Abstract
Aims
Preventing mitochondrial dysfunction and enhancing mitochondrial health and biogenesis is a crucial therapeutic approach to ameliorate injury following acute myocardial infarction. Although the antioxidant role of melatonin against ischemia/reperfusion injury has been reported, the exact mechanism of protection, in vivo, remains poorly understood. This study aims to identify and elaborate upon mechanism of melatonin protection of rat cardiac mitochondria against acute myocardial infarction.
Main methods
Rats were pre-treated with melatonin (10 mg/kg body weight (b.w.); intraperitoneally, i.p.) before isoproterenol bitartrate (ISO) administration (25 mg/kg body weight (b.w.) subcutaneously,s.c.) and their effect on rat heart mitochondrial structure and function was studied. Biochemical changes in activity of biomarkers of oxidative stress, antioxidant enzymes as well as Krebs' cycle enzymes were analyzed. Gene expression studies and Isothermal titration calorimetric studies with pure catalase and ISO were also carried out.
Key findings
Melatonin was shown to reduce ISO induced oxidative stress, by stimulating superoxide dismutase activity and removing the inhibition of Krebs' cycle enzymes. Herein we report for the first time in rat model that melatonin activates the SIRT1-PGC-1α-SIRT3 signaling pathways after ISO administration, which ultimately induces mitochondrial biogenesis. Melatonin exhibited significant protection of mitochondrial architecture and topology along with increased calcium ion permeability and reactive oxygen species (ROS) generation induced by ISO. Isothermal calorimetric studies revealed that melatonin binds to ISO molecules and sequesters them from the reaction thereby limiting their interaction with catalase along with occupying the binding sites of catalase themselves.
Significance
Activation of SIRT1-PGC-1α-SIRT3 pathway by melatonin along with its biophysical properties prevents ISO induced mitochondrial injury in rat heart.
Keywords: Melatonin, Oxidative stress, SIRT1, SIRT3, PGC 1α, Isothermal titration calorimetry, Food science, Environmental science, Veterinary medicine, Health sciences
Melatonin, Oxidative stress, SIRT1, SIRT3, PGC 1α, Isothermal titration calorimetry, Food science, Environmental science, Veterinary medicine, Health sciences.
1. Introduction
The global burden of cardiovascular diseases (CVDs) has increased at an alarming rate with well-developed countries leading in cases related to CVD associated disability and mortality [1]. Myocardial infarction as an important cause of majority of premature deaths with critical risk factors that include ischemia/reperfusion injury, hypertension, diabetes and obesity [2]. Myocardial ischemia, characterized by decreased oxygen supply to the cardiac tissue, leads to rampant generation of ROS such as superoxide anions (.O2-) and hydroxyl radicals (.OH), as well as reactive nitrogen species (RNS), all of which have been reported to arise from a malfunctioning electron transport chain (ETC) in mitochondria [3].
Mitochondria, the seat of oxidative phosphorylation and energy production, plays a pivotal role in ROS induced oxidative damage to important macromolecules like DNA, proteins and lipids eventually leading to cellular apoptosis [4, 5, 6]. ROS mediated damage to mitochondria leads to mitochondrial dysfunction [7, 8]which obliterates ionic homeostasis and contractile function of the heart. ISO, a β-adrenergic agonist, causes gross infarcts in the rat heart [9] and its pathophysiological effects were comparable to those found in humans [10]. Isoproterenol perpetrates myocardial damage by instigating a cascade reaction that generates huge amount of superoxide anion radicals, hydrogen peroxide and hydroxyl radicals [11, 12].
Melatonin (N-acetyl-5-methoxytryptamine), an evolutionarily conserved ubiquitous molecule, which regulates circadian rhythms and controls reproductive behavior and retinal function [13], has recently gained remarkable recognition as a broad spectrum antioxidant and a potent free radical scavenger [14]. Research studies have documented its ability to neutralize ROS such as singlet oxygen (1O2), superoxide anion radical (O2.-), hydrogen peroxide (H2O2), hydroxyl radical (.OH) [15, 16] along with up-regulating antioxidant enzymes and down-regulating pro-oxidant enzymes [17, 18]. Melatonin also chelates metal ions [19] and prevents electron leakage from ETC [20].
Melatonin induced activation of two important members of the Sirtuin (silent mating type information regulation 2 homolog) family, Sirtuin 1 (SIRT1) and Sirtuin 3 (SIRT3), along with PGC 1α, which stands for Peroxisome proliferator-activated receptor gamma coactivator 1-alpha, plays a key role in the regulation of mitochondrial biogenesis [21]. The sirtuins are a family of highly conserved NAD+-dependent deacetylases that act as cellular sensors to detect energy availability and also modulate various metabolic processes by activating important signaling pathways. Among the sirtuins, mammalian SIRT1 and SIRT3 specifically are known to coordinate cellular energy stores and ultimately maintain cellular energy homeostasis by deacetylating a variety of proteins that induce catabolic processes while inhibiting the anabolic processes. A study by Yu et al, 2017 proposed that melatonin protects against mitochondrial dysfunction in myocardial ischemia/reperfusion injury in type 1 diabetes by preserving mitochondrial function and enhancing biogenesis via the AMP-activated protein kinase (AMPK)-PGC1α-SIRT3 signaling pathway [22]. However, the significance of this pathway adopted by melatonin in providing protection against acute myocardial infarction has not been studied yet.
Apart from the receptor dependent and receptor independent pathways by which melatonin offers protection, we have previously demonstrated a third possible mechanism by which melatonin protects antioxidant enzymes against copper-ascorbate, in vitro, by binding to them and masking their binding sites [23]. However, the binding energetics between melatonin and isoproterenol is still unknown. Hence, the present study was designed to investigate whether melatonin could provide protection against ISO induced mitochondrial dysfunction in rat heart in vivo and to explore the role of SIRT1-PGC1α-SIRT3 signaling pathway in improving mitochondrial function and biogenesis. We have also tried to investigate the thermodynamic interaction between melatonin and ISO with a pure antioxidant enzyme, catalase, through isothermal calorimetric binding studies, to predict and propose a possible mechanism of action employed by melatonin in providing protection to rat heart against ISO induced oxidative damage.
2. Materials and methods
2.1. Chemicals and reagents
All the chemicals used for the present research are of analytical grade. The primary antibodies used in the experiments such as NF-kB p65 (ab16502), PGC 1α (ab54481), HSP70 (ab79852), SIRT 1 (ab110304), SIRT 3 (ab189860) and Goat anti-rabbit IgG alkaline phosphatase (ALP)-conjugated secondary antibody (ab97048) were purchased from Abcam Biotechnology Company, USA. Primary antibody against β-actin (sc-130657) was procured from Santa Cruz Biotechnology, Inc. Elisa kits for estimation of TNFα, IL-1β, IL-6 and IL-10 were purchased from Ray Biotech, Inc., USA. Isoproterenol bitartrate, 2,3,5-triphenyltetrazolium chloride (TTC), 2′,7′-Dichlorofluorescin di acetate (DCFDA), JC-1 (5, 5′, 6, 6′-tetrachloro-1, 1′, 3,3′tetraethylbenzimidazolcarbocyanine iodide), glutathione reductase (GR) and few other chemicals were purchased from Sigma Aldrich, USA. Melatonin, trichloro acetic acid (TCA), thiobarbituric acid (TBA) reduced glutathione (GSH),5-Bromo-4-chloro-3-indolyl phosphate (BCIP), nitro blue tetrazolium (NBT), cytochrome c, α-ketoglutarate, succinate and other important reagents were procured from Sisco Research Laboratories (SRL), Mumbai, India. Thiobarbituric acid (TBA) and other chemicals were purchased from Merck Limited, Delhi, India.
2.2. Animal experiments
Sixty male Wistar rats weighing between 150-180 gms were procured from a registered supplier under the Committee for the Purpose of Control and Supervision of Experimental Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India. The experimental design and treatment protocols were approved (IAEC/IV/Proposal/DB-01/2016) by the Institutional Animal Ethics Committee (IAEC) of the Department of Physiology, University of Calcutta.
All rats were allowed to acclimatize for at least 7 days and kept under standard laboratory conditions with water and food ad libitum followed by 2 days of treatment period.
The rats were divided into the following groups with 6 rats in each group (n = 6):
-
1)
Control (CON): Only vehicle (0.9% NaCl) treated rats.
-
2)
Only melatonin treated groups (M5, M10, M20 and M40): Rats were injected with melatonin intraperitoneally at a dose of 5(M5), 10 (M10), 20 (M20) and 40 mg/kg body weight (M40), respectively. These four graded doses of melatonin was chosen after referring to previous literature [11] in which these doses exhibited significant biological effects.
-
3)
Isoproterenol treated group (ISO): Rats were injected subcutaneously with 25 mg/kg b.w. twice at an interval of 24 h to induce myocardial infarction. This dose of ISO has already been documented earlier to produce significant infarcts in rats [11].
-
4)
Isoproterenol + melatonin treated groups (ISO + M5, ISO + M10, ISO + M20 and ISO + M40): Rats were injected with melatonin (by i.p) at different doses (5, 10, 20 and 40 mg/kg bw) respectively, 30 min prior to ISO injection.
All animals were kept at room temperature while maintaining 12 hrs of daylight and 12 hrs of darkness and sacrificed 24 hrs after the second ISO injection by cervical dislocation. Blood was collected by cardiac puncture, before sacrifice, for the preparation of the serum. Their hearts were collected and stored at -20°C for further biochemical analyses. For histological studies, a ventricular portion of cardiac tissue was fixed by 10% buffered formaldehyde and another small part of cardiac tissue was subjected to 3% glutaraldehyde immediately after collection for scanning electron microscopy (SEM) study.
2.3. Preparation of mitochondrial fractions of rat heart
Cardiac tissue was homogenized (10%) in ice cold 50 mM potassium phosphate buffer (pH 7.4) using a Potter- Elvenjem glass homogenizer (Belco Glass, Inc., Vineland, NJ,USA) for 20secs. The 10% homogenate was centrifuged at 600 g for 10 min in cold to pellet down nuclear debris. The supernatant was collected and again subjected to centrifugation at 16,000 g for 45 min in cold. Supernatant which contains cytosolic sample was discarded and the pellet was re-suspended in sucrose buffer (50 mM Tris HCl, pH 7.8 containing 250 mM sucrose) to obtain mitochondrial suspension. It was stored at -20 °C for future biochemical assays. All assays involving enzymatic analysis except SGOT (Serum glutamate oxaloacetate transaminase), LDH1 (Lactate dehydrogenase 1) and total LDH (Total lactate dehydrogenase) were done using mitochondrial suspension sample.
2.4. Measurement of serum injury markers
The SGOT activity of rat heart was assayed by the method of Reitman and Frankel [24]. The LDH1 activity was measured according to the method of Stritmatter [25] with some modifications [26] and total LDH activity was measured by the method of Levine et al. (1994) [27].
2.5. Measurement of oxidative stress biomarkers
Lipid peroxidaton level (LPO) in mitochondria was measured as nmoles of TBARS/mg of protein [28] with some modifications [29]. Protein carbonyl content (PCO) was measured by DNPH assay [27] and reduced glutathione (GSH) content was estimated by the method of Sedlack and Lindsay [30] with some modifications [29]. Oxidized glutathione (GSSG) level was determined from the difference between total and actual GSH levels [31]. The ratio of GSH:GSSG was also calculated [32].
2.6. Measurement of antioxidant and antioxidant metabolizing enzymes
Glutathione peroxidase (GPx) activity was measured by method of Paglia and Valentine [33] with some modifications [34]. Glutathione reductase (GR) and Manganese superoxide dismutase (MnSOD) activity was estimated by method of Krohne-Echrich et al [35] and Marklund and Marklund [36] respectively. Catalase enzyme activity was estimated according to the method of Beer and Sizer [37] with some modifications [10].
2.7. Measurement of pyruvate dehydrogenase (PDH) and other Krebs' cycle enzymes
PDH activity was measured spectrophotometrically at 340 nm [38] and aconitase activity was measured by monitoring the formation of cis-aconitate from iso-citrate (20 mM) at 240 nm following the method of Gardner et al. [39]. The activity of isocitrate dehydrogenase (ICDH) and alpha ketoglutarate dehydrogenase (α-KGDH) were measured according to Duncan et al. (1979) [40]. The method of Veeger et al. (1969) [41]was used to measure succinate dehydrogenase (SDH) activity. Fumarase activity was determined spectrophotometrically by measuring the increase in absorbance at 240 nm in the reaction mixture to which 30 mM potassium phosphate (pH 7.4) and 0.1 mM L-malate and suitable amount of mitochondrial suspension were added following the method of Racker et al [42]. One unit (U) was defined as the amount of enzyme necessary to produce 1 μmol fumarate per minute (e240 = 3.6 mM-1 cm-1). Malate dehydrogenase activity (MDH) was determined according to method of Dasika et al. 2015 [43] by measuring the decrease in absorbance at 340 nm resulting from the oxidation of NADH. One unit oxidizes one micromole of NADH per minute at pH 7.4 under the specified conditions. The enzyme activity was expressed as Units/min/mg protein. Citrate synthase activity was examined spectrophotometrically by the method of Parvin [44] at 420 nm. All enzyme activities were expressed as Units/min/mg protein.
2.8. Determination of the activities of respiratory chain enzymes
The activities of NADH-Cytochrome c oxidoreductase and cytochrome c oxidase were measured by the method of Goyal and Srivastava (1995) [45] at 565 and 550 nm, respectively.
2.9. Measurement of infarct area by TTC stain
Infarct area of rat heart was measured according to the method Yan et al. 2012 [46]. Briefly the ventricular portion of the hearts from each group were cut transversely into 1-mm-thick slices and stained with 2% triphenyltetrazolium chloride (TTC) in PBS (pH 7.4) for 20 min in a 37 °C water bath. After fixation for 4–6 h in 10% buffered formaldehyde, each slice was photographed. Viable myocardium stained brick red and infarct tissues appeared pale white. Infarct area were measured by automated planimetry using Image J software (version 1.46u, National Institutes of Health), with the infarct size expressed as a percentage of the total infarct area. The extent of the area of infarct was quantified by three independent and blind observers.
2.10. Studies on tissue morphology with light microscopy
The tissue sections of different groups were stained with haematoxylin-eosin (HE) to study the morphological changes. Rat heart tissue was fixed in 10% formalin and embedded in paraffin following routine histological procedure. Five μm thick cardiac tissue sections were prepared and stained with haematoxylin–eosin (H-E). The stained tissue sections were examined under a light microscope (Leica) at 400× magnification [47].
Masson's trichrome staining of cardiac tissue sections was performed by following routine staining procedure [48, 49, 50]. Briefly, deparaffinised tissue sections were brought to water medium and pre-heated Bouin's solution was applied to them and allowed to stand for 60 min. Then tissue sections were washed in running tap water to remove the picric acid. Thereafter, Weigert's working hematoxylin solution was applied for 10 min. Bluening was done under running tap water for 5 min and the sections were rinsed in distilled water once again. After that Biebrich scarlet solution was applied for 5 min. After rinsing the tissue sections in distilled water, phosphotungstic/phosphomolybdic acid was applied for 10 min. Without discarding the solution, aniline blue solution was directly applied to them for 5 min. Those tissue sections were again rinsed in distilled water and 1% acetic acid was applied to them for 1 min. After incubation, tissue sections were taken out of solution, dehydrated and cleaned with xylene and finally was mounted with DPX. The stained tissue sections were examined under a light microscope (Leica) at 400× magnification. The percentage area of fibrosis has been quantified using ImageJ software.
2.11. Measurement of mitochondrial intactness by Janus Green B stain
Fifty microlitre of mitochondrial suspensions of different groups were spread uniformly on a glass slide and air dried. 0.1% Janus green B stain was applied to cover the smear and kept in dark for 20 min. It was then rinsed with distilled water carefully to remove excess stain. The bluish green stained slides were mounted with distilled water and observed under Olympus fluorescence microscope under green filter (excited at 450 nm) [23]. The fluorescent intensity of each sample was calculated using ImageJ software.
2.12. Study of mitochondrial morphology through scanning electron microscopy (SEM)
Isolated cardiac mitochondria was fixed in 2.5% cold glutaraldehyde for 24–48 h immediately after dissection of the animals for SEM study following the method of Mukherjee et al. 2015 [51]. The prepared samples were evaluated by scanning electron microscopy (SEM; Zeiss Evo 18 model EDS 8100) at a magnification of 20,000 KX.
2.13. Determination of the mitochondrial membrane potential (Δψm)
Mitochondrial membrane potential was determined by using the mitochondria- specific lipophilic cationic fluorescent dye, JC-1, following a well calibrated method of Cossarizza et al. (2000) [52]. Mitochondria from all four groups of tissues were prepared as described above and were stained with JC-1 dye (0.2 μg/mL) and then incubated in dark at 37 °C for 20 min. The flow cytometer (BDFACS Versa, USA), with the excitation wavelength 488 nm and an emission wavelength of band pass filter 586/42 nm was used for the detection of mitochondrial membrane potential (Δψm) and the FITC-A median was expressed as depolarized population percentage (%).
2.14. Determination of mitochondrial membrane calcium ion permeability
Calcium ion permeability across the inner mitochondrial membrane was assessed by following the method of Bratosin et al [53]. Mitochondrial suspensions were incubated with Calcein-AM dye at 5 μM concentration at 37 °C for 30 min followed by flow cytometric analysis carried out at 515 nm wavelength (excitation wavelength 495 nm) using BD FACS Verse. Data were analyzed by FACSuite software, histogram overlays of Ca2+ fluorescence were done, and mean fluorescence intensity was plotted as a bar diagram.
2.15. Measurement of mitochondrial ROS levels
Mitochondrial ROS levels were measured by 2′,7′ –dichlorofluorescin diacetate (DCFDA, also known as H2DCFDA), a fluorogenic dye that measures hydroxyl, peroxyl and other reactive oxygen species (ROS) activity using a BD FACS Aria II flow cytometer, USA. Briefly, samples were incubated with the stable non-fluorescent DCFDA for 15 min which becomes oxidized to a highly fluorescent 2′,7′-dichlorofluorescein (DCF) in the presence of ROS [54]. Data was represented as histogram overlays of different groups plotted using FlowJo software (version 10) and mean fluorescence intensity was represented by bar diagram.
2.16. Measurement of some important inflammatory markers in serum
Some important inflammatory markers such as tumor necrosis factor alpha (TNFα) (ELR- TNFα), interleukin-1β (IL-1β) (ELR-IL1b), interleukin-6 (IL-6) (ELR-IL6) and interleukin-10 (IL-10) (ELR-IL10) were determined by standard ELISA kits purchased from RayBiotech, Inc. (USA). The rat TNF –α ELISA kit used for quantitative determination of rat TNF-α was in the range from 25 to 20000 pg/ml. This assay displayed a sensitivity of 25 pg/ml. Average recovery is estimated to be 95.43% from rat serum. The determination range of IL-1β rat ELISA kit was in the range 80–50000 pg/ml while that of rat IL-6 was 30–10000 pg/ml. IL-10 rat ELISA kit from raybiotech had a determination range from 10 to 6000 pg/ml.
2.17. Determination of mitochondrial levels of different proteins by western blot analysis
Western blot analysis was performed with cardiac mitochondrial suspensions which were prepared as described earlier by Bandyopadhyay et al. (2004) [29]. The samples were subjected to SDS–PAGE (10%) analysis according to the method of Laemmli (1970) [55]. Eighty microgram proteins were loaded in each lane for immunodetection of NFκB, PGC 1α, HSP70, SIRT1, SIRT3 and β ACTIN. After completion of the running, the gel was transferred to nitrocellulose membrane (Pall Corporation, USA), the immunoblot was then incubated in blocking solution (5% bovine serum albumin) for 1 h followed by washing thrice in TBS-T (15 min each). The blot was then incubated with respective primary antibodies (1:2000) overnight at 4°C. The blot was washed thrice in TBS-T and incubated with secondary antibody (Goat anti-rabbit IgG ALP conjugate 1:3000) for 2 h at 4°C. Finally, the membrane was washed again and incubated in BCIP/NBT substrate. Individual band intensity of respective immunoblots was normalized by the intensity of β-actin (NIH, Bethesda, MD, USA) and the relative density of the bands was quantified using ImageJ software and expressed in relative densitometric units [29, 55]. The relative pixel density of the bands obtained was quantified using ImageJ software (NIH, Bethesda, MD,USA).
2.18. Isothermal titration calorimetry (ITC) studies
The binding pattern of pure catalase with isoproterenol bitartrate and melatoin, alone and in combination, was analyzed by isothermal titration calorimetry using Microcal ITC-200, Malvern, UK. For this assay, in the sample cell, 0.3 ml of pure catalase enzyme (4 × 10−9 mM) was titrated with 0.04 ml of 2 mM ISO and 80 μM melatonin as ligands in specific combinations as follows: (i) only ISO, (ii) only melatonin and (iii) ISO and melatonin together. The interactions between melatonin and isoproterenol bitartrate was also studied. In this case, 0.3 ml of pure melatonin (80 μM) taken in sample cell was titrated against 0.04 ml of 2 mM ISO as ligand. For a single run, titration was conducted with twenty injections of each ligand (2 μL each) with 150 s spacing between two successive injections for approximately 1 h at 37 °C [56]. The amount of heat change per second (ΔH) is the area under the curve and is expressed in terms of kcal mol−1 of injectant against molar ratio as shown at the bottom of each curve.
2.19. Estimation of protein
The protein concentration in each mitochondrial sample was determined by the method of Lowry et al [57].
2.20. Statistical evaluation
Each experiment was repeated at least three times. All data are presented as Mean ± S.E. Significance of mean values of different parameters between the groups were analyzed using one-way analysis of variances (ANOVA) after ascertaining the homogeneity of variances between the groups. Pair wise comparisons were done by calculating the least significance (P < 0.001). Statistical tests were performed using Microcal Origin version 7.0 designed for Windows (OriginLab Corporation, Northampton, MA, USA).
3. Results
Figure 1A, B and C shows a significant increase in SGOT, LDH1 and total LDH activity respectively in ISO (25 mg/kg bw) treated rat serum (∗p < 0.001 vs control) and the subsequent dose-dependent protection offered by pre-treatment with melatonin (#p < 0.001 vs ISO), suggests that melatonin at a minimum effective dose of 10 mg/kg bw can significantly protect against the rise in serum SGOT levels as compared to ISO. Serum from only melatonin treated rats at the doses of 5, 10, 20, 40 mg/kg body weight did not show any significant change in SGOT levels as compared to control group.
Figure 1.
Graphical representation of the changes in the levels of (A) SGOT (B) LDH1 and (C) Total LDH in serum of rats pre-treated with different doses of melatonin (M) (5, 10, 20 and 40 mg/kg bw; i.p.) with/without ISO administration (25 mg/kg bw; s.c.). Values are expressed as means ± S.E. for six samples for each group. (∗p < 0.001 versus control, #p < 0.001 versus ISO treated cardiac mitochondria, using one way ANOVA).
Figure 2A, B and C depicts a significant increase in LPO and PCO levels along with decrease in GSH content in cardiac mitochondria upon ISO treatment respectively. Interestingly, dose-dependent melatonin treatment showed a significant protection of all these parameters in comparison to ISO group with 10 mg/kg bw dose being the minimum effective dose of protection. A significant increase in GSSG level (Figure 2D) and decrease in GSH:GSSG ratio (Figure 2E) after ISO treatment was observed, however, dose- dependent pre-treatment of mitochondria with melatonin protected the levels from being altered. The only melatonin treated groups showed no significant difference as compared to control group.
Figure 2.
Graphical representation of the changes in the levels of (A) LPO (B) PCO content and (C) GSH content (D) GSSG and (E) GSH:GSSG ratio in cardiac mitochondria from rats pre-treated with different doses of melatonin (M) (5, 10, 20 and 40 mg/kg bw; i.p.) with/without ISO administration (25 mg/kg bw; s.c.). Values are expressed as means ± S.E. for six samples for each group. (∗p < 0.001 versus control, #p < 0.001 versus ISO treated cardiac mitochondria, using one way ANOVA).
Cardiac mitochondrial GPx, GR and CAT enzyme activities (Figure 3A, B and D respectively) were found to be significantly decreased whereas a significant increase in Mn SOD activity (Figure 3C) was observed upon ISO treatment. Pre-treatment with melatonin could, however, dose-dependently provide protection against all such alterations. Only melatonin treated groups did not show any significant difference when compared to control group.
Figure 3.
Diagrammatic representation of the changes in the enzymatic activities of (A) GPx (B) GR (C) Mn SOD and (D) Catalase in cardiac mitochondria from rats pre-treated with different doses of melatonin (M) (5, 10, 20 and 40 mg/kg bw; i.p.) with/without ISO administration (25 mg/kg bw; s.c.). Values are expressed as means ± S.E. for six samples for each group. (∗p < 0.001 versus control, #p < 0.001 versus ISO treated cardiac mitochondria, using one way ANOVA).
Enzyme activities of mitochondrial PDH (Figure 4A) and all Krebs' cycle enzymes namely, aconitase, ICDH, αKGDH, SDH, fumerase, MDH and citrate synthase (Figure 4B-H) were significantly inhibited upon ISO treatment, however, dose-dependent pre-treatment with melatonin showed a remarkable ability to prevent such inhibition and protected these enzymes from being altered. There was no significant difference as compared to control in the activities of the above enzymes in the mitochondrial samples isolated from only melatonin treated rats.
Figure 4.
Graphical representation of the changes in the activities of Krebs' cycle enzymes namely (A) Pyruvate dehydrogenase (PDH) (B) Aconitase (C) Isocitrate dehydrogenase (ICDH) (D) alpha ketoglutarate dehydrogenase (α KGDH) (E) Succinate dehydrogenase (SDH) (F) Fumerase (G) Malate dehydrogenase (MDH) and (H) Citrate Synthase activity in cardiac mitochondria from rats pre-treated with different doses of melatonin (M) (5, 10, 20 and 40 mg/kg bw; i.p.) with/without ISO administration (25 mg/kg bw; s.c.). Values are expressed as means ± S.E. for six samples for each group. (∗p < 0.001 versus control, #p < 0.001 versus ISO treated cardiac mitochondria, using one way ANOVA).
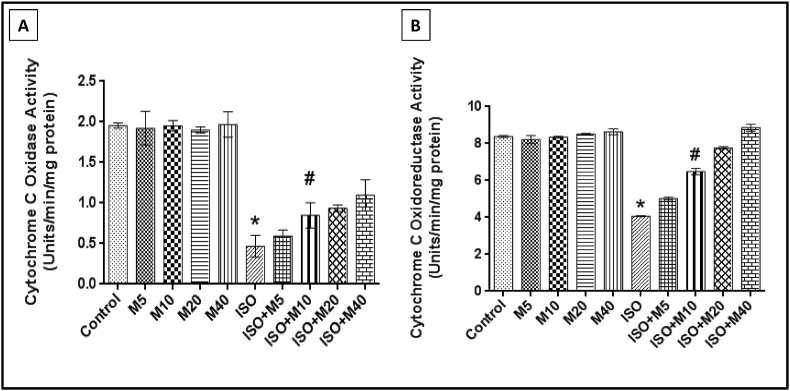
Figure 5A and B depicts the significant decrease in Cytochrome c oxidase and Cytochrome c oxidoreductase activity after ISO treatment which was dose-dependently found to be protected by melatonin pre-treatment with 10 mg/kg bw being the minimum effective dose of protection. Only melatonin treated rats did not show any remarkable difference when compared to control in the above parameters.
Figure 5.
Diagrammatic representation of the changes in the enzymatic activities of respiratory chain enzymes: (A) Cytochrome c oxidase and (B) Cytochrome c oxidoreductase activity in cardiac mitochondria from rats pre-treated with different doses of melatonin (M) (5, 10, 20 and 40 mg/kg bw; i.p.) with/without ISO administration (25 mg/kg bw; s.c.). Values are expressed as means ± S.E. for six samples for each group. (∗p < 0.001 versus control, #p < 0.001 versus ISO treated cardiac mitochondria, using one way ANOVA).
After analyzing all the above mentioned parameters in a dose-response approach, it was found that melatonin pre-treatment at a minimum dose of 10 mg/kg bw prior to ISO treatment showed significant results as compared to only ISO treated groups and hence was the dose of choice for further investigation. Thus, a more detailed investigation was carried out using only the minimum effective dose of 10 mg/kg bw of melatonin alone and also thirty minutes prior to ISO treatment.
Figure 6A shows rat hearts stained with TTC stain in which infarcted area is shown by a white patch in ISO treated heart section which shows a significantly increased percentage of risk area in Figure 6B whereas pre-treatment with melatonin at a minimum effective dose of 10 mg/kg bw could effectively reduce the extent of infarction caused by ISO. There was no significant occurrence of an infarct in heart sections from only melatonin treated (10 mg/kg bw) rats in comparison to control.
Figure 6.
Identification of infarct area by TTC staining of rat heart. Panel (A) shows the representative images of rat heart sections of different groups. Infarct area is a white patch in the histological section of heart from ISO (25 mg/kg bw; s.c.) treated rat shown by black arrows. Pre-treatment of rats with melatonin with 10 mg/kg bw; i.p (ISO + M10) prior to ISO treatment was identified as the minimum effective dose of protection. M10 represents only melatonin treated (10 mg/kg bw) group. Panel B shows the graphical representation of the percentage of risk area in each tissue. Values are expressed as means ± S.E. for each group. (∗p < 0.001 versus control, #p < 0.001 versus ISO treated heart, using one way ANOVA).
H&E staining of heart tissue sections revealed severe myodegeneration and necrosis of muscle fibers, interstitial edema, and heavy neutrophil infiltration in ISO treated tissues as is evident from Figure 7A. All these changes were found to be reduced in 10 mg/kg bw melatonin pre-treated group. Masson's trichrome staining (Figure 7B1), revealed that collagen deposition was higher in ISO treated groups as compared to control along with a higher percentage of fibrosis as seen in Figure 7B2. However, melatonin pre-treatment was found to significantly decrease collagen deposition and it also reduced the percentage of fibrosis at a minimum effective dose of 10 mg/kg bw. Tissue sections from only melatonin (10 mg/kg bw) treated group did not show any significant difference as compared to control.
Figure 7.
Representative images of H&E stained rat heart tissue sections (panel A) sections along with Masson trichrome stained heart tissue sections (panel B1) of control (Control), only melatonin treated -10 mg/kg bw only (M10), only ISO (25 mg/kg bw) treated and melatonin pretreated + ISO administered group (ISO + M10). Black arrows in H&E stained tissue sections indicate ISO induced infiltration of neutrophils and myofibril degeneration in cardiac tissue. Masson trichrome stained ISO treated heart sections indicated increased collagen content and marked area of fibrosis (black arrow). (40× magnification). B2) Graphical representation of percentage of fibrosis area from the images of Panel B1 of control (CON), melatonin only (M10), ISO treated (ISO) and ISO + melatonin treated (ISO + M10) groups. Values are expressed as means ± S.E. for six samples for each group. (∗p < 0.001 versus control, #p < 0.001 versus ISO treated heart, using one way ANOVA).
Janus Green B stain was used to assess the intactness of rat cardiac mitochondria (Figure 8A). A significantly lower level of fluorescent intensity percentage in ISO group was observed, however, melatonin pre-treated rats with 10 mg/kg bw dose were effectively protected from such damage as quantified in Figure 8B. Mitochondria isolated from the hearts of only melatonin treated group (10 mg/kg bw) did not show any significant decrease in fluorescence intensity as compared to control.
Figure 8.
Representative images of Janus green B stained mitochondrial samples of rat heart tissue (20× magnification) is shown in Panel A. Panel B shows the graphical representation of percentage of fluorescent intensity from the images of Panel A of control (CON), melatonin only (M10), ISO treated (ISO) and ISO + melatonin treated (ISO + M10) groups. Values are expressed as means ± S.E. for six samples for each group. (∗p < 0.001 versus control, #p < 0.001 versus ISO treated heart, using one way ANOVA).
SEM images of ISO treated cardiac mitochondria displayed increased irregularities in surface topology along with blebbing and convolution of outer membrane as shown by the peaks in Figure 9. Melatonin pre-treatment, however, prevented these changes and protected the surface morphology of mitochondria from being altered. Melatonin alone however did not show any significant effect.
Figure 9.
Representative SEM images of isolated rat heart mitochondria with their corresponding surface roughness being displayed alongside the image (magnification 20 KX) of different groups: control (CON), melatonin only (M10), ISO treated (ISO) and ISO + melatonin treated (ISO + M10) group. Red arrow heads indicate increased roughness and ISO induced blebbing of mitochondrial surface in the green peaks shown alongside the images.
Flow cytometric analysis of JC-1 stained mitochondria revealed that majority of control mitochondria are polarized (Figure 10A), however, ISO treated mitochondria showed predominantly depolarized populations. Surprisingly, mitochondria from melatonin pre-treated group showed a significantly lower percentage of depolarized population as illustrated in Figure 10B. Mitochondria isolated from only melatonin treated rats did not exhibit any significant change in JC-1 analysis as compared to control.
Figure 10.
Flurometric analysis of rat heart mitochondria after JC-1 staining. Panel A shows the FACS distribution pattern of cardiac mitochondria stained with JC-1 dye according to change in membrane potential of the different groups: control (CON), melatonin only (M10), ISO treated (ISO) and ISO + melatonin treated (ISO + M10) group. Panel B represents the histogram showing the fluorescent intensity (%) of JC-1 (percent of depolarized population of mitochondrial membrane) of the different groups. Values are expressed as means ± S.E. for six samples for each group. (∗p < 0.001 versus control, #p < 0.001 versus ISO treated heart, using one way ANOVA).
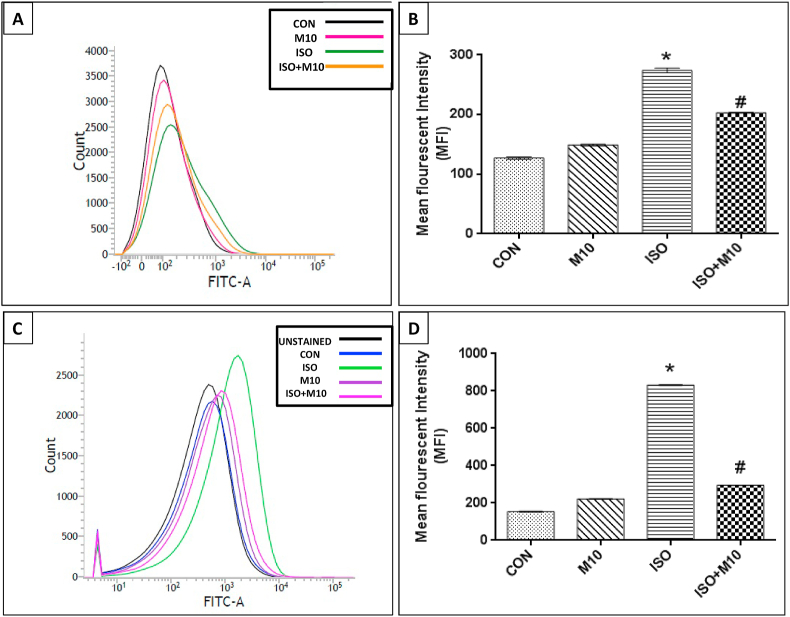
The Mean Fluorescence intensity (MFI) of calcein dye due to Ca2+ accumulation as well as DCFH-DA stained ROS in ISO treated mitochondria showed increased levels with a significant rightward shift. However, mitochondria isolated from melatonin pre-treated rats showed a reduced permeability of calcium ions across the mitochondrial membrane (Figure 11B) along with decreased ROS levels (Figure 11D) and hence significantly lower levels of fluorescence with a leftward shift of MFI in the histogram suggesting that melatonin prevents calcium ion loss and ROS generation and hence prevents cellular apoptosis. The mitochondria isolated from only melatonin treated group did not show any significant difference in either of the two parameters as compared to control.
Figure 11.
Representative images of ISO induced changes in rat heart mitochondria by FACS analysis after (A) Calcein staining. Panel B shows the graphical representation of the mean fluorescent intensity (MFI) obtained after calcein staining of the different groups: control (CON), melatonin only (M10), ISO treated (ISO) and ISO + melatonin treated (ISO + M10) group. Panel C represents the curve showing DCFDA staining of mitochondria from control (CON), melatonin only (M10), ISO treated (ISO) and ISO + melatonin treated (ISO + M10) group respectively and panel D represents the quantification of ROS in terms of mean fluorescence intensity in rat cardiac mitochondria of the different groups. Values are expressed as means ± S.E. for six samples for each group. (∗p < 0.001 versus control, #p < 0.001 versus ISO treated heart, using one way ANOVA).
Figure 12A,B, C and D shows a significant increase in serum pro-inflammatory cytokines like TNF α, IL 1β and IL-6 and decrease in an anti-inflammatory cytokine, IL-10 respectively after ISO administration which were found to be restored to near normal levels upon melatonin pre-treatment. Melatonin treatment alone showed no significant changes in cytokines levels when compared to control.
Figure 12.
Diagrammatic representation of the changes in the inflammatory levels of pro-inflammatory (A) TNF α, (B) IL - 1β, (C) IL - 6 and an anti inflammatory cytokine (D) IL - 10 in different groups: control (CON), melatonin only (M10), ISO treated (ISO) and ISO + melatonin treated (ISO + M10) group. Values are expressed as means ± S.E. for six samples for each group. (∗p < 0.001 versus control, #p < 0.001 versus ISO treated cardiac mitochondria, using one way ANOVA).
Figure 13A shows that mitochondria from ISO treated rats expressed significantly higher protein levels of NFκB (Figure 13B), and HSP70 (Figure 13D). However, melatonin pre-treatment significantly declined their expression. Significant upregulation in gene expression of PGC 1α, SIRT1 and SIRT3 (Figure 13C, E and F respectively) by melatonin pre-treatment was also observed. No significant changes was observed in gene expression of the above parameters in only melatonin treated rats in comparison to control.
Figure 13.
Diagrammatic representation of the immunoblots [A] and their relative densities of (A) NFKB, (B)PGC 1 α, (C) HSP70, (D) SIRT1, (E) SIRT3 and (F) β ACTIN of rat cardiac mitochondria from different groups: control (CON), melatonin only (M10), ISO treated (ISO) and ISO + melatonin treated (ISO + M10) group. Values are expressed as means ± S.E. for six samples for each group. (∗p < 0.001 versus control, #p < 0.001 versus ISO treated cardiac mitochondria, using one way ANOVA).
Figure 14 shows the representative images of isothermal titration calorimetric (ITC) data showing heat change vs time titration curve. A highly exothermic reaction between ISO and catalase was observed with a three site sequential binding pattern with rapid opening of the sites accompanied by fast heat exchange per second which does not tends towards saturation as is suggested by the downward sharp peaks seen in Figure 14A. In Figure 14B, a fairly good thermodynamic interaction with a one site rapid binding pattern between catalase and melatonin was observed with no free binding sites and hence the reaction goes towards saturation. However, when ISO and melatonin together interacted with catalase (Figure 14D), a highly exothermic reaction occurred, as ISO binds to catalase rapidly, shown by sharp downward peaks with a gradual decrease in heat change. However, the second half of the curve displays characteristics similar to that of melatonin and catalase ITC curve, which suggests that melatonin binds to both ISO and catalase very quickly and thereby protects the enzyme from damage. The initial binding pattern of ISO and melatonin only (Figure 14C) shows a very rapid heat change per second and opening of sequential binding sites making it a highly exothermic reaction by nature.
Figure 14.
A representative isothermal titration calorimetric dataset of pure Catalase (CAT). In the heat change vs. time titration curve, each peak represents an injection of ligand into the sample cell that contains pure CAT, where the ligand used is (A) ISO, (B) melatonin (D) catalase, ISO and melatonin. Panel C represents ITC curve showing the interaction between ISO as sample and melatonin as ligand. The amount of heat change per second (ΔH) is represented by the area under the curve (top curve) and the heat change in terms of kcal mol−1 of injectant against molar ratio is shown in bottom curve of each figure respectively. Values of ΔH, ΔS and no. of sites were expressed in terms of mean ± S.E.
4. Discussion
Melatonin has been shown to protect against ROS induced mitochondrial dysfunction in various disease conditions [58, 59, 60, 61, 62]. In, the present study, ISO administration (25 mg/kg bw) caused acute myocardial infarction through the β-adrenergic pathway as it acts as a β adrenergic agonist [11]. Elevated levels of serum and mitochondrial oxidative stress biomarkers along with changes in GSH, GSSG and GSH: GSSG ratio after ISO treatment suggests substantial free radical production and disturbances in the cellular redox buffer [32]. The oxidation of ISO to semiquinones, which in turn react with oxygen to produce O2•- and H2O2 [63], and the susceptibility of cardiolipin present in inner mitochondrial membrane to lipid peroxidation are the primary causative factors that lead to ETC inactivation thereby signaling increased ROS and RNS production [64, 65]. Decrease in GPx activity, which protects against oxidative damage by catalyzing the removal of hydrogen peroxide via oxidation of GSH that is recycled from oxidized glutathione by the NADPH-dependent GR [66], along with increased MnSOD activity which catalyzes the removal of superoxide radicals and converts it to hydrogen peroxide which is neutralized by CAT enzyme [66] are the consequences of ISO administration. Melatonin has been reported to upregulate the gene expression of antioxidant enzymes in the face of oxidative stress [67]. In agreement with previous findings, we concluded that melatonin protected mitochondrial enzymes from being altered via its free radical scavenging and antioxidant properties.
The inhibition of Krebs' cycle enzymes, which regulate the energy metabolism of the cell, by ISO, is consistent with previous studies [11, 51] and it was perhaps due the fact that the oxygen involved in ETC may directly oxidize and inactivate iron-sulfur cluster proteins thus affecting respiratory chain activity. It may also lead to electron leakage along with the generation of the highly reactive •OH and the peroxy nitrite radical (ONOO_), a potent oxidizing agent that can cause oxidative damage to mitochondrial proteins and function. By scavenging these free radical, melatonin protects against the inhibition of Krebs' cycle enzymes and also prevents the ETC from malfunctioning, thereby reducing the risk of free radical mediated damage.
Another plausible explanation for inactivation of the above enzymes could be due to the physical binding of ISO to these enzymes. However, in order to bind to the enzymes present in the mitochondria, ISO molecules must first enter the mitochondria by passing through the double membrane which is possible only if the mitochondrial membrane gets damaged. Histopathological analysis of ISO treated rat hearts after TTC staining displayed marked gross infarcts along with H&E and Masson trichrome stained tissue sections that displayed marked histological changes suggesting that tissue injury upon ISO treatment is distinct as reported earlier [11, 51]. Notably, vital staining with Janus Green B and SEM analysis of isolated mitochondria indicated a disruption of membrane integrity and surface topology upon ISO treatment which is consistent with previous confocal and biochemical findings which showed increased mitochondrial membrane roughness and appearance of blebs which were found to be prevented upon pre-treatment with melatonin [51]. In this study, melatonin pre-treatment of the rat heart mitochondria was found to protect the mitochondria from ISO induced changes.
Mitochondrial membrane potential (MMP), measured by JC-1 staining, is an indicator for cell death or apoptosis. Decrease in MMP coincides directly with the opening of the mitochondrial membrane permeability transition pores (MPTP), resulting in the release of cytochrome c into the cytosol, which in turn leads to downstream events in the apoptotic cascade. JC-1 is a mitochondria-specific dual-fluorescent probe that exhibits a potential-dependent accumulation in mitochondria showing higher levels of accumulation in control or polarised and decreased accumulation in depolarized mitochondria [68] that have undergone ISO treatment. Pre- treatment with melatonin at 10 mg/kg bw protected the mitochondria by scavenging ROS, decreasing MMP and thereby inhibiting the opening of the MPTP.
Elevated levels of mitochondrial Ca2+ plays an important role in initiation of programmed cell death or apoptosis [69]. Increased calcein fluorescence observed in ISO treated mitochondria indicates high calcium load which may possibly disturb the mitochondrial function by altering MMP and increasing ROS production [70, 71]. Notably, in the present study, the fourfold increase in ROS production in ISO treated mitochondria and mitochondrial calcium overload was significantly reduced by melatonin, which by virtue of its antioxidant property, could alleviate ROS mediated mitochondrial dysfunction.
The redox status of the cell is regulated by an important transcription factor called NF-kB, which has been shown to modulate several pathways related to cellular immune response, inflammation, and proliferation in the skeletal muscle and it also plays a key role in the expression of the inducible inflammatory genes, including TNF α and interleukin 1β [72]. TNF-α induced production of mitochondrial ROS also creates a positive feedback loop whereby the increased ROS levels cause further activation of NF-kB [73]. In line with the literature, we observed increased gene expression and activation of NF-kB in response to increased production of pro-inflammatory cytokines such as IL-1β, TNF-α and IL-6 in ISO treated rats along with decrease in IL-10, an anti-inflammatory cytokine. Conversely, melatonin pre-treatment not only lowered the levels of pro-inflammatory cytokines but also protected the IL -10 level, thus confirming melatonin's e anti-inflammatory influence reported earlier [74].
In the present study we found that melatonin induced activation of SIRT1, a NAD-dependent class III histone deacetylase, upon ISO treatment, along with SIRT1 mediated deacetylation and activation of PGC-1α, a metabolic regulator of mitochondrial biogenesis [21] which has previously been reported to protect against cadmium hepatotoxity, cardiovascular disease, neurodegeneration, and liver injury [75, 76, 77, 78]. Interestingly, we also observed up-regulation of SIRT3, the principal mitochondrial deacetylase and downstream target of SIRT1- PGC-1α signaling, known to regulate mitochondrial biogenesis and promote mitochondrial function by deacetylating and activating SOD2(MnSOD) [79] which in turn improves the GSH:GSSG redox balance thus preventing oxidative injury in a number of diseases conditions [80, 81]. Hence, the SIRT1-PGC-1α–SIRT3 signaling mechanism adopted by melatonin indirectly activates the downstream signaling pathways that not only induce mitochondrial biogenesis but also prevents mitochondrial dysfunction in rat heart against ISO induced oxidative stress.
Amongst all the observations of the study, perhaps, the most interesting and fascinating finding of the study was the thermodynamic binding pattern observed between ISO and melatonin and their pattern of heat change with catalase. The ITC binding dynamics between ISO and catalase shows a sequential and rapid binding pattern suggesting that the binding of one molecule of ISO to a single binding site of catalase opens up numerous new binding sites for ISO to attach thereby aggravating the oxidative damage caused to the antioxidant enzyme. However, unlike ISO, melatonin binds to catalase in a one site binding pattern with a positive net enthalpy change which indicates that melatonin binds to catalase and saturates all the sites available without opening new binding sites which is consistent with our earlier finding [23]. This protects and preserves the structure and function of catalase. Surprisingly, the isothermal calorimetric binding study between ISO and melatonin gave us results that are novel in its approach as well are being reported, perhaps, for the first time. The reaction between melatonin and ISO is highly exothermic with a net negative enthalpy change suggesting a spontaneous and very rapid binding pattern with unprecedented affinity between the molecules which tend to attain saturation very quickly. This observation indicates that melatonin's ability to rapidly bind to and neutralize these oxidant molecules as well as restrain them from binding to other cellular structures or macromolecules stands in testimony to its proficient anti-oxidative capability. A previous report on ITC studies involving melatonin mentions that melatonin was thought to dispense its potential antioxidant benefits via its MT3 receptor which has been recently identified as QR2 and inhibition of QR2 by melatonin may render the cells to be protected from ROS [82]. Although the cardioprotective nature of melatonin against ISO induced myocardial injury in rats has previously been shown [83], this new mechanism of dual protection afforded by melatonin to antioxidant enzymes, evident from the ITC pattern between ISO, melatonin and catalase, which shows that melatonin sequesters ISO molecules and prevents it from attacking the binding sites of catalase along with occupying and masking the active sites of catalase enzyme themselves thereby preventing any possibility of interaction between ISO and catalase is unique to this study and highlights the beneficial role of melatonin. Although many medicinal plants with antioxidant properties have been used as a cardioprotective agent against ISO [84], melatonin remains the most potent and promising candidate of all. The therapeutic role of exogenous melatonin in cardiovascular diseases has been discussed previously [85] suggesting it to be consumed through plant foods or by direct supplementation to promote various health benefits [86].
5. Conclusion
The most crucial findings of the current study reported a significant protection and stimulation of SIRT1, PGC 1α and SIRT3 genes upon melatonin pre-treatment which suggests that that perhaps melatonin engages in the activation of the SIRT1-PGC 1α-SIRT3 signaling pathway to exhibit its antioxidant and cardioprotective effects along with promoting mitochondrial biogenesis against ISO mediated mitochondrial myocardial injury. Also, perhaps, we are the first to report and demonstrate the novel and dual nature of protection provided by melatonin to antioxidant enzymes from oxidant molecules, like ISO, thereby preventing oxidative stress induced injury to cardiac tissue as well as mitochondria. The important findings of the study have been summarized in a graphical abstract as depicted in Figure 15. This unique nature of melatonin warrants further investigation and analysis to truly understand and appreciate this extraordinary attribute of this ubiquitous indole.
Figure 15.
Pre-treatment of rats with melatonin (10 mg/kg bw) prior to ISO treatment (25 mg/kg bw) prevented occurrence of myocardial infarction and averted mitochondrial dysfunction caused by ISO mediated oxidative stress. Melatonin prevented ISO induced changes in mitochondrial LPO, PCO, GSH,GPX,GR, Kreb's cycle enzyme activity along with preventing the release of inflammatory cytokines thereby checking mitochondrial oxidative stress. Melatonin was also shown to inhibit ISO induced oxidative stress mediated increase in NFκβ by stimulating SIRT1 release which in turn activated the downstream cascade of PGC 1α and SIRT3 signalling. Activated or upregulated SIRT3 signals MnSOD production which ultimately was shown to improve the GSH/GSSG ratio which could effectively prevent mitochondrial oxidative stress to prevent cell death and necrosis. Activation of PGC 1α and SIRT3 is also known to prevent mitochondrial dysfunction and furthermore upregulation of PGC 1α promotes mitochondrial biogenesis. The red arrows indicate the effect of ISO treatment alone and the green arrows indicate the effect of melatonin pretreatment before ISO treatment.
Declarations
Author contribution statement
Shamreen Naaz: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Sanatan Mishra, Palash K Pal: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Aindrila Chattopadhyay: Conceived and designed the experiments; Analyzed and interpreted the data.
Asish R. Das: Analyzed and interpreted the data.
Debasish Bandyopadhyay: Conceived and designed the experiments; Wrote the paper.
Funding statement
This work was supported by the UGC Major Research Project under CPEPA Scheme of UGC awarded to University of Calcutta (Grant No: 8-2/2008(NS/PE) DATED 14.12.2011) (Available to Debasish Bandyopadhyay) as well as the UPE–II Scheme of UGC, Govt. of India awarded to University of Calcutta. Shamreen Naaz and Sanatan Mishra were supported by the BI-92 of Department of Physiology University of Calcutta (Teacher's Research Grant) (Available to Debasish Bandyopadhyay) Palash Kumar Pal was supported by the UGC Dr. D. S. Kothari Post Doctoral Fellowship (BL/16-17/0502), Govt. of India. Debasish Bandyopadhyay was supported by the DST PURSE Grant awarded to Calcutta University.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thankfully acknowledge the technical help of Dr. Ritesh Kumar Tiwari, Center of Excellence for Nanobiotechnology (CRNN), University of Calcutta for the flowcytometric studies along with Souvik Roy and Arijit Pal from DBT-IPLS scheme of University of Calcutta, Department of Biotechnology (DBT), Govt. of India are also acknowledged for their support in helping to carry out Isothermal calorimetric studies.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Reiter R.J. Melatonin, a novel protective agent against oxidative injury of the ischemic reperfused heart. Cardiovasc. Res. 2003;58:10–19. doi: 10.1016/s0008-6363(02)00827-1. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S., Hawken S., Ounpuu S., Dans T., Avezum A., Lanas F., McQueen M., Budaj A., Pais P., Varigos J., Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 3.Petrosillo G., Di Venosa N., Pistolese M., Casanova G., Tiravanti E., Colantuono G., Federici A., Paradies G., Ruggiero F.M. Protective effect of melatonin against mitochondrial dysfunction associated with cardiac ischemia-reperfusion: role of cardiolipin. Faseb. J. 2006;20:269–276. doi: 10.1096/fj.05-4692com. [DOI] [PubMed] [Google Scholar]
- 4.Mariani E., Polidori M.C., Cherubini A., Mecocci P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005;827(1):65–75. doi: 10.1016/j.jchromb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Scheibye-Knudsen M., Fang E.F., Croteau D.L., Wilson D.M., III, Bohr V.A. Protecting the mitochondrial powerhouse. Trends Cell Biol. 2015;25(3):158–170. doi: 10.1016/j.tcb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weidinger A., Kozlov A.V. Biological activities of reactive oxygen and nitrogen species: oxidative stress versus signal transduction. Biomolecules. 2015;5(2):472–484. doi: 10.3390/biom5020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heusch G., Boengler K., Schulz R. Inhibition of mitochondrial permeability transition pore opening: the Holy Grail of cardioprotection. Basic Res. Cardiol. 2010;105:151–154. doi: 10.1007/s00395-009-0080-9. [DOI] [PubMed] [Google Scholar]
- 8.Li T., Zhang P., Liu J., Zhou R., Li Q., You Z., Dian K. Protective effects of hemoglobin-based oxygen carrier given to isolated heart during ischemia via attenuation of mitochondrial oxidative damage. Free Radic. Biol. Med. 2010;48(8):1079–1089. doi: 10.1016/j.freeradbiomed.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 9.Bindoli A., Rigobello M.P., Deeble D.J. Biochemical and toxicological properties of the oxidation products of catecholamines. Free Radic. Biol. Med. 1992;13(4):391–405. doi: 10.1016/0891-5849(92)90182-g. [DOI] [PubMed] [Google Scholar]
- 10.Chattopadhyay A., Biswas S., Bandyopadhyay D., Sarkar C., Datta A.G. Effect of isoproterenol on lipid peroxidation and antioxidant enzymes of myocardial tissue of mice and protection by quinidine. Mol. Cell. Biochem. 2003;245:43–49. doi: 10.1023/a:1022808224917. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee D., Roy S.G., Bandyopadhyay A., Chattopadhyay A., Basu A., Mitra E., Ghosh A.K., Reiter R.J., Bandyopadhyay D. Melatonin protects against isoproterenol-induced myocardial injury in the rat: antioxidative mechanisms. J. Pineal Res. 2010;48(3):251–262. doi: 10.1111/j.1600-079X.2010.00749.x. [DOI] [PubMed] [Google Scholar]
- 12.Khalil Md., Ahmmed I., Ahmed R., Tanvir E.M., Afroz R., Paul S., Gan S.H., Alam N. Amelioration of isoproterenol-induced oxidative damage in rat myocardium by Withania somnifera leaf extract. BioMed Res. Int. 2015:1–10. doi: 10.1155/2015/624159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reiter R.J., Tan D.X., Galano A. Melatonin: exceeding expectations. Physiology. 2014;29(5):325–333. doi: 10.1152/physiol.00011.2014. [DOI] [PubMed] [Google Scholar]
- 14.Tan D.X., Reiter R.J., Manchester L.C., Yan M.T., El-Saw M., Sainz R.M., Mayo J.C., Kohen R., Allegra M., Hardeland R. Chemical and physical properties and potential mechanism: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002;2(2):181–197. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- 15.Manchester L.C., Coto-Montes A., Boga J.A., Andersen L.P., Zhou Z., Galano A., Vriend J., Tan D.X., Reiter R.J. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015;59(4):403–419. doi: 10.1111/jpi.12267. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez C., Mayo J.C., Sainz R.M., Antolín I., Herrera F., Martín V., Reiter R.J. Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal Res. 2004;36(1):1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 17.Beyer C.E., Steketee J.D., Saphier D. Antioxidant properties of melatonin--an emerging mystery. Biochem. Pharmacol. 1998;56(10):1265–1272. doi: 10.1016/s0006-2952(98)00180-4. [DOI] [PubMed] [Google Scholar]
- 18.Kotler M., Rodríguez C., Sáinz R.M., Antolín I., Menéndez-Peláez A. Melatonin increases gene expression for antioxidant enzymes in rat brain cortex. J. Pineal Res. 1998;24(2):83–89. doi: 10.1111/j.1600-079x.1998.tb00371.x. [DOI] [PubMed] [Google Scholar]
- 19.Galano A., Medina M.E., Tan D.X. Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: a physicochemical analysis. J. Pineal Res. 2015;58(1):107–116. doi: 10.1111/jpi.12196. [DOI] [PubMed] [Google Scholar]
- 20.Tan D.X., Manchester L.C., Terron M.P., Flores L.J., Reiter R.J. One molecule, many derivatives; a never-ending interaction of melatonin with reactive oxygen and nitrogen species. J. Pineal Res. 2007;42(1):28–42. doi: 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 21.Kahn B.B., Alquier T., Carling D., Hardie D.G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metabol. 2005;1(1):15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Yu L., Gong B., Duan W., Fan C., Zhang J., Li Z., Xue X., Xu Y., Meng D., Li B., Zhang B.M. Melatonin ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic rats by preserving mitochondrial function: role of AMPK-PGC-1α-SIRT3 signaling. Sci. Rep. 2017;7(1):1–13. doi: 10.1038/srep41337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh A.K., Naaz S., Bhattacharjee B., Ghosal N., Chattopadhyay A., Roy S., Reiter R.J., Bandyopadhyay D. Mechanism of melatonin protection against copper-ascorbate-induced oxidative damage in vitro through isothermal titration calorimetry. Life Sci. 2017;180:123–136. doi: 10.1016/j.lfs.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 25.Strittmatter C.F. Studies on avian xanthine dehydrogenases, properties and patterns of appearance during development. J. Biol. Chem. 1965;240(6):2557–2564. [PubMed] [Google Scholar]
- 26.Varcoe J.S. World Scientific Publishing Company; 2001. Clinical Biochemistry: Techniques and Instrumentation-A Practical Approach. [Google Scholar]
- 27.Levine R.L., Williams J.A., Stadtman E.P., Shacter E. Carbonyl assays for determination of oxidatively modified. Methods Enzymol. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 28.Buege J.A., Aust S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 29.Bandyopadhyay D., Ghosh G., Bandyopadhyay A., Reiter R.J. Melatonin protects against piroxicam-induced gastric ulceration. J. Pineal Res. 2004;36(3):195–203. doi: 10.1111/j.1600-079x.2004.00118.x. [DOI] [PubMed] [Google Scholar]
- 30.Sedlak J., Lindsay R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968;25(1):192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 31.Wendell P.L. Measurement of oxidized glutathione and total glutathione in the perfused rat heart. Biochem. J. 1970;117:661–665. doi: 10.1042/bj1170661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schafer F.Q., Buetner G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30(11):1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 33.Paglia D.E., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967;70(1):158–169. [PubMed] [Google Scholar]
- 34.Chattopadhyay A., Choudhury T.D., Bandyopadhyay D., Datta A.G. Protective effect of erythropoietin on the oxidative damage of erythrocyte membrane by hydroxyl radical. Biochem. Pharmacol. 2000;59(4):419–425. doi: 10.1016/s0006-2952(99)00277-4. [DOI] [PubMed] [Google Scholar]
- 35.Krohne-Ehrich G., Schirmer R.H., Untucht-Grau R. Glutathione reductase from human erythrocytes. Isolation of the enzyme and sequence analysis of the redox-active peptide. Eur. J. Biochem. 1977;80(1):65–71. doi: 10.1111/j.1432-1033.1977.tb11856.x. [DOI] [PubMed] [Google Scholar]
- 36.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1947;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 37.Beers R.F., Sizer I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952;195(1):133–140. [PubMed] [Google Scholar]
- 38.Chretien D., Pourrier M., Bourgeron T., Séné M., Rötig A., Munnich A., Rustin P. An improved spectrophotometric assay of pyruvate dehydrogenase in lactate dehydrogenase contaminated mitochondrial preparations from human skeletal muscle. Clin. Chim. Acta. 1995;240(2):129–136. doi: 10.1016/0009-8981(95)06145-6. [DOI] [PubMed] [Google Scholar]
- 39.Gardner P.R., Nguyen D.H., White C.W. Aconitase is a sensitive and critical target of oxygen poisoning in cultured mammalian cells and in rat lungs. Proc. Nat. Acad. Sci. U. S. A. 1994;91:12248–12252. doi: 10.1073/pnas.91.25.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duncan M.J., Fraenkel D.G. alpha-Ketoglutarate dehydrogenase mutant of Rhizobium meliloti. J. Bacteriol. 1979;137(1):415–419. doi: 10.1128/jb.137.1.415-419.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veeger C., DerVartanian D.V., Zeylemaker W.P. Succinate dehydrogenase:[EC 1.3.99.1 Succinate: (acceptor) oxidoreductase] Methods Enzymol. 1969;13:81–90. [Google Scholar]
- 42.Racker E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim. Biophys. Acta. 1950;4:211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- 43.Dasika S.K., Santosh K.C., Beard D.A. Determination of the catalytic mechanism for mitochondrial malate dehydrogenase. Biophys. J. 2015;108(2):408–419. doi: 10.1016/j.bpj.2014.11.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parvin R. Citrate synthase from yeast: [EC 4.1.3.7 Citrate oxaloacetage-lyase (CoA-acetylating)] Methods Enzymol. 1969;13:16–19. [Google Scholar]
- 45.Goyal N., Srivastava V.M. Oxidation and reduction of cytochrome c by mitochondrial enzymes of Setaria cervi. J. Helminthol. 1995;69(1):13–17. doi: 10.1017/s0022149x00013778. [DOI] [PubMed] [Google Scholar]
- 46.Yan X., Shichita T., Katsumata Y., Matsuhashi T., Ito H. Deleterious effect of the IL-23/IL-17A axis and γδT cells on left ventricular remodeling after myocardial infarction. J. Am. Heart Assoc. 2012;1(5) doi: 10.1161/JAHA.112.004408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mukherjee D., Ghosh A.K., Bandyopadhyay A., Basu A., Dutta S., Pattari S.K., Reiter R.J., Bandyopadhyay D. Melatonin protects against isoproterenol-induced alterations in cardiac mitochondrial energy-metabolizing enzymes, apoptotic proteins, and assists in complete recovery from myocardial injury in rats. J. Pineal Res. 2012;53(2):166–179. doi: 10.1111/j.1600-079x.2012.00984.x. [DOI] [PubMed] [Google Scholar]
- 48.Sheehan D., Hrapchak B. second ed. Battelle Press; Ohio: 1980. Theory and Practice of Histotechnology; pp. 189–190. [Google Scholar]
- 49.Bancroft J., Stevens A. second ed. Churchill-Livingston; NY: 1982. Theory and Practice of Histological Techniques; pp. 131–135. [Google Scholar]
- 50.Luna L. third ed. McGraw-Hill; NY: 1968. Manual of Histologic Staining Methods of the AFIP; pp. 94–95. [Google Scholar]
- 51.Mukherjee D., Ghosh A.K., Dutta M., Mitra E., Mallick S., Saha B., Reiter R.J., Bandyopadhyay D. Mechanisms of isoproterenol-induced cardiacmitochondrial damage:protective actions of melatonin. J. Pineal Res. 2015;58(3):275–290. doi: 10.1111/jpi.12213. [DOI] [PubMed] [Google Scholar]
- 52.Cossarizza A., Salvioli S. Flow cytometric analysis of mitochondrial membrane potential using JC-1. Curr. Protoc. Cytometr. 2000;13(1):9–14. doi: 10.1002/0471142956.cy0914s13. [DOI] [PubMed] [Google Scholar]
- 53.Bratosin D., Mitrofan L., Palii C., Estaquier J., Montreuil J. Novel fluorescence assay using calcein-AM for the determination of human erythrocyte viability and aging. Cytometry. 2005;66A:78–84. doi: 10.1002/cyto.a.20152. [DOI] [PubMed] [Google Scholar]
- 54.Bhogal R.H., Curbishley S.M., Weston C.J., Adams D.H., Afford S.C. Reactive oxygen species mediate human hepatocyte injury during hypoxia/reoxygenation. Liver Transpl. 2010;16(11):1303–1313. doi: 10.1002/lt.22157. [DOI] [PubMed] [Google Scholar]
- 55.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;27:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 56.Rajarathnam K., Rosgen J. Isothermal titration calorimetry of membrane proteins - progress and challenges. Biochim. Biophys. Acta. 2014;1838:69–77. doi: 10.1016/j.bbamem.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lowry O.H., Rosenbrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 58.Escames G., López L.C., Tapias V., Utrilla P., Reiter R.J., Hitos A.B., Leon J., Rodriquez M.I., Acuña-Castroviejo D. Melatonin counteracts inducible mitochondrial nitric oxide synthase-dependent mitochondrial dysfunction in skeletal muscle of septic mice. J. Pineal Res. 2006;40(1):71–78. doi: 10.1111/j.1600-079X.2005.00281.x. [DOI] [PubMed] [Google Scholar]
- 59.Srinivasan V., Spence D.W., Pandi-Perumal S.R., Brown G.M., Cardinili D.P. Melatonin in mitochondrial dysfunction and related disorders. Int. J. Alzheimer's Dis. 2011:326320. doi: 10.4061/2011/326320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petrosillo G., DiVenosa N., Pistolese M., Casanova G., Tiravanti E., Colantuono G. Protective effect of melatonin against mitochondrial dysfunction associated with cardiac ischemia-reperfusion: role of cardiolipin. Faseb. J. 2006;20(2):269–276. doi: 10.1096/fj.05-4692com. [DOI] [PubMed] [Google Scholar]
- 61.Yang Y., Jiang S., Dong Y Y., Fan C., Zhao L., Yang X., Li J., Di S., Yue L. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT 1-dependent mechanism during ischemic-stroke in mice. J. Pineal Res. 2015;58(1):61–70. doi: 10.1111/jpi.12193. [DOI] [PubMed] [Google Scholar]
- 62.Paul S., Naaz S., Ghosh A.K., Mishra S., Chattopadhyay A., Bandyopadhyay D. Melatonin chelates iron and binds directly with phenylhydrazine to provide protection against phenylhydrazine induced oxidative damage in red blood cells along with its antioxidant mechanisms : an in vitro study. Melatonin Res. 2018;1(1):1–20. [Google Scholar]
- 63.Singal P.K., Kapur N., Dhillon K.S., Beamish R.E., Dhalla N.S. Role of free radicals in catecholamine-induced cardiomyopathy. Can. J. Physiol. Pharmacol. 1982;60(11):1390–1397. doi: 10.1139/y82-207. [DOI] [PubMed] [Google Scholar]
- 64.Paradies G., Petrosillo G., Paradies V., Reiter R.J., Ruggiero F.M. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J. Pineal Res. 2010;48(4):297–310. doi: 10.1111/j.1600-079X.2010.00759.x. [DOI] [PubMed] [Google Scholar]
- 65.Leon J., Acuna-Castroviejo D., Escames G., Tan D.X., Reiter R.J. Melatonin mitigates mitochondrial malfunctions. J. Pineal Res. 2005;38(1):104–117. doi: 10.1111/j.1600-079X.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 66.Dhalla N.S., Temsah R.M., Netticadan T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000;18(6):655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 67.Han L., Wang H., Li L., Li X., Ge J., Reiter R.J., Wang Q. Melatonin protects against maternal obesity-associated oxidative stress and meiotic defects in oocytes via the SIRT 3-SOD 2-dependent pathway. J. Pineal Res. 2017;63(3) doi: 10.1111/jpi.12431. [DOI] [PubMed] [Google Scholar]
- 68.Wang W.Z., Fang X.H., L Stephenson L. Melatonin attenuates I/R-induced mitochondrial dysfunction in skeletal muscle. J. Surg. Res. 2011;171(1):108–113. doi: 10.1016/j.jss.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 69.Orrenius S., Gogvadze V., Zhivotovsky B. Calcium and mitochondria in the regulation of cell death. Biochem. Biophys. Res. Commun. 2015;460:72–81. doi: 10.1016/j.bbrc.2015.01.137. [DOI] [PubMed] [Google Scholar]
- 70.Zhuo X.Z., Wu Y., Ni Y.J., Liu J.H., Gong M., Wang X.H. Isoproterenol instigates cardiomyocyte apoptosis and heart failure via AMPK inactivation-mediated endo-plasmic reticulum stress. Apoptosis. 2013;18:800-810. doi: 10.1007/s10495-013-0843-5. [DOI] [PubMed] [Google Scholar]
- 71.Yang J., Wang Z., Chen D.L. Shikonin ameliorates isoproterenol (ISO)-induced myocardial damage through suppressing fibro-sis, inflammation, apoptosis and ER stress. Biomed. Pharmacother. 2017;93:1343-1357. doi: 10.1016/j.biopha.2017.06.086. [DOI] [PubMed] [Google Scholar]
- 72.Titelbaum D.S., Degenhardt A., Kinkel R.P. Anti-tumor necrosis factor Alpha-associated multiple sclerosis. Am. J. Neuroradiol. 2005;26:1548–1550. [PMC free article] [PubMed] [Google Scholar]
- 73.Ghorbani A., Salari M., Shaygannejad V., Norouzi R. The role of melatonin in the pathogenesis of multiple sclerosis: a case-control study. Int. J. Prev. Med. 2013;4(2):S180–S184. [PMC free article] [PubMed] [Google Scholar]
- 74.Chahbouni M., Escames G., Venegas C., Selliva B., Garcia J.A. Melatonin treatment normalizes plasma pro-inflammatory cytokines and nitrosative/oxidative stress in patients suffering from Duchenne muscular dystrophy. J. Pineal Res. 2010;48(3):282–289. doi: 10.1111/j.1600-079X.2010.00752.x. [DOI] [PubMed] [Google Scholar]
- 75.Guo P., Pi H., Xu S., Zhang L., Li Y., Li M., Cao Z., Tian L. Melatonin improves mitochondrial function by promoting MT1/SIRT1/PGC-1 alpha-dependent mitochondrial biogenesis in cadmium-induced hepatotoxicity in vitro. Toxicol. Sci. 2014;142(1):182–195. doi: 10.1093/toxsci/kfu164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lagouge M., Argmann C., Gerhart-Hines Z.H., Meziane, Lerin C., Daussin F. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 77.Rodgers J.T., Lerin C., Gerhart-Hines Z., Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008;582:46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu H.R., Wang Z.Y., Zhu X.L., Wu X.X., Li E.G. Icariin protects against brain injury by enhancing SIRT1-dependent PGC-1alpha expression in experimental stroke. Neuropharmacology. 2010;59:70–76. doi: 10.1016/j.neuropharm.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 79.Hirschey M.D., Shimazo T., Goetzman E., Jing E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.You J., Yue Z., Chen S., Chen Y., Lu X., Zhang X., Shen P., Li J., Han Q., Li Z. Receptor-interacting Protein 140 represses Sirtuin 3 to facilitate hypertrophy, mitochondrial dysfunction and energymetabolic dysfunction in cardiomyocytes. Acta Physiol. 2016 doi: 10.1111/apha.12800. [DOI] [PubMed] [Google Scholar]
- 81.Matsushima S., Sadoshima J. The role of sirtuins in cardiac disease. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H1375–1389. doi: 10.1152/ajpheart.00053.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Calamini B., Santarsiero B.D., Boutin J.A., Mesecar A.D. Kinetic, thermodynamic and X-ray structural insights into the interaction of melatonin and analogues with quinone reductase 2. Biochem. J. 2008;413(1):81–91. doi: 10.1042/BJ20071373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Acikel M., Buyukokuroglu M.E., Aksoy H., Erdogan F., Erol M.K. Protective effects of melatonin against myocardial injury induced by isoproterenol in rats. J. Pineal Res. 2003;35(2):75–79. doi: 10.1034/j.1600-079x.2003.00056.x. [DOI] [PubMed] [Google Scholar]
- 84.Widyaningsih W., Pramono S., Zulaela S., Widyarini S. Protection by ethanolic extract from Ulva lactuca L. against acute myocardial infarction: antioxidant and antiapoptotic activities. Malays. J. Med. Sci. 2017;24(6):39–49. doi: 10.21315/mjms2017.24.6.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Imenshahidi M., Golamreza K., Hosseinzadeh H. Effects of melatonin on cardiovascular risk factors and metabolic syndrome: a comprehensive review. N. Schmied. Arch. Pharmacol. 2020;393:521–536. doi: 10.1007/s00210-020-01822-4. [DOI] [PubMed] [Google Scholar]
- 86.Favero G., Franceschetti L., Buffoli B., Moghadasian M.H., Reiter R.J., Rodella L.F., Rezzani R. Melatonin: protection against age-related cardiac pathology. Ageing Res. Rev. 2017;35:336–349. doi: 10.1016/j.arr.2016.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.