Abstract
Purpose
Myofibromas are benign soft tissue tumors commonly encountered in infancy and childhood. Developing usually within the first two years of life, they can be multicentric and involve deep visceral organs.
Observations
We present the rare occurrence of a solitary orbital myofibroma in an adult patient. The clinical, histopathologic and immunohistochemical findings of the tumor are documented.
Conclusions
A comprehensive review of pediatric and adult orbital and periocular involvement by myofibroma is presented. Its characteristic pathologic and molecular findings are reviewed.
Importance
Myofibromas are uncommon but important tumors that can occur in the head and neck region, including the orbit. Seen more often in children, they can rarely be encountered in adult patients. Diagnosis is possible with a panel of immunostains and molecular analysis can be further confirmatory.
Keywords: Myofibroma, Myofibromatosis, PDGFRB mutation, Myopericytoma
1. Introduction
Myofibromas are uncommon tumors typically occurring in infants in the head and neck region. The spectrum of clinical behavior is broad, ranging from spontaneous regression to multi-visceral involvement and death. Though common in the head and neck, the occurrence of myofibroma in the orbit is rare and its solitary occurrence in adult patients is even more rare. We present the case of a solitary adult-onset myofibroma in the orbit and discuss its differential diagnosis and pathologic findings. We present a comprehensive review of the literature of orbital and periocular myofibroma to place the case in its clinical and epidemiologic context.
2. Case report
A 24-year-old woman with no pertinent past medical history presented to the University of Iowa as a referral for magnetic resonance imaging. She initially presented to her primary care provider for a 6-month history of a swollen left eyelid and pressure behind her eye. No family history of ocular disease or tumors was reported.
On examination, her visual acuity was 20/20 without correction and intraocular pressure was 20 mmHg, bilaterally. She was noted to have a slightly proptotic and inferiorly displaced left eye. Exophthalmometer measured the right eye at 13 mm and the left eye at 15 mm. Visual fields and extraocular movements were full and intact. No afferent pupillary defect was noted. The remainder of the anterior segment exam for both eyes was normal.
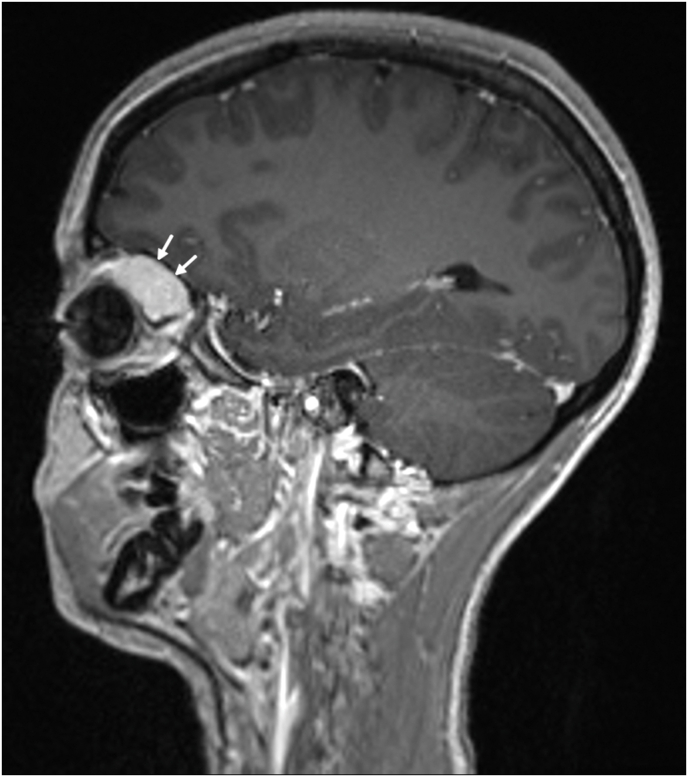
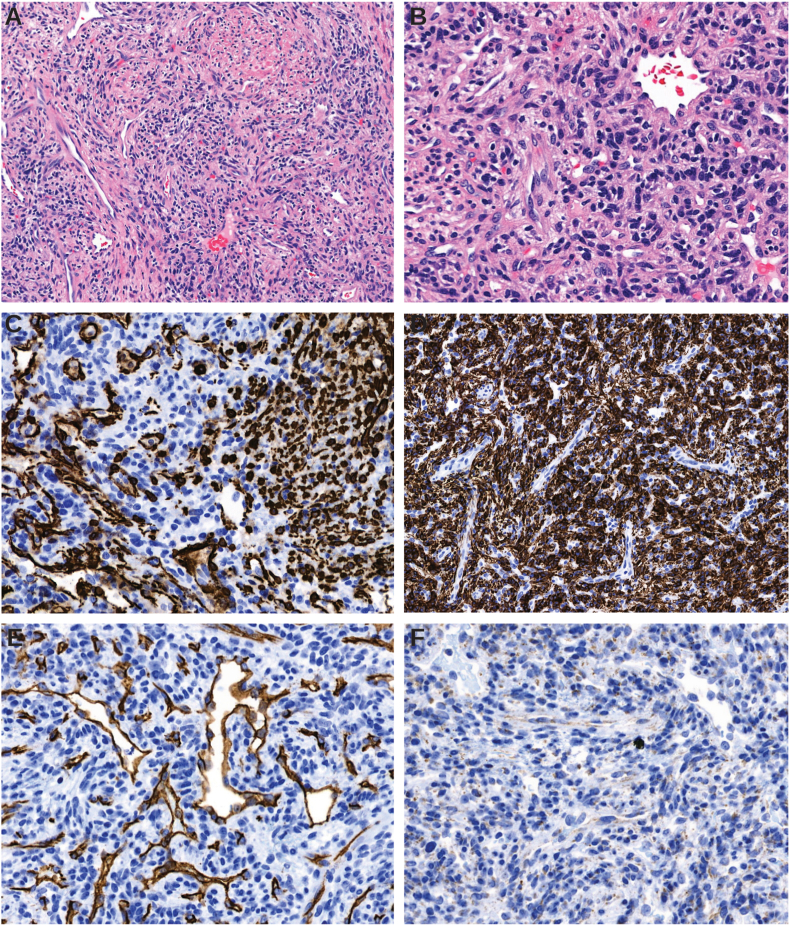
On dilated exam, the vitreous was clear. Optic nerves were normal in size and slightly asymmetric, with a right cup-to-disc ratio of 0.3 and a left cup-to-disc ratio of 0.2. The right macula was flat. The retinal vessels appeared normal in the right eye and were slightly congested in the left eye. The remainder of the retinal exam was within normal limits bilaterally. Optical coherence tomography (OCT) demonstrated a horizontal fold in the left macula. MR imaging showed an enhancing, well defined, solid, extraconal orbital mass in the left orbit (Fig. 1). The patient subsequently underwent a left lateral orbitotomy and excisional biopsy of the mass. Pathology of the left eye tumor revealed a spindle cell neoplasm composed of cells with plump oval nuclei and scant cytoplasm arranged in short, haphazard fascicles. There was a rich vascular network throughout the tumor with numerous thin-walled, branched, and staghorn-like vessels. There was a concentric perivascular arrangement of tumor cells around larger vessels that exhibited more abundant lightly eosinophilic cytoplasm. There were no areas of necrosis and no mitotic activity was identified. On immunohistochemistry, the spindle cells showed diffuse cytoplasmic positivity for desmin (Fig. 2, D) and were negative for CD34, STAT6 and myogenin. TLE1 was positive in rare tumor cells and endothelial cells. CD34 highlighted the vasculature within tumor, and SMA was positive in vessel walls and spindle cells (Fig. 2, C). Molecular analysis was performed utilizing DNA and RNA based next-generation sequencing with an expanded cancer mutation profiling assay that evaluated for presence of substitutions, insertion/deletions and gene fusions in a large panel of genes (n = 214), and for copy number alterations in a subset of them, both inclusive of PDGFRB (depth of coverage >1000x). No gene alterations of established, potential, or uncertain significance were found in PDGFRB or any of the other studied genes, excepting a PIK3CA c.3062A>G mutation. The morphologic and immunohistochemical features were most consistent with a diagnosis of myofibroma/myopericytoma.
Fig. 1.
Sagittal T1-weighted post-contrast magnetic resonance image (MR) showing an orbital mass involving the left orbit, denoted by white arrows.
Fig. 2.
A – H&E stain, 100x original magnification, low-power photomicrograph showing architectural features of myofibroma with haphazard bundles of spindled cells arranged around numerous vascular channels. B–H&E stain, 200x original magnification, higher-power showing bland oval-to-spindle tumor cells with minimal pleomorphism. C – Immunohistochemistry for smooth muscle actin (SMA) highlights smooth muscle in vascular walls and tumor cells. D – Desmin shows strong cytoplasmic positivity in tumor. E − CD34 highlights endothelial cells in vascular channels and is negative in tumor. F – STAT6 shows weak cytoplasmic expression and is negative in tumor nuclei.
3. Discussion
Myofibromas are uncommon benign tumors of mesenchymal cells exhibiting myofibroblastic differentiation. They share several morphologic features with myopericytomas and are grouped together in the WHO classification of soft tissue tumors. The occurrence of multifocal myofibromas, occasionally in association with systemic disturbances is known as myofibromatosis.1 Myofibromatosis and visceral involvement usually shows a familial pattern of occurrence and exhibits an autosomal dominant pattern of inheritance.2 On the other hand, most solitary myofibromas tend to be sporadic.
In the literature, there are only two reported cases of solitary adult orbital myofibroma to date. The present case is the first with comprehensive immunohistochemical and molecular studies in adult orbital myofibroma. Servat et al.3 described a large orbital mass (~8 cm) in a 47-year old man, with erosion and destruction of orbital bone and extension into the anterior cranial fossa. Hemalatha et al.4 described the occurrence of 3 cm orbital mass in a 28-year old woman which showed adipose tissue-like areas and a hemangiopericytomatous vascular pattern that was diagnosed as myofibroma. STAT6 immunohistochemistry was not performed in the first case, and in the second, immunostains to exclude solitary fibrous tumor (CD34 or STAT6) were not performed. Notably, some histologic features seen in the second case, including adipose differentiation, can be seen in solitary fibrous tumor. Both CD34 and STAT6 were performed in the present case and were negative, excluding the most applicable differential diagnostic consideration of solitary fibrous tumor given the histologic findings. Even outside the confines of the orbit, only a few myofibromas have been reported in adults in the periocular soft tissues. The reported adult orbital and periocular cases are summarized in Table 1.
Table 1.
Adult orbital and periocular myofibroma in the literature.
| Article | Year | Age | Sex | Site | Comments |
|---|---|---|---|---|---|
| Servat et al3 | 2011 | 47 | Male | Right orbit | Solitary 8 cm mass with bony erosion |
| Hemalatha et al4 | 2013 | 28 | Female | Left orbit | Solitary 3 cm mass; associated with bilateral microphthalmos |
| Beham et al30 | 1993 | 64 | Male | Lower eyelid | 1.1 cm mass; part of a case series |
| Kim SJ31 | 2003 | 45 | Female | Eyelid | Solitary painless tumor |
| Choopong et a32 | 2007 | 19 | Female | Sclera; supranasal limbus | Solitary tumor; 0.5 cm mass |
| Heath et al33 | 2018 | 71 | Male | Right lower eyelid | 2 cm violaceous nodule; showed spontaneous regression to become a plaque |
| Present case | 2020 | 34 | Female | Left orbit | Solitary ~2.2 cm mass; painless |
The head and neck is the most common anatomic sub-region involved by myofibroma and the tumor is mainly seen in infancy and childhood. There are several case reports and series of infantile/childhood orbital myofibroma5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 and involvement of periocular soft tissues.20, 21, 22 However, though myofibroma is the most common fibrous tumor of infancy, the tumor remains rare in the orbit. In a review encompassing a 60-year period at the Mayo Clinic, out of 340 cases of soft tissue tumors in children involving the orbit, just one was myofibroma.23 A review of 315 orbital soft tissue tumors at a referral children's hospital over a 20-year period found 11.4% (n = 36) to be mesenchymal tumors, including myofibromas though the exact number is not known (Drobysheva A et al. Ped Dev Pathol 2017; 20 (6); SPP Abstract 7). A retrospective series of 1264 patients with orbital masses over a 30-year period found two cases,24 both patients were less than 6 years of age. Mynatt et al. summarized25 reviewed cases of orbital infantile myofibroma in the English language literature from 1960 to 2011, accounting for changes in terminology. They found 24 cases in an age range of 0–12 years, the most common occurrence of which (n = 7) was at birth.
Rare as they are in children, the occurrence of adult orbital myofibroma is rarer. In two large hospital-based surveys of orbital tumors26,27 no definitive cases of myofibroma were found: the first study identified 55 out of 2480 consecutive patients with ‘myogenic lesions’ but none were diagnosed as myofibroma; the second examined 268 records of referred patients over a 9-year period at a cancer center: of the total, 18 were mesenchymal and none were myofibroma. A nationwide survey of orbital mass lesions in the Netherlands28 identified 965 tumors over a 24-year period; of these one was diagnosed as ‘fibroma’ and no myofibroma was identified.
The histopathologic appearance of myofibroma is characteristic: there are plump spindled cells in a moderately cellular distribution dispersed amidst prominent intratumoral vascular channels (Fig. 2A and 2B). Typically, a pericytic distribution of tumor cells is noted; this pattern is more accentuated in myopericytoma. Although some mitoses could be seen, features of malignancy such as increased mitotic activity, atypical mitotic figures, necrosis, vascular invasion, or locally infiltrative growth are not identified. The typical immunophenotype of myofibroma includes frequent positivity with smooth muscle actin and desmin less commonly. Interestingly, the present tumor showed variable immunoreactivity for SMA with positivity observed in patchy areas of tumor and in perivascular tumor cells and vessel walls (Fig. 2, C). This finding is noteworthy for myofibroma which is described to be uniformly positive for SMA in the literature. Desmin, however, showed strong areas of cytoplasmic immunopositivity (Fig. 2, D). Vascular, neural, histiocytic markers CD34, S100 and CD68 are almost always negative in myofibroma as was observed. Solitary fibrous tumor is an important differential diagnostic consideration in the orbit and has overlapping features with myofibroma, particularly the branched vasculature that can be seen in both tumors. There was no nuclear STAT6 expression in contrast to solitary fibrous tumor (SFT) which is STAT6 positive.
The molecular findings in the literature on myofibroma are summarized in Table 2. Characteristic gain-of-function PDGFRB mutations have been described in both the familial and sporadic forms of infantile/childhood myofibroma. A recent large-scale multi-institutional study examining 69 patients with myofibromas found no PDGFRB mutations from tumors in patients age >18 years.29 The reported mutations in myopericytomas are variable with one study findings similar PDGFRB mutations and another reporting a lack in them. A subset of cellular myofibroma/myopericytomas have been shown to harbor SRF-RELA gene fusions. Our findings of a lack of PDGFRB mutations are in line with recent findings indicating a virtual absence of activating PDGFRB mutations in adults. The absence of any other alterations in genes commonly encountered in soft tissue tumors lends further support to the diagnosis. Given that orbital soft tissue is a deep site, one possibility worth considering (and one that cannot be completely excluded) is that the tumor in the present case existed in infancy or childhood and presented late by being slow growing. But the solitary (non-multifocal) nature of the tumor, combined with the lack of molecular alterations that are more frequently seen pediatric and multicentric myofibromatosis (in which deep-seated lesions such as in the orbit may occur) make it more likely that the lesion was sporadic and occurred in adulthood.
Table 2.
Molecular abnormalities described in myofibroma/myofibromatosis.
| Condition | Genetic abnormality | Mutations found |
|---|---|---|
| Familial infantile myofibromatosis16,34 | Recurrent PDGFRB mutation | c.1681C > T |
| c.1978C > A | ||
| c.1998C > A | ||
| Sporadic myofibroma29,35, 36, 37, 38, 39 | PDGFRB mutation | c.1681C > T |
| c.1685A > G | ||
| c.1957A > G | ||
| Subset of cellular myofibroma/myopericytoma40 | SRF-RELA fusion |
4. Conclusion
We hereby report the rare occurrence of adult myofibroma in the orbit and the second such case to be fully characterized by immunohistochemistry. Though rare, myofibroma should be considered in the differential diagnosis of orbital spindle cell mesenchymal neoplasms in adults and children.
Patient consent
Written consent to publish this case has not been obtained. This report does not contain any personal identifying information.
Funding
No funding or grant support
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Intellectual property
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
Research ethics
We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
IRB approval was obtained (required for studies and series of 3 or more cases).
Written consent to publish potentially identifying information, such as details or the case and photographs, was obtained from the patient(s) or their legal guardian(s).
Declaration of competing interest
Anand Rajan KD previously served as a member of a Scientific Advisory Board to Roche Diagnostic Corporation.
The following authors have no financial disclosures: NCM, MRT, NAS.
Acknowledgements
None.
References
- 1.Wiswell T.E., Sakas E.L., Stephenson S.R., Lesica J.J., Reddoch S.R. Infantile myofibromatosis. Pediatrics. 1985;76:981–984. [PubMed] [Google Scholar]
- 2.Martignetti J.A., Tian L., Li D. Mutations in PDGFRB cause autosomal-dominant infantile myofibromatosis. Am J Hum Genet. 2013;92:1001–1007. doi: 10.1016/j.ajhg.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Servat J., Williamson J., Piepmeier J., Sinard J., Bernardino R.C. Giant myofibroma of the orbit in an adult male. Orbit. 2011;31:21–23. doi: 10.3109/01676830.2011.605501. [DOI] [PubMed] [Google Scholar]
- 4.Hemalatha A.L., Sindhuram V.A., Asha U. Myfibroma which was associated with A rudimentary eyeball, which masqueraded as an ocular palpebral cyst. J Clin Diagn Res. 2013;7:557–559. doi: 10.7860/JCDR/2013/5095.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffy M.T., Harris M., Hornblass A. Infantile myofibromatosis of orbital bone A case report with computed tomography, magnetic resonance imaging, and histologic findings. Ophthalmology. 1997;104:1471–1474. doi: 10.1016/s0161-6420(97)30114-6. [DOI] [PubMed] [Google Scholar]
- 6.Shields C.L., Husson M., Shields J.A., Mercado G., Eagle R.C., Jr. Solitary intraosseous infantile myofibroma of the orbital roof. Archives of ophthalmology (Chicago, Ill. 1960;116:1528–1530. doi: 10.1001/archopht.116.11.1528. 1998. [DOI] [PubMed] [Google Scholar]
- 7.Ikediobi N.I., Iyengar V., Hwang L., Collins W.E., Metry D.W. Infantile myofibromatosis: support for autosomal dominant inheritance. J Am Acad Dermatol. 2003;49:S148–S150. doi: 10.1067/mjd.2003.333. [DOI] [PubMed] [Google Scholar]
- 8.Cruz A.V., Maia E.M., Burmamm TGet al Involvement of the bony orbit in infantile myofibromatosis. Ophthalmic Plast Reconstr Surg. 2004;20:252. doi: 10.1097/01.iop.0000123501.30336.2c. [DOI] [PubMed] [Google Scholar]
- 9.Nam D., Moon H., Chung D., Baek S. Solitary infantile myofibroma of the orbital bone. Clin Exp Ophthalmol. 2005;33:549–552. doi: 10.1111/j.1442-9071.2005.01084.x. [DOI] [PubMed] [Google Scholar]
- 10.Persaud T.O., Nik N.A., Keating R.F. Solitary orbital infantile myofibroma: a case report and review of the literature. J Am Assoc Pediatr Ophthalmol Strabismus. 2006;10:283–284. doi: 10.1016/j.jaapos.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues E.B., Shields C.L., Eagle R.C., Marr B.P., Shields J.A. Solitary intraosseous orbital myofibroma in four cases. Ophthalmic Plast Reconstr Surg. 2006;22:292. doi: 10.1097/01.iop.0000226900.79557.cf. [DOI] [PubMed] [Google Scholar]
- 12.Lope L.A., Hutcheson K.A., Khademian Z.P. Magnetic resonance imaging in the analysis of pediatric orbital tumors: utility of diffusion-weighted imaging. Journal of AAPOS. 2010;14:257–262. doi: 10.1016/j.jaapos.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Larsen A.C., Prause Ju, Petersen B.L., Heegaard S. Solitary infantile myofibroma of the orbit. Acta Ophthalmol. 2011;89 doi: 10.1111/j.1755-3768.2010.02003.x. [DOI] [PubMed] [Google Scholar]
- 14.Yazici B., Bilge A.D., Yazici Z., Yalcinkaya U. Congenital cranio-orbital myofibroma. Ophthalmic Plast Reconstr Surg. 2011;27:e108–111. doi: 10.1097/IOP.0b013e3181fc05f8. [DOI] [PubMed] [Google Scholar]
- 15.Bloom R.I., Schwarcz R.M., Zhang C., Rosenberg J.B. A case of congenital myofibroma of the orbit presenting at birth. Orbit. 2013;32:33–35. doi: 10.3109/01676830.2012.736594. [DOI] [PubMed] [Google Scholar]
- 16.Cheung Y., Gayden T., Campeau P.M. A recurrent PDGFRB mutation causes familial infantile myofibromatosis. Am J Hum Genet. 2013;92:996–1000. doi: 10.1016/j.ajhg.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi K., Katori N., Otsuki Y., Ohno-Matsui K. Clinicopathological study of three cases of infantile fibromatosis of the orbit. Int Ophthalmol. 2014;34:1097–1106. doi: 10.1007/s10792-014-9915-y. [DOI] [PubMed] [Google Scholar]
- 18.MacIntosh P.W., Grob S.R., Stagner A.M. Multicentric myofibromatosis presenting as a large congenital eyelid myofibroma. Journal of AAPOS. 2016;20:70–73. doi: 10.1016/j.jaapos.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Eshraghi B., Dehghani S., Saeedi-Anari G. A rare erosive orbital mass in a child: case report of myofibroma. Journal of Current Ophthalmology. 2017;29:224–227. doi: 10.1016/j.joco.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witschel H. Angeborene isolierte Fibromatose des Lides. Ophthalmologica. 1982;185:1–6. doi: 10.1159/000309215. [DOI] [PubMed] [Google Scholar]
- 21.Friis J., Daugaard S., Heegaard S., Larsen L., Prause J., Schmidt P. Solitary myofibroma of the eyelid. Acta Ophthalmol Scand. 2004;82:109–111. doi: 10.1111/j.1395-3907.2004.0189e.x. [DOI] [PubMed] [Google Scholar]
- 22.Lascaratos G., Gupta M., Bridges L., MacRae M. Myofibroma of the conjunctiva invading the cornea in infancy. J Pediatr Ophthalmol Strabismus. 2010;47 doi: 10.3928/01913913-20100510-05. Online:3. [DOI] [PubMed] [Google Scholar]
- 23.Kodsi S.R., Shetlar D.J., Campbell J.R., Garrity J.A., Bartley G.B. A review of 340 orbital tumors in children during a 60-year period. Am J Ophthalmol. 1994;117:177–182. doi: 10.1016/s0002-9394(14)73074-0. [DOI] [PubMed] [Google Scholar]
- 24.Shields J.A., Shields C.L., Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions the 2002 Montgomery Lecture, part 1. Ophthalmology. 2004;111:997–1008. doi: 10.1016/j.ophtha.2003.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Mynatt C.J., Feldman K.A., Thompson L.D.R. Orbital infantile myofibroma: a case report and clinicopathologic review of 24 cases from the literature. Head and Neck Pathology. 2011;5:205. doi: 10.1007/s12105-011-0260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonavolontà G., Strianese D., Grassi P. An analysis of 2,480 space-occupying lesions of the orbit from 1976 to 2011. Ophthalmic Plast Reconstr Surg. 2013;29:79–86. doi: 10.1097/IOP.0b013e31827a7622. [DOI] [PubMed] [Google Scholar]
- 27.Shinder R., Al‐Zubidi N., Esmaeli B. Survey of orbital tumors at a comprehensive cancer center in the United States. Head Neck. 2011;33:610–614. doi: 10.1002/hed.21498. [DOI] [PubMed] [Google Scholar]
- 28.Johansen S., Heegaard S., Bøgeskov L., Prause Ju. Orbital space‐occupying lesions in Denmark 1974–1997. Acta Ophthalmol Scand. 2000;78:547–552. doi: 10.1034/j.1600-0420.2000.078005547.x. [DOI] [PubMed] [Google Scholar]
- 29.Dachy G., de Krijger R.R., Fraitag S. Association of PDGFRB mutations with pediatric myofibroma and myofibromatosis. JAMA Dermatology. 2019;155 doi: 10.1001/jamadermatol.2019.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beham A., Dve S., Suster S., Fletcher C.D.M. Solitary myofibroma in adults: clinicopathological analysis of a series. Histopathology. 1993;22:335–341. doi: 10.1111/j.1365-2559.1993.tb00132.x. [DOI] [PubMed] [Google Scholar]
- 31.Kim S.J. A case of solitary adult myofibroma in eyelid. Korean J Dermatol. 2003;41 [Google Scholar]
- 32.Choopong P., Nielsen P.G., Perlman E.M., Huang J.J., Dryja T.P., Foster S.C. Solitary myofibroma of the sclera. Cornea. 2007;26:114–116. doi: 10.1097/01.ico.0000243951.07096.fd. [DOI] [PubMed] [Google Scholar]
- 33.Heath M., Hajar T., Korcheva V., Leitenberger J. Spontaneous involution (regression) of a solitary cutaneous myofibroma in an adult patient. J Cutan Pathol. 2018;45:159–161. doi: 10.1111/cup.13071. [DOI] [PubMed] [Google Scholar]
- 34.Arts F.A., Chand D., Pecquet C. PDGFRB mutants found in patients with familial infantile myofibromatosis or overgrowth syndrome are oncogenic and sensitive to imatinib. Oncogene. 2016;35:3239. doi: 10.1038/onc.2015.383. [DOI] [PubMed] [Google Scholar]
- 35.Agaimy A., Bieg M., Michal M. Recurrent somatic PDGFRB mutations in sporadic infantile and solitary adult myofibromas but not in angioleiomyomas and myopericytomas. Am J Surg Pathol. 2017;41:195–203. doi: 10.1097/PAS.0000000000000752. [DOI] [PubMed] [Google Scholar]
- 36.Hung Y.P., Fletcher C. Myopericytomatosis. The American Journal of Surgical Pathology. 2017;41:1034–1044. doi: 10.1097/PAS.0000000000000862. [DOI] [PubMed] [Google Scholar]
- 37.Qawahmed R., Sawyer S.L., Vassilyadi M. Infantile myofibromatosis with intracranial extradural involvement and PDGFRB mutation: a case report and review of the literature. Pediatr Dev Pathol. 2018;22(3):258–264. doi: 10.1177/1093526618787736. [DOI] [PubMed] [Google Scholar]
- 38.Dereure O. Myofibromatose infantile sporadique : mutations avec gain de fonction de PDGFRB. Ann Dermatol Vénéréol. 2017;144:574–575. doi: 10.1016/j.annder.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Guimier A., Gordon C.T., Hully M. A novel de novo PDGFRB variant in a child with severe cerebral malformations, intracerebral calcifications, and infantile myofibromatosis. Am J Med Genet Part A. 2019;179A:1304–1309. doi: 10.1002/ajmg.a.61151. [DOI] [PubMed] [Google Scholar]
- 40.Antonescu C.R., Sung Y.-S.S., Zhang L., Agaram N.P., Fletcher C.D. Recurrent SRF-RELA fusions define a novel subset of cellular myofibroma/myopericytoma: a potential diagnostic pitfall with sarcomas with myogenic differentiation. Am J Surg Pathol. 2017;41:677–684. doi: 10.1097/PAS.0000000000000811. [DOI] [PMC free article] [PubMed] [Google Scholar]