Abstract
Colorectal cancer screening saves lives and is cost-effective. It allows early detection of the pathology, and enables earlier medical intervention. Despite clinical practice guidelines promoting screening for average risk individuals, uptake remains suboptimal in many populations. Few studies have examined how sociobehavioural factors influence screening uptake in the context of behaviour change theories such as the health belief model. This systematic review therefore examines how the health belief model’s constructs are associated with colorectal cancer screening.
Four databases were systematically searched from inception to September 2019. Quantitative observational studies that used the health belief model to examine colorectal screening history, intention or behaviour were included.
A total of 30 studies met the criteria for review; all were of cross-sectional design. Perceived susceptibility, benefits and cues to action were directly associated with screening history or intention. Perceived barriers inversely associated with screening history or intention. The studies included also found other modifying factors including sociodemographic and cultural norms. Self-report of screening history, intention or behaviour, convenience sampling and lack of temporality among factors were common limitations across studies.
The health belief model’s associations with colorectal cancer screening uptake was consistent with preventive health behaviours in general. Future studies should examine how theory-based behavioural interventions can be tailored to account for the influence of socioecological factors.
Keywords: Colorectal cancer, Cancer screening, Barriers to screening, Health belief model, Health behaviour
1. Introduction
Colorectal cancer is one of the most commonly occurring cancers globally, with some 1.8 million incident cases in 2018 (World Cancer Research Fund, n.d., World Health Organisation, 2020). In Singapore, for example, colorectal cancer is the top cancer with 9807 incident cases diagnosed from 2011 to 2015 (National Registry of Diseases Office Singapore, 2015). Overall survival has improved over the years and has been attributed to advances in colorectal cancer screening, multimodality treatment and surgical techniques over the years. Nonetheless, screening allows for early detection of the pathology and enables intervention to be performed earlier before further progression (Hewitson et al., 2008). When detected early, the disease can be treated with a better prognosis and quality of life for patients (O'Connell et al., 2004). In addition, extant literature has demonstrated the cost-effectiveness of colorectal cancer screening (Ran et al., 2019). Colorectal cancer screening is therefore recommended by health authorities and the World Health Organisation as an effective way to reduce incidence and mortality (Elmunzer et al., 2015, Centers for Disease Control and Prevention, 2020, HealthHub, 2020).
Under the current clinical practice guidelines on cancer screening from the Singapore Ministry of Health as well as recommendations from the United States Preventive Services Task Force, the modalities for colorectal cancer screening for “average risk” individuals (i.e. those without personal and family history or colorectal-related comorbidities) include the faecal occult blood tests (guaiac faecal occult blood test; faecal immunochemical test), colonoscopy, flexible sigmoidoscopy, and computed tomographic colonography (Ministry of Health Singapore, 2010, U.S. Preventive Services Task Force, 2016). However, despite a range of clinical practice guidelines and public health advisories promoting the use of colorectal cancer screening in the general population, screening rates remain less than ideal in many countries (U.S. Preventive Services Task Force, 2016, Gimeno García, 2012). Colorectal cancer screening uptake in the US in 2018 for example, was at 66.8%, lower than the 2020 target of 70.5% (Institute and Screening, 2020). This was already much higher than the uptake in other European national colorectal cancer screening programs, such as the UK (52.0%) and France (34.3%) (Navarro et al., 2017).
While much effort has been made to better understand the factors that influence colorectal cancer screening behaviour, most studies have structured their assessment of these factors without the application of intrapersonal theories or frameworks of behaviour change (Cooke and French, 2008, Kiviniemi et al., 2011, Macrae et al., 1984). This is a missed opportunity as the use of established theoretical frameworks has been crucial in the development of effective, evidence-based interventions to change health behaviour (Glanz et al., 2008). The health belief model is one such framework that has been commonly applied to explain intrapersonal decision-making processes on a wide range of health behaviours, including vaccination and screening (Johnson et al., 2008, Brewer and Fazekas, 2007, Yarbrough and Braden, 2001). In its most commonly utilised version – by Champion and Skinner – the model consists of six sociobehavioural constructs: perceived benefits, barriers, susceptibility, severity, the presence of cues to action, and self-efficacy (Glanz et al., 2008, Champion and Skinner, 2008). These constructs influence health behaviour, often alongside other intrapersonal modifying factors (e.g. age, gender, health literacy) (Champion and Skinner, 2008). The health belief model has already been widely used in breast cancer screening research, and studies have shown that mammography compliance in female populations is significantly associated with higher perceived susceptibility to breast cancer, higher perceived benefits of screening, lower barriers to getting screened, and the presence of cues to action (e.g. recommendations) from health professionals (Champion et al., 2008, Phillips et al., 1998). Behavioural change interventions based on the health belief model have shown to effectively increase mammography uptake by tailoring intervention components to address the constructs most relevant to the target audience (Yarbrough and Braden, 2001, Champion et al., 2006, Han et al., 2009, Darvishpour et al., 2018, Wang et al., 2014). Comparatively, colorectal cancer screening is not breast cancer screening; uptake between genders, for example, has sometimes been shown to be different in colorectal cancer screening (Gimeno García, 2012, Kang and Son, 2017), again emphasizing the need to understand how these modifying factors interact with the health belief model’s constructs.
Given the utility of the health belief model in informing interventions to improve mammography uptake, it seems almost intuitive that a similar effort should be made to study how the model influences colorectal cancer screening. This review aims to systematically examine the literature to better understand how the health belief model’s constructs are associated with colorectal cancer screening intention and behaviour in the screening-eligible general population.
2. Methods
2.1. Review protocol and search strategy
An a priori protocol was developed prior to commencing this review, in which the research question was first defined using the PICOS framework. Specifically, we were interested in all observational quantitative or mixed methods studies (S) that examined how the health belief mode’s constructs (I) were associated with colorectal cancer screening intention or behaviour (O) in an average risk general population (P). Based on the protocol, one reviewer (GJW) then conducted a comprehensive search of published literature from each of the four selected databases (PubMed, Scopus, PsycINFO, CINAHL), from database inception to September 2019. The concepts searched were relevant to colorectal cancer, colorectal cancer screening, and the health belief model. Search terms were kept broad-based and applied to all fields in order to minimise the likelihood of excluding any relevant articles during the initial review of titles and abstracts. The search strategy was constructed using free text key words and Boolean operators. An example of the search strategy utilised in this review is provided here:
(“colorectal cancer” OR “colon cancer” OR “rectal cancer”) AND (“health belief model” OR “beliefs” OR “health behaviour” OR “perception” OR “barriers” OR “susceptibility” OR “benefits” OR “knowledge” OR “attitudes”) AND (“screening” OR “prevention” OR “health screening” OR “colonoscopy” OR “guaiac faecal occult blood test” OR “faecal immunochemical test” OR “flexible sigmoidoscopy” OR “sigmoidoscopy” OR “CT colonography”)
The search excluded grey literature (e.g. news articles, lecture slides, unpublished theses) and was limited to English language publications. To further reduce the likelihood of missing relevant studies, we supplemented this search with manual hand-searches of the reference lists from included articles.
2.2. Study selection
Articles were included if (I) the study design was observational and included a quantitative or mixed methodology, (II) participants included average risk general population eligible for screening (aged 50 – 75 years), (III) exposure variables of interest were health belief model sociobehavioural constructs, and (IV) outcome of interest was general colorectal cancer screening uptake, defined as completion of an investigation using either a stool-based test (guaiac faecal occult blood test; faecal immunochemical test) or direct visualisation test (flexible sigmoidoscopy; colonoscopy; computed tomographic colonography), or intention to screen for colorectal cancer using the abovementioned test modalities.
Articles were excluded if they (I) were interventional studies or review articles, (II) did not include quantitative methodology in the study design, (III) did not use the health belief model, or (IV) were not related to general colorectal cancer screening uptake.
2.3. Data extraction, analysis, and assessment of quality
Based on the search strategy, articles were extracted from each of the four databases by one reviewer (GJW). Duplicates were removed via EndNote (EndNote [computer program], 2013). Titles and abstracts were shortlisted for full text review by two reviewers (JL and GJW), and full texts were independently reviewed in a blinded manner by two reviewers (JL and TZL) using the aforementioned inclusion and exclusion criteria. Disputes on article inclusion in the full text review phase were resolved through unblinded consultation with a third reviewer (GJW).
Quality assessment and risk of bias was performed for each included study by two reviewers (JL and TZL) using the appropriate Joanna Briggs Institute Critical Appraisal Tools checklist (based on the study design) (Joanna Briggs Institute, 2020). An example of the checklist utilised for cross-sectional studies can be found in Supplementary File 1. Disputes on quality assessment and risk of bias were resolved through unblinded discussion and mutual agreement with a third reviewer (GJW). Relevant data from the included articles were extracted by one reviewer (JL) into a standardised data spreadsheet; this consisted of the country of study, study design and methodology, sample characteristics, exposure and outcome variable(s), and key findings. A descriptive summary was performed to organise findings, implications, and limitations across the included articles.
3. Results
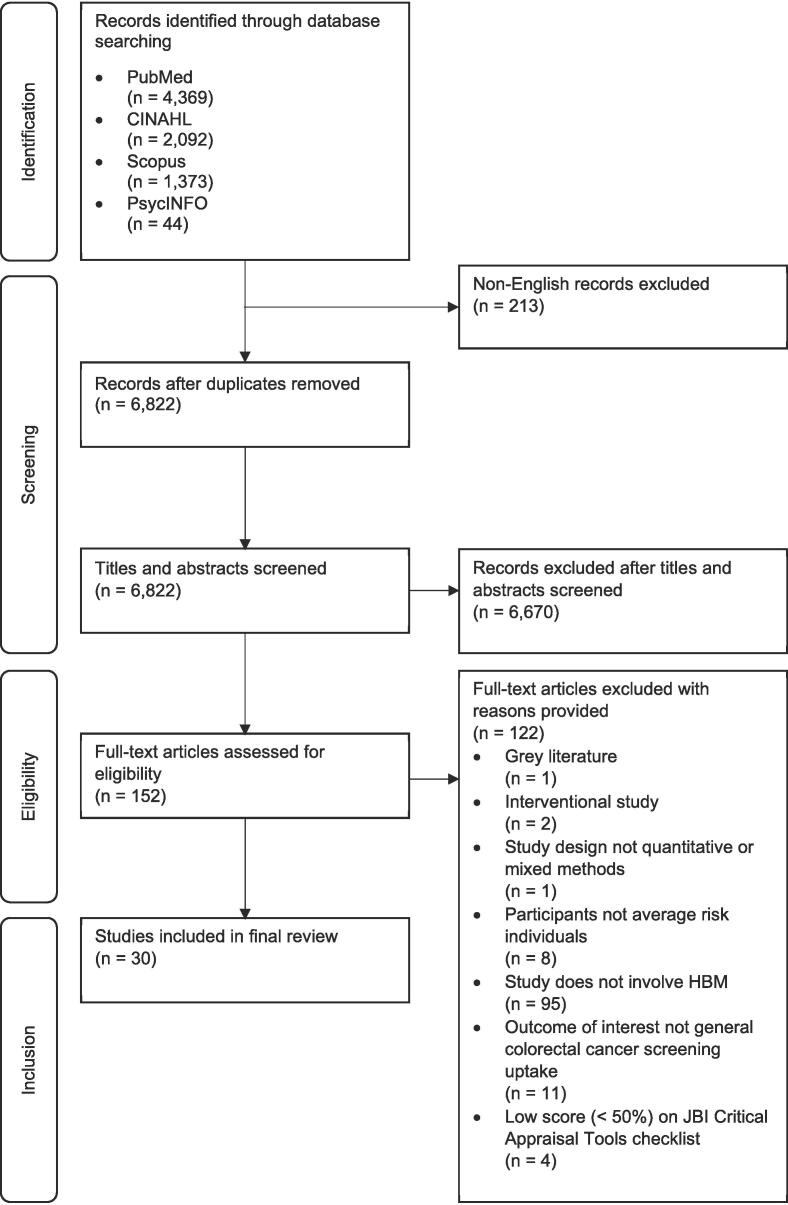
The initial search yielded 7667 records, of which 1676 were removed as duplicates, leaving 6822 articles for evaluation. Of these, the initial review of titles and abstracts excluded 6659 records based on the selection criteria described above. Full texts of the remaining 163 articles were evaluated with an emphasis on the design, exposure and outcome variables of interest, and the quality of the study (defined as having satisfied more than 50% of the critical appraisal criteria in the relevant Joanna Briggs Institute checklist). A description of the country of origin, sample size and age range, sample ethnicity/nationality, study design, sampling strategy, health belief model instrument used (or references, if specific subscales/items were adapted from other sources), exposure and outcome variables of interest, and major findings in relation to colorectal cancer screening, of the final 30 articles included in this review can be found in Table 1. A flow diagram of the selection process can be found in Fig. 1.
Table 1.
Key characteristics, variables of interest and major findings of studies included in this review.
| Authors | Country | Sample size | Sample age range | Ethnic description of sample | Study design | Sampling strategy | HBM instrument(s) used | Exposure variable(s) | Outcome variable(s) | Major findings (directly associated with CRC screening) | Major findings (inversely associated with CRC screening) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (Almadi et al., 2015) | Saudi Arabia | 500 | 18–75 (Mean age 41 years) | 500 Saudi Arabians | Cross-sectional | One-stage cluster sampling of malls, convenience sampling within clusters | Questionnaire designed based on HBM, using a 5-point Likert scale | Sociodemographics Family history of CRC Knowledge of CRC symptoms, risk factors, screening HBM constructs (Perceived severity, perceived susceptibility, perceived barriers) |
Intention to screen for CRC | Knowledge of CRC risk factors (age, male gender as risk factors) were positively associated with intention to screen Perceived barrier (i.e. colonoscopy is harmful) positively associated with intention to screen |
Perceived barrier (i.e. not wanting to know about cancer) negatively associated with intention to screen |
| (Arnold et al., 2012) | USA | 975 | 50–89 (Median age 57 years) | 654 African Americans, 315 Whites, 3 Hispanics | Cross-sectional | Random sample from eight federally-qualified health centres (as part of RCT) Study comprised baseline measure for the RCT |
46-item questionnaire designed using HBM, validated in previous studies (Dolan et al., 2004 and Wolf et. al., 2005), using a 3-point scale | Sociodemographics Literacy Knowledge of CRC screening HBM constructs (Perceived susceptibility, perceived barriers, perceived benefits, cues to action, self-efficacy) |
Past CRC screening behaviour | Low literacy negatively associated with past CRC screening | |
| (Azaiza and Cohen, 2008) | Israel | 520 | 50–75 (Mean age 60 years) | 358 Jews, 162 Arabs | Cross-sectional | Random digit dialling of households from general population | 15-item questionnaire based on Becker's Health Belief Questionnaire (1980), using a 5-point scale | Sociodemographics Level of CRC worry HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, perceived benefits, cues to action) |
Past CRC screening behaviour | Demographics (age, educational attainment, first degree relative with CRC) positively associated with past CRC screening Cues to action (i.e. physician's recommendation), perceived susceptibility, perceived benefits positively associated with past CRC screening |
|
| (Bae et al., 2014) | South Korea | 237 | 50 and above (Mean age 60 years) | 237 South Koreans | Cross-sectional | Unspecified | A 36-item instrument was adapted from the Jacob's HBM scale for colon cancer screening (2002), using a 5-point Likert scale | Sociodemographics HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, perceived benefits, self-efficacy) Health motivation |
Adherence to CRC screening (i.e. annual FOBT completion between 2002 and 2011) | Perceived susceptibility positively associated with CRC screening adherence | Monthly household income negatively associated with CRC screening adherence Perceived barriers and severity negatively associated with CRC screening adherence |
| (Ben Natan et al., 2019) | Israel | 200 | 50–79 (Mean age 57 years) | 200 Israeli Arabs | Cross-sectional | Snowball sampling from general population | A 16-item questionnaire based on the questionnaire constructed by Azaiza and Cohen (Azaiza and Cohen, 2008), using a 5-point Likert scale | Sociodemographics HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, perceived benefits, cues to action) |
Past CRC screening behaviour (FOBT only) Intention to screen for CRC (FOBT only) |
Family history of CRC positively associated with intention to screen Perceived susceptibility, severity, benefits and cues to action positively associated with intention to screen |
Perceived barriers negatively associated with intention to screen |
| (Frank et al., 2004) | USA | 49 | 50 and above | 49 African American (Women) | Cross-sectional | Random sampling from four churches | 45-item Champion's HBM Scale (1999), using a 5-point scale | Sociodemographics HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, perceived benefits) Perceived confidence Health motivation |
Past CRC screening behaviour | Perceived susceptibility and benefits positively associated with past CRC screening Perceived confidence positively associated with past CRC screening |
Perceived barriers and severity negatively associated with past CRC screening Health motivation negatively associated with past CRC screening |
| (Dashdebi et al., 2016) | Iran | 600 | 50 and above | 600 Iranians | Cross-sectional | One-stage cluster sampling of laboratories, convenience sampling within clusters | 52-item instrument based on Satia et. al., 2007, Shokar et. al., 2008 and Chen et. al., 2010 was used, using a 5-point Likert scale | Sociodemographics Knowledge of CRC HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, perceived benefits, self-efficacy) |
Past CRC screening behaviour (FOBT only) | Perceived benefits and self-efficacy positively associated with past CRC screening | Perceived barriers negatively associated with past CRC screening |
| (Gorin, 2005) | USA | 950 | 49 and above | 950 Hispanics | Cross-sectional (FOBT provided post-survey) | Convenience sampling of women from hospital-based national breast and cervical screening program | 2-item on barriers based on Manne et. al., 2002 using a 4-point Likert scale 5-item on supports based on Manne et. al., 2002 and Rakowski et. al., 1992, 1996, using a 4-point Likert scale 2-item on cues to action, based on Myers et. al., 1994, and Manne et. al., 2002, using a binary scale 1-item on susceptibility based on Lipkus et. al., 2000, using a 5-point scale 2-item on perceived severity based on Aiken et. al., 1994, using a 4-point Likert scale 7-item on fatalism based on Lerman et. al., 1991 and Powe, 1995. |
Sociodemographics Family and personal history of CRC Knowledge of CRC risk factors, symptoms, screening HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, cues to action) Cancer worry Fatalism |
Intention to screen for CRC (FOBT only) CRC screening uptake (completion of FOBT provided post-survey) |
Fatalism positively associated with CRC screening uptake | Perceived barriers negatively associated with CRC screening uptake Cancer worry negatively associated with CRC screening uptake |
| (Hay et al., 2003) | USA | 280 | 50–75 (mean age 62 years) | 44 African Americans, 213 Caucasians, 11 Latinos/Hispanics, 6 Asians, 6 Others | Cross-sectional | Convenience sampling of women from a large, urban breast cancer diagnostic facility | 1-item on perceived susceptibility based on Weinstein, 1980, 1987, using a 5-point scale 3-item on perceived severity using Aiken et. al., 1994, using a 5-point scale 3-item on self-efficacy, using a 5-point scale 27-item on perceived benefits and barriers based on Rakowski et. al., 1993, using a 5-point scale |
Sociodemographics Family history of CRC HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, perceived benefits, cues to action, self-efficacy) |
Past CRC screening behaviour | Perceived benefits, cues to action and self-efficacy positively associated with past CRC screening | Perceived barriers negatively associated with past CRC screening |
| (Hughes et al., 2015) | USA | 393 | 50–75 (Mean age 62 years) | 194 Rural Whites, 179 Urban Whites, 5 Rural Non-Whites, 12 Urban Non-Whites | Cross-sectional | Random sampling of patient population from two regional medical centres | 22-item instrument based on James et. al., 2002, Menon et. al., 2007, Ueland et. al., 2006 and Janz et. al., 2003, using a 5-point Likert scale | Sociodemographics Personal history of CRC HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, perceived benefits, cues to action, self-efficacy) |
Past CRC screening behaviour | Perceived benefits and susceptibility positively associated with past CRC screening | Perceived barriers negatively associated with past CRC screening |
| (James et al., 2002) | USA | 397 | 50 and above (Mean age 63 years) | 397 African Americans | Cross-sectional | Convenience sampling from a larger study involving 12 churches | Barrier and Benefit items were derived from focus groups conducted during the pilot studies, using a Likert-type scale | Sociodemographics HBM constructs (Perceived barriers, perceived benefits) |
Past CRC screening behaviour | Perceived benefits positively associated with past CRC screening (all screening modalities) | Perceived barriers negatively associated with past CRC screening (all screening modalities) |
| (Janz et al., 2003) | USA | 355 | 50–79 | 74 Black Male, 98 Black Female, 105 White Male, 98 White Female | Cross-sectional | Random sampling of household telephone numbers from general population | 18-item instrument on benefits and barriers based on Rawl et. al. 10-item instrument on perceived severity and susceptibility based on Myers et. al., |
Sociodemographics HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, perceived benefits) Salience and coherence of CRC screening |
Past CRC screening behaviour | Age positively associated with past CRC screening (FOBT and flexible sigmoidoscopy) Perceived susceptibility positively associated with past CRC screening (flexible sigmoidoscopy only) |
Perceived barriers negatively associated with past CRC screening (all screening modalities) |
| (Javadzade et al., 2012) | Iran | 196 | 50 and above | 196 Iranians | Cross-sectional | Random sampling of referral patients from four FOBT laboratories; one-stage cluster sampling from general population | 26-item instrument on perceived susceptibility, severity, benefits and barriers designed based on resources, books and papers, using a 5-point Likert scale 5-item instrument on self-efficiency designed based on resources, books and papers, using a 4-point Likert scale. |
Sociodemographics Knowledge of CRC screening Group assignment (referral or general population) HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, perceived benefits, cues to action, self-efficacy) |
Past CRC screening behaviour Intention to screen for CRC |
Referral group positively associated with past CRC screening | |
| (Khani Jeihooni et al., 2017) | Iran | 240 | 50 and above | 240 Iranians | Cross-sectional | Random sampling of referral patients from two FOBT laboratories; convenience sampling from general population | 26-item instrument on perceived susceptibility, severity, benefits and barriers designed based on Javadzade et. al., 2012, using a 5-point Likert scale 5-item instrument on self-efficiency designed based on Javadzade et. al., 2012, using a 4-point Likert scale. |
Sociodemographics Knowledge of CRC screening Group assignment (referral or general population) HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, perceived benefits, cues to action, self-efficacy) Perceived social support |
Past CRC screening behaviour (FOBT only) Intention to screen for CRC (FOBT only) |
Knowledge of CRC screening positively associated with past CRC screening Perceived severity, susceptibility, and benefits positively associated with past CRC screening Self-efficacy and perceived social support positively associated with past CRC screening |
Perceived barriers negatively associated with past CRC screening |
| (Koo et al., 2012) | Multinational | 2990 | 50 and above | 311 Australians, 161 Bruneians, 275 Chinese, 93 Filipinos, 289 Hong Kongers, 65 Indians, 203 Indonesians, 313 Japanese, 399 Koreans, 99 Malaysians, 93 Pakistanis, 436 Singaporeans, 90 Taiwanese, 163 Thais | Cross-sectional | Random sampling from outpatient clinics within each participating hospital | HBM Questionnaire based on Sung et. al., 2008 | Sociodemographics Knowledge of CRC symptoms, risk factors, and screening HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, perceived benefits, cues to action) Access to healthcare |
Past CRC screening behaviour Intention to screen for CRC |
Knowledge of CRC screening positively associated with past CRC screening Cues to action (physician's recommendation) positively associated with past CRC screening |
|
| (Lee et al., 2019) | USA | 121 | 50–75 (Mean age 61 years) | 121 Thais in USA | Cross-sectional | Convenience sampling from Thai community service agency and two temples | HBM subscale questionnaire based on Menon et. al., 2003, 2007, using a 5-point Likert scale | Sociodemographics Perceived health status HBM constructs (Perceived susceptibility, perceived barriers, perceived benefits, self-efficacy) Spousal support |
Past CRC screening behaviour | Age positively associated with past CRC screening Self-efficacy positively associated with past CRC screening |
|
| (Lee and Im, 2013) | USA | 281 | 50–88 (Mean age 67 years) | 281 Korean Americans | Cross-sectional | Convenience sampling from two Korean senior centres and two Korean churches | 33-item instrument adapted from Champion's original scale, using a 4-point Likert scale | Sociodemographics Family and personal history of CRC HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, perceived benefits, self-efficacy) Motivation to go for CRC screening Cultural factors (fatalism, modesty, family support, use of eatern medicine, helplessmess) |
Past CRC screening behaviour (colonoscopy and flexible sigmoidoscopy) | Perceived severity positively associated with past CRC screening (females only) Motivation positively associated with past CRC screening (females only) Self-efficacy positively associated with past CRC screening (both males and females) |
Fatalism negatively associated with past CRC screening (males only) |
| (Leung et al., 2016) | Hong Kong SAR | 240 | 60 and above (Mean age 75 years) | 240 Chinese | Cross-sectional | Convenience sampling from three non-governmental organisations' elderly centres | 35-item CRC Perception and Screening instrument was based on Green and Kelly, 2004, Leung et. al., 2014, using a 5-point Likert scale 4-item on self-efficacy based on von Wagner et. al., 2009, using a 5-point scoring scale 3-item on cue to action based on Sung et. al., 2008, using a binary yes/no format |
Sociodemographics Knowledge of CRC symptoms, risk factors, and screening HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, perceived benefits, cues to action, self-efficacy) Fear of CRC |
Past CRC screening behaviour | Cues to action positively associated with past CRC screening | Perceived severity and barriers negatively associated with past CRC screening |
| (Lin et al., 2019) | Taiwan | 391 | 50–75 | 391 Taiwanese | Cross-sectional | Unspecified | HBM subscale questionnaire based on Wu et. al., 2013, Wong et. al., 2013, using a 5-point Likert scale | Sociodemographics Family and personal history of CRC HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, perceived benefits, cues to action, self-efficacy) |
Intention to screen for CRC (FOBT only) | Perceived severity, benefits and self-efficacy positively associated with intention to screen | Perceived barriers negatively associated with intention to screen |
| (Macrae et al., 1984) | Australia | 581 | 40–75 | 523 Australians, 58 undefined | Cross-sectional (FOBT provided post-survey) | Convenience sampling from 14 clinical outpatient practices | 11-item instrument constructed using specifications based on Rosenstock, 1975, using a 5-point scale | Sociodemographics Family and personal history of CRC HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, perceived benefits) Health motivation Efficacy of treatment |
CRC screening uptake (completion of FOBT provided post-survey) | Perceived susceptibility positively associated with CRC screening uptake | Perceived barriers negatively associated with CRC screening uptake |
| (Menon et al., 2007) | USA | 206 | 50 and above (Mean age 61 years) | 167 White, 39 Nonwhite | Cross-sectional | Convenience sampling from large health maintenance organisation | 55-item instrument validated previously by author, using Likert scales | Sociodemographics Knowledge of CRC risk factors and screening HBM constructs (Perceived susceptibility, perceived barriers, perceived benefits, self-efficacy) |
Transtheoretical Model constructs (Precontemplation, contemplation, action) (FOBT and sigmoidoscopy) Note: Participants in “action” phase counted as ever having completed CRC screening |
Perceived susceptibility and benefits positively associated with past CRC screening (FOBT only) Perceived susceptibility and self-efficacy positively associated with past CRC screening (sigmoidoscopy only) |
Perceived barriers negatively associated with past CRC screening (FOBT and sigmoidoscopy) |
| (Ng et al., 2007) | Singapore | 514 | 50 and above | 514 Singaporean-Chinese | Cross-sectional | Random sampling from general population | 22-item instrument adapted from Green and Kelly, 2004, which was based on Stretcher and Rosenstock's HBM, 1997, using a 5-point likert scale | Sociodemographics Knowledge of CRC and screening HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, perceived benefits, cues to action) |
Past CRC screening behaviour | Knowledge of CRC and screening positively associated with past CRC screening Perceived benefits and cues to action positively associated with past CRC screening |
Perceived severity and barriers negatively associated with past CRC screening |
| (Palmer et al., 2011) | USA | 504 | 50–75 | 504 African Americans | Cross-sectional | Random digit dialling of households from general population | 3-item on perceived susceptibility based on Lipkus, using a 4-point Likert scale 3-item on self-efficacy based on Rakowski et. al., 2004 4-item on perceived barriers and benefits based on Vernon et.al., 1997 and Jacobs, 2002, using a 5-point Likert scale |
Sociodemographics Personal history of CRC Sources of health information Knowledge of CRC HBM constructs (Perceived susceptibility, perceived barriers, perceived benefits) |
Past CRC screening behaviour Intention to screen for CRC |
Perceived susceptibility and cues to action positively associated with past CRC screening | |
| (Sammut et al., 2019) | Malta | 245 | 57–61 | 245 Maltese | Cross-sectional | Random sampling from national screening database | Instrument based on Dome Le et. al., 2013 and Champion et. al., 2014 | Sociodemographics HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, perceived benefits) Fear of CRC |
Past CRC screening behaviour | Perceived barriers negatively associated with past CRC screening | |
| (Sohler et al., 2015) | USA | 1101 | 50–75 (Mean age 57 years) | 112 Hispanic, 67 Black, 60 Non-hispanic White, 11 Other | Cross-sectional | Convenience sampling from primary care clinics in four states; study comprised baseline measure for CRC screening RCT | 13-item Instrument based on EHBM | Sociodemographics Knowledge of CRC risk factors and screening HBM constructs (Perceived barriers, cues to action, self-efficacy) |
CRC screening uptake (at 12-month follow-up in RCT) | Cues to action and self-efficacy positively associated with CRC screening uptake (colonoscopy only) | |
| (Taheri-Kharameh et al., 2016) | Iran | 200 | 50 and above (Mean age 62 years) | 200 Iranians | Cross-sectional | Convenience sampling from outpatient clinics in three teaching hospitals | 36-item Champion's Health Belief Model Scale using a 5-point Likert scale | Sociodemographics Family history of CRC Knowledge of CRC and screening HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, perceived benefits) Health motivation |
Past CRC screening behaviour | Knowledge of CRC and screening positively associated with past CRC screening | Perceived barriers negatively associated with past CRC screening |
| (Taş et al., 2019) | Turkey | 235 | 50–70 (Mean age 59 years) | 235 Turks | Cross-sectional | Convenience sampling from one family health center | 33-item instrument based on Health Belief Model Scale for Protection from Colorectal Cancer, evaluated in Tureky by Ozsoy et. al., 2007, using a 5-point Likert scale | Sociodemographics Family and personal history of CRC Knowledge of CRC risk factors, symptoms, screening HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, perceived benefits) Health motivation |
Past CRC screening behaviour | Knowledge of CRC screening positively associated with past CRC screening | |
| (Tastan et al., 2013) | Turkey | 160 | 50 and above (Mean age 61 years) | 160 Turks | Cross-sectional | Convenience sampling from one family medicine clinic | 33-item instrument derived from Champion's Health Belief Model Scale | Sociodemographics Personal history of CRC Knowledge of CRC risk factors and screening HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, perceived benefits) Health motivation |
Past CRC screening behaviour | Perceived benefits positively associated with past CRC screening | |
| (Wong et al., 2013) | Singapore | 1763 | 50 and above (Mean age 61 years) | 1410 Chinese, 157 Indians, 136 Malays, 40 Others | Cross-sectional | Stratified random sampling of residential households from general population | 24-item instrument designed based on HBM, piloted on 10 subjects | Sociodemographics Family and personal history of CRC Knowledge of CRC, symptoms and screening HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, perceived benefits, cues to action) |
Past CRC screening behaviour | Perceived susceptibility and cues to action positively associated with past CRC screening (males and females) Family history of CRC positively associated with past CRC screening (females) |
Perceived barriers negatively associated with past CRC screening (males and females) |
| (Yoo et al., 2013) | USA | 5586 | 50 and above (Mean age 63 years) | 2769 Caucasians, 718 Non-Caucasians | Cross-sectional | Random digit dialling from general population | 18-item instrument based on HBM constructs was used | Family and personal history of CRC Perceived health status HBM constructs (Perceived severity, perceived susceptibility, perceived barriers, perceived benefits) |
Past CRC screening behaviour (FOBT only) | Perceived threat (composite of severity and susceptibility) positively associated with past CRC screening Positive expectations (composite of benefits minus barriers) positively associated with past CRC screening |
Fig. 1.
PRISMA flow chart illustrating search strategy used to identify eligible studies for inclusion within the current review. HBM refers to health belief model; JBI refers to Joanna Briggs Institute.
Through descriptive summary of the included articles’ key findings, we identified four broad categories of factors. These were 1) health belief model constructs and 2) other modifying factors that tended to have a direct association, as well as 3) health belief model constructs and 4) other modifying factors that tended to have an inverse association, with colorectal cancer screening behaviour or intention.
3.1. Descriptive characteristics of the included articles
The final 30 articles comprised 21,010 participants from 18 countries, with nearly half (14 articles) from western settings (USA and Australia). All but three articles recruited participants aged 50 years and above (Macrae et al., 1984, Almadi et al., 2015, Gorin, 2005). Despite our broad selection criteria, all included articles were of cross-sectional design.
3.2. Health belief model constructs directly associated with colorectal cancer screening intention or behaviour
Of the six health belief model constructs, perceived benefits (in 13 articles) (Azaiza and Cohen, 2008, Ben Natan et al., 2019, Frank et al., 2004, Dashdebi et al., 2016, Hay et al., 2003, Hughes et al., 2015, James et al., 2002, Khani Jeihooni et al., 2017, Lin et al., 2019, Menon et al., 2007, Ng et al., 2007, Tastan et al., 2013, Yoo et al., 2013), susceptibility (in 12 articles) (Macrae et al., 1984, Hughes et al., 2015, Khani Jeihooni et al., 2017, Menon et al., 2007, Azaiza and Cohen, 2008, Ben Natan et al., 2019, Frank et al., 2004, Yoo et al., 2013, Bae et al., 2014, Janz et al., 2003, Palmer et al., 2011, Wong et al., 2013), and cues to action (in nine articles) (Azaiza and Cohen, 2008, Ben Natan et al., 2019, Hay et al., 2003, Ng et al., 2007, Palmer et al., 2011, Wong et al., 2013, Koo et al., 2012, Leung et al., 2016, Sohler et al., 2015) were the three most commonly demonstrated to have a direct association with screening intention or behaviour. Self-efficacy was found to be directly associated with screening intention or behaviour in eight (Dashdebi et al., 2016, Hay et al., 2003, Khani Jeihooni et al., 2017, Lin et al., 2019, Menon et al., 2007, Sohler et al., 2015, Lee et al., 2019, Lee and Im, 2013) articles, and perceived severity in five (Ben Natan et al., 2019, Yoo et al., 2013, Lee and Im, 2013, Khani Jeihooni et al., 2017, Lin et al., 2019) articles. The most common health beliefs directly associated with colorectal cancer screening can be found in Table 2.
Table 2.
Most common health belief model constructs directly associated with colorectal cancer screening intention or behaviour.
3.3. Other modifying factors directly associated with colorectal cancer screening intention or behaviour
Participants’ knowledge of colorectal cancer (e.g. risk factors, symptoms, screening modalities) was directly associated with screening intention or behaviour in six articles (Almadi et al., 2015, Khani Jeihooni et al., 2017, Ng et al., 2007, Koo et al., 2012, Taheri-Kharameh et al., 2016, Taş et al., 2019). The next most common modifying factors with a direct association were the age of the participant (in three articles) (Azaiza and Cohen, 2008, Janz et al., 2003, Lee et al., 2019), and a family or personal history of colorectal cancer (in three articles) (Azaiza and Cohen, 2008, Ben Natan et al., 2019, Wong et al., 2013). Fatalism was directly associated with colorectal cancer screening in one article (Gorin, 2005).
3.4. Health belief model constructs inversely associated with colorectal cancer screening intention or behaviour
Perceived barriers (in 19 articles) (Macrae et al., 1984, Almadi et al., 2015, Gorin, 2005, Bae et al., 2014, Janz et al., 2003, Wong et al., 2013, Leung et al., 2016, Taheri-Kharameh et al., 2016, Frank et al., 2004, Dashdebi et al., 2016, Hay et al., 2003, Hughes et al., 2015, James et al., 2002, Khani Jeihooni et al., 2017, Lin et al., 2019, Menon et al., 2007, Ng et al., 2007) was the dominant health belief model construct inversely associated with screening intention or behaviour. The most common barriers to colorectal cancer screening can be found in Table 3. Interestingly, perceived severity was also found to be inversely associated with screening intention or behaviour in four articles (Frank et al., 2004, Ng et al., 2007, Bae et al., 2014, Leung et al., 2016).
Table 3.
Most common health belief model constructs inversely associated with colorectal cancer screening intention or behaviour.
3.5. Other modifying factors inversely associated with colorectal cancer screening intention or behaviour
In addition to perceived barriers and severity, low literacy (one article) (Arnold et al., 2012), monthly household income (one article) (Bae et al., 2014), and fatalism (one article) (Lee and Im, 2013) were modifying factors also found to be inversely associated with screening intention and behaviour. Notably, fatalism was a significant factor only in male participants (Lee and Im, 2013).
3.6. Risk of bias within included studies
As all included studies were of cross-sectional design, we assessed risk of bias using the Joanna Briggs Institute Critical Appraisal Tools checklist for analytical cross-sectional studies (Moola et al., 2017). Seven studies either failed to provide clear sample inclusion criteria or describe the study setting and population (Macrae et al., 1984, Ben Natan et al., 2019, Tastan et al., 2013, Taş et al., 2019, Sammut et al., 2019). Nearly half the included studies utilised non-random sampling (Macrae et al., 1984, Almadi et al., 2015, Gorin, 2005, Ben Natan et al., 2019, Dashdebi et al., 2016, Hay et al., 2003, Lin et al., 2019, Menon et al., 2007, Sohler et al., 2015, Lee et al., 2019, Lee and Im, 2013, Taheri-Kharameh et al., 2016). Only two studies were able to objectively establish participants’ screening, medical and family history (Macrae et al., 1984, Sammut et al., 2019); and only three studies were able to measure their samples’ colorectal cancer screening uptake reliably without using participant self-report (Macrae et al., 1984, Gorin, 2005, Sohler et al., 2015). Nonetheless, the included studies generally utilised validated health belief model scales, reported possible confounding, and adjusted for confounders in their analyses.
4. Discussion
To our knowledge, this was the first systematic review of quantitative studies evaluating the association between the health belief model’s constructs and colorectal cancer screening in screening-eligible general populations. The articles included in our review generally suggest that the health belief model can be useful in understanding the facilitators and barriers to colorectal cancer screening, and could be used to guide future tailored interventions to improve colorectal cancer screening intention and adherence to screening recommendations, such as annual faecal immunochemical testing or colonoscopy every 10 years.
The included articles observed that higher perceived susceptibility and benefits were the two constructs most commonly associated with screening intention or behaviour. This is consistent the health belief model in general, which suggests that individuals are more likely to perform a preventive health behaviour when they perceive themselves to be at risk of a negative health outcome, and can see a benefit to performing the recommended health behaviour (Champion and Skinner, 2008, Didarloo et al., 2017, Shirazi Zadeh Mehraban et al., 2018). Similarly, we found that cues to action consistently associated with colorectal cancer screening adherence, and it was interesting to note that the most common cue across studies was the presence of a physician’s recommendation to screen and advice from family or friends. These findings emphasize the important role physicians play in continuing to advise their patients to be screening-adherent, especially for those identified to have a family history of colorectal cancer or personal medical history of colorectal (Pornet et al., 2014, Chua and Koh, 2014).
As expected, perceived barriers was the major health belief model construct that was inversely associated with screening intention or behaviour within most of the studies in this review. As observed in Table 3, perceived barriers encompass a wide spectrum of factors, ranging from the structural (e.g. lack of access to care, cost of screening) to the psychosocial (e.g. embarrassment to go for screening, fear of knowing screening results), and it is likely that the relative significance of these barriers may differ between screening modalities, individuals, communities and health systems (Talaat, 2015, Ng and Wong, 2013). However, perceived severity was found to be inversely associated with screening intention and behaviour in four of the thirty studies. This is interesting as high perceived severity should instead predict an increased likelihood of performing the behaviour (Champion and Skinner, 2008). Three of these articles involved Asian (Korean, Hong Kong Chinese and Singaporean Chinese) populations, and suggested that these findings could be due to cultural norms of wanting to deny the knowledge of serious health conditions to avoid associated psychological consequences, an interpretation that is supported elsewhere in the literature (Sun et al., 2004, Sung et al., 2008).
Building on this point, we recognise that the health belief model inherently explains a predominantly intrapersonal set of influences on health behaviour. While other intra- (e.g. knowledge, fatalism, low literacy) and interpersonal (e.g. family history, monthly household income) modifying factors have emerged in the present review; these are non-exhaustive and beyond the scope of the present review to consider in full. Nonetheless, other studies have demonstrated wider, socioecological considerations surrounding colorectal cancer screening uptake and adherence. These include access to health services, as well as ethnic and socioeconomic disparities that vary across countries and subpopulations (Power et al., 2009, Partin et al., 2017).
With this in mind, future studies should strive to better understand how intrapersonal theories of behaviour change like the health belief model can be augmented to concurrently account for interpersonal, provider-based and socioecological influences on colorectal cancer screening behaviour (Seeff et al., 2013, Park and Kim, 2014). In applied settings, public health professionals should also consider the role of sociocultural norms when designing theoretically-grounded screening interventions to ensure that these are tailored appropriately to their target populations.
4.1. Limitations across included studies
Three crucial limitations were identified across the studies included in this review. The first was the lack of any prospective observational designs. As cross-sectional studies are only able to analyse associations between the health belief model and participants’ screening history or intention to screen for colorectal cancer at a single time point, this precludes the establishment of any temporal relationships and prevented us from evaluating the existence of an “intention-behaviour gap” (where intention does not necessarily translate to future behaviour).
The second limitation was the lack of random sampling in nearly half the studies. Due to the increased risk of selection biases in convenience and other non-random sampling strategies, this will no doubt affect any attempt at the generalisation of findings (Sedgwick, 2013). Moreover, most of the included studies that measured past screening behaviour did so using participant self-report, raising the possibility of recall or social desirability biases.
Lastly, the majority of studies were situated in Western (Macrae et al., 1984, Gorin, 2005, Frank et al., 2004, Menon et al., 2007, Yoo et al., 2013, Janz et al., 2003, Palmer et al., 2011, Sammut et al., 2019, Hughes et al., 2015, James et al., 2002, Sohler et al., 2015, Lee et al., 2019, Lee and Im, 2013) or Middle Eastern (Almadi et al., 2015, Azaiza and Cohen, 2008, Ben Natan et al., 2019, Dashdebi et al., 2016, Khani Jeihooni et al., 2017, Tastan et al., 2013, Taheri-Kharameh et al., 2016, Sammut et al., 2019, Javadzade et al., 2012) settings, with only six studies conducted in Asia (Lin et al., 2019, Ng et al., 2007, Bae et al., 2014, Wong et al., 2013, Koo et al., 2012, Leung et al., 2016). As three studies on Asian populations within this review have already demonstrated slightly different associations compared to what is commonly known about the health belief model, future research may wish to contribute to the comparative dearth of Asian studies in the literature, in order to examine how sociocultural contexts influence or change established theoretical understanding of health behaviour (Dong and Simon, 2018).
4.2. Limitations of the current review
The present review aimed to capture all observational studies utilising a quantitative or mixed methodology that examined associations between the health belief model and colorectal cancer screening, using a multi-database search strategy. However, it is possible that some relevant articles may have been missed as we only included publications which had full texts that were available in English.
Interestingly, despite our comparatively broad inclusion criteria, none of the articles included in the final review featured mixed-methods designs. As this review therefore only included quantitative studies, we were also unable to assess the potential contributions of the qualitative body of literature on this topic.
5. Conclusions
In spite of these limitations, our review found that the health belief model’s constructs’ association with screening intention and behaviour are generally consistent across countries and with the existing literature (Glanz et al., 2008). By identifying commonly relevant perceived risks, benefits, barriers and cues to action, we hope that theoretically-grounded behavioural change interventions can be tailored to be more effective at increasing colorectal cancer uptake in the general population.
We reiterate that future studies should also seek to understand how the health belief model influences individuals’ screening behaviour over time with the use of prospective study designs. Further research should also consider how the health belief model interacts with the cultural and social norms, as well as interpersonal, community and other socioecological factors that may also influence colorectal cancer screening.
6. Funding Statement:
This work was supported by the Singapore Population Health Improvement Centre (SPHERiC) [NMRC/CG/C026/2017_NUHS].
The funders had no role in the study design; collection, analysis and interpretation of the data; writing of the report; and in the decision to submit the article for publication.
CRediT authorship contribution statement
Jerrald Lau: Conceptualization, Methodology, Writing - original draft, Writing - review & editing. Tian-Zhi Lim: Conceptualization, Writing - review & editing. Gretel Jianlin Wong: Methodology, Visualization. Ker-Kan Tan: Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Dr Miny Samuel at the Yong Loo Lin School of Medicine, National University of Singapore for valuable discussions and assistance in revising the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2020.101223.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Almadi M.A., Mosli M.H., Bohlega M.S. Effect of public knowledge, attitudes, and behavior on willingness to undergo colorectal cancer screening using the health belief model. Saudi J. Gastroenterol. 2015;21(2):71–77. doi: 10.4103/1319-3767.153814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold C.L., Rademaker A., Bailey S.C., Esparza J.M., Reynolds C., Liu D., Platt D., Davis T.C. Literacy barriers to colorectal cancer screening in community clinics. J. Health Commun. 2012;17(sup3):252–264. doi: 10.1080/10810730.2012.713441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azaiza F., Cohen M. Colorectal cancer screening, intentions, and predictors in Jewish and Arab Israelis: a population-based study. Health Educ. Behav. 2008;35(4):478–493. doi: 10.1177/1090198106297045. [DOI] [PubMed] [Google Scholar]
- Bae N., Park S., Lim S. Factors associated with adherence to fecal occult blood testing for colorectal cancer screening among adults in the Republic of Korea. Eur. J. Oncol. Nurs. 2014;18(1):72–77. doi: 10.1016/j.ejon.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Ben Natan M., Abu Husayn A., Haj Muhamad R. Intention to undergo faecal occult blood testing in an ethnic minority. Int. J. Nurs. Pract. 2019;25(2):e12721. doi: 10.1111/ijn.12721. [DOI] [PubMed] [Google Scholar]
- Brewer N.T., Fazekas K.I. Predictors of HPV vaccine acceptability: a theory-informed, systematic review. Prev. Med. 2007;45(2-3):107–114. doi: 10.1016/j.ypmed.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. What Should I Know About Screening? https://www.cdc.gov/cancer/colorectal/basic_info/screening/index.htm. Published 2020. Accessed.
- Champion V.L., Skinner C.S. The health belief model. Health Behav. Health Educ.: Theory, Res. Practice. 2008;4:45–65. [Google Scholar]
- Champion V.L., Skinner C.S. Health Behavior and Health Education: Theory, Research, and Practice. fourth ed. Jossey-Bass; San Francisco, CA, US: 2008. The health belief model; pp. 45–65. [Google Scholar]
- Champion V.L., Springston J.K., Zollinger T.W., Saywell R.M., Jr., Monahan P.O., Zhao Q., Russell K.M. Comparison of three interventions to increase mammography screening in low income African American women. Cancer Detect. Prev. 2006;30(6):535–544. doi: 10.1016/j.cdp.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Champion V.L., Monahan P.O., Springston J.K., Russell K., Zollinger T.W., Saywell R.M., JR, Maraj M. Measuring mammography and breast cancer beliefs in African American Women. J. Health Psychol. 2008;13(6):827–837. doi: 10.1177/1359105308093867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua A.H., Koh G.C. Does patient education and recommendation result in increased uptake of colorectal cancer screening using the fecal occult blood test? Ann. Acad. Med. Singapore. 2014;43(10):517–518. [PubMed] [Google Scholar]
- Cooke R., French D.P. How well do the theory of reasoned action and theory of planned behaviour predict intentions and attendance at screening programmes? A meta-analysis. Psychol. Health. 2008;23(7):745–765. doi: 10.1080/08870440701544437. [DOI] [PubMed] [Google Scholar]
- Darvishpour A., Vajari S.M., Noroozi S. Can health belief model predict breast cancer screening behaviors? Open Access Maced J Med Sci. 2018;6(5):949–953. doi: 10.3889/oamjms.2018.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashdebi K.G., Noroozi A., Tahmasebi R. Factors predicting fecal occult blood testing among residents of Bushehr, Iran, based on the health belief model. Asian Pac. J. Cancer Prev. 2016;17(sup3):17–22. doi: 10.7314/apjcp.2016.17.s3.17. [DOI] [PubMed] [Google Scholar]
- Didarloo A., Nabilou B., Khalkhali H.R. Psychosocial predictors of breast self-examination behavior among female students: an application of the health belief model using logistic regression. BMC Public Health. 2017;17(1):861. doi: 10.1186/s12889-017-4880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X., Simon M.A. Achieving health equity in Asian populations. Gerontol. Geriatric Med. 2018;4 doi: 10.1177/2333721418778169. 2333721418778169-2333721418778169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmunzer B.J., Singal A.G., Sussman J.B. Comparing the effectiveness of competing tests for reducing colorectal cancer mortality: a network meta-analysis. Gastrointest. Endosc. 2015;81(3) doi: 10.1016/j.gie.2014.10.033. 700-709.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EndNote [computer program]. Version EndNote X9. Philadelphia, PA: Clarivate Analytics; 2013.
- Frank D., Swedmark J., Grubbs L. Colon cancer screening in African American women. ABNF J. 2004;15(4):67–70. [PubMed] [Google Scholar]
- Gimeno García A.Z. Factors influencing colorectal cancer screening participation. Gastroenterol. Res. Pract. 2012;2012 doi: 10.1155/2012/483417. 483417-483417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanz K., Rimer B.K., Viswanath K. fourth ed. Jossey-Bass; 2008. Health Behaviour and Health Education: Theory, Research, and Practice. [Google Scholar]
- Gorin S.S. Correlates of colorectal cancer screening compliance among urban hispanics. J. Behav. Med. 2005;28(2):125–137. doi: 10.1007/s10865-005-3662-5. [DOI] [PubMed] [Google Scholar]
- Han H.-R., Lee J.-E., Kim J., Hedlin H.K., Song H., Kim M.T. A meta-analysis of interventions to promote mammography among ethnic minority women. Nurs. Res. 2009;58(4):246–254. doi: 10.1097/NNR.0b013e3181ac0f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay J.L., Ford J.S., Klein D. Adherence to colorectal cancer screening in mammography-adherent older women. J. Behav. Med. 2003;26(6):553–576. doi: 10.1023/a:1026253802962. [DOI] [PubMed] [Google Scholar]
- HealthHub. Colorectal Cancer. https://www.healthhub.sg/a-z/diseases-and-conditions/24/colorectalcancer. Published 2020. Accessed.
- Hewitson P., Glasziou P., Watson E., Towler B., Irwig L. Cochrane systematic review of colorectal cancer screening using the Fecal occult blood test (Hemoccult): an update. Am. J. Gastroenterol. 2008;103(6):1541–1549. doi: 10.1111/j.1572-0241.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- Hughes A.G., Watanabe-Galloway S., Schnell P., Soliman A.S. Rural–urban differences in colorectal cancer screening barriers in Nebraska. J. Community Health. 2015;40(6):1065–1074. doi: 10.1007/s10900-015-0032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute. Colorectal Cancer Screening. https://progressreport.cancer.gov/detection/colorectal_cancer. Published 2020. Accessed.
- James A.S., Campbell M.K., Hudson M.A. Perceived barriers and benefits to colon cancer screening among African Americans in North Carolina: how does perception relate to screening behavior? Cancer Epidemiol., Biomarkers Prevention. 2002;11(6):529–534. [PubMed] [Google Scholar]
- Janz N.K., Wren P.A., Schottenfeld D., Guire K.E. Colorectal cancer screening attitudes and behavior: a population-based study. Prev. Med. 2003;37(6):627–634. doi: 10.1016/j.ypmed.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Javadzade H., Hasanzade A., Reisi M., Sharifirad G.H., Mostafavi F., Shahnazi H. Factors associated with the fecal occult blood testing for colorectal cancer screening based on health belief model structures in moderate risk individuals, Isfahan, 2011. J. Edu. Health Promot. 2012;1(1) doi: 10.4103/2277-9531.99218. 18-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joanna Briggs Institute. Critical Appraisal Tools. https://joannabriggs.org/critical_appraisal_tools. Accessed.
- Johnson C.E., Mues K.E., Mayne S.L., Kiblawi A.N. Cervical cancer screening among immigrants and ethnic minorities: a systematic review using the health belief model. J. Lower Genital Tract Dis. 2008;12(3):232–241. doi: 10.1097/LGT.0b013e31815d8d88. [DOI] [PubMed] [Google Scholar]
- Kang Y., Son H. Gender differences in factors associated with colorectal cancer screening: a national cross-sectional study in Korea. Asia Pac. J. Public Health. 2017;29(6):495–505. doi: 10.1177/1010539517718336. [DOI] [PubMed] [Google Scholar]
- Khani Jeihooni A., Kashfi S.M., Shokri A., Kashfi S.H., Karimi S. Investigating factors associated with FOBT screening for colorectal cancer based on the components of health belief model and social support. Asian Pac. J. Cancer Prevention. 2017;18(8):2163–2169. doi: 10.22034/APJCP.2017.18.8.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi M.T., Bennett A., Zaiter M., Marshall J.R. Individual-level factors in colorectal cancer screening: a review of the literature on the relation of individual-level health behavior constructs and screening behavior: decision making about colorectal cancer screening. Psycho-Oncology. 2011;20(10):1023–1033. doi: 10.1002/pon.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo J.H., Leong R.W.L., Ching J. Knowledge of, attitudes toward, and barriers to participation of colorectal cancer screening tests in the Asia-Pacific region: a multicenter study. Gastrointest. Endosc. 2012;76(1):126–135. doi: 10.1016/j.gie.2012.03.168. [DOI] [PubMed] [Google Scholar]
- Lee H.Y., Im H. Colorectal cancer screening among Korean American Immigrants: unraveling the influence of culture. J. Health Care Poor Underserved. 2013;24(2):579–598. doi: 10.1353/hpu.2013.0087. [DOI] [PubMed] [Google Scholar]
- Lee E., Natipagon-Shah B., Sangsanoi-Terkchareon S., Warda U.S., Lee S.-Y. Factors influencing colorectal cancer screening among Thais in the U.S. J. Commun. Health. 2019;44(2):230–237. doi: 10.1007/s10900-018-0578-x. [DOI] [PubMed] [Google Scholar]
- Leung D.Y.P., Wong E.M.L., Chan C.W.H. Determinants of participation in colorectal cancer screening among community-dwelling Chinese older people: testing a comprehensive model using a descriptive correlational study. Eur. J. Oncol. Nurs. 2016;21:17–23. doi: 10.1016/j.ejon.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Lin T.-Y., Chuang S.-T., Huang S.-F., Hsu H.-P., Lu L.-T., Guo J.-L. Likelihood of a fecal occult blood test uptake among older adults: comparisons between health professionals and healthcare volunteers based on the health belief model. BMC Geriatr. 2019;19(1):51. doi: 10.1186/s12877-019-1067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae F.A., Hill D.J., St. John D.J.B., Ambikapathy A., Garner J.F. Predicting colon cancer screening behavior from health beliefs. Prev. Med. 1984;13(1):115–126. doi: 10.1016/0091-7435(84)90044-6. [DOI] [PubMed] [Google Scholar]
- Menon U., Belue R., Sugg Skinner C., Rothwell B.E., Champion V. Perceptions of colon cancer screening by stage of screening test adoption. Cancer Nurs. 2007;30(3):178–185. doi: 10.1097/01.NCC.0000270706.80037.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health Singapore. Cancer Screening. MOH Clinical Practice Guidelines 1/2010 Web site. https://www.moh.gov.sg/docs/librariesprovider4/guidelines/cpg_cancer-screening.pdf. Published 2010. Accessed.
- Moola S., Munn Z., Tufanaru C. Chapter 7: systematic reviews of etiology and risk. In: Aromataris E., Munn Z., editors. Joanna Briggs Institute Reviewer's Manual. The Joanna Briggs Institute; 2017. [Google Scholar]
- Navarro M., Nicolas A., Ferrandez A., Lanas A. Colorectal cancer population screening programs worldwide in 2016: an update. World J. Gastroenterol. 2017;23(20):3632. doi: 10.3748/wjg.v23.i20.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng E.S.T., Tan C.H., Teo D.C.L., Seah C.Y.E., Phua K.H. Knowledge and perceptions regarding colorectal cancer screening among Chinese—a community-based survey in Singapore. Prev. Med. 2007;45(5):332–335. doi: 10.1016/j.ypmed.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Ng S.C., Wong S.H. Colorectal cancer screening in Asia. Br. Med. Bull. 2013;105(1):29–42. doi: 10.1093/bmb/lds040. [DOI] [PubMed] [Google Scholar]
- O'Connell J.B., Maggard M.A., Ko C.Y. Colon cancer survival rates with the New American Joint Committee on Cancer Sixth Edition Staging. JNCI J. Natl. Cancer Inst. 2004;96(19):1420–1425. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- Palmer R.C., Chhabra D., McKinney S. Colorectal cancer screening adherence in African–American Men and Women 50 years of age and older living in Maryland. J. Community Health. 2011;36(4):517–524. doi: 10.1007/s10900-010-9336-4. [DOI] [PubMed] [Google Scholar]
- Park S.H., Kim G.S. Colorectal cancer screening in Korean Workers: using a stage model approach to examine the ecological predictors of behavior. Cancer Nurs. 2014;37(4):278–291. doi: 10.1097/NCC.0b013e31829bc913. [DOI] [PubMed] [Google Scholar]
- Partin M.R., Gravely A.A., Burgess J.F., Jr Contribution of patient, physician, and environmental factors to demographic and health variation in colonoscopy follow-up for abnormal colorectal cancer screening test results: colorectal Cancer Screening Follow-Up. Cancer. 2017;123(18):3502–3512. doi: 10.1002/cncr.30765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips K.A., Kerlikowske K., Baker L.C., Chang S.W., Brown M.L. Factors associated with women's adherence to mammography screening guidelines. Health Serv. Res. 1998;33(1):29–53. [PMC free article] [PubMed] [Google Scholar]
- Pornet C., Denis B., Perrin P., Gendre I., Launoy G. Predictors of adherence to repeat fecal occult blood test in a population-based colorectal cancer screening program. Br. J. Cancer. 2014;111(11):2152–2155. doi: 10.1038/bjc.2014.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power E., Miles A., von Wagner C., Robb K., Wardle J. Uptake of colorectal cancer screening: system, provider and individual factors and strategies to improve participation. Future Oncol. 2009;5(9):1371–1388. doi: 10.2217/fon.09.134. [DOI] [PubMed] [Google Scholar]
- Ran T., Cheng C.-Y., Misselwitz B., Brenner H., Ubels J., Schlander M. Cost-effectiveness of colorectal cancer screening strategies—a systematic review. Clin. Gastroenterol. Hepatol. 2019;17(10) doi: 10.1016/j.cgh.2019.01.014. 1969-1981.e15. [DOI] [PubMed] [Google Scholar]
- National Registry of Diseases Office Singapore. Singapore Cancer Registry Annual Registry Report 2015. https://www.nrdo.gov.sg/docs/librariesprovider3/Publications-Cancer/cancer-registry-annual-report-2015_web.pdf?sfvrsn=10. Published 2015. Accessed.
- Sammut R., Camilleri S., Trapani J. The knowledge and attitudes of persons who participate and do not participate in colorectal cancer screening: a comparative survey. Appl. Nurs. Res. 2019;49:29–34. doi: 10.1016/j.apnr.2019.07.004. [DOI] [PubMed] [Google Scholar]
- Sedgwick P. Convenience sampling. BMJ. 2013;347:f6304. [Google Scholar]
- Seeff L.C., DeGroff A., Joseph D.A. Moving forward: using the experience of the CDCs' Colorectal Cancer Screening Demonstration Program to guide future colorectal cancer programming efforts: Moving Forward. Cancer. 2013;119:2940–2946. doi: 10.1002/cncr.28155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi Zadeh Mehraban S., Namdar A., Naghizadeh M.M. Assessment of preventive behavior for cervical cancer with the health belief model. Asian Pac. J. Cancer Prevention. 2018;19(8):2155–2163. doi: 10.22034/APJCP.2018.19.8.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohler N.L., Jerant A., Franks P. Socio-psychological factors in the Expanded Health Belief Model and subsequent colorectal cancer screening. Patient Educ. Couns. 2015;98(7):901–907. doi: 10.1016/j.pec.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W.Y., Basch C.E., Wolf R.L., Li X.J. Factors associated with colorectal cancer screening among Chinese-Americans. Prev. Med. 2004;39(2):323–329. doi: 10.1016/j.ypmed.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Sung J.J.Y., Choi S.Y.P., Chan F.K.L., Ching J.Y.L., Lau J.T.F., Griffiths S. Obstacles to colorectal cancer screening in Chinese: a study based on the health belief model. Am. J. Gastroenterol. 2008;103(4):974–981. doi: 10.1111/j.1572-0241.2007.01649.x. [DOI] [PubMed] [Google Scholar]
- Taheri-Kharameh Z., Noorizadeh F., Sangy S., Zamanian H., Shouri-Bidgoli A.R., Oveisi H. Factors associated with adherence to colorectal cancer screening among moderate risk individuals in Iran. Asian Pac. J. Cancer Prev. 2016;16(18):8371–8375. doi: 10.7314/apjcp.2015.16.18.8371. [DOI] [PubMed] [Google Scholar]
- Talaat N. Adherence and barriers to colorectal cancer screening varies among Arab Americans from different countries of origin. Arab J. Gastroenterol. 2015;16(3-4):116–120. doi: 10.1016/j.ajg.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Taş F., Kocaöz S., Çirpan R. The effect of knowledge and health beliefs about colorectal cancer on screening behaviour. J. Clin. Nurs. 2019;28(23-24):4471–4477. doi: 10.1111/jocn.15032. [DOI] [PubMed] [Google Scholar]
- Tastan S., Andsoy I.I., Iyigun E. Evaluation of the knowledge, behavior and health beliefs of individuals over 50 regarding colorectal cancer screening. Asian Pac. J. Cancer Prev. 2013;14(9):5157–5163. doi: 10.7314/apjcp.2013.14.9.5157. [DOI] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force. Final Recommendation Statement, Colorectal Cancer: Screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/colorectal-cancer-screening2#consider. Published 2016. Accessed.
- Wang W.-L., Hsu S.-D., Wang J.-H., Huang L.-C., Hsu W.-L. Survey of breast cancer mammography screening behaviors in Eastern Taiwan based on a health belief model. Kaohsiung J. Med. Sci. 2014;30(8):422–427. doi: 10.1016/j.kjms.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R.K., Wong M.L., Chan Y.H., Feng Z., Wai C.T., Yeoh K.G. Gender differences in predictors of colorectal cancer screening uptake: a national cross sectional study based on the health belief model. BMC Public Health. 2013;13(1):677. doi: 10.1186/1471-2458-13-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Cancer Research Fund. Colorectal cancer statistics. https://www.wcrf.org/dietandcancer/cancer-trends/colorectal-cancer-statistics. Published n.d. Accessed.
- World Health Organisation. World Cancer Report: Cancer Research for Cancer Prevention. https://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-Cancer-Research-For-Cancer-Prevention-2020. Published 2020. Accessed.
- Yarbrough S.S., Braden C.J. Utility of health belief model as a guide for explaining or predicting breast cancer screening behaviours. J. Adv. Nurs. 2001;33(5):677–688. doi: 10.1046/j.1365-2648.2001.01699.x. [DOI] [PubMed] [Google Scholar]
- Yoo W., Kwon M.-W., Pfeiffer L.J. Influence of communication on colorectal cancer screening: revisiting the Health Belief Model. J. Commun. Healthcare. 2013;6(1):35–43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.