Abstract
Amauroderma s.lat. has been defined mainly by the morphological features of non-truncate and double-walled basidiospores with a distinctly ornamented endospore wall. In this work, taxonomic and phylogenetic studies on species of Amauroderma s.lat. are carried out by morphological examination together with ultrastructural observations, and molecular phylogenetic analyses of multiple loci including the internal transcribed spacer regions (ITS), the large subunit of nuclear ribosomal RNA gene (nLSU), the largest subunit of RNA polymerase II (RPB1) and the second largest subunit of RNA polymerase II (RPB2), the translation elongation factor 1-α gene (TEF) and the β-tubulin gene (TUB). The results demonstrate that species of Ganodermataceae formed ten clades. Species previously placed in Amauroderma s.lat. are divided into four clades: Amauroderma s.str., Foraminispora, Furtadoa and a new genus Sanguinoderma. The classification of Amauroderma s.lat. is thus revised, six new species are described and illustrated, and eight new combinations are proposed. SEM micrographs of basidiospores of Foraminispora and Sanguinoderma are provided, and the importance of SEM in delimitation of taxa in this study is briefly discussed. Keys to species of Amauroderma s.str., Foraminispora, Furtadoa, and Sanguinoderma are also provided.
Keywords: Ganodermataceae, morphology, phylogeny, Polyporales, ultrastructure
INTRODUCTION
Amauroderma was established by Murrill (1905) and is usually classified in Ganodermataceae. The type species, A. schomburgkii was described from Guyana. The species of the genus often emerged from the roots of living or dead trees on the ground (Ryvarden 2004), and as traditional circumscription the genus is distributed mainly in Neotropics and tropical or subtropical areas of Africa, Asia and Oceania. The taxonomy of the genus was mostly based on traditional morphological characteristics. Macroscopically, it is usually characterized by annual and stipitate to sessile basidiomata, dull to laccate pilei, fresh pore surface colour changing or not when bruised or dry. Microscopically, the genus presents dimitic to trimitic hyphal system, globose to ellipsoid basidiospores, without truncate apex, which are double-walled with thick and ornamented endospore wall (rarely smooth) (Furtado 1981, Corner 1983, Aime et al. 2003, Ryvarden 2004).
Taxonomic and phylogenetic studies of Ganodermataceae have been carried out in recent years, but most studies focused on Ganoderma (Smith & Sivasithamparam 2000, Hong & Jung 2004, Pilotti et al. 2004, Wang et al. 2009, Cao et al. 2012, Cao & Yuan 2013, Li et al. 2014b, Lima Júnior et al. 2014, Coetzee et al. 2015, Hapuarachchi et al. 2015, Xing et al. 2016, Xing et al. 2018, Thawthong et al. 2017) and only a few studies dealt with Amauroderma (Gomes-Silva et al. 2015, Costa-Rezende et al. 2016, 2017, Song et al. 2016). Regarding this phylogenetic context, Amauroderma has been pointed as polyphyletic and regarded as Amauroderma s.lat., in which Amauroderma s.str. has been defined as a Neotropical clade (Gomes-Silva et al. 2015, Costa-Rezende et al. 2016); as for the relevance of hyphal system composition and basidiospore ultrastructure for generic delimitation in the group was observed, as well as two new genera called Foraminispora and Furtadoa were proposed (Costa-Rezende et al. 2017). Furthermore, the generic status of Amauroderma s.lat. species from Asia and Oceania still was questioned (Song et al. 2016), such as ‘Amauroderma rude clade’ composed by A. rude, A. rugosum and A. perplexum; and ‘Amauroderma yunnanense clade’ composed by A. yunnanense (Costa-Rezende et al. 2017), as well as a variety of Asian and African species which were not phylogenetically tested. In this study, taxonomic and phylogenetic analyses of Amauroderma s.lat. were carried out based on more samples from areas outside the neotropics, such as Africa, Asia and Oceania. Based on the abundant specimens, morphological descriptions including ultrastructural features combined with six gene markers provided convincing evidence to clarify the taxonomic status of the species in Amauroderma s.lat.
Nine genera are accepted in Ganodermataceae, of which Amauroderma s.str., Foraminispora, Furtadoa, Haddowia and Magoderna have non-truncate basidiospores, while Ganoderma, Tomophagus, Humphreya and Trachyderma have basidiospores with obvious truncate apex (Karsten 1881, Imazeki 1939, Imazeki 1952, Steyaert 1972, Corner 1983, Moncalvo & Ryvarden 1997, Costa-Rezende et al. 2017). Amauroderma s.str. is distinguished by di-trimitic hyphal system and endospore wall with solid columnar to semi-reticulated ornamentation (Costa-Rezende et al. 2017). Foraminispora has the unique ultrastructure features of basidiospores with hollow columnar endospore ornamentation, which persist to the exospore wall forming holes sometimes under SEM (Costa-Rezende et al. 2017). Furtadoa is distinguished by a monomitic hyphal system in context, with clamped and simple-septate generative hyphae which are different from other genera (Costa-Rezende et al. 2017). Haddowia has distinctive basidiospores, of which the exospore wall with longitudinal ridges partly connected with short transverse walls (Steyaert 1972, Corner 1983). Magoderna can be distinguished by woody hard basidiomata with slightly shiny pileus and fibrotic context, and its hyphae in pileal cover anticlinal (Steyaert 1972). Tomophagus has light basidiomata, with a pale and soft context (Le et al. 2012). Humphreya can be characterized by basidiospores with reticular or erratic irregular ridged double walls (Steyaert 1972). Trachyderma is mainly characterized by a fleshy succulent context when growing (Imazeki 1939, 1952).
In this study, in order to clarify the taxonomy and phylogeny of taxa in Amauroderma s.lat., specimens from different regions of the world were studied using macro-morphology, microscopic examinations together with ultrastructural observations and molecular phylogenetic analyses of six genes (including ITS, nLSU, RPB1, RPB2, TEF and TUB). According to the integrative research, Amauroderma s.lat. is divided into four genera including one new genus; furthermore, six new species are described and eight new combinations are proposed and re-described.
MATERIALS AND METHODS
Morphological studies
All samples examined in this study were deposited at the herbaria of the Institute of Microbiology, Beijing Forestry University, China (BJFC), the Institute of Microbiology, Chinese Academy of Sciences, China (HMAS), the Universidade Federal de Pernambuco, Brazil (URM) and the National Herbarium of Victoria, Australia (MEL). Macro-morphological descriptions were based on field notes and herbarium specimens. Special colour terms followed Petersen (1996). Micro-morphological data were obtained from dried specimens and observed under a light microscope following methods in Li et al. (2014a) and Han et al. (2016). Microscopic features and measurements were made from slide preparations stained with Cotton Blue and Melzer’s reagent. Spores were measured from sections cut from the tubes. To represent variation in the size of spores, 5 % of measurements were excluded from each end of the range and are given in parentheses. The following abbreviations were used: IKI = Melzer’s reagent; IKI– = neither amyloid nor dextrinoid; KOH = 5 % potassium hydroxide; CB = Cotton Blue; CB+ = cyanophilous; L = mean spore length (arithmetic average of all spores); W = mean spore width (arithmetic average of all spores); Q = variation in the L/W ratios between the specimens studied; n (a/b) = number of spores: (a) measured from given number, (b) of specimens. Microscopic features, such as basidiospores, basidia, cystidioles and hyphae, were observed and photographed at a magnification of up to ×1000 with a Nikon Digital Sight DS-Fi2 microscope (Nikon Corporation, Tokyo, Japan), and quantified by the Image-Pro Plus 6.0 software (Media Cybernetics, Silver Spring, USA). Ultrastructures of basidiospores were observed by Scanning Electron Microscopy (SEM) at Beijing Forestry University, China (BJFU) and Tsinghua University, China (THU). Firstly, the fragments of the tubes or spore powder on the pileal surface were slightly broken by Tissue-Prep (Bio-explorer International Limited, Beijing, China) to observe the endospore ornamentation in detail. These tissues were metal sprayed and observed using a Field Emission Scanning Electron Microscope (FESEM) Hitachi SU-8010 (Hitachi, Ltd, Tokyo, Japan).
DNA extraction, amplification and sequencing
A CTAB rapid plant genome extraction kit-DN14 (Aidlab Biotechnologies Co., Ltd, Beijing, China) and an FH plant DNA kit II (Demeter Biotech Co., Ltd., Beijing, China) were used to extract total genomic DNA from dried specimens and to perform the polymerase chain reaction (PCR) according to the manufacturer’s instructions with some modifications (Cui et al. 2019, Shen et al. 2019).
The internal transcribed spacer regions (ITS) was amplified with primer pairs ITS5 and ITS4 (White et al. 1990); for the large subunit of nuclear ribosomal RNA gene (nLSU) we used LR0R and LR5 (Vilgalys & Hester 1990). The primer pairs RPB1-Af and RPB1-Cr (Matheny et al. 2002) were used to amplify the largest subunit of RNA polymerase II (RPB1), and primer pairs fRPB2-5F and fRPB2-7CR (Liu et al. 1999) were used to amplify the second subunit of RNA polymerase II (RPB2). The translation elongation factor 1-α gene (TEF) was amplified with primer pairs EF1-983F and EF1-1567R (Rehner & Buckley 2005). Primer pairs Bt-1a and Bt-1b (Glass & Donaldson 1995) were used to amplify the β-tubulin gene (TUB). The primer LR7 (Vilgalys & Hester 1990) was used sometimes as an alternative to LR5; i2.2F and aCr (Binder et al. 2010) were used as alternatives to RPB1-Af and RPB1-Cr; RPB2-6F and bRPB2-7R (Liu et al. 1999) were sometimes used as alternatives to fRPB2-5F and fRPB2-7CR.
The final PCR volume was 30 μl; each tube contained 1 μl each primer, 1 μl extracted DNA, 12 μl ddH2O and 15 μl 2 × EasyTaq PCR Supermix (TransGen Biotech Co., Ltd., Beijing, China). PCRs were performed on S1000™ Thermal Cycler (Bio-Rad Laboratories, California, USA). The PCR procedure for ITS and TUB was: initial denaturation at 95 °C for 3 min, followed by 34 cycles of denaturation at 94 °C for 40 s, annealing at 54 °C for 45 s and extension at 72 °C for 1 min, and a final extension at 72 °C for 10 min. The PCR procedure for TEF was: initial denaturation at 95 °C for 3 min, followed by 34 cycles of denaturation at 94 °C for 40 s, annealing at 55 °C for 45 s and extension at 72 °C for 1 min, and a final extension at 72 °C for 10 min. The PCR procedure for nLSU was: initial denaturation at 94 °C for 1 min, followed by 34 cycles of denaturation at 94 °C for 30 s, annealing at 50 °C for 1 min and extension at 72 °C for 1.5 min, and a final extension at 72 °C for 10 min. The PCR procedure for RPB1 and RPB2 was an initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 1 min, annealing at 55 °C for 2 min and extension at 72 °C for 1.5 min, and a final extension at 72 °C for 10 min. The PCR products were purified and sequenced at the Beijing Genomics Institute (BGI), China, with the same primers. All sequences analysed in this study were deposited at GenBank and listed in Table 1.
Table 1.
Taxa information and GenBank accession numbers of the sequences used in this study.
aNewly generated sequences for this study.
Bold species = new species.
Phylogenetic analyses
The sequences generated in this study and retrieved from GenBank were combined with ITS, nLSU, RPB1, RPB2, TEF and TUB sequences of Ganodermataceae and outgroups. Perenniporiella chaquenia and P. pendula were selected as outgroups following Costa-Rezende et al. (2016). The datasets were aligned in MAFFT 7 (Katoh & Toh 2008; https://mafft.cbrc.jp/alignment/server/) and manually adjusted in BioEdit (Hall 1999). Two combined matrixes were reconstructed for phylogenetic analyses as a 4-gene dataset (ITS+nLSU+RPB1+TEF) and a 6-gene dataset (ITS+nLSU+RPB1+RPB2+TEF+TUB); their congruences were evaluated with the partition homogeneity test (PHT) by PAUP* v. 4.0b10 (Swofford 2003) under 1 000 homogeneity replicates. The best-fit evolutionary model was selected by hierarchical likelihood ratio tests (hLRT) and Akaike information criterion (AIC) in MrModeltest2 v. 2.3 (Nylander 2008) after scoring 24 models of evolution by PAUP* v. 4.0b10. The maximum likelihood (ML) and Bayesian Inference (BI) analyses were performed based on the two datasets respectively. Settings for phylogenetic analyses followed Song & Cui (2017) and Zhu et al. (2019). ML researches were conducted with RAxML-HPC v. 8.2.3 (Stamatakis 2014) involved 1 000 ML searches under the GTRGAMMA model, and only the maximum likelihood best tree from all searches was kept. In addition, 1 000 rapid bootstrap replicates were run with the GTRCAT model to assess ML bootstrap values (ML) of the nodes. BI were performed using MrBayes v. 3.2 (Ronquist & Huelsenbeck 2003) with four simultaneous independent chains for two datasets, performing 10 million generations until the split deviation frequency value < 0.01, and sampled every 100th generation. The first 25 % sampled trees were discarded as burn-in, while the remaining ones were used to calculate Bayesian posterior probabilities (BPP) of the clades. The ML bootstrap (ML) ≥ 50 % and Bayesian posterior probabilities (BPP) ≥ 0.95 were presented on topologies from ML analyses, respectively. All trees were viewed in FigTree v. 1.4.3 (http://tree.bio.ed.ac.uk/software/figtree/). The final alignments and the retrieved topologies were deposited in TreeBASE (http://www.treebase.org), under accession ID: 24765 (http://purl.org/phylo/treebase/phylows/study/TB2:S24765).
RESULTS
Molecular phylogeny
In this study, 545 sequences derived from six gene loci (ITS, nLSU, RPB1, RPB2, TEF and TUB) were used to reconstruct phylogenetic trees of Ganodermataceae, 308 of them were newly generated, including 66 sequences of ITS, 69 of nLSU, 12 of RPB1, 57 of RPB2, 67 of TEF, and 37 of TUB. The phylogenetic reconstruction performed with maximum likelihood (ML) and Bayesian inference (BI) analyses for two combined datasets showed similar topology and few differences in statistical support. The partition homogeneity test indicated all the six different genes display a congruent phylogenetic signal (P value = 1.00). The best-fit evolutionary model selected by MrModeltest2 v. 2.3 for each region of six genes were HKY+I+G (ITS1), K80 (5.8S), HKY+I+G (ITS2), GTR+I+G (nLSU), HKY+G (RPB1 introns), F81 (RPB1 1st codon), GTR+G (RPB1 2nd codon), GTR+G (RPB1 3rd codon), SYM+G (RPB2 introns), K80+I+G (RPB2 1st codon), GTR+I+G (RPB2 2nd codon), GTR+I+G (TEF introns), HKY+I+G (TEF 1st codon), SYM+I+G (TEF 2nd codon), GTR+G (TEF 3rd codon) and HKY+I+G (TUB). These models were applied in Bayesian analyses for two combined datasets.
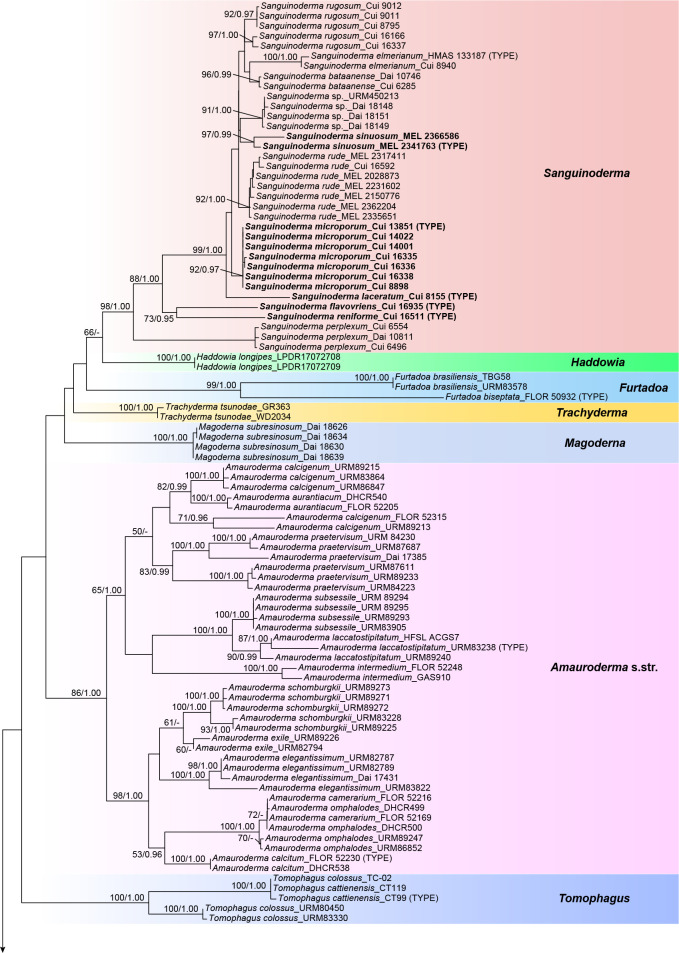
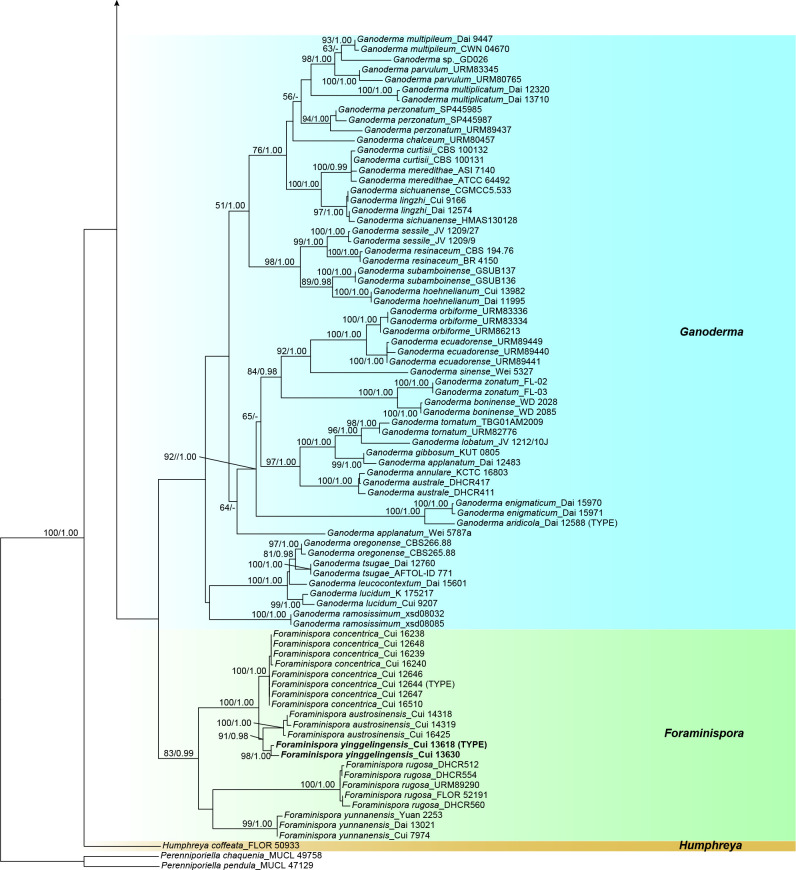
The combined 4-gene dataset (ITS+nLSU+RPB1+TEF) included sequences from 175 specimens representing 69 taxa of Ganodermataceae and Perenniporiella chaquenia and P. pendula as outgroups. The dataset had an aligned length of 3 354 characters, of which 2 366 were constant, 196 were variable and parsimony-uninformative, and 792 were parsimony-informative. The average standard deviation of split frequencies in the Bayesian analyses reached 0.008332. The calculated values based on the dataset are shown in Fig. 1. The clades obtained in this topology, besides the outgroup, were Amauroderma s.str. (86 % ML, 1.00 BPP), Foraminispora (83 % ML, 0.99 BPP), Furtadoa (99 % ML, 1.00 BPP), Ganoderma, Haddowia (100 % ML, 1.00 BPP), Humphreya, Magoderna (100 % ML, 1.00 BPP), Sanguinoderma (98 % ML, 1.00 BPP), Tomophagus (100 % ML, 1.00 BPP) and Trachyderma (100 % ML, 1.00 BPP).
Fig. 1.
ML analyses of Ganodermataceae based on dataset of ITS+nLSU+RPB1+TEF. ML bootstrap values higher than 50 % and Bayesian posterior probabilities values more than 0.95 are shown. New species are in bold.
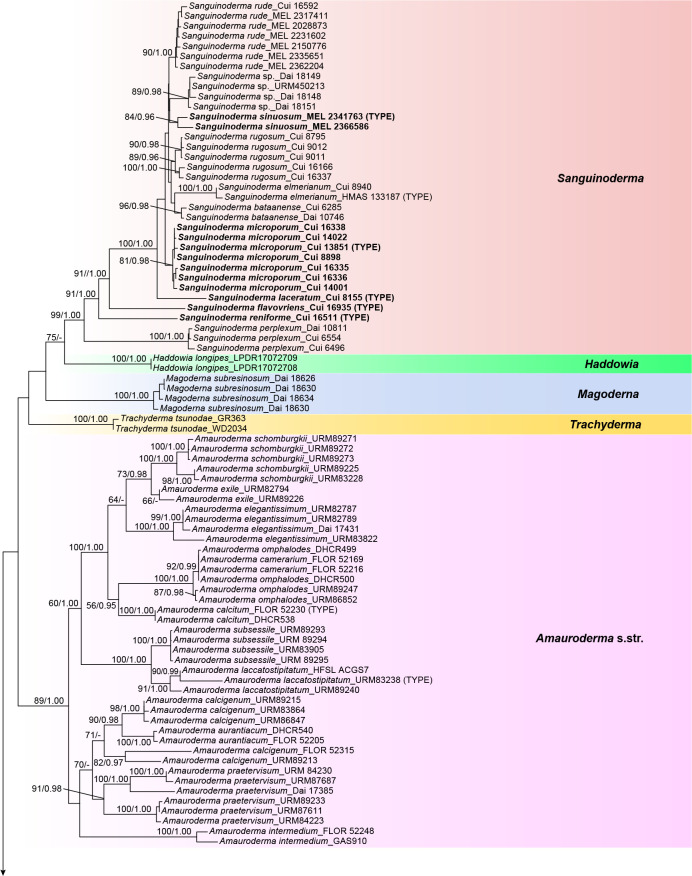
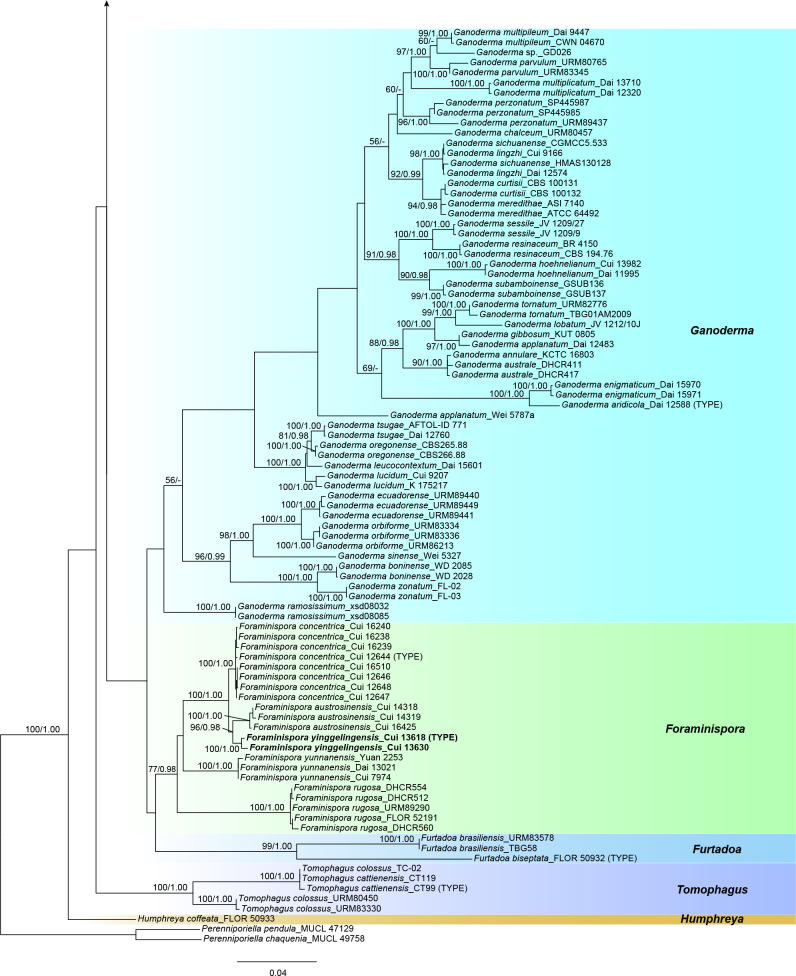
The combined 6-gene dataset (ITS+nLSU+RPB1+RPB2+ TEF+TUB) also included sequences from 175 specimens representing 69 taxa of Ganodermataceae and Perenniporiella chaquenia and P. pendula as outgroups. The dataset had an aligned length of 4 810 characters, of which 3 168 were constant, 256 were variable and parsimony-uninformative, and 1 386 were parsimony-informative. The average standard deviation of split frequencies in the Bayesian analyses reached 0.009067. The calculated values based on the dataset are shown in Fig. 2. The clades obtained in this topology were Amauroderma s.str. (89 % ML, 1.00 BPP), Foraminispora (77 % ML, 0.98 BPP), Furtadoa (99 % ML, 1.00 BPP), Ganoderma, Haddowia (100 % ML, 1.00 BPP), Humphreya, Magoderna (100 % ML, 1.00 BPP), Sanguinoderma (99 % ML, 1.00 BPP), Tomophagus (100 % ML, 1.00 BPP) and Trachyderma (100 % ML, 1.00 BPP).
Fig. 2.
ML analyses of Ganodermataceae based on dataset of ITS+nLSU+RPB1+RPB2+TEF+TUB. ML bootstrap values higher than 50 % and Bayesian posterior probabilities values more than 0.95 are shown. New species are in bold.
The phylogenetic trees (Fig. 1, 2) show that the species of Amauroderma s.lat. are divided into four distinct clades:
The Amauroderma s.str. clade: All species in this clade were derived from neotropical areas, including A. aurantiacum, A. calcigenum, A. calcitum, A. camerarium, A. elegantissimum, A. exile, A. intermedium, A. laccatostipitatum, A. omphalodes, A. praetervisum, A. schomburgkii and A. subsessile.
The Foraminispora clade: Previously, only one species, Fo. rugosa, from the Neotropics was included in this clade. According to our study, one new species: Fo. yinggelingensis, and three new combinations: Fo. austrosinensis, Fo. concentrica and Fo. yunnanensis from East Asia are also included in this clade.
The Furtadoa clade: Two species: Fu. biseptata and Fu. brasiliensis from the Neotropics clustered in this clade.
The Sanguinoderma clade: The species in this clade were mainly found in warm temperate to subtropical or tropical regions of Asia, Africa and Oceania rather than the Neotropics. Until now, this clade included five new species: S. flavovirens, S. laceratum, S. microporum, S. reniforme and S. sinuosum; five new combinations: S. bataanense, S. elmerianum, S. perplexum, S. rude and S. rugosum; and one undetermined taxon: Sanguinoderma sp.
TAXONOMY
Key to genera of Amauroderma s.lat.
1. Fresh pore surface colour changing to blood red when bruised. . . . . . . . . . . . . . . . . . . . . . . . . . . Sanguinoderma
1. Fresh pore surface colour unchanging when bruised . . . . 2
2. Pileal surface dull, tomentose, yellowish brown to reddish brown . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Foraminispora
2. Pileal surface dull to laccate, glabrous to tomentose, dark brown. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
3. Pileal surface dull to laccate; hyphal system dimitic in the context, generative hyphae clamped . Amauroderma s.str.
3. Pileal surface dull; hyphal system monomitic in the context, generative hyphae clamped to simple-septate . . . Furtadoa
Amauroderma s.str. Murrill, Bull. Torrey Bot. Club 32: 366. 1905 — MycoBank MB17052
Type species. Amauroderma schomburgkii (Mont. & Berk.) Torrend, Brotéria, Sér. Bot. 18 (no. 2): 140. 1920.
Basidiomata annual, centrally or laterally stipitate to sessile or subsessile, coriaceous, corky to woody hard. Pileus single, fan-shaped, suborbicular or funnel-shape. Pileal surface in shades of dark brown, vinaceous brown to almost black, dull, glittery or laccate, glabrous to tomentose, concentrically zonate or furrowed. Pore surface pale white, yellowish brown, orange or brown when dry, colour unchanging when bruised; pores circular to angular. Context pale white, yellowish brown, pinkish brown, brown, corky. Hyphal system dimitic; generative hyphae colourless, with clamp connections, thin-walled; skeletal hyphae colourless to pale yellow to brown, thick-walled, arboriform. Basidiospores globose or ellipsoid to obovoid, colourless to pale yellow, double-walled, endospore wall smooth or ornamented, IKI– or dextrinoid.
Notes — So far, all species in Amauroderma s.str. are from the Neotropics and they are morphologically variable. The geographical distribution and the combination of morphological characteristics, such as a colour-unchanging pore surface when bruised, presence of skeletal hyphae in both context and tubes, basidiospores with solid columnar endospore ornamentation can be used to distinguish this genus from other genera of Amauroderma s.lat. Based on morphological characteristics, 24 species were included in Amauroderma s.str., of which 14 have DNA sequences. Amauroderma trichodematum presents a monomitic hyphal system and is probably related to Furtadoa (Robledo et al. 2015). Since this feature is not in accordance with the current circumscription of Amauroderma s.str., this species was not included in this key.
Key to accepted species of Amauroderma s.str.
1. Pileal surface laccate . . . . . . . . . . . . . . . . . . . A. renidens
1. Pileal surface non-laccate . . . . . . . . . . . . . . . . . . . . . . . 2
2. Basidiomata with a laccate stipe . . . . . . . . . . . . . . . . . . 3
2. Basidiomata without a laccate stipe . . . . . . . . . . . . . . . 4
3. Pores angular, 7–8 per mm; basidiospores 8–10 × 7–8 μm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A. laccatostipitatum
3. Pores circular, 5–6 per mm; basidiospores 9–11 × 7–8 μm. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A. picipes
4. Basidiomata sessile to substipitate . . . . . . . . . . . . . . . . . 5
4. Basidiomata distinctly stipitate . . . . . . . . . . . . . . . . . . . . 6
5. Basidiomata sessile, pileal surface azonate, pores angular . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A. sessile
5. Basidiomata substipitate, pileal surface zonate, pores circular. . . . . . . . . . . . . . . . . . . . . . . . . . . . A. subsessile
6. Stipe white . . . . . . . . . . . . . . . . . . . . . . A. albostipitatum
6. Stipe different coloured . . . . . . . . . . . . . . . . . . . . . . . . . . 7
7. Pileus with 2–9 lobes . . . . . . . . . . . . . . . . . A. floriformum
7. Pileus entire . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
8. Basidiospores with smooth inner layer . . . A. coltricioides
8. Basidiospores with ornamented inner layer. . . . . . . . . . 9
9. Context white to cream . . . . . . . . . . . . . . . . . . . . . . . . .10
9. Context cinnamon to dark brown . . . . . . . . . . . . . . . . . 11
10. Pileal surface tomentose, pores 3–4 per mm; basidiospores 7–10 × 7–9 μm . . . . . . . . . . . . . . . A. boleticeum
10. Pileal surface glabrous, pores 1–3 per mm; basidiospores 10–13 × 8–10 μm . . . . . . . . . . . . . . . . . . . . . . A. partitum
11. Pileus ≤ 2 mm thick, context without black lines . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A. elegantissimum
11. Pileus > 2 mm thick, context with or without black lines . . . . . . . . . . . . . . . . 12
12. Basidiospores distinctly dextrinoid . . . . . . . . . . . . . . . . . . . . 13
12. Basidiospores non-dextrinoid . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
13. Pores 4–6 per mm; basidiospores obovoid . . . . . . . . . . . . . . . . . . . . . . . . . . . A. ovisporum
13. Pores 7–8 per mm; basidiospores globose to subglobose . . . . . . . . . . . . . . . . . . . . . . A. unilaterum
14. Basidiomata woody hard; basidiospores oblong, 6–7.5 μm wide. . . . . . . . . . . . . . . . . . . . . . . . . . . . . A. oblongisporum
14. Basidiomata corky to woody hard; basidiospores globose to ellipsoid, > 7.5 μm wide . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
15. Pores ≤ 3 per mm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
15. Pores > 3 per mm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
16. Basidiomata woody hard; basidiospores 9–11 μm long . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A. intermedium
16. Basidiomata corky; basidiospores > 11 μm long . . . . . 17
17. Pileal surface slightly shiny; basidiospores with slightly ornamented inner layer . . . . . . . . . . . . . . . . . . A. calcitum
17. Pileal surface dull; basidiospores with distinctly ornamented inner layer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
18. Basidiospores globose to subglobose, 14–17 × 13–17 μm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A. aurantiacum
18. Basidiospores broadly ellipsoid to ellipsoid, 13.5–16 × 10–12 μm . . . . . . . . . . . . . . . . . . . . . . . . . A. calcigenum
19. Context without black lines . . . . . . . . . . . . . . . . . . . . . . 20
19. Context with black lines . . . . . . . . . . . . . . . . . . . . . . . . 21
20. Pileal surface yellowish brown, pores 5–7 per mm; basidiospores broadly ellipsoid . . . . . . . . . . . . . . A. camerarium
20. Pileal surface reddish brown, pores 3–5 per mm; basidiospores globose to subglobose . . . . . . . A. pseudoboletus
21. Basidiomata coriaceous to corky, pileal surface slightly shiny . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A. exile
21. Basidiomata woody hard, pileal surface dull . . . . . . . . 22
22. Pore surface pale white; basidiospores pale yellow . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A. schomburgkii
22. Pore surface cinnamon; basidiospores yellow to yellowish brown . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
23. Basidiospores distinctly thick-walled, with distinctly ornamented inner layer . . . . . . . . . . . . . . . . . . A. omphalodes
23. Basidiospores medially thick-walled, with faintly ornamented inner layer . . . . . . . . . . . . . . . . . A. praetervisum
Foraminispora Robledo, D.H. Costa & Drechsler-Santos, Persoonia 39: 258. 2017 — MycoBank MB819015
Type species. Foraminispora rugosa (Berk.) D.H. Costa, Drechsler-Santos & Robledo, Persoonia 39: 262. 2017.
Basidiomata annual, centrally to laterally stipitate, corky to woody hard. Pileus single, suborbicular to umbelliform. Pileal surface yellowish brown to reddish brown, dull, tomentose, concentrically zonate or furrowed, radially rugose. Pore surface white to straw colour when dry, colour unchanging when bruised; pores circular to angular; dissepiments thin to thick, entire. Context white to pale yellow, without dark resinous lines, corky. Hyphal system dimitic to trimitic; all hyphae IKI–, CB+; tissues darkening in KOH; generative hyphae colourless, thin-walled, with clamp connections; skeletal hyphae colourless to pale yellow, thick-walled, arboriform branched and flexuous; binding hyphae colourless, subsolid, branched and flexuous. Basidiospores globose to ellipsoid, colourless to pale yellow, double and distinctly thick-walled, exospore wall uneven or foveolate, endospore wall with hollow and columnar spinules which persist until the exospore wall forming holes visible under SEM, IKI– or slightly dextrinoid, CB+.
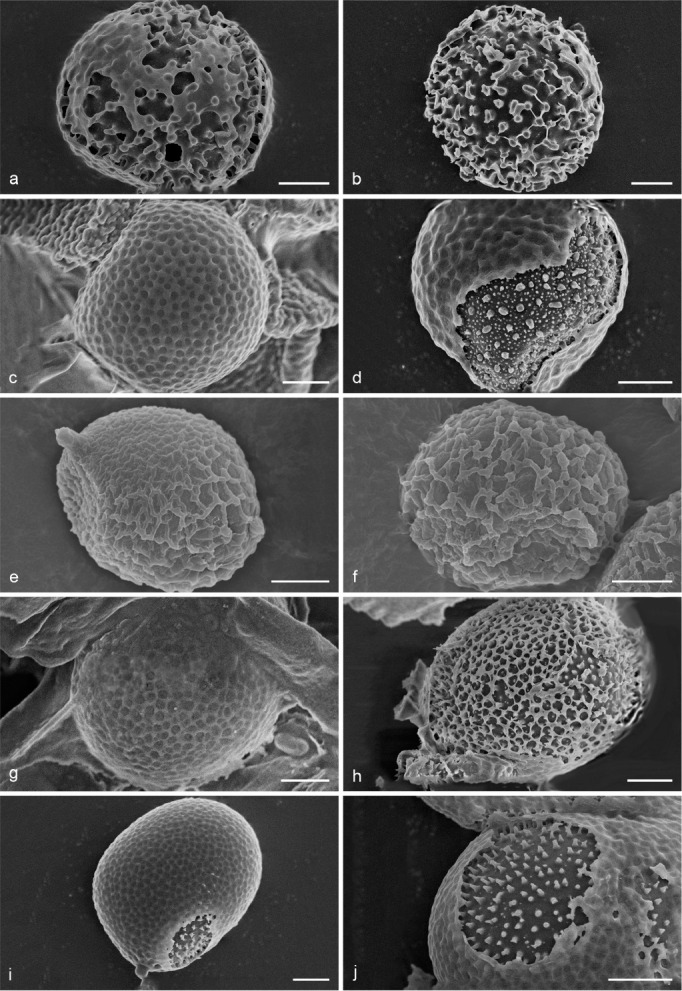
Notes — Foraminispora was established by Costa-Rezende et al. (2017) and only Fo. rugosa from the Neotropics was included. In our current study, three taxa previously belonging to Amauroderma s.lat. and one new species grouped together with Fo. rugosa and formed the Foraminispora clade in both the 4-gene and 6-gene based phylogenetic analyses (Fig. 1, 2). All these five species share similar spore ultrastructural characters such as uneven exospore wall with holes causing by hollow and columnar spinules on endospore wall which are unique within Ganodermataceae (Fig. 3).
Fig. 3.
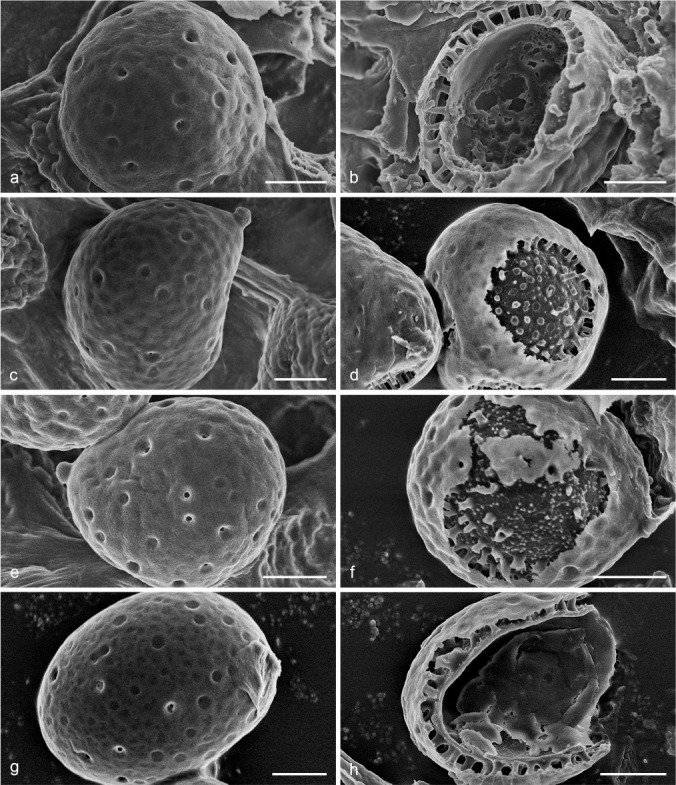
Scanning Electron Micrograph (SEM) of basidiospores of Foraminispora. a–b. Fo. austrosinensis (Cui 16425); c–d. Fo. concentrica (Cui 12648); e–f. Fo. yinggelingensis (Cui 13618); g–h. Fo. yunnanensis (Cui 7974). a, c, e, g: General view showing exospore wall; b, d, f, h: detail in endospore ornamentation. — Scale bars: a–h = 2 μm.
Key to accepted species of Foraminispora
1. Basidiospores > 10 μm long . . . . . . . . . . . . . . . . . . . . . . . 2
1. Basidiospores < 10 μm long . . . . . . . . . . . . . . . . . . . . . . . 3
2. Pores 2–3 per mm, dissepiments lacerate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Fo. yunnanensis
2. Pores 5–8 per mm, dissepiments entire . . . . . . Fo. rugosa
3. Pores 3–5 per mm; basidiospores subglobose to broadly ellipsoid . . . . . . . . . . . . . . . . . . . . . . . . . . . Fo. concentrica
3. Pores > 5 per mm; basidiospores globose to subglobose . 4
4. Basidiomata corky; cystidioles clavate, basidiospores IKI– . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Fo. austrosinensis
4. Basidiomata woody hard; cystidioles absent, basidiospores slightly dextrinoid . . . . . . . . . . . . . . . . Fo. yinggelingensis
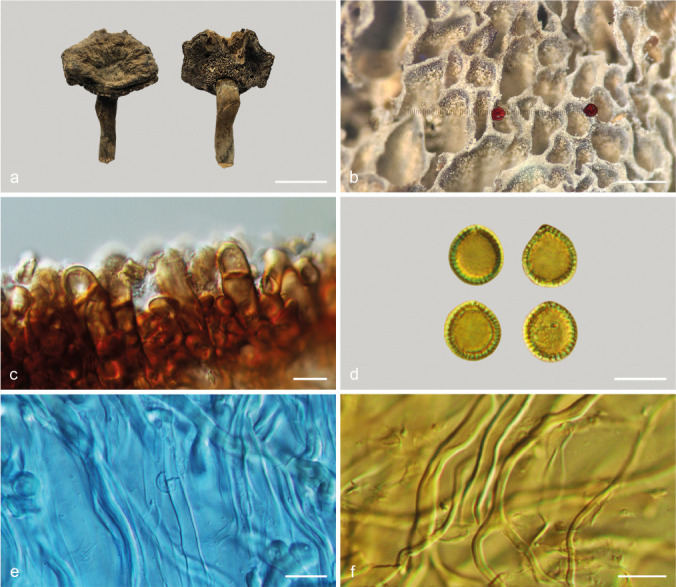
Foraminispora austrosinensis (J.D. Zhao & L.W. Hsu) Y.F. Sun & B.K. Cui, comb. nov. — MycoBank MB828440; Fig. 3a–b, 4
Fig. 4.
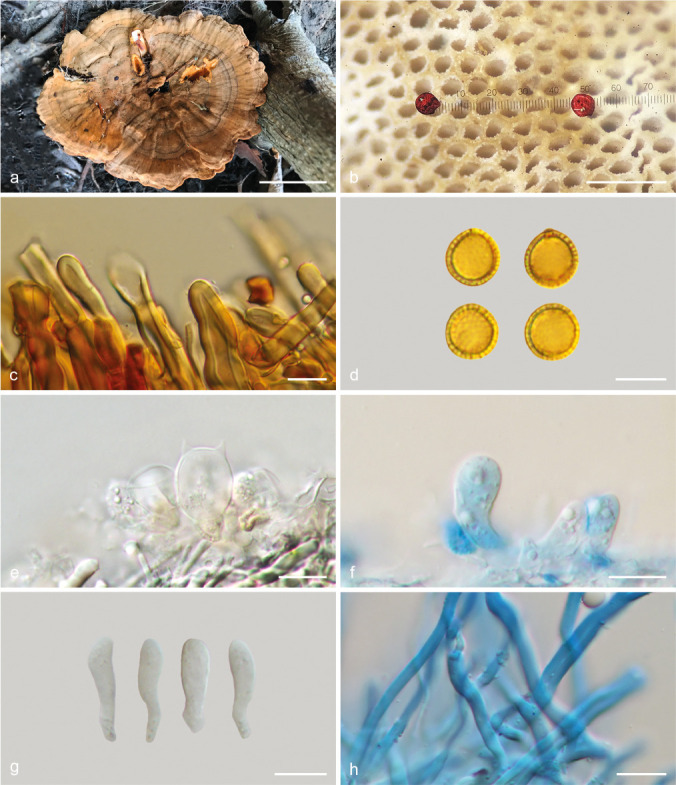
Basidiomata and microscopic structures of Foraminispora austrosinensis (Cui 16425). a. Basidiomata; b. pores; c. apical cells from pileal cover; d. basidiospores; e. basidia; f. basidioles; g. cystidioles; h. skeletal hyphae from context. — Scale bars: a = 2.5 cm; b = 0.5 mm; c, e–h = 10 μm; d = 7 μm.
Basionym. Amauroderma austrosinense J.D. Zhao & L.W. Hsu, Acta Mycol. Sin. 3(1): 20. 1984.
Basidiomata annual, centrally to laterally stipitate, coriaceous to corky. Pileus single, suborbicular to umbelliform, up to 8.5 cm diam and 5 mm thick. Pileal surface yellow to yellowish brown when dry, dull, tomentose, with concentric furrows and radial wrinkles, sagging at the centre; margin acute to obtuse, entire, wavy and incurved when dry. Pore surface white to cream buff when fresh, colour unchanging when bruised, pale yellow when dry, even ferruginous in old specimens; pores circular to angular, 6–7 per mm; dissepiments slightly thick, entire. Context white to cream, without dark resinous lines, corky, up to 2 mm thick. Tubes concolorous with pore surface, hard corky, up to 3 mm long. Stipe concolorous with pileal surface, cylindrical and hollow, swollen at base, up to 7.5 cm long and 1 cm diam. Hyphal system trimitic; generative hyphae with clamp connections, all hyphae IKI–, CB+; tissues darkening in KOH. Generative hyphae in context colourless, thin-walled, 2–4 μm diam; skeletal hyphae in context colourless to pale yellow, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 3–6 μm diam; binding hyphae in context colourless, subsolid, branched and flexuous, 1–2 μm diam. Generative hyphae in tubes colourless, thin-walled, 2–3 μm diam; skeletal hyphae in tubes colourless to pale yellow, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 2–5 μm diam; binding hyphae in tubes colourless, subsolid, branched and flexuous, 1–2 μm diam. Pileal cover composed of clamped generative hyphae, thin- to thick-walled, apical cells clavate and inflated, often slanting to one side, yellowish brown, about 60–80 × 5–10 μm, forming a regular palisade. Cystidia absent; cystidioles clavate, colourless, thin-walled, 18–20 × 4–5 μm. Basidia barrel-shaped to clavate, colourless, thin-walled, 13–23 × 11–14 μm; basidioles in shape similar to basidia, colourless, thin-walled, 13–17 × 6–10 μm. Basidiospores globose to subglobose, pale yellow, IKI–, CB+, with double and distinctly thick walls, exospore wall smooth, endospore wall with conspicuous spinules, (7–)7.2–8.5(–8.7) × (5.8–)6.7–8(–8.3) μm, L = 7.87 μm, W = 7.37 μm, Q = 1.06–1.07 (n = 60/2). Under SEM, exospore wall uneven or foveolate; endospore wall with some hollow and columnar spinules which persist to exospore wall forming holes.
Specimens examined. China, Hainan Province, Changjiang County, Yajia Forest Farm, on ground, 20 Apr. 1977, S.J. Han, HMAS 42695 (holotype, HMAS); Yunnan Province, Lincang County, 5 Aug. 2015, B.K. Cui, Cui 14318 (BJFC); ibid., Cui 14319 (BJFC). – Vietnam, Lam Dong Province, Bidoup-Nuiba National Park, 15 Oct. 2017, B.K. Cui, Cui 16425 (BJFC).
Notes — Amauroderma austrosinense was described from the tropical part of China (Zhao et al. 1984). It has the typical features such as white to straw colour pore surface when fresh and colour unchanging when bruised, cream context, hollow and columnar spinules on endospore wall which persist to the exospore wall forming the holes (Fig. 3a–b) that characterise Foraminispora (Costa-Rezende et al. 2017). In the phylogenetic analyses, A. austrosinense was shown as a distinct lineage in Foraminispora with high support (100 % ML, 1.00 BPP). Therefore, we transferred A. austrosinense to Foraminispora as a new combination.
Foraminispora concentrica (J. Song, Xiao L. He & B.K. Cui) Y.F. Sun & B.K. Cui, comb. nov. — MycoBank MB828441; Fig. 3c–d, 5
Fig. 5.
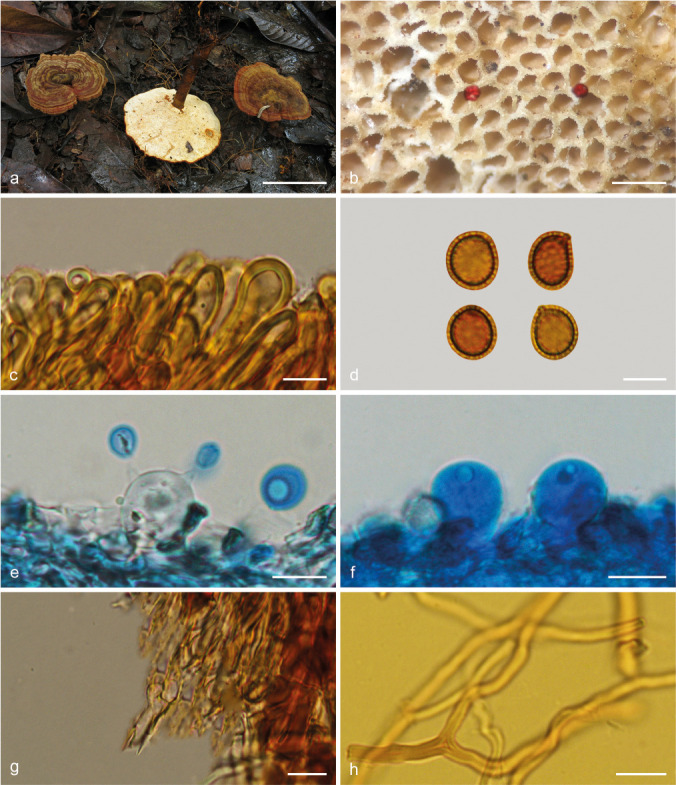
Basidiomata and microscopic structures of Foraminispora concentrica (Cui 12648). a. Basidiomata; b. pores; c. apical cells from pileal cover; d. basidiospores; e. basidia; f. basidioles; g. generative hyphae from context; h. skeletal hyphae from context. — Scale bars: a = 3 cm; b = 0.5 mm; c, e–h = 10 μm; d = 7 μm.
Basionym. Amauroderma concentricum J. Song, Xiao L. He & B.K. Cui, Phytotaxa 260: 47. 2016.
Basidiomata annual, centrally to laterally stipitate, coriaceous to corky. Pileus single, orbicular to suborbicular, up to 11 cm diam and 1 cm thick. Pileal surface yellowish brown to reddish brown, dull, tomentose, with obvious concentric zones and radial wrinkles, slightly sagging at the centre; margin acute, entire, faintly wavy and incurved when dry. Pore surface white when fresh, colour unchanging when bruised, pale yellow to straw colour when dry; pores circular to angular, 3–5 per mm; dissepiments thin to slightly thick, entire. Context white to cream, without dark resinous lines, corky, up to 4 mm thick. Tubes concolorous with pore surface, hard corky, up to 6 mm long. Stipe concolorous with pileal surface, cylindrical and hollow, slightly swollen at base, up to 7.5 cm long and 6 mm diam. Hyphal system trimitic; generative hyphae with clamp connections, all hyphae IKI–, CB+; tissues darkening in KOH. Generative hyphae in context colourless, thin-walled, 3–4 μm diam, often collapsed; skeletal hyphae in context colourless to pale yellow, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 2–6 μm diam; binding hyphae in context colourless, subsolid, branched and flexuous, 1–2 μm diam. Generative hyphae in tubes colourless, thin-walled, 2–3 μm diam; skeletal hyphae in tubes pale yellow, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 2–5 μm diam; binding hyphae in tubes colourless, subsolid, branched and flexuous, 1–2 μm diam. Pileal cover composed of clamped generative hyphae, thin- to thick-walled, apical cells clavate, inflated or constricted, faintly slanting to one side, yellowish brown, about 30–60 × 6–11 μm, forming a regular palisade. Cystidia or cystidioles absent. Basidia barrel-shaped to clavate, colourless, thin-walled, 17–25 × 10–14 μm; basidioles in shape similar to basidia, colourless, thin-walled, 15–22 × 7–12 μm. Basidiospores subglobose to broadly ellipsoid, pale yellow, slightly dextrinoid, CB+, with double and distinctly thick walls, exospore wall smooth, endospore wall with conspicuous echinules, (6.8–)7.7–9.5(–9.6) × (6–)6.8–8.3(–9) μm, L = 8.6 μm, W = 7.48 μm, Q = 1.13–1.17 (n = 60/2). Under SEM, exospore wall uneven or foveolate, endospore wall with some hollow and columnar spinules which persist to exospore wall forming holes.
Specimens examined. China, Sichuan Province, Mianning County, Lingshansi Park, on ground of angiosperm forest, 13 Sept. 2015, B.K. Cui, Cui 12644 (holotype, BJFC); ibid., Cui 12646 (paratype, BJFC); ibid., Cui 12647 (paratype, BJFC); ibid., Cui 12648 (paratype, BJFC); Yunnan Province, Lanping County, Luoguqing, 18 Sept. 2017, B.K. Cui, Cui 16238 (BJFC); ibid., Cui 16239 (BJFC); ibid., Cui 16240 (BJFC); Guizhou Province, Liupanshui, Lingshansi Park, on stump of angiosperm tree, 15 Oct. 2018, H.J. Li, Cui 1510 (BJFC).
Notes — Amauroderma concentricum is distributed in temperate and subtropical areas of China. Based on its typical features and phylogenetic support, we transfer A. concentricum to Foraminispora as a new combination. Foraminispora concentrica is similar to Fo. austrosinensis by the yellow brown and tomentose concentrically zonate pileal surface, white context and pore surface, but the pores (6–7 per mm) and basidiospores (7.2–8.5 × 6.7–8 μm) of Fo. austrosinensis are relatively smaller than Fo. concentrica; moreover, the basidiospores of Fo. concentrica are slightly dextrinoid, while Fo. austrosinensis has non-dextrinoid basidiospores.
Foraminispora yinggelingensis Y.F. Sun & B.K. Cui, sp. nov. — MycoBank MB828434; Fig. 3e–f, 6
Fig. 6.
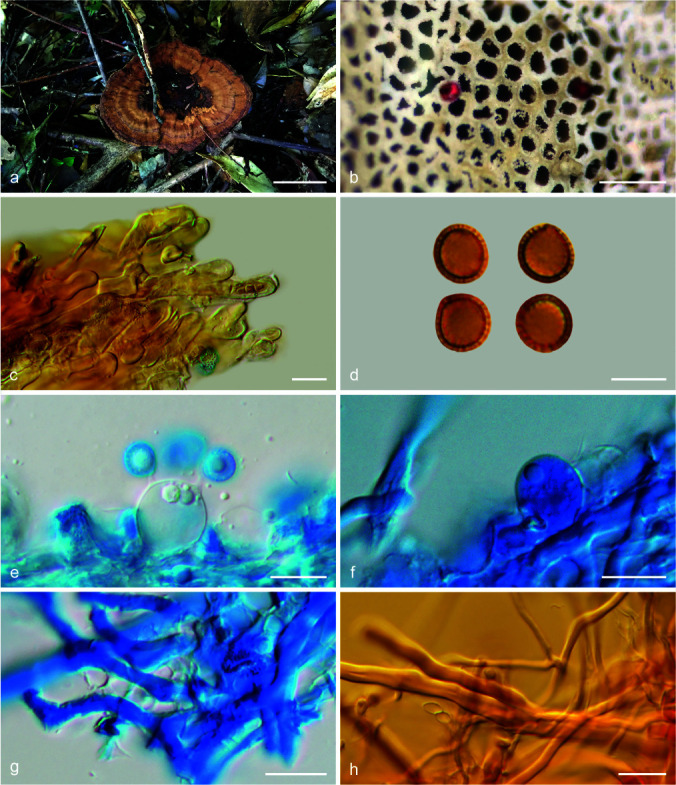
Basidiomata and microscopic structures of Foraminispora yinggelingensis (Cui 13618). a. Basidiomata; b. pores; c. apical cells from pileal cover; d. basidiospores; e. basidia; f. basidioles; g. skeletal hyphae from tubes; h. skeletal and binding hyphae from context. — Scale bars: a = 2.5 cm; b = 0.5 mm; c, e–h = 10 μm; d = 7 μm.
Etymology. Yinggelingensis (Lat.), refers to Yinggeling, the type locality of this species.
Holotype. China, Hainan Province, Baisha County, Yinggeling Nature Reserve, on ground of angiosperm forest, 17 Nov. 2015, B.K. Cui, Cui 13618 (BJFC).
Diagnosis. Foraminispora yinggelingensis is characterized by its woody hard basidiomata, umbelliform pileus with obvious concentric dark zones, bright yellow generative hyphae in pileal cover, and slightly dextrinoid basidiospores.
Basidiomata annual, centrally stipitate, woody hard. Pileus single, suborbicular to umbelliform, up to 7 cm diam and 4 mm thick. Pileal surface yellowish brown to ferruginous when dry, dull, tomentose, with obvious concentric dark zones and radial wrinkles, slightly sagging at the centre; margin obtuse, entire, flat or incurved and wavy when dry, extending to pore surface. Pore surface white when fresh, colour unchanging when bruised, pale yellow when dry; pores subcircular to angular or irregular, 5–7 per mm; dissepiments slightly thick, entire. Context pale buff, without dark resinous lines, hard corky, up to 2 mm thick. Tubes straw colour, woody hard, up to 2 mm long. Stipe concolorous with pileal surface, cylindrical and hollow, up to 6 cm long and 6 mm diam. Hyphal system trimitic; generative hyphae with clamp connections, all hyphae IKI–, CB+; tissues darkening in KOH. Generative hyphae in context colourless, thin-walled, 2–3 μm diam; skeletal hyphae in context colourless to pale yellow, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 3–5 μm diam; binding hyphae in context colourless, subsolid, branched and flexuous, up to 3 μm diam. Generative hyphae in tubes colourless, thin-walled, 2–3 μm diam; skeletal hyphae in tubes colourless to pale yellow, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 2–4 μm diam; binding hyphae in tubes colourless, subsolid, branched and flexuous, 1–2 μm diam. Pileal cover composed of clamped generative hyphae, thin- to thick-walled, apical cells clavate, faintly slanting to one side and tortuous, bright yellow, about 50–80 × 5–12 μm, forming an irregular palisade. Cystidia or cystidioles absent. Basidia barrel-shaped to clavate, colourless, thin-walled, 16–21 × 11–13 μm; basidioles in shape similar to basidia, colourless, thin-walled, 11–15 × 5–11 μm. Basidiospores globose to subglobose, pale yellow, slightly dextrinoid, CB+, with double and distinctly thick walls, exospore wall smooth, endospore wall with conspicuous spinules, (7–)7.2–8.5(–8.7) × 6.6–8 μm, L = 7.89 μm, W = 7.17 μm, Q = 1.1 (n = 60/1). Under SEM, exospore wall uneven or foveolate, endospore wall with some hollow and columnar spinules which persist to exospore wall forming holes.
Additional specimen (paratype) examined. China, Hainan Province, Baisha County, Yinggeling Nature Reserve, on ground of angiosperm forest, 17 Nov. 2015, B.K. Cui, Cui 13630 (BJFC).
Notes — Foraminispora yinggelingensis was collected from the tropical part of China. It is similar to Fo. austrosinensis in the umbelliform basidiomata, white to cream context and pore surface, and similar basidiospores, but the woody hard basidiomata, the absence of cystidioles and the slightly dextrinoid basidiospores of Fo. yinggelingensis make it different from Fo. austrosinensis. In phylogenetic analyses, these two species nested in two well-supported lineages (Fig. 1, 2).
Foraminispora yunnanensis (J.D. Zhao & X.Q. Zhang) Y.F. Sun & B.K. Cui, comb. nov. — MycoBank MB828442; Fig. 3g–h, 7
Fig. 7.

Basidiomata and microscopic structures of Foraminispora yunnanensis (Cui 7974). a. Basidiomata; b. pores; c. apical cells from pileal cover; d. basidiospores; e. basidia; f. basidioles; g. generative hyphae from context; h. skeletal hyphae from tubes. — Scale bars: a = 2 cm; b = 0.5 mm; c, e–h = 10 μm; d = 7 μm.
Basionym. Amauroderma yunnanense J.D. Zhao & X.Q. Zhang, Acta Mycol. Sin. S1: 268. 1986.
Basidiomata annual, centrally to laterally stipitate, coriaceous to corky. Pileus single, suborbicular to flabelliform, up to 6.5 cm diam and 6 mm thick. Pileal surface cinnamon to reddish brown when dry, dull, tomentose, with faintly concentric furrows at the margin and irregular wrinkles; margin subacute to obtuse, entire and wavy when dry. Pore surface white to pale straw, colour unchanging when bruised; pores circular to angular, 2–3 per mm; dissepiments thin, fragile when dry. Context white to pale yellow, without dark resinous lines, corky, up to 2 mm thick. Tubes concolorous with pore surface, occasionally fascicular, woody hard, up to 4 mm long. Stipe concolorous with pileal surface, cylindrical and solid, slightly swollen at base, up to 8 cm long and 6 mm diam. Hyphal system trimitic; generative hyphae with clamp connections, all hyphae IKI–, CB+; tissues darkening in KOH. Generative hyphae in context colourless, thin-walled, 3–4 μm diam; skeletal hyphae in context pale yellow, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 3–6 μm diam; binding hyphae in context colourless, subsolid, branched and flexuous, 1–3 μm diam. Generative hyphae in tubes colourless, thin-walled, 2–4 μm diam; skeletal hyphae in tubes colourless to pale yellow, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 2–5 μm diam; binding hyphae in tubes colourless, subsolid, branched and flexuous, 1–2 μm diam. Pileal cover composed of clamped generative hyphae, thin- to thick-walled, apical cells clavate, inflated or constricted, arranged loosely, yellowish brown, about 40–60 × 6–9 μm, forming an irregular palisade. Cystidia or cystidioles absent. Basidia barrel-shaped to clavate, colourless, thin-walled, 20–35 × 11–18 μm; basidioles in shape similar to basidia, colourless, thin-walled, 13–23 × 6–10 μm. Basidiospores broadly ellipsoid to ellipsoid, pale yellow, IKI–, CB+, with double and distinctly thick walls, exospore wall smooth, endospore wall with conspicuous spinules, 8–10.7(–11.2) × (6.8–)7–8.3(–8.7) μm, L = 9.35 μm, W = 7.63 μm, Q = 1.18–1.27 (n = 60/2). Under SEM, exospore wall uneven or foveolate, endospore wall with some hollow and columnar spinules which persist to exospore wall forming holes.
Specimens examined. China, Yunnan Province, Xichou County, Xiaoqiaogou, on ground, 14 May 1959, Q.Z. Wang, HMAS 48231 (holotype, HMAS); Kunming, Qiongzhusi Park, on ground of angiosperm forest, 21 Oct. 2009, B.K. Cui, Cui 7974 (BJFC).
Notes — Foraminispora yunnanensis can be distinguished by large lacerate pores (2–3 per mm), occasionally fascicular tubes and broadly ellipsoid to ellipsoid basidiospores. It is also consistent with Foraminispora in the unique ultrastructural characteristics of the exospore wall with obvious holes caused by hollow and columnar spinules on the endospore wall which persist to the exospore wall (Fig. 3g–h). In the phylogenetic analyses, Fo. yunnanensis was shown to be a distinct lineage in Foraminispora with high support (100 % ML, 1.00 BPP).
Furtadoa D.H. Costa, Robledo & Drechsler-Santos, Persoonia 39: 263. 2017 — MycoBank MB819014
Type species. Furtadoa biseptata D.H. Costa, Drechsler-Santos & Reck, Persoonia 39: 265. 2017.
Basidiomata annual, centrally to laterally stipitate, soft to corky. Pileus single, orbicular to flabelliform or infundibuliform. Pileal surface yellowish brown to greyish brown, dull, glabrous to tomentose, obviously concentrically zonate. Pore surface white to straw colour when dry; pores circular to angular; dissepiments thin to thick, entire to lacerate. Context yellow brown, with dark resinous lines, soft corky. Hyphae system monomitic to dimitic; context composed of clamped to simple-septate generative hyphae, thin- to slightly thick-walled; tubes composed of clamped generative hyphae and arboriform skeletal hyphae. Basidiospores subglobose to ellipsoid, colourless, double-walled with conspicuous endospore spinules, IKI–.
Notes — Furtadoa was established by Costa-Rezende et al. (2017) and all species (including Fu. biseptata, Fu. brasiliensis, Fu. corneri) have so far been found in the Neotropics. The genus is distinguished from the other genera in Amauroderma s.lat. by presenting a monomitic context. Based on the 4-gene and 6-gene phylogenetic analyses (Fig. 1, 2), we confirm the status of Fu. biseptata and Fu. brasiliensis. However, Gomes-Silva et al. (2015) considered that Amauroderma corneri was the synonymy of A. brasiliense according to the morphological features of funnel- to fan-shaped, soft basidiomata almost whitish when fresh, large pores (1–2 per mm) and non-dextrinoid basidiospores (8–10 × 7–9 μm). Therefore, we treated Fu. corneri as a synonymy of Fu. brasiliensis until molecular evidence is provided.
Key to accepted species of Furtadoa
1. Generative hyphae in context with both clamps and simple septa . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Fu. biseptata
1. Generative hyphae in context with clamps only . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Fu. brasiliensis
Sanguinoderma Y.F. Sun, D.H. Costa & B.K. Cui, gen. nov. — MycoBank MB828433
Etymology. Sanguinoderma (Lat.), refers to the genus producing Amauroderma-like basidiomata but with a fresh pore surface that changes to blood red when bruised.
Type species. Sanguinoderma rude (Berk.) Y.F. Sun, D.H. Costa & B.K. Cui.
Diagnosis. Sanguinoderma is characterized by basidiomata corky to woody hard, pileus dark, pore surface colour changing to blood red when bruised, basidiospores double-walled in which exospore wall semi-reticulate or vermiculate to verrucose, endospore wall with solid and columnar to coniform spinules under SEM.
Basidiomata annual, central or lateral stipitate to almost sessile, corky to woody hard. Pileus single, suborbicular to flatly reniform. Pileal surface dark brown to nearly black, dull, glabrous to tomentose, concentrically zonate or furrowed and radially rugose. Pore surface greyish white to dark grey when fresh, colour changing to blood red when bruised, then quickly darkening; pores circular to angular or irregular; dissepiments thin to thick, entire. Context pale brown to dark brown, with resinous lines, hard corky. Hyphal system trimitic; all hyphae IKI–, CB+; tissues darkening in KOH; generative hyphae colourless, thin-walled, with clamp connections; skeletal hyphae colourless to yellowish brown, thick-walled, arboriform branched and flexuous; binding hyphae colourless to pale yellow, subsolid, branched and flexuous. Basidiospores subglobose or ellipsoid to reniform, pale yellow, double and slightly to distinctly thick-walled, exospore wall semi-reticulate or vermiculate to verrucose, endospore wall with solid and columnar to coniform spinules under SEM, IKI–, CB+.
Notes — All examined specimens in Sanguinoderma were found in the Paleotropics and in Oceania. In the 4-gene and 6-gene based phylogenetic analyses (Fig. 1, 2) these species nested in eleven highly supported lineages. Sanguinoderma presents similar macro- and micro-morphology to Amauroderma s.str.; however, it can be distinguished by its conspicuous reaction in the pore surface, which rapidly changes to blood red when bruised. With the addition of morphological evidence, five new species are described and five new combinations are proposed and re-described. One taxon with insufficient morphological characters to separate it from other taxa is treated as Sanguinoderma sp.
Key to accepted species of Sanguinoderma
1. Pores ≤ 4 per mm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
1. Pores > 4 per mm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
2. Pore dissepiments lacerate, tubes fascicular when dry . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. laceratum
2. Pore dissepiments entire, tubes unchanged when dry . . . 3
3. Basidiospores globose to subglobose, exospore wall verrucose with long and columnar endospore spinules . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. bataanense
3. Basidiospores subglobose to ellipsoid, exospore wall semi-reticulate to vermiculate with short to long and columnar to coniform endospore spinules . . . . . . . . . . . . . . . . . . . . . . 4
4. Basidiospores subglobose to broadly ellipsoid, medially thick-walled, exospore wall semi-reticulate with small and deep pits . . . . . . . . . . . . . . . . . . . . . . S. rude
4. Basidiospores ellipsoid, distinctly thick-walled, exospore wall uneven with distinctly circular hollows . . . . . . S. sinuosum
5. Pores surface yellowish green; basidiospores 8–9.2 × 6.4–7.2 μm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. flavovirens
5. Pores surface pale white to dark grey; basidiospores 9–14 × 8–12 μm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
6. Basidiomata almost sessile or with a very short and thick stipe . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. perplexum
6. Basidiomata with distinct stipe . . . . . . . . . . . . . . . . . . . . . 7
7. Basidiomata laterally stipitate, pileus flatly reniform . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. elmerianum
7. Basidiomata centrally or laterally stipitate, pileus suborbicular to flabelliform . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
8. Pileal cover derived from loose generative hyphae; basidiospores reniform . . . . . . . . . . . . . . . . . . . . . . . S. reniforme
8. Pileal cover derived from compact generative hyphae; basidiospores subglobose to broadly ellipsoid . . . . . . . . . . . . 9
9. Pores 6–7 per mm with extremely thick dissepiments, yellowish brown . . . . . . . . . . . . . . . . . . . . . . . . S. microporum
9. Pores 5–7 per mm with slightly thick dissepiments, greyish white to dark grey . . . . . . . . . . . . . . . . . . . . . . . S. rugosum
Sanguinoderma bataanense (Murrill) Y.F. Sun & B.K. Cui, comb. nov. — MycoBank MB828443; Fig. 8a–b, 9
Fig. 8.
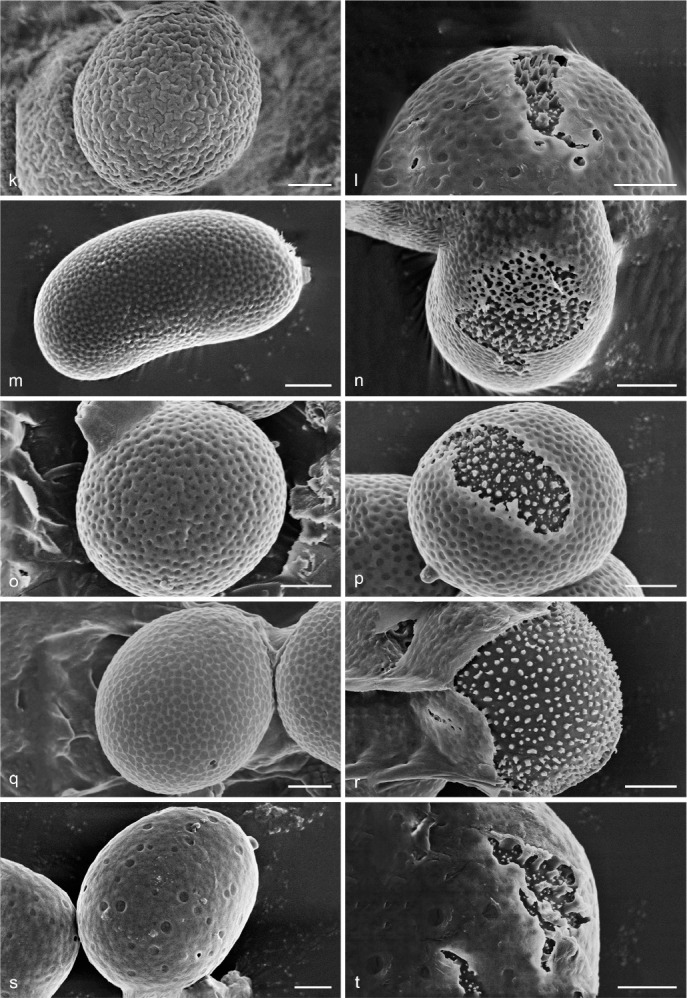
Scanning Electron Micrograph (SEM) of basidiospores of Sanguinoderma. a–b. S. bataanense (Dai 10746); c–d. S. elmerianum (HMAS 133187); e–f. S. flavovirens (Cui 16935); g–h. S. laceratum (Cui 8155); i–j. S. microporum (Cui 13851); k–l. S. perplexum (Dai 10811); m–n. S. reniforme (Cui 16511); o–p. S. rude (MEL 2150776); q–r. S. rugosum (Cui 16377); s–t. S. sinuosum (MEL 2366586). a, c, e, g, i, k, m, o, q, s: General view showing exospore wall; b, d, f, h, j, l, n, p, r, t: detail in endospore ornamentation. — Scale bars: a–t = 2 μm.
Fig. 9.
Basidiomata and microscopic structures of Sanguinoderma bataanense (Dai 10746). a. Basidiomata; b. pores; c. apical cells from pileal cover; d. basidiospores; e. generative hyphae from tubes; f. skeletal hyphae from context. — Scale bars: a = 2 cm; b = 0.5 mm; c–f = 10 μm.
Basionym. Amauroderma bataanense Murrill, Bull. Torrey Bot. Club 35: 407. 1908.
≡ Ganoderma bataanense (Murrill) Sacc. & Trotter, Syll. Fung. 21: 307. 1912.
≡ Polystictus bataanensis (Murrill) Sacc. & Trotter, Syll. Fung. 21: 322. 1912.
Basidiomata annual, laterally stipitate, soft to hard corky. Pileus single, suborbicular to flabelliform, up to 3.5 cm diam and 8 mm thick. Pileal surface dark yellowish brown when dry, dull, glabrous, with concentric furrows and radial wrinkles; margin obtuse, entire, wavy and incurved when dry. Pore surface greyish white when fresh, colour changing to blood red when bruised, then quickly darkening; pores subcircular to irregular, 1–4 per mm; dissepiments thin, entire. Context dark brown, with thick black stratum, corky, up to 2 mm thick. Tubes darker than context, woody hard, up to 6 mm long. Stipe concolorous with pileal surface, cylindrical and hollow, slightly swollen at the base, up to 4.5 cm long and 3 mm diam. Hyphal system trimitic; generative hyphae with clamp connections, all hyphae IKI–, CB+; tissues darkening in KOH. Generative hyphae in context colourless, thin-walled, 2–4 μm diam; skeletal hyphae in context pale yellow, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 2–6 μm diam; binding hyphae in context pale yellow, subsolid, branched and flexuous, 1–2 μm diam. Generative hyphae in tubes colourless, thin-walled, 2–3 μm diam; skeletal hyphae in tubes pale yellow, thick-walled with a wide or narrow lumen to subsolid, arboriform branched and flexuous, up to 7 μm diam; binding hyphae in tubes pale yellow, subsolid, branched and flexuous, 1–2 μm diam. Pileal cover composed of clamped generative hyphae, thin- to thick-walled, apical cells clavate with obvious septa, slightly inflated, reddish brown, about 30–70 × 5–9 μm, forming a regular palisade. Cystidia or cystidioles absent. Basidia barrel-shaped to clavate, colourless, thin-walled, 30–40 × 12–16 μm; basidioles in shape similar to basidia, colourless, thin-walled, 25–30 × 8–13 μm. Basidiospores globose to subglobose, pale yellow, IKI–, CB+, with double and distinctly thick walls, exospore wall smooth, endospore wall with conspicuous spinules, (9.6–)10–12.5(–13.1) × (8.5–)9–11(–11.3) μm, L = 11.13 μm, W = 9.84 μm, Q = 1.13 (n = 60/2). Under SEM, exospore wall thin and badly worn, endospore wall with long and thick columnar spinules which are loosely arranged and cause conspicuous warts on exospore wall (Fig. 8a–b).
Specimens examined. China, Hainan Province, Danzhou, on stump of Litchi chinensis, 7 May 2009, B.K. Cui, Cui 6285 (BJFC); ibid., Y.C. Dai, Dai 10746 (BJFC); Guangxi Autonomous Region, Baise, Jinzhongshan Nature Reserve, on rotten wood, 21 Oct. 1957, L.W. Xu, HMAS 23492 (HMAS).
Notes — Amauroderma bataanense was described from the Philippines (Murrill 1908). We collected it from the tropical areas of China. It can be distinguished by its fresh pore surface colour that changes to blood red when bruised, subcircular to irregular pores (1–4 per mm) with thin and entire dissepiments, and distinctly thick-walled basidiospores. In the phylogenetic analyses, A. bataanense was shown to be a distinct well-supported lineage in Sanguinoderma with strong support (Fig. 1, 2). Therefore, we transferred A. bataanense to Sanguinoderma.
Sanguinoderma elmerianum (Murrill) Y.F. Sun & B.K. Cui, comb. nov. — MycoBank MB828444; Fig. 8c–d, 10
Fig. 10.
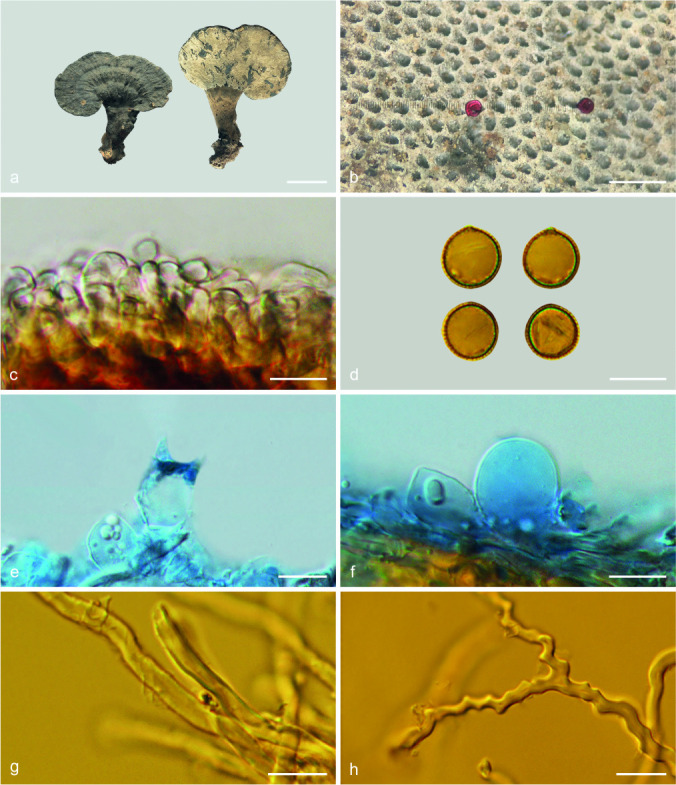
Basidiomata and microscopic structures of Sanguinoderma elmerianum (HMAS 133187). a. Basidiomata; b. pores; c. apical cells from pileal cover; d. basidiospores; e. basidia; f. basidioles; g. skeletal hyphae from tubes; h. binding hyphae from context. — Scale bars: a = 5 cm; b = 0.5 mm; c–h = 10 μm.
Basionym. Amauroderma elmerianum Murrill, Bull. Torrey Bot. Club 34: 475. 1907.
≡ Ganoderma elmerianum (Murrill) Sacc. & Trotter, Syll. Fung. 21: 305. 1912.
Basidiomata annual, lateral stipitate to almost sessile, coriaceous to soft corky. Pileus single, flatly reniform, up to 14.5 cm diam and 1 cm thick. Pileal surface dark brown to nearly black, dull, glabrous, with concentric furrows and radial wrinkles; margin obtuse, entire, slightly wavy and flat when dry. Pore surface pale brown to pale grey when fresh, colour changing to blood red when bruised, then quickly darkening; pores circular to angular, 5–7 per mm; dissepiments slightly thick, entire. Context pale brown to cinnamon, with two dark melanoid lines, soft corky, up to 4 mm thick. Tubes slightly darker than context, concolorous with pore surface, hard corky, up to 6 mm long. Stipe concolorous with pileal surface, cylindrical, swollen at base, about 9 cm long and 3 cm diam. Hyphal system trimitic; generative hyphae with clamp connections, binding hyphae usually serrated, all hyphae IKI–, CB+; tissues darkening in KOH. Generative hyphae in context colourless, thin-walled, 2–4 μm diam, usually collapsed; skeletal hyphae in context colourless to pale yellow, thick-walled with a wide or narrow lumen to subsolid, arboriform branched and flexuous, 3–6 μm diam; binding hyphae in context colourless, subsolid, branched and flexuous, 1–2 μm diam. Generative hyphae in tubes colourless, thin-walled, 2–4 μm diam; skeletal hyphae in tubes pale yellow, with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 3–7 μm diam; binding hyphae in tubes colourless to pale yellow, subsolid, branched and flexuous, 1–2 μm diam. Pileal cover composed of clamped generative hyphae, thin- to thick-walled, apical cells clavate with obvious septa, inflated and slightly tortuous, pale brown to reddish brown, about 30–50 × 5–9 μm, forming a regular palisade. Cystidia or cystidioles absent. Basidia barrel-shaped to clavate, colourless, thin-walled, about 22–25 × 10–17 μm; basidioles in shape similar to basidia, colourless, thin-walled, 14–22 × 6–13 μm. Basidiospores globose to subglobose, pale yellow, IKI–, CB+, with double and medially thick walls, exospore wall smooth, endospore wall with conspicuous spinules, (8.4–)9.2–11.1(–11.2) × (7.8–)9–10.1(–10.5) μm, L = 10.26 μm, W = 9.5 μm, Q = 1.07–1.09 (n = 60/2). Under SEM, exospore wall alveolate to semi-reticulate, endospore wall with long and medially thick coniform spinules loosely arranged.
Specimens examined. China, Yunnan Province, Xishuangbanna, Baka Xiao Zhai Nature Reserve, 5 Aug. 2003, T.Z. Wei, HMAS 133187 (HMAS); Guangdong Province, Zhaoqing, Dinghushan Nature Reserve, dead angiosperm tree, 30 June 2010, B.K. Cui, Cui 8940 (BJFC).
Notes — Amauroderma elmerianum was described from the Philippines (Murrill 1907). The specimens collected from China have soft basidiomata with a flat reniform pileus, and globose to subglobose basidiospores with an ornamented endospore wall (Fig. 8c–d); characters which are consistent with the previous descriptions (Murrill 1907, Furtado 1981). In the phylogenetic analyses, A. elmerianum was shown as a distinct well-supported lineage in Sanguinoderma with high support (100 % ML, 1.00 BPP). Therefore, we transferred A. elmerarium to Sanguinoderma.
Sanguinoderma flavovirens Y.F. Sun & B.K. Cui, sp. nov. — MycoBank MB828435; Fig. 8e–f, 11
Fig. 11.
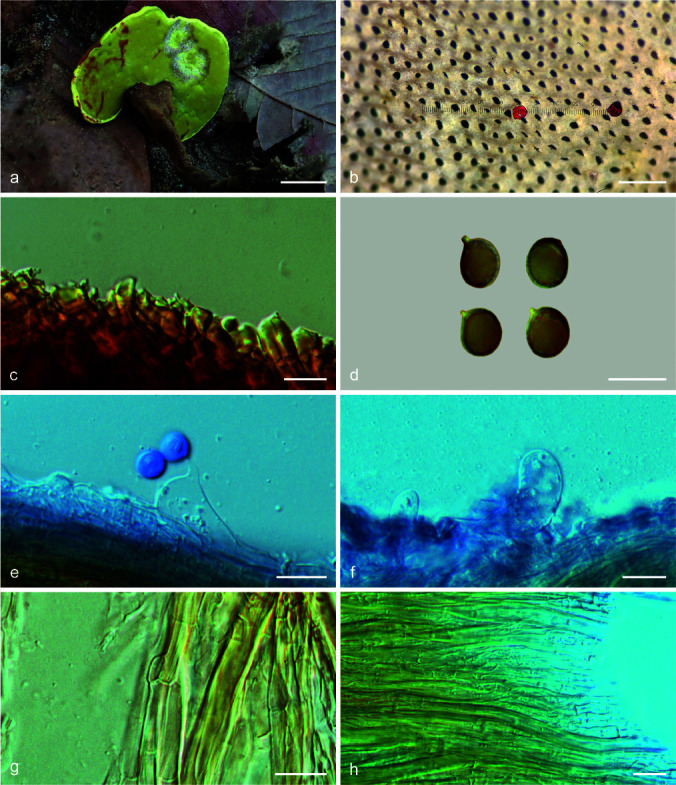
Basidiomata and microscopic structures of Sanguinoderma flavovirens (Cui 16935). a. Basidiomata; b. pores; c. apical cells from pileal cover; d. basidiospores; e. basidia; f. basidioles; g. generative hyphae from tubes; h. regularly arranged hyphae from context. — Scale bars: a = 1 cm; b = 0.5 mm; c–h = 10 μm.
Etymology. Flavovirens (Lat.), refers to the yellowish green pore surface when fresh.
Holotype. Zambia, Ndola, Ngosa Village, on ground of angiosperm forest, 5 Jan. 2018, Bo Zhang, Cui 16935 (BJFC; isotype IFP).
Diagnosis. Sanguinoderma flavovirens differs from other species in this genus by its yellowish green pore surface with distinctly thick dissepiments, almost regularly arranged hyphae in context, and relatively small basidiospores.
Basidiomata annual, laterally stipitate, hard corky. Pileus single, suborbicular, up to 4.5 cm diam and 5 mm thick. Pileal surface dark yellowish brown when dry, dull, glabrous, with obvious concentric furrows and faint radial wrinkles; margin obtuse, entire, wavy and incurved when dry. Pore surface yellowish green when fresh, colour changing to blood red when bruised, then quickly darkening; pores circular to angular, 5–6 per mm; dissepiments distinctly thick, entire. Context straw colour, with dark melanoid lines, corky, up to 2 mm thick. Tubes nearly black when dry, hard corky, up to 3 mm long. Stipe concolorous with pileal surface, cylindrical and hollow, tapering at the base, up to 6 cm long and 7 mm diam. Hyphal system trimitic; hyphae almost regularly arranged in context, generative hyphae with clamp connections, all hyphae IKI–, CB+; tissues darkening in KOH. Generative hyphae in context colourless, thin-walled, 3–4 μm diam; skeletal hyphae in context pale yellow, thick-walled with a wide to narrow lumen, slightly flexuous, 3–7 μm diam; binding hyphae in context colourless, subsolid, rarely branched and slightly flexuous, 1–2 μm diam. Generative hyphae in tubes colourless, 2–4 μm diam; skeletal hyphae in tubes pale yellow to bright yellow, thick-walled with a wide to narrow lumen, slightly flexuous, up to 7 μm diam; binding hyphae in tubes pale yellow, subsolid, branched and flexuous, 1–2 μm diam. Pileal cover composed of clamped generative hyphae, thin- to thick-walled, apical cells clavate, faintly slanting to one side and flexuous, yellowish brown to reddish brown, about 30–50 × 4–7 μm, forming a regular palisade. Cystidia or cystidioles absent. Basidia clavate, colourless, thin-walled, 18–27 × 9–18 μm; basidioles in shape similar to basidia, colourless, thin-walled, 15–20 × 5–11 μm. Basidiospores subglobose to broadly ellipsoid, pale yellow, IKI–, CB+, with double and slightly thick walls, exospore wall smooth, endospore wall with indistinct spinules, 8–9.2(–9.3) × (6–)6.4–7.2 μm, L = 8.61 μm, W = 6.85 μm, Q = 1.26 (n = 60/1). Under SEM, exospore wall reticulate to vermiculate with obvious warts.
Notes — Sanguinoderma flavovirens was collected from Zambia. It is a distinct species on account of the yellowish green pore surface when fresh, colour changing to blood red when bruised, which is the typical feature of Sanguinoderma. In addition, S. flavovirens was shown to be a distinct lineage in Sanguinoderma through 4-gene and 6-gene phylogenetic analyses (Fig. 1, 2).
Sanguinoderma laceratum Y.F. Sun & B.K. Cui, sp. nov. — MycoBank MB828436; Fig. 8g–h, 12
Fig. 12.
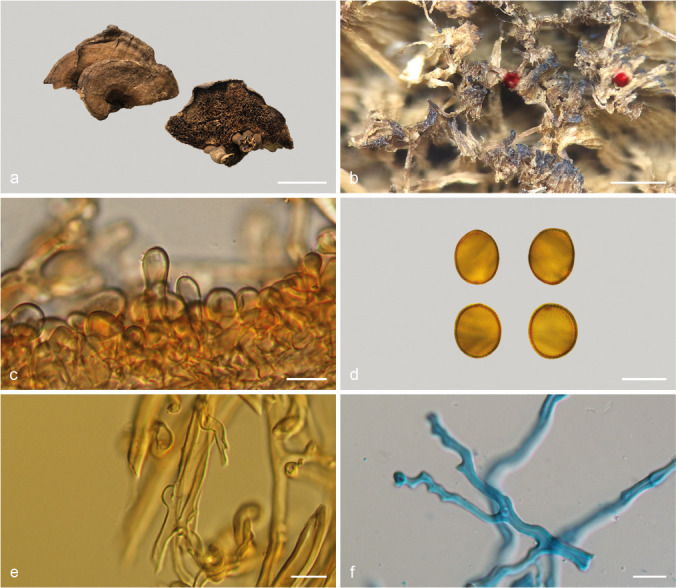
Basidiomata and microscopic structures of Sanguinoderma laceratum (Cui 8155). a. Basidiomata; b. pores; c. apical cells from pileal cover; d. basidiospores; e. generative hyphae from tubes; f. skeletal hyphae from context. — Scale bars: a = 3.5 cm; b = 1.5 mm; c–f = 10 μm.
Etymology. Laceratum (Lat.), refers to the lacerate pores.
Holotype. China, Yunnan Province, Baoshan, Gaoligongshan Nature Reserve, on fallen angiosperm trunk, 25 Sept. 2009, B.K. Cui, Cui 8155 (BJFC; isotype IFP).
Diagnosis. Sanguinoderma laceratum differs from other species in this genus by a combination of soft basidiomata with superposed pileus, lacerate pores with thin dissepiments, and tubes becoming fascicular when dry.
Basidiomata annual, laterally stipitate, soft corky. Pileus multiple and superposed, flabelliform or reniform, up to 7.5 cm diam and 1 cm thick. Pileal surface dark cinnamon when dry, dull, tomentose, with faint concentric zones and radial wrinkles; margin acute, entire, wavy and incurved when dry. Pore surface pale buff to greyish brown when fresh, colour changing to blood red when bruised, becoming dark brown to nearly black; pores irregular, 2–3 per mm; dissepiments thin, lacerate when dry. Context straw colour to pale brown, with pale grey melanoid lines, soft corky, up to 4 mm thick. Tubes concolorous with context, woody hard, becoming fascicular when dry, up to 6 mm long. Stipe concolorous with pileal surface, cylindrical and hollow, up to 8.5 cm long and 4 mm diam. Hyphal system trimitic; generative hyphae with clamp connections, all hyphae IKI–, CB+; tissues darkening in KOH. Generative hyphae in context colourless, slightly thick-walled, 3–4 μm diam; skeletal hyphae in context pale yellow, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 3–7 μm diam; binding hyphae in context pale yellow, subsolid, branched and flexuous, up to 2 μm diam. Generative hyphae in tubes colourless, slightly thick-walled, 3–4 μm diam; skeletal hyphae in tubes pale yellow, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 3–6 μm diam; binding hyphae in tubes pale yellow, subsolid, branched and flexuous, 1–2 μm diam. Pileal cover composed of clamped generative hyphae, thin- to thick-walled, apical cells clavate, inflated and flexuous, pale yellow to yellowish brown, about 25–40 × 6–13 μm, forming a regular palisade. Cystidia or cystidioles absent. Hymenium collapsed in the studied sample, basidia and basidioles not seen. Basidiospores subglobose to broadly ellipsoid, pale yellow, IKI–, CB+, with double and slightly thick walls, exospore wall smooth, endospore wall with spinules, (10.2–)10.5–12.3(–12.5) × (8.6–)9–10.5(–11.1) μm, L = 11.38 μm, W = 9.71 μm, Q = 1.17 (n = 60/1). Under SEM, exospore wall semi-reticulate, badly worn, endospore wall with long and slightly thick coniform spinules tightly arranged.
Notes — Sanguinoderma laceratum can be distinguished by the superposed pileus with slight concentric zones and lacerate pores with thin dissepiments. Sanguinoderma laceratum shares a deeply worn exospore wall with S. bataanense, but the latter has globose to subglobose basidiospores with distinctly thick walls with a verrucose exospore wall (Fig. 8a–b). In the phylogenetic analyses, S. laceratum was shown to be a distinct lineage in Sanguinoderma (Fig. 1, 2).
Sanguinoderma microporum Y.F. Sun & B.K. Cui, sp. nov. — MycoBank MB828437; Fig. 8i–j, 13
Fig. 13.
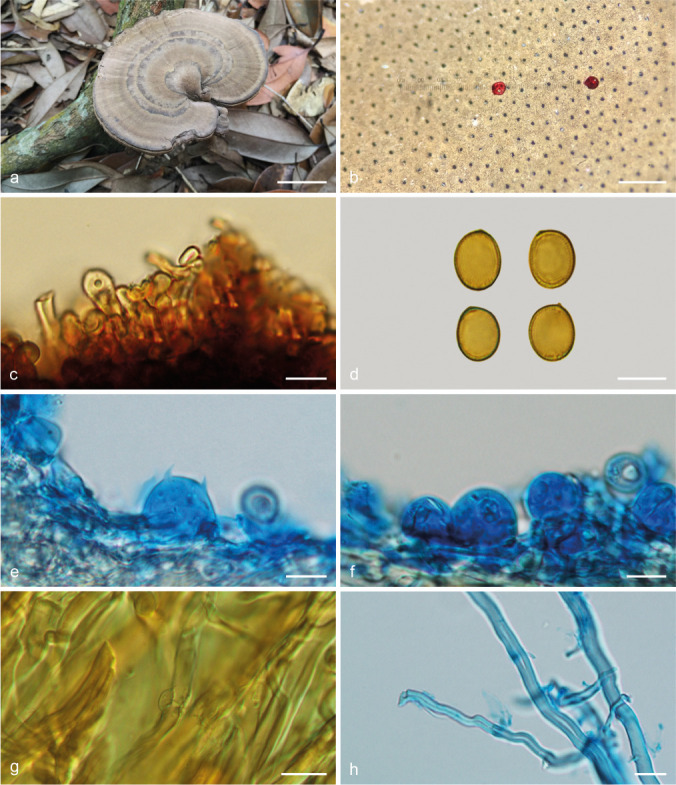
Basidiomata and microscopic structures of Sanguinoderma microporum (Cui 13851). a. Basidiomata; b. pores; c. apical cells from pileal cover; d. basidiospores; e. basidia; f. basidioles; g. generative hyphae from tubes; h. skeletal hyphae from context. — Scale bars: a = 3 cm; b = 0.5 mm; c–h = 10 μm.
Etymology. Microporum (Lat.), refers to the small pores.
Holotype. China, Hainan Province, Qiongzhong County, Limushan National Forest Park, on ground of angiosperm forest, 16 June 2016, B.K. Cui, Cui 13851 (BJFC).
Diagnosis. Sanguinoderma microporum differs from other species in this genus by its woody hard basidiomata with concentrically zonate or furrowed pileus surface, and small pores with extremely thick dissepiments.
Basidiomata annual, centrally to laterally stipitate, woody hard. Pileus single, suborbicular to flabelliform, up to 10.5 cm diam and 8 mm thick. Pileal surface pale yellowish brown to greyish brown, dull, glabrous, with concentric dark zones or furrows and radial wrinkles; margin acute to obtuse, entire, wavy and incurved when dry. Pore surface yellowish brown to dark brown when fresh, colour changing to blood red when bruised and becoming greyish brown when dry; pores circular, 6–7 per mm; dissepiments extremely thick (about 0.12–0.16 mm thick), entire. Context straw colour, with dark melanoid lines, corky, up to 4 mm thick. Tubes pale brown, up to 4 mm long. Stipe concolorous with pileal surface, cylindrical and hollow, up to 10.5 cm long and 9 mm diam. Hyphal system trimitic; generative hyphae with clamp connections, all hyphae IKI–, CB+; tissues darkening in KOH. Generative hyphae in context colourless, thin-walled, 3–4 μm diam; skeletal hyphae in context pale yellow, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 3–8 μm diam; binding hyphae in context colourless, subsolid, branched and flexuous, up to 2 μm diam. Generative hyphae in tubes colourless, thin-walled, 2–3 μm diam; skeletal hyphae in tubes pale yellow, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 3–6 μm diam; binding hyphae in tubes colourless, subsolid, branched and flexuous, 1–2 μm diam. Pileal cover composed of clamped generative hyphae, thin- to thick-walled, apical cells clavate, faintly inflated and flexuous, pale yellow to dark brown, about 25–40 × 6–13 μm, forming an irregular palisade. Cystidia or cystidioles absent. Basidia barrel-shaped to clavate, colourless, thin-walled, 18–22 × 13–18 μm; basidioles in shape similar to basidia, colourless, thin-walled, 16–20 × 10–15 μm. Basidiospores broadly ellipsoid, pale yellow, IKI–, CB+, with double and slightly thick walls, exospore wall smooth, endospore wall with conspicuous spinules, (10.7–)11–12(–12.3) × (8.5–)8.7–9.8(–10) μm, L = 11.52 μm, W = 9.18 μm, Q = 1.23–1.28 (n = 60/2). Under SEM, exospore wall semi-reticulate, endospore wall with long and slightly thin coniform spinules tightly arranged.
Additional specimens (paratypes) examined. China, Guangdong Province, Zhaoqing, Dinghushan Nature Reserve, on ground of angiosperm forest, 29 June 2010, B.K. Cui, Cui 8898 (BJFC); Guangxi Autonomous Region, Fangchenggang, Shiwandashan Nature Reserve, on ground of angiosperm forest, 6 July 2016, J.L. Zhou, Cui 14001 (BJFC); ibid., Cui 14022 (BJFC); Nanning, Qingxiushan Park, on ground of angiosperm forest, 3 Aug. 2017, J.L. Zhou, Cui 16335 (BJFC); ibid., Cui 16336 (BJFC); ibid., Cui 16338 (BJFC).
Notes — Sanguinoderma microporum is a distinct species on account of its distinctly small pores with extremely thick dissepiments. It has broadly ellipsoid basidiospores with slightly thick walls which are similar to S. laceratum. But S. laceratum differs from S. microporum by its superposed pileus and lacerate pores with thin dissepiments. In the phylogenetic analyses, S. microporum was shown to be a distinct well-supported lineage in Sanguinoderma (Fig. 1, 2).
Sanguinoderma perplexum (Corner) Y.F. Sun & B.K. Cui, comb. nov. — MycoBank MB828445; Fig. 8k–l, 14
Fig. 14.
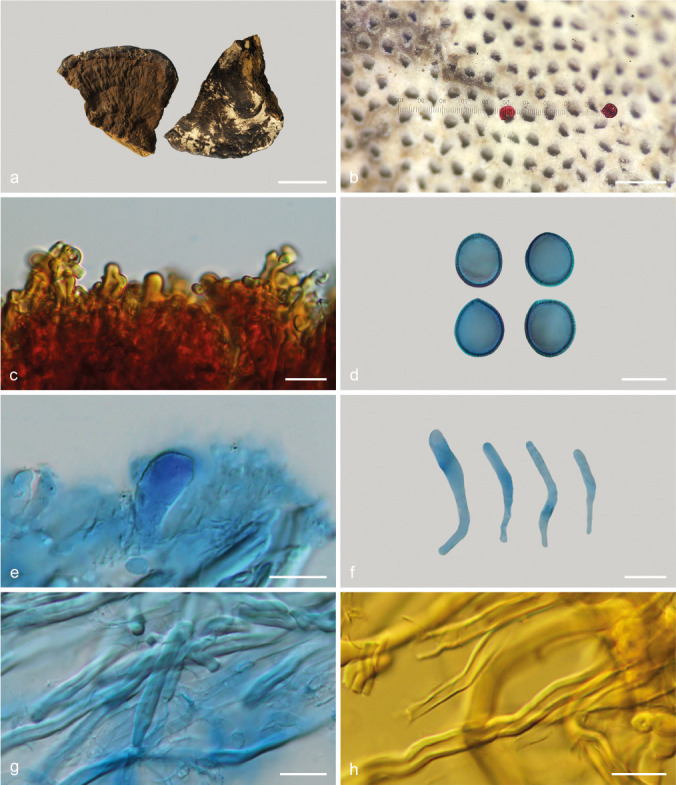
Basidiomata and microscopic structures of Sanguinoderma perplexum (Dai 10811). a. Basidiomata; b. pores; c. apical cells from pileal cover; d. basidiospores; e. basidioles; f. cystidioles; g. generative and skeletal hyphae from tubes; h. skeletal hyphae from context. — Scale bars: a = 3 cm; b = 0.5 mm; c–h = 10 μm.
Basionym. Amauroderma perplexum Corner, Beih. Nova Hedwigia 75: 82. 1983.
Basidiomata annual, almost sessile or with a very short and thick stipe, hard corky to woody hard. Pileus single, suborbicular to flabelliform, applanate, up to 11.5 cm long, 8 cm wide and 3 cm thick. Pileal surface reddish brown to ferruginous, dull, glabrous, with concentric furrows and radial wrinkles; margin obtuse, entire, slightly wavy when dry. Pore surface pale white to cream when fresh, colour changing to blood red when bruised, then quickly darkening; pores circular to angular, 5–6 per mm; dissepiments medially thick, entire. Context yellowish brown to cinnamon, with a bunch of melanoid lines, corky, up to 2.4 cm thick. Tubes concolorous with pore surface, hard corky, up to 6 mm long. Stipe concolorous with pileal surface, lateral-attached, up to 2 cm long and 3 cm diam. Hyphal system trimitic; generative hyphae with clamp connections, all hyphae IKI–, CB+; tissues darkening in KOH. Generative hyphae in context colourless, thin-walled, 3–5 μm diam; skeletal hyphae in context pale yellow to pale brown, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 3–7 μm diam; binding hyphae in context colourless, subsolid, branched and flexuous, 1–3 μm diam. Generative hyphae in tubes colourless, thin-walled, 2–4 μm diam; skeletal hyphae in tubes pale yellow to yellowish brown, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 3–6 μm diam; binding hyphae in tubes colourless, subsolid, branched and flexuous, 1–3 μm diam. Pileal cover composed of clamped generative hyphae, thin- to thick-walled, apical cells gelatinized, flexuous, yellowish brown, about 30–50 × 4–8 μm, forming an irregular palisade. Cystidia absent; cystidioles clavate, colourless, thin-walled, 27–30 × 2–3 μm. Basidia barrel-shaped to clavate, colourless, thin-walled, 30–44 × 22–28 μm; basidioles in shape similar to basidia, colourless, thin-walled, 19–30 × 5–11 μm. Basidiospores subglobose to broadly ellipsoid, pale yellow, IKI–, CB+, with double and medially thick walls, exospore wall smooth, endospore wall with conspicuous spinules, (11.1–)11.5–14(–14.8) × (9–)10–12(–13.9) μm, L = 12.75 μm, W = 10.84 μm, Q = 1.16–1.19 (n = 60/2). Under SEM, exospore wall densely vermiculate to semi-reticulate, endospore wall with long and thick columnar spinules loosely arranged.
Specimens examined. China, Hainan Province, Changjiang County, Bawangling Nature Reserve, on stump of Acmena acuminatissima, 9 May 2009, B.K. Cui, Cui 6496 (BJFC); on angiosperm stump, 9 May 2009, Y.C. Dai, Dai 10811 (BJFC); ibid., 10 May 2009, B.K. Cui, Cui 6554 (BJFC).
Notes — Amauroderma perplexum was described from Malaysia (Corner 1983). It can be mainly characterized by sessile to substipitate and woody hard basidiomata, clavate cystidioles and a vermiculate to semi-reticulate exospore wall with thick columnar endospore spinules (Fig. 8k–l). In addition, A. perplexum presents a pore surface that changes to blood red when bruised and is shown to be a distinct lineage in Sanguinoderma with high phylogenetic support (100 % ML, 1.00 BPP). Therefore, we transferred A. perplexum to Sanguinoderma. Sanguinoderma perplexum is similar to S. laceratum by sharing broadly ellipsoid basidiospores with slightly thick walls, but S. laceratum differs from S. perplexum by its thin and lacerate dissepiments and semi-reticulate exospore wall with slightly thin and coniform endospore spinules (Fig. 8g–h).
Sanguinoderma reniforme Y.F. Sun & B.K. Cui, sp. nov. — MycoBank MB828438; Fig. 8m–n, 15
Fig. 15.
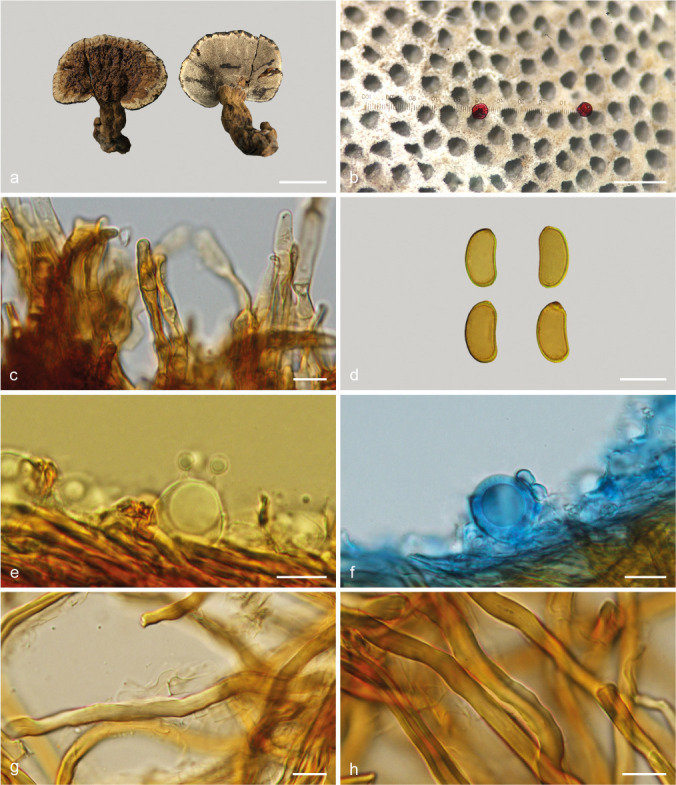
Basidiomata and microscopic structures of Sanguinoderma reniforme (Cui 16511). a. Basidiomata; b. pores; c. apical cells from pileal cover; d. basidiospores; e. basidia; f. basidioles; g. generative hyphae from tubes; h. skeletal hyphae from context. — Scale bars: a = 1.5 cm; b = 0.5 mm; c–h = 10 μm.
Etymology. Reniforme (Lat.), refers to the reniform basidiospores.
Holotype. Zambia, Ndola, Ngosa Village, on ground of angiosperm forest, 6 Jan. 2018, B. Zhang, Cui 16511 (BJFC; isotype IFP).
Diagnosis. Sanguinoderma reniforme differs from other species by its tomentose pileal surface with tumid wrinkles, and reniform basidiospores.
Basidiomata annual, laterally stipitate, hard corky. Pileus single, flabelliform to reniform, up to 4 cm diam and 5 mm thick. Pileal surface yellowish brown to dark yellowish brown, pale yellow in the edge, dull, tomentose, with concentric zones and irregular tumid wrinkles; margin obtuse, entire, slightly wavy and flat when dry. Pore surface pale grey when fresh, colour changing to blood red when bruised, then quickly darkening; pores circular to angular, 4–5 per mm; dissepiments slightly thick, entire. Context pale yellowish brown, with variegated resinous stratum, hard corky, up to 3 mm thick. Tubes concolorous with pore surface, corky, up to 2 mm long. Stipe concolorous with pileal surface, cylindrical, swollen at the base, up to 5 cm long and 1 cm diam. Hyphal system trimitic; generative hyphae with clamp connections, all hyphae IKI–, CB+; tissues darkening in KOH. Generative hyphae in context colourless, thin-walled, 3–5 μm diam; skeletal hyphae in context pale yellow to yellowish brown, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 3–7 μm diam; binding hyphae in context pale yellow, subsolid, branched and flexuous, 1–2 μm diam. Generative hyphae in tubes colourless, thin-walled, 3–4 μm diam; skeletal hyphae in tubes pale yellow to yellowish brown, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 3–6 μm diam; binding hyphae in tubes pale yellow, subsolid, branched and flexuous, 1–2 μm diam. Pileal cover composed of clamped generative hyphae, thin- to thick-walled, apical cells clavate, constricted, flexuous, pale yellowish brown, about 40–70 × 5–7 μm, forming an irregular palisade. Cystidia or cystidioles absent. Basidia barrel-shaped to clavate, colourless, thin-walled, 18–23 × 14–16 μm; basidioles in shape similar to basidia, colourless, thin-walled, 18–20 × 7–15 μm. Basidiospores reniform, pale yellow, IKI–, CB+, with double and slightly thick walls, exospore wall smooth, endospore wall with faint spinules, (11–)11.2–13 (–13.3) × (5.5–)5.7–7(–7.3) μm, L = 12.12 μm, W = 6.35 μm, Q = 1.91 (n = 60/1). Under SEM, exospore wall obviously verrucose or semi-reticulate, endospore wall with short and slightly thick coniform spinules tightly arranged.
Notes — Sanguinoderma reniforme was collected from Zambia and it is unique in the genus due to its reniform basidiospores. In addition, S. reniforme was shown to be a distinct lineage in Sanguinoderma (Fig. 1, 2).
Sanguinoderma rude (Berk.) Y.F. Sun, D.H. Costa & B.K. Cui, comb. nov. — MycoBank MB828446; Fig. 8o–p, 16
Fig. 16.
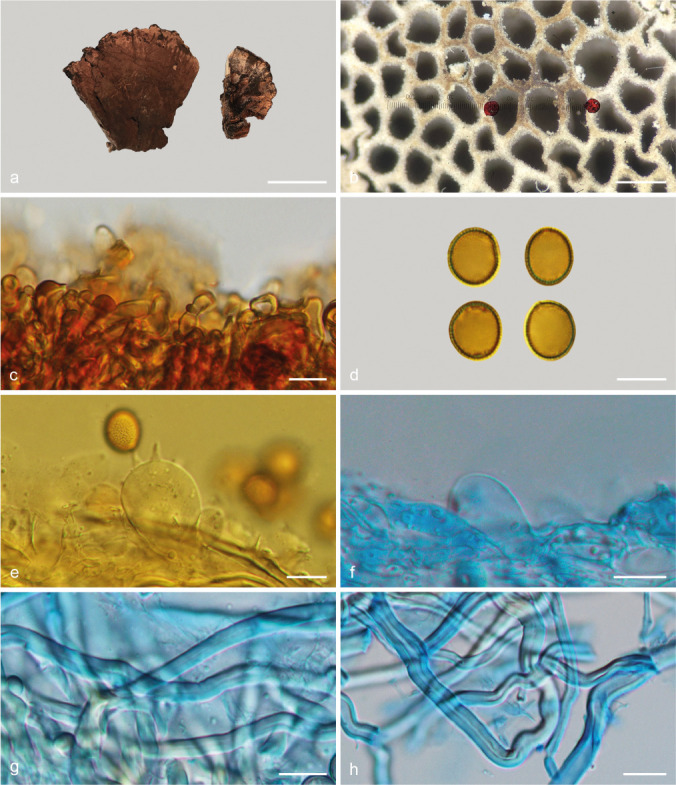
Basidiomata and microscopic structures of Sanguinoderma rude (MEL 2335651). a. Basidiomata; b. pores; c. apical cells from pileal cover; d. basidiospores; e. basidia; f. basidioles; g. skeletal hyphae from tubes; h. skeletal hyphae from context. — Scale bars: a = 3 cm; b = 0.5 mm; c–h = 10 μm.
Basionym. Polyporus rudis Berk., Ann. Mag. Nat. Hist. 3: 323. 1839.
≡ Amauroderma rude (Berk.) Torrend, Brotéria, Sér. Bot. 18: 127. 1920.
Basidiomata annual, lateral stipitate to almost sessile, corky. Pileus single, suborbicular to flabelliform, up to 7.5 cm diam and 1.7 cm thick. Pileal surface dark brown to ferruginous, dull, glabrous, with faintly concentric zones and radial wrinkles; margin acute to obtuse, entire, wavy and incurved when dry. Pore surface pale grey to greyish yellow, colour changing to blood red when bruised, then quickly darkening; pores subcircular to irregular, 1–4 per mm; dissepiments slightly thick, entire. Context woody colour to pale brown, without resinous lines, soft corky, up to 9 mm thick. Tubes darker than pore surface, hard corky, up to 8 mm long. Stipe concolorous with pileal surface, cylindrical and hollow, up to 5 cm long and 4 mm diam. Hyphal system trimitic; generative hyphae with clamp connections, all hyphae IKI–, CB+; tissues darkening in KOH. Generative hyphae in context colourless, thin- to slightly thick-walled, 2–4 μm diam; skeletal hyphae in context pale yellow, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 2–8 μm diam; binding hyphae in context pale yellow, subsolid, branched and flexuous, 1–2 μm diam. Generative hyphae in tubes colourless, thin- to slightly thick-walled, 2–4 μm diam; skeletal hyphae in tubes pale yellow, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 3–6 μm diam; binding hyphae in tubes pale yellow, subsolid, 1–2 μm diam. Pileal cover composed of clamped generative hyphae, thin- to thick-walled, apical cells clavate, faintly inflated, flexuous, yellowish brown, about 40–80 × 4–6 μm, forming an irregular palisade. Cystidia or cystidioles absent. Basidia barrel-shaped to clavate, colourless, thin-walled, 13–33 × 12–28 μm; basidioles suborbicular to clavate, colourless, thin-walled, 15–25 × 8–19 μm. Basidiospores subglobose to broadly ellipsoid, pale yellow, IKI–, CB+, with double and medially thick walls, exospore wall smooth, endospore wall with conspicuous spinules, (9–)9.5–12.5(–12.9) × 8–10.7(–11) μm, L = 11.18 μm, W = 9.5 μm, Q = 1.17–1.19 (n = 60/2). Under SEM, exospore wall alveolate to semi-reticulate with small and deep pits, endospore wall with short and slightly thick columnar spinules tightly arranged.
Specimens examined. Australia, Victoria State, Eastern Highlands, on rotten stump, 20 Mar. 1994, N.H. Sinnott, MEL 2028873 (MEL); East Gippsland, on buried wood, 27 Mar. 2002, K.R. Thiele, MEL 2150776 (MEL); Eastern Highlands, on stump of Eucalyptus, 12 May 2003, S.H. Lewis, MEL 2231602 (MEL); Gippsland Plain, on rotten stump of shrub, 4 May 2007, H. Strand, MEL 2317411 (MEL); Fairfield, on rotten stump of Acacia, 23 Apr. 2009, N.H. Sinnott, MEL 2335651 (MEL); Valley Reserve, on rotten wood, 14 July 2012, N.G. Karunajeewa, MEL 2362204 (MEL); Tasmania, on base of dead Eucalyptus, 12 May 2018, B.K. Cui, Cui 16592 (BJFC).
Notes — Amauroderma rude was described from Tasmania as Polyporus rudis (Berkeley 1839). We have examined the specimens collected from the type locality of Tasmania and mainland in Australia. It can be characterized by its big pores (1–4 per mm) with slightly thick and entire dissepiments, and alveolate to semi-reticulate exospore wall with small and deep pits (Fig. 8o–p). Amauroderma rude was shown as a distinct well-supported lineage in Sanguinoderma (Fig. 1, 2). Sanguinoderma rude is similar to S. perplexum by the subglobose to broadly ellipsoid basidiospores, but S. perplexum differs from S. rude by the woody hard basidiomata, small pores (5–6 per mm) with medially thick dissepiments and vermiculate to semi-reticulate exospore wall with long and thick endospore spinules (Fig. 8k–l). Amauroderma intermedium is quite similar to S. rude both in macro- and micro-morphology (Gomes-Silva et al. 2015), but it can be distinguished by its Neotropical distribution, darker context and colour-unchanging pore surface when bruised (Torrend 1920, Ryvarden 2004).
Sanguinoderma rugosum (Blume & T. Nees) Y.F. Sun, D.H. Costa & B.K. Cui, comb. nov. — MycoBank MB828447; Fig. 8q–r, 17
Fig. 17.

Basidiomata and microscopic structures of Sanguinoderma rugosum (Cui 16337). a. Basidiomata; b. pores; c. apical cells from pileal cover; d. basidiospores; e. basidioles; f. cystidioles; g. generative hyphae from tubes; h. skeletal hyphae from context. — Scale bars: a = 4 cm; b = 0.5 mm; c–h = 10 μm.
Basionym. Polyporus rugosus Blume & T. Nees, Nova Acta Acad. Caes. Leop.-Carol. German. Nat. Cur. 13: 21. 1826.
≡ Amauroderma rugosum (Blume & T. Nees) Torrend, Brotéria, Sér. Bot. 18: 127. 1920.
Basidiomata annual, centrally to laterally stipitate, corky to woody hard. Pileus single, suborbicular to flabelliform, up to 12 cm diam and 8 mm thick. Pileal surface dark brown to near black, dull, glabrous, with obviously concentric furrows and radial wrinkles, centre navel-shaped; margin acute to obtuse, entire, wavy and incurved when dry. Pore surface greyish white when fresh, colour changing to blood red when bruised, then quickly darkening; pores circular to angular, 5–7 per mm; dissepiments slightly thick, entire. Context cinnamon to dark brown, with two black resinous lines, corky, up to 5 mm thick. Tubes concolorous with pore surface, woody hard, up to 3 mm long. Stipe concolorous with pileal surface, cylindrical and hollow, slightly swollen at base, up to 12 cm long and 1 cm diam. Hyphal system trimitic; generative hyphae with clamp connections, all hyphae IKI–, CB+; tissues darkening in KOH. Generative hyphae in context colourless, thin-walled, 4–6 μm diam; skeletal hyphae in context yellowish brown to dark brown, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 4–6 μm diam; binding hyphae in context pale yellow, subsolid, branched and flexuous, 1–2 μm diam. Generative hyphae in tubes scanty; skeletal hyphae in tubes dark brown, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 3–7 μm diam; binding hyphae in tubes pale brown, subsolid, branched and flexuous, 1–2 μm diam. Pileal cover composed of clamped generative hyphae, thin- to thick-walled, apical cells clavate with obvious septa at the base, inflated, dark brown, about 20–50 × 6–10 μm, forming a regular palisade. Cystidia absent; cystidioles clavate and apexes constricted, colourless, thin-walled, 20–28 × 3–5 μm. Basidia barrel-shaped to clavate, colourless, thin-walled, 18–25 × 9–20 μm; basidioles in shape similar to basidia, colourless, thin-walled, 15–20 × 10–16 μm. Basidiospores broadly ellipsoid, pale yellow, IKI–, CB+, with double and slightly thick walls, exospore wall smooth, endospore wall with conspicuous spinules, (9.9–)10.2–11.3(–11.7) × (8–)8.3–9.2(–9.5) μm, L = 10.75 μm, W = 8.86 μm, Q = 1.21–1.22 (n = 60/2). Under SEM, exospore wall alveolate to semi-reticulate, endospore wall with short and thin columnar spinules tightly arranged.
Specimens examined. China, Guangdong Province, Shaoguan, Chebaling Nature Reserve, on ground of angiosperm forest, 25 June 2010, B.K. Cui, Cui 8795 (BJFC); Zhaoqing, Heishiding Nature Reserve, on ground, 1 July 2010, B.K. Cui, Cui 9011 (BJFC); ibid., Cui 9012 (BJFC); Guangxi Autonomous Region, Nanning, Liangfengjiang National Forest Park, on ground, 3 Aug. 2017, J.L. Zhou, Cui 16337 (BJFC); ibid., 4 Aug. 2017, Cui 16166 (BJFC). – Indonesia, West Java, Banten, on Acacia mangium, Bougher, E7079 (PERTH).
Notes — Amauroderma rugosum is a widespread species distributed in tropical and subtropical areas of South East Asia. It seems to be a species of difficult delimitation due to its great spectrum of morphologic variation (colour, texture and basidiospores size), exhibiting a broadly distribution (although some of the records are dubious), being one of the most cited Amauroderma species (Furtado 1981, Corner 1983). Based on its colour-changing pore surface when bruised and good phylogenetic support, it is proposed as a new combination in Sanguinoderma. It is similar to S. microporum in the dark brown pileal surface with obvious concentric furrows and radial wrinkles, but S. microporum differs from S. rugosum by the extremely thick dissepiments and slightly bigger basidiospores (11–12 × 8.7–9.8 μm) with long and thin endospore coniform spinules (Fig. 8i–j). The distinction between S. rugosum and S. rude is difficult, but the latter has a softer pileus and slightly larger spores (9.5–12.5 × 8–10.7 μm).
Sanguinoderma sinuosum Y.F. Sun & B.K. Cui, sp. nov. — MycoBank MB828439; Fig. 8s–t, 18
Fig. 18.
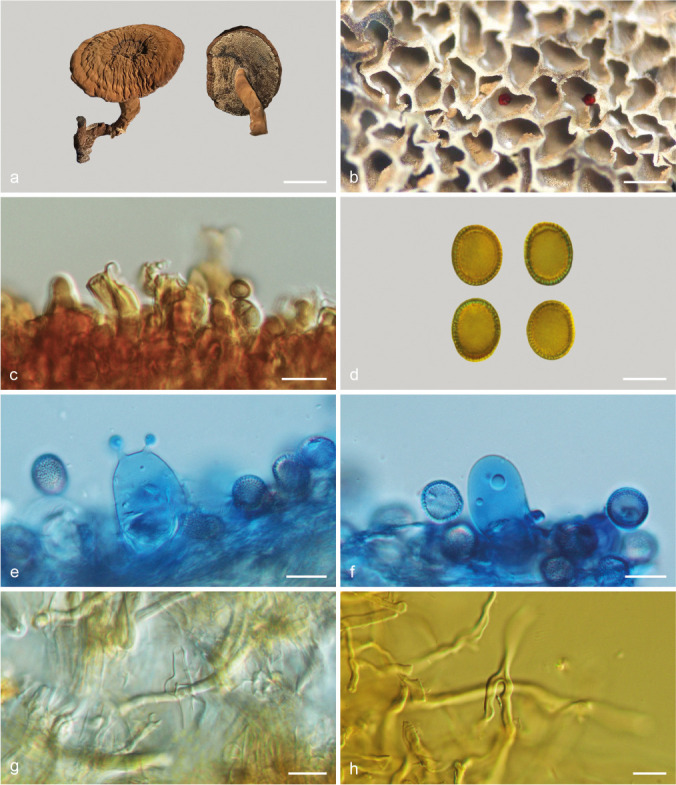
Basidiomata and microscopic structures of Sanguinoderma sinuosum (MEL 2366586). a. Basidiomata; b. pores; c. apical cells from pileal cover; d. basidiospores; e. basidia; f. basidioles; g. generative hyphae from tubes; h. skeletal hyphae from context. — Scale bars: a = 2.5 cm; b = 0.5 mm; c–h = 10 μm.
Etymology. Sinuosum (Lat.), refers to the sinuate pores.
Holotype. Australia, Queensland State, Sunshine Coast, on sandy ground, 7 Mar. 2010, P. Leonard, MEL 2366586 (MEL).
Diagnosis. Sanguinoderma sinuosum differs from other species in this genus by its sinuate pores with thin dissepiments, big and broadly ellipsoid to ellipsoid basidiospores.
Basidiomata annual, centrally to laterally stipitate, soft to hard corky. Pileus single, suborbicular to flabelliform, up to 6.5 cm diam and 1.6 cm thick. Pileal surface dark brown to ferruginous when dry, dull, glabrous, with obvious concentric furrows and strong radial wrinkles; margin obtuse, entire, wavy and incurved when dry. Pore surface pale white when fresh, colour changing to blood red when bruised, then quickly darkening; pores sinuate, 2–3 per mm; dissepiments thin, entire. Context straw colour to cinnamon, with pale white lines, soft corky, up to 7 mm thick. Tubes cinnamon, woody hard, up to 9 mm long. Stipe concolorous with pileal surface, cylindrical and flexuous, up to 6 cm long and 1.5 cm diam. Hyphal system trimitic; generative hyphae with clamp connections, all hyphae IKI–, CB+; tissues darkening in KOH. Generative hyphae in context colourless, thin-walled, 2–4 μm diam; skeletal hyphae in context pale yellow, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, 3–6 μm diam; binding hyphae colourless to pale yellow, subsolid, branched and flexuous, 1–2 μm diam. Generative hyphae in tubes colourless, up to 3 μm diam; skeletal hyphae in tubes colourless to pale yellow, thick-walled with a wide to narrow lumen or subsolid, arboriform branched and flexuous, up to 5 μm diam; binding hyphae in tubes pale yellow, subsolid, branched and flexuous, 1–2 μm diam. Pileal cover composed of clamped generative hyphae, thin- to thick-walled, apical cells clavate, faintly inflated, pale yellowish brown, about 50–70 × 4–6 μm, forming an irregular palisade. Cystidia or cystidioles absent. Basidia barrel-shaped to clavate, colourless, thin-walled, 25–33 × 15–19 μm; basidioles in shape similar to basidia, colourless, thin-walled, 23–28 × 13–16 μm. Basidiospores broadly ellipsoid to ellipsoid, pale yellow to yellowish brown, IKI–, CB+, with double and slightly thick walls, exospore wall smooth, endospore wall with conspicuous spinules, (12.3–)12.5–13.7(–14.3) × 9.1–10.8(–11.1) μm, L = 13.07 μm, W = 10.08 μm, Q = 1.3 (n = 60/1). Under SEM, exospore wall uneven, with distinctly circular hollows, endospore wall with long and thick columnar spinules tightly arranged.
Additional specimen (paratype) examined. Australia, Victoria State, Langwarrin Flora and Fauna Reserve, on rotten stump of Acacia, 6 June 2010, N.H. Sinnott, MEL 2341763 (MEL).
Notes — Sanguinoderma sinuosum was collected from Australia. It is characterized by its sinuate pores with thin dissepiments, large and broadly ellipsoid to ellipsoid basidiospores. Sanguinoderma sinuosum is similar to S. rude by the dark brown and corky pileus with concentric furrows and radial wrinkles, but S. rude has subcircular pores with slightly thick dissepiments, and smaller basidiospores (9.5–12.5 × 8–10.7 μm) with short and slightly thick columnar endospore spinules (Fig. 8o–p). In the phylogenetic analyses, these two species nested in two lineages with good support (Fig. 1, 2).
DISCUSSION
Costa-Rezende et al. (2017) provided a phylogenetic analysis on Amauroderma s.lat. based on 4-gene sequences (ITS+nLSU+ TEF+RPB1) with main focus on samples from Neotropics and indicated that Amauroderma was polyphyletic. In the current study, taxonomic and phylogenetic analyses of Amauroderma s.lat. were carried out based on samples from Africa, Asia and Oceania and more gene markers (ITS+nLSU+TEF+RPB1+ RPB2+TUB). Our results also confirmed the polyphyly of Amauroderma and that species previously belonging to Amauroderma s.lat. are divided into four clades representing four genera: Amauroderma s.str., Foraminispora and Furtadoa, as already shown by Costa-Rezende et al. (2017), and the new genus Sanguinoderma. This division was also supported by morphology and ultrastructural features, and the classification system of Amauroderma s.lat. was revised. The distribution and main morphological differences of accepted genera in Amauroderma s.lat. are shown in Table 2. Besides the new genus, six new species and eight new combinations have been proposed and described.
Table 2.
The distribution and main morphological differences of accepted genera in Amauroderma s.lat.
| Genus | Distribution | Pileal surface | Pore surface | Hyphal system | Shape of basidiospores | Ornamentation of basidiospores | References |
|---|---|---|---|---|---|---|---|
| Amauroderma s.str. | most neotropical areas | brown to near black, dull to laccate, glabrous to tomentose | pale white to yellowish brown, colour unchanged when bruised | dimitic | globose or ellipsoid to obovoid | endospore wall smooth or ornamented | Gomes-Silva et al. 2015, Costa-Rezende et al. 2016 |
| Foraminispora | South China, Central and South America | yellowish brown to reddish brown, dull, tomentose | white to straw colour, colour unchanged when bruised | dimitic to trimitic | globose to ellipsoid foveolate, endospore wall with hollow and columnar spinules which persist to exospore wall forming holes | exospore wall uneven or | Costa-Rezende et al. 2017, present study |
| Furtadoa | Central and South America | yellowish brown to greyish brown, dull, glabrous to tomentose | white to straw colour, colour unchanged when bruised | monomitic to dimitic | subglobose to ellipsoid | endospore wall with conspicuous spinules | Gomes-Silva et al. 2015, Costa-Rezende et al. 2017 |
| Sanguinoderma | Asia, South Africa and Oceania | dark brown to near black, dull, glabrous to tomentose | grey white to dark grey, colour changed to blood red when bruised | trimitic | subglobose or ellipsoid to reniform | exospore wall semi-reticulate or vermiculate to verrucose, endospore wall with solid and columnar to coniform spinules | present study |
The examination of fresh specimens previously classified in Amauroderma and some undescribed taxa showed the presence of a striking reaction on pore surface, which changes almost instantaneously to blood red when bruised, and then turns to a dark coloration. This reaction was already reported by Corner (1983) for A. atrum, A. perplexum and A. rugosum. The phylogenetic results showed that all the sampled species which present this reaction grouped together. Then, we proposed the genus Sanguinoderma to accommodate those species. Some macro-fungi present the amino acid tyrosine, which is enzymatically oxidized to a red intermediate and further to melanin and acts as a bioactive agent against microbes and harming substances (Halbwachs et al. 2016), which could be the case of Sanguinoderma species.
The new genus comprises the species from tropical Asia, Africa and Oceania. Sanguinoderma and Amauroderma s.str. share stipitate to almost sessile and corky to woody hard basidiomata, glabrous to tomentose and concentrically zonate or furrowed pileal surface and double-walled basidiospores. However, Sanguinoderma may be distinguished from Amauroderma s.str. by the fresh pore surface colour that changes to blood red when bruised. In addition, Amauroderma s.str. has a neotropical distribution (Gomes-Silva et al. 2015, Costa-Rezende et al. 2016, Costa-Rezende et al. 2017, Song et al. 2016), while Sanguinoderma is distributed in the Paleotropical region and Oceania.
The species in Amauroderma s.str. have a dull to laccate pileal surface, a fresh pore surface colour unchanging when bruised, a dimitic hyphal system and non-dextrinoid or dextrinoid basidiospores with solid endospore ornamentation (Ryvarden 2004, Gomes-Silva et al. 2015, Song et al. 2016, Costa-Rezende et al. 2017). Up to now, Foraminispora was considered as a monotypic genus, composed only by F. rugosa from Neotropics (Costa-Rezende et al. 2017). During the study of basidiospores ultrastructure of tropical and subtropical species previously classified in Amauroderma (A. austrosinense, A. concentricum and A. yunnanense) and one undescribed species, we observed that those species presented basidiospores with the typical morphology of Foraminispora. Besides that, those species grouped together with F. rugosa in our phylogenetic study. Thus, we propose here three new combinations and one new species for Foraminispora. All these five species are similar to Amauroderma s.str. species by stipitate and corky to woody hard basidiomata, fresh pore surface colour unchanging when bruised, similar hyphal system and non-truncate, double-walled, indextrinoid or dextrinoid basidiospores. But Foraminispora is unique on account of the globose to ellipsoid basidiospores with hollow and columnar endospore spinules, which persist to the exospore wall forming holes sometimes which can be viewed under SEM (Fig. 3). So far, Foraminispora is composed of five species, of which four are found in temperate or subtropical regions of East Asia and one in neotropical areas.
Furtadoa comprises two species from Neotropics, which have stipitate and fleshy basidiomata with a dull pileal surface, a fresh pore surface colour unchanging when bruised, and a monomitic context with both clamped and simple septate generative hyphae (Gomes-Silva et al. 2015, Costa-Rezende et al. 2017). Based on these features, Furtadoa can be easily distinguished from the other three genera.
Scanning electron microscopy (SEM) had been widely used in the taxonomy of Basidiomycota, such as Russulales (Lebel & Trappe 2000), Bondarzewia (Chen et al. 2016) and Phylloporus (Zeng et al. 2013). Costa-Rezende et al. (2017) established Foraminisprora based on its unique ultrastructural characters of the basidiospores, as mentioned above. In our study and in addition to the molecular analyses, the basidiospores of Foraminispora and Sanguinoderma were also scanned by SEM and we observed the ornamentations on the endospore and exospore walls of Sanguinoderma are quite different from those of Foraminispora. Spores of Sanguinoderma have a semi-reticulate or vermiculate to verrucose exospore wall and an endospore wall with solid and columnar to coniform spinules (Fig. 8). According to our observations and previous descriptions, we consider that SEM is a far more important taxonomic method in the identification of genera than species within Ganodermataceae.
Acknowledgements
We express our gratitude to the curators of IFP, JV, HMAS and MEL herbaria for the loan of specimens. Special thanks are due to Drs. Shuang-Hui He (Beijing Forestry University, China), Hai-Sheng Yuan (Institute of Applied Ecology, Chinese Academy of Sciences, China), Tom May (Royal Botanic Gardens Victoria, Australia) for assistance during field collections. The research was financed by the National Natural Science Foundation of China (Project No. 31670016), the Biodiversity Survey and Assessment Project of the Ministry of Ecology and Environment of China (No. 2019HJ2096001006), the Fundamental Research Funds for the Central Universities (Project No. 2016ZCQ04), Beijing Forestry University Outstanding Young Talent Cultivation Project (No. 2019JQ03016) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. DHCR acknowledges the Australian Academy of Science by the funding for participation in the Australia-Brazil PhD Exchange program.
REFERENCES
- Aime MC, Henkel TW, Ryvarden L. 2003. Studies in neotropical polypores 15: new and interesting species from Guyana. Mycologia 95: 614–619. doi: https://doi.org/10.1080/15572536.2004.11833065. [DOI] [PubMed] [Google Scholar]
- Berkeley MJ. 1839. Contributions towards a Flora of Van Diemen’s Land; from collections sent by R.W. Lawrence and Ronald Gaunn, Esqrs., to Sir W.J. Hooker. Annals and Magazine of Natural History 3: 322–327. [Google Scholar]
- Binder M, Larsson KH, Matheny PB, et al. 2010. Amylocorticiales ord. nov. and Jaapiales ord. nov.: early diverging clades of Agaricomycetidae dominated by corticioid forms. Mycologia 102: 865–880. doi: https://doi.org/10.3852/09-288. [DOI] [PubMed] [Google Scholar]
- Cao Y, Wu SH, Dai YC. 2012. Species clarification of the prize medicinal Ganoderma mushroom “Lingzhi”. Fungal Diversity 56: 49–62. doi: https://doi.org/10.1007/s13225-012-0178-5. [Google Scholar]
- Cao Y, Yuan HS. 2013. Ganoderma mutabile sp. nov. from southwestern China based on morphological and molecular data. Mycological Progress 12: 121–126. doi: https://doi.org/10.1007/s11557-012-0819-9. [Google Scholar]
- Chen JJ, Cui BK, He SH, et al. 2016. Molecular phylogeny and global diversity of the remarkable genus Bondarzewia (Basidiomycota, Russulales), Mycologia 108: 697–708. doi: https://doi.org/10.3852/14-216. [DOI] [PubMed] [Google Scholar]
- Coetzee MPA, Marincowitz S, Muthelo VG, et al. 2015. Ganoderma species, including new taxa associated with root rot of the iconic Jacaranda mimosifolia in Pretoria, South Africa. IMA Fungus 6: 249–256. doi: https://doi.org/10.5598/imafungus.2015.06.01.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corner EJH. 1983. Ad Polyporaceas I. Amauroderma and Ganoderma. Beihefte zur Nova Hedwigia 75: 1–182. [Google Scholar]
- Costa-Rezende DH, Gugliotta AM, Góes-Neto A, et al. 2016. Amauroderma calcitum sp. nov. and notes on taxonomy and distribution of Amauroderma species (Ganodermataceae). Phytotaxa 244: 101–124. doi: https://doi.org/10.11646/phytotaxa.244.2.1. [Google Scholar]
- Costa-Rezende DH, Robledo GL, Góes-Neto A, et al. 2017. Morphological reassessment and molecular phylogenetic analyses of Amauroderma s.lat. raised new perspectives in the generic classification of the Ganodermataceae family. Persoonia 39: 254–269. doi: https://doi.org/10.3767/persoonia.2017.39.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui BK, Li HJ, Ji X, et al. 2019. Species diversity, taxonomy and phylogeny of Polyporaceae (Basidiomycota) in China. Fungal Diversity 97: 137–392. doi: https://doi.org/10.1007/s13225-019-00427-4. [Google Scholar]
- Furtado JS. 1981. Taxonomy of Amauroderma (Basidiomycetes, Polyporaceae). Memoirs of the New York Botanical Garden 34: 1–109. [Google Scholar]
- Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous Ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes-Silva AC, De Lima NC, Malosso E, et al. 2015. Delimitation of taxa in Amauroderma (Ganodermataceae, Polyporales) based in morphology and molecular phylogeny of Brazilian specimens. Phytotaxa 227: 201–228. doi: https://doi.org/10.11646/phytotaxa.227.3.1. [Google Scholar]
- Halbwachs H, Simmel J, Bässler C. 2016. Tales and mysteries of fungal fruiting: How morphological and physiological traits affect a pileate lifestyle. Fungal Biology Reviews 30: 36–61. doi: https://doi.org/10.1016/j.fbr.2016.04.002. [Google Scholar]
- Hall TA. 1999. Bioedit: a user-friendly biological sequence alignment editor and analyses program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Han ML, Chen YY, Shen LL, et al. 2016. Taxonomy and phylogeny of the brown-rot fungi: Fomitopsis and its related genera. Fungal Diversity 80: 343–373. doi: https://doi.org/10.1007/s13225-016-0364-y. [Google Scholar]
- Hapuarachchi KK, Wen TC, Deng CY, et al. 2015. Mycosphere Essays 1: Taxonomic confusion in the Ganoderma lucidum species complex. Mycosphere 6: 542–559. doi: https://doi.org/10.5943/mycosphere/6/5/4. [Google Scholar]
- Hong SG, Jung HS. 2004. Phylogenetic analysis of Ganoderma based on nearly complete mitochondrial small-subunit ribosomal DNA sequences. Mycologia 96: 742–755. doi: https://doi.org/10.1080/15572536.2005.11832922. [DOI] [PubMed] [Google Scholar]
- Imazeki R. 1939. Studies in Ganoderma of Nippon. Bulletin of the Tokyo Science Museum 1: 29–52. [Google Scholar]
- Imazeki R. 1952. A contribution to the fungus flora of Dutch New Guinea. Bulletin of the Government Forest Experimental Station Meguro 57: 87–128. [Google Scholar]
- Karsten PA. 1881. Enumeratio Boletinearum et Polyporearum Fennicarum, systemate novo dispositarum. Revue Mycologique Toulouse 3: 16–19. [Google Scholar]
- Katoh K, Toh H. 2008. Recent developments in the MAFFT multiple sequence alignment program. Briefings In Bioinformatics 9: 286–298. doi: https://doi.org/10.1093/bib/bbn013. [DOI] [PubMed] [Google Scholar]
- Le XT, Le QHN, Pham ND, et al. 2012. Tomophagus cattienensis sp. nov., a new Ganodermataceae species from Vietnam: evidence from morphology and ITS DNA barcodes. Mycological Progress 11: 775–780. doi: https://doi.org/10.1007/s11557-011-0789-3. [Google Scholar]
- Lebel T, Trappe JM. 2000. Type studies of sequestrate Russulales. I. generic type species. Mycologia 92: 1188–1205. doi: https://doi.org/10.2307/3761486. [Google Scholar]
- Li HJ, Cui BK, Dai YC. 2014a. Taxonomy and multi-gene phylogeny of Datronia (Polyporales, Basidiomycota). Persoonia 32: 170–182. doi: https://doi.org/10.3767/003158514X681828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TH, Hu HP, Deng WQ, et al. 2014b. Ganoderma leucocontextum, a new member of the G. lucidum complex from southwestern China. Mycoscience 56: 81–85. doi: https://doi.org/10.1016/j.myc.2014.03.005. [Google Scholar]
- Lima Júnior NC de, Gibertoni TB, Malosso E. 2014. Delimitation of some neotropical laccate Ganoderma (Ganodermataceae): molecular phylogeny and morphology. Revista de Biología Tropical 62: 1197–1208. doi: https://doi.org/10.15517/RBT.V62I3.12380. [PubMed] [Google Scholar]
- Liu YJ, Whelen S, Hall BD. 1999. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology & Evolution 16: 1799–1808. doi: https://doi.org/10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- Matheny PB, Liu YJ, Ammirati JF, et al. 2002. Using RPB1 sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales). American Journal of Botany 89: 688–698. doi: https://doi.org/10.3732/ajb.89.4.688. [DOI] [PubMed] [Google Scholar]
- Moncalvo JM, Ryvarden L. 1997. A nomenclatural study of the Ganodermataceae Donk. Synopsis Fungorum 11: 1–114. [Google Scholar]
- Murrill WA. 1905. The Polyporaceae of North America: XI. A synopsis of the brown pileate species. Bulletin of the Torrey Botanical Club 32: 353–371. [Google Scholar]
- Murrill WA. 1907. Some Philippine Polyporaceae. Bulletin of the Torrey Botanical Club 34: 465–481. [Google Scholar]
- Murrill WA. 1908. Additional Philippine Polyporaceae. Bulletin of the Torrey Botanical Club 35: 391–416. [Google Scholar]
- Nylander J. 2008. MrModeltest2 v. 2.3. Program distributed by the author. Evolutionary Biology Centre, Uppsala University. [Google Scholar]
- Petersen JH. 1996. Farvekort. The Danish Mycological Society’s colour-chart. Foreningen til Svampekundskabens Fremme, Greve. [Google Scholar]
- Pilotti CA, Sanderson FR, Aitken AB, et al. 2004. Morphological variation and host range of two Ganoderma species from Papua New Guinea. Mycopathologia 158: 251–265. doi: https://doi.org/10.1023/B:MYCO.0000041833.41085.6f. [DOI] [PubMed] [Google Scholar]
- Rehner SA, Buckley E. 2005. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97: 84–98. doi: https://doi.org/10.1080/15572536.2006.11832842. [DOI] [PubMed] [Google Scholar]
- Robledo GL, Newman DS, Popoff OF, et al. 2015. Amauroderma trichodermatum (Ganodermataceae, Basidiomycota): first record from Bolivia and geographic distribution map, with notes on nomenclature and morphology. Check List 11: 1–5. doi: https://doi.org/10.15560/11.4.1671. [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. doi: https://doi.org/10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Ryvarden L. 2004. Neotropical polypores 1. Introduction, Hymenochaetaceae and Ganodermataceae. Synopsis Fungorum 19: 1–227. [Google Scholar]
- Shen LL, Wang M, Zhou JL, et al. 2019. Taxonomy and phylogeny of Postia. Multi-gene phylogeny and taxonomy of the brown-rot fungi: Postia (Polyporales, Basidiomycota) and related genera. Persoonia 42: 101–126. doi: https://doi.org/10.3767/persoonia.2019.42.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BJ, Sivasithamparam K. 2000. Internal transcribed spacer ribosomal DNA sequence of five species of Ganoderma from Australia. Mycological Research 104: 943–951. doi: https://doi.org/10.1017/S0953756200002458. [Google Scholar]
- Song J, Cui BK. 2017. Phylogeny, divergence time and historical biogeography of Laetiporus (Basidiomycota, Polyporales). BMC Evolutionary Biology 17: 102. doi: https://doi.org/10.1186/s12862-017-0948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Xing JH, Decock C, et al. 2016. Molecular phylogeny and morphology reveal a new species of Amauroderma (Basidiomycota) from China. Phytotaxa 260: 47–56. doi: https://doi.org/10.11646/phytotaxa.260.1.5. [Google Scholar]
- Stamatakis A. 2014. RAxML Version 8: a tool for phylogenetic analyses and post analyses of large phylogenies. Bioinformatics 30: 1312–1313. doi: https://doi.org/10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyaert RL. 1972. Species of Ganoderma and related genera mainly of the Bogor and Leiden Herbaria. Persoonia 7: 55–118. http://www.repository.naturalis.nl/document/569803. [Google Scholar]
- Swofford DL. 2003. Paup*. Phylogenetic analyses using parsimony (*and other methods) version 4.0b10. Sinauer Associates, Massachusetts. [Google Scholar]
- Thawthong A, Hapuarachchi KK, Wen TC, et al. 2017. Ganoderma sichuanense (Ganodermataceae, Polyporales) new to Thailand. MycoKeys 22: 27–43. doi: https://doi.org/10.3897/mycokeys.22.13083. [Google Scholar]
- Torrend C. 1920. Les polyporacées du Brésil 1. Polyporacées stipités. Brotéria Série Botânica 18: 121–143. [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. doi: https://doi.org/10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DM, Wu SH, Su CH, et al. 2009. Ganoderma multipileum, the correct name for ‘G. lucidum’ in tropical Asia. Botanical Studies 50: 451–458. [Google Scholar]
- White TJ, Bruns T, Lee S, et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al. (eds), PCR protocols, a guide to methods and applications. Academic Press, San Diego, 315–322. doi: https://doi.org/10.1016/B978-0-12-372180-8.50042-1. [Google Scholar]
- Xing JH, Song J, Decock C, et al. 2016. Morphological characters and phylogenetic analyses reveal a new species within the Ganoderma lucidum complex from South Africa. Phytotaxa 266: 115–124. doi: https://doi.org/10.11646/phytotaxa.266.2.5. [Google Scholar]
- Xing JH, Sun YF, Han YL, et al. 2018. Morphological and molecular identification of two new Ganoderma species on Casuarina equisetifolia from China. MycoKeys 34: 93–108. doi: https://doi.org/10.3897/mycokeys.34.22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng NK, Tang LP, Li YC, et al. 2013. The genus Phylloporus (Boletaceae, Boletales) from China: morphological and multilocus DNA sequence analyses. Fungal Diversity 58: 73–101. doi: https://doi.org/10.1007/s13225-012-0184-7. [Google Scholar]
- Zhao JD, Xu LW, Zhang XQ. 1984. Taxonomic studies on Ganodermataceae of China 3. Mycosystema 3: 15–23. [Google Scholar]
- Zhu L, Song J, Zhou JL, et al. 2019. Species diversity, phylogeny, divergence time and biogeography of the genus Sanghuangporus (Basidiomycota). Frontiers in Microbiology 10: 812. doi: https://doi.org/10.3389/fmicb.2019.00812. [DOI] [PMC free article] [PubMed] [Google Scholar]