Abstract
Basal cell carcinoma is the most common malignancy in the United States. However, metastasis of basal cell carcinoma is exceedingly rare, with incidence estimates of 0.0028–0.055%. When it does metastasize, basal cell carcinoma most commonly spreads to regional lymph nodes and lungs, although other sites of disease can occur. This case report presents multi-modality imaging of a 54-year-old male who developed multifocal metastatic basal cell carcinoma approximately three years after initial presentation with an ulcerated groin lesion. Ultimately, metastases included many common and uncommon sites, including lymph nodes, lung, duodenum, spleen, and adrenal glands. This case provides an interesting example of an uncommon pattern of spread and associated symptoms of treatment-resistant metastatic basal cell carcinoma.
Keywords: Metastatic basal cell carcinoma, Non-melanoma skin cancer imaging, Dermatology
Introduction
Basal cell carcinoma (BCC) is the most common malignancy worldwide [1], with an increasing incidence in the United States [2]. Metastatic BCC is an exceptionally rare [3] and fatal disease [4]. Radiologists’ role in evaluation of advanced BCC is often focused on local involvement, but may expand to full-body imaging in rare circumstances when distant metastatic disease occurs. The paucity of metastatic BCC cases reported in the literature has resulted in limited knowledge of the potential patterns for metastatic spread. Presented here is a case of treatment-resistant, progressively metastatic BCC with an atypical pattern of spread.
Case report
A 54-year-old Caucasian male with a remote history of BCC on his nose in the 1980s and melanoma in 1994 presented to his dermatologist in 2016 for a 3-year history of an expanding, tender ulcer in the left suprapubic area near the base of the penis. Biopsy was recommended by his dermatologist, but the patient declined. Aerobic culture was positive for Streptococcus and Pseudomonas. The lesion did not resolve following antibiotic treatment. Soft tissue ultrasound demonstrated a 4.4 × 5.2 × 7.5 cm lobulated solid mass with internal vascularity and surrounding hyperemia. A subsequent skin biopsy was positive for pleomorphic nodular-infiltrating type BCC. Immunostains performed at this time ruled out other possible neuroendocrine tumors, including Merkel cell carcinoma.
Following the ultrasound and biopsy, a contrast-enhanced CT of the chest, abdomen, and pelvis demonstrated enlarged mediastinal lymph nodes, scattered subcentimeter bilateral pulmonary nodules, multiple enlarged inguinal and iliac chain lymph nodes, and a 6.9 × 2.8 cm left inguinal lymph node mass (Fig. 1). He was started on a smoothened inhibitor, vismodegib (Erivedge; Genentech - San Francisco, CA), by his local oncologist.
Fig. 1.
Initial staging with axial contrast-enhanced CT of the chest, abdomen, and pelvis demonstrating an enlarged mediastinal lymph node (a) small pulmonary nodule in the lingula (b), enlarged left external iliac lymph nodes (c), and a large left inguinal mass (d).
At follow-up, the patient demonstrated radiologic evidence for progression of disease, with enlargement of lymph nodes in the chest, abdomen, and pelvis despite four months of vismodegib therapy. Pulmonary nodules were unchanged. Vismodegib was discontinued with a plan to switch to immunotherapy, and the patient underwent a debulking procedure plus inguinofemoral lymphadenectomy. Tissue pathology again demonstrated pleomorphic nodular-infiltrating type BCC with lymphovascular invasion, involvement of 2 of 5 lymph nodes, and extra-nodal extension. Surgical margins were positive.
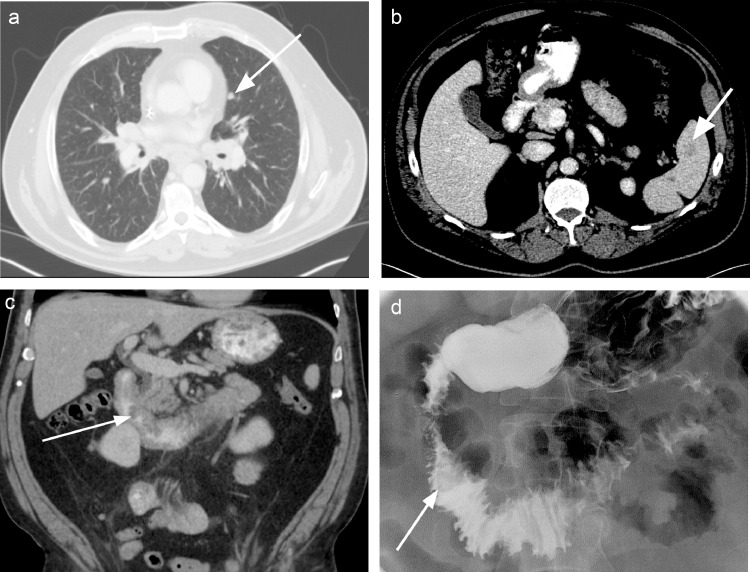
The patient was briefly lost to follow-up, having initially achieved an improved quality of life after local debulking. Over the course of 3 months, the patient developed symptoms of weight loss, abdominal pain, nausea, and early satiety, and returned to clinic. Restaging CT demonstrated marked interval progression of disease, with new and enlarging bilateral pulmonary nodules, new masses in the spleen and adrenal glands, new duodenal masses, and multifocal enlarging lymph nodes (Fig. 2).
Fig. 2.
Follow-up axial contrast-enhanced CT of the chest, abdomen, and pelvis demonstrating interval enlargement of the lingular pulmonary nodule (a) and a new splenic mass (b). Coronal reformatted CT image showing a new irregular duodenal mass protruding into the duodenal lumen (c). Subsequent fluoroscopic upper gastrointestinal series redemonstrating the periampullary lesion protruding into duodenal lumen (d).
Due to the patient's symptoms of nausea and early satiety in the setting of a duodenal mass, a fluoroscopic upper gastrointestinal series was performed. This redemonstrated a 2.5 cm periampullary lesion protruding into the second portion of the duodenum (Fig. 3). However, no high-grade obstruction was seen. Given a remote history of melanoma, upper endoscopy with biopsy of the duodenal lesion was performed to confirm that these distant metastases represented BCC and not melanoma. This confirmed the presence of 2 mucosal lesions arising from the second portion of the duodenum (Fig. 3). Pathology revealed poorly differentiated carcinoma, most consistent with metastatic BCC.
Fig. 3.
Images from upper endoscopy confirming 2 mucosal masses in the second portion of the duodenum.
The patient was started on immunotherapy with pembrolizumab (Keytruda; Merck – Kenilworth, NJ). Approximately 2 weeks later, he returned to clinic with complaints of worsened abdominal pain, new onset joint pain, and dyspnea with minimal exertion. The patient was offered hospital admission but declined and passed away 1 week later.
Discussion
Nonmelanoma skin cancer is estimated to affect more than 3.3 million people annually in the United States, with BCC representing more than half [5]. BCC is most common in older patients, particularly men over the age of 60, and is increasing in incidence [1]. The vast majority (70%) of BCC present on the face, while less than 1% present in the genital region, likely due to lower exposure to sunlight and UV radiation [6]. The primary risk factor for BCC is a history of UV radiation exposure, although fair skin, arsenic exposure, radiation therapy, and immunosuppression also play a role [7]. Surgical excision is typically diagnostic and curative for superficial BCC lesions. The only grading system currently available is the “high-risk” designation per the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology, although these aim to assist in establishing surgical margins rather than identifying patients at risk for metastasis or death [8].
Metastatic BCC is exceedingly rare, with current understanding of risk factors, incidence, pattern of spread, and prognosis limited by a paucity of literature. Estimates of incidence vary widely, ranging from 0.0028% to 0.055% of all BCC lesions [9]. One main risk factor for metastatic BCC is neglecting a primary lesion for many years [1] as was seen in this patient. Risk for metastatic BCC has also been associated with head/neck location, larger tumor diameter, increased tumor depth beyond fat, male sex, history of prior radiation at tumor site, and perineural invasion [3,8,10], although there is limited ability to predict metastatic BCC based on any of these features [10].
The spread of metastatic BCC usually begins via the lymphatic system before transitioning to hematogenous routes [11]. As a result, metastatic BCC has an early predilection for regional lymph nodes, and radiologists may encounter requests to evaluate local disease spread more frequently than examinations of more distant body parts [5]. It is important for radiologists to know that advanced metastatic BCC may behave like melanoma or other more aggressive skin cancers. When distant metastatic disease does occur, lungs and bone are likely among more common sites [5], with adrenal, duodenal, and splenic involvement (as presented here) vanishingly rare [11,12].
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2020.09.054.
Appendix. Supplementary materials
References
- 1.Cameron MC, Lee E, Hibler BP. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80(2):303–317. doi: 10.1016/j.jaad.2018.03.060. [DOI] [PubMed] [Google Scholar]
- 2.Mudigonda T, Pearce DJ, Yentzer BA, Williford P, Feldman SR. The economic impact of non-melanoma skin cancer: a review. J Natl Compr Cancer Netw. 2010;8(8):888–896. doi: 10.6004/jnccn.2010.0066. [DOI] [PubMed] [Google Scholar]
- 3.Tang S, Thompson S, Smee R. Metastatic basal cell carcinoma: case series and review of the literature. Australas J Dermatol. 2017;58(2):e40–e43. doi: 10.1111/ajd.12459. [DOI] [PubMed] [Google Scholar]
- 4.Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149(5):615–616. doi: 10.1001/jamadermatol.2013.3064. [DOI] [PubMed] [Google Scholar]
- 5.Kim JYS, Kozlow JH, Mittal B. Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol. 2018;78(3):540–559. doi: 10.1016/j.jaad.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Wang YJ, Tang TY, Wang JY, Huang YK, Wu YH. Genital basal cell carcinoma, a different pathogenesis from sun-exposed basal cell carcinoma? A case-control study of 30 cases. J Cutan Pathol. 2018 doi: 10.1111/cup.13304. [DOI] [PubMed] [Google Scholar]
- 7.Didona D, Paolino G, Bottoni U, Cantisani C. Non melanoma skin cancer pathogenesis overview. Biomedicines. 2018;6(1) doi: 10.3390/biomedicines6010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan FC, Ruiz ES, Karia PS, Besaw RJ, Neel VA, Schmults CD. Factors predictive of recurrence, metastasis, and death from primary basal cell carcinoma 2 cm or larger in diameter. J Am Acad Dermatol. 2019 doi: 10.1016/j.jaad.2019.09.075. [DOI] [PubMed] [Google Scholar]
- 9.McCusker M, Basset-Seguin N, Dummer R. Metastatic basal cell carcinoma: prognosis dependent on anatomic site and spread of disease. Eur J Cancer. 2014;50(4):774–783. doi: 10.1016/j.ejca.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Laga AC, Schaefer IM, Sholl LM, French CA, Hanna J. Metastatic basal cell carcinoma. Am J Clin Pathol. 2019;152(6):706–717. doi: 10.1093/ajcp/aqz089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alimoglu Y, Kilic E, Mercan H, Inci E. Metastatic basal cell carcinoma. J Craniofac Surg. 2011;22(3):1134–1136. doi: 10.1097/SCS.0b013e3182108fa6. [DOI] [PubMed] [Google Scholar]
- 12.Kleinberg C, Penetrante RB, Milgrom H, Pickren JW. Metastatic basal cell carcinoma of the skin. Metastasis to the skeletal system producing myelophthisic anemia. J Am Acad Dermatol. 1982;7(5):655–659. doi: 10.1016/s0190-9622(82)70146-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.