Abstract
Purpose
Traumatic brain injury (TBI) is one of the leading causes of disability and death in modern times, whose evaluation and prognosis prediction have been one of the most critical issues in TBI management. However, the existed models for the abovementioned purposes were defective to varying degrees. This study aims to establish an ideal brain injury state clinical prediction model (BISCPM).
Methods
This study was a retrospective design. The six-month outcomes of patients were selected as the end point event. BISCPM was established by using the split-sample technology, and externally validated via different tests of comparison between the observed and predicted six-month mortality in validating group. TBI patients admitted from July 2006 to June 2012 were recruited and randomly divided into establishing model group and validating model group. Twenty-one scoring indicators were included in BISCPM and divided into three parts, A, B, and C. Part A included movement, pupillary reflex and diameter, CT parameters, and secondary brain insult factors, etc. Part B was age and part C was medical history of the patients. The total score of part A, B and C was final score of BISCPM.
Results
Altogether 1156 TBI patients were included with 578 cases in each group. The score of BISCPM from validating group ranged from 2.75 to 31.94, averaging 13.64 ± 5.59. There was not statistical difference between observed and predicted mortality for validating group. The discrimination validation showed that the BISCPM is superior to international mission for prognosis and analysis of clinical trials (IMPACT) lab model.
Conclusion
BISCPM is an effective model for state evaluation and prognosis prediction of TBI patients. The use of BISCPM could be of great significance for decision-making in management of TBI.
Keywords: Traumatic brain injuries, Prognosis, International mission for prognosis and analysis of clinical trials
Introduction
Nowadays, traumatic brain injury (TBI) caused by traffic accidents, construction accidents and geological disasters becomes more and more frequent. It was reported that about 1.5 million people around the world died of TBI each year and there were much higher TBI incidence rates in developing countries.1 TBI is becoming a serious threat to human health. In 2014, a survey carried out in 47 Chinese hospitals involving 11,937 hospitalized TBI patients indicated that the mortality of patients with severe TBI was 27.23%, and the sum of mortality and severe disability was more than 53.17%.2
One of the most concerned issues by both physicians and patients is how to evaluate injury state and predict prognosis effectively in management of TBI. One survey in 2005 showed that about 80% of the visited doctors thought prognosis prediction is important for deciding whether to adopt excessive ventilation, sedation and mannitol treatment. However since there are a variety of factors affecting the outcome of patients with TBI, only about one third of the surveyed doctors thought it possible to reliably predict the prognosis.3
Many researchers have been trying to establish ideal models for injury state evaluation and prognosis prediction of TBI. Of them, the earlier widely accepted model is acute physiology and chronic health evaluation II (APACHE II), which was introduced in 1985 and adopted the worst values of 12 physiology index within 24 h after admission.4 Another model introduced from 2008 is international mission for prognosis and analysis of clinical trials (IMPACT), which has good prediction efficiency for poor six-month outcome by scoring the scale with instant value after admission. It is reported that more than 100 models have been established till now. However, one systematic review published in 2006 indicated that most of these models were of methodological defects and rarely applied in low-income countries.5
Many studies have showed that the factors including older age, lower Glasgow coma scale (GCS) score, poor pupillary response, and extra-cranial injury are all independent prognostic predictors for poor outcomes.6 Computed tomography (CT) signs, such as compression or disappearance of lateral ventricle and ambient cistern, traumatic subarachnoid hemorrhage (tSAH), etc., are also considered as prognostic predictors of TBI.7,8 Although most models included almost all the known predictors as mentioned above, they ignored the secondary brain insult factors (SBIF). In addition, the scores in many models like IMPACT or APACHE II were calculated with the values at admission or the worst time within 24 h after admission, which may lead to maladjustments for not considering the possible roles of medical management in the prognosis and the dynamic course of TBI. Therefore, we believe that the most qualified model should meet the following criteria: (1) including all the possible TBI prognostic predictors; and (2) considering the included predictors dynamically. Therefore we designed a retrospective study to establish a new brain injury state evaluation and prognosis prediction system (BISCPM), which may be beneficial to make a clinical decision, optimize the distribution of medical resources, and support the basic or clinical research.
Methods
Patients
Patients with TBI in 2006–2012 admitted to the neurosurgical intensive care unit (NICU) of Xijing Hospital affiliated to FMMU, the largest medical center in northwest China, were continuously recruited in the study. The including criteria were: (1) patients arrived at the hospital within 24 h after occurrence of TBI; (2) such brain CT signs as skull fracture, intracranial hematoma, cerebral edema, infarction, diffused axonal injury and tSAH, etc. If CT scan did not show any abnormalities, the patients must display one of the following symptoms such as loss of consciousness for more than 30 min, frequent seizures, or unilaterally or bilaterally decerebrate vegetative or decortical state. The excluding criteria were: (1) patients younger than 2 years old; (2) patients with bilateral mydriasis and absence of pupillary reflex; (3) patients or their immediate family members unwilling to accept any invasive treatment; (4) the duration from injury to admission being more than 24 h. All the included patients were randomly divided into two groups for establishing model (EM) and validating model (VM) (Fig. 1).
Fig. 1.
Schematic diagram of the study process.
Treatment and monitoring
The patients were surgically managed according to the expert consensus on the surgical treatment of TBI written by Chinese Neurosurgeons Association and Expert Committee of Neurotrauma.9 Non-surgical treatments were taken with reference to the guidelines for the management of severe TBI published by Brain Trauma Foundation.10 The monitoring after admission included: (1) physical examination such as consciousness, pupil diameter and reflex, and extremities movement, once per hour for patients with GCS score of eight or less, and once per 2–4 h for patients with GCS score of more than eight; (2) brain CT, at least once per day within 3 days after admission, and followed by immediate CT scan when the patients showed mydriasis or sharp elevated intracranial pressure (ICP) (CT parameters including hematoma volume, shift distance of midline and width of ambient cistern as well as whether there is tSAH determined by experienced radiologists and neurosurgeons together. The hematoma volume was calculated by Kothari RU's formula.)11; (3) arterial blood pressure (BP), heart rate (HR), blood oxygen, and body temperature being measured and recorded continuously by physiological monitors; (4) for patients with GCS score of 8 and less at admission, the invasive ICP monitoring (Codman & Shurtleff, Inc., Massachusetts, USA) applied post-operatively for at least 6 d; (5) cerebral perfusion pressure (CPP) being calculated and recorded; (6) serum sodium, potassium, creatinine and blood glucose (BG) being measured 4 to 6 times per day. All the data collected were transported to a computer with a special software from monitoring devices automatically or by human hand typing.
Follow-up
All the patients were followed up for at least six months after discharge via outpatient inquiry, telephone counseling, or questionnaires, and the outcomes were classified according to the Glasgow Outcome Scale (GOS).
Establishment and validation of BISCPM
BISCPM was established by using the split-sample technology. Firstly, all the possible TBI prognostic factors were included in the primary BISCPM, and then the final indicators and scoring method of BISCPM were obtained by using logistic regression with six-month mortality as prediction value. Subsequently, the BISCPM was validated by re-sample bootstrap technique, of which external calibration validation was performed via different test of comparison between the observed six-month and predicted six-month mortality calculated by BISCPM. In addition, the discrimination validation was taken via comparison of six-month mortality prediction between the impact lab model12 and BISCPM by the ROC analysis of area under the curve (AUC).
Statistical analysis
Statistical analysis was carried out by using SPSS 17.0 for Windows (SPSS, Inc., Chicago, USA) and p < 0.05 was considered as statistical significance. Classified data were presented as frequency or percentage, and continuous data were expressed as a mean ± standard deviation. Comparison between different groups was made by chi-square test for classified data, and by the Mann-Whitney U test or ttest for continuous data.13
Results
Clinical characters of patients
A total of 1156 patients with TBI were recruited, among which 765 were male, 391 female. The ages of patients ranged from 2 to 85 years with an average of (38.7 ± 4.8) years. The patients were randomly divided into EM and VM group with 578 cases in each group. The CT signs of patients at admission included brain stem injury, tSAH, epidural hematoma, subdural hematoma, cerebral hematoma, infarction, edema, diffused axonal injury, skull fracture, or mixture of several different types. The detailed distribution of patients’ age, gender, traumatic causes, GCS and CT signs at admission were shown in Table 1. Six-month mortality in EM group was 25.3%, of which 47.5% died within 14 days after injury, and the incidence of adverse outcomes including dead and persistent vegetative state was 29.1%. Six-month mortality in VM group was 23.9%, of which 45.5% died within 14 days after injury, and the incidence of adverse outcomes was 27.4%.
Table 1.
Population characteristics in both groups.
| Variables | Establishing model group (n = 578) | Validating model group (n = 578) | p valuea |
|---|---|---|---|
| Age (years) | 0.873 | ||
| ≤10 | 40 | 38 | |
| 11-20 | 41 | 39 | |
| 21-30 | 77 | 79 | |
| 31-40 | 65 | 70 | |
| 41-50 | 113 | 118 | |
| 51-60 | 112 | 104 | |
| 61-70 | 82 | 88 | |
| 71-80 | 36 | 37 | |
| >80 | 12 | 5 | |
| Gender | 0.852 | ||
| Males | 381 | 384 | |
| Female | 197 | 194 | |
| GCS score at admission | 0.446 | ||
| 3-8 | 440 | 428 | |
| 9-12 | 52 | 65 | |
| 13-15 | 86 | 85 | |
| Causes of TBI | 0.586 | ||
| Traffic accident | 316 | 330 | |
| Falling | 58 | 48 | |
| Beated by hard object | 131 | 119 | |
| Crushing | 25 | 27 | |
| Stabbing | 18 | 16 | |
| Injury from geological disasters | 5 | 5 | |
| Others | 25 | 38 | |
| CT signs | 0.649 | ||
| Brain stem injury | 21 | 17 | |
| tSAH | 18 | 22 | |
| Diffused axonal injury | 16 | 18 | |
| Cerebral infarction | 12 | 9 | |
| Skull fracture | 56 | 49 | |
| Intracranial hematoma | 238 | 219 | |
| Mixture | 217 | 244 |
GCS: Glasgow coma scale, TBI: traumatic brain injury, CT: computed tomography, tSAH: traumatic subarachnoid hemorrhage.
All p values were calculated with Chi-square test.
Establishment and validation of BISCPM
All indicators definitely included in the BISCPM were validated as significant variables by the univariate analysis followed by multivariate regression analysis, and goodness of fit for BISCPM was tested by Hosmer-Lemeshow test (p = 0.112). The BISCPM was described as follows.
-
(1)
Framework and indicators: the entire BISCPM includes part A, B and C (Table 2), and part A is subsequently divided into A1 and A2. The indicators of part A1 include movement (A1-1), pupillary light reflex (A1-2), pupil diameter (A1-3), and four CT parameters including hematoma volume (A1-4), shift distance of midline (A1-5), width of ambient cistern (A1-6) and whether there is tSAH (A1-7). The part A2 is composed of 13 SBIF, including high ICP (A2-1), low BP (A2-2), hypertension (A2-3), low CPP (A2-4), high fever (A2-5), hyperglycemia (A2-6), tachycardia (A2-7), hypoxemia (A2-8), hypernatremia (A2-9), hyponatremia (A2-10), hyperkalemia (A2-11), hypokalemia (A2-12), and high serum creatinine (A2-13). Part B and C are age and medical history of patient respectively.
-
(2)Scoring of every indicator and the total of BISCPM (Table 2): The worst value of 24 h after admission is used to score the indicator of part A1. The scoring of part A2 indicator was obtained by extracting square root of the sum of indicator score from Tab 2 multiplying by time score. The time score was acquired based on the duration of every indicator within 24 h after admission as follows: 1 point for equal or less than 6 h, 2 points for between 6 and 12 h, 3 points for between 12 and 18 h, and 4 points for between 18 and 24 h. Therefore, the score of every indicator of part A2 is calculated by the following formula:
For example, there is a patient X with ICP value of 17.6 mm Hg for 8 h within 24 h after admission, 21.8 mm Hg for 4 h, and 14.2 mm Hg for 12 h, so that the calculation of A2-1 score for patient X is:
At last, the total score of BISCPM is the sum of scores of part A, B and C, while the score of part A is the sum of score of part A1 and A2. Accordingly, the score of part A1 is the sum from A1-1 to A1-7, and score of part A2 is the sum from A2-1 to A2-13.
Table 2.
Indicators and scores of BISCPM.
| Indicators | Scores (points) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||||||
| A | A1 | A1-1 | Movement | Obeys | Localized pain | Withdrawal | Abnormal flexion | None | ||
| A1-2 | Pupillary light reflex | Bilateral normal | Unilateral reflex | Bilateral abnormal | ||||||
| A1-3 | Pupil diameter | Bilateral normal | Unilateral myosis | Bilateral myosis | Unilateral mydriasis | Bilateral mydriasis | ||||
| A1-4 | Hematoma volume (mL) | ≤30 | >30 | >60 | >90 | >120 | ||||
| A1-5 | Midline shift distance (cm) | ≤0.5 | >0.5 | >1.0 | >1.5 | >2.0 | ||||
| A1-6 | Ambient cistern width (mm) | >3 | ≤3 | ≤1 | ||||||
| A1-7 | traumatic subarachnoid hemorrhage | None | Yes | |||||||
| A2 | A2-1 | High ICP (mmHg) | <15 | ≥15 | ≥18 | ≥22 | ≥25 | |||
| A2-2 | Low BP (mmHg) | >70 and < 110 | ≤70 | ≤55 | ≤40 | ≤25 | ||||
| A2-3 | Hypertension (mmHg) | >70 and < 110 | ≥110 | ≥130 | ≥150 | ≥170 | ||||
| A2-4 | Low CPP (mmHg) | >60 | ≤60 | ≤50 | ≤40 | ≤30 | ||||
| A2-5 | High fever (°C) | <38 | ≥38 | ≥39 | ≥40 | ≥41 | ||||
| A2-6 | Hyperglycemia (mmol/L) | <7.0 | ≥7.0 | ≥12.0 | ≥17 | ≥22 | ||||
| A2-7 | Tachycardia (bpm) | <120 | ≥120 | ≥130 | ≥140 | ≥150 | ||||
| A2-8 | Hypoxemia (kPa) | >8.0 | ≤8.0 | ≤7.0 | ≤6.0 | ≤5.0 | ||||
| A2-9 | Hypernatremia (mmol/L) | >130 and < 150 | ≥150 | ≥160 | ≥170 | ≥180 | ||||
| A2-10 | Hyponatremia (mmol/L) | >130 and < 150 | ≤130 | ≤120 | ≤110 | ≤100 | ||||
| A2-11 | Hyperkalemia (mmol/L) | >3.5 and < 5.5 | ≥5.5 | ≥6.5 | ≥7.5 | ≥8.5 | ||||
| A2-12 | Hypokalemia (mmol/L) | >3.5 and < 5.5 | ≤3.5 | ≤2.5 | ≤1.5 | ≤0.5 | ||||
| A2-13 | HSCa (mg/100 mL) | <1.5 | ≥1.5 | ≥2.5 | ≥3.5 | ≥4.5 | ||||
| B | Age (years) | 1 | 2 | 3 | 5 | 7 | 9 | |||
| ≤30 | 31–40 | 41–50 | 51–60 | 61–70 | >70 | |||||
| C | Medical history | 0 | 3 | |||||||
| None | Chronic diseaseb | |||||||||
☆The total score is the sum of score of part A, B and C.
HSC: high serum creatinine.
The chronic diseases include: Cardio vascular system: New York Heart Association Class Ⅳ. Liver: biopsy proven cirrhosis and documented portal hypertension; prior episodes of hepatic failure, encephalopathy, or coma. Respiratory system: chronic restrictive, obstructive, or vascular disease resulting in severe exercise restriction; or documented chronic hypoxia, hypercapnia, secondary polycythemia, severe pulmonary hypertension (>40 mmHg), or respiratory dependency. Kidney: receiving chronic dialysis. Immuno-compromised: The patient has received therapy that suppresses resistance to infection, e.g., immune-suppression, chemotherapy, radiation, long term or recent high dose steroids, or has a disease that is sufficiently advanced to suppress resistance to infection, e.g., leukemia, lymphoma, AIDS.
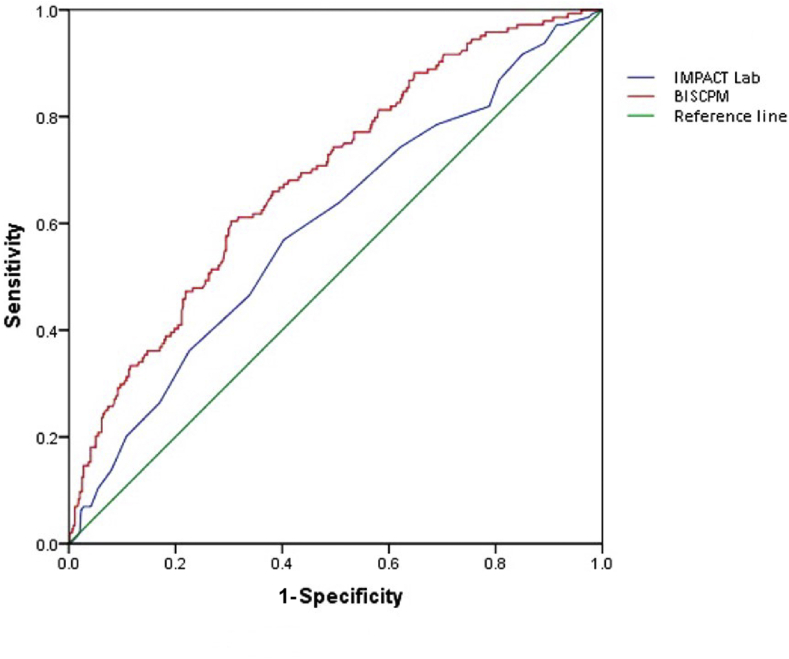
The possible minimum and maximum of BISCPM are 1 and 92. In the VM group, the total scores of BISCPM ranged from 2.75 to 31.94 with an average score of 13.64 ± 5.59 (Fig. 2). The formula to predict the individual six-month death risk (DR) by BISCPM is: ln (DR/1-DR) = 2.912 + (BISCPM∗ 0.125) (C = 0.85). The statistical analysis showed that the predicted six-month mortality in VM group was 21.8%, the observed mortality rate 23.9%, and there was not significant difference between these two mortalities (χ2 = 0.707, p = 0.40). ROC analysis via AUC method showed that the prediction discrimination of six-month mortality by BISCPM was superior to that by the IMPACT Lab model (0.788 vs. 0.694, p = 0.04) (Fig. 3), which also indicated a good discrimination of BISCPM.
Fig. 2.
The distribution of total brain injury state clinical prediction model (BISCPM) scores in validating model group.
Fig. 3.
Comparison of prediction discrimination of six-month mortality between BISCPM and IMPACT Lab. The AUCs are compared between BISCPM and IMPACT Lab with a concomitant p-value (p < 0.05 indicates a significant difference). BISCPM showed significantly higher AUC compared to IMPACT Lab for six-month mortality prediction.
Discussion
Although many studies have been carried out to establish an ideal injury evaluation and prognosis prediction model for TBI, most of them are of small sample size or lack of external validation.5 In addition, due to the diversity of factors influencing the prognosis of TBI, it is difficult to select the indictors of models. Researchers have to face a dilemma that with many indicators in one model usually makes it difficult to weight these items and leads to complex calculation; while with few indicators of too simple models, it can not lead to a reliable prediction undoubtedly despite of easy use.
Superior to other models, the BISCPM in our study almost included all the known prognostic factors of TBI, such as age, GCS score, pupil light reflex and SBIF.14,15 It was indicated from our data that the increase of patients' age was positively related to worse prognosis, and even a linear relationship was formed when patients’ ages were 50 years or older, so we expanded the scoring intervals of part B from 50 years old on. Maas et al.7 found that absence of the third ventricle and basal cistern in CT scan is strong indicators of higher six-month mortality. Other studies also detected some prognosis predictors from CT scan including tSAH, ventricle bleeding, absence of ambient cistern, and shift of midline.8,16 However, the prognostic significance of CT for children patients was unclear till now.17 By multivariate regression analysis, four CT indicators were screened for BISCPM and confirmed to be strong prognosis predictors of not only for adult but also for children patients in this study.
It is well known that a variety of SBIF are able to affect the prognosis, so it is reasonable to include the SBIF into the prediction models of TBI. The most frequent one of SBIF is low BP, whose close relationship with poor patients' outcomes has been detected. A retrospective study suggested that patients at admission with systolic BP between 120 and 150 mmHg, or mean arterial BP between 85 and 110 mmHg had a better outcome.18 High fever is definitely one of SBIF and closely related to the lower GCS score at discharge and longer ICU staying time of patients.19 Hyperglycemia can aggravate the cerebral microcirculation damage after TBI and is associated with poor prognosis, which has been confirmed by a number of randomized, controlled, clinical trials. Hypoxemia, another frequent SBIF, is able to aggravate brain edema and lead to prolonged hospital stay and higher mortality.18 In addition, some studies showed that the patients with lower or higher serum sodium level after TBI tend to have poor outcomes.19 ICP and CCP are strong indicators of patients’ prognosis, which has been validated by many studies. For example, Harhangi et al.20 concluded that whether the patients are able to survive or recover functionally at 1 year after injury could be reliably predicted by ICP determined at 72 h after admission. More and more studies emphasized the roles of SBIF on the outcomes of TBI; however, we noticed almost none of present models included enough SBIF.21 To the best of our knowledge, the BISCPM in our study included nearly all the known SBIF. Furthermore, another critical issue for consideration is the duration of SBIF as scoring a SBIF indicator in a model. Because the effects of medical management on the final outcome may be almost ignored, we believed it was not reasonable to score a SBIF indicator only by a static admitting value or a worst one during 24 h after admission. For this reason, we tried to establish an ideal BISCPM with dynamic consideration. A large sample size is necessary to establish an ideal model; however, consisting of a variety of databases in a model may lead to errors for potential disconformity of sampling among different databases.
Although the sample size in this paper was not very large, all the values included in model were collected by trained physicians under serious quality control or transmitted to special computers through monitors, and this study included a complete spectrum of TBI ranging from mild to severe, which may ensure that the established model is highly qualified and can be used among patients with different severity of TBI. In addition, most previous similar studies came from developed countries,22 but this model from the data of our hospital may be more applicable to the low-incoming or developing countries.
In conclusion, it is indicated that our BISCPM can reliably evaluate brain injury state and predict prognosis. However, it must be acknowledged that any evaluation and prediction system, even established ideally, is only an effective supplement to medical decisions for physicians, and cannot replace physician's experience and clinical guidelines.
Funding
Dr. Xia Li has received two grants from Xijing Hospital (No. XJZT10Y15 and No. XJZT14R15), and one grant from the Scientific Department of Shaanxi Province, China (No. 2014SF314).
Ethical Statement
This study was approved by the Ethics Committee of the Fourth Military Medical University.
Declaration of Competing Interest
The authors declared no conflicts of interest.
Footnotes
Peer review under responsibility of Daping Hospital and the Research Institute of Surgery of the Third Military Medical University.
References
- 1.Maas A.I., Stocchetti N., Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 2.Jiang J.Y. Chinese head trauma cooperators: head trauma in China. Injury. 2013;44:1453–1457. doi: 10.1016/j.injury.2012.08.045. [DOI] [PubMed] [Google Scholar]
- 3.Perel P., Wasserberg J., Ravi R.R. Prognosis following head injury: a survey of doctors from developing and developed countries. J Eval Clin Pract. 2007;13:464–465. doi: 10.1111/j.1365-2753.2006.00713. [DOI] [PubMed] [Google Scholar]
- 4.Knaus W.A., Draper E.A., Wagner D.P. Apache II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 5.Perel P., Edwards P., Wentz R. Systematic review of prognostic models in traumatic brain injury. BMC Med Inf Decis Making. 2006;6:38. doi: 10.1186/1472-6947-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brain Trauma Foundation (US) AAoNS Management and prognosis of severe traumatic brain injury. J Neurotrauma. 2000;17:451. doi: 10.1089/neu.2000.17.451. [DOI] [Google Scholar]
- 7.Maas A.I., Hukkelhoven C.W., Marshall L.F. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57:1173–1182. doi: 10.1227/01.neu.0000186013.63046.6b. [DOI] [PubMed] [Google Scholar]
- 8.Wardlaw J.M., Easton V.J., Statham P. Which CT features help predict outcome after head injury? J Neurol Neurosurg Psychiatry. 2002;72:188–192. doi: 10.1136/jnnp.72.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chinese Neurosurgeons Association Chinese Expert Committee of Neural Trauma. Expert consensus on the surgical treatment of patients with TBI in China. Chin J Neurosurg. 2009;25:100–102. [Google Scholar]
- 10.Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24:S1–S106. doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- 11.Kothari R.U., Brott T., Broderick J.P. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 12.Marmarou A., Lu J., Butcher I. IMPACT database of traumatic brain injury: design and description. J Neurotrauma. 2007;24:239–250. doi: 10.1089/neu.2006.0036. [DOI] [PubMed] [Google Scholar]
- 13.Harrell F.E., Jr., Lee K.L., Mark D.B. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Hukkelhoven C.W., Steyerberg E.W., Rampen A.J. Patient age and outcome following severe traumatic brain injury: an analysis of 5600 patients. J Neurosurg. 2003;99:666–673. doi: 10.3171/jns.2003.99.4.0666. [DOI] [PubMed] [Google Scholar]
- 15.Signorini D.F., Andrews P.J.D., Jones P.A. Predicting survival using simple clinical variables: a case study in traumatic brain injury. J Neurol Neurosurg Psychiatry. 1999;66:20–25. doi: 10.1136/jnnp.66.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maas A.I., Steyerberg E.W., Butcher I. Prognostic value of computerized tomography scan characteristics in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24:303–314. doi: 10.1089/neu.2006.0033. [DOI] [PubMed] [Google Scholar]
- 17.Ducrocq S.C., Meyer P.G., Orliaguet G.A. Epidemiology and early predictive factors of mortality and outcome in children with traumatic severe brain injury: experience of a French pediatric trauma center. Pediatr Crit Care Med. 2006;7:461–467. doi: 10.1097/01.PCC.0000235245.49129.27. [DOI] [PubMed] [Google Scholar]
- 18.Jeremitsky E., Omert L., Dunham C.M. Harbingers of poor outcome the day after severe brain injury: hypothermia, hypoxia, and hypoperfusion. J Trauma. 2003;54:312–319. doi: 10.1097/01.TA.0000037876.37236.D6. [DOI] [PubMed] [Google Scholar]
- 19.Natale J.E., Joseph J.G., Helfaer M.A. Early hyperthermia after traumatic brain injury in children: risk factors, influence on length of stay, and effect on short-term neurologic status. Crit Care Med. 2000;28:2608–2615. doi: 10.1097/00003246-200007000-00071. [DOI] [PubMed] [Google Scholar]
- 20.Harhangi B.S., Kompanje E.J., Leebeek F.W. Coagulation disorders after traumatic brain injury. Acta Neurochir. 2008;150:165–175. doi: 10.1007/s00701-007-1475-8. [DOI] [PubMed] [Google Scholar]
- 21.Raj R., Siironen J., Kivisaari R. Predicting outcome after traumatic brain injury: development of prognostic scores based on the IMPACT and the Apache II. J Neurotrauma. 2014;31:1721–1732. doi: 10.1089/neu.2014.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MRC Crash Trial Collaborators. Perel P., Arango M. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008;336:425–429. doi: 10.1136/bmj.39461.643438.25. [DOI] [PMC free article] [PubMed] [Google Scholar]