Existing: numerical cognition, visual cognition New: numerical cognition (e.g. numerosity). | 31 |
| Self | Assess the Self-Reference Effect. | Existing: recognition. | 32 |
| New: Self (e.g. self-reference effect, episodic memory). | |||
| Bang | Assess speech comprehension during movie watching. | Existing: language New: perception (e.g. action perception) auditory cognition (e.g. auditory-scene analysis). | 33 |
The list contains a short description of every task along with a brief summary of the psychological domains addressed by its experimental conditions. The bibliographic references pertaining to their original studies are also provided in the right most column.
All material used for stimulus presentation have been made publicly available (see Section “Code Availability”), together with video annotations of the corresponding protocols. Video annotations refer to video records of complete runs that are meant to be consulted for a better comprehension of the task paradigms. For each subject, the paradigm-descriptors’ files describing the occurrence of the events are part of the dataset, following BIDS Specification.
Mental time travel (MTT) task battery
The Mental Time Travel (MTT) task battery was developed following previous studies conducted at the NeuroSpin platform on chronosthesia and mental space navigation24–26. In these studies, participants judged the ordinality of real historical events in time and space by mentally project oneself, i.e. through egocentric mapping. In contrast, the present task was intended to assess the neural correlates underlying both mental time and space judgment involved in allocentric mapping implemented in narratives. To this end, and in order to remove confounds associated with prior subject-specific mental representations linked to the historical events, fictional scenarios were created with fabricated stories and characters.
Concretely, this battery is composed of two tasks –MTT WE and MTT SN– that were employed, each of them, in two different sessions. The stimuli of each task referred to a different island plotting different stories and characters. There were two stories per island and they were created based on a two-dimensional mesh of nodes. Each node corresponded to a specific action. The stories of each island evolved both in time and in one single cardinal direction. The cardinal directions, cued in the task, differed between sessions. Thus, space judgment was performed according to the cardinal directions West-East and South-North for tasks MTT WE and MTT SN, respectively. In addition, the stories of each island evolved spatially in opposite ways. For instance, the two stories plotted in the West-East island evolved across time from west to east and east to west, respectively.
Prior to each session, participants were to learn the story of the corresponding session. To prevent any retrieval of graphical memories referring to the schematic representation of the stories, they were presented as audio narratives. Additionally, the participants were also instructed to learn the stories chronographically, i.e. as they were progressively referred to in the narrative, and to refrain from doing (visual) notes, which could be encoded as mental judgments.
The task was organized as a block-design paradigm, composed of trials with three conditions of audio stimuli: (1) Reference, statement of an action in the story to serve as reference for the time or space judgment in the same trial; (2) Cue, question concerning the type of mental judgment to be performed in the same trial, i.e. “Before or After?” for the time judgment or “West or East?” and “South or North?” for the space judgment in the first and second sessions, respectively; and (3) Event, statement of an action to be judged with respect to the Reference and according to the Cue.
Every trial started with an audio presentation of the Reference followed by silence, with a duration of two and four seconds, respectively. The audio presentation of the Cue came next, followed by a silence period; they had respectively a duration of two and four seconds. Afterwards, a series of four Events were presented for two seconds each; all of them were interspersed by a Response condition of three seconds. Every trial ended with a silent period of seven seconds, thus lasting thirty nine seconds in total.
A black fixation cross was permanently displayed on the screen across conditions and the participants were instructed to never close their eyes. At the very end of each trial, the cross turned to red during half of a second in order to signal the beginning of the next trial; such cue facilitated the identification of the next audio stimulus as the upcoming Reference to be judged.
During the Response period, the participants had to press one of the two possible buttons, placed in their respective left and right hand. If the Cue presented in the given trial hinted at time judgment, the participants were to judge whether the previous Event occurred before the Reference, by pressing the button of the left hand, or after the Reference, by pressing the button of the right hand. If the Cue concerned with space judgment, the participants were to judge, in the same way, whether the Event occurred west or east of the Reference in the first session and south or north of the Reference in the second session.
One session of data collection comprised three runs; each of them included twenty trials. Half of the trials for a given run were about time navigation and the other half, space navigation. Five different references were shared by both types of navigation and, thus, there were two trials with the same reference for each type of navigation. Within trials, half of the Events related to past or western/southern actions and the other half to future or eastern/northen actions with respect to the Reference.
The order of the trials was shuffled within runs, only to ensure that each run would feature a unique sequence of trials according to type of reference (both in time and space) and cue. No pseudo-randomization criterion was imposed as the trials’ characterization was already very rich. Since there were only two types of answers, we also randomized events according to their correct answer within each trial. The same randomized sequence for each run was employed for all participants. The code of this randomization is provided together with the protocol of the task in a public repository on GitHub (see Section “Code Availability”). Note that the randomized sequence of trials for all runs is pre-determined and, thus, provided as inputs to the protocol for a specific session.
For sake of clarity, Online-only Table 1 contains a full description of all conditions employed in the experimental design of this task.
Online-only Table 1.
Regressors-of-Interest implemented in the design matrices of the tasks for the main contrasts of this dataset release.
| Regressor-of-Interest | Description of the effect modeled |
|---|---|
| MTT task battery* | |
| we/sn_average_reference | action in the story to serve as reference for the time or space judgment in the same trial in the west-east/south-north island |
| we/sn_all_space_cue | cue indicating a question about spatial orientation in the west-east/south-north island |
| we/sn_all_time_cue | cue indicating a question about time orientation in the west-east/south-north island |
| westside/southside_event | action to be judged whether it takes place west/south or east/north from this reference, that actually takes place west/south from this reference |
| eastside/northside_event | action to be judged whether it takes place west/south or east/north from this reference, that actually takes place east/north from this reference |
| we/sn_before_event | action to be judged whether it takes place before or after this reference, that actually takes place before this reference, in the west-east/south-north island |
| we/sn_after_event | action to be judged whether it takes place before or after this reference, that actually takes place after this reference, in the west-east/south-north island |
| we/sn_all_event_response | motor responses performed after every event condition in the west-east/south-north island |
| Preference task battery§ | |
| preference_constant | main effect of condition concerning the classification of the level of pleasantness of an item displayed on the screen |
| preference_linear | parametric effect concerning the rating provided by the participant |
| preference_quadratic | parametric effect of the squared rating |
| TOM localizer | |
| belief | main effect of condition concerning the reading of a false-belief story, that portrayed characters with false beliefs about their own reality |
| photo | main effect of condition concerning the reading of a story related to a false photograph, map or sign |
| Emotional Pain localizer | |
| emotional_pain | main effect of condition concerning the reading of a story that portrayed characters suffering from emotional pain |
| physical_pain | main effect of condition concerning the reading of a story that portrayed characters suffering from physical pain |
| Pain Movie localizer | |
| movie_mental | main effect of condition concerned with watching a movie scene whose characters experience changes in beliefs, desires and/or emotions |
| movie_pain | main effect of condition concerned with watching a movie scene whose characters experience physical pain |
| VSTM task | |
| vstm_constant | main effect of condition concerned with judging whether any bar changed orientation within two consecutive displays of bar sets on the screen |
| vstm_linear | parametric effect of the number of bars displayed during two consecutive displays† |
| vstm_quadratic | quadratic effect of the number of bars |
| Enumeration task | |
| enumeration_constant | main effect of condition concerned with judging the number of bars displayed on the screen |
| enumeration_linear | parametric effect of the number of bars displayed |
| enumeration_quadratic | quadratic effect of the number of bars |
| Self task | |
| instruction | main effect of condition concerning the presentation of a question related to the succeeding block‡ |
| encode_self1 | main effect of condition concerned with judging whether a certain adjective —displayed on the screen— qualifies oneself |
| encode_other1 | main effect of condition concerned with judging whether a certain adjective —displayed on the screen— qualifies someone else |
| recognition_self_hit2 | main effect of condition concerning the successful recognition of an adjective —displayed on the screen— as having been already presented during one “encode_self” trial of the preceding encoding phase |
| recognition_other_hit2 | main effect of condition concerning the successful recognition of an adjective —displayed on the screen— as having been already presented during one “encode_other” trial of the preceding encoding phase |
| correct_rejection2 | main effect of condition concerning the successful identification that a new adjective has never been presented before |
| Bang task | |
| talk | main effect of condition concerned with watching contiguous scenes of speech |
| no_talk | main effect of condition concerned with watching contiguous scenes of non-speech |
*MTT-task battery comprises two tasks that differ in their cardinal-orientation judgment –West-East and South-North– as detailed in Section “Mental Time Travel (MTT) task battery”. The experimental paradigm is the same and regressors-of-interest are equivalent with respect to cardinality.
In every trial, the same number of bars was kept between the two consecutive displays.
Questions were according to the type of the succeeding block. Thus, “encode_self” blocks were preceded by the question “Are you?”; “encode_other” blocks were preceded by the question “Is < name_of_famous_person > ?”; and recognition-phase blocks were preceded by the question “Have you seen?”.
1Regressor modeling condition from the Encoding phase.
2Regressor modeling condition from the Recognition phase.
Preference task battery
The Preference task battery was adapted from the Pleasantness Rating task (Study 1a) described in27, in order to capture the neural correlates underlying decision-making for potentially rewarding outcomes (aka “positive-incentive value”) as well as the corresponding level of confidence.
The whole task battery is composed of four tasks, each of them pertaining to the presentation of items of a certain kind. Therefore, Food, Painting, Face and House tasks were dedicated to “food items”, “paintings”, “human faces” and “houses”, respectively.
All tasks were organized as a block-design experiment with one condition per trial. Every trial started with a fixation cross, whose duration was jittered between 0.5 seconds and 4.5 seconds, after which a picture of an item was displayed on the screen together with a rating scale and a cursor. Participants were to indicate how pleasant the presented stimulus was, by sliding the cursor along the scale. Such scale ranged between 1 and 100. The value 1 corresponded to the choices “unpleasant” or “indifferent”; the middle of the scale corresponded to the choice “pleasant”; and the value 100 corresponded to the choice “very pleasant”. Therefore, the ratings related only to the estimation of the positive-incentive value of the items displayed.
One full session was dedicated to the data collection of all tasks. It comprised eight runs with sixty trials each. Although each trial had a variable duration, according to the time spent by the participant in the assessment, no run lasted longer than eight minutes and sixteen seconds. Every task was presented twice in two fully dedicated runs. The stimuli were always different between runs of the same task. As a consequence, no stimulus was ever repeated in any trial and, thus, no item was ever assessed more than once by the participants. To avoid any selection bias in the sequence of stimuli, the order of their presentation was shuffled across trials and between runs of the same type. This shuffle is embedded in the code of the protocol and, thus, the sequence was determined upon launching it. Consequently, the sequence of stimuli was also random across subjects. For each run (of each session), this sequence was properly registered in the logfile generated by the protocol.
Theory-of-mind and pain matrices task battery
This battery of tasks was adapted from the original task-fMRI localizers of Saxe Lab, intended to identify functional regions-of-interest in the Theory-of-Mind network and Pain Matrix regions. These localizers rely on a set of protocols along with verbal and non-verbal stimuli, whose material was obtained from https://saxelab.mit.edu/localizers.
Minor changes were employed in the present versions of the tasks herein described. Because the cohort of this dataset is composed solely of native French speakers, the verbal stimuli were thus translated to French. Therefore, the durations of the reading period and the response period within conditions were slightly increased.
Theory-of-mind localizer (TOM localizer)
The Theory-of-Mind Localizer (TOM localizer) was intended to identify brain regions involved in theory-of-mind and social cognition, by contrasting activation during two distinct story conditions: (1) belief judgments, reading a false-belief story that portrayed characters with false beliefs about their own reality; and (2) fact judgments, reading a story about a false photograph, map or sign28.
The task was organized as a block-design experiment with one condition per trial. Every trial started with a fixation cross of twelve seconds, followed by the main condition that comprised a reading period of eighteen seconds and a response period of six seconds. Its total duration amounted to thirty six seconds. There were ten trials in a run, followed by an extra-period of fixation cross for twelve seconds at the end of the run. Two runs were dedicated to this task in one single session.
The designs, i.e. the sequence of conditions across trials, for two possible runs were pre-determined by the authors of the original study and hard-coded in the original protocol (see Section “Theory-of-Mind and Pain Matrices task battery”). The IBC-adapted protocols contain the exactly same designs. For all subjects, design #1 was employed for the PA-run and design #2 for the AP-run.
Theory-of-mind and pain-matrix narrative localizer (Emotional Pain localizer)
The Theory-of-Mind and Pain-Matrix Narrative Localizer (Emotional Pain localizer) was intended to identify brain regions involved in theory-of-mind and Pain Matrix areas, by contrasting activation during two distinct story conditions: reading a story that portrayed characters suffering from (1) emotional pain and (2) physical pain29.
The experimental design of this task is identical to the one employed for the TOM localizer, except that the reading period lasted twelve seconds instead of eighteen seconds. Two different designs were pre-determined by the authors of the original study and they were employed across runs and participants, also in the same way as described for the TOM localizer (see Section “Theory-of-Mind Localizer (TOM localizer)”).
Theory-of-mind and pain matrix movie localizer (Pain Movie localizer)
The Theory-of-Mind and Pain Matrix Movie Localizer (Pain Movie localizer) consisted in the display of “Partly Cloud”, a 6-minute movie from Disney Pixar, in order to study the responses implicated in theory-of-mind and pain-matrix brain regions29,30.
Two main conditions were thus hand-coded in the movie, according to30, as follows: (1) mental movie, in which characters were experiencing changes in beliefs, desires, and/or emotions; and (2) physical pain movie, in which characters were experiencing physical pain. Such conditions were intended to evoke brain responses from theory-of-mind and pain-matrix networks, respectively. All moments in the movie not focused on the direct interaction of the main characters were considered as a baseline period.
Visual short-term memory (VSTM) and enumeration task battery
This battery of tasks was adapted from the control experiment described in31. They were intended to investigate the role of the Posterior Parietal Cortex (PPC) involved in the concurrent processing of a variable number of items. Because subjects can only process three or four items at a time, this phenomenon may reflect a general mechanism of object individuation34,35. On the other hand, PPC has been implicated in studies of capacity limits, during Visual Short-Term Memory (VSTM)36 and Enumeration35. While the former requires high encoding precision of items due to their multiple features, like location and orientation, the latter requires no encoding of object features. By comparing the neural response of the PPC with respect to the two tasks, the original study demonstrated a non-linear increase of activation, in this region, along with the increasing number of items. Besides, this relationship was different in the two tasks. Concretely, PPC activation started to increase from two items onward in the VSTM task, whereas such increase only happened from three items onward in the Enumeration task.
For both tasks, the stimuli consisted of sets of tilted dark-gray bars displayed on a light-gray background. Additionally, minor changes were employed in their present versions herein described: (1) both the response period and the period of the fixation dot at the end of each trial were made constant in both tasks; and (2) for the Enumeration task, answers were registered via a button-press response box instead of an audio registration of oral responses as in the original study.
Visual short-term memory task (VSTM)
In the VSTM task, participants were presented with a certain number of bars, varying from one to six.
Every trial started with the presentation of a black fixation dot in the center of the screen for 0.5 seconds. While still on the screen, the black fixation dot was then displayed together with a certain number of tilted bars –variable between trials from one to six– for 0.15 seconds. Afterwards, a white fixation dot was shown for 1 second. It was next replaced by the presentation of the test stimulus for 1.7 seconds, displaying identical number of tilted bars in identical positions together with a green fixation dot. The participants were to remember the orientation of the bars from the previous sample, and answer with one of the two possible button presses, depending on whether one of the bars in the current display had changed orientation by 90 °, which was the case in half of the trials. The test display was replaced by another black fixation dot for a fixed duration of 3.8 seconds. Thus, the trial was 7.15 seconds long. There were 72 trials in a run and four runs in one single session. Pairs of runs were launched consecutively. To avoid selection bias in the sequence of stimuli, the order of the trials was shuffled according to numerosity and change of orientation within runs and across participants.
Enumeration task
In the Enumeration task, participants were presented with a certain number of bars, varying from one to eight.
Every trial started with the presentation of a black fixation dot in the center of the screen for 0.5 seconds. While still on the screen, the black fixation dot was then displayed together with a certain number of tilted bars –variable between trials from one to eight– for 0.15 seconds. It was followed by a response period of 1.7 s, in which only a green fixation dot was being displayed on the screen. The participants were to remember the number of the bars that were shown right before and answer accordingly, by pressing the corresponding button. Afterwards, another black fixation dot was displayed for a fixed duration of 7.8 seconds. The trial length was thus 9.95 seconds. There were ninety six trials in a run and two (consecutive) runs in one single session. To avoid selection bias in the sequence of stimuli, the order of the trials was shuffled according to numerosity within runs and across participants.
Self task
The Self task was adapted from the study32, originally developed to investigate the Self-Reference Effect in older adults. This effect pertains to the encoding mechanism of information referring to the self, characterized as a memory-advantaged process. Consequently, memory-retrieval performance is also better for information encoded in reference to the self than to other people, objects or concepts.
The present task was thus composed of two phases, each of them relying on encoding and recognition procedures. The encoding phase was intended to map brain regions related to the encoding of items in reference to the self, whereas the recognition one was conceived to isolate the memory network specifically involved in the retrieval of those items. The phases were interspersed, so that the recognition phase was always related to the encoding phase presented immediately before.
The encoding phase had two blocks. Each block was composed of a set of trials pertaining to the same condition. For both conditions, a different adjective was presented at every trial on the screen. The participants were to judge whether or not the adjective described themselves –self-reference encoding condition– or another person –other-reference encoding condition. The other person was a public figure in France around the same age range as the cohort, whose gender matched the gender of every participant. Two public figures were mentioned, one at the time, across all runs; four public figures –two of each gender– were selected beforehand. By this way, we ensured that all participants were able to successfully characterize the same individuals, holding equal the levels of familiarity and affective attributes with respect to these individuals.
In the recognition phase, participants were to remember whether or not the adjectives had also been displayed during the previous encoding phase. This phase was composed of a single block of trials, pertaining to three categories of conditions. New adjectives were presented during one half of the trials whereas the other half were in reference to the adjectives displayed in the previous phase. Thus, trials referring to the adjectives from “self-reference encoding” were part of the self-reference recognition category and trials referring to the “other-reference encoding” were part of the other-reference recognition category. Conditions were then defined according to the type of answer provided by the participant for each of these categories (see Online-only Table 1 for details).
There were four runs in one session. The first three ones had three phases; the fourth and last run had four phases (see Table 2). Their total durations were twelve and 15.97 seconds, respectively. Blocks of both phases started with an instruction condition of five seconds, containing a visual cue. The cue was related to the judgment that should be performed next, according to the type of condition featured in that block. A set of trials, showing different adjectives, were presented afterwards. Each trial had a duration of five seconds, in which a response was to be provided by the participant. During the trials of the encoding blocks, participants had to press the button with their left or right hand, depending on whether they believed or not the adjective on display described someone (i.e. self or other, respectively for “self-reference encoding” or “other-reference encoding” conditions). During the trials of the recognition block, participants had to answer in the same way, depending on whether they believed or not the adjective had been presented before. A fixation cross was always presented between trials, whose duration was jittered between 0.3 seconds and 0.5 seconds. A rest period was introduced between encoding and recognition phases, whose duration was also jittered between ten and fourteen seconds. Long intervals between these two phases, i.e. longer than ten seconds, ensured the measurement of long-term memory processes during the recognition phase, at the age range of the cohort37,38. Fixation-cross periods of three and fifteen seconds were also introduced in the beginning and end of each run, respectively.
All adjectives were presented in the lexical form according to the gender of the participant. There were also two sets of adjectives. One set was presented as new adjectives during the recognition phase and the other set for all remaining conditions of both phases. To avoid cognitive bias across the cohort, sets were switched for the other half of the participants. Plus, adjectives never repeated across runs but their sequence was fixed for the same runs and across participants from the same set. Yet, pseudo-randomization of the trials for the recognition phase was pre-determined by the authors of the original study, according to their category (i.e. “self-reference recognition”, “other-reference recognition” or “new”), such that no more than three consecutive trials of the same category were presented within a block.
For sake of clarity, Online-only Table 1 contains a full description of all main conditions employed in the experimental design of this task.
Bang task
The Bang task was adapted from the study33, dedicated to investigate aging effects on neural responsiveness during naturalistic viewing.
The task relies on watching –viewing and listening– of an edited version of the episode “Bang! You’re Dead” from the TV series “Alfred Hitchcock Presents”. The original black-and-white, 25-minute episode was condensed to seven minutes and fifty five seconds while preserving its narrative. The plot of the final movie includes scenes with characters talking to each other as well as scenes with no verbal communication. Conditions of this task were thus set by contiguous scenes of speech and no speech.
This task was performed during a single run in one unique session. Participants were never informed of the title of the movie before the end of the session. Ten seconds of acquisition were added at the end of the run. The total duration of the run was thus eight minutes and five seconds.
Data Acquisition
Data across participants were acquired throughout six MRI sessions, whose structure is described in Table 2. Deviations from this structure were registered for two MRI sessions. Besides and as referred in Section “Data quality” as well as Fig. 1, a drop of the tSNR was identified for some MRI sessions. Additionally, data of the tasks featuring this release were not yet collected for subject 8 (consult Section “Participants” for further details). These anomalies in the data are summarized on Online-only Table 2.
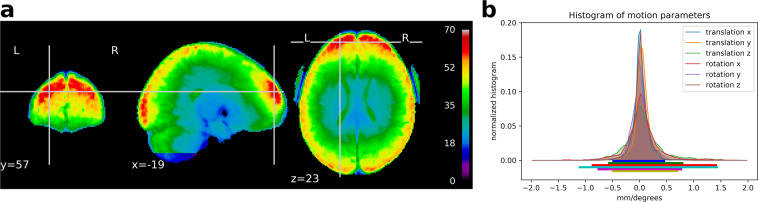
Fig. 1.
Global quality indices of the acquired data: tSNR map and motion magnitude distribution. (a) The tSNR map displays the average of tSNR across all tasks and subjects. This shows values mostly between 30 and 70, with larger tSNR in cortical regions, except for the bottom of the cerebellum, that was acquired at a lesser extent in this data release. (b) Density of within-run motion parameters, pooled across subjects and tasks. Six distributions are plotted, for the six rigid-body parameter of head motion (translations and rotations are in mm and degrees, respectively). Each distribution is based on ∼101k EPI volumes of 11 subjects, corresponding to all time frames for all acquisitions and subjects. Bold lines below indicate the 99% coverage of all distributions and show that motion parameters mostly remain confined to 1.5 mm/1degree across 99% of all acquired images.
Online-only Table 2.
Data anomalies.
| Participants | sub-01 | sub-04 | sub-05 | sub-06 | sub-07 | sub-08 | sub-09 | sub-11 | sub-12 | sub-13 | sub-14 | sub-15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tasks | |||||||||||||
| MTT 1 | tSNR drop at the bottom of cerebellum | No data | tSNR drop at the bottom of cerebellum | tSNR drop at the bottom of cerebellum | |||||||||
| MTT 2 | tSNR drop at the bottom of cerebellum | tSNR drop at the bottom of cerebellum | tSNR drop at the bottom of cerebellum | No data | |||||||||
| Preference | tSNR drop at the bottom of cerebellum | tSNR drop at the bottom of cerebellum | tSNR drop at the bottom of cerebellum | No data | tSNR drop at the bottom of cerebellum | tSNR drop at the bottom of cerebellum | tSNR drop at the bottom of cerebellum | tSNR drop at the bottom of cerebellum | tSNR drop at the bottom of cerebellum | Run “Food-PA” contains only the first 50 trials | |||
| Run “Painting-AP” is missing and Run “Faces-AP” was acquired twice | |||||||||||||
| TOM | tSNR drop at the bottom of cerebellum | No data | |||||||||||
| Enumeration | tSNR drop at the bottom of cerebellum | No data | tSNR drop at the bottom of cerebellum | tSNR drop at the bottom of cerebellum | |||||||||
| Self | No data | ||||||||||||
Four anomalies are summarized over MRI sessions and participants: tSNR drop at the bottom of the cerebellum; acquisition of two Runs “Faces-AP” in detriment of no acquisition of Run “Painting-AP” of the Preference battery for subject 11; only the first fifty trials of Run “Food-PA” of the Preference battery were registered for subject 15, since this participant could not complete the sixty trials within the pre-established duration of the run; no data for the tasks featuring this release were collected for subject 8, which will integrate a future release upon announcement.
Behavioral Data
Active responses were required from the participants in all tasks. The registry of all behavioral data, such as the qualitative responses to different conditions and corresponding response times, was held in log files generated by the stimulus-delivery software.
Imaging Data
FMRI data were collected using a Gradient-Echo (GE) pulse, whole-brain Multi-Band (MB) accelerated39,40 Echo-Planar Imaging (EPI) T2*-weighted sequence with Blood-Oxygenation-Level-Dependent (BOLD) contrasts. Two different acquisitions for the same run were always performed using two opposite phase-encoding directions: one from Posterior to Anterior (PA) and the other from Anterior to Posterior (AP). The main purpose was to ensure within-subject replication of the same tasks, while mitigating potential limitations concerning the distortion-correction procedure.
Spin-Echo (SE) EPI-2D image volumes were acquired in order to compensate for spatial distortions. Similarly to the GE-EPI sequences, two different phase-encoding directions, i.e. PA and AP, were employed in different runs pertaining to this sequence. There were four runs per session: one pair of PA and AP SE EPI-2D before the start of the GE-EPI sequences and another pair at the end.
The parameters for all types of sequences employed are provided in19 as well as in the documentation available on the IBC website: https://project.inria.fr/IBC/data/
Data Analysis
Image conversion
The acquired DICOM images were converted to NIfTI format using the dcm2nii tool, which can be found at https://www.nitrc.org/projects/dcm2nii. During conversion to NIfTI, all images were fully anonymized, i.e. pseudonyms were removed and images were defaced using the mri_deface command line from the Freesurfer-6.0.0 library.
Preprocessing
Source data were preprocessed using the same pipeline employed for the first release of the IBC dataset. Thus, refer to19 for more complete information about procedures undertaken during this stage.
In summary, raw data were preprocessed using PyPreprocess
(https://github.com/neurospin/pypreprocess), dedicated to launch in the python ecosystem pre-compiled functions of SPM12 software package v6685 and FSL library v5.0.
Firstly, susceptibility-induced off-resonance field was estimated from four SE EPI-2D volumes, each half acquired in opposite phase-encoding directions (see Section “Imaging Data” for details). The images were corrected based on the estimated deformation model, using the topup tool41 implemented in FSL42.
GE-EPI volumes of each participant were then aligned to each other, using a rigid body transformation, in which the average volume of all images across runs (per session) was used as reference43.
The mean EPI volume was also co-registered onto the T1-weighted MPRAGE (anatomical) volume of the corresponding participant44, acquired during the Screening session (consult19 for details).
The individual anatomical volumes were then segmented into tissue types in order to allow for the normalization of both anatomical and functional data into the standard MNI152 space, which was performed using the Unified Segmentation probabilistic framework45. Concretely, the segmented volumes were used to compute the deformation field for normalization to the standard MNI152 space.
FMRI model specification
FMRI data were analyzed using the General Linear Model (GLM). Regressors-of-interest in the model were designed to capture variations in BOLD signal, which are in turn coupled to neuronal activity pertaining to task performance. To this end, the temporal profile of task stimuli is convolved with the Hemodynamic Response Function (HRF) –defined according to46,47– in order to obtain the theoretical neurophysiological profile of brain activity in response to behavior. The temporal profiles of stimuli, for block-design experiments, are typically characterized by boxcar functions defined by triplets –onset time, duration and trial type– that can be extracted from log files’ registries generated by the stimulus-delivery software.
Because the present release encompasses tasks with different types of experimental designs, regressors-of-interest can refer to either conditions, wherein main effects of stimuli span a relatively long period, or parametric effects of those stimuli. Online-only Table 1 contains a complete description of all regressors-of-interest implemented in the models of every task.
Nuisance regressors were also modeled in order to account for different types of spurious effects arising during acquisition time, such as fluctuations due to latency in the HRF peak response, movements, physiological noise and slow drifts within run. We also account for another type of regressors-of-no-interest, referring to either no responses or non-correct behavioral responses, implemented in the model of the Self task. Concretely, the regressors “encode_self_no_response”, “encode_other_no_response”,
“recognition_self_no_response” and “recognition_other_no_response” –related to absence of responses in each condition– plus “recognition_self_miss” and “recognition_other_miss” –related to the unsuccessful recognition of an adjective previously presented– as well as “false_alarm” –related to the misrecognition of a new adjective as one already presented– were modeled separately as means to grant an accurate isolation of the effects pertaining to “recognition” and “self reference” in the regressors-of-interest (see Section “Self task” for further details about the design of this task).
A complete description of the general procedures concerned with the GLM implementation of the IBC data can be found in the first data-descriptor article19. Such implementation was performed using Nistats python module v0.0.1b (https://nistats.github.io), leveraging Nilearn python module v0.6.048 (https://nilearn.github.io/).
Model estimation
In order to restrict GLM parameters estimation to voxels inside functional brain regions, a brain mask was extracted from the normalized mean GE-EPI volume thresholded at a liberal level of 0.25, using Nilearn. This corresponds to a 25% average probability of finding gray matter in a particular voxel across subjects. A mass-univariate GLM fit was then applied to the preprocessed EPI data, for every run in every task, using Nistats. For this fit, we set a spatial smoothing of 5 mm full-width-at-half-maximum as a regularization term of the model; spatial smoothing is a standard procedure that ensures an increase of the Signal-to-Noise Ratio (SNR) at the same time that facilitates between-subject comparison. Parameter estimates for all regressors implemented in the model were computed, along with the respective covariance, at every voxel. Linear combinations between parameter estimates computed for the regressors-of-interest (listed on Online-only Table 1) as well as for the baseline were performed in order to obtain contrast maps with the relevant evoked responses.
More details about model estimation can be found in the first data-descriptor article19. Its implementation and the ensuing statistical analyses were performed using Nistats (about Nistats, see Section “FMRI Model Specification”).
Summary statistics
Because data were collected per task and subject in –at least– two acquisitions with opposite phase-encoding directions (see Section “Imaging Data” for details), statistics of their joint effects were calculated under a Fixed-Effects (FFX) model.
t-tests were then computed at every voxel for each individual contrast, in order to assess for statistical significance in differences among evoked responses. To assure standardized results that are independent from the number of observations, t-values were directly converted into z-values.
Data derivatives are thus delivered as individual contrast maps containing standard scores in all voxels that are confined to a grey-matter-mask with an average threshold >25% across subjects. We note that these postprocessed individual maps were obtained from the GLM fit of preprocessed EPI maps and, thus, they are represented in the standard MNI152 space. For more information about the access to the data derivatives, refer to Section “Derived statistical maps”.
Data Records
Both raw fMRI data (aka “source data”) as well as derived statistical maps of the IBC dataset are publicly available.
Source data
Source data of the present release (plus first and third releases) can be accessed via the public repository OpenNeuro49 under the data accession number ds00268550. This collection comprises ∼1.1 TB of MRI data. A former collection only referring to source data from the first release is still available in the same repository51.
The NIfTI files as well as paradigm descriptors and imaging parameters are organized per run for each session according to BIDS Specification:
the data repository is organized in twelve main directories sub-01 to sub-15; we underline that sub-02, sub-03 and sub-10 are not part of the dataset and corresponding data from sub-08 will be made available in further releases (see Table 1);
data from each subject are numbered on a per-session basis, following the chronological order of the acquisitions; we also note that this order is not the same for all subjects; the IBC documentation can be consulted for the exact correspondence between session number and session id for every subject on https://project.inria.fr/IBC/data/ (session id’s of the first and second releases are respectively provided on Table 2 of 19 and Table 2 of the present article);
acquisitions are organized within session by modality;
- different identifiers are assigned to different types of data as follows:
- gzipped NIfTI 4D image volumes of BOLD fMRI data are named as sub-XX_ses-YY_task-ZZZ_dir-AA_bold.nii.gz, in which XX and YY refer respectively to the subject and session id, ZZZ refers to the name of the task, and AA can be either ‘PA’ or ‘AP’ depending on the phase-encoding direction;
- event files are named as sub-XX_ses-YY_task-ZZZ_dir-AA_event.tsv;
- single-band, reference images are named as sub-XX_ses-YY_task-ZZZ_dir-AA_sbref.nii.gz.
Although BIDS v1.2.1 does not provide support for data derivatives, a similar directory tree structure was still preserved for this content.
Derived statistical maps
The unthresholded-statistic, contrast maps have been released in the public repository NeuroVault52 with the id = 661853. This collection comprises data from both releases. A former collection only referring to data derivatives from the first release is still available in the same repository (id = 443854).
Technical Validation
Behavioral data
Response accuracy of behavioral performance was calculated for those tasks requiring overt responses. It aims at providing a quantitative assessment of the quality of the imaging data in terms of subjects’ compliance. Because imaging data reflect herein brain activity related to behavior, scores of response accuracy across trials are good indicators of faithful functional representations regarding the cognitive mechanisms involved in the correct performance of the task. Individual scores are provided as percentages of correct responses with respect to the total number of responses in every run of a given task. The average of these scores is also provided as an indicator of the overall performance of the participant for that specific task.
Mental time travel task battery
Scores for MTT WE and MTT SN tasks are provided on Online-only Table 3. Participants were to give one answer out of two possible answers during the Response condition (see Section “Mental Time Travel (MTT) task battery” for details); one of the answers was the correct one. Nevertheless, trials were composed of one series of four consecutive Events interspersed by the corresponding Response. As a result, participants sometimes anticipated or delayed their answers when they were still listening the action during the corresponding Event condition or already in the next Event condition, respectively. To account for correct answers provided under these circumstances, responses given during the first half of an Event (except for the first one of the series) were considered as answers pertaining to the previous Event; on the other hand, responses given during the second half of an Event were considered as answers pertaining to the current Event. The average ± SD across participants for the two tasks are 76 ± 13% and, thus, higher than chance level (50%). We conclude that participants not only learnt correctly the stories plotted in both tasks but also performed successfully the time and space shifts upon listening the Event conditions.
Online-only Table 3.
Response accuracy (%) of behavioral performance for the MTT tasks during the Event + Response conditions.
| Number of trials per run = 20 / Chance level = 50% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Task | Run id | sub-01 | sub-04 | sub-05 | sub-06 | sub-07 | sub-09 | sub-11 | sub-12 | sub-13 | sub-14 | sub-15 |
| MTT WE | 0 | 81 | 83 | 76 | 71 | 57 | 82 | 42 | 76 | 57 | 70 | n/a |
| 1 | 81 | 92 | 78 | 83 | 60 | 90 | 51 | 76 | 58 | 78 | 95 | |
| 2 | 91 | 100 | 68 | 80 | 63 | 86 | 55 | 77 | 63 | 76 | 98 | |
| Mean | 85 | 92 | 75 | 78 | 60 | 86 | 50 | 77 | 60 | 75 | 97 | |
| MTT SN | 0 | 86 | 93 | 76 | 73 | 62 | 83 | 65 | 71 | 65 | 73 | n/a |
| 1 | 88 | 96 | 80 | 73 | 72 | 92 | 67 | 76 | 53 | 68 | n/a | |
| 2 | 91 | 100 | 56 | 82 | 68 | 87 | 70 | 70 | 57 | 72 | n/a | |
| Mean | 89 | 97 | 71 | 77 | 68 | 88 | 68 | 72 | 59 | 72 | n/a | |
| Total | 87 | 94 | 73 | 78 | 64 | 87 | 59 | 75 | 59 | 73 | 97 | |
These scores were estimated considering the answers provided during the Event + Response conditions; the time window of the answers for a certain Event were considered from the second half of presentation of the event itself up to the first half of presentation of the next event, totalizing a period of 5 seconds. The mean of the individual scores across runs per task is also presented along with the total mean comprising both tasks. The average ± SD comprising both tasks across participants are 76 ± 13%.
*Low scores for subject 15 relate to loss of behavioral data during acquisition time in both tasks; we stress this issue is due to loss of the log files generated by the stimulus-delivery software and, thus, agnostic to subject’s performance.
Theory-of-mind
In the Theory-of-Mind task, participants were to read stories involving either false-beliefs about the state of the world or scenery representations that were misleading or outdated. Afterwards they were to answer a question pertaining to the plot, in which one out of two possible answers was correct (see Section “Theory-of-Mind Localizer (TOM localizer)” for details). Online-only Table 4 provides the individual scores achieved for this task. The average ± SD across participants are 74 ± 16%, i.e. higher than chance level (50%). These results show that overall the participants understood the storylines and thus they were able to successfully judge the facts pointed out in the questions.
Online-only Table 4.
Response accuracy (%) of behavioral performance for the Theory-of-Mind localizer.
| Number of trials per run = 10 / Chance level = 50% | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Run id | sub-01 | sub-04 | sub-05 | sub-06 | sub-07 | sub-09 | sub-11 | sub-12 | sub-13 | sub-14 | sub-15 |
| 0 | 90 | 90 | 70 | 70 | 80 | n/a | 90 | 70 | 90 | 80 | 90 |
| 1 | 80 | 80 | 80 | 90 | 60 | 50 | 70 | 80 | 70 | 70 | 70 |
| Mean | 85 | 85 | 75 | 80 | 70 | 50 | 80 | 75 | 80 | 75 | 80 |
The mean of the scores for the two runs is also provided for every subject. The average ± SD across participants are 74 ± 16%.
Visual short-term memory task
In the VSTM task, participants were asked to identify, for every trial, whether there had been a change in the orientation of one of the bars during two consecutive displays of the same number of bars (see Section “Visual Short-Term Memory task (VSTM)” for details). There were thus two possible answers. Online-only Table 5 provides the individual scores for every run and the average across runs, grouped by numerosity of the visual stimuli (measured by the number of bars); numerosity ranged from one to six. In line with the behavioral results reported in the original study (Fig. 2 - plot E of 31), the scores start decreasing more prominently for numerosity >3 (see Online-only Table 6).
Online-only Table 5.
Response accuracy (%) of behavioral performance per numerosity for the VSTM task.
| Number of trials per run = 144 / Chance level = 50% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Numerosity | Run id | sub-01 | sub-04 | sub-05 | sub-06 | sub-07 | sub-09 | sub-11 | sub-12 | sub-13 | sub-14 | sub-15 |
| All numerosities | 0 | 36 | 51 | 28 | 53 | 44 | 49 | 58 | 35 | 29 | 33 | 40 |
| 1 | 49 | 49 | 12 | 57 | 36 | 56 | 50 | 40 | 35 | 18 | 38 | |
| 2 | 40 | 49 | 42 | 58 | 46 | 44 | 56 | 49 | 42 | 39 | 42 | |
| 3 | 43 | 58 | 39 | 57 | 39 | 56 | 57 | 47 | 43 | 43 | 44 | |
| Mean | 42 | 52 | 30 | 56 | 41 | 51 | 55 | 43 | 37 | 33 | 41 | |
| 1 | 0 | 33 | 57 | 38 | 56 | 45 | 78 | 77 | 38 | 36 | 54 | 60 |
| 1 | 60 | 59 | 6 | 60 | 31 | 60 | 64 | 55 | 31 | 18 | 56 | |
| 2 | 43 | 62 | 36 | 64 | 58 | 29 | 83 | 44 | 62 | 33 | 25 | |
| 3 | 70 | 73 | 54 | 60 | 50 | 71 | 58 | 88 | 62 | 42 | 50 | |
| Mean | 52 | 63 | 34 | 60 | 46 | 60 | 70 | 56 | 48 | 37 | 48 | |
| 2 | 0 | 50 | 82 | 54 | 54 | 80 | 58 | 59 | 36 | 38 | 43 | 31 |
| 1 | 58 | 38 | 18 | 55 | 29 | 75 | 57 | 40 | 27 | 40 | 27 | |
| 2 | 27 | 50 | 58 | 73 | 67 | 41 | 58 | 54 | 31 | 38 | 20 | |
| 3 | 31 | 83 | 17 | 77 | 58 | 43 | 67 | 55 | 45 | 45 | 56 | |
| Mean | 42 | 63 | 37 | 65 | 58 | 54 | 60 | 46 | 35 | 42 | 34 | |
| 3 | 0 | 27 | 50 | 36 | 100 | 64 | 38 | 17 | 46 | 42 | 50 | 50 |
| 1 | 44 | 64 | 23 | 60 | 54 | 55 | 42 | 82 | 42 | 12 | 42 | |
| 2 | 42 | 45 | 40 | 62 | 58 | 44 | 75 | 85 | 62 | 33 | 50 | |
| 3 | 58 | 38 | 71 | 45 | 33 | 47 | 50 | 45 | 27 | 27 | 36 | |
| Mean | 43 | 49 | 42 | 67 | 52 | 46 | 46 | 64 | 43 | 30 | 44 | |
| 4 | 0 | 30 | 31 | 20 | 61 | 36 | 25 | 100 | 25 | 20 | 14 | 20 |
| 1 | 50 | 88 | 11 | 50 | 40 | 67 | 60 | 31 | 21 | 20 | 36 | |
| 2 | 23 | 42 | 29 | 75 | 22 | 64 | 27 | 50 | 38 | 36 | 38 | |
| 3 | 9 | 67 | 40 | 62 | 27 | 54 | 56 | 43 | 45 | 50 | 45 | |
| Mean | 28 | 57 | 25 | 62 | 31 | 52 | 61 | 37 | 31 | 30 | 35 | |
| 5 | 0 | 50 | 58 | 17 | 33 | 50 | 46 | 62 | 40 | 18 | 14 | 62 |
| 1 | 42 | 8 | 8 | 50 | 31 | 36 | 55 | 7 | 46 | 0 | 44 | |
| 2 | 62 | 54 | 33 | 46 | 40 | 40 | 40 | 11 | 23 | 42 | 62 | |
| 3 | 45 | 45 | 33 | 55 | 33 | 67 | 64 | 33 | 36 | 50 | 36 | |
| Mean | 50 | 41 | 23 | 46 | 38 | 47 | 55 | 23 | 31 | 26 | 51 | |
| 6 | 0 | 29 | 44 | 8 | 18 | 17 | 54 | 38 | 21 | 20 | 33 | 21 |
| 1 | 30 | 38 | 9 | 62 | 33 | 36 | 31 | 40 | 44 | 20 | 20 | |
| 2 | 44 | 36 | 54 | 38 | 25 | 46 | 55 | 36 | 42 | 50 | 67 | |
| 3 | 47 | 46 | 9 | 36 | 33 | 45 | 46 | 38 | 33 | 50 | 47 | |
| Mean | 38 | 41 | 20 | 38 | 27 | 45 | 42 | 34 | 35 | 38 | 39 | |
The number of bars presented in the visual stimuli was different trial-by-trial, ranging from 1 to 6. For each level of numerosity, scores in every run are related to the trials referring to visual stimuli matching the specified numerosity. The mean of the scores across runs is also provided for every subject and numerosity.
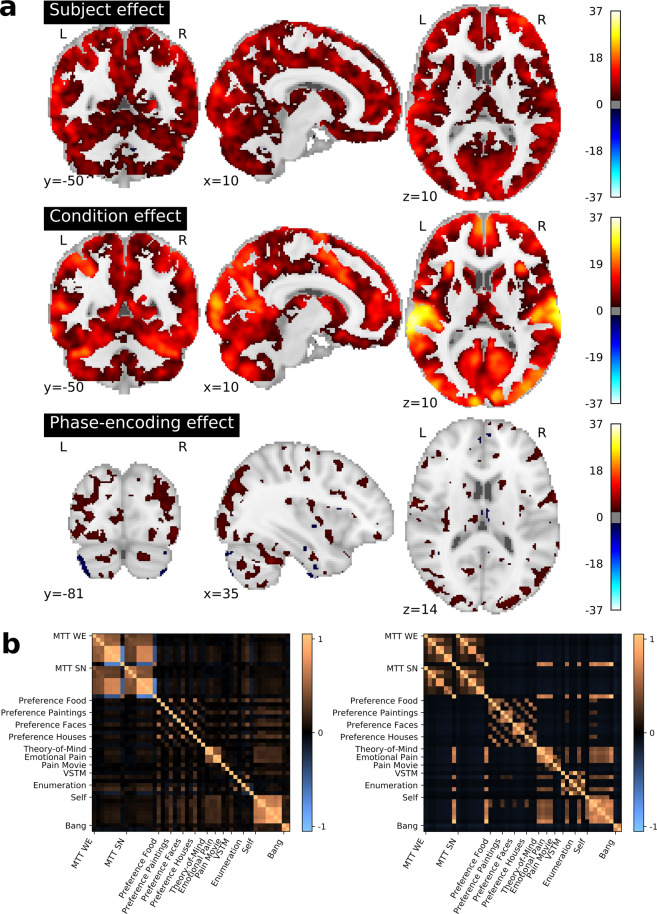
Fig. 2.
Overview of the information conveyed by the activation maps resulting from a first-level analysis. (a) Effects of subject, experimental condition and phase-encoding direction. A per-voxel ANOVA breaks the variance of the set of brain maps into subject, experimental condition, and phase-encoding direction related effects. All maps are given in z-scale and thresholded at an FDR level of 0.05. We note that these results strictly follow the gray-matter structure, as an anatomically-defined gray-matter mask was used in the first-level GLM model (see Section “Model Estimation”). (b) The similarity between condition-related activation maps, averaged across subjects (left), is related to the similarity of the same conditions, when these are characterized in terms of the Cognitive Atlas (right).
Online-only Table 6.
Group-level scores (%) of behavioral performance per numerosity for the VSTM task.
| Numerosity | Mean | SD |
|---|---|---|
| All numerosities | 44 | 8 |
| 1 | 52 | 10 |
| 2 | 49 | 11 |
| 3 | 48 | 10 |
| 4 | 41 | 14 |
| 5 | 39 | 11 |
| 6 | 36 | 7 |
The number of bars presented in the visual stimuli ranged from 1 to 6. For each level of numerosity, the mean of the scores across subjects is provided along with its standard deviation. Although there is a successive decrease of the scores as numerosity increases, one can see a prominent step decrease for numerosity >3.
Enumeration task
In the Enumeration task, participants were asked to identify, for every trial, the exact number of bars displayed on the screen (see Section “Enumeration task” for details). The number of bars ranged from one to eight; there were thus eight possible answers. Online-only Table 7 provides the individual scores for every run and the average across runs, grouped by numerosity of the visual stimuli (measured by the number of bars). Following the overall trend of the behavioral results reported in the original study (Fig. 2 - plot D of31), the scores decrease substantially for numerosity >4 (see Online-only Table 8).
Online-only Table 7.
Response accuracy (%) of behavioral performance per numerosity for the Enumeration task.
| Number of trials per run = 192 / Chance level = 12.5% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Numerosity | Run id | sub-01 | sub-04 | sub-05 | sub-06 | sub-07 | sub-09 | sub-11 | sub-12 | sub-13 | sub-14 | sub-15 |
| All numerosities | 0 | 72 | 67 | 49 | 58 | 69 | 69 | 58 | 69 | 65 | 34 | 54 |
| 1 | 70 | 71 | 47 | 69 | 73 | 69 | 51 | 66 | 64 | 48 | 50 | |
| Mean | 71 | 69 | 48 | 64 | 71 | 69 | 54 | 68 | 64 | 41 | 52 | |
| 1 | 0 | 100 | 77 | 93 | 89 | 100 | 93 | 100 | 86 | 100 | 82 | 75 |
| 1 | 92 | 100 | 60 | 100 | 92 | 70 | 100 | 100 | 100 | 82 | 83 | |
| Mean | 96 | 88 | 76 | 94 | 96 | 82 | 100 | 93 | 100 | 82 | 79 | |
| 2 | 0 | 100 | 93 | 83 | 80 | 100 | 73 | 92 | 85 | 90 | 80 | 88 |
| 1 | 100 | 85 | 92 | 100 | 94 | 78 | 94 | 100 | 100 | 56 | 100 | |
| Mean | 100 | 89 | 88 | 90 | 97 | 76 | 93 | 92 | 95 | 68 | 94 | |
| 3 | 0 | 100 | 78 | 100 | 69 | 83 | 100 | 69 | 92 | 87 | 25 | 77 |
| 1 | 100 | 100 | 64 | 73 | 100 | 100 | 42 | 91 | 89 | 80 | 64 | |
| Mean | 100 | 89 | 82 | 71 | 92 | 100 | 56 | 92 | 88 | 52 | 70 | |
| 4 | 0 | 100 | 87 | 33 | 47 | 62 | 90 | 0 | 92 | 89 | 18 | 40 |
| 1 | 92 | 89 | 33 | 67 | 88 | 79 | 0 | 83 | 100 | 36 | 64 | |
| Mean | 96 | 88 | 33 | 57 | 75 | 84 | 0 | 88 | 94 | 27 | 52 | |
| 5 | 0 | 17 | 27 | 18 | 23 | 58 | 64 | 45 | 36 | 0 | 18 | 14 |
| 1 | 42 | 47 | 31 | 45 | 58 | 80 | 20 | 38 | 17 | 33 | 30 | |
| Mean | 30 | 37 | 24 | 34 | 58 | 72 | 32 | 37 | 8 | 26 | 22 | |
| 6 | 0 | 18 | 31 | 8 | 29 | 54 | 18 | 60 | 45 | 44 | 0 | 11 |
| 1 | 23 | 62 | 17 | 20 | 64 | 38 | 44 | 62 | 20 | 27 | 33 | |
| Mean | 20 | 46 | 12 | 24 | 59 | 28 | 52 | 54 | 32 | 14 | 22 | |
| 7 | 0 | 50 | 58 | 13 | 80 | 71 | 55 | 55 | 50 | 69 | 12 | 36 |
| 1 | 36 | 60 | 22 | 89 | 60 | 69 | 67 | 36 | 73 | 36 | 8 | |
| Mean | 43 | 59 | 18 | 84 | 66 | 62 | 61 | 43 | 71 | 24 | 22 | |
| 8 | 0 | 67 | 78 | 50 | 71 | 30 | 44 | 38 | 50 | 36 | 50 | 73 |
| 1 | 93 | 56 | 50 | 47 | 29 | 47 | 33 | 33 | 10 | 47 | 38 | |
| Mean | 80 | 67 | 50 | 59 | 30 | 46 | 36 | 42 | 23 | 48 | 56 | |
The number of bars presented in the visual stimuli ranged from 1 to 8. For each level of numerosity, scores in every run are related to the trials referring to visual stimuli matching the specified numerosity. The mean of the scores across runs is also provided for every subject and numerosity.
Online-only Table 8.
Group-level scores (%) of behavioral performance per numerosity for the Enumeration task.
| Numerosity | Mean | SD |
|---|---|---|
| All numerosities | 61 | 10 |
| 1 | 90 | 8 |
| 2 | 89 | 9 |
| 3 | 81 | 16 |
| 4 | 63 | 30 |
| 5 | 35 | 17 |
| 6 | 33 | 16 |
| 7 | 50 | 21 |
| 8 | 49 | 16 |
The number of bars presented in the visual stimuli ranged from 1 to 8. For each level of numerosity, the mean of the scores across subjects is provided along with its standard deviation. Although there is a successive decrease of the scores as numerosity increases, one can see a prominent step decrease for numerosity >4.
Self task – recognition phase
The Self-task paradigm comprised two different phases: the encoding and recognition phases (see Section “Self task” for details). Both of them pertained to overt responses, although only the recognition phase required correct answers. In this particular phase, participants were to judge whether the adjective under display had already been presented in the previous encoding phase. Online-only Table 9 provides the individual scores for every run and the average across runs. The average ± SD across participants are 83 ± 8%, i.e. higher than chance level (50%), showing that participants successfully recognized either familiar or new adjectives in the majority of the “recognition” trials. Despite some low behavioral scores registered (particularly in run 3 for participant 1 and runs 0 and 1 for participant 5), we have only included trials with active and correct responses in the regressors-of-interest and, thus, neuroimaging results are not impacted by spurious effects potentially derived from occasional poor performances.
Online-only Table 9.
Response accuracy (%) of behavioral performance for the recognition phase of the Self task.
| Chance level = 50% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Run id | No. of trials* | sub-01 | sub-04 | sub-05 | sub-06 | sub-07 | sub-09 | sub-11 | sub-12 | sub-13 | sub-14 | sub-15 |
| 0 | 72 | 100 | 91 | 47 | 91 | 91 | 94 | 87 | 86 | 81 | 87 | 90 |
| 1 | 72 | 90 | 84 | 50 | 84 | 91 | 90 | 88 | 90 | 72 | 73 | 84 |
| 2 | 72 | 94 | 87 | 81 | 90 | 90 | 86 | 81 | 84 | 76 | 80 | 83 |
| 3 | 96 | 2 | 90 | 88 | 79 | 93 | 98 | 82 | 82 | 76 | 81 | 94 |
| Mean | — | 72 | 89 | 67 | 86 | 92 | 92 | 85 | 86 | 77 | 81 | 88 |
The mean of the scores for the four runs is also provided for every subject. The average ± SD across participants are 83 ± 8%.
*“No. of trials” refer (in this table) to the number of trials only for the recognition phase in the specified run and, thus, not to the total number of trials in the run. Because run #3 was longer than the remainder ones, the number of trials for the recognition phase was therefore greater. Concretely, this run included four blocks for the recognition phase with twenty four trials each, whereas all others comprised three blocks.
Imaging Data
Data quality
In order to provide an approximate estimate of data quality, measurements of the preprocessed data are presented in Fig. 1 and described as follows:
The temporal SNR (tSNR), defined as the mean of each voxels’ time course divided by their standard deviation, on normalized and unsmoothed data averaged across all acquisitions. Its values go up to 70 in the cortex. Given the high resolution of the data (1.5 mm isotropic), such values are indicative of a good image quality55;
The histogram of the six rigid body motion estimates of the brain per scan, in mm and degrees (for translational and rotational motion, respectively), together with their 99% coverage interval. One can notice that this interval ranges approximately within [−1.1, 1.5] mm as well as degrees, showing that motion excursions beyond 1.5 mm or degrees are rare. No acquisition was discarded due to excessive motion (>2 mm or degrees).
We observe an overall improvement of the tSNR with respect to the first release (see Section “Data quality” and Fig. 1 in19) in cortical regions, wherein functional activity in response to behavior is greatly covered (see Fig. 3). However, a poor coverage was attained for the bottom of the cerebellum (for more details about what sessions contributed specifically for this tSNR deviation, consult Online-only Table 2). Yet, as shown by Fig. 2, this issue is not driven by subject, condition or phase-encoding direction effects. It refers instead to a transient irregularity that affected the field-of-view during the acquisition period.
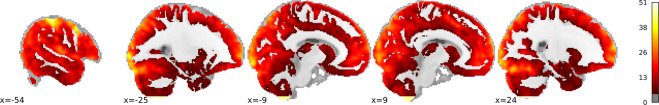
Fig. 3.
Brain coverage of the IBC-dataset second-release. Group-level F-map, at a threshold of p < 0.05, Bonferroni-corrected, representing the total area of the brain significantly covered by the tasks featuring solely the second release of the IBC dataset (FFX across tasks and subjects). One can easily observe an extensive brain coverage, with higher effects in lateral cortical areas by comparison with medial cortical areas and sub-cortical areas. We note that these results strictly follow the gray-matter structure, as an anatomically-defined gray-matter mask was used in the first-level GLM model (see Section “Model Estimation”).
Relevance of the IBC dataset for brain mapping
Effect of subject identity and task stimuli on activation
Taking into account the output of the GLM analysis for each acquisition, an assessment was performed at every voxel concerning how much variability of the signal could be explained by the effect of: (1) subject identity, (2) condition, and (3) phase-encoding direction. To assess the impact of these three factors, a one-way Analysis-of-Variance (ANOVA) of all contrast maps –1782 maps from the 11 subjects– was computed and results from the first-level analysis of the data were obtained for the aforementioned factors. The resulting statistical maps are displayed on the top of the Fig. 2. They show that both subject and condition effects are uniformly significant at p < 0.05, corrected for multiple comparisons using False Discovery Rate (FDR). Condition effects are overall higher than subject effects, particularly in sensory cortices like visual, auditory and somato-sensory regions. Effects pertaining to the phase-encoding direction are only significant in smaller areas comprising superior cortical regions, with special emphasis in the occipital lobe. We hypothesize that such results derive from the fact that PA data accounts for a larger amount than AP data in this dataset (see Table 2).
Similarity of brain activation patterns fits between-task similarity
Within-subject correlation matrices of all FFX contrast-maps pertaining to experimental conditions vs. baseline were computed as means to summarize the similarity between the functional responses to these conditions. The average of the correlation matrices across subjects was then estimated in order to assess the pattern of similarity between tasks. Because this second release accounts for a total of 49 conditions (and, consequently, 49 elementary contrasts) among all tasks, the average of correlation matrices from all participants is represented as a 49 × 49 correlation matrix (see Fig. 2, bottom-left). Note that this approach is different from performing a second-level analysis across subjects per task and compute the corresponding correlation matrix between tasks. Besides, experimental conditions were also encoded according to the Cognitive Atlas’ ontology (https://www.cognitiveatlas.org, see also21 for the link between each condition and cognitive labels). This labeling is listed in detail on Online-only Table 10, accounting for a total of 59 different cognitive components shared by the elementary contrasts. (Note that the number of cognitive components for the total amount of contrasts is 63 and, thus, slightly higher than the amount reported for the elementary contrasts only.) The 49 × 49 correlation matrix of the conditions defined in terms of occurrences of these cognitive descriptions was computed, since such labeling offers an approximate characterization of the tasks (see Fig. 2, bottom-right). They show clear similarities, together with discrepancies that are worthy of further investigation.
Online-only Table 10.
Cognitive labels associated with the model features, involved in the main contrasts, implemented for each task of the second-dataset release.
| Task | Description of the Condition§ | Cognitive Labels |
|---|---|---|
| MTT battery* | all references in we/sn island | auditory perception |
| memory retrieval | ||
| spatial localization | ||
| cardinal-direction judgment | ||
| temporal cognition | ||
| space cue on all references in we/sn island | auditory perception | |
| semantic categorization | ||
| cardinal orientation | ||
| time cue on all references in we/sn island | auditory perception | |
| semantic categorization | ||
| time orientation | ||
| westside/southside events | auditory perception | |
| spatial localization | ||
| west/south cardinal-direction judgment | ||
| eastside/northside events | auditory perception | |
| spatial localization | ||
| east/north cardinal-direction judgment | ||
| we/sn past events | auditory perception | |
| temporal cognition | ||
| past time | ||
| we/sn future events | auditory perception | |
| temporal cognition | ||
| future time | ||
| responses to events in we/sn island | response selection | |
| response execution | ||
| Preference Food | food evaluation | food cue reactivity |
| judgment | ||
| food preference | food cue reactivity | |
| reward valuation | ||
| incentive salience | ||
| confidence in food preference | food cue reactivity | |
| confidence judgment | ||
| Preference Painting | painting evaluation | visual form discrimination |
| visual color discrimination | ||
| judgment | ||
| painting preference | visual form discrimination | |
| visual color discrimination | ||
| reward valuation | ||
| incentive salience | ||
| confidence in painting preference | visual form discrimination | |
| visual color discrimination | ||
| confidence judgment | ||
| Preference Face | face evaluation | visual face recognition |
| face perception | ||
| facial attractiveness recognition | ||
| judgment | ||
| face preference | visual face recognition | |
| face perception | ||
| facial attractiveness recognition | ||
| reward valuation | ||
| incentive salience | ||
| confidence in face preference | visual face recognition | |
| face perception | ||
| facial attractiveness recognition | ||
| confidence judgment | ||
| Preference House | house evaluation | visual place recognition |
| judgment | ||
| house preference | visual place recognition | |
| reward valuation | ||
| incentive salience | ||
| confidence in house preference | visual place recognition | |
| confidence judgment | ||
| TOM localizer | fact judgments | semantic processing |
| reading | ||
| response execution | ||
| response selection | ||
| reasoning | ||
| theory-of-mind | ||
| belief judgments | semantic processing | |
| reading | ||
| response execution | ||
| response selection | ||
| reasoning | ||
| EP localizer | emotional-pain story | reading |
| response execution | ||
| response selection | ||
| empathy | ||
| imagined emotional pain | ||
| theory-of-mind | ||
| physical-pain story | reading | |
| response execution | ||
| response selection | ||
| empathy | ||
| imagined physical pain | ||
| MOV localizer | mental movie | visual attention |
| empathy | ||
| imagined emotional pain | ||
| theory-of-mind | ||
| physical-pain movie | visual attention | |
| empathy | ||
| imagined physical pain | ||
| VSTM | vstm response to constant numerosity | visual orientation |
| visual form discrimination | ||
| shape recognition | ||
| short-term memory | ||
| response selection | ||
| response execution | ||
| vstm response to numerosity | visual working memory | |
| numerosity | ||
| vstm response to numerosity interaction | visual buffer | |
| numerosity | ||
| Enumeration | enumeration response to constant numerosity | enumeration |
| numerosity | ||
| shape recognition | ||
| response selection | ||
| response execution | ||
| enumeration response to numerosity | visual working memory | |
| numerosity | ||
| enumeration response to numerosity interaction | visual buffer | |
| numerosity | ||
| Self | instructions | reading |
| self-reference encoding | judgment | |
| reading | ||
| response selection | ||
| response execution | ||
| self-reference effect | ||
| other-reference encoding | judgment | |
| reading | ||
| response selection | ||
| response execution | ||
| self-reference recognition | recognition | |
| reading | ||
| response selection | ||
| response execution | ||
| self-reference effect | ||
| other-reference recognition | recognition | |
| reading | ||
| response selection | ||
| response execution | ||
| other-reference effect | ||
| correct rejection | memory | |
| reading | ||
| response selection | ||
| response execution | ||
| no recognition | response selection | |
| response execution | ||
| Bang | speech | speech perception |
| speech processing | ||
| language comprehension | ||
| language processing | ||
| action preception | ||
| audiovisual perception | ||
| auditory scene analysis | ||
| social cognition | ||
| no speech | action perception | |
| audiovisual perception | ||
| auditory scene analysis | ||
| social cognition |
Tags were obtained from the Cognitive Atlas: https://www.cognitiveatlas.org. These labels provide an approximate description of the underlying cognitive components that are involved in the performance of the tasks.
*“MTT battery” comprises both MTT WE and MTT SN tasks. Conditions and their cognitive labels were defined in the same way for both of them.
In order to assess the feasibility of our cognitive descriptions, we then tested how similar the cross-condition characterizations using the cognitive labels and the activation maps are from each other. A Representational Similarity Analysis56 was performed between the activation-map and the cognitive-occurrence correlation matrices by means of the Spearman correlation between their upper-triangular coefficients, which amounted to 0.21 with . We then repeated this analysis for tasks of the first and second releases all together. They both combine 109 elementary conditions with 106 cognitive components. The Spearman correlation between the two different matrices is, in this case, 0.23 with , which is not only higher than the correlation obtained from the tasks of the second release but also from those of the first release (Spearman correlation: 0.21, with )19. We conclude that similarity tends to increase as more data is included in the dataset because new cognitive elements are added to the description of all tasks.
Brain coverage
Figure 3 displays all brain areas significantly covered by the tasks of this dataset extension. Most cortical and sub-cortical regions are altogether represented by this group of tasks. By comparison with the first release, the present one shows a prominent improvement of significant coverage in the cingulate cortex, namely its posterior section in the parietal lobe and its anterior section in the prefrontal lobe. Similarly, a better coverage of the right anterior temporal lobe can also be observed in this release.
On the other hand, a prominent deviation of the results at the bottom of the cerebellum in comparison with its neighboring regions is present due to a lesser coverage of this area, as reported in Section “Data quality” and Fig. 1.
As the dataset becomes larger, one may expect a progressively improvement of brain coverage. However, as referred already in19, this coverage may not be fully attained due to MR-related technical restrictions. Some locations are particularly sensitive to e.g. coil sensitivity or intra-voxel dephasing, which can result in a reduced tSNR in certain functional brain regions.
Usage Notes
The IBC project keeps promoting the open access and encouraging the community in adhering to practices concerned with data sharing and reproducibility in neuroimaging. Thus, free online access of raw data and derivatives is respectively assured by OpenNeuro50 and NeuroVault53.
This second release, along with the first one, brings together a variety of tasks –featuring in total 221 independent contrasts– allowing at the same time for an increase of the brain area significantly covered by these tasks (see Section “Brain Coverage” for more details).
The collection of new data continues till year 2022 and more releases are expected in the next years. The third release is already planned for the present year and it will be fully dedicated to the visual system; it will contain tasks pertaining to retinotopy, movie watching and viewing of naturalistic scenes. Future releases will also address in greater depth the auditory system through tonotopy as well as tasks on auditory language comprehension, listening of naturalistic sounds and music perception. Other tasks on biological motion, stimulus salience, working memory, motor inhibition, risk-based decision making and spatial navigation will also integrate these future releases. Finally, although IBC stands foremost as a task-fMRI dataset with a strong emphasis in the task dimension, future releases will be also dedicated to resting-state fMRI data as well as to other MRI modalities, concretely high-resolution T1- and T2- weighted, diffusion-weighted, T1- and T2- relaxometry and myelin water fraction.
The official website of the IBC project (https://project.inria.fr/IBC/) can be consulted anytime for a continuous update about the latest and forthcoming releases.
Acknowledgements
We thank Rebecca Saxe and colleagues for making publicly available the TOM and Pain Matrices task battery and for the availability to further clarify their implementation, designs and analyses. In particular, we would like to thank Hilary Richardson for the assistance regarding the implementation of the Pain Movie localizer and extraction of its paradigm descriptors for data analysis. We also thank Karen L. Campbell and Lorraine K. Tyler for having kindly provided the video and descriptors of the “Bang” task, which were respectively necessary for a successful re-implementation of the protocol and analysis of data. We thank the Center for Magnetic Resonance Research, University of Minnesota for having kindly provided the Multi-Band Accelerated EPI Pulse Sequence and Reconstruction Algorithms. We are grateful to Kamalaker Dadi, Loubna El Gueddari and Darya Chyzhyk for their assistance in some MRI acquisitions as well as Isabelle Denghien for the advisory and technical support in setting the task protocols. At last, we are especially thankful to all volunteers who have accepted to be part of this challenging study, with many repeated MRI scans over a long period of time. This project has received funding from the European Union’s Horizon 2020 Framework Program for Research and Innovation under Grant Agreement No 720270 (Human Brain Project SGA1) and 785907 (Human Brain Project SGA2).
Online-only Tables
Author contributions
A.L.P. set the task protocols, designed the MTT task battery and wrote the corresponding protocol, rewrote the protocols of the Self and “Bang” tasks, performed the MRI acquisitions, performed the analysis of the neuroimaging data, post-processed the behavioral data, produced video annotations, contributed to Nistats, wrote the documentation and wrote the paper. A.A. set the MRI sequences. B.G. designed the MTT task battery. N.C. designed the Preference task battery. A.K. designed the VSTM and Enumeration tasks. S.G. designed the Self task. E.D. developed Pypreprocess. J.J.T. performed some MRI acquisitions, set the video file for the “Bang” task, produced video annotations and wrote the documentation. C.G., S.B.-D., S.R.,Y.L. and V.B. performed the MRI acquisitions plus visual inspection of the neuroimaging data for quality-checking. L.L., V.J.-T. and G.M.-C. recruited the participants and managed routines related to appointment scheduling and ongoing medical assessment. C.D. recruited the participants and managed the scientific communication of the project with them. B.M. managed regulatory issues. E.S. designed the Self task. M.Pi. and D.M. designed the VSTM and Enumeration tasks. M.Pe. designed the Preference task battery. V.v-W. advised on the design of the MTT task battery. E.E. advised on the study design. G.V. developed Nilearn and advised on the study design plus analysis pipeline. S.D. conceived the general design of the study. L.H.-P. conceived the general design of the study and wrote the ethical protocols. B.T. conceived the general design of the study, managed the project, wrote the ethical protocols, performed some MRI acquisitions, performed the analysis of the neuroimaging data, developed Nistats + Nilearn and wrote the paper.
Code availability
Metadata concerning the stimuli presented during the BOLD fMRI runs are publicly available at https://github.com/hbp-brain-charting/public_protocols. They include: (1) the task-stimuli protocols; (2) demo presentations of the tasks as video annotations; (3) instructions to the participants; and (4) scripts to extract paradigm descriptors from log files for the GLM estimation.
Task-stimuli protocols from Preference, TOM and VSTM + Enumeration batteries were adapted from the original studies in order to comply with the IBC experimental settings, without affecting the design of the original paradigms. MTT battery pertains to an original protocol developed in Python under the context of the IBC project. Protocols of Self and Bang tasks were re-written from scratch in Python with no change of the design referring to the original paradigms.
The scripts used for data analysis are available on GitHub under the Simplified BSD license:
https://github.com/hbp-brain-charting/public_analysis_code. Additionally, a full description of all contrasts featuring data derivatives (see Section “Derived statistical maps” for details) as well as a list of the main contrasts are also provided under the folder hbp-brain-charting/public_analysis_code/ibc_data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wager TD, Lindquist M, Kaplan L. Meta-analysis of functional neuroimaging data: current and future directions. Soc Cogn Affect Neurosci. 2007;2:150–158. doi: 10.1093/scan/nsm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costafreda S. Meta-Analysis, Mega-Analysis, and Task Analysis in fMRI Research. Philosophy, Psychiatry, & Psychology. 2011;18:275–277. doi: 10.1353/ppp.2011.0049. [DOI] [Google Scholar]
- 3.Schwartz, Y. et al. Improving Accuracy and Power with Transfer Learning Using a Meta-analytic Database. In Ayache, N., Delingette, H., Golland, P. & Mori, K. (eds.) Med Image Comput Comput Assist Interv. 2012 (Springer, Berlin, Heidelberg), 15, 248–255. 10.1007/978-3-642-33454-2_31 (2012). [DOI] [PubMed]
- 4.Schwartz, Y., Thirion, B. & Varoquaux, G. Mapping cognitive ontologies to and from the brain. In NIPS’13: Proceedings of the 26th International Conference on Neural Information Processing Systems (Curran Associates Inc., Red Hook, NY, USA), 2, 1673–1681. https://arxiv.org/abs/1311.3859 (2013).
- 5.Varoquaux, G., Schwartz, Y., Pinel, P. & Thirion, B. Cohort-Level Brain Mapping: Learning Cognitive Atoms to Single Out Specialized Regions. In Gee, J. C., Joshi, S., Pohl, K. M., Wells, W. M. & Zöllei, L. (eds.) Inf Process Med Imaging (Springer, Berlin, Heidelberg), 23, 438–449. 10.1007/978-3-642-38868-2_37 (2013). [DOI] [PubMed]
- 6.Wager TD, et al. An fMRI-Based Neurologic Signature of Physical Pain. N Engl J Med. 2013;368:1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurevitch J, Koricheva J, Nakagawa S, Stewart G. Meta-analysis and the science of research synthesis. Nature. 2018;555:175–182. doi: 10.1038/nature25753. [DOI] [PubMed] [Google Scholar]
- 8.Müller VI, et al. Ten simple rules for neuroimaging meta-analysis. Neurosci Biobehav Rev. 2018;84:151–161. doi: 10.1016/j.neubiorev.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varoquaux, G. et al. Atlases of cognition with large-scale brain mapping. PLoS Comput Biol14. 10.1371/journal.pcbi.1006565 (2018). [DOI] [PMC free article] [PubMed]
- 10.Barch DM, et al. Function in the human connectome: Task-fMRI and individual differences in behavior. Neuroimage. 2013;80:169–89. doi: 10.1016/j.neuroimage.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glasser MF, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536:171–178. doi: 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinel P, et al. Fast reproducible identification and large-scale databasing of individual functional cognitive networks. BMC Neurosci. 2007;8:91. doi: 10.1186/1471-2202-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinel P, et al. The functional database of the ARCHI project: Potential and perspectives. Neuroimage. 2019;197:527–543. doi: 10.1016/j.neuroimage.2019.04.056. [DOI] [PubMed] [Google Scholar]
- 14.Hanke, M. et al. A high-resolution 7-Tesla fMRI dataset from complex natural stimulation with an audio movie. Sci Data1. 10.1038/sdata.2014.3 (2014). [DOI] [PMC free article] [PubMed]
- 15.Hanke M, et al. High-resolution 7-tesla fmri data on the perception of musical genres–an extension to the studyforrest dataset. F1000Res. 2015;4:174. doi: 10.12688/f1000research.6679.1. [DOI] [Google Scholar]
- 16.Hanke, M. et al. A studyforrest extension, simultaneous fMRI and eye gaze recordings during prolonged natural stimulation. Sci Data3. 10.1038/sdata.2016.92 (2016). [DOI] [PMC free article] [PubMed]
- 17.Sengupta, A. et al. A studyforrest extension, retinotopic mapping and localization of higher visual areas. Sci Data3. 10.1038/sdata.2016.93 (2016). [DOI] [PMC free article] [PubMed]
- 18.Nee DE. fMRI replicability depends upon sufficient individual-level data. Commun Biol. 2019;2:1–4. doi: 10.1038/s42003-018-0073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinho AL, et al. Individual Brain Charting, a high-resolution fMRI dataset for cognitive mapping. Sci Data. 2018;5:180105. doi: 10.1038/sdata.2018.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humphries C, Binder JR, Medler DA, Liebenthal E. Syntactic and Semantic Modulation of Neural Activity During Auditory Sentence Comprehension. J Cogn Neurosci. 2006;18:665–679. doi: 10.1162/jocn.2006.18.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poldrack R, et al. The Cognitive Atlas: Toward a Knowledge Foundation for Cognitive Neuroscience. Front Neuroinform. 2011;5:17. doi: 10.3389/fninf.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorgolewski K, et al. The brain imaging data structure: a standard for organizing and describing outputs of neuroimaging experiments. Sci Data. 2016;3:160044. doi: 10.1038/sdata.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 24.Gauthier B, van Wassenhove V. Cognitive mapping in mental time travel and mental space navigation. Cognition. 2016;154:55–68. doi: 10.1016/j.cognition.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Gauthier B, van Wassenhove V. Time Is Not Space: Core Computations and Domain-Specific Networks for Mental Travels. J Neurosci. 2016;36:11891–11903. doi: 10.1523/JNEUROSCI.1400-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gauthier B, Pestke K, van Wassenhove V. Building the Arrow of Time. Over Time: A Sequence of Brain Activity Mapping Imagined Events in Time and Space. Cereb Cortex. 2018;29:4398–4414. doi: 10.1093/cercor/bhy320. [DOI] [PubMed] [Google Scholar]
- 27.Lebreton M, Abitbol R, Daunizeau J, Pessiglione M. Automatic integration of confidence in the brain valuation signal. Nat Neurosci. 2015;18:1159–67. doi: 10.1038/nn.4064. [DOI] [PubMed] [Google Scholar]
- 28.Dodell-Feder D, Koster-Hale J, Bedny M, Saxe R. fMRI item analysis in a theory of mind task. Neuroimage. 2011;55:705–712. doi: 10.1016/j.neuroimage.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 29.Jacoby N, Bruneau E, Koster-Hale J, Saxe R. Localizing Pain Matrix and Theory of Mind networks with both verbal and non-verbal stimuli. Neuroimage. 2016;126:39–48. doi: 10.1016/j.neuroimage.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richardson, H., Lisandrelli, G., Riobueno-Naylor, A. & Saxe, R. Development of the social brain from age three to twelve years. Nat Commun9. 10.1038/s41467-018-03399-2 (2018). [DOI] [PMC free article] [PubMed]
- 31.Knops A, Piazza M, Sengupta R, Eger E, Melcher D. A Shared, Flexible Neural Map Architecture Reflects Capacity Limits in Both Visual Short-Term Memory and Enumeration. J Neurosci. 2014;34:9857–9866. doi: 10.1523/JNEUROSCI.2758-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genon S, et al. Cognitive and neuroimaging evidence of impaired interaction between self and memory in Alzheimer’s disease. Cortex. 2014;51:11–24. doi: 10.1016/j.cortex.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Campbell KL, et al. Idiosyncratic responding during movie-watching predicted by age differences in attentional control. Neurobiol Aging. 2015;36:3045–3055. doi: 10.1016/j.neurobiolaging.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luck S, Vogel E. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- 35.Piazza M, Mechelli A, Butterworth B, Price CJ. Are Subitizing and Counting Implemented as Separate or Functionally Overlapping Processes? Neuroimage. 2002;15:435–446. doi: 10.1006/nimg.2001.0980. [DOI] [PubMed] [Google Scholar]
- 36.Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- 37.Newell, A. & Simon, H. A. Human problem solving. 1st edn. (Prentice-Hall, NJ, 1972).
- 38.Ericsson KA, Kintsch W. Long-term working memory. Psychol Rev. 1995;102:211–245. doi: 10.1037/0033-295x.102.2.211. [DOI] [PubMed] [Google Scholar]
- 39.Moeller S, et al. Multiband multislice GE-EPI at 7 Tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. 2010;63:1144–53. doi: 10.1002/mrm.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feinberg DA, et al. Multiplexed Echo Planar Imaging for Sub-Second Whole Brain fMRI and Fast Diffusion Imaging. PLoS One. 2010;5:1–11. doi: 10.1371/journal.pone.0015710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20:870–888. doi: 10.1016/S1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- 42.Smith S, et al. Advances in functional and structural {MR} image analysis and implementation as {FSL} Neuroimage. 2004;23(Supplement 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 43.Friston K, Frith C, Frackowiak R, Turner R. Characterizing Dynamic Brain Responses with fMRI: a Multivariate Approach. Neuroimage. 1995;2:166–172. doi: 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- 44.Ashburner J, Friston K. Multimodal Image Coregistration and Partitioning - A Unified Framework. Neuroimage. 1997;6:209–217. doi: 10.1006/nimg.1997.0290. [DOI] [PubMed] [Google Scholar]
- 45.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 46.Friston K, et al. Event-related fMRI: Characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- 47.Friston K, Josephs O, Rees G, Turner R. Nonlinear event-related responses in fMRI. Magn Reson Med. 1998;39:41–52. doi: 10.1002/mrm.1910390109. [DOI] [PubMed] [Google Scholar]
- 48.Abraham A, et al. Machine learning for neuroimaging with scikit-learn. Front Neuroinform. 2014;8:14. doi: 10.3389/fninf.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poldrack, R. et al. Toward open sharing of task-based fMRI data: the openfMRI project. Front Neuroinform 7, 12. 10.3389/fninf.2013.00012(2013). [DOI] [PMC free article] [PubMed]
- 50.Pinho AL, 2020. IBC. OpenNeuro. https://openneuro.org/datasets/ds002685/versions/1.0.0
- 51.Pinho AL, 2017. Individual Brain Charting. OpenNeuro. [DOI]
- 52.Gorgolewski K, et al. NeuroVault.org: a web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Front Neuroinform. 2015;9:8. doi: 10.3389/fninf.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pinho AL, 2020. IBC release 2. NeuroVault. 6618
- 54.Pinho AL, 2018. Individual Brain Charting (IBC): Activation maps per contrast, session and individual. NeuroVault. 4438
- 55.Murphy K, Bodurka J, Bandettini PA. How long to scan? the relationship between fMRI temporal signal to noise ratio and necessary scan duration. Neuroimage. 2007;34:565–574. doi: 10.1016/j.neuroimage.2006.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kriegeskorte, N., Mur, M. & Bandettini, P. Representational similarity analysis–connecting the branches of systems neuroscience. Front Syst Neurosci2. 10.3389/neuro.06.004.2008 (2008). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Pinho AL, 2020. IBC. OpenNeuro. https://openneuro.org/datasets/ds002685/versions/1.0.0
- Pinho AL, 2017. Individual Brain Charting. OpenNeuro. [DOI]
- Pinho AL, 2020. IBC release 2. NeuroVault. 6618
- Pinho AL, 2018. Individual Brain Charting (IBC): Activation maps per contrast, session and individual. NeuroVault. 4438
Data Availability Statement
Metadata concerning the stimuli presented during the BOLD fMRI runs are publicly available at https://github.com/hbp-brain-charting/public_protocols. They include: (1) the task-stimuli protocols; (2) demo presentations of the tasks as video annotations; (3) instructions to the participants; and (4) scripts to extract paradigm descriptors from log files for the GLM estimation.
Task-stimuli protocols from Preference, TOM and VSTM + Enumeration batteries were adapted from the original studies in order to comply with the IBC experimental settings, without affecting the design of the original paradigms. MTT battery pertains to an original protocol developed in Python under the context of the IBC project. Protocols of Self and Bang tasks were re-written from scratch in Python with no change of the design referring to the original paradigms.
The scripts used for data analysis are available on GitHub under the Simplified BSD license:
https://github.com/hbp-brain-charting/public_analysis_code. Additionally, a full description of all contrasts featuring data derivatives (see Section “Derived statistical maps” for details) as well as a list of the main contrasts are also provided under the folder hbp-brain-charting/public_analysis_code/ibc_data.