Summary
3D in vitro cancer models are important therapeutic and biological discovery tools, yet formation of matrix-embedded multicellular spheroids prepared in high-throughput (HTP), and in a highly controlled manner, remains challenging. This is important to achieve robust and statistically relevant data. Here, we developed an enabling technology consisting of a bespoke drop-on-demand 3D bioprinter capable of HTP printing of 96-well plates of spheroids. 3D multicellular spheroids are embedded inside a hydrogel matrix with precise control over size and cell number, with the intra-experiment variability of embedded spheroid diameter coefficient of variation being between 4.2% and 8.7%. Application of 3D bioprinting HTP drug screening was demonstrated with doxorubicin. Measurements of IC50 values showed sensitivity to spheroid size, embedding, and how spheroids conform to the embedding, revealing parameters shaping biological responses in these models. Our study demonstrates the potential of 3D bioprinting as a robust HTP platform to screen biological and therapeutic parameters.
Subject Areas: Biotechnology, Cell Biology, Biomaterials
Graphical Abstract
Highlights
-
•
3D bioprinter for the high-throughput production of embedded 3D multicellular spheroids
-
•
Bioprinted spheroids are biologically similar to manually produced spheroids
-
•
Parameters such as spheroid size and embedding affect the response toward doxorubicin
-
•
High-throughput screening applications with high content imaging and viability assay
Biotechnology; Cell Biology; Biomaterials
Introduction
3D cell cultures have a number of advantages over 2D cell cultures and should be the workhorse of modern cell biology (Griffith and Swartz, 2006). For example, in cancer cell biology, a number of cellular properties, such as cell proliferation, differentiation, and response to drugs, are fundamentally different for cells in 2D and 3D environments (Fennema et al., 2013; Hoarau-Véchot et al., 2018). Furthermore, 3D models, compared with 2D cell culture models, exhibit more in vivo tumor-like features including hypoxic regions, gradient distribution of chemical and biological factors, and expression of pro-angiogenic and multidrug resistance proteins (Nyga et al., 2011). These in vivo features are observed in 3D spheroids, even though they are derived from a single immortal cell line. It is easy to envisage even more realistic 3D cell models, including cells embedded in active extracellular matrix (ECM) mimics (Lu et al., 2012) and incorporation of multiple cell types to form organoids (Fatehullah et al., 2016). And yet 2D cell culture is still commonly employed (Breslin and O’Driscoll, 2013) because of the ease of use and reproducibility of culturing cells on plastic or glass surfaces. Thus, the challenge is how to produce large quantities of ECM-embedded 3D cell cultures in an efficient and high-throughput (HTP) manner such that statistically relevant data can be obtained (Sant and Jhonston, 2017).
For 3D cell culture to be used for quantitative investigations including fundamental studies and drug screening (Breslin and O’Driscoll, 2013), control over additional culture parameters is required. For example, it is known that volume and shape of spheroids influences their sensitivity to anti-cancer drugs (Virgone-Carlotta et al., 2017; Zanoni et al., 2016) and that the ECM mimic that embeds spheroids determines the rate of growth and drug responses (Lam et al., 2014; Loessner et al., 2013). There are a variety of methods for producing multicellular spheroids using rotation, microfluidics, non-cell-adherent surfaces, magnetic levitation, or pealing confluent layers of cells off a surface (Costa et al., 2016; Souza et al., 2010; Wu et al., 2008). The use of non-adherent surfaces is the most popular approach (Fennema et al., 2013). This method employs ultra-low-attachment, round-bottomed well plates where gravity promotes cell aggregation to form a multicellular spheroid. To obtain the spheroid embedded within a hydrogel matrix, a more realistic model of the in vivo situation, requires the manual transfer of the spheroid into the appropriate hydrogel matrix (McCarroll et al., 2014). This is a process that is labor intensive and comes with a significant failure rate. An approach that overcomes the embedding challenge involves growing spheroids inside hydrogel matrices (Koledova, 2017), such as Matrigel, collagen, alginate, synthetic peptides, and polymers but at the cost of control over the spatial distribution and size of the spheroids. Thus, there is an urgent need for an HTP method for producing embedded spheroids that is compatible with many cell types and ECM mimics. The method should also have capabilities to control other culture parameters such as spheroid size and shape so that statistically reliable data on drug responses can be obtained.
Recently, 3D bioprinting has emerged as a promising method to create reproducible but complex biological constructs by printing cell-laden hydrogel matrix precursors or bioinks (Kang et al., 2016; Kolesky et al., 2016). For example, some exquisitely sophisticated cardiac implants have been developed with extrusion 3D bioprinting (Lee et al., 2019; Noor et al., 2019). However, none of the existing 3D bioprinters have been designed specifically for producing 3D cell cultures, and as such, are not ideal for this application. Drop-on-demand technologies, such as inkjet printing, have high cell compatibility and have been used extensively to print geometrically simple 3D hydrogel matrix constructs (Knowlton et al., 2015; Ng et al., 2017). Due to the droplet ejection mechanism, this technology shows very high cell viability but is limited to printing low-viscosity bioinks (Gudapati et al., 2016), which effectively means bioinks with low cell numbers, whereas high cell loadings are required for forming most 3D cell cultures. In contrast, the more popular microextrusion bioprinting process is used primarily in tissue engineering to print soft and hard tissues (Noor et al., 2019; Grigoryan et al., 2019). It is compatible with more viscous or semi-gel materials carrying a high density of cells. However, the imparted shear stress on the cells during printing affects cell viability (Murphy and Atala, 2014).
Here, we establish a bespoke drop-on-demand bioprinter that gives high cell number and high cell viability made possible using a solenoid microvalve printhead. We demonstrate the HTP bioprinting of embedded spheroids derived from various cell types. The printing process provided control over cell number, spheroid size, and dimensions of the embedding cup without compromising cell viability. We performed extensive studies to confirm that the structural and biological characteristics of the 3D bioprinted spheroids were similar to manually prepared spheroids, including cell proliferation, apoptosis, cytoskeletal structure, hypoxia, and stem-cell presentation. Furthermore, we show that the bioprinting approach for embedded spheroid production enables HTP drug response analysis and effectiveness of a drug in inhibiting cell proliferation through determination of half maximal inhibitory concentration (IC50 values).
Results
3D Bioprinting of Tumor Spheroids Inside a Hydrogel Matrix
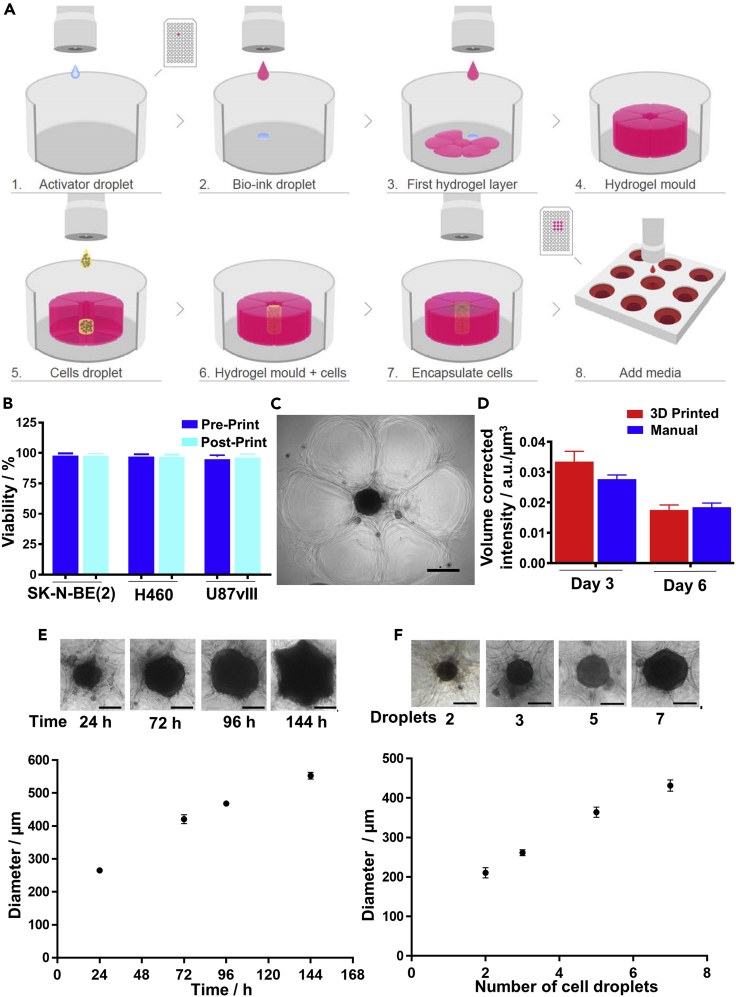
The bespoke 3D bioprinter for the preparation of embedded 3D spheroids prints a high density of cells in a single droplet, with high viability directly into a hydrogel matrix (Figure 1A and Video S1). The bespoke 3D bioprinter is composed of a solenoid microvalve printhead, 2-axis robotics, a pressure regulation system, a printing stage for a 96-well microtiter plate, and custom-written software for biological assay design and printing. Here, alginate bioink and CaCl2 activator were used as the hydrogel matrix. Previously reported printing parameters that affect cell viability (Ng et al., 2017; Lepowsky et al., 2018), such as pressure and bioink/activator concentrations, were optimized to ensure that the biological properties of the ejected cells were not affected by fluidic shear stress or the ink materials. To examine whether printed cells remained viable, a trypan blue exclusion cell viability assay was performed to rapidly assess the viability of neuroblastoma (SK-N-BE(2)), non-small cell lung cancer (H460), and glioblastoma (U87vIII) cells (Figure 1B). Cell viability of greater than 98% was observed for cells immediately before printing (pre-print) and after 3D bioprinting (post-print). This is consistent with the finding that the printing process did not induce apoptotic cell death in the sample (Figure S1A). To investigate the long-term biocompatibility of the hydrogel matrix, a live/dead assay was performed on the 3D encapsulated cells after a 72-h incubation, which confirmed the biocompatibility of the chosen hydrogel matrix, with viability of greater than 95% observed in all cases (Figures S1B–S1D).
Figure 1.
3D Bioprinting of Embedded Spheroids
(A) A schematic of the bioprinting process. Steps 1–4: A hydrogel matrix is bioprinted to form a cup (pink), (Steps 5–6) into which the desired number of cells (yellow) are deposited. Steps 6–8: The top half of the cup is then bioprinted to complete the embedding and incubated at 37°C and 5% CO2 until spheroids are formed.
(B) Cell viability of neuroblastoma (SK-N-BE(2)), non-small cell lung cancer (H460), and glioblastoma (U87vIII) cells in the bioink (Post-print) compared with cells before bioprinting (Pre-print) (n = 3).
(C) A microscopic image of a bioprinted spheroid (middle black circle) surrounded by hydrogel matrix (outer flower-like structures).
(D) Cell viability of bioprinted SK-N-BE(2) spheroids at days 3 and 6 compared with manually formed spheroids (n = 3, one-way ANOVA; day 3 = non-significant (n.s.), day 6 = n.s.).
(E) The formation and growth of bioprinted SK-N-BE(2) spheroids over a period of 144 h with 23,750 cells (5 cell droplets) initially seeded.
(F) Size of bioprinted SK-N-BE(2) spheroids after 3 days varied with the number of droplets containing cells that were seeded (E and F, n = 3 spheroids, scale bars, 200 μm).
Results are means ± SEM. See also Figures S1 and S2 and Videos S1, S2, S3, and S4
Video representation of the 3D bioprinting process of embedded 3D spheroids in a 96-well plate, depicting the bioprinting of the hydrogel matrix cup structure and the deposition of high cell concentration droplets inside the cup.
To generate a single tumor spheroid embedded inside an alginate 3D matrix structure, an alginate cup that had the structural stability to support gravity-based spheroid formation was printed (Figure 1A, steps 1–4 and Video S1). Next, cell-laden ink at 250 million cells/mL, which does not interact with the formation of the hydrogel, was prepared and printed into the cup (Figure 1A, steps 5–6). The embedded spheroid was completed by printing the top layer of the hydrogel matrix mold (Figure 1A, step 7). This process was then repeated, taking approximately 70 min to print the alginate cups and 10 min to print the cells in an entire 96-well plate without any effects on cell viability, such that each well contained a single spheroid in a hydrogel matrix (Figure 1A, step 8). A flyby printing logic was used to enable rapid production of bioprinted samples (Video S2). Upon incubation, a combination of gravitational forces and ECM secreted by cells promoted cell migration, adhesion and proliferation, and subsequent spheroid formation (Fennema et al., 2013). Phase contrast microscopic images of a printed spheroid of SK-N-BE(2) cells (Figure 1C) showed that the cells formed a dense ball within the cup with the individual alginate droplets forming the cup (flower-like arrangement). The formation of the spheroids over a 72 h period are shown in Videos S3 and S4 for the 3D bioprinted and manually prepared spheroids using a low-attachment, round-bottomed well plate (Vinci et al., 2012), respectively. The viability of SK-N-BE(2) cells within the spheroids were similar for printed and manually prepared spheroids after both 3 and 6 days (Figure 1D) indicating neither the printing process nor the bioink were influencing the cells deleteriously. Similar observations were made for H460 and U87vIII cells (Figures S2A and S2B).
Timecode in minutes.
Scale bar, 200 μm. Time code in hour.
Scale bar, 200 μm. Time code in hour.
The capability of the 3D bioprinter to accurately dispense discrete droplets with a high concentration of viable cells is critical for the formation of spheroids and unattainable using other printing technologies. Typically, five 19-nL droplets with approximately 4,750 cells per droplet were deposited inside the hydrogel matrix mold. Monitoring the growth of the 3D bioprinted SK-N-BE(2) cells over 144 h (Figure 1E) showed that the 3D bioprinted spheroids began to form after only 24 h. The rapid rate of spheroid formation was attributed to the high number of cells deposited inside a confined space. Similar observations were made for lung cancer cells (H460) and glioblastoma cells (U87vIII), respectively (Figures S2C and S2D). All bioprinted spheroids increased in diameter linearly with time. Importantly, as the cup was completely filled by the growing spheroid, the spheroid began to conform to the shape of the cup, which highlights the capability of the bioprinter to also produce spheroids with controlled shape by matching cup size, shape, and cell volume. The size of the spheroids can be controlled via the cell density in each droplet or the number of droplets printed. It was demonstrated that increasing the number of SK-N-BE(2) cell droplets from two (∼9,500 cells) to seven (∼33,250 cells) led to an increase in spheroid diameter from 190 ± 13 to 420 ± 19 μm after 3 days of incubation (Figure 1F). Thus the 3D bioprinting platform enabled the rapid formation of embedded spheroids with high cell viability and control over spheroid size and shape.
The In Vivo Tumor-like Characteristics of the 3D Bioprinted Spheroids
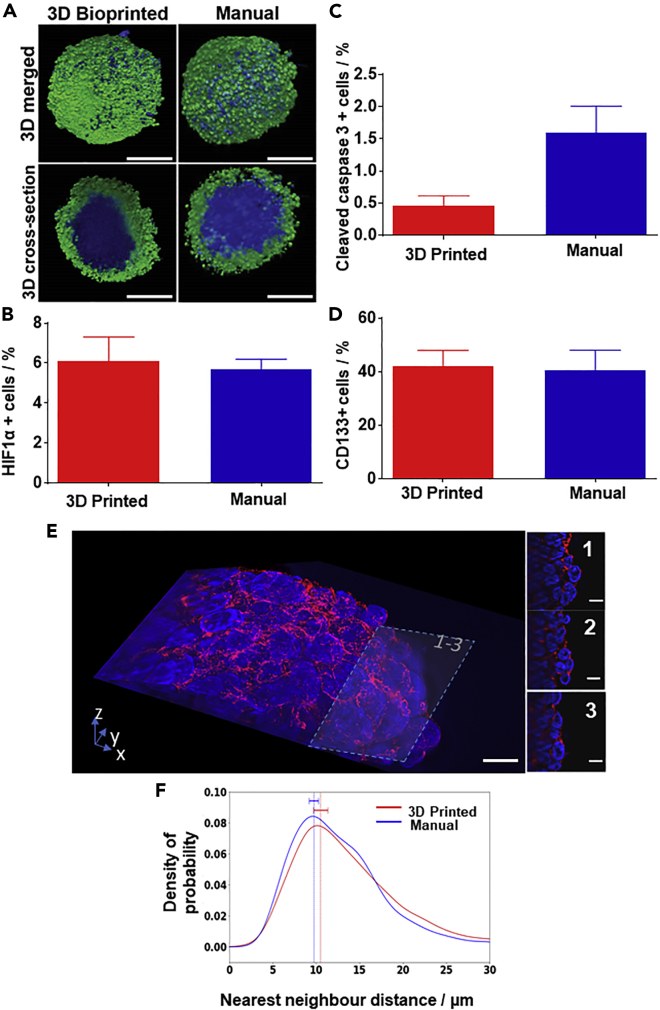
We next addressed whether 3D printed matrix-embedded spheroids also possess important in vivo tumor-like characteristics found in manually prepared spheroids. To visualize the organization of the 3D bioprinted SK-N-BE(2) spheroids, we immunostained for Ki67, a protein associated with cell proliferation (Breslin and O’Driscoll, 2013) and stained the nucleus (Figure 2A). Proliferating cells were consistently found on the periphery of the spheroid during the entire 6 days of investigation (Figure S3A). Similarly, quantitative analysis of the light-sheet microscope images (Figure S3B) located proliferating cells in both 3D bioprinted and manually prepared spheroids at the periphery (0.8–1 normalized distance, with 0 being the center and 1 the edge of the spheroid). The presence of hypoxic cells in the 3D bioprinted spheroids was demonstrated using the well-established hypoxia marker, HIF1α. Dissociating and immuno-labeling the spheroids allowed analysis via fluorescence-activated cell sorting (FACS), which showed equivalent HIF1α-positive cell populations of ∼5% in both the 3D bioprinted and manual spheroids (Figure 2B), confirming that 3D bioprinted spheroids carry the important hypoxia characteristic of a 3D spheroid model (Breslin and O’Driscoll, 2013). Manually prepared spheroids treated with CoCl2, an inducer of hypoxia, were used as positive controls for hypoxia (Triantafyllou et al., 2006).
Figure 2.
3D Bioprinted Spheroids Have a Similar Organization as Manually Produced Spheroids
(A) 3D rendered and 3D cross-sectioned (via optical sectioning) light-sheet microscopy images of the 3D bioprinted (left) and manual (right) SK-N-BE(2) spheroids, labeled with α-Ki67 antibody (green), indicating cell proliferation and the DNA dye Hoechst 33,342 (blue). Scale bars, 200 μm.
(B–E) (B) Percentage of HIF1α-positive, (C) cleaved caspase-3-positive, and (D) CD133-positive cells as determined by FACS. (In B–D, n = 3, unpaired t test; n.s.). (E) Lattice light-sheet images of a bioprinted spheroid, stained with phalloidin-568 (red) and SYTOX green (blue). The images were further sliced at 34.95 μm (E1), 27.45 μm (E2), and 19.95 μm (E3) from the top to show the high-resolution cellular arrangement of the spheroids. Scale bars, 10 μm.
(F) Quantification of the cell-cell density was conducted by measuring the average distance between the nearest-neighboring nuclei in 3 dimensions for both manually prepared and 3D bioprinted SK-N-BE(2) spheroids (n = 3).
Results are means ± SEM. See also Figures S3–S5.
Next, we investigated apoptosis in 3D bioprinting spheroids. 3D bioprinted and manually prepared spheroids were dissociated and stained for the apoptotic marker cleaved caspase-3 before FACS analysis. Spheroids treated with doxorubicin (0.4 mM) were used as a positive control. The percentage of cleaved caspase-3-positive cells was 0.4% and 1.7% for the 3D bioprinted and manual spheroid, respectively, and not statistically significantly different (Figure 2C). Apoptotic cells could also be seen in light-sheet microscopy images without a discernible difference in the number and location of cleaved caspase-3-positive cells between 3D bioprinted and manually assembled spheroids after 3 and 6 days (Figure S4).
We also assessed the percentage of cells with cancer stem-like properties. These are key features that make in vitro 3D models more tumor like than 2D models. Neuroblastoma cells express the cancer stem cell marker CD133, and CD133-positive neuroblastoma cells have the ability to form tumors (Garner and Beierle, 2015). To determine whether CD133-positive cancer stem-like cells can be found in the 3D bioprinted spheroids, FACS analyses using anti-CD133 antibody were conducted. The results showed that nearly 40% of the cells of the 3D bioprinted and the manually prepared spheroids were CD133 positive (Figure 2D), demonstrating the preservation of cancer stemness in both types of spheroids.
To evaluate how the cells were arranged inside the 3D bioprinted spheroids, H&E staining was performed on spheroid cross sections. Micrographs of the sectioned spheroids showed that the cell arrangements and populations were very similar in both 3D bioprinted and manual spheroids at both days 3 and 6 (Figure S5). Lattice light-sheet microscopy of the spheroids stained with phalloidin for F-actin organization (red) and SYTOX green for nuclei (blue) was used to explore the cell arrangement (Figure 2E). Cell-cell compactness was quantified by calculating the nearest-neighbor distance for each nucleus in both 3D bioprinted and manual spheroids at day 3, with no significant difference found (Figure 2F). Thus, both qualitative 3D images and quantitative analysis confirmed that 3D bioprinting does not significantly alter the arrangement and compactness of cells within spheroids compared with manually prepared spheroids. In summary, we demonstrated that 3D bioprinted spheroids had the desired cellular organization of cancer stem-like cells, apoptotic cells, and hypoxic cells at the core and proliferating cells at the periphery.
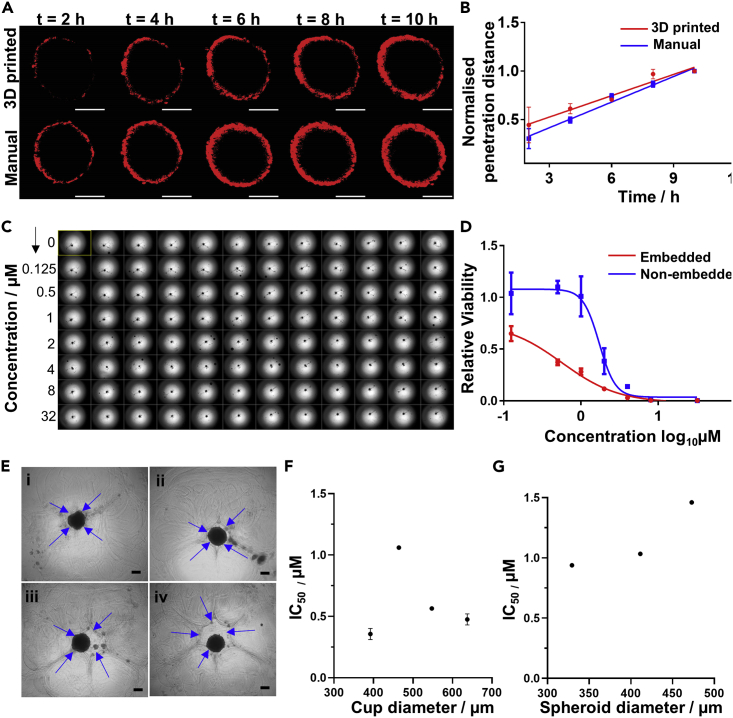
Having shown that the compactness of 3D bioprinted and manual spheroids was similar, we investigated whether this was reflected in the drug penetration into spheroids. Drug penetration into 3D bioprinted spheroids (compared with manual spheroids) was performed using a previously reported method (Sagnella et al., 2014). To conduct a direct comparison between the 3D bioprinted and manual spheroid, 3D bioprinted spheroid was recovered from the hydrogel matrix using the chelating solution. Both recovered 3D bioprinted and manually prepared spheroids were treated with 3.68 μM doxorubicin for 2 h. Doxorubicin diffusion into the spheroids was monitored with microscopy every 2 h for 10 h and quantified using a bespoke Python script. In brief, the image plane with maximum intensity for every time point was identified and used to calculate the penetration distance over time. For both types of spheroids, doxorubicin was only able to penetrate the periphery (Figure 3A), which is attributed to the compact cellular arrangements, an observation that is commonly found in tumors. Over time, doxorubicin slowly penetrates and accumulates in the spheroids in both recovered 3D bioprinted and manual samples (Figure 3B). These observations confirmed that the 3D bioprinted spheroids capture some of the biological behavior of tumors, making them suitable for HTP 3D drug discovery experiments.
Figure 3.
Bioprinted SK-N-BE(2) Spheroids Are Efficient 3D Models for High-Throughput Drug Discovery
(A) Confocal microscopic images at 2-h intervals over 10 h showing the penetration of doxorubicin (red) into the bioprinted and manually prepared spheroids. Scale bars, 200 μm.
(B) Penetration depth profile of doxorubicin into bioprinted and manual spheroids over 10 h (n = 2, 3 spheroids group).
(C) Operetta image of a full 96-well plate of 3D bioprinted embedded spheroids before doxorubicin treatment. Plate was then treated with eight different doxorubicin concentrations with 12 replicates for each concentration.
(D) Dose-response curve of bioprinted spheroids, embedded in the hydrogel matrix and non-embedded (n = 2, 6 spheroids per condition and per repeat).
(E) Bioprinted spheroids encapsulated inside varying cup sizes. Spheroid diameter was kept similar at 400 ± 18 μm, whereas cup sizes were varied as (i) 376 ± 27 μm, (ii) 464 ± 29 μm, (iii) 548 ± 27 μm, and (iv) 637 ± 20 μm. Blue arrows indicate the cup wall. Scale bars, 200 μm.
(F) Effect of the hydrogel matrix cup diameter on IC50 of doxorubicin on bioprinted spheroids. (n = 2. 6 spheroids per group).
(G) Effect of spheroid size on IC50 of doxorubicin on bioprinted spheroids with a constant cup size (n = 3 experimental repeats per condition).
Results are means ± SEM. See also Figure S6.
3D Bioprinted Spheroids as a High-Throughput Tool for Evaluating Drug Responses
The capability of 3D bioprinting of spheroids affords the opportunity of HTP drug screening. To show compatibility of the 3D bioprinted matrix-embedded single spheroids for HTP screening, we exposed a 96-well plate of neuroblastoma spheroids, grown to an average diameter of 400 μm (72 h incubation post-printing), to eight concentrations of doxorubicin (0–32 μM), with 12 repeats for each concentration, for a further 72 h (Figure 3C). The drug response was measured directly in the microtiter plate using the CellTiter-Glo assay, from which robust IC50 data were derived (Figure 3D). To demonstrate bioprinting reproducibility, the coefficient of variation (CV%) of the embedded spheroid diameter across all 3D bioprinted SK-N-BE(2) HTP experiments was determined to be between 4.2% and 8.7%, which is comparable to the values obtained from spheroids formed using an ultra-low attachment 96-well round-bottomed plate (Vinci et al., 2012). The quality of the 3D bioprinted embedded spheroids was tested with the statistically robust standardized mean difference (SSMD) analysis (Richter et al., 2017; Zhang, 2008). We conducted this analysis on all HTP screening experiments, with the results indicating that 95% of our printed embedded spheroids, and hence the assays, were of good or greater quality, when compared with the acceptable criteria of the SSMD analysis (Figure S6).
We next determined whether the embedding matrix itself has an impact on the drug response of spheroids, a parameter that has not been widely investigated. A direct comparison of the drug response was conducted between 3D bioprinted spheroids embedded in the hydrogel matrix and 3D bioprinted spheroids recovered from the hydrogel matrix. Interestingly, the presence of a hydrogel matrix increased doxorubicin sensitivity of the spheroids (Figure 3D) compared with free floating spheroids. This is possibly due to the hydrogel matrix acting as a sink for the drug, thus accumulating the drugs from the media into the inner gel region. This in turn increases the availability of the drug to the spheroid thus increasing the drug efficacy.
As the previous experiment highlighted the importance of the hydrogel matrix on drug efficacy, we next looked at another variable, the size of the hydrogel matrix cup in which the cells are deposited, and the spheroid grows (Figure 3E). Four different cup sizes were bioprinted while keeping the spheroid diameter constant such that the space between the spheroid and the embedding hydrogel matrix varied. Surprisingly, doxorubicin IC50 values were highly sensitive to cup size, indicating that matrix properties are an integral part of cancer biology (Figure 3F).
Finally, by exploiting the capability of the 3D bioprinter to adjust spheroid size on demand (Figure 3G), we quantified the effect of SK-N-BE(2) spheroid size on the drug response. Previous qualitative data suggest that MCF-7 spheroid size influences drug resistance (Gong et al., 2015). Three different spheroid diameters were generated, 329 ± 18 μm, 411 ± 32 μm, and 473 ± 32 μm, by changing the number of cell droplets deposited into the cup (3, 5, and 7 droplets, respectively). We found an increase in doxorubicin IC50 values ranging from 1.06 to 1.48 μM as the spheroid sizes increase (Figure 3G). Our data thus indicated that doxorubicin responses were inversely proportional to the surface area of the spheroids. The 3D bioprinting system presented herein is the ideal platform to create appropriate in vitro 3D cancer models and screen drug responses in a HTP manner.
Discussion
Despite the fact that the importance of 3D cancer cell models and 3D ECM mimics has been well established, their use in cancer research is not as widely adopted as it could be because of the difficulties involved in scaling up production of reproducible 3D cancer models embedded in a hydrogel matrix, and analyzing current embedded 3D cancer models in HTP. For the purpose of HTP and reliable production of 3D tumor spheroids, we developed a bespoke 3D bioprinter capable of printing droplets with cell concentrations up to 250 million cells/mL that maintains cell viability at greater than 98%. The combination of the chosen bioinks and non-contact, drop-on-demand, microvalve-based bioprinting technology used herein allowed the printing of high-cell-density bioinks without exposing the cells to detrimental levels of shear stress during the droplet ejection process. The droplet-based bioprinting is easily scalable, in a manner analogous to inkjet printing, and facilitates simultaneous production of multiple embedded, multicellular, spheroid-containing 3D tissue culture models, making it suitable for rapid production of 3D in vitro samples. Using this technology, 3D multicellular spheroids encapsulated in a hydrogel matrix were consistently printed into individual wells on a 96-well plate, demonstrating a novel method for HTP embedded 3D spheroid production. The bioprinted spheroids exhibited identical biological and architectural properties to manually prepared spheroids including overall viability, apoptosis, proliferation, cancer stemness, and compactness was conducted to confirm that they can be used to replace widely used, labor-intensive manual spheroid culture methods.
The bioprinted single spheroid in hydrogel matrix per well enables precise control over the spheroid size, uniformity, and scaffold properties. Moreover, the bioprinting process produces spheroids with consistent spatial location in each well, enabling rapid high-content analysis. Using the HTP bioprinting of spheroids and analysis we began a process of determining some of the important parameters in defining the drug response of spheroids, a thus far underexplored aspect of 3D cell cultures. Our results demonstrate that the larger the spheroid the higher the IC50 for the model drug doxorubicin. Furthermore, the embedding process was shown to make the spheroid more sensitive to the drug, presumably because the matrix used herein accumulated the drug. Finally, we showed that the spheroid shape formed by the contact area between the spheroid and the hydrogel matrix affected doxorubicin effectiveness. Taken together, the capability of 3D bioprinted spheroids opens up many opportunities to create more relevant 3D cancer models in an HTP manner. In particular, primary cell mixtures and stem cells can be incorporated, which is of particular interest to precision medicine and regenerative medicine. The most powerful aspect of this 3D bioprinting platform is the ability for end users, such as cancer researchers, to intuitively design innovative assays that will open up endless possibilities that can be expected to revolutionize biomedical research, including drug research and development.
Limitation of the Study
-
•
Drug screening of 3D bioprinted embedded spheroids was performed with the commonly used chemotherapeutic agent doxorubicin, and further studies using other chemotherapeutic drugs will establish the biocompatibility of the hydrogel matrix with different agents.
-
•
Scalability of our system to 384-well plates, as this would be valuable for use in drug screening facilities. The current hardware, printhead, and the 3D spheroid model are compatible with a 384-well plate format; however, further studies are required to investigate the bioprinting physics in relation to ejecting droplets into a 384-well plate well reproducibly.
-
•
Drug response assays using the CellTiter-Glo assay were performed directly in the 96-well bioprinted plates, whereas the hypoxia and caspase assays performed in this study involved removing the 3D spheroids from gels for processing. Our technology can be adapted to a range of future applications “in situ,” where removal of spheroids is not required by using detection probes for hypoxia and cell death via direct imaging applications.
Resource Availability
Lead Contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, J. Justin Gooding (justin.gooding@unsw.edu.au).
Materials Availability
The study did not generate new unique reagents.
Data and Code Availability
All data are available from the Lead Contact upon request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors would like to thank the Biomedical Imaging Facility (BMIF) within the Mark Wainwright Analytical Center at UNSW for use of the facility and their technical assistance, in particular from Dr. Elvis Pandzic, Dr. Michael Carnell, and Dr. Sandra Fok. The authors acknowledge the Children's Cancer Institute, which is affiliated with the Sydney Children's Hospital and School of Women's & Children's Health, UNSW Sydney. The authors acknowledge the following funding sources: Australian Research Council (ARC) Linkage Grant (LP130101035 to J.J.G., M.K., and J.C.C.R.); ARC Laureate Fellowship (FL150100060 to J.J.G.); National Health and Medical Research Council (NHMRC) Program Grant (APP1091261 to M.K. and J.J.G.); NHMRC Principal Research Fellowship (APP1119152 to M.K.) and an NHMRC Senior Research Fellowship (1059278 to K.G.), support from ARC Centre of Excellence in Convergent Bio-Nano Science and Technology (CE140100036 to J.J.G. and M.K.), and ARC Centre of Excellence in Advanced Molecular Imaging (CE140100011 to K.G.).
Author Contributions
R.H.U., L.A., and C.M.F. designed, performed, and analyzed experiments. L.A., A.P.O.M., and T.A. established and performed the image analysis. J.C.C.R., A.P.O.M., and K.J.O.M. designed and built the 3D bioprinter. J.B. performed and analyzed the lattice light-sheet experiments. K.G. designed the lattice light-sheet experiments and contributed to the data analysis and interpretation. J.C.C.R., M.K., and J.J.G. designed the project strategy and experiments. All authors contributed to the writing of the manuscript.
Declaration of Interests
A.P.O.M., T.A., K.J.O.M., and J.C.C.R. are consultants, employees, shareholders, and/or optionees of Inventia Life Science Pty. Ltd. Inventia has an interest in commercializing the 3D bioprinting technology. R.H.U., L.A., C.M.F., A.P.O.M., J.C.C.R., M.K., and J.J.G. are co-inventors of a patent (WO2017/011854) related to this work.
Published: October 23, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101621.
Contributor Information
Maria Kavallaris, Email: m.kavallaris@ccia.unsw.edu.au.
J. Justin Gooding, Email: justin.gooding@unsw.edu.au.
Supplemental Information
References
- Breslin S., O’Driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug Discov. 2013;18:240–249. doi: 10.1016/j.drudis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Costa E.C., Moreira A.F., Melo-Diogo D.D., Gaspar V.M., Carvalho M.P., Correia I.J. 3D tumor spheroids: an overview on the tools and techniques used for their analysis. Biotechnol. Adv. 2016;34:1427–1441. doi: 10.1016/j.biotechadv.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Fatehullah A., Tan S.H., Barker N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 2016;18:246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- Fennema E., Rivron N., Rouwkema J., van Blitterswijk C., de Boer J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013;31:108–115. doi: 10.1016/j.tibtech.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Garner E.F., Beierle E.A. Cancer stem cells and their interaction with the tumor microenvironment in neuroblastoma. Cancers. 2015;8:5. doi: 10.3390/cancers8010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X., Lin C., Cheng J., Su J., Zhao H., Liu T., Wen X., Zhao P. Generation of multicellular tumor spheroids with microwell-based agarose scaffolds for drug testing. PLOS ONE. 2015;10:e0130348. doi: 10.1371/journal.pone.0130348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith L.G., Swartz M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- Grigoryan B., Paulsen S.J., Corbett D.C., Sazer D.W., Fortin C.L., Zaita A.J., Greenfield P.T., Calafat N.J., Gounley J.P., Ta A.H. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science. 2019;364:458–464. doi: 10.1126/science.aav9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudapati H., Dey M., Ozbolat I. A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials. 2016;102:20–42. doi: 10.1016/j.biomaterials.2016.06.012. [DOI] [PubMed] [Google Scholar]
- Hoarau-Véchot J., Rafii A., Touboul C., Pasquier J. Halfway between 2D and animal models: are 3D cultures the ideal tool to study cancer-microenvironment interactions? Int. J. Mol. Sci. 2018;19:181. doi: 10.3390/ijms19010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H.W., Lee S.J., Ko I.K., Kengla C., Yoo J.J., Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016;34:312–319. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- Knowlton S., Onal S., Yu C.H., Zhao J.J., Tasoglu S. Bioprinting for cancer research. Trends Biotechnol. 2015;33:504–513. doi: 10.1016/j.tibtech.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Koledova Z. 3D cell culture: an introduction. In: Koledova Z., editor. 3D Cell Culture: Methods and Protocols. Springer New York; 2017. pp. 1–11. [Google Scholar]
- Kolesky D.B., Homan K.A., Skylar-Scott M.A., Lewis J.A. Three-dimensional bioprinting of thick vascularized tissues. PNAS. 2016;113:3179–3184. doi: 10.1073/pnas.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam C.R.I., Wong H.K., Nai S., Chua C.K., Tan N.S., Tan L.P. A 3D biomimetic model of tissue stiffness interface for cancer drug testing. Mol. Pharm. 2014;11:2016–2021. doi: 10.1021/mp500059q. [DOI] [PubMed] [Google Scholar]
- Lee A., Hudson A.R., Shiwarski D.J., Tashman J.W., Hinton T.J., Yerneni S., Bliley J.M., Campbell P.G., Feinberg A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science. 2019;365:482–487. doi: 10.1126/science.aav9051. [DOI] [PubMed] [Google Scholar]
- Lepowsky E., Muradoglu M., Tasoglu S. Towards preserving post-printing cell viability and improving the resolution: past, present, and future of 3D bioprinting theory. Bioprinting. 2018;11:e00034. [Google Scholar]
- Loessner D., Flegg J.A., Byrne H.M., Clements J.A., Hutmacher D.W. Growth of confined cancer spheroids: a combined experimental and mathematical modelling approach. Integr. Biol. 2013;5:597–605. doi: 10.1039/c3ib20252f. [DOI] [PubMed] [Google Scholar]
- Lu P., Weaver V.M., Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll J.A., Gan P.P., Erlich R.B., Liu M., Dwarte T., Sagnella S.S., Akerfeldt M.C., Yang L., Parker A.L., Chang M.H. TUBB3/βIII-tubulin acts through the PTEN/AKT signaling axis to promote tumorigenesis and anoikis resistance in non-small cell lung cancer. Cancer Res. 2014;75:415–425. doi: 10.1158/0008-5472.CAN-14-2740. [DOI] [PubMed] [Google Scholar]
- Murphy S.V., Atala A. 3D bioprinting of tissues and organs. Nat. Biotech. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- Ng W.L., Lee J.M., Yeong W.Y., Win-Naing M. Microvalve-based bioprinting – process, bio-inks and applications. Biomater. Sci. 2017;5:632–647. doi: 10.1039/c6bm00861e. [DOI] [PubMed] [Google Scholar]
- Noor N., Shapira A., Edri R., Gal I., Wertheim L., Dvir T. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv. Sci. 2019;6:1900344. doi: 10.1002/advs.201900344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyga A., Cheema U., Loizidou M. 3D tumour models: novel in vitro approaches to cancer studies. J. Cell Commun. Signal. 2011;5:239. doi: 10.1007/s12079-011-0132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter S., Schulze U., Tomancak P., Oates A.C. Small molecule screen in embryonic zebrafish using modular variations to target segmentation. Nat. Commun. 2017;8:1901. doi: 10.1038/s41467-017-01469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagnella S.M., Duong H., MacMillan A., Boyer C., Whan R., McCarroll J.A., Davis T.P., Kavallaris M. Dextran-based doxorubicin nanocarriers with improved tumor penetration. Biomacromol. 2014;15:262–275. doi: 10.1021/bm401526d. [DOI] [PubMed] [Google Scholar]
- Sant S., Johnston P.A. The production of 3D tumor spheroids for cancer drug discovery. Drug Discov. Today Technol. 2017;23:27–36. doi: 10.1016/j.ddtec.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza G.R., Molina J.R., Raphael R.M., Ozawa M.G., Stark D.J., Levin C.S., Bronk L.F., Ananta J.S., Mandelin J., Georgescu M.M. Three-dimensional tissue culture based on magnetic cell levitation. Nat. Nanotechnol. 2010;5:291–296. doi: 10.1038/nnano.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafyllou A., Liakos P., Tsakalof A., Georgatsou E., Simos G., Bonanou S. Cobalt induces hypoxia-inducible factor-1alpha (HIF-1alpha) in HeLa cells by an iron-independent, but ROS-, PI-3K- and MAPK-dependent mechanism. Free Radic. Res. 2006;40:847–856. doi: 10.1080/10715760600730810. [DOI] [PubMed] [Google Scholar]
- Vinci M., Gowan S., Boxall F., Patterson L., Zimmermann M., Court W., Lomas C., Mendiola M., Hardisson D., Eccles S.A. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012;10:29. doi: 10.1186/1741-7007-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgone-Carlotta A., Lemasson M., Mertani H.C., Diaz J.J., Monnier S., Dehoux T., Delanoe-Ayari H., Riviere R., Rieu J.P. In-depth phenotypic characterization of multicellular tumor spheroids: effects of 5-Fluorouracil. PLOS ONE. 2017;12:e0188100. doi: 10.1371/journal.pone.0188100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.Y., Di Carlo D., Lee L.P. Microfluidic self-assembly of tumor spheroids for anticancer drug discovery. Biomed. Microdevices. 2008;10:197–202. doi: 10.1007/s10544-007-9125-8. [DOI] [PubMed] [Google Scholar]
- Zanoni M., Piccinini F., Arienti C., Zamagni A., Santi S., Polico R., Bevilacqua A., Tesei A. 3D tumor spheroid models for in vitro therapeutic screening: a systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016;6:19103. doi: 10.1038/srep19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.D. Novel analytic criteria and effective plate designs for quality control in genome-scale RNAi screens. J. Biomol. Screen. 2008;13:363–377. doi: 10.1177/1087057108317062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video representation of the 3D bioprinting process of embedded 3D spheroids in a 96-well plate, depicting the bioprinting of the hydrogel matrix cup structure and the deposition of high cell concentration droplets inside the cup.
Timecode in minutes.
Scale bar, 200 μm. Time code in hour.
Scale bar, 200 μm. Time code in hour.
Data Availability Statement
All data are available from the Lead Contact upon request.