Abstract
Strobilomyces is broadly distributed geographically and serves an important ecological function. However, it has been difficult to delimit species within the genus, primarily due to developmental variations and phenotypic plasticity. To elucidate phylogenetic relationships among species within the genus and to understand its species diversity, especially in Asia, materials of the genus collected from five continents (Africa, Asia, Australia, Europe, and North/Central America) were investigated. The phylogeny of Strobilomyces was reconstructed based on nucleotide sequences of four genes coding for: the largest and the second largest subunits of the RNA polymerase II (RPB1 and RPB2); the translation elongation factor subunit 1-α (TEF1); and the mitochondrial cytochrome oxidase subunit 3 (COX3). The combined results based on molecular phylogenetics, morphological characters, host tree associations, and geographical distribution patterns support a new classification consisting of two sections, sect. Strobilomyces and sect. Echinati. Using the genealogical concordance phylogenetic species recognition (GCPSR) approach, at least 33 phylogenetic species in Asia can be delimited, all of which are supported by morphological features, and five phylogenetic species remain to be described. The mountainous region of Southwest China is especially special, containing at least 21 species and likely represents a centre of diversification. We further compared our specimens with the type specimens of 25 species of Strobilomyces. Our comparisons suggest that, there are a total of 31 distinct species, while S. sanmingensis, S. verruculosus, S. subnigricans, and S. zangii/S. areolatus, are synonyms of S. mirandus, S. giganteus, S. alpinus and S. seminudus, respectively. Eight new species, namely, S. albidus, S. anthracinus, S. calidus, S. cingulatus, S. densisquamosus, S. douformis, S. microreticulatus and S. pinophilus, are described. A dichotomous key to the Asian Strobilomyces species is provided.
Keywords: Boletes, ectomycorrhizal fungi, infrageneric treatment, morphological characters, taxonomy
INTRODUCTION
Strobilomyces Berk. (1851) is one of the most conspicuous members of Boletaceae and can be easily recognized in the field. The genus is characterized by a blackish, black-brown or yellow-brown pileus covered with scales and a distinct reddening or blackening discolouration on exposure (Corner 1972, Singer 1986, Sato et al. 2011, Han et al. 2017, 2018). Strobilomyces has a broad geographical distribution and has been reported from all continents except South America (Sato et al. 2017, Han et al. 2018). All known species in this genus are known to form ectomycorrhizal associations with Casuarinaceae, Dipterocarpaceae, Fabaceae, Fagaceae, Myrtaceae and Pinaceae which are distributed from tropical to subtropical and temperate regions (Sato et al. 2017, Han et al. 2018). A previous study suggested that Strobilomyces likely originated in Africa during the early Eocene and subsequently dispersed to and speciated in other regions of the southern and northern hemisphere (Han et al. 2018).
Morphologically, besides the aforementioned macro-morphological characters, the genus Strobilomyces is characterized by subglobose to elliptic basidiospores with reticulate, semireticulate, flat-roofed conical or echinate ornamentation. Singer (1975) once broadened the circumscription of Strobilomyces and divided it into two sections: sections Strobilomyces and Pterospori. Section Pterospori incorporated those species whose spores are ornamented with a thickened rim around the apiculus and widely spaced longitudinal costae that sometimes possess intercostal ridging. A few years later, Pegler & Young (1981) erected a new genus, Afroboletus, to accommodate sect. Pterospori which was supported by recent molecular evidence (Nuhn et al. 2013, Wu et al. 2014, 2016, Sato et al. 2017, Han et al. 2017, 2018). The phylogenetic analyses further indicate that both Afroboletus and Strobilomyces are monophyletic and they are sister groups.
Taxonomically, a number of species have been published in Strobilomyces but clearly do not have the current diagnostic morphological features of Strobilomyces. Those species belong to genera such as Afroboletus, Austroboletus, Boletellus and Boletinus and have already been transferred to their corresponding modern genera of the family Boletaceae based on the evidences from both morphology and molecular phylogeny (Singer 1938, Wolfe 1980, Pegler & Young 1981, Singer et al. 1992). The current narrow circumscription of Strobilomyces has received broad recognition among mycologists (Nuhn et al. 2013, Wu et al. 2014, 2016, Sato et al. 2017, Han et al. 2017, 2018).
Partly due to their mycorrhizal associations with diverse host plants, the genus Strobilomyces contains a high species diversity, especially in Asia (Sato et al. 2017, Han et al. 2017, 2018). Up to now, a total of about 30 species of Strobilomyces have been described worldwide (Berkeley 1851, Cooke 1889, Beeli 1926, Singer 1945, Chiu 1948, Corner 1972, Horak 1980, 2011, Hongo 1982, Ying & Ma 1985, Zang 1985, Ying 1986, Wen & Ying 2001, Huang 2002, Ge & Yang 2005, Sato et al. 2005, 2011, Sato & Murakami 2009, Petersen et al. 2012, Gelardi et al. 2013, Antonín et al. 2015, Terashima et al. 2016, Tibpromma et al. 2017; see Table 1). However, despite over one and a half centuries of study, the taxonomy and systematics of this genus is still poorly resolved, especially in Asia. The majority of Strobilomyces species have only been described by their morphological characters. Furthermore, despite the large number of species described from Asia, few researchers have compared Asian materials from those outside of Asia.
Table 1.
List of reported Strobilomyces species worldwide.
| Reported Strobilomyces species | Type locality | References | Treatment in this study |
|---|---|---|---|
| S. alpinus M. Zang, Y. Xuan & K.K. Chen | China | Zang (1985) | – |
| S. annulatus Corner | Malaysia | Corner (1972) | – |
| S. areolatus H.A. Wen & J.Z. Ying | China | Wen & Ying (2001) | Synonym of S. seminudus |
| S. atrosquamosus J.Z. Ying & H.A. Wen | China | Wen & Ying (2001) | – |
| S. brunneolepidotus Har. Takah. & Taneyama | Japan | Terashima et al. (2016) | – |
| S. confusus Singer | USA | Singer (1945) | – |
| S. echinocephalus Gelardi & Vizzini | China | Gelardi et al. (2013) | – |
| S. echinatus Beeli | Congo | Beeli (1926) | – |
| S. foveatus Corner | Malaysia | Corner (1972) | – |
| S. giganteus M. Zang | China | Zang (1985) | – |
| S. glabellus J.Z. Ying | China | Ying & Ma (1985) | – |
| S. glabriceps W.F. Chiu | China | Chiu (1948) | – |
| S. hongoi Hirot. Sato | Japan | Sato et al. (2011) | – |
| S. latirimosus J.Z. Ying | China | Ying & Ma (1985) | – |
| S. longistipitatus D. Chakr., K. Das & S. Adhikari | India | Tibpromma et al. (2017) | – |
| S. mirandus Corner | Malaysia | Corner (1972) | – |
| S. mollis Corner | Malaysia | Corner (1972) | – |
| S. montosus Berk. | India | Berkeley (1851) | – |
| S. nigricans Berk. | India | Berkeley (1852) | – |
| S. parvirimosus J.Z. Ying | China | Ying (1986) | – |
| S. polypyramis Hook.f. | India | Berkeley (1851) | – |
| S. pteroreticulosporus Antonín & Vizzini | Korea | Antonín et al. (2015) | – |
| S. sanmingensis N.L. Huang | China | Huang (2002) | Synonym of S. mirandus |
| S. seminudus Hongo | Japan | Hongo (1982) | – |
| S. strobilaceus (Scop.) Berk. | Slovakia | Berkeley (1851), Petersen et al. (2012) | – |
| S. subnigricans J.Z. Ying | China | Ying (1986) | Synonym of S. alpinus |
| S. subnudus J.Z. Ying | China | Ying & Ma (1985) | – |
| S. velutinus J.Z. Ying | China | Ying & Ma (1985) | – |
| S. velutipes Cooke & Massee | Australia | Cooke (1889) | – |
| S. verruculosus Hirot. Sato | Japan | Sato & Murakami (2009) | Synonym of S. giganteus |
| S. zangii Gelardi | China | Gelardi (2013) | Synonym of S. seminudus |
One main reason for the taxonomic confusion in Strobilomyces is the limited microscopic morphological differences among species within this genus, including pileipellis, basidia and cheio-/pleurocystidia, etc. Indeed there is only one microscopically diagnosable character, viz. subglobose to elliptic basidiospores with different ornamentations including reticulate, semireticulate, flat-roofed conical or echinate ones. Therefore, the species delimitation of Strobilomyces has mainly depended on macro-morphological and ecological traits, i.e., the size and shape of the pileus, colour, size and morphology of the scales on the pileus and stipe, hymenophoral pore size of the tubes, colour changes of the exposed context, presence or absence of an annulus or an annular zone, association with host plants and geographical distributions (Corner 1972, Singer 1986, Sato et al. 2011, Han et al. 2018). However, the variation of morphological characters in this genus has not been comprehensively evaluated relative to molecular phylogenetic evidence.
Molecular techniques have revolutionized fungal phylogenetics and fungal species delimitation. In Strobilomyces, previous molecular phylogenetic studies suggested that not only some species were misidentified but the species diversity in Strobilomyces is also remarkably high (Sato et al. 2007, 2011, 2017, Sato & Murakami 2008, Han et al. 2018). Recently, Han et al. (2018) revealed 49 phylogenetic species in Strobilomyces worldwide, with 26 potential new species, and indicated that Asia could be the centre of species diversity of Strobilomyces. Among these, at least 20 described species and 13 new species were from East Asia (Han et al. 2018). The study also suggested that a multigene phylogenetic approach coupled with detailed morphological comparisons are needed in order to resolve the taxonomy and relationships among species in Strobilomyces in Asia.
In order to bring the diversity of Asian Strobilomyces into a sharper focus, this study has three specific goals:
to resolve the classification system in Strobilomyces;
to delimit the Strobilomyces species in Asia based on morphological observations, phylogenetic analyses and ecological data; and
to provide a dichotomous key to the Strobilomyces species of Asia.
MATERIALS AND METHODS
Sample collections
Samples were collected from the tropical, subtropical, and temperate regions of many parts of the world and deposited in the Cryptogamic Herbarium of Kunming Institute of Botany, Chinese Academy of Sciences (HKAS), the Mycological Herbarium of Microbiology Institute, Chinese Academy of Sciences (HMAS), the New York Botanical Garden (NY), Forest Research Institute Malaysia (FRIM), Makino Herbarium (MAK), Universität Wien (WU), the Farlow Herbarium (FH), National Herbarium of Victoria (MEL), and Muséum National d’Histoire Naturelle (PC). The colour codes in the descriptions refer to Kornerup & Wanscher (1981). Macroscopic characters were described from fresh basidiomes or dried specimens. Microscopic structures were observed under compound microscope with dried materials revived in 5 % KOH and dyed with Congo red when necessary. Methods for microscopic studies followed those in Cai et al. (2016) and Wu et al. (2014, 2016). A scanning electron microscope (SEM) was used for observing spore ornamentations following the methods in Wu et al. (2014, 2016). The descriptions of species are arranged in alphabetical order of the epithets. Exemplars for species used in this study along with corresponding vouchers, GenBank accession numbers, geographical locations and host plants are listed in Appendix S1 (Table S1.2) of Han et al. (2018).
Molecular phylogenetics
Protocols for genomic DNA extraction, PCR amplification, and sequencing followed those of Wu et al. (2014, 2016) and references therein. To estimate the species diversity of Strobilomyces in Asia, a four-locus (RPB1 and RPB2, the genes for partial polymerase II largest and the second largest subunits; TEF1, the gene for translation elongation factor subunit 1-α; and COX3, the gene for mitochondrial cytochrome oxidase subunit 3) phylogeny was constructed. Nucleotide sequences from each gene were aligned using MAFFT v. 7.245 (Katoh & Standley 2013) and then refined manually with Bioedit v. 7.2.5 (Hall 1999). The ambiguously aligned regions were arranged manually for the phylogenetic analyses. The resulting alignment containing all four loci (RPB1, RPB2, TEF1 and COX3) can be accessed at TreeBASE (Study Accession URL: http://purl.org/phylo/treebase/phylows/study/TB2:S22532).
Single-gene analyses were conducted to test for potential incongruencies among the four-gene fragments using maximum likelihood (ML) analyses and Bayesian inference (BI). The four-gene fragments were combined by Phyutility (Smith & Dunn 2008) for phylogenetic analysis, on the premise that no well-supported (BS > 70 %, Nuhn et al. 2013) conflict was detected. ML and BI analyses were conducted on RAxML v. 7.2.6 (Stamatakis 2006) and MrBayes v. 3.2 (Ronquist et al. 2012), respectively. The best-fitting evolutionary models were determined by MrModeltest v. 2.2 (Nylander 2004) via the Akaike information criterion (AIC). Under ML optimization, the GTR+I+G model was selected for RAxML searches, and the bootstrap values were calculated with 1 000 replicates. For BI analyses, four Markov Chain Monte Carlo (MCMC) chains were run simultaneously for 20 million generations with trees sampled every 100 generations. We considered the sampling of the posterior distribution to be adequate when the average standard deviation of split frequencies was < 0.01. Chain convergence was further assessed with Tracer v. 1.5 (Rambaut & Drummond 2009) to confirm sufficient effective sampling size (ESS > 200). We discarded the first 25 % of trees before a majority rule consensus tree was generated. All gene regions were analysed. For the best partition schemes and evolutionary models see Appendix S1 (Table S1.5–1.7) of Han et al. (2018).
Phylogenetic species delimitation
We delimited species boundaries using the Genealogical Concordance Phylogenetic Species Recognition (GCPSR) method (Taylor et al. 2000). Similar to the criteria proposed by Dettman et al. (2003), a clade was recognized as an independent evolutionary lineage if it was well supported by at least one single-locus genealogy, and was not contradicted by any other single-locus genealogy at the same level of support. Such clades were judged by both ML Bootstrap (MLB ≥ 70 %) and Bayesian posterior probabilities (BPP ≥ 0.95). When assigning independent evolutionary lineages to phylogenetic species, the combined four-locus analysis was also considered. For any divergent terminal branch represented by only one specimen, the branch was considered as a putative phylogenetic species if it also showed morphological difference(s) from its closely related sister groups.
Morphological studies
Based on a four-locus dataset (RPB1-RPB2-TEF1-COX3), a phylogeny with one representative collection from each species of Strobilomyces (exception: five collections from S. strobilaceus) is utilized for scoring and summarizing trends in morphological and ecological characters. The following three microscopic, eleven macroscopic characters and two ecological data were considered, viz. size, ornamentation and mesh size of the basidiospores; size and shape of the pileus; colour, size and morphology of the scales on the pileus and stipe; pore size of the tubes; colour changes of the exposed context; presence or absence of an annulus and an annular zone; association with host plants; and geographical distributions. These morphological characters for individual species were based on our own observations of mature fruiting bodies.
In accordance with Bas (1969), the size of basidiomes of Strobilomyces is coded as: 1) tiny (pileus diam < 30 mm); 2) small (pileus diam 30–60 mm); 3) medium-sized (pileus diam 60–100 mm); or 4) large (pileus diam > 100 mm). The shape of the pileus can be: 1) subconical; or 2) subhemispherical. The scale colour on the pileus showed five types: 1) black; 2) grey-black; 3) grey to dirty white; 4) black-brown; or 5) red-brown or golden-tawny. The scale size at its base in diam is treated using three states: 1) small (< 3 mm); 2) medium-sized (3–5 mm); or 3) large (> 5 mm). The scale morphology of the pileus when mature is coded as: 1) more or less erect conical or pyramidal scales; 2) fluffy floss (Th: thick floss, T: thin floss, S: floss arranged in spiral); 3) patch-like to appressed scales; or 4) granular scales. The pore size of the tubes is divided into two types: 1) small (S: 0.5–1 mm diam); or 2) large (L: 1–3 mm diam). The presence or absence of annulus has two states: 1) annulus present (Y); or 2) annulus absent (N). The presence or absence of annular zone has two types: 1) annular zone present (Y); or 2) annular zone absent (N). The context discolouration on exposure possess three types: 1) rusty red; 2) orange-red; or 3) grey-black. Species of Strobilomyces are ectomycorrhizal and their association with plants was coded as: 1) Fagaceae; 2) Pinaceae; 3) Fagaceae and Pinaceae; 4) Casuarinaceae, Myrtaceae and Dipterocarpaceae; or 5) Fabaceae. The geographical distribution can be: 1) tropical (Tr); 2) subtropical (St); 3) temperate (T); or 4) subalpine (Sa).
The characters of basidiospores in Strobilomyces, viz. the shape, size, ornamentation and mesh size, used in the previous studies, are more important than other microscopic features in delimitating species. For the description of the shape of basidiospores, the terminology follows Bas (1969). Basidiospore size in this study is divided into three states based on spore length: 1) small (< 9 μm); 2) medium-sized (9–12 μm); or 3) large (> 12 μm). The ornamentation of mature basidiospores is coded as four types: 1) reticulate; 2) semireticulate; 3) flat-roofed conical; or 4) echinate. Mesh size of mature reticulate basidiospores is here treated using two states: 1) small (1–2 μm diam); or 2) large (2–4 μm diam).
RESULTS
Phylogenetic analyses
A total of 587 sequences were obtained from GenBank, in which the aligned length of the concatenated four-gene fragments was 2 712 bp, including 1 004 parsimony informative sites. No obvious differences in topology were observed between the ML and Bayesian analyses (Fig. 1, Table S1). Four major clades (I, II, III and IV) in Strobilomyces were inferred based on a four-locus matrix (RPB1-RPB2-TEF1-COX3) (Fig. 1). According to the grouping and ranking criteria of GCPSR method, 26 phylogenetic species were recognized in Asia. All of those except for S. alpinus and S. densisquamosus, were strongly supported as monophyletic lineages by MLB (≥ 70 %) and BPP (≥ 0.95) in at least three of the single gene trees, and 14 were robustly supported by all four genealogies (Fig. 1, S2–S5). In the combined analyses of the four-locus matrix, all 26 species except for S. echinocephalus were supported as monophyletic by 100 % MLB and 1.0 BPP (Fig. 1, Table 2). As for the remaining seven lineages in Asia represented by single collections, they were recognized as potential phylogenetic species because they were genetically divergent and morphologically distinct from their sister groups. In total 33 phylogenetic species (26 from GCPSR method and seven belong to potential phylogenetic species) were delimitated from Asia, and most of them can be circumscribed by a certain group of morphological characters (Fig. 2). Although S. albidus consisted of two subgroups, no significant genetic divergence, morphological characters or geographical distribution patterns were found between them. Therefore, we treated the two subgroups as a single taxon. Strobilomyces anthracinus, S. brunneolepidotus, S. densisquamosus and S. subnudus showed similar phylogenetic structure, with one subgroup sistering to other collections. All the short branches displayed in the four lineages suggested that they represent a single species. Phylogenetic species outside Asia, were also marked for comparison by morphological characters (Fig. 2).
Fig. 1.
Phylogeny inferred from four-locus dataset (RPB1-RPB2-TEF1-COX3), with branch lengths based on the Maximum Likelihood analysis. Only MLB over 70 % and BPP over 0.95 are given in the tree. Bold black and red branches represented independent evolutionary lineages that were well supported by at least one locus and not contradicted by any other locus. Bold red branches showed phylogenetic species. Eight newly described species are indicated in black bold letters. The Roman numerals (I, II, III and IV) indicated the four major clades of Strobilomyces. The two sections are displayed on the right of the phylogram.
Table 2.
Criteria for phylogenetic species of Strobilomyces in analyses of support values in individual gene partitions and the combined four-locus dataset.
| Independent evolutionary lineage | rpb1 | rpb2 | tef1α | cox3 | Combined four-locus dataset II | 1Phylogenetic species |
|---|---|---|---|---|---|---|
| S. albidus (HKAS89104, HKAS74924, MAKs305, HKAS84695, NY1393538, HKAS52621) | 100/1.00 | 92/1.00 | 99/1.00 | –/0.96 | 100/1.00 | S. albidus (HKAS89104, HKAS74924, MAKs305, HKAS84695, NY1393538, HKAS52621) |
| S. albidus (HKAS89104, HKAS74924, MAKs305) | 98/1.00 | 81/1.00 | 100/0.95 | –/– | 100/1.00 | |
| S. albidus (HKAS84695, NY1393538, HKAS52621) | 100/1.00 | –/– | 98/1.00 | –/– | 100/1.00 | |
| S. albidus (HKAS84695, NY1393538) | 100/1.00 | –/– | –/– | –/– | 100/1.00 | |
| S. alpinus | 100/1.00 | –/– | 94/0.95 | –/– | 100/1.00 | S. alpinus |
| S. annulatus | 100/1.00 | 100/1.00 | 100/1.00 | #/# | 100/1.00 | S. annulatus |
| S. anthracinus (HKAS83740, HKAS74532, HKAS52570) | 100/1.00 | 98/1.00 | 99/1.00 | 91/0.96 | 100/1.00 | S. anthracinus (HKAS83740, HKAS74532, HKAS52570) |
| S. anthracinus (HKAS83740, HKAS74532) | 77/1.00 | 79/– | 100/– | –/– | 74/1.00 | |
| S. atrosquamosus | 100/1.00 | 99/1.00 | 85/1.00 | –/– | 100/1.00 | S. atrosquamosus |
| S. brunneolepidotus (MAKs309, HKAS81935, HKAS80689, HKAS84683) | 100/1.00 | 100/1.00 | 100/1.00 | 99/1.00 | 100/1.00 | S. brunneolepidotus (MAKs309, HKAS81935, HKAS80689, HKAS84683) |
| S. brunneolepidotus (HKAS80689, HKAS84683) | –/– | 87/0.99 | 86/0.99 | –/– | 100/1.00 | |
| S. calidus | 100/1.00 | 100/1.00 | 100/1.00 | #/# | 100/1.00 | S. calidus |
| S. cingulatus | 100/1.00 | –/– | 100/1.00 | 77/1.00 | 100/1.00 | S. cingulatus |
| S. confusus | 87/1.00 | 70/– | 100/1.00 | #/# | 100/1.00 | S. confusus |
| S. densisquamosus (HKAS82354, HKAS84945, HKAS83112, HKAS83112, HKAS61701, HKAS74932, HKAS91250) | 99/1.00 | 70/0.95 | –/– | –/– | 100/1.00 | S. densisquamosus (HKAS82354, HKAS84945, HKAS61701, HKAS74932, HKAS91250) |
| S. densisquamosus (HKAS82354, HKAS84945, HKAS83112) | –/– | –/– | 71/1.00 | 90/1.00 | 100/1.00 | |
| S. douformis | 100/1.00 | 100/1.00 | 99/1.00 | 98/1.00 | 100/1.00 | S. douformis |
| S. echinatus | 100/1.00 | 100/1.00 | 100/1.00 | #/# | 100/1.00 | S. echinatus |
| S. echinocephalus | 94/1.00 | 94/– | 100/1.00 | 89/0.99 | 80/1.00 | S. echinocephalus |
| S. foveatus | 100/1.00 | 100/1.00 | 100/1.00 | #/# | 100/1.00 | S. foveatus |
| S. giganteus | 100/1.00 | 100/1.00 | 100/1.00 | 99/1.00 | 100/1.00 | S. giganteus |
| S. glabriceps | 98/1.00 | 87/0.98 | 99/1.00 | –/– | 100/1.00 | S. glabriceps |
| S. hongoi | 100/1.00 | 100/1.00 | #/# | 93/1.00 | 100/1.00 | S. hongoi |
| S. latirimosus | 100/1.00 | 100/1.00 | 100/1.00 | 100/1.00 | 100/1.00 | S. latirimosus |
| S. microreticulatus | 100/1.00 | 97/1.00 | 100/1.00 | 98/– | 100/1.00 | S. microreticulatus |
| S. mirandus | 100/1.00 | 100/1.00 | 100/1.00 | 86/0.99 | 100/1.00 | S. mirandus |
| S. montosus | 100/1.00 | 100/1.00 | 100/1.00 | 90/– | 100/1.00 | S. montosus |
| S. parvirimosus | 100/1.00 | 100/1.00 | 100/1.00 | –/– | 100/1.00 | S. parvirimosus |
| S. pinophilus | 100/1.00 | 100/1.00 | 100/1.00 | 100/1.00 | 100/1.00 | S. pinophilus |
| S. pteroreticulosporus | 100/1.00 | 85/1.00 | 83/– | 74/0.95 | 100/1.00 | S. pteroreticulosporus |
| S. seminudus | 100/1.00 | 100/1.00 | 100/1.00 | –/– | 100/1.00 | S. seminudus |
| S. strobilaceus | 99/1.00 | 94/1.00 | –/1.00 | 75/1.00 | 100/1.00 | S. strobilaceus |
| S. subnudus (HKAS59435, HKAS83404, HKAS84811, HKAS82418) (HKAS59435, HKAS83404, HKAS84811, HKAS82418) | 100/1.00 | 86/– | 100/1.00 | 94/1.00 | 100/1.00 | S. subnudus |
| S. subnudus (HKAS59435, HKAS83404, HKAS84811) | –/– | 93/1.00 | –/– | –/– | 100/0.97 | |
| S. velutinus | 100/1.00 | 87/– | 100/1.00 | –/– | 100/1.00 | S. velutinus |
| S. velutipes | 100/1.00 | 100/0.98 | 89/0.98 | 91/0.98 | 100/1.00 | S. velutipes |
| S. sp.4 | 100/1.00 | 99/1.00 | 100/1.00 | 83/0.99 | 100/1.00 | S. sp.4 |
| S. sp.11 | 100/1.00 | 100/1.00 | 100/1.00 | 100/1.00 | 100/1.00 | S. sp.11 |
| S. sp.16 | 100/1.00 | 100/1.00 | 100/1.00 | 100/1.00 | 100/1.00 | S. sp.16 |
| S. sp.18 (NY1034410, NY1034411, NY1034409) | 95/1.00 | 96/1.00 | 100/1.00 | 100/1.00 | 100/1.00 | S. sp.18 (NY1034410, NY1034411, NY1034409) |
| S. sp.18 (NY1034410, NY1034411) | 94/0.99 | –/– | –/– | –/– | 100/1.00 |
Support values are shown as MLB/BPP. – and # represent phylogenetic species with low support (MLB < 70 %, BPP < 0.95) and lacking counterpart sequences, respectively.
1Support values are not acquired for the following 16 potential phylogenetic species represented by single collection, which are therefore not included in the table: S. glabellus, S. mollis, S. sp. 1–3, S. sp. 5–10, S. sp. 12–15, S. sp. 17, because every potential phylogenetic species was genetically divergent from its sister or in a relatively isolated phylogenetic position.
Fig. 2.
Selected morphological character states and ecological features in Strobilomyces were mapped on the consensus tree from four-locus dataset (RPB1-RPB2-TEF1-COX3), mostly including one representative collection for each species. The BPP values over 0.95 from the Bayesian analysis were shown above the branch. Two sections of Strobilomyces were enclosed in grey and pink frames. Four clades (Clades I–IV) were labelled for discussion. Traits and states were given under the consensus tree. Uncertain state for a taxon was given as ‘–’, not applicable was given as ‘/’.
Morphological observations
Type materials of 25 species (Table 1) were re-examined in our study. Specimens of Strobilomyces from Africa, Asia, Australia, North/Central America, and Europe were studied and compared with these type specimens. Finally, 31 species (including 23 known and eight novel species described here) from Asia were recognized and elucidated mainly based on the characters (size, ornamentation and mesh size) of basidiospores, size and shape of pileus, colour, size and morphology of the scales on the pileus and stipe, pore size of tubes, colour changes of the exposed context, presence or absence of an annulus or an annular zone and ecological parameters (Fig. 2). The remaining five Asian phylogenetic species will be circumscribed when additional collections are available.
TAXONOMY
Infrageneric classification system
Two sections in Strobilomyces are inferred based on molecular data, scoring of morphological and ecological traits (Fig. 1, 2). The shape of the pileus, ornamentation and shape of the basidiospores, host plant associates and the geographical distribution patterns appear as constant characters support the following proposal of two sections.
Strobilomyces sect. Echinati L.H. Han, Zhu L. Yang & Ndolo Ebika, sect. nov. — MycoBank MB824861; Fig. 1, 2
Etymology. From Latin ‘Echinati’, referring to the echinate ornamentation of basidiospores.
Basidiomes stipitate-pileate, fleshy. Pileus conical to subconical, dry, covered with small, more or less erect conical to pyramidal scales; margin appendiculate. Context dirty white to grey-white, becoming rusty red then grey-black to black on exposure. Hymenophore tubular, whitish to grey, becoming black-brown or smoky black when mature; pores angular. Stipe subcylindrical, covered with granular or warty scales; annulus or annular zone at stipe absent. Basidiospores broadly ellipsoid to ellipsoid, surface ornamented with echinate warts. Cheilocystidia and pleurocystidia present. Hymenophoral trama boletoid. Surface of pileus and scales composed of filamentose hyphae.
Type of section. Strobilomyces echinatus Beeli.
Species in sect. Echinati. Strobilomyces echinatus Beeli.
The only species in this section putatively forms an ectomycorrhizal relationship with plants of Gilbertiodendron (Fabaceae). Only known from tropical Africa (Beeli 1926).
Strobilomyces sect. Strobilomyces
Basidiomes stipitate-pileate, fleshy. Pileus hemispherical to applanate, dry, covered with small to large, more or less erect conical to pyramidal scales, or patch-like to appressed scales or floss; margin appendiculate. Context dirty white to grey-white, becoming red then black or directly grey-black on exposure. Hymenophore tubular, whitish to grey, becoming black-brown or smoky black when mature; pores angular. Stipe subcylindrical, covered with thin to thick fluffy floss or granular scales; sometimes with an annulus or an annular zone at the apex. Basidiospores subglobose, broadly ellipsoid, ellipsoid to long ellipsoid, with reticulate, semireticulate or flat-roofed conical ornamentation. Cheilocystidia and pleurocystidia common. Hymenophoral trama boletoid. Surface of pileus and scales composed of filamentose hyphae.
Type of section. Strobilomyces strobilaceus (Scop.) Berk.
Species of sect. Strobilomyces usually form ectomycorrhizal relationships with plants of Dipterocarpaceae, Myrtaceae, Casuarinaceae, Fagaceae or Pinaceae. Mainly in Asia, Australasia, Europe and North/Central America.
Species of Strobilomyces sect. Strobilomyces
1. Strobilomyces albidus L.H. Han, J. Xu & Zhu L. Yang (see below)
2. Strobilomyces alpinus M. Zang, Y. Xuan & K.K. Chen (synonym: S. subnigricans J.Z. Ying)
3. Strobilomyces annulatus Corner
4. Strobilomyces anthracinus L.H. Han, J. Xu & Zhu L. Yang (see below)
5. Strobilomyces atrosquamosus J.Z. Ying & H.A. Wen
6. Strobilomyces brunneolepidotus Har. Takah. & Taneyama
7. Strobilomyces calidus L.H. Han, J. Xu & Zhu L. Yang (see below)
8. Strobilomyces cingulatus L.H. Han & Zhu L. Yang (see below)
9. Strobilomyces confusus Singer
10. Strobilomyces densisquamosus L.H. Han & Zhu L. Yang (see below)
11. Strobilomyces douformis L.H. Han & Zhu L. Yang (see below)
12. Strobilomyces echinocephalus Gelardi & Vizzini
13. Strobilomyces foveatus Corner
14. Strobilomyces giganteus M. Zang (synonym: S. verruculosus Hirot. Sato)
15. Strobilomyces glabellus J.Z. Ying
16. Strobilomyces glabriceps W.F. Chiu
17. Strobilomyces hongoi Hirot. Sato
18. Strobilomyces latirimosus J.Z. Ying
19. Strobilomyces longistipitatus D. Chakr., K. Das & S. Adhikari
20. Strobilomyces microreticulatus L.H. Han & Zhu L. Yang (see below)
21. Strobilomyces mirandus Corner (synonym: S. sanmingensis N.L. Huang)
22. Strobilomyces mollis Corner
23. Strobilomyces montosus Berk.
24. Strobilomyces nigricans Berk.
25. Strobilomyces parvirimosus J.Z. Ying
26. Strobilomyces pinophilus L.H. Han & Zhu L. Yang (see below)
27. Strobilomyces polypyramis Hook.f.
28. Strobilomyces pteroreticulosporus Antonín & Vizzini
29. Strobilomyces seminudus Hongo (synonyms: S. areolatus H.A. Wen & J.Z. Ying, S. zangii Gelardi)
30. Strobilomyces strobilaceus (Scop.) Berk.
31. Strobilomyces subnudus J.Z. Ying
32. Strobilomyces velutinus J.Z. Ying
33. Strobilomyces velutipes Cooke & Massee
The species of Strobilomyces recorded in Asia
Strobilomyces albidus L.H. Han, J. Xu & Zhu L. Yang, sp. nov. — MycoBank MB824853; Fig. 2, 3a1–a2, 4a, 5
Fig. 3.

Representative basidiomes of Strobilomyces species with reticulate basidiospores. — a1. S. albidus (HKAS 89104, holotype); a2. S. albidus (HKAS 74924); b. S. alpinus (HKAS 94051); c1–c2. S. anthracinus (HKAS 83740, holotype); d1–d2. S. atrosquamosus (HKAS 84736); e1–e2. S. brunneolepidotus (HKAS 80689); f1. S. cingulatus (HKAS 73175, holotype); f2. S. cingulatus (HKAS 91330); g1–g2. S. douformis (HKAS 87097, holotype); h1–h2. S. echinocephalus (HKAS 77546); i: S. glabellus (HKAS 74887); j1–j2. S. glabriceps (HKAS 74762); j3. S. glabriceps (HKAS 78573); k1–k2. S. latirimosus (HKAS 84793); l. S. microreticulatus (HKAS 74863, holotype); m. S. mirandus (HKAS 87083); n1–n2. S. montosus (HKAS 74809); o. S. parvirimosus (HKAS 74547); p. S. pinophilus (HKAS 80300, holotype); q1–q3. S. pteroreticulosporus (HKAS 81881); r1–r2. S. strobilaceus (HKAS 95079); s1–s2. S. sp.11 (NY 2072541). — Scale bars = 20 mm.
Fig. 4.

Reticulate basidiospores of Strobilomyces species under scanning electron microscope (SEM). — a. S. albidus (HKAS 89104, holotype); b1. S. alpinus (HMAS 47645, holotype); b2. S. alpinus (HMAS 47645, holotype of S. subnigricans); c. S. anthracinus (HKAS 83740, holotype); d1. S. atrosquamosus (HMAS 72079, holotype); d2. S. atrosquamosus (HKAS 84736); e. S. brunneolepidotus (HKAS 80689); f. S. cingulatus (HKAS 73175, holotype); g. S. douformis (HKAS 87097, holotype); h. S. echinocephalus (HKAS 75765, isotype); i. S. glabellus (HMAS 26736, holotype); j1–j2. S. glabriceps (HKAS 74762); k1. S. latirimosus (HMAS 43748, holotype); k2. S. latirimosus (HKAS 84793); l. S. microreticulatus (HKAS 74863, holotype); m. S. mirandus (HKAS 87083); n. S. mollis (HKAS 59833); o. S. montosus (HKAS 74809); p1. S. parvirimosus (HMAS 27590, holotype); p2. S. parvirimosus (HKAS 74547); q. S. pinophilus (HKAS 80300, holotype); r. S. pteroreticulosporus (HKAS 81881); s1. S. strobilaceus (HKAS 95079); s2. S. strobilaceus (WU 16537); s3. S. strobilaceus (WU 10210); t1–t2. S. sp.11 (NY 2072541).
Fig. 5.
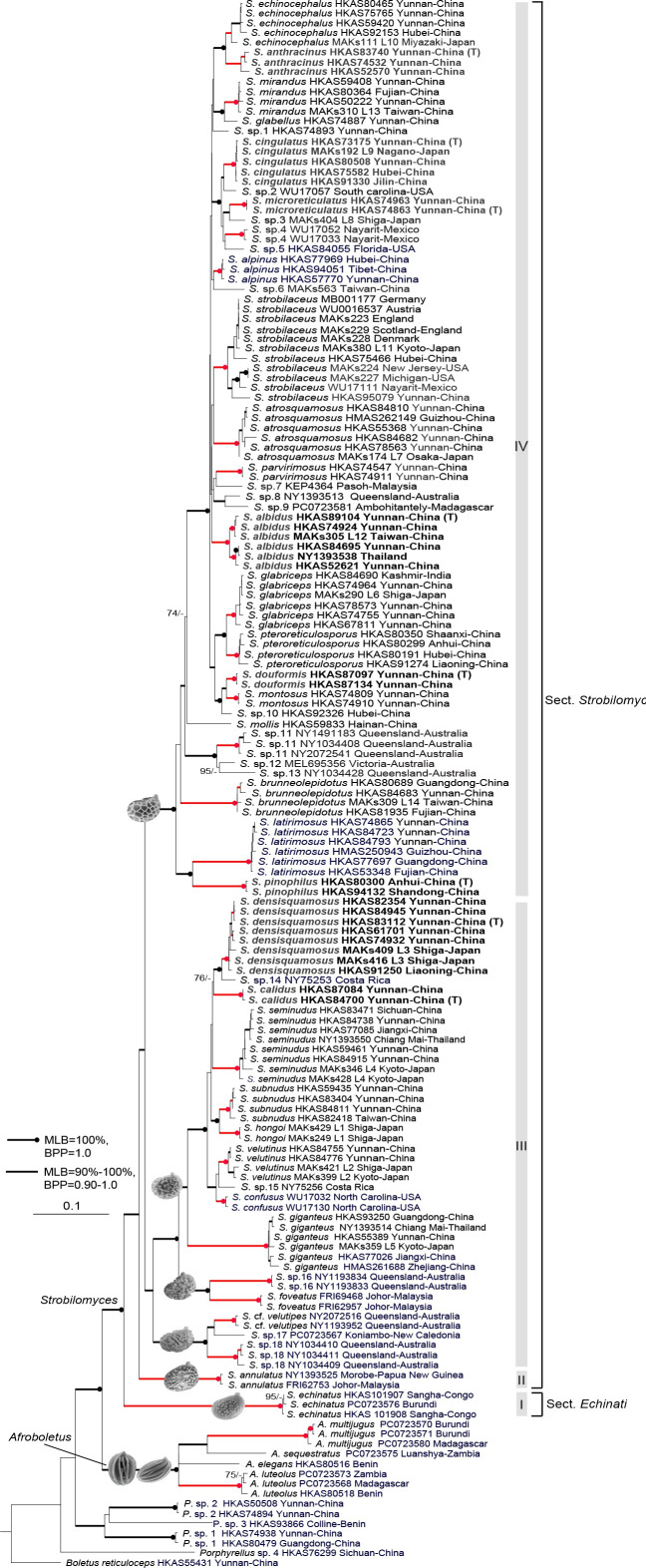
Strobilomyces albidus (HKAS 89104, holotype). a. Basidia and pleurocystidia; b. cheilocystidia; c. pileipellis. — Scale bars = 10 μm.
Etymology. From Latin ‘albidus’ = off-white, referring to the dirty white scales on the stipe.
Holotype. China, Yunnan Province, Puer City (previously called Simao), Laiyang River Nature Reserve, 1400 m elev., 28 June 2014, K. Zhao 441 (HKAS 89104).
Basidiomes (Fig. 3a1–a2) small to medium-sized. Pileus 50–70 mm diam, hemispherical or applanate, dry, covered with black-brown (5D8) to dark chocolate (6E7), patch-like to appressed scales or floss, 2–4 mm high, 3–5 mm diam at base, ground whitish (2A1); margin more or less appendiculate with triangular veil remnants concolorous with pileal ground; context white (8A1), staining rusty red (9C4) then black (10E1) on exposure. Tubes emarginate with decurrent tooth, white (6A1) then smoky grey (8C1); hymenophoral pores angular, small, 0.5–1 mm diam; pores and tubes concolorous, whitish (14A1) then smoky grey (4D1), immediately staining rudty red (6D8) then black (17F8) on exposure. Stipe 50–112 mm long, 4–13 mm diam, subcylindrical, curved; surface reticulated by extended tubes at top, entirely covered with whitish (1B2) thin fluffy floss; context white (8A1), discolouration similar to that of tubes; annulus or annular zone absent; basal mycelium dirty white (1B1) to grey-white (6B1).
Basidiospores (Fig. 4a) [180/9/5] (6.5–)7–9 × 6–7(–8) μm (Q = 1.1–1.29(–1.38), Qm = 1.18 ± 0.04) excluding ornamentation, subglobose to broad ellipsoid, dark brown (7D5), completely reticulate with meshes 2–3 μm diam and 1–2 μm high; apiculus 0.5 μm long. Basidia (Fig. 5a) 37–45 × 13–17 μm, narrowly clavate to clavate, 4-spored; sterigmata 3–6 μm long. Hymenophoral trama boletoid; hyphae cylindrical, 7–10 μm diam. Cheilocystidia (Fig. 5b) 48–76 × 15–20 μm, subfusiform to conical or sublageniform, hyaline or with yellow-brown (5B7) plasmatic pigment, thin-walled. Pleurocystidia (Fig. 5a) 44–63 × 9–14 μm, numerous, narrowly lageniform to subfusiform with long beak, thin-walled. Pileipellis (Fig. 5c) an intricate trichodermium, composed of 6–16 μm wide cylindric to submoniliform hyphae; hyphae roughly intertwined into clusters, with short obtuse terminal cells; cell wall dark brown (5E8), slightly thickened (< 1 μm). Pileal trama composed of 5–10 μm wide interwoven hyphae. Hyphae of scales on stipe similar to those on pileus. Stipe trama composed of 3–12 μm wide cylindrical hyphae. Clamp connections absent.
Habitat & Distribution — Solitary or scattered on soil in forests dominated by Lithocarpus spp. and Castanopsis spp.; currently recorded from tropical China and Thailand.
Additional specimens examined. China, Yunnan Province, Jinghong City, Dadugang town, 1300 m elev., 22 July 2007, Y.C. Li 934 (HKAS 52621); same location, 10 July 2014, L.H. Han 426 (HKAS 84722); Baoshan City, Tengchong County, longteng secondary road X193-52, 1650 m elev., 4 Aug. 2013, G. Wu 610 (HKAS 74924); Xishuangbanna City, Mengla County, Xishuangbanna Nature Reserve, 1300 m elev., 8 July 2014, L.H. Han 399 (HKAS 84695); Taiwan Province, Nantoh, H. Sato (MAK s305). – Thailand, Chiang Mai Province, Highway 1095, 1150 m elev., 10 June 2006, R.E. Halling et al. 8800 (NY1393538).
Notes — Strobilomyces albidus is characterized by its small to medium-sized basidiomes (50–70 mm diam), pileus with black-brown to dark chocolate, medium-sized, patch-like to appressed scales or floss (2–4 mm high, 3–5 mm diam at base), stipe entirely with whitish thin fluffy floss, small hymenophoral pores (0.5–1 mm diam), small subglobose to broad ellipsoid basidiospores (7–9 × 6–7 μm) with large meshes (2–3 μm diam × 1–2 μm in height), rusty red discolouration of the context on exposure (Fig. 3a1–a2, 4a) and tropical distribution in Asia. This species occupies a relatively isolated position in the phylogenetic tree (Fig. 1). It resembles S. echinocephalus by its pileus with black-brown patch-like to appressed scales or floss. However, S. echinocephalus has slightly larger basidiospores (8.5–10 × 6.5–8 μm), stipe entirely covered with black-brown to black thick floss, and context changing directly to grey-black on exposure (Gelardi et al. 2013; Fig. 3h1–h2, 4h). Furthermore, the known distribution range of S. echinocephalus is restricted to subtropical regions of East Asia (Gelardi et al. 2013, Han et al. 2018, this study).
Strobilomyces alpinus M. Zang, Y. Xuan & K.K. Chen, Acta Bot. Yunnan. 7: 386. 1985 — MycoBank MB104808; Fig. 2, 3b, 4b1–b2
= Strobilomyces subnigricans J.Z. Ying, Acta Mycol. Sin., Suppl. 1: 306. 1987.
Habitat & Distribution — Solitary or scattered on soil or on trunk of trees in forests dominated by Abies species; currently recorded from subalpine regions of southwestern and central China.
Specimens examined. China, Yunnan Province, Diqing City, Xianggelila County, 3900 m elev., 24 Aug. 1983, K.K. Chen & Y. Xuan 24 (HKAS 14247, holotype of S. alpinus); Lijiang City, Yulong County, Laojun Mountain, 3800 m elev., 3 Sept. 2009, G. Wu 238 (HKAS 57770); Hubei Province, Yichang City, Xingshan County, Shennongjia National Nature Reserve, Houzishi reserve station, 2800 m elev., 29 July 1984, Y.B. Peng 152 (HMAS 47645, holotype of S. subnigricans); Hubei Province, Yichang City, Xingshan County, Shennongding Nature Reserve, 2500 m elev., 17 July 2012, J. Qin 568 (HKAS 77969); Tibet, Linzhi County, Bujiu Village, 3600 m elev., 31 June 2014, B. Feng 1667 (HKAS 94051).
Notes — Strobilomyces alpinus is characterized by its medium-sized to large basidiomes (60–120 mm diam), black-brown to black-purple pileus with more or less erect pyramidal to appressed scales densely arranged, concolorous stipe with thick fluffy floss, large hymenophoral pores (1–2 mm diam), rusty red discolouration of the context on exposure, large reticulate basidiospores (holotype of S. alpinus: 11.5–14 × 9.5–11 μm, Q = 1.2–1.35, Qm = 1.27 ± 0.09; Fig. 4b1) with large meshes (2–4 μm diam) and selective association with Abies spp. (Zang 1985; Fig. 3b). The macro-morphological characters and host plants of some samples observed by the authors are consistent with those of S. alpinus reported by Zang (1985) from the subalpine regions of southwestern China, and recall those of S. subnigricans, which was described from the subalpine regions of central China (Ying 1986).
The basidiospore ornamentation of S. alpinus was originally described as spiny or verrucose (Zang 1985). However, based on comparative studies of the type material of S. alpinus and S. subnigricans, we find that both possess large and broadly ellipsoid to ellipsoid basidiospores ornamented with complete reticulations and large meshes (2–4 μm diam) (holotype of S. alpinus: 11.5–14 × 9.5–11 μm, Q = 1.2–1.35, Qm = 1.27 ± 0.09, Fig. 4b1; holotype of S. subnigricans: 11–14 × 9–11 μm, Q = 1.16–1.33, Qm = 1.24 ± 0.08, Fig. 4b2). In addition, our phylogenetic analyses indicated that morphologically similar samples collected from the type localities of S. alpinus and S. subnigricans are clustered together with strong support (Fig. 1). Thus, S. subnigricans is treated as a synonym of S. alpinus. Strobilomyces alpinus seems to be a distinct species in the genus, because of its exclusively subalpine distribution with a high host preference for Abies spp., and largest basidiospores compared to all other Strobilomyces species. Phylogenetically, none closely allied species with S. alpinus is recognized (Fig. 1).
Strobilomyces longistipitatus, recently described from northern India (Tibpromma et al. 2017) was collected under Abies densa. It is characterized by a long stipe (3–4 times longer, or more, than pileus diameter), almost blackish appressed squamules densely arranged and large basidiospores (10–13 × 8–10 μm) with a complete reticulum. The morphological and ecological features of S. longistipitatus are consistent with those of S. alpinus. There is no variation in LSU nucleotide sequence between S. longistipitatus and S. alpinus. However, ITS nucleotide sequence comparison reveals 23 base pairs differences between them. Thus, it remains open whether or not the two taxa are conspecific. Accordingly, sequences with higher resolution (e.g., RPB1, RPB2 and TEF1) referring to the holotype and/or additional collections of S. longistipitatus are required.
Strobilomyces nigricans (Berkeley 1852), originally described from northern India, morphologically and ecologically resembles S. alpinus and S. longistipitatus. Both Horak (1980) and Pegler & Young (1981) examined the holotype of S. nigricans, and obtained the identical result concerning the size of the basidiospores of S. nigricans (9.5–12 × 7.5–9.5 μm, Q = 1.1), which is significantly smaller than those reported for S. alpinus and S. longistipitatus. Horak (1980) described the basidiospore ornamentation as an irregular, crest-like and often disconnected net, while Pegler & Young (1981) regarded it as a complete reticulum. Owing to the poor condition of the holotype specimen of S. nigricans, further study of additional materials from the type location is needed.
Strobilomyces annulatus Corner, Boletus in Malaysia: 58. 1972 — MycoBank MB324272; Fig. 2, 6a
Fig. 6.

Non-reticulate basidiospores of Strobilomyces species under scanning electron microscopy (SEM). — a. S. annulatus (NY 1393525); b. S. calidus (HKAS 84700); c1. S. confusus (F 2782, holotype); c2. S. confusus (F 2531, co-type); d. S. densisquamosus (HKAS 83112, holotype); e1. S. echinatus (NEST 1818); e2. S. echinatus NEST 1818); f1–f2. S. foveatus (FRI62957); g1. S. giganteus (HKAS 11755, holotype); g2. S. giganteus (MAK s693, holotype of S. verruculosus); g3. S. giganteus (HKAS 93250); h. S. hongoi (MAK s429); i1. S. seminudus (HMAS 72949, holotype of S. areolatus); i2. S. seminudus (HKAS 3224, holotype of S. zangii); i3. S. seminudus (HKAS 80459); j. S. subnudus (HMAS 32706, holotype); k. S. velutinus (HMAS 45911, holotype); l1–l2. S. cf. velutipes (NY 2072516).
Habitat & Distribution — Solitary or scattered on soil in tropical forests dominated by Dipterocarpaceae; currently recorded from Malaysia and Papua New Guinea.
Specimens examined. Malaysia, Johor, Gunong Panti, Sungei Dohol, 12 July 1931, Corner (E 83831, holotype); Sabah, Mt Kinabalu, Mesilau, 1700 m elev., 7 Mar. 1964, RSNB 5654 (E); Pahang, Tasik Cini (KEP FRI 62579); Johor, Endau-Rompin, Pulau Bertam (KEP FRI 62753); same location as above (KEP FRI 62275); same location as above (KEP FRI 6267). – Papua New Guinea, Lae, 13 Feb. 1992, R.E. Halling 6786 (NY 1393525).
Notes — Strobilomyces annulatus is one of the largest species of the genus found in Asia. We re-examined the holotype of S. annulatus and it can be recognized by the ample ring on the stipe with fuscous vinaceous purplish thick pulverulent floss and squamules, black-brown to vinaceous pileus with small floccose-pulverulent erect conical scales (2–4 mm high, 2–3 mm diam at the base) which are easily brushed off, large hymenophoral pores (1–2 mm diam), context reddening on exposure and the medium-sized echinate-subreticulate basidiospores (holotype of S. annulatus: 9.5–11.5 × 7–10 μm, Q = 1.19–1.32, Qm = 1.3 ± 0.04; Fig. 6a) (Corner 1972, Horak 2011, this study). Strobilomyces confusus, S. cingulatus, S. glabriceps, S. microreticulatus, S. pinophilus, S. pteroreticulosporus and S. strobilaceus, share the character of annulus. However, the latter six species are entirely different from S. annulatus in their reticulate basidiospores; S. confusus possesses smaller basidiospores (8.5–10 × 7–8 μm) than S. annulatus and more or less erect pyramidal scales on the pileus without vinaceous tint (Berkeley 1851, Singer 1945, Chiu 1948, Corner 1972, Pegler & Young 1981, Horak 2011, Petersen et al. 2012, Antonín et al. 2015, this study).
Strobilomyces anthracinus L.H. Han, J. Xu & Zhu L. Yang, sp. nov. — MycoBank MB824854; Fig. 2, 3c1–c2, 4c, 7
Fig. 7.
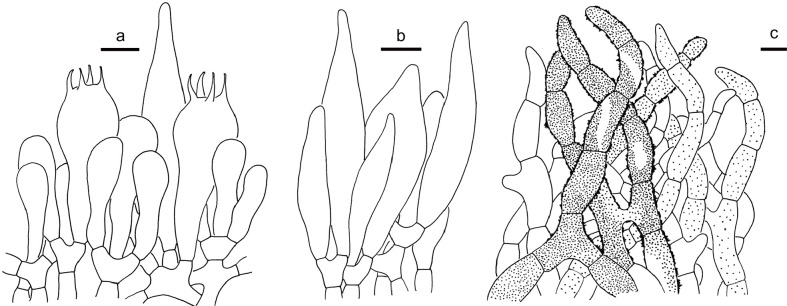
Strobilomyces anthracinus (HKAS 83740, holotype). a. Basidia and pleurocystidia; b. cheilocystidia; c. pileipellis. — Scale bars = 10 μm.
Etymology. From Latin ‘anthracinus’ = charcoal black, referring to the colour of the scales on pileus and stipe.
Holotype. China, Yunnan Province, Dali City, Nanjian County, Ailao Mountain Nature Reserve, 2580 m elev., 7 Aug. 2014, Q. Cai 1271 (HKAS 83740).
Basidiomes (Fig. 3c1–c2) tiny to small. Pileus 25–50 mm diam, at first subhemispherical or convex, then applanate, dry, covered with charcoal black (17F2–8) to black (17F1), more or less erect pyramidal scales, 3–5 mm high, 3–5 mm diam at base, exposing dirty white (1B1) subpellis when breaking up; margin partially appendiculate with triangular fragments of thick floccose veil remnants concolorous with pileal surface; context white (8A1), quickly changing to rusty red (9C4) then black (10E1) on exposure. Tubes adnexed to narrowly adnate, white (6A1) then smoky grey (8C1); hymenophoral pores angular, small, 0.5–1 mm diam; pores and tubes concolorous, whitish (14A1) then fuscous (12D3), immediately staining rusty red (11D4) then black (17F8) on exposure. Stipe 50–80 mm long, 4–10 mm diam, subcylindrical or slightly tapering downwards; surface poroid reticulate with elongate meshes at apex, covered with thin fluffy floss, evenly distributed and projecting 1–2 mm from surface of stem, concolorous with pileus; context white (8A1), discolouration similar to that of tubes; annulus or annular zone absent; basal mycelium grey-white (6B1).
Basidiospores (Fig. 4c) [60/3/3] (7.5–)8.5–10 × 6.5–8(–9) μm (Q = 1.2–1.41(–1.5), Qm = 1.32 ± 0.05) excluding ornamentation, broad ellipsoid to ellipsoid, dark brown (7D5), completely reticulate with meshes 2–3.5 μm diam and 1–1.5 μm high; apiculus 0.5 μm long. Basidia (Fig. 7a) 35–45 × 10–16 μm, narrowly clavate to clavate, 4-spored; sterigmata 4–6 μm long. Hymenophoral trama boletoid; hyphae cylindrical, 6–11 μm wide. Cheilocystidia (Fig. 7b) 48–70 × 14–25 μm, abundant, narrowly lageniform to narrowly conical, thin-walled, hyaline or with dark brown plasmatic pigment. Pleurocystidia (Fig. 7a) 41–65 × 13–18 μm, numerous, narrowly lageniform to conical, thin-walled. Pileipellis (Fig. 7c) an intricate trichodermium, composed of 4–16 μm wide cylindric to submoniliform hyphae; loosely interwoven in clusters, with short attenuate terminal cells; cell wall dark brown (5E8), more or less thickened (< 1 μm). Pileal trama composed of 4–12 μm wide interwoven hyphae. Hyphae of scales on stipe similar to those of pileus. Stipe trama composed of 3–10 μm wide cylindrical hyphae. Clamp connections absent.
Habitat & Distribution — Solitary or scattered on soil in forests dominated by Lithocarpus spp.; currently recorded from subtropical China.
Additional specimens examined. China, Yunnan Province, Puer City, Jingdong County, the Ailaoshan Station for Subtropical Forest Ecosystem Studies in Yunnan, 1450 m elev., 18 July 2007, Y.C. Li 885 (HKAS 52570); Yunnan Province, Baoshan City, Tengchong County, Gaoligongshan, 2100 m elev., 9 Aug. 2011, B. Feng 1052 (HKAS 74532).
Notes — Strobilomyces anthracinus is characterized by its tiny to small basidiomes (25–50 mm diam), charcoal black scales on the pileus and stipe, small hymenophoral pores (0.5–1 mm diam), small to medium-sized basidiospores (8.5–10 × 6.5–8 μm) with large meshes (2–3.5 μm diam), rusty red discolouration of the context on exposure (Fig. 3c1–c2, 4c) and subtropical distribution in China. Phylogenetically, S. anthracinus is closely related to S. echinocephalus (Fig. 1). However, the latter species differs from the former by its larger basidiomes (50–120 mm diam), pileus with black-brown thin and scattered scales and context changing to grey-black on exposure (Gelardi et al. 2013; Fig. 3h1–h2). Morphologically, S. anthracinus is similar to S. calidus described below, because of their black more or less erect pyramidal scales on the pileus. However, S. calidus has smaller scales (1–3 mm diam) on the pileus, echinate basidiospores with confluent tubercles and irregular incomplete reticulum, and a predominant distribution in tropical China (Fig. 2).
Strobilomyces atrosquamosus J.Z. Ying & H.A. Wen, Myco-systema 20: 298. 2001 — MycoBank MB484866; Fig. 2, 3d1–d2, 4d1–d2
Habitat & Distribution — Solitary or scattered on soil in forests dominated by Fagaceae; currently recorded from tropical and subtropical regions of China and Japan.
Specimens examined. China, Yunnan Province, Puer City, 11 Sept. 1986, Y. Li 325 (HMAS 72079, holotype); Yunnan Province, Puer City, Changlinggang, 1600 m elev., 30 July 2008, B. Feng 257 (HKAS 55368); Yunnan Province, Kunming City, Qiongzhu Temple, 6 Sept. 2012, L.H. Han 4 (HKAS 78563); Yunnan Province, Puer City, Weather Station of Land and Transport, national road 404-K14, 1400 m elev., 11 July 2014, L.H. Han 440 (HKAS 84736); Yunnan Province, Wenshan City, Donggua Village, alt. 1150 m, 5 Aug. 2014, L.H. Han 514 (HKAS 84810). – Japan, Osaka Prefecture, H. Sato (MAK s174); same location and collector (MAK s322).
Notes — Strobilomyces atrosquamosus is characterized by its medium-sized basidiomes (60–80 mm diam), brown pileus with dark brown to black-brown (upper part) to red-brown or vinaceous brown (lower part), small to medium-sized, more or less erect pyramidal scales (2–4 mm high, 2–4 mm diam at base), stipe with dark red-brown to dark black-brown thick fluffy floss, large hymenophoral pores (1–2 mm diam), context becoming rusty red on exposure and small to medium-sized reticulate basidiospores with small meshes (1–2 μm diam) (Wen & Ying 2001; holotype of S. atrosquamosus: 8–10 × 6–8 μm, Q = 1.17–1.36, Qm = 1.26 ± 0.08; Fig. 3d1–d2, 4d1–d2). Morphologically, it resembles S. brunneolepidotus in having more or less red-brown basidiomes. However, S. brunneolepidotus possesses unicoloured (red-brown) erect conical scales on the pileus and grey-black discolouration of the context on exposure (Corner 1972, Terashima et al. 2016; Fig. 3e1–e2).
Strobilomyces brunneolepidotus Har. Takah. & Taneyama, The fungal flora in southwestern Japan, agarics and boletes 1: 303. 2016 — MycoBank MB809939; Fig. 2, 3e1–e2, 4e
Habitat & Distribution — Solitary or scattered on soil in forests dominated by Fagaceae; currently known from tropical China and Japan.
Specimens examined. China, Guangdong Province, Zhaoqing City, Fengkai County, Heishiding Nature Reserve, 600 m elev., 3 June 2013, K. Zhao 264 (HKAS 80689); Fujian Province, Sanming City, Geshikao National Forest Garden, 200 m elev., 8 July 2013, T. Guo 733 (HKAS 81935); Yunnan Province, Xishuangbanna City, Mengla County, 1039 m elev., 6 July 2014, H.L. Han 387 (HKAS 84683); Taiwan Province, Fushan Botanical Garden, H. Sato (MAK s309). – Japan, Okinawa Prefecture, 9 June 2012, Y. Taneyama (TNS-F-48210, holotype).
Notes — Strobilomyces brunneolepidotus is characterized by its medium-sized basidiomes (60–100 mm diam), dirty white pileus with red-brown, small to medium-sized, erect conical scales (1–3 mm high, 2–5 mm diam at base), apically reticulated stipe with concolorous thick fluffy floss and erect conical scales, large hymenophoral pores (1–3 mm diam), context becoming grey-black on exposure, small reticulate basidiospores (7.5–9 × 6.5–8 μm, Q = 1.15–1.29, Qm = 1.23 ± 0.06; Fig. 4e) with small meshes (1–2 mm diam) and tropical to subtropical distribution (Terashima et al. 2016; Fig. 3e1–e2). Strobilomyces brunneolepidotus, S. atrosquamosus and S. glabellus are the three known species with red-brown basidiomes. However, these three species have a separate position in the phylogenetic tree (Fig. 1). Strobilomyces atrosquamosus possesses brown pileus with dark brown to black-brown (upper part) to red-brown or vinaceous brown (lower part), more or less erect pyramidal scales, stipe with dark red-brown to dark black-brown thick fluffy floss, larger basidiospores (8–10 × 6–8 μm) and rusty red discolouration of the context on exposure (Wen & Ying 2001; Fig. 3d1–d2, 4d1–d2). Finally, S. glabellus is characterized by its grey to light red-brown to red-brown, patch-like to appressed scales or floss on the pileus, stipe with thin floss or subglabrous, smaller hymenophoral pores (0.5–1 mm diam) and larger meshes (2–3.5 μm diam) on the surface of basidiospores (Ying & Ma 1985; Fig. 3i, 4i).
Strobilomyces calidus L.H. Han, J. Xu & Zhu L. Yang, sp. nov. — MycoBank MB824855; Fig. 2, 6b, 8a1–a2, 9
Fig. 8.

Representative basidiomes of Strobilomyces species with non-reticulate basidiospores. — a1–a2. S. calidus (HKAS 84700); b1–b2. S. densisquamosus (HKAS 83112, holotype); c1. S. echinatus (NEST 1597); c2. S. echinatus (NEST 1818); d1. S. giganteus (HKAS 59637); d2. S. giganteus (HKAS 74967); e1–e3. S. seminudus (HKAS 80459); f1–f2. S. subnudus (HKAS 83823); g1–g2. S. velutinus (HKAS 84776); h1–h2. S. cf. velutipes (NY 2072516). — Scale bars = 20 mm.
Fig. 9.
Strobilomyces calidus (HKAS 84700, holotype). a. Basidia and pleurocystidium; b. cheilocystidia; c. pileipellis. — Scale bars = 10 μm.
Etymology. From Latin ‘calidus’ = having a warm climate, referring to its tropical habit.
Holotype. China, Yunnan Province, Xishuangbanna City, Menghai County, Xishuangbanna Nature Reserve, 1200 m elev., 9 July 2014, L.H. Han 404 (HKAS 84700).
Basidiomes (Fig. 8a1–a2) medium-sized. Pileus 60–90 mm diam, subhemispherical, dry, densely covered with black (9F7), more or less erect conical to pyramidal scales, small, 1–3 mm high, 1–3 mm diam at base, subpellis context dirty white (1B1) to brown-black (9E4); margin occasionally appendiculate with a few slender ciliate veil remnants concolorous with pileal surface; context white (8A1), quickly changing to orange-red (6B8) then black (10E1) on exposure. Tubes adnate with decurrent tooth, white (6A1) then smoky grey (8C1); hymenophoral pores angular, large, 1–2 mm diam; pores and tubes concolorous, white (14A1) then cinnamon grey (5D1), immediately staining brown-black (6E6), then black (17F8) on exposure. Stipe 65–110 mm long, 6–15 mm diam, subcylindrical or slightly thickening to base; surface with elongate reticulum at upper, entirely with black granular scales; context white (8A1), discolouration similar to that of pileus; annulus and annular zone absent; basal mycelium grey-white (6B1).
Basidiospores (Fig. 6b) [60/3/2] (7.5–)8.5–10 × 7–8(–9) μm (Q = 1.18–1.25(–1.28), Qm = 1.21 ± 0.04) excluding ornamentation, broad ellipsoid, dark brown (7D5), echinate with confluent tubercles and irregular incomplete reticulation, ornamentation 0.5–1 μm high; apiculus 0.5 μm long. Basidia (Fig. 9a) 32–44 × 11–17 μm, narrowly clavate to clavate, 4-spored; sterigmata 4–6 μm long. Hymenophoral trama boletoid; hyphae cylindrical, 6–10 μm wide. Cheilocystidia (Fig. 9b) 35–65 × 15–25 μm, abundant, narrowly lageniform to narrowly conical, hyaline or with dark brown (5C8) plasmatic pigment, thin-walled. Pleurocystidia (Fig. 9a) 45–65 × 15–18 μm, numerous, narrowly lageniform to narrowly utriform, thin-walled. Pileipellis (Fig. 9c) an intricate trichodermium, wrapped in bundles, composed of 4–18 μm wide subradially arranged hyphae, with short attenuated terminal cells, cell wall dark brown (5E8) and slightly thickened (< 1 μm). Pileal trama composed of 4–11 μm wide interwoven hyphae. Hyphae of scales on stipe similar to those on pileus. Stipe trama composed of 3–13 μm wide cylindrical hyphae. Clamp connections absent.
Habitat & Distribution — Solitary or scattered on soil in forests dominated by Fagaceae; presently recorded from tropical China.
Additional specimen examined. China, Yunnan Province, Puer City, Taiyanghe National Forest Park, 1326 m elev., 2 Aug. 2014, X.B. Liu 438 (HKAS 87084).
Notes — Strobilomyces calidus is characterized by its medium-sized basidiomes (60–90 mm diam), pileus with black, small, more or less erect conical to pyramidal scales (1–3 mm diam), stipe entirely granulated with black scales, large hymenophoral pores (1–2 mm diam), small to medium-sized echinate basidiospores (8.5–10 × 7–8 μm) with confluent tubercles and irregular incomplete reticulum and orange-red discolouration of the context on exposure (Fig. 8a1–a2, 6b). The taxon is recorded from tropical China only. Phylogenetically, S. calidus is closely related to S. densisquamosus, a species described in the present paper (Fig. 1). Morphologically, S. calidus resembles S. anthracinus because of their black scales on the pileus and stipe. However, S. anthracinus has a charcoal black to black basidiome, a stipe with fluffy floss, smaller hymenophoral pores 0.5–1 mm diam, reticulate basidiospores, rusty red discolouration of the context on exposure and subtropical distribution (Fig. 3c1–c2, 4c).
Strobilomyces cingulatus L.H. Han & Zhu L. Yang, sp. nov. — MycoBank MB824856; Fig. 2, 3f1–f2, 4f, 10
Fig. 10.
Strobilomyces cingulatus (HKAS 73175, holotype). a. Basidia and pleurocystidia; b. cheilocystidia; c. pileipellis. — Scale bars = 10 μm.
Etymology. From Latin ‘cingulatus’, referring to the annulus of the species.
Holotype. China, Yunnan Province, Dali City, Binchuan County, Jizu Mountain, 2200 m elev., 3 Aug. 2013, L.H. Han 184 (HKAS 73175).
Basidiomes (Fig. 3f1–f2) small to medium-sized. Pileus 40–70 mm diam, hemispherical to subhemispherical and finally applanate, dry, covered with black-brown (6D5) at apex and light brown (6C2) to dirty white (1B1) at base, small, thin, patch-like to appressed scales or floss, 1–3 mm diam at base, sometimes showing whitish patches of subpellis; context white (8A1), staining grey-black (13B1) then black (10E1) on exposure. Tubes narrowly adnate with decurrent tooth, white (6A1) then smoky grey (8C1) with age; hymenophoral pores angular, small, 0.5–1 mm diam; pores and tubes concolorous, white (14A1) then smoky grey (4D1), immediately staining rusty red (6D8) then black (17F8) on exposure. Stipe 50–180 mm long, 5–14 mm diam, subcylindrical, curved; conspicuously with elongate reticulum at apex, membranous annulus thick and floccose; surface of stipe entirely covered with thick fluffy floss arranged in spiral, upper and lower halves of stipe composed of grey-white (6B1) and dark black-brown (7D5) floss, respectively; context white (8A1), then dark brick red (9D8) on exposure; basal mycelium dirty white (1B1) to grey-white (6B1).
Basidiospores (Fig. 4f) [80/4/4] (8.5–)9–11 × (6–)7–8.5(–9) μm (Q = (1.13–)1.18–1.3(–1.36), Qm = 1.23 ± 0.05) excluding ornamentation, broad ellipsoid to ellipsoid, dark brown (7D5), completely reticulate with meshes 1–2 μm diam and 1–2 μm high; apiculus 0.5 μm long. Basidia (Fig. 10a) 22–42 × 13–17 μm, narrowly clavate to clavate, 4-spored; sterigmata 3–5 μm long. Hymenophoral trama boletoid; hyphae cylindrical, 3–15 μm wide. Cheilocystidia (Fig. 10b) 25–70 × 10–25 μm, abundant, narrowly conical or sublageniform, usually containing brown-yellow plasmatic pigment (6B5), thin-walled. Pleurocystidia (Fig. 10a) 30–65 × 10–25 μm, numerous, subfusiform or narrowly to broadly lageniform with subacute apex, thin-walled. Pileipellis (Fig. 10c) an intricate trichodermium, composed of 6–16 μm wide cylindric to submoniliform hyphae; hyphae loosely interwoven, often separating at septa, with obtuse terminal cells; cell wall dark brown (5E8), more or less thickened (< 1 μm). Pileal trama composed of 3–11 μm wide interwoven hyphae. Hyphae of scales on stipe similar to those on pileus. Stipe trama composed of 3–12 μm wide cylindrical hyphae. Clamp connections absent.
Habitat & Distribution — Solitary or scattered on soil in forests dominated by Castanopsis spp. or Quercus spp.; presently known from subtropical to temperate China and Japan.
Additional specimens examined. China, Hubei Province, Yichang City, Muyu Town, Shennongjia Village, 1900 m elev., 18 July 2012, Q. Cai 848 (HKAS 75582); Yunnan Province, Kunming City, Kunming Botanic Garden, 1900 m elev., 4 July 2014, G. Wu 1134 (HKAS 80508); Jilin Province, Yanbian City, Antu County, Dayangcha Nature Reserve, 927 m elev., 25 Aug. 2015, J. Li 302 (HKAS 91330). – Japan, Nagano Prefecture, Susaka, H. Sato (MAK s192).
Notes — Strobilomyces cingulatus is characterized by its small to medium-sized basidiomes (40–70 mm diam), pileus with black-brown at apex and light brown to dirty white at base, small, thin, patch-like to appressed scales (1–3 mm diam at base) or floss, stipe with an annulus at apex and thick fluffy floss arranged in spiral, small hymenophoral pores (0.5–1 mm diam), medium-sized reticulate basidiospores (9–11 × 7–8.5 μm) with small meshes (1–2 μm diam) and grey-black discolouration of the context on exposure (Fig. 3f1–f2, 4f). In addition, S. cingulatus is widely distributed in fagalean forests of the subtropical to temperate regions in East Asia. Phylogenetically and morphologically, S. cingulatus is closely related to S. microreticulatus, a species described below. For a comparison between them see the notes of S. microreticulatus.
Strobilomyces densisquamosus L.H. Han & Zhu L. Yang, sp. nov. — MycoBank MB824857; Fig. 2, 6d, 8b1–b2, 11
Fig. 11.
Strobilomyces densisquamosus (HKAS 83112, holotype). a. Basidia and pleurocystidia; b. cheilocystidia; c. pileipellis. — Scale bars = 10 μm.
Etymology. From Latin ‘dense’ = close, ‘squamosus’ = scaly, referring to the densely compacted scales on the pileus.
Holotype. China, Yunnan Province, Qiubei County, Xiangqi Village, 1569 m elev., 10 Aug. 2014, L.H. Han 578 (HKAS 83112).
Basidiomes (Fig. 8b1–b2) medium-sized to large. Pileus 60–120 mm diam, subhemispherical to applanate, dry, densely covered with grey-black (2E1), more or less erect conical to pyramidal scales, small, 1–3 mm high, 1–3 mm diam at base; margin mostly appendiculate with thick and triangular or irregular lacy veil remnants concolorous with pileal surface; context white (8A1), quickly changing to orange-red (6B8) then black (10E1) on exposure. Tubes adnate with decurrent tooth, white (6A1) then smoky grey (8C1); hymenophoral pores angular, small, 0.5–1 mm diam; pores and tubes concolorous, white (14A1) then cinnamon grey (5D1), immediately staining rusty red (8C7) then black (17F8) on exposure. Stipe 40–130 mm long, 4–12 mm diam, subcylindrical or slightly tapering to base, beset by grey to dirty white (6B1) floccose squamules around annular zone at apex; stipe surface entirely with elongate reticulum, covered with thin fluffy floss, grey-white and black at upper and lower part respectively; context white (6A1), discolouration similar to that of tubes; annulus absent; basal mycelium grey-white (6B1).
Basidiospores (Fig. 6d) [60/3/2] (8–)8.5–10.5 × 7–9(–10) μm (Q = (1.06–)1.11–1.28(–1.44), Qm = 1.19 ± 0.06) excluding ornamentation, subglobose to broad ellipsoid, dark brown (7D5), echinate with irregular short ribs, ornamentation 0.5–1 μm high; apiculus 0.5 μm long. Basidia (Fig. 11a) 32–44 × 11–17 μm, narrowly clavate to clavate, 4-spored; sterigmata 4–6 μm long. Hymenophoral trama boletoid; hyphae cylindrical, 6–12 μm wide. Cheilocystidia (Fig. 11b) 30–65 × 15–25 μm, abundant, narrowly lageniform to narrowly conical, hyaline or with dark brown (5C8) plasmatic pigment, thin-walled. Pleurocystidia (Fig. 11a) 45–70 × 14–18 μm, scarce, narrowly lageniform to narrowly utriform, thin-walled. Pileipellis (Fig. 11c) an intricate trichodermium, composed of 4–15 μm wide cylindric to submoniliform hyphae; hyphae subradially arranged, with short attenuated terminal; cell wall dark brown (5E8), slightly thickened (< 1 μm). Pileal trama composed of 4–12 μm wide interwoven hyphae. Hyphae of scales on stipe similar to those on pileus. Stipe trama composed of 3–12 μm wide cylindrical hyphae. Clamp connections absent.
Habitat & Distribution — Solitary or scattered on soil or on trunk in forests dominated by Fagaceae and Pinaceae; presently known from subtropical and temperate China and Japan.
Additional specimens examined. China, Yunnan Province, Chuxiong City, Zixi Mountain, 1800 m elev., 18 Sept. 2010, Z.W. Ge 2778 (HKAS 61701); Yunnan Province, Baoshan City, Tengchong County, X193-52 national road, 1650 m elev., 11 Aug. 2011, G. Wu 618 (HKAS 74932); Yunnan Province, Kunming City, Yeya Lake, 2000 m elev., 18 Aug. 2012, B. Feng 1212 (HKAS 82354); Yunnan Province, Wenshan City, Qiubei County, Xiangqi Village, 1569 m elev., 10 Aug. 2014, H.L. Han 585 (HKAS 84945); Liaoning Province, Benxi City, Changbai Mountain, 432 m elev., 21 Aug. 2015, J. Li 222 (HKAS 91250). – Japan, Shiga Prefecture, Nagara, H. Sato (MAK s409); same location and collector (MAK s416).
Notes — Strobilomyces densisquamosus is characterized by its medium-sized to large basidiomes (60–120 mm diam), pileus densely covered with grey-black, small, more or less erect conical to pyramidal scales (1–3 mm high, 1–3 mm diam at base), stipe with an annular zone and grey-white (upper) and black (lower) thin fluffy floss, small hymenophoral pores (0.5–1 mm diam), small to medium-sized echinate basidiospores (8.5–10.5 × 7–9 μm) with irregular short ribs and orange-red discolouration of the context on exposure (Fig. 6d, 8b1–b2). This species often grows on the basal trunk of trees of Fagaceae and Pinaceae. Phylogenetically, S. densisquamosus is related to S. calidus and S. sp. 14 (Fig. 1). Both S. densisquamosus and S. calidus have comparable basidiospores and colour reaction of the context. However, S. calidus differs from S. densisquamosus by its black scales on the pileus, larger hymenophoral pores (1–2 mm), and stipe entirely with granular scales. In addition, the geographical distribution of S. calidus is restricted to tropical China (Fig. 2). Strobilomyces confusus, originally described from south-eastern North America (Singer 1945), resembles S. densisquamosus by the grey-black to black-brown more or less erect pyramidal scales on the pileus and small to medium-sized echinate basidiospores with irregular short ribs (Fig. 6c1–c2, d). However, S. confusus differs from S. densisquamosus by its relatively thinner and scattered scales on the pileus, minor veil remnants and a shaggy-woolly stipe (Singer 1945, this study).
Strobilomyces douformis L.H. Han & Zhu L. Yang, sp. nov. — MycoBank MB824858; Fig. 2, 3g1–g2, 4g, 12
Fig. 12.
Strobilomyces douformis (HKAS 87097, holotype). a. Basidia and pleurocystidia; b. cheilocystidia; c. pileipellis. — Scale bars = 10 μm.
Etymology. ‘douformis’ refers to the shape of the basidiospore meshes similar to the traditional Chinese volumetric pot ‘dou’.
Holotype. China, Yunnan Province, Baoshan City, Longling County, Daxue Mountain, 2500 m elev., 29 July 2014, X.B. Liu 451 (HKAS 87097).
Basidiomes (Fig. 3g1–g2) small to medium-sized. Pileus 50–97 mm diam, subhemispherical then applanate, dry, covered with black (10E1), large, more or less erect pyramidal scales 5–10 mm diam at base, with their interstices showing light grey to dirty white context of subpellis; margin appendiculate with triangular fragments of thick floccose veil remnants concolorous with pileal surface; context white (8A1), quickly changing to rusty red (9C4) then black (10E1) on exposure. Tubes narrowly adnate with slightly decurrent tooth, white (6A1) then smoky grey (8C1); hymenophoral pores angular, small, 0.5–1 mm diam; pores and tubes concolorous, white (14A1) then grey-black (12E1), immediately staining dull red (11D4), then black (17F8) on exposure. Stipe 80–132 mm long, 9–13 mm diam, subcylindrical; surface roughly with elongate reticulum, covered with thick fluffy floss arranged in spiral, concolorous with pileus; context white (8A1), discolouration similar to that of tubes; annulus and annular zone absent; basal mycelium greywhite (6B1).
Basidiospores (Fig. 4g) [40/2/2] (8–)9–10.5(–11) × (7–)8–9 μm (Q = 1.11–1.25(–1.31), Qm = 1.18 ± 0.06) excluding ornamentation, subglobose to broad ellipsoid, dark brown (7D5), completely reticulate with meshes 1–1.5 μm high and 2.5–4 μm diam at base; apiculus 0.5 μm long. Basidia (Fig. 12a) 30–49 × 11–15 μm, narrowly clavate to clavate, 4-spored; sterigmata 4–6 μm long. Hymenophoral trama boletoid; hyphae cylindrical, 6–13 μm wide. Cheilocystidia (Fig. 12b) 35–65 × 15–25 μm, abundant, broadly fusoid or utriform with blunt appendage, hyaline or with dark brown (5C8) plasmatic pigment, thin-walled. Pleurocystidia (Fig. 12a) 45–70 × 15–19 μm, broadly fusoid with obtuse apex or narrowly lageniform, thin-walled. Pileipellis (Fig. 12c) an intricate trichodermium, composed of 5–14 μm wide cylindric to submoniliform hyphae; hyphae densely packed in clusters, erect or loosely interwoven, terminal cells attenuate towards apex; cell wall slightly thickened (< 1 μm). Pileal trama composed of 4–12 μm wide interwoven hyphae. Hyphae of scales on stipe similar to those on pileus. Stipe trama composed of 5–10 μm wide cylindrical hyphae. Clamp connections absent.
Habitat & Distribution — Solitary or scattered on soil in forests dominated by Fagaceae; currently known from subtropical China.
Additional specimen examined. China, Yunnan Province, Baoshan City, Longling County, Daxue Mountain, 2500 m elev., 3 Aug. 2014, X.B. Liu 498 (HKAS 87134).
Notes — Strobilomyces douformis is characterized by its small to medium-sized basidiomes (50–97 mm diam), pileus with black, large, more or less erect pyramidal scales (5–10 mm diam), stipe with concolorous thick fluffy floss arranged in spiral, small hymenophoral pores (0.5–1 mm diam), medium-sized basidiospores (9–10.5 × 8–9 μm) with large meshes (2.5–4 μm diam) and rusty red discolouration of the context on exposure (Fig. 3g1–g2, 4g). Phylogenetically, S. douformis is closely related to S. montosus and S. sp.10 (Fig. 1). However, the latter two taxa differ by their grey-black erect conical scales on the pileus and thick fluffy floss evenly distributed on the stipe (Berkeley 1851, Horak 1980; Fig. 3n1–n2).
Strobilomyces echinocephalus Gelardi & Vizzini, Mycol. Progr. 12: 578. 2013 — MycoBank MB801553; Fig. 2, 3h1–h2, 4h
Habitat & Distribution — Solitary or scattered on soil in forests dominated by Fagaceae; currently known from subtropical China and Japan.
Specimens examined. China, Yunnan Province, Dehong City, Yingjiang County, 2170 m elev., 17 July 2009, Y.C. Li 1673 (HKAS 59420); Yunnan Province, Kunming City, Qiongzhu Temple, 2200 m elev., 5 Oct. 2011, G. Wu 768 (HKAS 75765, isotype); Yunnan Province, Kunming City, Yeya Lake, 2000 m elev., 10 Aug. 2013, L.H. Han 235 (HKAS 80465); Hubei Province, Yichang City, Muyu County, Guanmen Mountain, 1650 m elev., 8 Aug. 2015, Y.Y. Cui 252 (HKAS 92153). – Japan, Miyazaki Prefecture, Takaoka, H. Sato (MAK s111).
Notes — Strobilomyces echinocephalus is characterized by its small to large basidiomes (50–120 mm diam), pileus with medium-sized, thin, scattered, more or less erect pyramidal to appressed scales (3–5 mm high, 3–5 mm diam at base), stipe densely with dark black-brown to black thick fluffy floss, small hymenophoral pores (0.5–1 mm diam), grey-black discolouration of the context on exposure, small to medium-sized reticulate basidiospores with large meshes (2–3.5 μm diam) and subtropical distribution (Gelardi et al. 2013; isotype of S. echinocephalus: 8.5–10 × 6.5–8 μm, Q = 1.18–1.43, Qm = 1.28 ± 0.07; Fig. 3h1–h2, 4h). Phylogenetically, S. echinocephalus is closely related to S. anthracinus, but S. anthracinus differs from S. echinocephalus by its charcoal black scales on the pileus and stipe, smaller basidiomes (25–50 mm diam) and rusty red discolouration of the context on exposure (Fig. 1, 2). Morphologically, S. echinocephalus is somewhat similar to S. albidus and S. cingulatus in sharing black-brown, thin, and scattered scales on the pileus (Gelardi et al. 2013; Fig. 3a1–a2, f1–f2, h1–h2). However, S. albidus differs from S. echinocephalus by its whitish stipe, smaller basidiospores (7–9 × 6–7 μm), and context staining rusty red on exposure (Fig. 3a1–a2, 4a). Strobilomyces cingulatus differs from S. echinocephalus by its larger basidiospores (9–11 × 7–8 μm) with smaller meshes (1–2 μm diam) and stipe with fluffy floss arranged in spiral (Fig. 3f1–f2, 4f).
Strobilomyces foveatus Corner, Boletus in Malaysia: 60. 1972 — MycoBank MB324273; Fig. 2, 6f1–f2
Habitat & Distribution — Solitary or scattered on soil in tropical forests dominated by Dipterocarpaceae; currently only known from Malaysia.
Specimens examined. Malaysia, Sarawak, Bako National Park, 31 Jan. 1959, Corner (E 83831, holotype); Johor, Endau-Rompin, Pulau Bertam (KEP FRI 62957); Pasoh, Negeri Sembilan (KEP FRI 69410); same location as above (KEP FRI 69468).
Notes — Strobilomyces foveatus is readily recognized by the medium-sized basidiomes (60–100 mm diam), pileus with black-brown to fuscous umber, small, firm, erect conical scales (1.5–2.5 mm high, 1.5–3 mm diam at base), small hymenophoral pores (0.5–1 mm diam), stipe with whitish poroid reticulation at the apex and fuliginous umber fluffy floss downwards, context reddening on exposure and the small to medium-sized basidiospores densely with isolated flat-roofed conial ornamentation (holotype of S. foveatus: 8–10 × 6–8 μm, Q = 1.12–1.25, Qm = 1.17 ± 0.04; Fig. 6f1–f2) (Corner 1972, Pegler & Young 1981, Horak 2011). The shape and size of the basidiospores densely with isolated flat-roofed cones recall S. velutipes, originally described from Australia. However, the pileus of S. velutipes is covered with tomentose-villous to appressed scales (Cooke 1889, Watling & Li 1999). Strobilomyces cf. velutipes in this study is macro-morphologically similar to S. velutipes, while differs by the basidiospores with semireticulate ornamentation (Fig. 6l1–l2, 8h1–h2). Macroscopically, however, S. foveatus resembles both S. mollis and S. montosus in the erect conical scales on the pileus, but the latter two species have reticulate basidiospores (Fig. 4n–o).
Strobilomyces giganteus M. Zang, Acta Bot. Yunnan. 7: 385. 1985 — MycoBank MB104809; Fig. 2, 6g1–g3, 8d1–d2
= Strobilomyces verruculosus Hirot. Sato, Mycoscience 50: 175. 2009.
Habitat & Distribution — Solitary or scattered on soil in forests dominated by Fagaceae; recorded from tropical to subtropical China, Japan and Thailand.
Specimens examined. China, Sichuan Province, Xichang City, Luoji Mountain, 2000 m elev., 17 Aug. 1983, M.S. Yuan 146 (HKAS 11755, holotype of S. giganteus); Zhejiang Province, Kaihua City, Gutian Mountain, July 2009, C. Guo et al. 854 (HMAS 261688); Jiangxi Province, Ganzhou City, Jiulianshan National Nature Reserve, 500 m elev., 12 June 2012, G. Wu 854 (HKAS 77026). – Japan, Kyoto Prefecture, Joyo-shi, Mito-shrine, 25 July 2007, H. Sato (MAK s693, holotype of S. verruculosus); same location and collector (MAK s359). – Thailand, Chiang Mai Province, Mae Sai Town, 55 km on Highway 1095, 10 June 2006, 982 m elev., R.E. Halling 8803 (NY 1393514).
Notes — Both S. giganteus and S. verruculosus are characterized in having black large basidiomes (usually more than 100 mm diam) with small hard erect conical scales (1–2 mm high, 1–3 mm diam at base), small hymenophoral pores (0.5–1 mm diam), thick stipe with black minutely conical scales and fluffy floss, context reddening on exposure and small to medium-sized semireticulate basidiospores (Zang 1985, Sato & Murakami 2009; Fig. 6g1–g3, 8d1–d2). We carefully re-examined the type specimens of these two species, and found that in the protologue of S. giganteus the size of the basidiospores is inaccurate in Zang (1985). Our study revealed that the basidiospores measure 8.5–10 × 7–8.5 μm (Q = 1.18–1.29, Qm = 1.24 ± 0.08; Fig. 6g1), and thus are in agreement with those of S. verruculosus, 8–10 × 7–9 μm (Q = 1.08–1.2, Qm = 1.16 ± 0.06; Fig. 6g2). In addition, the samples collected in China and recognized as S. giganteus are clustering together with S. verruculosus. Thus, S. verruculosus is treated as a synonym of S. giganteus. Strobilomyces giganteus occupies an isolated position in the phylogenetic tree with a long branch. We suspect that this taxon represents a relic of Strobilomyces spp. in the Palaeotropics (Han et al. 2018). Morphologically, it is similar to S. annulatus in having large basidiomes, erect conical scales on the pileus, and incomplete reticulate basidiospores. However, S. annulatus differs from S. giganteus by its distinct large annulus, soft and friable scales with brownish vinaceous tint and larger basidiospores (9.5–11.5 × 7–10 μm) (Corner 1972; Fig. 6a, g1–g3).
Strobilomyces glabellus J.Z. Ying, Acta Mycol. Sin. 4: 96. 1985 — MycoBank MB104810; Fig. 2, 3i, 4i
Habitat & Distribution — Solitary or scattered on soil in forests dominated by Fagaceae; currently recorded from subtropical China.
Specimens examined. China, Yunnan Province, Wenshan City, Guangnan County, 30 June 1959, Q.Z. Wang 760 (HMAS 26736, holotype); Yunnan Province, Baoshan City, Pumanshao, 1800 m elev., 8 Aug. 2011, G. Wu 573 (HKAS 74887).
Notes — We carefully re-examined the holotype specimen of S. glabellus. It is characterized by the medium-sized basidiomes (70–95 mm diam), pileus with light red-brown to red-brown patch-like to appressed scales or floss, stipe subglabrous or with light red-brown to dirty white floss, small hymenophoral pores (0.5–1 mm diam), small basidiospores (holotype of S. glabellus: 7–9 × 6–7 μm, Q = 1.11–1.3, Qm = 1.2 ± 0.07; Fig. 3i, 4i) with large meshes (2–3.5 μm) and subtropical distribution (Ying & Ma 1985; Fig. 3i). It is closely related to and morphologically resembles S. mirandus, but S. mirandus differs by its golden-tawny to brown-yellow, more or less erect pyramidal scales on the pileus, smaller basidiospores (6–8 × 5.5–7 μm) with smaller meshes (1–2 μm diam) and tropical habitats (Corner 1972, Ying & Ma 1985; Fig. 4i, m).
Strobilomyces glabriceps W.F. Chiu, Mycologia 40: 229. 1948 — MycoBank MB291242; Fig. 2, 3j1–j3, 4j1–j2
Habitat & Distribution — Solitary or scattered on soil in forests dominated by Fagaceae or mixed forest of Fagaceae and Pinaceae; currently recorded from subtropical China, Japan and India.
Specimens examined. China, Yunnan Province, Kunming City, Xi Mountain, 7 June 1936, T.K. Yien 8034 (HMAS 530101, holotype); Yunnan Province, Nujiang City, Bingzhongluo Town, 1600 m elev., 2 Aug. 2010, Q. Cai 249 (HKAS 67811); same location, 30 July 2011, G. Wu 444 (HKAS 74755); Yunnan Province, Baoshan City, Wayao Town, 1900 m elev., 13 Aug. 2011, G. Wu 650 (HKAS 74964); Yunnan Province, Kunming City, Yunnan Academy of Forestry, 2000 m elev., 9 Sept. 2012, L.H. Han 14 (HKAS 78573). – India, Jammu and Kashmir, Poonch, Krishna Ghat, 1554 m elev., 16 July 2014, Y.P. Sharma. – Japan, Shiga Prefecture, Ohmi-Shrine, Jingu-cho, Otsu-shi, H. Sato (MAK s290); same location and collector (MAK s293); same location and collector (MAK s363); same location and collector (MAK s379).
Notes — Strobilomyces glabriceps is characterized by the medium-sized to large basidiomes (90–150 mm diam), subglabrous pileus with grey-black, large, patch-like to appressed scales (5–14 mm diam) or floss, stipe with cottony annulus at apex and grey to dirty white and grey-black thin fluffy floss on the upper and lower part, large hymenophoral pores (1–3 mm diam), grey-black discolouration of the context on exposure, medium-sized reticulate basidiospores with large meshes (2–4 μm diam) and the subtropical distribution (Chiu 1948; holotype of S. glabriceps: 9–11 × 7.5–9.5 μm, Q = 1.1–1.36, Qm = 1.21 ± 0.08; Fig. 3j1–j3, 4j1–j2). Phylogenetically, S. glabriceps is closely related to S. pteroreticulosporus (Fig. 1). However, the latter species differs by its smaller erect conical scales (1–3 mm high, 1–3 mm diam at base) on the pileus, rusty red discolouration on exposure, and preferable association with Pinus spp. (Antonín et al. 2015; Fig. 3q1–q3). Strobilomyces glabriceps is morphologically similar to S. douformis (see notes under S. douformis).
Strobilomyces hongoi Hirot. Sato, Mycologia 103: 603. 2011 — MycoBank MB518940; Fig. 2, 6h
Habitat & Distribution — Scattered on the ground in mixed forests of Castanopsis cuspidata and evergreen Quercus spp., in mixed forests of deciduous Quercus spp., or in mixed forests of deciduous Quercus spp. and Pinus densiflora; currently known from subtropical Japan.
Specimens examined. Japan, Kyoto Prefecture, Yoshida Hill, 4 Aug. 2009, H. Sato (TNS-F-36013, holotype, TNS F-36014, TNS-F-36015); same location and collector, 24 July 2003 (MAK s180, MAK s182); same location and collector, 31 Aug. 2004 (MAK s281); same location and collector, 13 Sept. 2005 (MAK s371, MAK s372); same location and collector, 19 Sept. 2005 (MAK s386, MAK s391); same location and collector, 24 Sept. 2005 (MAK s425, MAK s426, MAK s429, TNS-F-36009); same location and collector, 11 July 2006 (MAK s447, MAK s451); Shiga Prefecture, Nagara, Otsu, 11 July 2004, H. Sato (MAK s249); Osaka Prefecture, Mino, 21 July 2002, H. Sato (MAK s17); same location and collector, 4 July 2006 (MAK s473, MAK s478, MAK s501, MAK s509, MAK s520, MAK s524); Miyazaki Prefecture, Kawanaka, Aya, 8 July 2006, H. Sato (MAK s535, MAK s536).
Notes — This species is characterized by its small to medium-sized basidiomes (30–80 mm diam), pileus with grey-black patch-like to appressed scales (3–8 mm diam at base), stipe with coarsely reticulum on its upper half and with fluffy floss on its lower half, small hymenophoral pores (0.5–1 μm diam), context turning red on exposure and small semireticulate basidiospores (holotype of S. hongoi: 7–9 × 6–7 μm, Q = 1.07–1.31, Qm = 1.17 ± 0.07) with irregular tubercles often confluent and subcristate (Sato et al. 2011; Fig. 6h). Phylogenetically, S. hongoi and S. subnudus are closely related (Fig. 1). However, the basidiospores of S. subnudus are larger (8–10 × 7.5–8.5 μm) and the stipe is entirely covered with thin fluffy floss (Fig. 6j, 8f1–f2).
Strobilomyces latirimosus J.Z. Ying, Acta Mycol. Sin. 4: 97. 1985 — MycoBank MB124474; Fig. 2, 3k1–k2, 4k1–k2
Habitat & Distribution — Solitary or scattered on soil in tropical to subtropical forests dominated by Fagaceae and Pinaceae; currently recorded from tropical and subtropical China.
Specimens examined. China, Guangxi Province, Hechi City, Donglan County, 2000 m elev., 19 June 1970, Y.G. Zong 146 (HMAS 43748, holotype); Fujian Province, Sanming City, Geshikao National Forest Garden, 200 m elev., 25 July 2007, Y.C. Li 1003 (HKAS 53348); Guangdong Province, Zhaoqing City, Fengkai County, Heishiding Mountain, 185 m elev., 13 Aug. 2012, F. Li 788 (HKAS 77697); Guizhou Province, Xingyi City, Zerong County, Naju Village, 1200 m elev., 4 Aug. 2010, G.J. Li et al. 10006 (HMAS 250943); Yunnan Province, Nujiang City, Lushui County, 1700 m elev., 7 Aug. 2011, G. Wu 551 (HKAS 74865); Yunnan Province, Jinghong City, Dadugang County, 1100 m elev., 10 July 2014, L.H. Han 427 (HKAS 84723); Yunnan Province, Wenshan City, Funing County, Xinhe Village, 1195 m elev., 3 Aug. 2014, L.H. Han 497 (HKAS 84793).
Notes — Strobilomyces latirimosus is characterized by its medium-sized basidiomes (60–90 mm diam), pileus with grey-black, medium-sized to large, patch-like to appressed scales (4–18 mm diam at base), large hymenophoral pores (1–2 mm diam), stipe with a slightly enlarged apex and entirely with whitish thick fluffy floss, context turning grey-black on exposure, small reticulate basidiospores with small meshes (1–2 μm diam) and tropical to subtropical distribution (Ying & Ma 1985; holotype of S. latirimosus: 7–9 × 6–7 μm, Q = 1.15–1.41, Qm = 1.27 ± 0.08; Fig. 3k1–k2, 4k1–k2). Phylogenetically, S. latirimosus is closely related to S. pinophilus, described below (Fig. 1). However, S. pinophilus differs by its small to medium-sized more or less erect pyramidal scales (1–3 mm high, 2–4 mm diam at base) on the pileus, larger basidiospores (9–11 × 7–8 μm) with larger meshes (2.5–4 μm), smaller hymenophoral pores (0.5–1 mm), and temperate distribution (Fig. 2, 3p, 4q). Morphologically, S. latirimosus resembles S. glabriceps in their patch-like to appressed scales or floss on the pileus, large hymenophoral pores (1–2 mm), grey-black discolouration of the context on exposure and reticulate basidiospores (Chiu 1948, Ying & Ma 1985; Fig. 2, 3j1–j3, k1–k2). However, S. glabriceps differs by its annulus on the stipe and larger basidiospores (9–11 × 7.5–9.5 μm) with larger meshes (2–4 μm diam) (Chiu 1948; Fig. 3j1–j3, 4j1–j2).
Strobilomyces longistipitatus D. Chakr., K. Das & S. Adhikari, Fungal Diversity 83: 200. 2017 — MycoBank MB817357
Habitat & Distribution — Under Abies densa in subalpine mixed forests (broad-leaved and coniferous); currently recorded from northern India.
Notes — See the notes of S. alpinus.
Strobilomyces microreticulatus L.H. Han & Zhu L. Yang, sp. nov. — MycoBank MB824859; Fig. 2, 3l, 4l, 13
Fig. 13.
Strobilomyces microreticulatus (HKAS 74863, holotype). a. Basidia and pleurocystidia; b. cheilocystidia; c. pileipellis. — Scale bars = 10 μm.
Etymology. From Latin ‘micro’ = small and ‘reticulatus’ = reticulated, referring to the small mesh of basidiospores.
Holotype. China, Yunnan Province, Lushui County, Laowo County, Chongren Village, 1700 m elev., 7 Aug. 2011, G. Wu 549 (HKAS 74863).
Basidiomes (Fig. 3l) small. Pileus 30–40 mm diam, hemispherical to subhemispherical, dry, covered with black-brown (5D3) scales in centre and dirty white (1B1) to light black-brown (7B2) scales on periphery, scales more or less erect pyramidal, small to medium-sized, 1–3 mm high, 2–4 mm diam at base; margin appendiculate from thick membranous veil remnants concolorous with pileal surface; context white (8A1), staining rusty red (9C4) then black (10E1) on exposure. Tubes adnate with decurrent tooth, white (6A1) then fuliginous (9E2); hymenophoral pores angular, small, 0.5–1 mm diam; pores and tubes concolorous, white (14A1) then smoky grey (4D1), immediately staining rusty red (6D8), then black (17F8) on exposure. Stipe 40–60 mm long, 6 mm diam, subcylindrical, curved; surface reticulated by outstretched tubes and a woolly and delicate annulus at apex; consisting of grey to dirty white (6B1) and grey-black (9E1) fluffy floss above and below annulus respectively; context white (8A1), then vivid purple black (14E7) on exposure; basal mycelium dirty white (1B1) to grey-white (6B1).
Basidiospores (Fig. 4l) [60/2/2] 9–11(–12) × (6.5–)7–8 μm (Q = (1.20–)1.25–1.42(–1.57), Qm = 1.34 ± 0.06) excluding ornamentation, broad ellipsoid to ellipsoid, dark brown (7D5), completely reticulate with meshes 1–1.5 μm diam and 1 μm high; apiculus 0.5 μm long. Basidia (Fig. 13a) 29–45 × 12–17 μm, narrowly clavate to clavate, 4-spored; sterigmata 4–6 μm long. Hymenophoral trama boletoid; hyphae cylindrical, 5–12 μm wide. Cheilocystidia (Fig. 13b) 32–60 × 12–24 μm, abundant, fusiform to narrowly lageniform or conical, usually containing brown-yellow plasmatic pigment (6B5), thin-walled. Pleurocystidia (Fig. 13a) 30–60 × 9–20 μm, scarce, broadly fusiform or broadly lageniform with subacute apex, thin-walled. Pileipellis (Fig. 13c) an intricate trichodermium, composed of 8–17 μm wide cylindric to submoniliform hyphae; hyphae laxly interwined, with short terminal cells; cell wall dark brown (5E8), slightly thickened (< 1 μm). Pileal trama composed of 3.5–10 μm wide interwoven hyphae. Hyphae of scales on stipe similar to those on pileus. Stipe trama composed of 4–13 μm wide cylindrical hyphae. Clamp connections absent.
Habitat & Distribution — Solitary or scattered on soil in forests of Fagaceae; presently recorded from subtropical China.
Additional specimen examined. China, Yunnan Province, Baoshan City, Wayao Village, 1900 m elev., 13 Aug. 2011, G. Wu 649 (HKAS 74963).
Notes — Strobilomyces microreticulatus is characterized by its small basidiomes (30–40 mm diam), pileus with small to medium-sized black-brown scales (2–4 mm diam in centre) and dirty white to light black-brown scales on periphery, stipe with grey-white and grey-black floss above and below annulus, respectively, small hymenophoral pores (0.5–1 mm diam), medium-sized reticulate basidiospores (9–11 × 7–8 μm) with small meshes (1–1.5 μm diam) and rusty red discolouration of the context on exposure (Fig. 3l, 4l). Strobilomyces microreticulatus is phylogenetically related to S. cingulatus (Fig. 1), but S. cingulatus differs by its pileus with black-brown small thin scales, stipe with thick floss in spiral, and grey-black discolouration of the context on exposure (Fig. 2, 3f1–f2, l). Further, S. cingulatus occupies a wide distribution from subtropical to temperate China. Morphologically, S. microreticulatus is similar to S. pteroreticulosporus in their more or less whitish pileus, stipe with dirty white to grey to grey-black floss and reticulate basidiospores. However, S. pteroreticulosporus is distinguished by its medium-sized to large basidiomes, erect conical scales with grey-black top and whitish base, stipe with thick floss arranged in spiral, large hymenophoral pores (1–2 mm diam) and temperate distribution (Antonín et al. 2015; Fig. 3q1–q3).
Strobilomyces mirandus Corner, Boletus in Malaysia: 61. 1972 — MycoBank MB324274; Fig. 2, 3m, 4m
= Strobilomyces sanmingensis N.L. Huang, Mycosystema 21: 6. 2002.
Habitat & Distribution — Solitary or scattered on soil in forests dominated by Fagaceae; currently recorded from Malaysia, Japan and China.
Specimens examined. China, Yunnan Province, Jinghong City, Dadugang County, 1300 m elev., 7 July 2006, Y.C. Li 468 (HKAS 50222); Yunnan Province, Dehong City, Yingjiang County, 200 m elev., 17 July 2009, Y.C. Li 1661 (HKAS 59408); Fujian Province, Sanming City, Geshikao National Forest Garden, 200 m elev., 8 July 2013, L.H. Han 134 (HKAS 80364); Taiwan Province, Taipei City, Fushan Botanical Garden, H. Sato (MAK s310). – Japan, Kyusyu, Miyazaki Prefecture, 200 m elev., 10 July 2002, S. Kurogi (CBM, FB-33928); same location and collector, 12 July 2003 (CBM, FB-33929); same location and collector, 6 July 2004 (CMB, FB-34452). – Malaysia, Johore, Tanjong Sedili Kechil, 2 Apr. 1934, Corner (E, holotype).
Notes — Strobilomyces mirandus can be easily distinguished by its tiny to small basidiomes (15–60 mm diam), pileus with golden-tawny to golden-orange, small, more or less erect pyramidal scales (1–3 mm high, 1–3 mm diam at base) and slender veil remnants, stipe with concolorous thin fluffy to floccose-squamulose floss, small hymenophoral pores (0.5–1 mm diam), small reticulate basidiospores with small meshes (1–2 μm diam) and mainly tropical distribution (Corner 1972, Horak 2011; holotype of S. mirandus: 6–8 × 5.5–7 μm, Q = 1.08–1.2, Qm = 1.12 ± 0.05; Fig. 3m, 4m).
Strobilomyces mirandus and S. glabellus are clustered in the same strongly supported clade (Fig. 1). Strobilomyces glabellus differs from S. mirandus by its pileus with light red-brown to red-brown patch-like to appressed scales or floss and larger basidiospores (7–9 × 6–7 μm) with larger meshes (2–3.5 μm) (Corner 1972, Ying & Ma 1985, Ge & Yang 2005, Sato et al. 2005, Horak 2011; Fig. 3i, m, 4i, m). Morphologically, S. mirandus resembles S. echinatus by the golden-tawny pileus and the tropical geographical distribution, but S. echinatus has medium-sized basidiomes (60–100 mm diam), pileus with dark red-brown erect conical to pyramidal scales, stipe with thick dark red-brown to black-brown fluffy floss, larger basidiospores (9–11 × 8–10 μm) with uniform echinate warts as ornamentation and association with plants of Fabaceae (Beeli 1926, Corner 1972, Pegler & Young 1981; Fig. 3m, 4m, 6e1–e2, 8c1–c2).
Strobilomyces sanmingensis, originally described from Sanming City, Fujian Province located in south-eastern China, is extremely close to S. mirandus morphologically (Huang 2002, Ge & Yang 2005; this study). Unfortunately, the holotype specimen of S. sanmingensis is lost. However, specimens collected from the type locality and regarded as S. sanmingensis (HKAS 80364) clustered with S. mirandus display no differences in the phylogenetic analyses. Therefore, we treat S. sanmingensis as a synonym of S. mirandus.
Strobilomyces mollis Corner, Boletus in Malaysia: 63. 1972 — MycoBank MB324275; Fig. 2, 4n
Habitat & Distribution — Solitary or scattered on soil in forests of Fagaceae; currently known from Malaysia, Singapore, India and China.
Specimens examined. China, Hainan Province, Ledong County, Jianfengling, 850 m elev., 3 Aug. 2009, N.K. Zeng 488 (HKAS 59833). – Malaysia, Sabah, Mt Kinabalu, Mesilau, 1500–1700 m elev., 6 Apr. 1964, Corner RSNB 8137 (E 83832, holotype). – Singapore, Mandai Road, 21 Sept. 1930, Corner (E 83834).
Notes — Strobilomyces mollis was recorded from Malaysia, Singapore, India and southern China (Corner 1972, Zang 1985, Horak 2011, Kour et al. 2013). It is characterized by the tiny to medium-sized basidiomes (20–70 mm diam), pileus with black-brown to vinaceous, medium-sized, soft erect conical scales (3–5 mm high, 2–4 mm diam at base), stipe with concolorous thin fluffy floss, large hymenophoral pores (1–1.5 mm diam), context becoming rusty red then black on exposure, small to medium-sized reticulate basidiospores (holotype of S. mollis: 7.5–9.5 × 6.5–8 μm, Qm = 1.15; Fig. 4n) with large meshes (2–3.5 μm) and the occurrence of tropical habitat (Corner 1972, this study). Phylogenetically, it is located in an isolated position and seems most closely related to Chinese samples (Fig. 1). Morphologically, S. mollis, S. montosus and S. pteroreticulosporus share erect conical scales on the pileus. However, the latter two species have hard and scattered scales on the pileus, and larger basidiospores (9–12 × 7–11 μm) (Horak 1980, 2011, Pegler & Young 1981, Antonín et al. 2015; Fig. 3n1–n2, q1–q3, 4o, r).
Strobilomyces montosus Berk., Hooker’s J. Bot. Kew Gard. Misc. 3: 78. 1851 — MycoBank MB104810; Fig. 2, 3n, 4o
Habitat & Distribution — Solitary or scattered on soil in mixed forests of Fagaceae and Pinaceae; currently recorded from India and southwestern China.
Specimens examined. China, Yunnan Province, Baoshan City, Houqiao Town, 1700 m elev., 10 Aug. 2011, G. Wu 596 (HKAS 74910); Yunnan Province, Nujiang City, Ekeluo Village, 1600 m elev., 4 Aug. 2011, G. Wu 495 (HKAS 74809). – India, Darjeeling, Jillapahar, Hooker 121 (K, holotype).
Notes — Strobilomyces montosus is characterized by its small to medium-sized basidiomes (30–70 mm diam), pileus with grey-black, medium-sized, erect conical scales (2–4 mm high, 3–5 mm diam at base), stipe with grey-black thick fluffy floss, small hymenophoral pores (0.5–1 mm diam), medium-sized to large reticulate basidiospores (holotype of S. montosus: 9–13 × 7–8.5 μm, Qm = 1.12; Fig. 4o) with large meshes (2–4 μm diam and 0.5–1 μm high) and grey-black discolouration of the context on exposure (Berkeley 1851, Horak 1980, Pegler & Young 1981; Fig. 3n1–n2). Horak (1980) examined the holotype of S. montosus, and pointed out that the type material is in bad condition. The size of basidiospores recorded by Pegler & Young (1981) is slightly larger than that observed by Horak (1980). Strobilomyces montosus is phylogenetically related to S. douformis (Fig. 1). However, S. douformis differs from S. montosus by its black, large (5–10 mm diam at base), more or less erect pyramidal scales on the pileus and rusty red discolouration of the context on exposure (Fig. 2, 3g1–g2, n1–n2). Morphologically, S. montosus resembles S. pteroreticulosporus. Diagnostic characters of these two species are discussed in the notes of S. pteroreticulosporus.
Strobilomyces nigricans Berk., Hooker’s J. Bot. Kew Gard. Misc. 4: 139. 1852 — MycoBank MB146282
Habitat & Distribution — On fir-cone; currently only known from Northern India.
Specimen examined. India, Khasia Hills, 27 June 1850, Hooker 4 (K, holo-type).
Notes — See the notes of S. alpinus.
Strobilomyces parvirimosus J.Z. Ying, Acta Mycol. Sin., Suppl. 1: 305. 1986 — MycoBank MB127539; Fig. 2, 3o, 4p1–p2
Habitat & Distribution — Solitary or scattered on soil in forests dominated by Fagaceae; currently recorded from subtropical China.
Specimens examined. China, Yunnan Province, Wenshan City, Qiubei County, Qingping Village, 1700 m elev., 16 July 1959, Q.Z. Wang 833 (HMAS 27590, holotype); Yunnan Province, Baoshan City, Houqiao Town, 1700 m elev., 10 Aug. 2011, G. Wu 597 (HKAS 74911); same location and date, B. Feng 1067 (HKAS 74547).
Notes — Strobilomyces parvirimosus is characterized by its small to medium-sized basidiomes (30–70 mm diam), pileus with black-brown, small to medium-sized, more or less erect pyramidal scales (3–5 mm diam at base), concolorous stipe with thin fluffy floss, small hymenophoral pores (0.5–1 mm diam), small to medium-sized reticulate basidiospores (holotype of S. parvirimosus: 8–10 × 6.5–8 μm, Q = 1.15–1.38, Qm = 1.29 ± 0.1; Fig. 4p1–p2) with small meshes (1–2 μm diam, 1 μm high) and subtropical distribution (Ying 1986; Fig. 3o). Morphologically, S. parvirimosus resembles S. montosus in their pileus with medium-sized more or less erect pyramidal scales. However, S. montosus differs by its grey-black basidiomes, larger basidiospores (9–13 × 7–8.5 μm) with larger meshes (1–2 μm diam), context changing to grey-black on exposure and subtropical to tropical distribution (Berkeley 1851; Fig. 3n1–n2, 4o).
Strobilomyces pinophilus L.H. Han & Zhu L. Yang, sp. nov. — MycoBank MB824860; Fig. 2, 3p, 4q, 14
Fig. 14.
Strobilomyces pinophilus (HKAS 80300, holotype). a. Basidia and pleurocystidia; b. cheilocystidia; c. pileipellis. — Scale bars = 10 μm.
Etymology. From Latin ‘pinus’ = pine, and Latin ‘philus’ = preferring, named for its association with Pinus.
Holotype. China, Anhui Province, Huoshan County, Taiyang Village, 600 m elev., 9 Sept. 2012, L.H. Han 69 (HKAS 80300).
Basidiomes (Fig. 3p) small to medium-sized. Pileus 40–70 mm diam, subhemispherical, then convex, dry, covered with grey-black (8B1), more or less erect pyramidal scales, small to medium-sized, 1–3 mm high, 2–4 mm diam at base, ground and subpellis context dirty white (1B1); margin appendiculate with triangular or strip shaped fragments of floccose veil remnants concolorous with pileal surface; context white (8A1), quickly changing to rusty red (9C4) then black (10E1) on exposure. Tubes emarginate with slightly decurrent tubes, white (6A1) then smoky grey (8C1); hymenophoral pores angular, small, 0.5–1 mm diam; pores and tubes concolorous, white (14A1) then fuscous vinaceous (12D3), immediately staining brick red (11C7) then black (17F8) on exposure. Stipe 90–100 mm long, 7–10 mm diam, subcylindrical or slightly attenuating towards base; surface with elongate reticulum at apex, covering grey (8C1), dirty white (1B1) to grey-black (1D1) thick fluffy floss arranged in spiral; context white (8A1), discolouration similar to that of tubes; annulus floccose-membranous, soft, delicate, irregularly spreading then pendent, at first dirty white (1B1) then grey-black (1D1); basal mycelium grey-white (6B1).
Basidiospores (Fig. 4q) [60/3/2] (8–)9–11 × 7–8(–9) μm (Q = 1.2–1.29 (1.33), Qm = 1.25 ± 0.03) excluding ornamentation, broad ellipsoid, dark brown (7D5), completely reticulate with meshes 2.5–4 μm diam and 1–2 μm high; apiculus 0.5 μm long. Basidia (Fig. 14a) 30–42 × 10–14 μm, narrowly clavate to clavate, 4-spored; sterigmata 3–5 μm long. Hymenophoral trama boletoid; hyphae cylindrical, 4–10 μm wide. Cheilocystidia (Fig. 14b) 35–54 × 12–18 μm, narrowly lageniform to narrowly conical, hyaline or with dark brown (5C8) plasmatic pigment, thin-walled. Pleurocystidia (Fig. 14a) 43–60 × 15–20 μm, narrowly lageniform to conical, thin-walled. Pileipellis (Fig. 14c) an intricate trichodermium, composed of 5–15 μm wide laxly interwoven hyphae accumulated in clusters, terminal cells cylindric or subclavate, slightly with dark brown (5E8) plasmatic pigment, cell wall more or less thickened (< 1 μm). Pileal trama composed of 6–12 μm wide interwoven hyphae. Hyphae of scales on stipe similar to those on pileus. Stipe trama composed of 4–10 μm wide cylindrical hyphae. Clamp connections absent.
Habitat & Distribution — Solitary or scattered on soil in forests dominated by Pinus thunbergii; currently known only in temperate China.
Additional specimen examined. China, Shandong Province, Weihai City, Shengjing Mountain, 300 m elev., 22 Sept. 2014, B. Feng 1746 (HKAS 94132).
Notes — Strobilomyces pinophilus is characterized by its small to medium-sized basidiomes (40–70 mm diam), pileus with grey-black (apex) and whitish (base), small to medium-sized, more or less erect pyramidal scales (2–4 mm diam), stipe with an annulus at apex and grey, dirty white to grey-black thick fluffy floss arranged in spiral, small hymenophoral pores (0.5–1 mm diam), medium-sized basidiospores (9–11 × 7–8 μm) with large meshes (2.5–4 μm diam), rusty red discolouration of the context on exposure and preferable association with Pinus thunbergii in temperate forests (Fig. 3p, 4q).
Phylogenetically, S. pinophilus and S. latirimosus form a sister relationship with strong statistical support (Fig. 1). Both of them are recorded from Pinus forests. However, S. latirimosus has pileus with medium-sized to large patch-like to appressed scales (4–18 mm diam at base), smaller basidiospores (7–9 × 6–7 μm) with smaller meshes (1–2 μm), grey-black discolouration of the context on exposure and tropical to subtropical distribution (Ying & Ma 1985, Han et al. 2018; Fig. 3k1–k2, 4k1–k2).
Morphologically, S. cingulatus, S. glabriceps, S. microreticulatus, S. pinophilus, S. densisquamosus, S. seminudus and S. subnudus have a similar stipe with grey to dirty white (above) and grey-brown to grey-black (below) scales. However, the latter three species have incomplete reticulate basidiospores and stipe without annulus. Finally, S. cingulatus and S. glabriceps are distinguished from S. pinophilus by the pileus with patch-like to appressed scales or floss and the blackening context on exposure (Chiu 1948; Fig. 3f1–f2, j1–j3). Strobilomyces microreticulatus has smaller basidiomes (30–40 mm diam), pileus with black-brown scales in centre and dirty white to light black-brown scales on periphery, basidiospores with smaller meshes (1–1.5 μm) and subtropical distribution (Fig. 3l, 4l).
Strobilomyces pteroreticulosporus, another temperate species originally described from the Republic of Korea, is very similar to S. pinophilus in macro- and microscopic characters. However, S. pteroreticulosporus is characterized by the larger basidiomes (70–110 mm diam), pileus with erect conical dirty white scales with grey-black apex, larger hymenophoral pores (1–2 mm diam) and the context generally exudes oily secretion after cutting (Antonín et al. 2015; Fig. 3q1–q3).
Strobilomyces polypyramis Hook.f., Hooker’s J. Bot. Kew Gard. Misc. 3: 78. 1851 — MycoBank MB146703
Habitat & Distribution — Solitary or scattered on soil in tropical forests; currently known from India.
Specimen examined. India, Sikkim, Jillapahar, Hooker 104 (K, holotype).
Notes — Strobilomyces polypyramis is characterized by the large basidiomes (150–175 mm diam), pileus with black-brown erect conical scales, black-brown to brown purple smooth stipe, large hymenophoral pores (1–2 mm diam), context reddening on exposure and the medium-sized echinate basidiospores with irregular warts and short flanges (holotype of S. polypyramis: 9–10.5 × 7–9 μm, Qm = 1.16) (Berkeley 1851, Horak 1980, Pegler & Young 1981, this study). Three species, namely S. annulatus, S. densisquamosus and S. calidus, have basidiospores whose shape, size and ornamentation are similar to those of S. polypyramis (Fig. 6a–b, d). Unfortunately, the holotype material of S. polypyramis is in fragmentary condition and some morphological structures cannot be examined and evaluated properly (Horak 1980, this study).
Strobilomyces pteroreticulosporus Antonín & Vizzini, Phytotaxa 219: 81. 2015 — MycoBank MB812008; Fig. 2, 3q1–q3, 4r
Habitat & Distribution — Solitary or scattered on soil in forests dominated by Pinus spp.; currently recorded from temperate regions of the Republic of Korea and China.
Specimens examined. China, Anhui Province, Liuan City, Huoshan County, Taiyang Village, 600 m elev., 2 June 2013, L.H. Han 68 (HKAS 80299); Hubei Province, Shiyan City, Yingtaogou Village, 800 m elev., 1 July 2013, Y.J. Hao 911 (HKAS 80191); Shaanxi Province, Ankang City, Zhenping County, Shuijingping Village, 800 m elev., 4 July 2013, L.H. Han 120 (HKAS 80350); Liaoning Province, Shenyang City, Tianzhu Mountain, 200 m elev., 23 Aug. 2015, J. Li 246 (HKAS 91274).
Notes — Strobilomyces pteroreticulosporus is characterized by its medium-sized to large basidiomes (70–110 mm diam), pileus with dirty white, small, erect conical scales (1–3 mm high, 1–3 mm diam at base) with grey-black apex, stipe with an annulus at apex and dirty white (upper part) to grey-black (lower part) thick fluffy floss arranged in spiral, large hymenophoral pores (1–2 mm diam), context becoming rusty red then black and exuding oily secretion on exposure and medium-sized reticulate basidiospores with large meshes (2–4 μm) (Antonín et al. 2015; 9–11 × 7–8.5 μm, Q = 1.13–1.29, Qm = 1.21 ± 0.07; Fig. 3q1–q3, 4r). Phylogenetically, S. pteroreticulosporus is closely related to S. glabriceps (Fig. 1). However, S. glabriceps differs by its subglabrous pileus with patch-like to appressed scales or floss, grey-black discolouration of the context on exposure and subtropical distribution (Chiu 1948; Fig. 3j1–j3). Morphologically, S. pteroreticulosporus is similar to S. pinophilus and S. montosus due to their grey-black pileus and analogous basidiospores. However, a close phylogenetic relationship is not supported in our molecular analyses. Strobilomyces pinophilus differs from S. pteroreticulosporus by its small to medium-sized basidiomes (40–70 mm diam), pileus with grey-black more or less erect pyramidal scales and context with no oily secretion phenomenon (Fig. 3p, q1–q3). Strobilomyces montosus differs from S. pteroreticulosporus by its small to medium-sized basidiomes (30–70 mm diam), pileus with medium-sized erect conical scales (3–5 mm diam), and grey-black discolouration of the context on exposure (Berkeley 1851, Horak 1980, Pegler & Young 1981; Fig. 3n1–n2, q1–q3, 4o, r).
Strobilomyces seminudus Hongo, Trans. Mycol. Soc. Japan 23: 197. 1982 — MycoBank MB109255; Fig. 2, 6i1–i3, 8e1–e3
= Strobilomyces areolatus H.A. Wen & J.Z. Ying, Mycosystema 20: 297. 2001.
= Strobilomyces zangii Gelardi, Mycol. Progr. 12: 586. 2013.
≡ Heimiella nigricans M. Zang, Acta Bot. Yunnan. 7: 395. 1985. non Strobilomyces nigricans Berk., Hooker’s J. Bot. Kew Gard. Misc. 4: 139. 1852.
≡ Heimioporus nigricans (M. Zang) E. Horak, Sydowia 56: 238. 2004.
Habitat & Distribution — Solitary or scattered on soil in forests dominated by Pinaceae and Fagaceae; currently known from Japan, southern China and northern Thailand.
Specimens examined. China, Yunnan Province, Baoshan City, Zhonghegongshe, 3 Aug. 1977, X.J. Li 384 (HKAS 3224, holotype of Heimiella nigricans); same location and date, X.J. Li 389 (HKAS 3228); Yunnan Province, Tengchong City, Guangpojiao Village, 1900 m elev., 20 July 2009, Y.C. Li 1714 (HKAS 59461); Yunnan Province, Wenshan City, Qiubei County, Qingping Village, 1557 m elev., 9 Aug. 2014, L.H. Han 555 (HKAS 84915); Guizhou Province, Guiyang city, Huaxi, 1100 m elev., 8 July 1988, J.Z. Ying, Y.C. Zong & Y. Li 319 (HMAS 73379, holotype of S. areolatus); Jiangxi Province, Fuzhou City, Shuyuan Village, 40 m elev., 19 June 2012, G. Wu 913 (HKAS 77085); Sichuan Province, Luzhou City, Hutou Town, 500 m elev., 16 June 2014, Q. Cai 1009 (HKAS 83471). – Japan, Shiga Prefecture, Kokubo, Otsu, 3 Sept. 1973 (TNS F-174779, holotype); Kyoto Prefecture, Mizorogaike, 13 Sept. 2001, H. Sato (MAK s11); Shiga Prefecture, Nagara, Otsu, 22 Sept. 2005, H. Sato (MAK s406, MAK s411); Osaka Prefecture, Mino, 14 July 2006, H. Sato (MAK s495, MAK s500, MAK s508); Miyazaki Prefecture, Takakaracho, Nishimorokatagun, 9 July 2004, H. Sato (MAK s243); Kagoshima Prefecture, Yakushima, 28 June 2003, H. Sato (MAK s107); Kyoto Prefecture, Yoshida Hill, H. Sato (MAK s428). – Thailand, Chiang Mai Province, Doi Suthep National Park, 1150 m elev., 13 June 2006, R.E. Halling 8818 (NY 1393550).
Notes — Strobilomyces seminudus is characterized by its small to medium-sized basidiomes (30–90 mm diam), pileus with grey-black, small, patch-like to appressed scales or floss (1–3 mm diam at base), large hymenophoral pores (1–2 mm diam), stipe with an annular zone becoming distinctly thickened near the apex and whitish reticulum beset by grey to dirty white fluffy floss above and grey-black floccose-squamulose below, context becoming orange-red on exposure and small semireticulate basidiospores with irregular echinate and subcristate ornamentation (Hongo 1982, Sato et al. 2011; holotype of S. seminudus: 7–9 × 6.5–8.5 μm, Q = 1.07–1.31, Qm = 1.16 ± 0.07; Fig. 6i1–i3, 8e1–e3). It somewhat resembles S. densisquamosus, S. giganteus and S. sp.11 in having pileus with grey-black to black scales, annular zone at the apex of the stipe and incomplete reticulate basidiospores (Hongo 1982, Zang 1985; Fig. 3s1–s2, 4t1–t2, 6d, g1–g3, 8b1–b2, d1–d2). However, S. densisquamosus differs from S. seminudus by its small hymenophoral pores (0.5–1 mm diam), larger echinate basidiospores (8.5–10.5 × 7–9 μm) with irregular short ribs; S. giganteus differs by the larger basidiomes (> 100 mm diam), pileus with erect conical scales, smaller hymenophoral pores (0.5–1 mm diam), and larger semireticulate basidiospores (8–10 × 7–8.5 μm); finally, S. sp.11 has appressed scales, smaller hymenophoral pores (0.5–1 mm diam), reticulate basidiospores and association with plants of Myrtaceae (Fig. 3s1–s2, 4t1–t2, 6d, g1–g3, 8b1–b2, d1–d2).
Strobilomyces areolatus and S. zangii, described from southwestern China, are morphologically identical with S. seminudus according to the original description and our re-examination of the relevant type specimens. The size of semireticulate basidiospores (7–9 × 6.5–8 μm) are consistent with those of S. seminudus (Fig. 6i1–i2). Fortunately, we successfully extracted DNA from the holotype material of S. areolatus. In the single-gene (ITS) analysis (Table S1, Fig. S6), this species is clustered together with S. seminudus. In addition, we have collected several specimens of S. seminudus from the type locality of S. zangii. Therefore, we treat S. areolatus and S. zangii as synonyms of S. seminudus.
Strobilomyces strobilaceus (Scop.) Berk., Hooker’s J. Bot. Kew Gard. Misc. 3: 78. 1851 — MycoBank MB238002; Fig. 2, 3r1–r2, 4s1–s3
Basionym. Boletus strobilaceus Scop., Annus Hist.-Nat. 4: 148. 1770.
= Boletus strobiliformis Dicks., Fasc. Pl. Crypt. Brit. 1: 17. 1785.
= Boletus floccopus Vahl, Fl. Dan. 8: t. 1252. 1797.
≡ Strobilomyces floccopus (Vahl) P. Karsten, Bidrag Kannedom Finlands Natur Folk 3 37: 16. 1882.
Habitat & Distribution — Solitary or scattered on soil in forests dominated by Fagaceae or in mixed forests of Fagaceae and Pinaceae; currently known from Europe, East Asia and North/Central America.
Specimens examined. Austria, Niederösterreich, Hainfeld, Sonnleiten, Balsenhöhe, 10 Aug. 1991, W.S. Klofac (WU 10211); Niederösterreich, Porrau, Sandleiten, 11 Aug. 1991, A. Hausknecht (WU 10209); Steiermark, Wildon, Buchberg, 18 Sept. 1996, A. Hausknecht (WU 16537). – China, Hubei Province, Yichang City, Muyu Town, Shennongjia Village, 9 July 2012, 1500 m elev., Q. Cai 711 (HKAS 75466); Yunnan Province, Chuxiong City, Dazhong Mountain Nature Reserve, 24 Aug. 2015, 2200 m elev., J.W. Liu 384 (HKAS 95079). – England, H. Sato (MAK s223); same location and collector (MAK s229). – Denmark, Falster, H. Sato (MAK s228). – Germany, M. Weiss (MB 001177). – Mexico, Federal State Nayarit, 6 June 1996, I. Krisai-Greilhuber & H. Voglmayr (WU 17111). – USA, New Jersey, H. Sato (MAK s224); same location and collector (MAK s227).
Notes — Strobilomyces strobilaceus is characterized by its medium-sized to large basidiomes (60–120 mm diam), pileus with black-brown to grey-black, large, more or less erect conical to pyramidal scales (European materials) or patch-like to appressed scales (Asian materials) (3–5 mm high, 5–12 mm diam at base), stipe with grey-black to black thick floss evenly distributed and a cottony annulus at the apex, large hymenophoral pores (1–1.5 mm diam), rusty red discolouration of the context on exposure, medium-sized reticulate basidiospores (9–11 × 8–9.5 μm, Q = 1.22–1.33, Qm = 1.26 ± 0.07; Fig. 4s1–s3) with small meshes (1–2 μm high, 1–2 μm diam at base) and wider basidia (> 17 μm) (Berkeley 1851, Petersen et al. 2012; Fig. 3r1–r2).
The concept of S. strobilaceus was taxonomically not clarified yet due to the absence of original type material. Petersen et al. (2012) designated a neotype specimen (SAV 3214) collected in Slovakia and suggested that only one species occurs in Europe. In the S. strobilaceus subclade, there are two strongly supported lineages: one consisted of seven collections from Europe and East Asia, and the other lineage had four collections from North/Central America and East Asia. We observed no obvious morphological differences among these samples except for the variable shape of pileal scales. Based on the criteria of the GCPSR method applied in this study, the S. strobilaceus subclade is recognized here as a single phylogenetic species. Future analyses could possibly compromise the monophyly of the two lineages by the addition of more samples.
Morphologically, S. strobilaceus is similar to S. glabriceps or S. parvirimosus in their pileal scales. However, S. glabriceps differs by its stipe with grey to dirty white and grey-black thin fluffy floss on the upper and lower part, grey-black discolouration of the context on exposure, larger meshes (2–4 μm diam) of the basidiospores and subtropical distribution (Chiu 1948; Fig. 3j1–j3, 4j1–j2). Strobilomyces parvirimosus differs from S. strobilaceus by its small to medium-sized basidiomes (30–70 mm diam), pileus with black-brown medium-sized scales (3–5 mm diam), stipe with black-brown thin floss and without annulus, smaller hymenophoral pores (0.5–1 mm diam) and smaller basidiospores (8–10 × 6.5–8 μm) (Ying 1986; Fig. 3o, 4p1–p2).
Strobilomyces subnudus J.Z. Ying, Acta Mycol. Sin. 4: 99. 1985 — MycoBank MB104811; Fig. 2, 6j, 8f1–f2
Habitat & Distribution — Solitary or scattered on soil in forests dominated by Pinaceae and Fagaceae; currently recorded from subtropical China.
Specimens examined. China, Jiangsu Province, Nanjing City, 14 Sept. 1961, X.J. Liu 351 (HMAS 32706, holotype); same location, 6 June 1936, H.N. Shen 351 (HMAS 7670); Taiwan Province, Nantou County, Meifeng, 2350 m elev., 15 Sept. 2012, B. Feng 1276 (HKAS 82418); Yunnan Province, Baoshan City, Daxue Mountain, 2500 m elev., 31 July 2014, J. Qin 987 (HKAS 83404); Yunnan Province, Wenshan City, Qiubei County, Donggua Village, 1150 m elev., 5 Aug. 2014, L.H. Han 515 (HKAS 84811).
Notes — Strobilomyces subnudus is characterized by its small to medium-sized basidiomes (30–80 mm diam), pileus with grey-black, small, patch-like to appressed scales or floss (1–3 mm diam at base), appendiculate thin veil remnants along the margin, stipe with grey-white and grey-black thin fluffy floss at upper and lower halves, small hymenophoral pores (0.5–1 mm), rusty red discolouration of the context on exposure and small to medium-sized semireticulate basidiospores with confluent tubercles and subcristate (Ying & Ma 1985; holotype of S. subnudus: 8–10 × 7.5–8.5 μm, Q = 1.06–1.3, Qm = 1.17 ± 0.05; Fig. 6j, 8f1–f2).
Phylogenetically, S. subnudus is closely related to S. hongoi (Fig. 1). However, S. hongoi differs from S. subnudus by its pileus with larger scales or patches (3–8 mm diam), stipe with coarsely reticulum on its upper half and with fluffy floss on its lower half, and smaller basidiospores (7–9 × 6.5–8 μm) (Ying & Ma 1985, Sato et al. 2011; Fig. 6h, j, 8f1–f2). Morphologically, it is very similar to S. seminudus in sharing grey-black pileus with small appressed pyramidal scales (1–3 mm high, 2–3 mm diam at base), semireticulate basidiospores with confluent tubercles and subcristate ornamentation, and stipe with grey-white fluffy floss above and grey-black floccose-squamulose below. However, S. seminudus differs from S. subnudus by its smaller basidiospores (7–9 × 6.5–8.5 μm), stipe with an annular zone at the apex, larger hymenophoral pores (1–2 mm diam), orange-red discolouration of the context on exposure and tropical to subtropical distribution (Hongo 1982, Ying & Ma 1985, Sato et al. 2011; Fig. 6i1–i3, j, 8e1–e3, f1–f2).
Strobilomyces velutinus J.Z. Ying, Acta Mycol. Sin. 4: 100. 1985 — MycoBank MB104812; Fig. 6k, 8g1–g2
Habitat & Distribution — Solitary or scattered on soil in forests dominated by Fagaceae; currently recorded from subtropical China and Japan.
Specimens examined. China, Yunnan Province, Wenshan City, Guangnan County, 15 June 1959, Q.Z. Wang 811 (HMAS 45911, holotype); Wenshan City, Guangnan County, Muyi Reservoir, 1617 m elev., 31 July 2014, L.H. Han 459 (HKAS 82418); Wenshan City, Malipo County, Xinhe Village, 1398 m elev., 2 Aug. 2014, L.H. Han 480 (HKAS 84776). – Japan, Kyoto Prefecture, Kyoto-shi, Higashiyama-ku, H. Sato (MAK s120); Kyoto Prefecture, Kyoto-shi, Sakyo-ku, Mt Yoshida, H. Sato (MAK s399); same location and collector (MAK s381); Kyoto Prefecture, Kyoto-shi, Sakyo-ku, Takaragaike, H. Sato (MAK s370); Osaka Prefecture, Mino-shi, H. Sato (MAK s170); Shiga Prefecture, Otsu-shi, Mt Tanakami, H. Sato (MAK s405); Shiga Prefecture, Otsu-shi, Miidera-cho, H. Sato (MAK s421).
Notes — Strobilomyces velutinus is characterized by its small basidiomes (30–60 mm diam), pileus with grey-black, small, scattered, more or less erect pyramidal scales and velvety rimose-areolate patches to appressed scales (1–3 mm high, 1–3 mm diam at base), small hymenophoral pores (0.5–1 mm diam), stipe with black scattered granular scales and medium-sized semireticulate basidiospores (Ying & Ma 1985; holotype of S. velutinus: 9–11 × 7–9 μm, Q = 1.12–1.3, Qm = 1.21 ± 0.08; Fig. 6k, 8g1–g2). Strobilomyces velutinus, originally described from southwestern China, S. confusus from North America and S. sp. 15 of Costa Rica are closely related. However, S. confusus differs from S. velutinus by its pileus with thinner and more acute scales, stipe with an annulus and smaller basidiospores (8.5–10 × 7–8 μm) (Singer 1945, Ying & Ma 1985; Fig. 6c1–c2, k). A workable concept of S. sp. 15 needs to be formed with more samples. Morphologically, S. velutinus is similar to S. parvirimosus and S. pinophilus regarding the pileus with more or less pyramidal scales and small hymenophoral pores (0.5–1 mm diam) (Ying & Ma 1985; Fig. 3o–p, 4p1–p2, q, 6k, 8g1–g2). However, S. parvirimosus and S. pinophilus differs from S. velutinus by their reticulate basidiospores.
KEY TO THE SPECIES OF STROBILOMYCES IN ASIA
1. Pileus with black-brown, red-brown or golden-tawny scales. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
1. Pileus with black, grey-black, grey or dirty white scales 16
2. Pileus with black-brown or red-brown scales; basidiospores larger, more than 7 μm in length . . . . . . . . . . . . . . . . . . . 3
2. Pileus with golden-tawny to golden-orange scales; basidiospores smaller, 6–8 × 5.5–7 μm . . . . . . . . . S. mirandus
3. Pileus with red-brown scales . . . . . . . . . . . . . . . . . . . . . 4
3. Pileus with black-brown scales . . . . . . . . . . . . . . . . . . . . 6
4. Stipe with red-brown, vinaceous brown, dark brown to black-brown thick floss; hymenophoral pores larger, 1–3 mm diam; tropical to subtropical . . . . . . . . . . . . . . . . . . . 5
4. Stipe with dirty white to light red-brown thin floss; hymenophoral pores smaller, 0.5–1 mm diam; subtropical . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. glabellus
5. Pileus with red-brown, scattered, erect conical scales; stipe with concolorous fluffy floss and conical scales; context changing to grey-black on exposure . . . . . . . . . . . . . . . . . . . . . . . S. brunneolepidotus
5. Pileus with red-brown to vinaceous brown (lower part) to dark brown to black-brown (upper part), crowded, more or less erect pyramidal scales; stipe with dark brown to black-brown fluffy floss; context changing to rusty red on exposure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. atrosquamosus
6. Pileus with more or less erect conical to pyramidal scales . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
6. Pileus with patch-like to appressed scales or floccose floss . . . . . . . . . . . . . 14
7. Basidiospores with complete reticulum . . . . . . . . . . . 8
7. Basidiospores with incomplete reticulum . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
8. Pileus with thick and crowded scales; stipe with black-brown to grey-black floss; context changing to rusty red on exposure . . . . . . . . . . . . . . . . . . . . . . . 9
8. Pileus with thin and scattered scales; stipe with dark black-brown to black floss; context changing to grey-black on exposure. . . . . . . . . . . . . . . . . . . . . . . S. echinocephalus
9. Pileal scales with purple tint; meshes of basidiospores larger, 2–3.5 μm diam; pores larger, 1–2 mm diam . . . 10
9. Pileal scales without purple tint; meshes of basidiospores smaller, 1–2 μm diam; pores smaller, 0.5–1 mm diam 11
10. Basidiospores larger, 9.5–12 × 7.5–9.5 μm; associated with Abies spp.; mainly in subalpine areas in northern India . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. nigricans
10. Basidiospores smaller, 7.5–9.5 × 6.5–8 μm; associated with Fagaceae; mainly in tropical areas of Southeast Asia . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. mollis
11. Pileus with dirty white to light black-brown scales on periphery; stipe with an annulus, with grey to dirty white (upper part) and grey-black floss (lower part); basidiospores larger, 9–11 × 7–8 μm. . . . . . . . . . . . . . . . . . S. microreticulatus
11. Pileus with black-brown scales on periphery; stipe without an annulus, with black-brown floss; basidiospores smaller, 8–10 × 6.5–8 μm . . . . . . . . . . . . . . . . . . S. parvirimosus
12. Hymenophoral pores larger, 1–2 mm diam; basidiospores larger, 9–11.5 × 7–10 μm, with echinate-subreticulate ornamentation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13
12. Hymenophoral pores smaller, 0.5–1 mm diam; basidiospores smaller, 8–10 × 6–8 μm, with isolated flat-roofed cones . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. foveatus
13. Pileal scales with vinaceous tint; stipe with an annulus . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. annulatus
13. Pileal scales without vinaceous tint; stipe without an annulus. . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. polypyramis
14. Hymenophoral pores smaller, 0.5–1 mm diam; basidiospores smaller, less than 11 μm in length; associated with Fagaceae; distributed in other areas. . . . . . . . . . . . . . 15
14. Hymenophoral pores larger, 1–2 mm diam; basidiospores larger, 11–14 × 9–11 μm; associated with Abies spp.; mainly in subalpine areas of southwestern China . . . .S. alpinus
15. Stipe without an annulus, with dirty white thin floss evenly distributed; basidiospores smaller, 7–9 × 6–7 μm, meshes larger, 2–3 μm diam; context changing to rusty red on exposure; tropical . . . . . . . . . . . . . . . . . . . . . . . . . S. albidus
15. Stipe with an annulus, with grey to dirty white (upper part) and dark black-brown (lower part) thick floss arranged in spiral; basidiospores larger, 9–11 × 7–8.5 μm, meshes smaller, 1–2 μm diam; context changing to grey-black on exposure; subtropical to temperate. . . . . . S. cingulatus
16. Pileus with black scales . . . . . . . . . . . . . . . . . . . . . . . . 17
16. Pileus with grey-black, grey to dirty white scales . . . . 20
17. Basidiospores with complete reticulum . . . . . . . . . . . . 18
17. Basidiospores with incomplete reticulum . . . . . . . . . . . 19
18. Basidiomes larger, 50–97 mm diam; pileus with black, larger, more or less erect pyramidal scales 5–10 mm diam; stipe with thick floss arranged in spiral . . . . . S. douformis
18. Basidiomes smaller, 25–50 mm diam; pileus with charcoal black, smaller, more or less erect pyramidal scales 3–5 mm diam; stipe with thin floss evenly distributed . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. anthracinus
19. Pileus with hard, erect conical scales; stipe with an annular zone at apex, with conical scales to fluffy floss; basidiospores with semireticulate ornamentation; tropical to subtropical. . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. giganteus
19. Pileus with soft, more or less erect conical to pyramidal scales; stipe without an annular zone, with granular scales; echinate basidiospores with confluent tubercles and irregular incomplete reticulations; tropical. . . . . S. calidus
20. Pileus with more or less erect conical to pyramidal scales . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
20. Pileus with patch-like to appressed scales or floss . . . 26
21. Basidiospores with complete reticulum. . . . . . . . . . . . 22
21. Basidiospores with incomplete reticulum . . . . . . . . . . . 25
22. Pileus with small to large scales, 1–12 mm diam; stipe with an annulus; context changing to rusty red on exposure; subtropical to temperate . . . . . . . . . . . . . . . . . . . . . . . . 23
22. Pileus with medium-sized, erect conical scales 3–5 mm diam; stipe without an annulus; context changing to grey-black on exposure; tropical to subtropical. . . S. montosus
23. Pileus with smaller, erect conical or more or less erect pyramidal scales 1–4 mm diam; stipe with grey to dirty white (upper part) and grey-black (lower part) floss arranged in spiral; meshes of basidiospores larger, 2–4 μm diam; temperate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
23. Pileus with larger, more or less erect conical to pyramidal scales (European materials) or patch-like to appressed scales (Asian materials) 5–12 mm diam; stipe with grey-black to black floss evenly distributed; meshes of basidiospores smaller, 1–2 μm diam; subtropical to temperate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. strobilaceus
24. Basidiomes larger, 70–110 mm diam; pileus with grey-black at top and whitish at base, erect conical scales, ground whitish; hymenophoral pores larger, 1–2 mm diam. . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . S. pteroreticulosporus
24. Basidiomes smaller, 40–70 mm diam; pileus with grey-black, more or less erect pyramidal scales, ground dirty white to grey; hymenophoral pores smaller, 0.5–1 mm diam . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. pinophilus
25. Basidiomes larger, 60–120 mm diam; stipe with grey-white (upper part) and black (lower part) floss; echinate basidiospores with irregular short ribs; context changing to orange-red on exposure; associated with Fagaceae and Pinaceae; subtropical to temperate . S. densisquamosus
25. Basidiomes smaller, 30–60 mm diam; stipe with black granular scales; basidiospores with semireticulate ornamentation; context changing to rusty red on exposure; associated with Fagaceae; subtropical. . . . . . . S. velutinus
26. Basidiospores with complete reticulum . . . . . . . . . . . . 27
26. Basidiospores with incomplete reticulum . . . . . . . . . . . 28
27. Stipe without an annulus, with whitish floss; basidiospores smaller, 7–9 × 6–7 μm, meshes smaller, 1–2 μm diam; tropical to subtropical . . . . . . . . . . . . . . . . . S. latirimosus
27. Stipe with an annulus, with grey to dirty white (upper part) and grey-black (lower part) floss; basidiospores larger, 9–11 × 7.5–9.5 μm, meshes larger, 2–4 μm diam; subtropical . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. glabriceps
28. Stipe without an annular zone; hymenophoral pores smaller, 0.5–1 mm diam; context changing to rusty red on exposure; subtropical. . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
28. Stipe with an annular zone; hymenophoral pores larger, 1–2 mm diam; context changing to orange-red on exposure; tropical to subtropical . . . . . . . . . . . . . . . . . S. seminudus
29. Pileus with larger scales 3–8 mm diam; stipe with coarsely reticulate elongate meshes (upper part) and grey-black (lower part) floss; basidiospores smaller, 7–9 × 6–7 μm. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. hongoi
29. Pileus with smaller scales 1–3 mm diam; stipe with grey to dirty white (upper part) and grey-black (lower part) floss; basidiospores larger, 8–10 × 7.5–8.5 μm. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . S. subnudus
DISCUSSION
Phylogeny and infrageneric treatment of Strobilomyces
We provide here a robust phylogeny based on type and voucher collections of Strobilomyces from several continents. Our analyses identified that the examined species belong to four clades (Fig. 1). Clade I is basal to the remaining three clades. Morphologically, clade I is characterized by basidiomes with subconical to conical pileus and echinate basidiospores. Geographically, this clade is restricted to Africa, in putative association with Fabaceae (Beeli 1926, Singer 1986, Han et al. 2018). The remaining three clades (clades II, III and IV) form a statistically strongly supported group, circumscribed by taxa which share a hemispherical to applanate pileus, and basidiospores with reticulate, semireticulate or flat-roofed conical ornamentation, and they are widely distributed on several continents in association with plants of other families (Cooke 1889, Singer 1945, Corner 1972, Horak 1980, 2011, Petersen et al. 2012, Sato et al. 2017, Han et al. 2018, this study). Based on the above-mentioned evidences, two sections, namely, sect. Echinati and sect. Strobilomyces are erected to accommodate the observed differences and divergence (Fig. 1). Clades II, III and IV in sect. Strobilomyces roughly correspond to clades /annulatus, A and B in Sato et al. (2007, 2017), respectively.
Delimitation and geographical distribution patterns of Strobilomyces species
Our data revealed that two unique characters, the ornamentation of basidiospores and size of scales on the pileus, could help distinguish clades I, II, and III from clade IV. Specifically, species in clades I, II and III possess non-reticulate basidiospores and smaller scales on the pileus compared with clade IV (Fig. 2). Of the 33 phylogenetic species recognized from Asia based on the phylogenetic analyses, 26 phylogenetic species are represented by two or more collections satisfying the GCPSR criteria of Dettman et al. (2003) (Table 2). Two single collections delimitated as S. glabellus and S. mollis, respectively, were also included in our multi-gene phylogeny, while S. nigricans and S. polypyramis were not encompassed in the phylogenetic analyses due to paucity of materials. All the phylogenetic species correspond well with their morphological features (Fig. 2). The additional five Asian phylogenetic species (S. sp.1, S. sp.3, S. sp.6, S. sp.7 and S. sp.10) either require additional specimens for precise delimitation or will be described in future treatments.
Our data revealed that three microscopic and thirteen macroscopic/ecological characters are useful to delimitate Strobilomyces species, even though some of these features are difficult to score as a single state for a species. The three microscopic characters, i.e., the size, ornamentation and mesh size of the basidiospores, have taxonomic values in recognizing species. Our results suggest that the majority species from Asia have small to medium-sized basidiospores (8–11 × 7.5–9 μm). However, S. mirandus (6–8 × 5.5–7 μm) and S. alpinus (11–14 × 9–11 μm) have the smallest and the largest basidiospores in the genus (Corner 1972, Zang 1985). As for morphologically similar species with analogous basidiospore size, the size of the meshes provides an important criterion for distinguishing them. On the contrary, the structure of the pileipellis, form and size of the basidia and pleuro- and cheilocystidia, provide little taxonomic information.
The thirteen macroscopic characters, namely, the size and shape of the pileus, colour, size and morphology of the scales on the pileus and stipe, pore size of the tubes, colour changes of the exposed context, presence or absence of an annulus or an annular zone, association with host plants and geographical distributions, are quite valuable for recognition of Strobilomyces species in the field. In our study, the majority of Strobilomyces species in the world have small to medium-sized basidiomes (Fig. 2). As for the colour of basidiomes, S. mirandus is the only species with a golden-tawny pileus. Three species, namely, S. atrosquamosus, S. brunneolepidotus and S. glabellus, share pileal scales with red-brown tint, while S. alpinus and S. mollis possess a black-brown pileus with vinaceous tint. The remaining species with reticulate basidiospores, and all the species with non-reticulate basidiospores, show varying degrees of grey to blackish or black-brown scales on the pileus (Fig. 2). Although the shape of the scales on the pileus may be deformed in the development of the basidiomes, it is always stable when mature and thus could be used to delimitate species. Most species have a pileus with erect conical or more or less pyramidal scales, while nine species exhibit a pileus with patch-like to appressed scales or floss owing to their ontogenesis (Fig. 2). As for the stipe, S. densisquamosus, S. giganteus and S. seminudus possess a distinct annular zone at the apex. Nine species, i.e., S. albidus, S. cingulatus, S. microreticulatus, S. densisquamosus, S. glabriceps, S. latirimosus, S. pinophilus, S. seminudus and S. subnudus have whitish floss on the upper part or over the whole surface of the stipe, while other species have a stipe covered with blackish, black-brown or golden-tawny floss. In this study, most species from Asia show reddish discolouration of the context immediately on exposure, whereas only six species (S. brunneolepidotus, S. cingulatus, S. echinocephalus, S. glabriceps, S. latirimosus and S. montosus) exhibit blackish discolouration directly. However, we would like to note that minor variations of these characters may be observed among individuals within each species due to differences in developmental stages of the basidiomes and their environmental conditions during fruiting.
Based on the currently available data about the geographic distribution of Strobilomyces, the phylogenetic species identified in Asia constitute about 67 % (i.e., 33/49) of the global lineages in Strobilomyces. Seven species (S. albidus, S. annulatus, S. calidus, S. foveatus, S. mirandus, S. mollis and S. polypyramis) are restricted to tropical habitats and two species (S. pinophilus and S. pteroreticulosporus) are restricted to temperate environments in Asia. Strobilomyces cingulatus, S. densisquamosus and S. strobilaceus are distributed widely across subtropical and temperate parts of China. Strobilomyces alpinus, S. longistipitatus and S. nigricans all occur in the subalpine regions and are particularly associated with Abies. Harbouring at least 21 species, the region of the Mountains of Southwest China, a biodiversity hotspot, is probably a centre of diversification of Strobilomyces.
Plant associates and their significance in the evolution of Strobilomyces
Results from ancestral state reconstruction analyses showed that plant associates of the basal group in Strobilomyces appear to be Detarioideae/Phyllanthaceae/Monotoideae in Africa. The associations subsequently switched to Myrtaceae/Casuarinaceae/Caesalpinioideae and Fagaceae/Pinaceae in Australia and Asia (Han et al. 2018). The evolution of Strobilomyces species associated with Fagaceae/Pinaceae likely triggered a relatively recent and rapid radiation, possibly facilitated by habitat contraction and drastic climatic fluctuations in the Oligocene and Miocene, and then followed by expansion during the Miocene thermal optimum (Han et al. 2018). In addition, host-shift events with respect to Fagaceae/Pinaceae might have provided ecological opportunities for this rapid diversification (Sato et al. 2017).
Our field observations and field notes of herbarium collections indicates that most Strobilomyces species in China are putatively associated with trees of either Fagaceae or Pinaceae, and explicit molecular evidences of host plants in nine Strobilomyces species are known (Sato et al. 2007). Strobilomyces alpinus/S. nigricans/S. longistipitatus, and S. pteroreticulosporus/S. pinophilus are apparently endemic to subalpine and temperate Asia, and specific to Abies and Pinus, respectively. We speculate that these five species might have diversified due to host shift and then spread to other regions with the dispersal of other host plants. More information about plant associates and host specificity of Strobilomyces is vital to understand the dispersal and speciation patterns in this genus.
Acknowledgements
The authors are very grateful to the herbaria of FH, FRIM, HMAS, HMJAU, KUN, MAK, MEL, NY, PC and WU, the Ailaoshan Station for Subtropical Forest Ecosystem Studies in Yunnan, and Drs. Fang Li, Tie-Zheng Wei, Michael Weiss, Tolgor Bau, Nourou S. Yorou, Nian-Kai Zeng, Xiao-Dan Yu, Zai-Wei Ge, Li-Ping Tang, Yan-Chun Li, Bang Feng, Qing Cai, Jiao Qin, Yan-Jia Hao, Ting Guo, Kuan Zhao, Xi-Hui Du and Ms. Yang-Yang Cui, Jing Li, Mr. Xiao-Bin Liu, Jian-Wei Liu, Pan-Meng Wang, for providing specimens and images for this study. The authors thank Dr. Yan-Hui Zhao and Mr. Zhi-Jia Gu for helping scan basidiospores. Thanks are also given to the anonymous reviewers for their valuable comments and suggestions. Financial support was provided by the Strategic Priority Research Program of Chinese Academy of Sciences (grant no. XDB31000000), the International Partnership Program of Chinese Academy of Sciences (grant no. 151853KYSB20170026), the Yunling Scholar and the Youth Top-Notch Talent Funds of the Ten Thousand Talents Program of Yunnan Provincial Government. L.H. Han thanks the National Nature Science Foundation of China (grant no. 31860005). Gang Wu thanks the Science and Technology Research Program of Kunming Institute of Botany, the Chinese Academy of Sciences (KIB2017002), and the Youth Innovation Promotion Association, Chinese Academy of Sciences (grant no. 2017436). R.E. Halling thanks the National Science Foundation (USA) for funding under grants DEB-9972018, DEB-9300798, DEB-1020421 and DEB-0414665, and the National Geographic Society Committee for Research and Exploration in grants 7341-02 and 8457-08.
Supplementary material
Taxa used in the molecular phylogenetic study of ITS dataset, with voucher information and GenBank accession numbers. Sequences generated in this study are in bold.
Phylogenetic tree of Strobilomyces generated from a four-locus dataset (RPB1-RPB2-TEF1-COX3) using BI analysis. Bayesian posterior probabilities (BPP > 0.95) are indicated by numbers above branches.
Phylogenetic tree of Strobilomyces inferred from ML analysis with branch support obtained by ML and BI analyses based on RPB1 sequences. Branch support values are indicated by numbers above branches (MLB/BPP). – represent MLB < 70 % or BPP < 0.95.
Phylogenetic tree of Strobilomyces inferred from ML analysis with branch support obtained by ML and BI analyses based on RPB2 sequences. Branch support values are indicated by numbers above branches (MLB/BPP). – represent MLB < 70 % or BPP < 0.95.
Phylogenetic tree of Strobilomyces inferred from ML analysis with branch support obtained by ML and BI analyses based on TEF1 sequences. Branch support values are indicated by numbers above branches (MLB/BPP). – represent MLB < 70 % or BPP < 0.95.
Phylogenetic tree of Strobilomyces inferred from ML analysis with branch support obtained by ML and BI analyses based on COX3 sequences. Branch support values are indicated by numbers above branches (MLB/BPP). – represents MLB < 70 % or BPP < 0.95.
Phylogenetic tree of Strobilomyces inferred from ML analysis with branch support obtained by ML and BI analyses based on ITS sequences. – represents MLB < 70 % or BPP < 0.95.
REFERENCES
- Antonín V, Vizzini A, Ercole E, et al. 2015. Strobilomyces pteroreticulosporus (Boletales), a new species of the S. strobilaceus complex from the Republic of Korea and remarks on the variability of S. confusus. Phytotaxa 219: 78–86. [Google Scholar]
- Bas C. 1969. Morphology and subdivision of Amanita and a monograph of its section Lepidella. Persoonia 5: 285–573. [Google Scholar]
- Beeli M. 1926. Contribution nouvelle á l’étude de la flore mycologique du Congo. Bulletin de la Société Royale de Botanique de Belgique 58: 203–215. [Google Scholar]
- Berkeley MJ. 1851. Decades of fungi. XXXVI. Sikkim-Himalayan fungi collected by Dr. Hooker. Hooker’s Journal of Botany and Kew Garden Miscellany 3: 77–84. [Google Scholar]
- Berkeley MJ. 1852. Decades of fungi. XXXIXI-XL. Sikkim and Indian fungi. Hooker’s Journal of Botany and Kew Garden Miscellany 4: 130–142. [Google Scholar]
- Cai Q, Cui YY, Yang ZL. 2016. Lethal Amanita species in China. Mycologia 108: 993–1009. [DOI] [PubMed] [Google Scholar]
- Chiu WF. 1948. The boletes of Yunnan. Mycologia 40: 199–231. [Google Scholar]
- Cooke MC. 1889. New Australian fungi. Grevillea 18: 1–8. [Google Scholar]
- Corner EJH. 1972. Boletus in Malaysia. Singapore, Botanic Gardens. [Google Scholar]
- Dettman JR, Jacobson DJ, Taylor JW. 2003. A multilocus genealogical approach to phylogenetic species recognition in the model eukaryote Neurospora. Evolution 57: 2703–2720. [DOI] [PubMed] [Google Scholar]
- Ge ZW, Yang ZL. 2005. Strobilomyces mirandus, a species new to China. Mycosystema 24: 143–144. [Google Scholar]
- Gelardi M, Vizzini A, Ercole E, et al. 2013. Strobilomyces echinocephalus sp. nov. (Boletales) from south-western China, and a key to the genus Strobilomyces worldwide. Mycological Progress 12: 575–588. [Google Scholar]
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analyses program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Han LH, Buyck B, Yorou NS, et al. 2017. Afroboletus sequestratus (Boletales), the first species with sequestrate basidioma in the genus. Phytotaxa 305: 11–20. [Google Scholar]
- Han LH, Feng B, Wu G, et al. 2018. African origin and global distribution patterns: Evidence inferred from phylogenetic and biogeographical analyses of ectomycorrhizal fungal genus Strobilomyces. Journal of Biogeography, 45: 201–212. [Google Scholar]
- Hongo T. 1982. Materials for the fungus flora of Japan (32). Transactions of the Mycological Society of Japan 23: 195–200. [Google Scholar]
- Horak E. 1980. Indian Boletales and Agaricales. Revision and new taxa. Sydowia 33: 88–110. [Google Scholar]
- Horak E. 2011. Revision of Malaysian species of Boletales s.l. (Basidiomycota) described by E.J.H. Corner (1972, 1974). Forest Research Institute, Malaysia. [Google Scholar]
- Huang NL. 2002. A new species of Strobilomyces from China. Mycosystema 21: 6–8. [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornerup A, Wanscher JH. 1981. Taschenlexikon der Farben. Germany, Muster-Schmidt Verlag. [Google Scholar]
- Kour H, Kumar S, Sharma YP. 2013. Two species of Strobilomyces from Jammu and Kashmir, India. Mycosphere 4: 1006–1013. [Google Scholar]
- Nuhn ME, Binder M, Taylor AF, et al. 2013. Phylogenetic overview of the Boletineae. Fungal Biology 117: 479–511. [DOI] [PubMed] [Google Scholar]
- Nylander JAA. 2004. MrModeltest v2. Program distributed by the author, Evolutionary Biology Centre, Uppsala University. [Google Scholar]
- Pegler DN, Young TWK. 1981. A natural arrangement of the Boletales, with reference to spore morphology. Transactions of the British Mycological Society 76: 103–146. [Google Scholar]
- Petersen RH, Hughes KW, Adamčík S, et al. 2012. Typification of three European species epithets attributable to Strobilomyces (Boletales). Czech Mycology 64: 141–163. [Google Scholar]
- Rambaut A, Drummond AJ. 2009. Tracer. Version 1.5. Available at http://beast.bio.ed.ac.uk/Tracer.
- Ronquist F, Teslenko M, Van der Mark P, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Hattori T, Kurogi S, et al. 2005. Strobilomyces mirandus Corner, a new record from Japan. Mycoscience 46: 102–105. [Google Scholar]
- Sato H, Hattori T, Lee SS, et al. 2011. Two species of Strobilomyces (Boletaceae, Boletales), S. seminudus and S. hongoi sp. nov. from Japan. Mycologia 103: 598–609. [DOI] [PubMed] [Google Scholar]
- Sato H, Murakami N. 2008. Reproductive isolation among cryptic species in the ectomycorrhizal genus Strobilomyces: population-level CAPS marker-based genetic analysis. Molecular Phylogenetics and Evolution 48: 326–334. [DOI] [PubMed] [Google Scholar]
- Sato H, Murakami N. 2009. Strobilomyces verruculosus sp. nov. from Japan. Mycoscience 50: 173–178. [Google Scholar]
- Sato H, Tanabe AS, Toju H. 2017. Host shifts enhance diversification of ectomycorrhizal fungi: diversification rate analysis of the ectomycorrhizal fungal genera Strobilomyces and Afroboletus with an 80-gene phylogeny. New Phytologist 214: 443–454. [DOI] [PubMed] [Google Scholar]
- Sato H, Yumoto T, Murakami N. 2007. Cryptic species and host specificity in the ectomycorrhizal genus Strobilomyces (Strobilomycetaceae). American Journal of Botany 94: 1630–1641. [DOI] [PubMed] [Google Scholar]
- Singer R. 1938. Sur les genres Ixocomus, Boletinus, Phylloporus, Gyrodon et Gomphidius. 2. Les Boletinus. Revue de Mycologie 3: 157–177. [Google Scholar]
- Singer R. 1945. The Boletoideae of Florida with notes on extralimital species. I. The Strobilomycetaceae. Farlowia 2: 97–141. [Google Scholar]
- Singer R. 1975. The Agaricales in modern taxonomy (3rd ed). Vaduz, Cramer Press. [Google Scholar]
- Singer R. 1986. The Agaricales in modern taxonomy. Koenigstein, Germany, Koeltz Scientific Books. [Google Scholar]
- Singer R, Garcia J, Gomez LD. 1992. The Boletineae of Mexico and Central America IV. Beihefte zur Nova Hedwigia 105: 1–62. [Google Scholar]
- Smith SA, Dunn CW. 2008. Phyutility: a phyloinformatics tool for trees, alignments and molecular data. Bioinformatics 24: 715–716. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Taylor JW, Jacobson DJ, Kroken S, et al. 2000. Phylogenetic species recognition and species concepts in fungi. Fungal Genetics and Biology 31: 21–32. [DOI] [PubMed] [Google Scholar]
- Terashima Y, Takahashi H, Taneyama Y. 2016. The fungal flora in southwestern Japan: Agarics and boletes. Tokyo, Tokai University Press. [Google Scholar]
- Tibpromma S, Hyde KD, Jeewon R, et al. 2017. Fungal diversity notes 491–602: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 83: 1–261. [Google Scholar]
- Watling R, Li TH. 1999. Australian boletes. A preliminary survey. Edinburgh, Royal Botanic Garden Edinburgh. [Google Scholar]
- Wen HA, Ying JZ. 2001. Supplementary notes on the genus Strobilomyces from China II. Mycosystema 20: 297–300. [Google Scholar]
- Wolfe CB., Jr 1980. Austroboletus and Tylopilus subg. Porphyrellus, with emphasis on North American taxa. Bibliotheca Mycologica 69: 1–148. [Google Scholar]
- Wu G, Feng B, Xu J, et al. 2014. Molecular phylogenetic analyses redefine seven major clades and reveal 22 new generic clades in the fungal family Boletaceae. Fungal Diversity 69: 93–115. [Google Scholar]
- Wu G, Li YC, Zhu XT, et al. 2016. One hundred noteworthy boletes from China. Fungal Diversity 81: 25–188. [Google Scholar]
- Ying JZ. 1986. Supplement notes on genus Strobilomyces from China. Acta Mycologica Sinica Suppl. I: 305–308. [Google Scholar]
- Ying JZ, Ma QM. 1985. New taxa and records of the genus Strobilomyces in China. Acta Mycologica Sinica 4: 95–102. [Google Scholar]
- Zang M. 1985. Notes on the Boletales from eastern Himalayas and adjacent of China. Acta Botanica Yunnanica 7: 383–401. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Taxa used in the molecular phylogenetic study of ITS dataset, with voucher information and GenBank accession numbers. Sequences generated in this study are in bold.
Phylogenetic tree of Strobilomyces generated from a four-locus dataset (RPB1-RPB2-TEF1-COX3) using BI analysis. Bayesian posterior probabilities (BPP > 0.95) are indicated by numbers above branches.
Phylogenetic tree of Strobilomyces inferred from ML analysis with branch support obtained by ML and BI analyses based on RPB1 sequences. Branch support values are indicated by numbers above branches (MLB/BPP). – represent MLB < 70 % or BPP < 0.95.
Phylogenetic tree of Strobilomyces inferred from ML analysis with branch support obtained by ML and BI analyses based on RPB2 sequences. Branch support values are indicated by numbers above branches (MLB/BPP). – represent MLB < 70 % or BPP < 0.95.
Phylogenetic tree of Strobilomyces inferred from ML analysis with branch support obtained by ML and BI analyses based on TEF1 sequences. Branch support values are indicated by numbers above branches (MLB/BPP). – represent MLB < 70 % or BPP < 0.95.
Phylogenetic tree of Strobilomyces inferred from ML analysis with branch support obtained by ML and BI analyses based on COX3 sequences. Branch support values are indicated by numbers above branches (MLB/BPP). – represents MLB < 70 % or BPP < 0.95.
Phylogenetic tree of Strobilomyces inferred from ML analysis with branch support obtained by ML and BI analyses based on ITS sequences. – represents MLB < 70 % or BPP < 0.95.